Abstract
Introduction
Brachial artery aneurysm (BAA) following long-standing arteriovenous fistula (AVF) ligation after renal transplantation is odd.
Case presentation
Two cases of brachial artery aneurysm treated with bypass (a saphenous vein graft and a PTFE graft). In the first patient no complications were recorded whereas an infection was diagnosed after 6 months from the procedure in the second treatment.
Clinical discussion
Multiple factors activated by stress on the vessel wall followed by fistula ligation are the cause of vascular remodeling of the three layers making up the wall with possible evolution in aneurysmatic lesions. In literature the gold standard for this lesion is the surgical approach, only one endovascular procedure is reported. The traditional surgical approach uses the autologous vein or prosthetic PTFE grafts.
Conclusion
Brachial artery aneurysm is a complication that affects patients undergoing renal transplantation who have already undergone AVF ligation. In our experience autologous vein graft represented the best solution.
Keywords: Brachial artery aneurysm, Complication fistula, Renal transplantation, Dialysis, Surgical bypass
Highlights
-
•
Brachial Artery Aneurysm is a rare complication diagnosed often in renal transplant patients after closure of fistula.
-
•
The main factor responsible is a stress on the vessel wall with endothelial injuries.
-
•
The gold standard treatment is a surgical approach, only one endovascular procedure is reported in literature.
-
•
The autologous vein graft represented the best solution.
1. Introduction
BAA is defined as a dilatation of the artery having a diameter greater than 10 mm or an increase of diameter greater than 50 % of the longitudinal axis. BAA is a rare complication (incidence of 4.5 %) diagnosed often in renal transplant patients, treated with closure of the hemodialysis' fistula with an incidence of 4.5 % [1]. Possible complications are peripheral embolization, thrombosis of the sac, rupture and hand ischemia [[2], [3], [4], [5], [6]]. The normal diameter of the brachial artery in women is 3.5–4.3 mm, while in men 4.1–4.8 mm [2]. True aneurysms occur in 0.17 % of cases, but usually are secondary to injury, infection and congenital defects. Data from the literature show that aneurysm after fistula ligation is a rare event with a median time from fistula ligation to aneurysm development of 120 months [5]. Here, we report our experience with two cases of a huge brachial artery aneurysm after fistula ligation with review of the literature.
This work has been written in accordance with the SCARE criteria [7].
2. Case 1
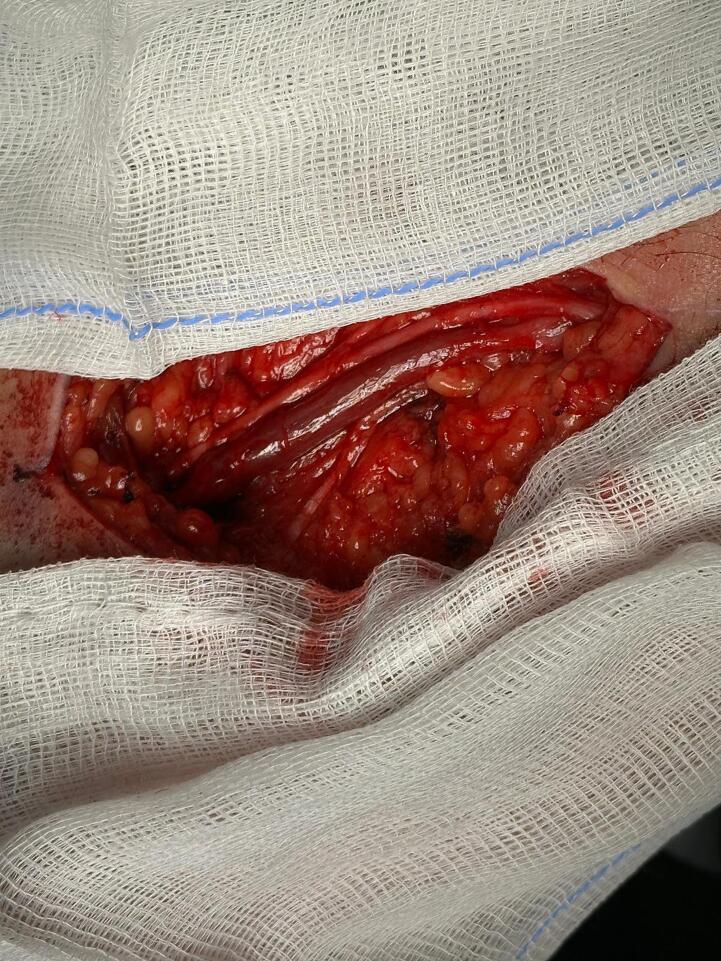
A 67-year-old man, referred to our hospital for a pulsating mass on the left forearm present from several months with increasing paresthesias and pain. CT angiography showed an aneurysmal dilatation of the brachial artery (25.5 mm × 19 mm) (Fig. 1). During the admission physical examination performance a medial pulsatile mass was shown on the ante cubital face of the left forearm. Axillary and brachial pulses were noticable to palpation, no radial flow was present due to thrombotic occlusion. Medical history reported terminal uremia, hypertension, previous gallbladder stones, cervical and lumbosacral spondylarthrosis. No-smoking patient, underwent left radio-cephalic AVF for chronic renal