Abstract
Background
People with psoriasis are at a higher risk for having central neurological problems, according to previous studies; however, it is unclear if there is a genetic link between the risk of developing psoriasis and developing central neurological disorders. In this study, the possible link between genetically predisposed psoriasis and the risk of common central nervous system disorders was comprehensively investigated.
Methods
There was no overlap in the participant populations between the psoriasis and central neurological disorders genome-wide association studies, which provide the genetic resources. Inverse variance weighting, often used as Mendelian randomization (MR) analysis, is the main method. To guarantee the accuracy of our findings, a number of sensitivity studies were carried out.
Results
MR analysis revealed that although psoriasis was reported to increase the risk of Parkinson's disease (OR = 4.42, 95%CI[-3.81~6.79], P = 0.58) and epilepsy (OR = 4.71, 95%CI[-2.20~5.30], P = 0.42) in this study, they did not reach statistical significance. At the same time, this study did not observe that psoriasis would increase the risk of multiple sclerosis (OR = 0.01, 95%CI [-12.61~3.83], P = 0.30) and migraine (OR = 0.99, 95%CI [0.94~1.05], P = 0.78), they also did not reach statistical significance. Under all sensitivity assessments, the results remained stable.
Conclusions
Psoriasis does not appear to raise the risk of migraine, Parkinson's disease, multiple sclerosis, or epilepsy, according to our study.
Keywords: Psoriasis, Central neurological disorders, Mendel randomization, Causality, Genome-wide association study
1. Background
Central nervous system disorders encompass a spectrum of disorders ranging from modest neurological impairment with motor, sensory, visual, linguistic, or cognitive functions to comatose states and brain death [1]. Nearly one-sixth of the global population is currently affected by central neurological disorders [2]. Common central neurological disorders, such as multiple sclerosis and Parkinson's disease, impose a substantial economic burden on society [3,4]. After decades of research, central nervous system disorders continue to pose diagnostic and therapeutic difficulties, with limited diagnostic and therapeutic options [5]. Central nervous system disorders have complex etiologies that defy reduction to a single cause. Genetic, environmental, and immunological disorders may contribute to the development and progression of various disorders [6].
Well-defined, red, scaly patches are characteristic of psoriasis, a persistent inflammatory skin condition [7]. Psoriasis affects more than 60 million individuals around the world, with the greatest prevalence in Europe and the least common in East Asia [8]. Approximately 30 % of the heredity of psoriasis was explained by the results of the genome-wide association studies (GWASs), which found more than 80 susceptibility loci [9]. Some studies showed a positive family history in approximately 35 % of patients [10].
In recent years, the relationship between psoriasis and central nervous system disorders has received a growing amount of study. Psoriasis patients are more likely to exhibit central nervous system disorders than the general population [11,12]. Notably, it remains unknown whether the risk of central nervous system disorders is directly attributable to psoriasis. This is because the risk factors for disorders of the central nervous system are also probable risk factors for psoriasis. These studies did not adequately control for these confounding variables, which may have led to erroneous associations. Mendelian randomization (MR), a nice method of epidemiological research, can explain observation bias. To determine whether or not an exposure caused a certain result, it uses genetic variants as instrumental variables(IVs) [13,14]. The instrumental advantages of MR rely on the fact that genetic diversity in a population is distributed at random as a consequence of the random assignment of genes during meiosis [13]. In genetics, the direction of causality is determined and genetic diversity leads to different phenotypes but not vice versa. In general, the environmental exposure factors we measure are more or less correlated with behavioral, social, psychological, and other factors, resulting in bias. However, genetic variants are not affected by these confounding factors. Therefore, compared with traditional observational studies, Mendelian randomization studies are less susceptible to reverse causality and confounding. The level of evidence is significantly higher than that of traditional observational studies. Additionally, the evolving GWAS provides a potent and dependable intravenous infusion for MR research [15,16].
In the present study, we examined whether genetic evidence of psoriasis is substantially associated with the risk of central neurological disorders using a two-sample MR analysis.
