Abstract
In the recent past Metal-organic frameworks (MOFs) based thin films have demonstrated superior performance in various technological applications such as optical and optoelectronic devices, electrochemical energy storage, catalysis, and sensing. Herein we report tuning the optical performance of stable complexes using Cu and Fe metal ions with carboxylate benzene dicarboxylic (BDC), leading toward the formation of novel MOF structures. The formation of Cu-BDC and Fe-BDC were confirmed by XRD and SEM studies. The thermal stability of two MOFs was investigated, indicating that, the Cu-BDC is more stable than Fe-BDC. Further, the optical properties were investigated in the wavelength range 325–1100 nm, and the Fe-BDC exhibited greater optical transmission properties than Cu-BDC by 33 %, as investigated by Wemple-DiDomenico and Tauc models. The dispersion parameters related to optical studies for Cu-BDC were better in comparison to Fe-BDC, which could be attributed to the increase in Cu valence electrons due to an increase in the number of cations. The electrochemical behavior in terms of CV measurements shows the presence of pseudo capacitance in both Fe-BDC and Cu-BDC MOFs. The improved CV performance of Cu-BDC MOF suggests that it could be used as a storage material. This work successfully demonstrates the tailoring of optical properties related to MOF thin films through the formation of stable complexes using BDC as a potential material for the fabrication of OLED's and Solar cells. The improved CV performance suggests that these MOF based materials could be used as anodes in fabrication of batteries or supercapacitors.
Keywords: Metal-organic frameworks, BDC, Fe-BDC, Cu-BDC, Optical properties
1. Introduction
Metal-organic frameworks (MOFs) are class of porous organic-inorganic hybrid crystalline materials with clusters or metal ions linked to the organic ligands to generate structures with 1D, 2D, or 3 dimensions [1,2]. These MOFs have attracted lot of interest from researchers owing to their superior properties, such as high porosity [3], improved surface area [4], excellent thermal compatibility [5], and good chemical tunability [6]. These unique property makes the MOFs potential materials for various technological applications such as gas/energy storage [7,8], photocatalysis [9], drug delivery [10,11], semiconductor electronics [12,13], and electrochemical/chemical sensing [14,15]. To improve the physiochemical properties of MOF-based materials, it is advantageous to introduce various functional groups into the MOFs. However, the synthesis of highly porous functional MOFs is largely been limited to chemical synthetic methods. Nevertheless, the post-synthesis methods have emerged as easy techniques to achieve the chemical modification in MOFs due to the abundant and readily available reactions for the intermediate linking ligand molecules [6]. Furthermore, the main function of metal ions is to create nodes that can effectively react and bind with the linkers' arms [16]. As such, these nodes are known to impact the basic structural formation of MOFs, leading to the development of specific target functional performances.
1,4-Benzenedicarboxylates are examples of highly promising MOF materials due to their chemical stability, high porosity, and modular decompositions [17]. Transition metals, specifically iron (Fe) and copper (Cu) have been implemented to improve the electrical conductivity of MOFs, as they possess high conductivity [18]. Furthermore, Cu and Fe have been widely studied as semiconductor materials, known for their distinguishable properties such as electrochemical stability, desirable electro-optical properties, low production costs, distinct electronic structures, good isoelectric properties, and large excitation binding energy [19]. According to literature surveys, Pedro et al. [20] investigated the role of cobalt and nickel addition in MOF-525 on charge transport rate, while Masoomi et al. [21] fabricated and studied a mixed complex of different metals within MOFs and found that the metal ions can act as an active site in various MOF applications. Zn dopant was also found to be effective in engineering the visible light absorption and electrical properties of MOF-5, ideal for optoelectronics and sensing applications. You et al. have investigated the co-doping method for BDC to improve the removal of Methylene Blue dye from polluted water [22]. Moreover, Abdollah et al. studied the physical, chemical and optical properties of Cu-BDC, which significantly enhances the photocatalytic degrading performance of about 98 % towards removal of Acid Orange 7 from wastewater samples [23]. Cheng et al. assured that Fe-BDC has excellent corrosion resistance to Cl in simulated seawater [24]. Interestingly, Syzgantseva et al. examined the effect of different metal centers (Mg(II), La(III), and Zr(IV)) on the electronic structure of two different MOFs (MIL-125 and UiO-66) [25]. Additionally, the synthesis and applications of pristine MOF, MOF composites, and their derivatives based on transition metals (Fe, Co, and Ni) for oxygen reduction were investigated and reported. The unique and versatile structural and physio-chemical properties of Fe-BDC MOFs makes them potential candidates for various technological applications [26]. This research aims towards synthesis and characterization of novel MOFs based on Cu-BDC/Fe-BDC, aiming towards improving their optical and electrochemical properties for potential optoelectronic and energy storage applications. Cu-BDC and Fe-BDC are considered Metal-Organic Frameworks (MOFs), which belong to a family of highly porous materials with a broad range of potential applications. By exploring their optical and electrochemical properties, we can gain a better understanding of their behavior and potential usefulness in different fields. The optical properties of our synthesized MOF could prove beneficial in the design and development of more efficient optoelectronic devices, like sensors, light-emitting diodes (LEDs) and solar cells. On the other hand, studying the electrochemical properties of these materials helps us comprehend their behavior in electrochemical devices, such as batteries and supercapacitors. Overall, investigating the optical and electrochemical properties of Cu-BDC and Fe-BDC can offer valuable insights for advancing their applications in various technological areas, ultimately resulting in the creation of more efficient and sustainable devices.