failure (CRF) due to IgA-deposited glomerulopathy, cadaver kidney transplantation in 2017 and subsequent fistula deafferentation. The patient's medication history includes immunosuppressive therapy with mycophenolic acid 360 mg and Tacrolimus, antiplatelet, antihypertensive and hypouricemic therapy. Despite the presence of CRF (creatinine 1.77 mg/dl preoperatively, GFR 38.9 ml/min), the patient did not required hemodialytic therapy. With the patient under supraclavicular block nerve anesthesia, a longitudinal incision was made in the bicipital groove as a way to expose the brachial artery until its terminal tract (Fig. 2). The aneurysm was isolated and sectioned at the ends in order to perform the aneurysmectomy. A brachial-brachial bypass using interposed reverse right saphenous was done (Fig. 3). Endovascular treatment was excluded due to thrombosis of the radial artery and the presence of neurological symptoms by compression on the median nerve. After treatment, no pain, edema or other significant symptoms was noticed and post-surgical therapy consisted of oral antibiotic (amoxicillin + clavulanic acid and LMWH). No complications were noticed during hospitalization and the patient was discharged on the 6th post-operative day. Follow-up lasted 9 months and was conducted with US scan which showed the long-term patency of the bypass.
Fig. 1.
CT Angiography 3-dimensional volume rendering showing left arm (A) with tortuosity and extension of brachial aneurysm (B). CT Angiography with maximum diameter of aneurysm.
Fig. 2.
Intraoperative brachial aneurysm before (A) and after aneurysmectomy (B).
Fig. 3.
Intraoperative image of Bypass in vein after aneurysmectomy.
3. Case 2
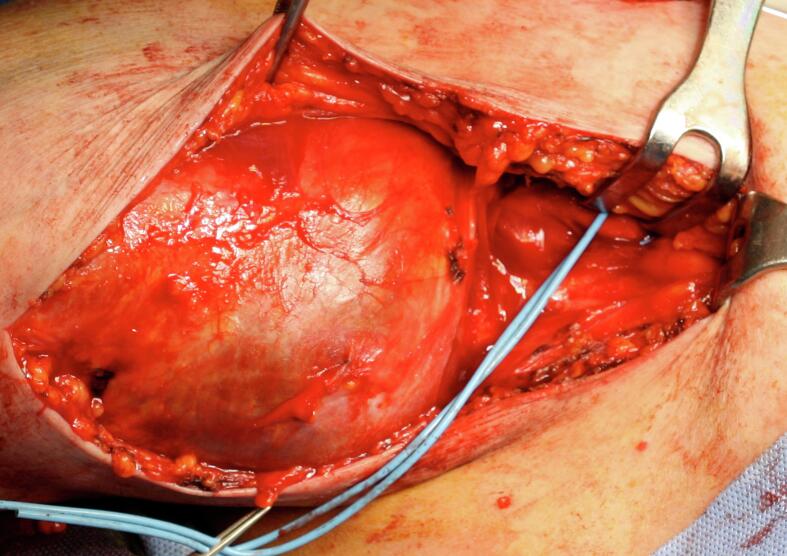
A 64-year-old man came to our attention for a progressive increase in the volume of right arm associated with pain and paresthesia in ipsilateral hand during the last month. On clinical examination, a large pulsatile mass was observed in a medial position towards the bicipital groove (Fig. 4). A duplex scan revealed an aneurysm approximately 8 cm in diameter. The patient had a history of renal failure due to IgA nephropathy and, as a result, he started hemodialysis therapy via a Cimino–Brescia AVF when he was 30 years old. The AVF was ligated after a successful cadaveric renal transplant which he underwent 13 years later. His past medical history included type 2 diabetes, hypertension, chronic obstructive pulmonary disease, cirrhosis due to chronic hepatitis C, and deforming arthrosis of the left hip treated with a hip replacement when he was 54 years old. Ever since he underwent renal transplantation, the patient had been on steroids and immunosuppressive drugs to prevent biocompatibility problems. Because of a history of allergy to contrast agent that had caused a severe respiratory crisis, the patient underwent a computed tomography (CT) scan without contrast. The images confirmed the presence of a BAA measuring 8.4 × 14 cm (Fig. 5). Endovascular treatment was not indicated because of the patient's allergy and the large size of the aneurysm, which caused symptoms of nerve compression. The aneurysm was therefore treated surgically. With the patient under general anesthesia, a longitudinal incision was made in the bicipital groove to expose the brachial artery (Fig. 6). The aneurysm was isolated and an incision