2. Methods
2.1. The Study's methodology and data sources
This is an MR study with two samples based on summary-level data. The results of a genome-wide association study (GWAS) were used to obtain genetic data on psoriasis and central nervous system disorders for this study. We used GWAS data for psoriasis from the IEU Open GWAS Project, which contained 5314 cases and 457,619 controls, for the exposure dataset. Data from the ieu open GWAS project's GWAS for numerous disorders of the central nervous system were also included in the result dataset. Table 1 provides a summary of the demographic characteristics of the study participants.
Table 1.
Data sources of the Exposure and Outcomes.
| Features |
Information sources |
cases/controls |
Samples |
Populations |
|---|---|---|---|---|
| Exposure | ||||
| psoriasis | ukb-b-10537 | 5314/457,619 | 462,933 | European |
| Outcomes | European | |||
| migraine | ukb-b-16868 | 13,597/449,336 | 462,933 | European |
| Parkinson's Disease | ieu-b-7 | 33,674/449,056 | 482,730 | European |
| multiple sclerosis | ieu-b-18 | 47,429/68,374 | 115,803 | European |
| epilepsy | ieu-b-8 | 15,212/29,677 | 44,889 | European |
Using a two-sample MR investigation, we assessed the potential causative relationship between psoriasis susceptibility genes and the likelihood of developing disorders of the central nervous system. SNPs were employed as IVs. Fig. 1 provides an overview of the research methodology. Everything fits well within the three fundamental MR analysis theories [17]: 1. IVs influenced exposure strongly; 2. IVs were not linked to potential confounding factors; and 3. The hazard of outcomes was directly impacted by exposure to IVs and not via any other mechanisms.
Fig. 1.
A diagram depicting how Mendelian randomization research might be set up The three theories upon which Mendelian randomization is based are: 1. There is a high degree of correlation between the exposure and the IVs; 2. the IVs are not influenced by other factors; and 3. the instrumental variables only influence the results via exposure and not in any other manner.
2.2. Selection of IVs
All genetic variants substantially associated with psoriasis (P < 5 × 10−8) were deemed to be IVs. The level of linkage disequilibrium (LD) was calculated to determine whether SNPs were in a state of LD. By removing SNPs within a 10,000 kb window with a r2 < 0.001 threshold, these SNPs were determined to be independent. To rule out the possibility of pleiotropic effects, we used Phenoscan V2 to seek for secondary phenotypes associated with each SNP. SNPs corresponding to the phenotypes associated with the outcomes were excluded from further analysis, while the remaining SNPs were utilized.
The power of the screened SNPs was evaluated using F-statistics to exclude the possibility of weak-tool bias. F = β2exposure/SE2exposure. If F > 10, the strength of the association between the IVs and exposure was judged to be high enough that weak-tool bias would not be a factor in the MR analysis's findings.
2.3. Statistical analyses
The aggregated SNP-psoriasis and SNP-nervous system disease statistics were harmonized to verify that the alleles of each SNP were consistent between psoriasis and central nervous system disorders. The MR study relied mainly on the analytical methodology of inverse variance weighting (IVW). Simultaneously, the causal relationship was inferred using Weighted median, MR-Egger, Simple mode, Weighted mode and MR-pleiotropy residual sum and outlier (MR-PRESSO). Each method makes some assertions about the efficacy of SNPs. The Weighted median is estimated when fifty percent of the IVs are invalid. Despite the low statistical power of the MR-Egger method, it provides an estimate after correcting for multiple effects. In order to offer an accurate MR estimate, the MR-PRESSO approach can automatically identify and remove abnormalities in IVW linear regression. All of these techniques were employed to investigate causality exhaustively.
2.4. Sensitivity analyses
In this investigation, various sensitivity analysis methodologies were introduced. Before choosing an analytical approach, Cochran's Q test was used to determine the degree of variation among SNP estimations. The fixed-effects IVW technique was prioritized if the p-value was more than 0.05, suggesting that there was no heterogeneity, and the random-effects model was employed otherwise. The horizontal pleiotropy of IVs was evaluated using the MR-Egger intercept technique. The intercept in the MR-Egger test was used to estimate the mean horizontal pleiotropic impact; if the p-value was less than 0.05, it indicated that the IVW estimate may have been skewed. Third, to see whether the result was due to a single SNP, we performed a leave-one-out sensitivity test. Finally, funnel and forest plots were developed for the express purpose of detecting pleiotropy.