In this study, we report the synthesis and fabrication of thin films using Cu-BDC and Fe-BDC on quartz and glass substrates using a thermal vapor deposition technique. The linear and nonlinear optical properties of these films were reported in detail. The transmission (T) and reflection (R) spectra were analyzed to determine the optical constants such as the extinction coefficient, refractive index, and dielectric constants. The CV measurements of both composites will be investigated. Further, these constants were theoretically compared using the Wemple-DiDomenico (WD) and Tauc models. The outcomes reported in this study facilitate towards understanding the effect of metal ions on the morphological, structural and optical properties of MOFs derived from BDC.
2. Experimental details
2.1. Materials and chemicals
Copper (II) nitrate trihydrate (Cu (NO3)2.2H2O, 99 % pure, Sigma-Aldrich), FeCl3, 6H2O (98 %, Sigma-Aldrich) N,N dimethylformamide (DMF, 99.8 %), hydrochloride acid (HCl, 37 %), ammonium hydroxide (NH4OH, 32 %), were procured from Merck Co. 1,4-Benzene dicarboxylic acid (H2BDC, 99 %, Sigma-Aldrich)) and 3-cyanotripropyltriethoxysilane were procured by sigma-Aldrich Co. The chemicals were directly used as procured without any further purification.
2.2. Syntheses of Cu-BDC and Fe-BDC MOFs
The chemicals used in this study such as Cu (CH3COO)2. H2O, FeCl3.6H2O, terephthalic acid or BDC, and ethanol were of analytical grade and used as received without further purification. To prepare Cu-BDC, 2.1597 g (13 mmol) BDC was dissolved in 50 ml ethanol, and 2.5955 (13 mmol) Cu (CH3COO)2.H2O was then added to the above solution (1:1 M ratio) and mixed to complete dissolution. A microwave oven was employed to enhance the formation of Cu-BDC by heating near dryness, then cooling and addition of 10 mL ethanol, while the microwave heating process was then repeated for three more trials. Finally, the dry Cu-BDC product was dried at 60–70 °C. The preparation of Fe-BDC was accomplished following the similar procedure mentioned above via different molar ratio (1.3:1) by dissolving and mixing 2.1597 g (13 mmol) BDC in 50 mL ethanol with 2.703 g (10 mmol) FeCl3.6H2O. This mixture was heated in a microwave oven to enhance the formation of Fe-BDC by heating near dryness, then cooling and addition of 10 mL ethanol, while the microwave heating process was then repeated for three more trials, and the dry Fe-BDC product was obtained at 60–70 °C. The schematic representation of Cu-BDC and Fe-BDC MOFs preparation and investigation of different properties conducted in this study is shown in Figure-1.
Fig. 1.
Schematic representation of Cu-BDC and Fe-BDC MOFs preparation and their applications in present investigation.
2.3. Instrumentations
The crystal structure of the prepared Cu-BDC and Fe-BDC MOFs were investigated through X-ray diffraction (XRD). The XRD spectra was obtained between 2θ = 4° and 2θ = 80° with Diffractometer (Rigaku RINT 2200, Japan) using Cu Kα as radiation source of wavelength 1.524 Ȧ. The surface morphology of the prepared MOFs thin films was studied using scanning electron microscope (SEM) [27]. A carbon tape with double coating was used to analyze the sample surface at 25 kV, using JEOL-SEM JFC-1100E's. The T and R spectra of the prepared MOFs films were analyzed through a dual beam spectrophotometer (Jenway model 6800 UV/Vis) with range of wavelength between 200 and 1100 nm, with a resolution ± 0.1 nm. Thermogravimetric analysis (TGA and DTGA) was studied through a Shimadzu, Japan, TGA-50 thermal analyzer in the temperature range of 50–700 °C, and the TGA thermograms were obtained with a ramping rate of 10 °C/min till 700 °C and flow rate of 20 mL min−1 in pure nitrogen atmosphere. The chemical features such as functional groups of thin films were studied through FTIR (Shimadzu Japan, FT-IR 8400S Spectrophotometer in KBr pellet media) in the spectral region of 400–4000 cm−1. The electrochemical performance was investigated through CHI608E electrochemical workstation, through a three-electrode system, using Fe-BDC and Cu-BDC modified GCE as the working electrode, platinum wire as a counter electrode, and Ag/AgCl as reference electrode, in 6 M KOH, in the potential window −0.6 to 0V (vs Ag/AgCl electrode).