was made in the aneurysm wall. A large concentric thrombus was removed. Since an autologous vein was not available as prosthetic material, a reinforced polytetrafluoroethylene (PTFE) prosthesis was used to re-establish blood flow in the arm (Fig. 7). A duplex scan performed in the postoperative period showed that the prosthetic graft was patent and that there was no peri-anastomotic stenosis. Histological examination using hematoxylin and eosin stain of the aneurysm wall showed that it consisted of intimal and medial degeneration with fibrous and inflammatory chronic tissue without signs of infection (Fig. 8). No complications were observed on clinical examination and duplex scanning performed 6 months after surgery but after 8 months an infection of prosthesis was diagnosed with wound dehiscence and graft explant in absence of ischemic symptoms for possible compensation coming from brachial profunda artery. The lesion was treated by closure for secondary intention.
Fig. 4.
Proximal brachial aneurysm before surgical procedure.
Fig. 5.
Basal CT scan showing huge brachial artery aneurysm with maximum diameter of 8.4 cm.
Fig. 6.
Intraoperative brachial aneurysm.
Fig. 7.
Intraoperative image of Bypass in PTFE after aneurysmectomy.
Fig. 8.
Histopathological features of the resected aneurysm. Hema- toxylin and eosin (H&E) stain at 925 magnification showing true aneurysm without infection/cells associated with an acute inflammatory process.
4. Clinical discussion
The first case of brachial artery aneurysm was described by Hunter in 1757 and today 49 cases are reported in the literature [8]. Several factors are responsible of occurrence of brachial artery aneurysm after AVF ligation. According to Eugster et al. the main factor responsible is a stress on the vessel wall because of an increased blood flow, which causes the release of endothelial factors, such as nitric oxide, and an increase in vessel wall resistance resulting from fistula ligation [9]. Texeira et al. identify the etiology of lesion in an up-regulation of vasodilatory factors, degradation of elastic fibers and deposition on the vessel wall of calcium and phosphate secondary to dialysis, causing an activation of a pro-inflammatory process, in addition to immunosuppression and long-term corticosteroid therapy [10]. Carrol et al. describe other factors that can cause an inflammation of the vessel wall, such as hemodialysis, diabetes, dyslipidemia, hypertension, infection, and repeated trauma [11]. The use of long-term immunosuppressive therapy is another determinant of brachial artery aneurysm because it results in vascular remodeling of the three layers of the vessel wall due to the establishment of a cytokine-mediated inflammatory process [12]. Diagnostics uses different techniques such as duplex ultrasonography, computed tomography, magnetic resonance imaging, and digital subtraction angiography [[13], [14], [15], [16]]. Regarding AVF ligation after transplantation, some authors advise against it if the fistula is functioning and should be reserved for hand ischemia cases, risk of pseudo-aneurysm rupture, severe venous hypertension or significant high-output heart failure [17,18]. In literature non significative data withstand the role of fistula's position in BAA etiopathogenesis although most of the cases were related to AVF with cephalic vein (Table 1).