In the R software, version 4.1.2, the packages “TwoSampleMR” and “MR-PRESSO” were used for all statistical testing and analysis. Moreover, every p-value was two-sided.
3. Results
3.1. Characteristics of selected SNP variants and outcomes of central neurological disorders
We extracted IVs from the GWAS that were substantially associated with psoriasis (P < 5 × 10−8) and removed LD (r2<0.001,10,000-kb). Additionally, we eliminated palindromic SNPs with moderate allele frequency and an incompatible allele. Two SNPs, rs12188300 and rs13191494, associated with palindromic SNPs were excluded. Additionally, we eliminated the incompatible allele rs9265203. The SNPs that passed screening were included in subsequent analyses (Supplemental Tables 1–4). The IVs strength test revealed no evidence of weak-tool bias (F-statistic >10) (Supplemental Tables 1–4).
3.2. Causal estimates of genetic psoriasis susceptibility and central neurological disorders risk
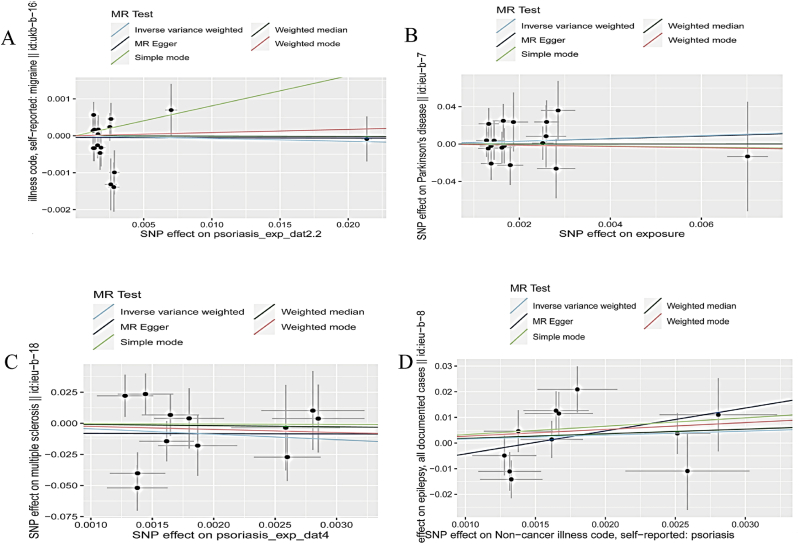
Although psoriasis was reported to increase the risk of Parkinson's disease (OR = 4.42, 95%CI[-3.81~6.79], P = 0.58) and epilepsy (OR = 4.71, 95%CI[-2.20~5.30], P = 0.42) in this study, they did not reach statistical significance. At the same time, this study did not observe that psoriasis would increase the risk of multiple sclerosis (OR = 0.01, 95%CI [−12.61~3.83], P = 0.30) and migraine (OR = 0.99, 95%CI [0.94~1.05], P = 0.78). The scatter plots were illustrated in Fig. 2(A-D). We eliminated some outliers when analyzing the relationship between psoriasis and multiple sclerosis using MR-PRESSO (Supplemental Table 5).
Fig. 2.
The scatter plots of Mendelian randomization results: (A) Psoriasis and Migraine; (B) Psoriasis and Parkinson's disease; (C) Psoriasis and Multiple Sclerosis; (D) Psoriasis and epilepsy. SNP, single-nucleotide polymorphism; MR, Mendelian randomization. The slope of the scatter plot represents the magnitude of the impact of psoriasis on central neurological disorders.
3.3. Sensitivity analyses of MR
When testing for heterogeneity among these SNPs, the p-value of the Cochran Q statistic was larger than 0.05, suggesting that there was no heterogeneity (Table 2). The randomized-effects IVW approach was the major analytic tool for this MR investigation, considering the existence of heterogeneity. Few pleiotropic associations between psoriasis and central neurological diseases were found using the MR-Egger regression intercept (Table 2). Furthermore, the findings did not significantly shift after progressively removing one SNP using the leave-one-out technique (Supplementary Fig. 1). More intuitive representations of heterogeneity, such as forest and funnel plots, are shown in Supplemental Figs. 2 and 3, respectively.