3. Results and discussions
3.1. Structural investigation
SEM (Scanning Electron Microscopy) is a useful tool for investigating MOFs as it facilitates the surface morphology visualization of the porous structure at high resolution. Figure-2a and 2b display the SEM micrographs of Cu-BDC and Fe-BDC, respectively. The SEM micrograph of the prepared Cu-BDC shows regular particles, high porosity, ordered structure, and homogeneity which proves that the Cu-BDCs were successfully synthesized [28]. Cu-BDC was characterized as a long bar-like and rod like structure via aggregation of spherical and semispherical particles. Furthermore, the grains will have a uniform distribution that could facilitate the optical properties and these properties can be exploited for a wide range of applications, such as optical sensors, optical filters, and photovoltaics. While the SEM image of Fe-BDC exhibits spike-like morphology and hence affects the light propagation in comparison to Cu-BDC. The SEM micrographs reveals that the Cu-BDC have particles of size ranging between 20 and 30 nm, whereas Fe-BDC contains particles of dimensions 15–20 nm.
Fig. 2.
SEM images of (a) Cu-BDC and (b) Fe-BDC MOFs.
The X-ray diffraction patterns related to Cu-BDC and Fe-BDC are depicted in Figure-3a and 3b, respectively. The Cu-BDC show characteristic XRD peaks at 2θ = 7.0°, 14.8°, 17.7°, 18.6°, 22.0°, 24.7°,28.8°, 40.5°, and 44.5° are related to (2 2 0), (2 2 2), (3 3 3), (4 2 0), (4 2 2), (7 7 3), (8 8 2), (4 4 0), and (5 3 3) crystal planes, respectively as previously reported [29,30]. Degree of crystallinity, Xc, for the fabricated Cu-BDC and Fe-BDC thin films can be calculated using [31]:
| (1) |
In the above equation Ac and Aa signifies total crystalline and amorphous regions respectively. Using Eq. (1), the crystallinity values were found 60.6 %, 43.2 %, 49.4 %, 57.2 %, and 43.2 % for Cu-BDC. Whereas, Fe-BDC show the characteristic XRD peaks at 2θ = 17.7°, 25°, 28. 8°, 29.8°, 35°, and 40.4° which are related to (2 1 0), (2 2 1), (3 3 3), (4 2 2), (4 2 1), and (4 4 0). The crystallinity values were identified as 45.6 %, 43.2 %, 59.4 %, 57.2 %, 37 %, and 44.2 % for Fe-BDC. Therefore, XRD analysis concludes that the average crystallinity of Cu-BDC and Fe-BDC are 50 % and 57.32 %, respectively. Therefore, we can expect that Fe-BDC will show a higher optical transparency. Furthermore, crystallinity can influence the diffusion of ions or molecules within the MOF structure. A higher crystallinity within Fe-BDC might enhance the mobility of charge carriers, leading to improved electrochemical behavior, such as faster charge transfer or higher conductivity. Various parameters related to crystal structures of Cu-BDC and Fe-BDC MOFs using XRD analysis have been reported in table-1.
Fig. 3.
XRD patterns of (A) Cu-BDC and (B) Fe-BDC MOFs.
Table 1.
The variation of crystal size, micro-strain, dislocation density, and crystallites per unit area for Cu-BDC and Fe-BDC MOFs.