Table 1.
Closed AVFs in patients with BAA reported in the literature.
| Type of AVF | N. patient |
|---|---|
| Brachio-cephalic | 21 |
| Radio-cephalic | 20 |
| Undefined | 6 |
| Brachio-basilic | 1 |
| Brachio/radio-basilic | 1 |
The gold standard for this lesion is a surgical approach with vein apposition when is possible thanks to the highest patency rate demonstrated in literature [[19], [20], [21]]. Only one case is reported with success as endovascular procedure where Maynar R. et al. treated a long BAA through the placement of several endoprosthesis in order to avoid a surgical approach because the patient was deemed “a poor candidate for surgical intervention due to the length of the aneurysm” [22].
The traditional surgical approach makes use of autologous veins and prosthetic PTFE grafts [[2], [3], [4]]. About a review of the literature, of the 49 reported cases of brachial artery aneurysm secondary arteriovenous fistula ligation, saphenous vein inverted was used in 21 patients (42.8 %), PTFE prostheses in 9 patients (18.4 %), cephalic vein or basilic vein inverted in 3 patients (6.2 %), other surgeries in 16 patients (32.6 %) (Table 2). In our experience the bypass in PTFE was complicated by an infection probably due to the lack of tissues necessary to protect a graft positioned less deeply than the skin, but in absence of adequate vein grafts for replacement, PTFE is a valid option [[23], [24], [25], [26]].
Table 2.
Cases of BAA reported in the literature.
| References | Age/sex | Clinical presentation | Graft | Aneurisma size (cm) | N. |
|---|---|---|---|---|---|
| Soares et al. [1] | 66/M | Pain and swelling | VGS inverted | 4.4 | 1 |
| Dinoto et al. [2] | 64/M | Pulsing swelling with discomfort and arm pain | PTFE protesis | 8.4 | 1 |
| Anastasiadou C et al. [13] | 49/M | Pain and swelling | Cephalic vein | 3.7 | 1 |
| Barac et al. [27] | 50.5/M | Pain and swelling | Varius (VGS inv, dacron graft) | 6.5 | 2 |
| Satoshi T et al. [28] | 60/M | Pain and swelling | end-to-end anastomosis | 3.5 | 1 |
| Ferrara et al. [29] | 61/M | Pain | Basilic Vein | N/a | 1 |
| Nguyen et al. [30] | 51/M | Brachial and axillary pulse with a palpable supraclavicular thrill in the absence | VGS inverted | N/a | 1 |
| Battaglia et al. [31] | N/a | Pain | PTFE protesis | 5 | 1 |
| Murphy et al. [32] | 61/M | Swelling in arm with some discomfort with rapid increase of swelling and pain over the 2 weeks preceding arrival at the unit | Cephalic vein | 5 | 1 |
| Chemla et al. [33] | N/a | Pain | VGS inverted | N/a | 5 |
| Basile et al. [34] | 55/M | Development of a pulsatile swelling above the elbow | VGS inverted | 2.4 | 1 |
| Marzelle et al. [35] | 38.1/7 M-3F | Pain and swelling | Varius (VGS ins 2, Basilic Vein 1, PTFE 3, Allograft 1, Dacron 1) | 5.6 | 8 |
| Garza et al. [36] | N/a | Pain and swelling | VGS inverted | N/a | 1 |
| Bahia et al. [37] | 62.5/M | Pain with ischemia | VGS inverted | N/a | 2 |
| De Santis et al. [38] | 47/M | Pain | Plastic of arterial wall | N/a | 1 |
| Khalid et al. [39] | N/a | Pain and swelling | VGS inverted | N/a | 3 |
| Gardiner et al. [40] | 51.2 12 M-1F |
Pain and swelling | Varius Vein conduit | N/a | 13 |
| Fernandez et al. [41] | 49.5/M | Pain and swelling | VGS inverted | 4.5 | 2 |
| Correia et al. [42] | 47/M | Asymptomatic | PTFE protesis | 4.5 | 1 |
| Rodrigues et al. [43] | 54/F | Pain (rupture) | PTFE protesis | 4.1 | 1 |
| Lee et al. [44] | 51/M | Pain and swelling | VGS inverted | 3 | 1 |
| Frandri J et al. [45] | 26 | Pain and swelling | Arterial trasposizione | N/a | 5 |
N/a Not available.