Table 2.
Psoriasis IVs for pleiotropy and heterogeneity testing in relation to central nervous system disorders GWAS.
| Outcomes | Pleiotropy analysis |
Heterogeneity analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MR-Egger |
MR-Egger |
Inverse-variance weighted |
|||||||
| Intercept | SE | P | Q | Q_df | Q_pval | Q | Q_df | Q_pval | |
| migraine | −4.60E-05 | 0.14E-03 | 0.74 | 19.96 | 16.00 | 0.22 | 20.10 | 17.00 | 0.27 |
| Parkinson's Disease | 2.00E-04 | 0.01 | 0.99 | 9.73 | 15.00 | 0.84 | 9.73 | 16.00 | 0.88 |
| multiple sclerosis | −0.01 | 0.03 | 0.79 | 19.05 | 10.00 | 0.04 | 19.19 | 11.00 | 0.06 |
| epilepsy | −0.01 | 0.01 | 0.34 | 15.24 | 9.00 | 0.09 | 16.97 | 10.00 | 0.08 |
df, degree of freedom; MR, Mendelian randomization; Q, heterogeneity statistic Q.
4. Discussion
Previous epidemiological studies have suggested a link between psoriasis and an increased risk for central neurological disorders [18,19]. However, these observational studies may have been influenced by some confounding factors. Using MR, we examined the role of psoriasis in the risk of central neurological disorders. The findings revealed that psoriasis had no causal association with the risk of migraine, Parkinson's disease, multiple sclerosis, or epilepsy. To the best of our knowledge, this is the first study to investigate the causal relationship between psoriasis and the risk of central neurological disorders with a mendelian randomization study. These findings improved our understanding of the function of psoriasis in the progression of these diseases and had clinical implications for patients and physicians.
Observational investigations have demonstrated a correlation between psoriasis and migraine. Migraine attacks with or without aura (MA and MO) are marked by moderate to severe unilateral recurring pulsatile headaches. Le et al. reported that 23 and 12 comorbid diseases are linked to MA and MO, respectively [20]. Four studies have characterized the association between psoriasis and migraine [18,[20], [21], [22]]. Patients with psoriasis were shown to be more likely to get migraine headaches if they were female. More so than in the general population without psoriasis, those with the illness were more likely to have MA crises, and they occurred more often. However, there was no change in the incidence of MO crises. Psoriasis sufferers who had migraines tended to have far more severe instances of the skin condition, as measured by the psoriasis area and severity index (PASI) [21]. While some research has linked psoriasis to an increased incidence of migraines, another population-based study has shown no such link [23]. Different research found that moderate-to-severe psoriasis was the only level at which migraines were linked [22]. Given the paucity of existing research, it is clear that more must be done. To investigate this potential genetic link between psoriasis and migraine, we conducted a mendelian randomization study.
A total of three studies examined the association between psoriasis and the risk of developing Parkinson's disease (PD) [12,24,25]. Although all studies found an elevated risk, only two met the criteria for statistical significance [24,25]. In psoriasis, the risk of developing PD was 1.38 times higher than in healthy individuals [26]. Notably, the prevalence of psoriasis was not observed to be higher in those with Parkinson's disease, according to a population survey [27]. Generally speaking, more extensive study is needed to explain these contradictory findings.