| Samples | Size (nm) | Microstrain × 10−3 (line−2 m−4) | Dislocation Density × 10−3 (nm−2) |
Crystallites (N) per unit area × 10−10 (m−2) |
|---|---|---|---|---|
| Cu-BDC | 26.21± 2 nm | 3.64 | 0.73442 | 0.15922 |
| Fe-BDC | 17.45 ± 2 nm | 3.57 | 0.65915 | 0.13538 |
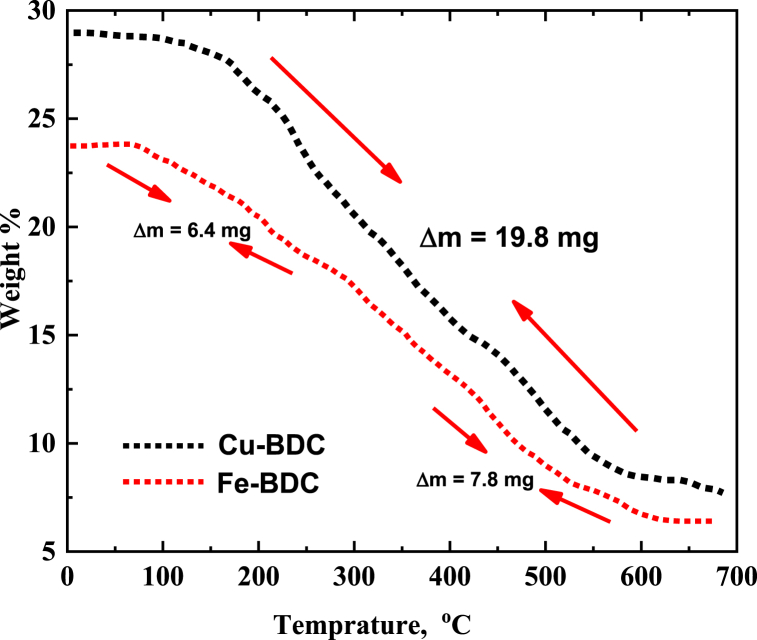
The thermal stability characteristics of the prepared Cu-BDC and Fe-BDC were studied and investigated in the temperature range 50–700 °C using the thermogravimetric analysis technique (TGA) and are represented in Figure-4. It is evident that the Cu-BDC structure was highly stable up to 200 °C with a minimum weight loss of about 0.97 %. Cu-BDC was then found to thermally decompose and produce a significant weight loss (190–540 °C) corresponding to 19.8382 mg out of the initial sample (30 mg) corresponds to 66.13 % weight loss. This sudden degradation is mainly related to the dissociation of organic compounds and loss of BDC moiety from Cu-BDC to finally yield the stable CuO. On the other hand, Fig. 4 shows the TGA thermogram of Fe-BDC with three successive degradation steps at (i) 50–280 °C with a weight loss of 6.43 mg (26.79 % loss), (ii) 280–360 °C with a weight loss of 8.40 mg (35.08 % loss), and (iii) 360–545 °C with weight loss of 5.90 mg (32.96 % loss). These three decomposition steps are mainly related to the loss of water molecules adsorbed on the surface of Fe-BDC, the intermediate step corresponds to thermal degradation of organic molecules from Fe-BDC, and finally, complete decomposition of Fe-BDC leaving only a residual mass corresponding to 3.27 mg (13.63 %). The outlined results of thermal analysis for both MOFs denote that Cu-BDC is thermally more stable than Fe-BDC under the investigated temperature range of 50–700 °C.
Fig. 4.
TGA thermograms of Cu-BDC and Fe-BDC MOFs.
The differential thermal gravimetric analysis (DTGA) for Cu-BDC and Fe-BDC is shown in Figure-5. The DTA figures reveal that, the weight losses of Cu-BDC are 3.79 %, 38 %, and 2.6 % at temperatures of 198 °C, 302 °C, and 472 °C, respectively. On the other hand, the weight loss of Fe-BDC is 41 %, 4.6 %, and 5.4 % at temperatures of 349 °C, 424 °C, and 518 °C, respectively. One possible explanation for the higher weight loss in Fe-BDC compared to Cu-BDC could be attributed to differences in the thermal stability of the two materials. It is possible that Fe-BDC experiences a more significant degradation or decomposition process within the temperature range analyzed in the DTGA (as shown in Figure-5), leading to a greater weight loss compared to Cu-BDC.
Fig. 5.
DTGA thermograms of Cu-BDC and Fe-BDC MOFs.
3.2. Optical measurement
The Transmission (T), and Reflection (R) spectra were recorded in the wavelength range of 200–1100 nm for the MOFs synthesized in the present study. Figure-6 shows T and R spectra with the wavelength (λ) in nm for the Cu-BDC and Fe-BDC samples. As shown, the T of Fe-BDC is greater than Cu-BDC by about 33 % in the wavelength region of 325–1100 nm. This could be related to the difference in the D shell electrons which affects the photon transmission [32]. Furthermore, the horizontal curve of the transmittance in this range for the two doped MOFs indicates its and good homogeneity and low surface roughness. In the wavelength region below 325 nm, a significant decrease in the transmittance of the two doped MOFs is recorded. Also, as seen in Fig. 6, we can divide the spectra into two regions: a highly transparent features noticed in visible and near-IR region whereas a zone of strong absorption is observed in the near-UV region. The reflection of our two doped MOFs shows a wavy shape at the wavelength of less than 400 nm and this could be attributed to the scattering within the material's defects and the degree of crystallinity of the material.
Fig. 6.
Transmission (T) and Reflection (R) spectra of Cu-BDC and Fe-BDC MOFs.