5. Conclusions
Brachial artery aneurysm is a complication linked to patients undergoing renal transplantation who already sustained an AVF ligation. Several factors may predispose patients to the development of this complication, such as immunosuppressive therapy, diabetes, high blood pressure, and long-term hemodialytic replacement therapy. The choice of treatment in these patients is still traditional surgical approach, there is only one case available in literature about a patient treated with endovascular technique and even if it was reported as a complete success we have no evidence about its efficacy in the medium and long term on a significant number of patients. In our experience autologous vein graft represented the best solution.
Institutional review board statement
All procedures followed were in accordance with the ethical standards of the Institutional Committee on Human Experimentation and with the Helsinki Declaration. According to the internal review board, the retrospective and anonymized nature of the study did not require medical ethical committee approval.
Informed consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
All procedures followed were in accordance with the ethical standards of the Institutional Committee on Human Experimentation and with the Helsinki Declaration. According to the internal review board, the retrospective and anonymized nature of the study did not require medical ethical committee approval.
This article does not require ethics committee approval due to the retrospective nature of the paper and the type of technique reported.
Funding
This research received no external funding.
Author contributions
Conceptualization, MA.L., E.D. and D.M.; methodology, E.D., D.T.; software, MA.L.; validation, D.M., E.D. and F.P.; formal analysis, MA.L. and E.D.; investigation, MA.L.; resources, MA.L., D.T.; data curation, E.D., D.M.; writing—original draft preparation, E.R.; writing—review and editing, E.D., E.R.; visualization, D.M., F.P.; supervision, F.P.; project administration, F.P. All authors have read and agreed to the published version of the manuscript.
Guarantor
Ettore Dinoto.
Research registration number
Not applicable
Declaration of competing interest
The authors declare no conflict of interest.
Data availability
The data presented in this study are available on request from the corresponding author.
References
- 1.Soares T., Castro-Fereira T., Rocha Neves J., et al. True brachial artery aneurysm after arteriovenous fistula for hemodialysis - case report. Rev. Port. Cir. Cardiotorac. Vasc. 2017;24(3–4):182. Jul-Dec. [PubMed] [Google Scholar]
- 2.Dinoto E., Bracale U.M., Vitale G., et al. Late, giant brachial artery aneurysm following hemodialysis fistula ligation in a renal transplant patient: case report and literature review. Gen. Thorac. Cardiovasc. Surg. 2012;11:60768–60770. doi: 10.1007/s11748-012-0075-6. [DOI] [PubMed] [Google Scholar]
- 3.Panagrosso M, Bracale UM, Del Guercio L, Viscardi A, Peluso A, Dinoto E. Case report of a large cephalic vein aneurysm inducing heart failure in a renal transplant patient with radio-cephalic fistula for haemodialysis. Int. J. Surg. Case Rep. 2020;77S(Suppl):S162-S165. doi: 10.1016/j.ijscr.2020.07.055. Epub 2020 Aug 27. PMID: 32888880; PMCID: PMC7876928. [DOI] [PMC free article] [PubMed]
- 4.Sapienza P., Borrelli V., Ciardi A., et al. Late occurrence of brachial artery aneurysm after the closure of a long-standing vascular access for hemodialysis: a pathogenetic hypothesis. J.CardiovascSurg. 2013;54:445–447. [PubMed] [Google Scholar]