Treatment with fumarates has shown potential for both multiple sclerosis (MS) and psoriasis, suggesting that the two conditions may have a shared etiology [28]. In fact, genetic risk variants and inflammatory pathways are similar in both of these inflammatory disorders. The prevalence of psoriasis in the MS community was 54 % greater than in matched individuals, and almost 5 % of MS patients were confirmed to have the skin disease [29]. On the other hand, psoriasis patients were also reported to have an increased incidence of MS[[30], [31]]. A recent research conducted in Denmark indicated that the severity of psoriasis was significantly correlated with an elevated risk of MS(30). In other words, the rates of MS incidence per 10000 person-years were 3.22 (95 % CI: 2.57–4.04) for those with moderate psoriasis, 4.55 (95 % CI: 2.52–8.22) for those with severe psoriasis, and 1.78 (95 % CI: 1.74–1.82) for those in the control. Even after accounting for confounding variables, the rate was still significantly greater in the psoriasis groups than in the control group. It is not completely understood how psoriasis and MS influence each other and affect the overall prognosis of patients. According to Miron et al. [32], Having psoriasis is linked to a slower rate of MS disease development, a later time to relapse, and a later time to substantial neurological impairment scores. In addition, our findings indicate that psoriasis may not be a risk factor for the progression of multiple sclerosis.
Epilepsy has been documented as a symptom of psoriasis in case-report studies [33]. However, in these patients, seizures were caused by progressive multifocal leukoencephalopathy due to efalizumab use. Psoriasis has been linked to the development of epileptic occur, a phenomenon initially reported by Lindegrd (1986)[34]. Psoriasis patients may have an increased chance of developing epilepsy, according to research [35]. Psoriasis patients were found to be using anticonvulsant drugs at a rate 4.37 times higher than the control group, according to a review of medications associated with psoriasis co-morbidities [odds ratio (95 % CI): 4.37 (2.81–6.79); adjusted odds ratio (95 % CI): 4.20 (2.68–6.60); p < 0.001] [36]. Analyzing data from 23,542 people with psoriasis, researchers found an elevated incidence of epilepsy compared to the general population [odds ratio (95 % confidence interval):1.9 (1.6–2.3)] [35]. Furthermore, an elevated incidence of epilepsy was linked to the presence of psoriasis in children [35,37]. Psoriasis patients' experiences with epilepsy are poorly understood on many fronts. We don't know much about the frequency with which seizures occur, their causes, or the most prevalent types.
Nevertheless, our research has some limitations. Firstly, due to the lack of detailed clinic information, we were unable to perform subgroup analysis among the European population. Secondly, according to ethnicity, psoriasis prevalence and mortality vary. Only the European populations participated in the study, so the causal relationship between psoriasis and central neurological disorders cannot be extrapolated to other populations.
In brief, this MR study does not provide evidence of a causal association between psoriasis and the risk of central neurological disorders in European populations. Most likely, psoriasis is not the cause of these diseases.
Fund support
This research did not receive any specific grant from funding agencies.
Ethics statement
The GWAS summary statistics data used in our study were publicly available, which obtained informed consent from all participating studies by following the protocols approved by their respective institutional review boards. No separate ethical approval was required for this study.
Availability of data proclaims
If you have any questions about the study's original contributions beyond what is included in the article/Supplementary Material, please contact the article's corresponding author.
Data availability statement
Data associated with this study was obtained from IEU Open GWAS project (mrcieu.ac.uk). All data included in article/supp. material/referenced in the article.
-
1.
Naval N, Chandolu S, Mirski M. Organ failure: central nervous system. Seminars in respiratory and critical care medicine 2011; 32(5):587-597.
-
2.
Zhou X, Smith QR, Liu X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology 2021; 13(4):e1695.