The optical absorption of the films ( extinction coefficient (k) and the refractive index (n) corresponds to two MOFs can be evaluated using equations (2), (3), (4) [33,34]
| (2) |
| (3) |
| (4) |
where d is the thin film thickness. Figure-7 depicts the spectra of k and n for Cu-BDC and Fe-BDC thin films, respectively. Fig. 7a, at the long wavelength, the values of k are exceedingly small because the films are transparent. Fig. 7b shows a slight change in the refractive index in the wavelength range between 500 nm and 1100 nm. Fe-BDC shows a rapid increase in the refractive index of the films in the wavelength range between 300 and 400 nm could be related to the electronic inter-band transition for Fe elements.
Fig. 7.
The spectra of (a) extinction coefficient and (b) refractive index for (a) Cu-BDC and (b) Fe-BDC MOFs films.
In designing optical devices, their performance mainly depends on the study of dispersion behavior. In our analysis, we utilized the single-electronic WDD. It is used to calculate the dispersion of thin films. The Wemple-DiDomenico model is used to analyze the complex dielectric function of materials and extract important optical parameters [35]. It describes the response of a material to external electromagnetic radiation in terms of its refractive index and extinction coefficient. According to the WDD, the refractive index is analyzed using the following equation [36]:
| (5) |
| (6) |
where Es is the single oscillator energy, Ed is the dispersion energy, ε∞ is the high-frequency dielectric constant, the carrier concentration is N, and the vacuum permittivity is εo and they act to describe the optical properties of our fabricated metal MOF film. According to Eq. (5), Figure-8 shows the relation between 1/(n2 -1) and E2 for Cu-BDC and Fe-BDC films. While the evolution of n2 with λ2 as represented in Eq. (6) is illustrated as inset of Fig. 8. The calculated optical parameters for Fe-BDC and Cu-BDC are listed in Table 2. The improved performance of optical parameters of Cu-BDC compared to Fe-BDC could be interpreted by ionization variation that occurs at the Cu center of Cu-BDC has more valence electrons than that in Fe. This is mainly due to increase in the interaction between cations and neighboring anions [37].
Fig. 8.
The refractive index variation for (a) Cu-BDC and (b) Fe-BDC MOFs.
Table 2.
Dispersion parameters of Cu-BDC and Fe-BDC MOFs.
| Samples | Eo (eV) | Ed (eV) | M-1 = Ed/Eo | M-3 = Ed/Eo3 | ε∞ | εL | N/m* (1047 g−1cm−3) |
|---|---|---|---|---|---|---|---|
| Cu-BDC MOF | 1.89 | 71.61 | 37.89 | 10.61 | 15.56 | 19.84 | 3.58 |
| Fe-BDC MOF | 1.97 | 30.37 | 15.42 | 3.97 | 6.59 | 7.94 | 1.15 |
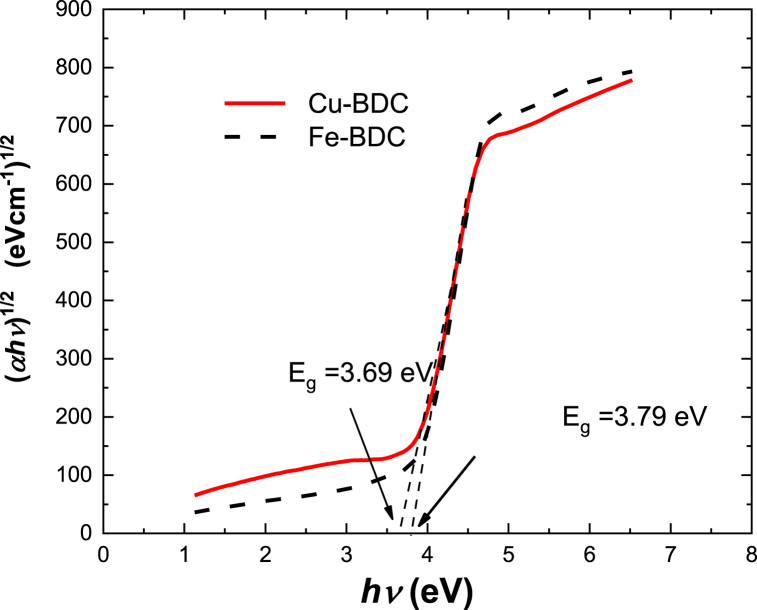
The regions between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular (HOMO) is commonly referred as optical energy band gap Eg, which is mainly responsible for the electronic transitions within the material. The Tauc model is used to determine the optical bandgap of a material. The model is based on the understanding that the absorption of light in solids occurs due to transitions of electrons between energy levels. It describes the absorption coefficient as a function of photon energy and involves fitting experimental data to determine the extrapolated Tauc plot. The Tauc plot is a graphical representation of the absorption coefficient as a function of energy of photon, allowing the determination of the bandgap energy indirectly. According to Tauc's relation, Eg can be calculated using Eq. (7) mentioned below [38]:
| (7) |
where X is a constant, E is the photon energy, and the values of q depends on the type of transition accordingly it takes the values ½ for an indirect transition and 2 for a direct transition respectively. Figure-9 shows the relation between (αhν)1/2 and E for Cu-BDC and Fe-BDC films and two calculated Eg are described in this figure. The values of Eg for Cu-BDC and Fe-BDC films are 3.7 eV and 3.8 eV. The value of Eg for Fe-BDC is much greater than Cu-BDC by about 0.1 eV, this could be related to dislocations and vacancies, within the microstructure film. The obtained values of Eg correspond with that determined by Lin et al. who measured the optical bandgap of several types of MOFs such as Zn-BDC and found Eg to be 3.8 eV [36]. The BDC–NH2 energy gap was about 2.95 and it was calculated by Musho et al. [38].