- 5.Kordzadeh A., D’Espiney Barbara R.M., Ahmad A.S., et al. Donor artery aneurysm formation following the ligation of haemodialysis arteriovenous fistula: a systematic review and case reports. J. Vasc. Access. 2015;16(1):5–12. doi: 10.5301/jva.5000297. [DOI] [PubMed] [Google Scholar]
- 6.Janeckova J., Bachleda P., Koleckova M., Utikal P. Brachial artery aneurysm as a late complication of arteriovenous fistula. J. Vasc. Access. 2023 Sep;24(5):926–932. doi: 10.1177/11297298211059326. Epub 2021 Nov 17. PMID: 34789043. [DOI] [PubMed] [Google Scholar]
- 7.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battaglia L., Bucci F., Battaglia M., Reddler A. Late occurrence of a large brachial artery aneurysm following closure of a hemodialysis arteriovenous fistula. Ann. Vasc. Surg. 2006 Jul;20(4):533–535. doi: 10.1007/s10016-006-9050-y. Epub 2006 May 27. PMID: 16732447. [DOI] [PubMed] [Google Scholar]
- 9.Eugster T., Wigger P., Bolter S., et al. Brachial artery dilatation after arteriovenous fistulae in patients after renal transplation. A 10 year-follow-up ultrasound scan. J. Vasc. Surg. 2003;37:564–567. doi: 10.1067/mva.2003.94. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira S., Pinto P.S., Veiga C., et al. Aneurysmal degeneration of the brachial artery after vascular access creation: surgical treatment results. Int. J. Angiol. 2017 Sep;26(3):186–190. doi: 10.1055/s-0037-1601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll G.T., McGloughlin T.M., Burke P.E., et al. Wall shear stresses remain elevated in mature arteriovenous fistulas: a case study. J. Biomech. Eng. 2011;133:02. doi: 10.1115/1.4003310. [DOI] [PubMed] [Google Scholar]
- 12.Reilly J.M., Savage E.B., Brophy C.M., et al. Hydrocortisone rapidly induces aortic rupture in a genetically susceptible mouse. Arch. Surg. 1990;125:707–709. doi: 10.1001/archsurg.1990.01410180025004. [DOI] [PubMed] [Google Scholar]
- 13.Anastasiadou C., Megalopoulos A., Tasiopoulou K., et al. A rare case of brachial artery aneurysm following hemodialysis fistula ligation in a transplanted patient. Vasc. Endovasc. Surg. 2019;53(1):71–74. doi: 10.1177/1538574418794078. [DOI] [PubMed] [Google Scholar]
- 14.Turchino D., Peluso A., Accarino G., Accarino G., De Rosa C., D’Angelo A., Machi P., Mirabella D., Pecoraro F., Del Guercio L., Bracale U.M., Dinoto E. A multicenter experience of three different “iliac branched” stent grafts for the treatment of aorto-iliac and/or iliac aneurysms. Ann. Vasc. Surg. 2023 Mar 13 doi: 10.1016/j.avsg.2023.02.033. S0890-5096(23)00148-6. Epub ahead of print. PMID: 36921795. [DOI] [PubMed] [Google Scholar]
- 15.Leite T., Pires M., Pires L., Chagas C., Oliveira A.C. Giant iatrogenic pseudoaneurysm of the brachial artery: a case report. Int. J. Surg. Case Rep. 2017;37:193–195. doi: 10.1016/j.ijscr.2017.06.044. Epub 2017 Jul 3. PMID: 28704745; PMCID: PMC5508487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gozzo C., Caruana G., Cannella R., Farina A., Giambelluca D., Dinoto E., Vernuccio F., Basile A., Midiri M. CT angiography for the assessment of EVAR complications: a pictorial review. Insights Imaging. 2022 Jan 15;13(1):5. doi: 10.1186/s13244-021-01112-4. PMID: 35032231; PMCID: PMC8761205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaffe H.C., Greenstein S.M. Should functioning AV fistulas be ligated after renal transplantation? J. Vasc. Access. 2012;13(4):405–408. doi: 10.5301/jva.5000086. [DOI] [PubMed] [Google Scholar]
- 18.Aitken E., Kingsmore D. The fate of the fistula following renal transplantation. Transpl. Int. 2014;27(9):e90–e91. doi: 10.1111/tri.12326. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen B.N., Neville R.F., Abugideiri M., Amdur R., Sidawy A.N. The effect of graft configuration on 30-day failure of infrapopliteal bypasses. J. Vasc. Surg. 2014 Apr;59(4):1003–1008. doi: 10.1016/j.jvs.2013.10.091. Epub 2013 Dec 19. PMID: 24360587. [DOI] [PubMed] [Google Scholar]