CRediT authorship contribution statement
Ya Li: Writing - original draft, Methodology. Jun Cai: Methodology. Huimin Chong: Writing - review & editing, Supervision, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
In any case, we appreciate everyone's help with this MR study. Analytical summaries are provided by the IEU Open GWAS project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e24774.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Naval N, Chandolu S, Mirski M. Organ failure: central nervous system. Semin. Respir. Crit. Care Med. 2011;32(5):587–597. doi: 10.1055/s-0031-1287867. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Smith QR, Liu X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip. Rev.Nanomedicine and nanobiotechnology. 2021;13(4):e1695. doi: 10.1002/wnan.1695. [DOI] [PubMed] [Google Scholar]
- 3.Malani Shukla N., Lotze T.E., Muscal E. Inflammatory diseases of the central nervous system. Neurol. Clin. 2021;39(3):811–828. doi: 10.1016/j.ncl.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical Neurology and epidemiology of the major Neurodegenerative diseases. Cold Spring Harbor Perspect. Biol. 2018;10(4) doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildner P., Stasiołek M., Matysiak M. Differential diagnosis of multiple sclerosis and other inflammatory CNS diseases. Multiple sclerosis and related disorders. 2020;37 doi: 10.1016/j.msard.2019.101452. [DOI] [PubMed] [Google Scholar]
- 6.Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths C.E.M., Armstrong A.W., Gudjonsson J.E., Barker J. Psoriasis. Lancet (London, England) 2021;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 8.Parisi R., Iskandar I.Y.K., Kontopantelis E., Augustin M., Griffiths C.E.M., Ashcroft D.M. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ (Clinical research ed) 2020;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsoi L.C., Stuart P.E., Tian C., Gudjonsson J.E., Das S., Zawistowski M., et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017;8 doi: 10.1038/ncomms15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E.B., Wu K.K., Lee M.P., Bhutani T., Wu J.J. Psoriasis risk factors and triggers. Cutis. 2018;102(5S):18–20. [PubMed] [Google Scholar]
- 11.Kim M., Park H.E., Lee S.H., Han K., Lee J.H. Increased risk of Alzheimer's disease in patients with psoriasis: a nationwide population-based cohort study. Sci. Rep. 2020;10(1):6454. doi: 10.1038/s41598-020-63550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheu J.J., Wang K.H., Lin H.C., Huang C.C. Psoriasis is associated with an increased risk of parkinsonism: a population-based 5-year follow-up study. J. Am. Acad. Dermatol. 2013;68(6):992–999. doi: 10.1016/j.jaad.2012.12.961. [DOI] [PubMed] [Google Scholar]
- 13.Smith G.D., Ebrahim S. Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? International journal of epidemiology. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 14.Burgess S., Foley C.N., Zuber V. Inferring causal relationships between risk factors and outcomes from genome-wide association study data. Annu. Rev. Genom. Hum. Genet. 2018;19:303–327. doi: 10.1146/annurev-genom-083117-021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J., Baird D., Borges M.C., Bowden J., Hemani G., Haycock P., et al. Recent developments in mendelian randomization studies. Current epidemiology reports. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawlor D.A., Harbord R.M., Sterne J.A., Timpson N., Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 17.Emdin C.A., Khera A.V., Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 18.Egeberg A., Mallbris L., Hilmar Gislason G., Skov L., Riis Hansen P. Increased risk of migraine in patients with psoriasis: a Danish nationwide cohort study. J. Am. Acad. Dermatol. 2015;73(5):829–835. doi: 10.1016/j.jaad.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Amanat M., Salehi M., Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev. Neurosci. 2018;29(7):805–813. doi: 10.1515/revneuro-2017-0108. [DOI] [PubMed] [Google Scholar]
- 20.Le H., Tfelt-Hansen P., Russell M.B., Skytthe A., Kyvik K.O., Olesen J. Co-morbidity of migraine with somatic disease in a large population-based study. Cephalalgia : an international journal of headache. 2011;31(1):43–64. doi: 10.1177/0333102410373159. [DOI] [PubMed] [Google Scholar]
- 21.Capo A., Affaitati G., Giamberardino M.A., Amerio P. Psoriasis and migraine. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2018;32(1):57–61. doi: 10.1111/jdv.14472. [DOI] [PubMed] [Google Scholar]