Fig. 9.
The dependence of (αhν)1/2 on hν for (a) Cu-BDC and (b) Fe-BDC MOFs.
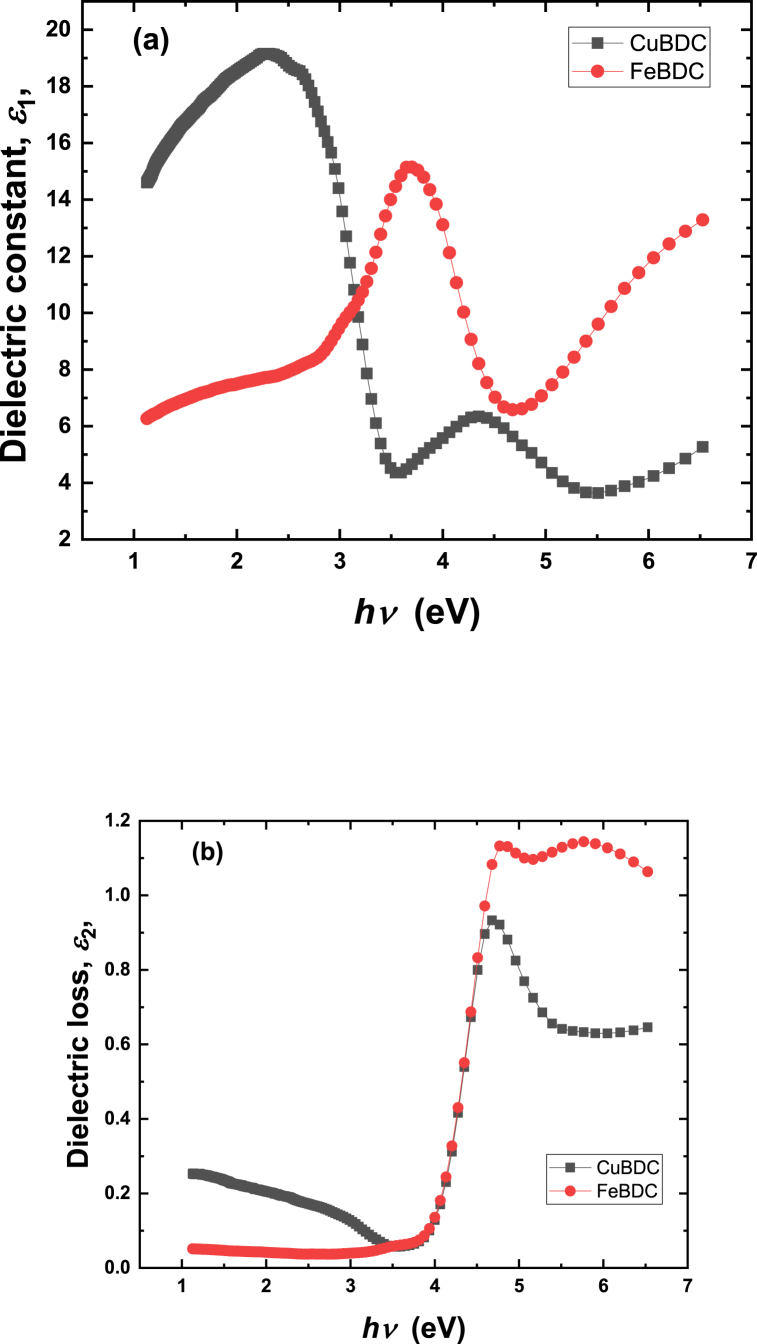
The synergetic interactions between electrons and photons inside the material can be realized by analyzing the complex dielectric constants. The complex dielectric constant of the material ε*, can be obtained by using the following relations (8-10) [39]:
| (8) |
| (9) |
| (10) |
where ε1 is the real dielectric constant and ε2 is imaginary constant (dielectric loss). Figure-10 shows the plot of the dielectric constant and the dielectric loss of Cu-BDC and Fe-BDC films. The difference that appears between Cu-BDC and Fe-BDC could be explained based on interactions of the central metal electrons.
Fig. 10.