- 20.Pecoraro F., Bajardi G., Dinoto E., Vitale G., Bellisi M., Bracale U.M. Endograft connector technique to treat popliteal artery aneurysm in a morbid obese patient. Vascular. 2015 Apr;23(2):165–169. doi: 10.1177/1708538114533961. Epub 2014 May 8. PMID: 24810759. [DOI] [PubMed] [Google Scholar]
- 21.Almasri J., Adusumalli J., Asi N., Lakis S., Alsawas M., Prokop L.J., Bradbury A., Kolh P., Conte M.S., Murad M.H. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J. Vasc. Surg. 2019 Jun;69(6S):126S–136S. doi: 10.1016/j.jvs.2018.01.071. Epub 2019 May 28. PMID: 31159976. [DOI] [PubMed] [Google Scholar]
- 22.Maynar Manuel, Sanchez-Alvarez E., Qian Z., López-Benitez R., Long D., Zerolo Ignacio. Percutaneous endovascular treatment of a brachial artery aneurysm. Ejves Extra. 2003;6:15–19. doi: 10.1016/S1533-3167(03)00066-9. [DOI] [Google Scholar]
- 23.Dinoto E., Ferlito F., Urso F., Mirabella D., Bajardi G., Pecoraro F. Iliac-femoral stent-graft infection after hybrid procedure redo: case report. Int. J. Surg. Case Rep. 2021 Jul;84 doi: 10.1016/j.ijscr.2021.106096. (Epub 2021 Jun 8. PMID: 34119935; PMCID: PMC8209074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinoto E., Bajardi G., La Marca M.A., Ferlito F., Mirabella D., Pecoraro F. Composite femoro-tibial bypass as alternative solution in complicated revascolarization: case report. Int. J. Surg. Case Rep. 2021 Jul;84 doi: 10.1016/j.ijscr.2021.106103. (Epub 2021 Jun 10. PMID: 34126580; PMCID: PMC8209681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecoraro F., Sabatino E.R., Dinoto E., La Rosa G., Corte G., Bajardi G. Late complication after superficial femoral artery (SFA) aneurysm: stent-graft expulsion outside the skin. Cardiovasc. Intervent. Radiol. 2015;38(5):1299–1302. doi: 10.1007/s00270-014-0970-6. Oct. (Epub 2014 Aug 26. PMID: 25156947) [DOI] [PubMed] [Google Scholar]
- 26.Leite T., Pires M., Pires L., Chagas C., Oliveira A.C. Giant iatrogenic pseudoaneurysm of the brachial artery: a case report. Int. J. Surg. Case Rep. 2017;37:193–195. doi: 10.1016/j.ijscr.2017.06.044. (Epub 2017 Jul 3. PMID: 28704745; PMCID: PMC5508487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barac S., Rata A.L., Popescu A.I., et al. True brachial artery aneurysm in patients with previous arterio-venous fistula ligation and immunosuppressant therapy for renal transplantation: case report and literature review. Healthcare (Basel). 2022 Mar 3;10(3):470. doi: 10.3390/healthcare10030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyota Satoshi, KentaroInoue Shun Kurose, et al. True brachial artery aneurysm after arteriovenous fistula closure following renal transplantation: a case report and literatu rereview. Surg Case Rep. 2019;5:188. doi: 10.1186/s40792-019-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara D., Di Filippo M., Spalla F., et al. Giant true brachial artery aneurysm after hemodialysis fistula closure in a renal transplant patient. Case Rep. Nephrol. Dial. 2016;6:128–132. doi: 10.1159/000452299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen D.Q.A., Ruddle A.C., Thompson J.F. Late axillo-brachial arterial aneurysm following ligated Brescia-Cimino haemodialysis fistula. Eur. J. Vasc. Endovasc. Surg. 2001;22:381–382. doi: 10.1053/ejvs.2001.1460. [DOI] [PubMed] [Google Scholar]
- 31.Battaglia L., Bucci F., Battaglia M., et al. Late occurrence of a large brachial artery aneurysm following closure of a hemodialysis arteriovenous fistula. Ann. Vasc. Surg. 2006;20:533–535. doi: 10.1007/s10016-006-9050-y. [DOI] [PubMed] [Google Scholar]