- 22.Galili E., Barzilai A., Shreberk-Hassidim R., Merdler I., Caspi T., Astman N. Neuropsychiatric comorbidity among adolescents with psoriasis. Br. J. Dermatol. 2018;178(4):910–916. doi: 10.1111/bjd.16031. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y.W., Keller J.J., Lin H.C. Medical comorbidity associated with psoriasis in adults: a population-based study. The British journal of dermatology. 2011;165(5):1037–1043. doi: 10.1111/j.1365-2133.2011.10494.x. [DOI] [PubMed] [Google Scholar]
- 24.Rugbjerg K., Friis S., Ritz B., Schernhammer E.S., Korbo L., Olsen J.H. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology. 2009;73(18):1462–1468. doi: 10.1212/WNL.0b013e3181c06635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Sundquist J., Sundquist K. Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener. Dis. 2012;10(1–4):277–284. doi: 10.1159/000333222. [DOI] [PubMed] [Google Scholar]
- 26.Ungprasert P., Srivali N., Kittanamongkolchai W. Risk of Parkinson's disease among patients with psoriasis: a systematic review and meta-analysis. Indian J. Dermatol. 2016;61(2):152–156. doi: 10.4103/0019-5154.177771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pupillo E., Cricelli C., Mazzoleni F., Cricelli I., Pasqua A., Pecchioli S., et al. Epidemiology of Parkinson's disease: a population-based study in Primary care in Italy. Neuroepidemiology. 2016;47(1):38–45. doi: 10.1159/000448402. [DOI] [PubMed] [Google Scholar]
- 28.Metz I., Traffehn S., Straßburger-Krogias K., Keyvani K., Bergmann M., Nolte K., et al. Glial cells express nuclear nrf2 after fumarate treatment for multiple sclerosis and psoriasis. Neurology(R) neuroimmunology & neuroinflammation. 2015;2(3):e99. doi: 10.1212/NXI.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrie R.A., Patten S.B., Tremlett H., Wolfson C., Leung S., Fisk J.D. Increased incidence and prevalence of psoriasis in multiple sclerosis. Multiple sclerosis and related disorders. 2017;13:81–86. doi: 10.1016/j.msard.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Egeberg A., Mallbris L., Gislason G.H., Skov L., Hansen P.R. Risk of multiple sclerosis in patients with psoriasis: a Danish nationwide cohort study. J. Invest. Dermatol. 2016;136(1):93–98. doi: 10.1038/JID.2015.350. [DOI] [PubMed] [Google Scholar]
- 31.Guido N., Cices A., Ibler E., Huynh T., Majewski S., Sable K., et al. Multiple sclerosis association with psoriasis: a large U.S. population, single centre, retrospective cross-sectional study. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2017;31(9):e397–e398. doi: 10.1111/jdv.14205. [DOI] [PubMed] [Google Scholar]
- 32.Miron G., Gurevich M., Baum S., Achiron A., Barzilai A. Psoriasis comorbidity affects multiple sclerosis neurological progression: a retrospective case - control analysis. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2017;31(12):2055–2061. doi: 10.1111/jdv.14403. [DOI] [PubMed] [Google Scholar]
- 33.Gadzia J., Turner J. Progressive multifocal leukoencephalopathy in two psoriasis patients treated with efalizumab. J. Drugs Dermatol. JDD : J. Drugs Dermatol. JDD. 2010;9(8):1005–1009. [PubMed] [Google Scholar]
- 34.Lindegård B. Diseases associated with psoriasis in a general population of 159,200 middle-aged, urban, native Swedes. Dermatol. 1986;172(6):298–304. doi: 10.1159/000249365. [DOI] [PubMed] [Google Scholar]
- 35.Ong M.S., Kohane I.S., Cai T., Gorman M.P., Mandl K.D. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014;71(5):569–574. doi: 10.1001/jamaneurol.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerdes S., Zahl V.A., Knopf H., Weichenthal M., Mrowietz U. Comedication related to comorbidities: a study in 1203 hospitalized patients with severe psoriasis. Br. J. Dermatol. 2008;159(5):1116–1123. doi: 10.1111/j.1365-2133.2008.08786.x. [DOI] [PubMed] [Google Scholar]
- 37.Kelati A., Baybay H., Najdi A., Zinoune S., Mernissi F.Z. Pediatric psoriasis: Should we be concerned with comorbidity? Cross-sectional study. Pediatr. Int. : official journal of the Japan Pediatric Society. 2017;59(8):923–928. doi: 10.1111/ped.13309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study was obtained from IEU Open GWAS project (mrcieu.ac.uk). All data included in article/supp. material/referenced in the article.
-
1.
Naval N, Chandolu S, Mirski M. Organ failure: central nervous system. Seminars in respiratory and critical care medicine 2011; 32(5):587-597.
-
2.
Zhou X, Smith QR, Liu X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology 2021; 13(4):e1695.