The spectra of (a) dielectric constant (ε1), and (b) dielectric loss (ε2) for (a) Cu-BDC and (b) Fe-BDC MOFs.
3.3. Nonlinear optical measurements
Nonlinear optical properties of the materials could be studied when the material is exposed to laser light with high intensity. The nonlinear optical properties are remarkably interesting due to the wide variety of applications ranging from optoelectronics to medicine such as optical fiber cables and optical gadgets modulators, etc.
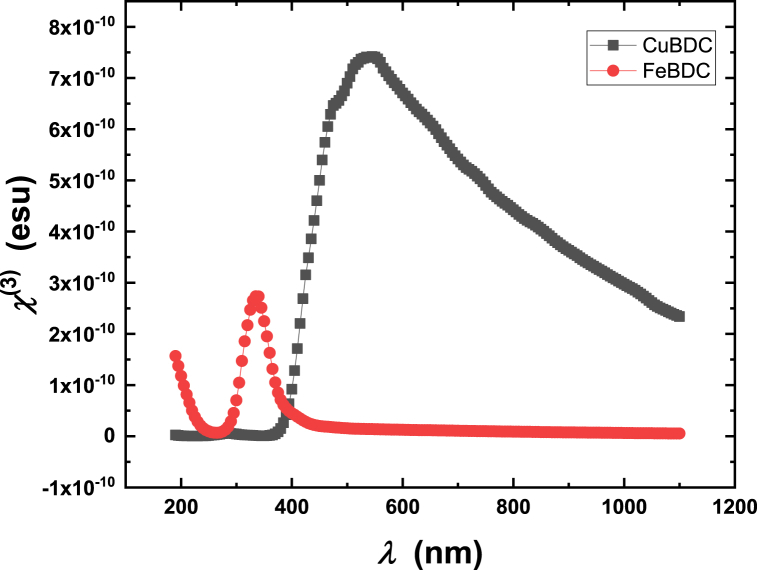
The nonlinear optical properties mainly depends on third order nonlinear susceptibility (χ(3)) and nonlinear refractive index (n2). Using the Miller's theoretical model, n2 and χ(3) can be obtained using the below mentioned relations (11–12) [40,41]:
| (11) |
| (12) |
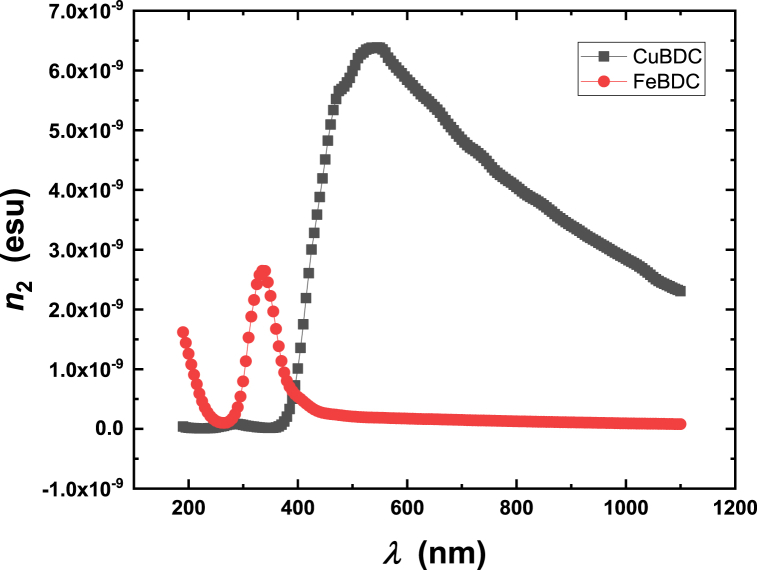
where no is the linear refractive index. Figure-11 depicts the spectra of χ(3) of Cu-BDC and Fe-BDC films. Fig. 12 shows the spectra of n2 of Cu-BDC and Fe-BDC MOF films. As shown in Fig. 11, Fig. 12, the values of χ(3) and n2 for Cu-BDC are much greater than that in Fe-BDC. The interpretation of the high nonlinearity in Cu-BDC is that it has valence electrons increase which can create more d-π interaction within MOFs that act to strengthen the nonlinear optical properties. The higher nonlinearity of Cu-BDC compared to Fe-BDC can have several potential applications such as frequency doubling, optical parametric amplification, or optical switching. Furthermore, the higher nonlinearity of Cu-BDC could be beneficial in devices such as optical switches, logic gates, or memory elements. These materials could enable faster and more efficient processing of data in electronic devices.
Fig. 11.
The spectra of third nonlinear optical susceptibility, χ(3), for (a) Cu-BDC and (b) Fe-BDC MOFs.
Fig. 12.
The spectra of nonlinear refractive index, n2, for (a) Cu-BDC and (b) Fe-BDC MOFs.