- 32.Murphy J., Bakran A. Late acute presentation of a large brachial artery aneurysm following ligation of a Brescia-Cimino arteriovenous fistula. EJVES Extra. 2009;18:73–75. [Google Scholar]
- 33.Chemla E., Nortley M., Morsy M. Brachial artery aneurysms associated with arteriovenous access for hemodialysis. Semin. Dial. 2010;23:440–444. doi: 10.1111/j.1525-139X.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- 34.Basile C., Antonelli M., Libutti P., et al. Is there a link between the late occurrence of a brachial arteryaneurysm and the ligation of an arteriovenous fistula? Semin. Dial. 2010;24:341–342. doi: 10.1111/j.1525-139X.2010.00719.x. [DOI] [PubMed] [Google Scholar]
- 35.Marzelle J., Gashi V., Nguyen H.D., et al. Aneurysmal degeneration of the donor artery after vascular access. J. Vasc. Surg. 2012;55:1052–1057. doi: 10.1016/j.jvs.2011.10.112. [DOI] [PubMed] [Google Scholar]
- 36.Garza R., Dangleben D.A., Welkie J.F. Brachial artery aneurysm with “blue finger syndrome” after ligation of a remote brachialartery-cephalicvein fistula. Vasc. Endovasc. Surg. 2013;47:479–481. doi: 10.1177/1538574413487441. [DOI] [PubMed] [Google Scholar]
- 37.Bahia S.S., Tomei F., Ozdemir B.A., et al. Acute limb ischaemia due to focal brachial artery aneurysms complicating brachio cephalic arteriovenous fistula ligation: two recent case reports. J. Vasc. Access. 2014;15:427–430. doi: 10.5301/jva.5000279. [DOI] [PubMed] [Google Scholar]
- 38.De Santis F., Martini G., Mani G., et al. Diffuse aneurysmal degeneration of the brachial artery after Long-standing high-flow arteriovenous fistula closure for hemodialysis at elbow level. Ann. Vasc. Surg. 2014;28(1315):e11–1315.e15. doi: 10.1016/j.avsg.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Khalid U., Parkinson F., Mohiuddin K., et al. Brachial artery aneurysms following brachio-cephalic AV fistula ligation. J. Vasc. Access. 2014;15:22–24. doi: 10.5301/jva.5000156. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner J., Smout J., Torella F. Repair of access-related brachial artery aneurysm with cadaveric homograft: mid-term follow-up. J. Vasc. Access. 2016;17:156–157. doi: 10.5301/jva.5000597. [DOI] [PubMed] [Google Scholar]
- 41.Fernández P.C., Al-Sibbai A.A.Z., González G.M., et al. Aneurisma humeral verdadero en relación con acceso vascular en paciente trasplantado renal: a propósito de 2 casosclínicos. Nefrologia. 2017;37:96–98. doi: 10.1016/j.nefro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Correia M., Marinho A., Mendes C., et al. True brachial artery aneurysm in a patient with vascular access for haemodialysis and kidney graft. Rev. Port. Cir. Cardiotorac. Vasc. 2017;24:184. [PubMed] [Google Scholar]
- 43.Rodrigues R., Aacleto F., Lima P., et al. Rupture of a true brachial artery aneurysm in a kidney transplant patient after arteriovenous fistula ligation: a rare presentation of an unusual disease. J. Vasc. Access. 2019;20:107. doi: 10.1177/1129729818776900. [DOI] [PubMed] [Google Scholar]
- 44.Lee H.Y., Roh Y.N., Kim H.T., et al. Arterial aneurysmal degeneration with venous varicosity following ligation of an arteriovenous fistula in a kidney transplant recipient. Vasc. Endovasc. Surg. 2019;53:242–245. doi: 10.1177/1538574418814058. [DOI] [PubMed] [Google Scholar]
- 45.Fendri J., Palcau L., Cameliere L., Coffin O., Felisaz A., Gouicem D., Dufranc J., Laneelle D., Berger L. True brachial artery aneurysm after arteriovenous fistula for hemodialysis: five cases and literature review. Ann. Vasc. Surg. 2017 Feb;39:228–235. doi: 10.1016/j.avsg.2016.05.115. (Epub 2016 Aug 12. PMID: 27531094) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.