3.4. Electrochemical studies
The electrochemical investigation is one of the important tools to understand the possible energy storage capabilities of a material. Figure-13 shows the electrochemical performance using GCE-modified Fe-BDC and Cu-BDC MOF electrodes in terms of CV measurements at the scan rate of 40 mVs−1. The existence of redox peaks in cathodic and anodic scans of the CV curves in both Fe-BDC and Cu-BDC MOFs indicates a presence of significant pseudo-capacitance in the material during the electrochemical reactions, which indicates the possibility of using these materials for energy storage. In Fig. 12 it can be noticed that the Cu-BDC shows improved electrochemical performance than the Fe-BDC indicating that it is better material for energy storage than the Fe-BDC. The improved electrochemical performance of the Cu-BDC could be related to its mesoporous and crystalline structure. The favorable structure such as porosity and crystallinity in Cu-BDC, facilitates the diffusion of electrolytes and improves electrochemical reactions inside the material [42]. Apart from that, these mesopores in the composite material acts as active sites to facilitate interactions between the surface atoms of the electrode and the electrolytic ions, due to improved charge density in the composite structure, it facilitates the higher charge transfer at the surface electrode. In conclusion, the improved diffusion rate and pore structures could be responsible for better CV performance of the Cu-BDC. The electrochemical performance indicates that Cu-BDC could possibly be used as energy storage materials in the fabrication of high-performance supercapacitors.
Fig. 13.
CV curves for GCE modified Fe-BDC and Cu-BDC MOFs electrodes at the scan rate of 40 mVs−1..
4. Conclusions
Herein we report the successful formation of Fe-BDC and Cu-BDC and to explore their linear and non-linear optical characteristics and improved electrochemical performance towards possible energy storage materials. The high porosity and the pore size of MOFs can be achieved by choosing appropriate metal nodes and linkers. The metal centers in MOFs play a vital role in improving their linear and non-linear optical properties. The optical parameters such as refractive index and dielectric constants were calculated by measuring the transmission spectra. Also, the optical bandgap values were calculated. Furthermore, the values of χ(3) and n2 for Cu-BDC are much greater than that in Fe-BDC. The interpretation of the high nonlinearity in Cu-MOF is mainly due to an increase in valence electrons that can create more d-π interactions in the MOFs. The electrochemical behavior of both Fe-BDC and Cu-BDC MOFs shows the presence of pseudocapacitance in their CV curves. The improved CV performance of Cu-BDC MOF in comparison to Fe-BDC MOFs associated with the presence of pseudo capacitance suggests that Cu-BDC MOF could be a promising material for energy storage applications. Hence, this research provides fundamental insights into the structural aspects, optical and electrochemical performance of the Fe-BDC and Cu-BDC MOFs. Owing to superior structural features, improved optical and electrochemical properties, through the findings of this study, we propose these Fe-BDC and Cu-BDC could be potentially used in optoelectronic and electrochemical energy storage applications. Further in-depth analysis of the materials properties of these novel MOFs could open new avenues in opto-electronic devices as well as energy storage industries.
Statements and declarations
The authors declare that there is no conflict of interest in the manuscript.
Data availability statement
The Authors confirm that the datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Taymour A. Hamdalla: Writing – review & editing, Writing – original draft, Supervision, Investigation, Data curation, Conceptualization. S. Alfadhli: Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization. Syed Khasim: Writing – review & editing, Writing – original draft, Validation, Supervision, Formal analysis, Data curation, Conceptualization. A.A.A. Darwish: Writing – original draft, Software, Investigation, Conceptualization. E.F.M. ElZaidia: Writing – original draft, Resources, Methodology, Investigation, Formal analysis. S.A. Al-Ghamdi: Writing – original draft, Software, Methodology, Investigation, Conceptualization. Meshari M. Aljohani: Writing – review & editing, Validation, Methodology, Investigation. Mohamed E. Mahmoud: Writing – original draft, Software, Methodology, Data curation, Conceptualization. Seleim M. Seleim: Writing – original draft, Supervision, Resources, Investigation, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number (0117-1443-S)
References
- 1.Hendon C.H., Rieth A.J., Korzy ′nski M.D., Dincă M. Grand challenges and future opportunities for metal-organic frameworks. ACS Cent. Sci. 2017;3:554–563. doi: 10.1021/acscentsci.7b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sindoro M., Yanai N., Jee A.Y., Granick S. Colloidal-sized metal-organic frameworks: synthesis and applications. Acc. Chem. Res. 2014;47:459–469. doi: 10.1021/ar400151n. [DOI] [PubMed] [Google Scholar]
- 3.Khalil Islam E., Xue Cong, Liu Wenjing, Li Xiaohan, Shen Yu, Li Sheng, Zhang Weina, Huo Fengwei. The role of defects in metal–organic frameworks for nitrogen reduction reaction: when defects switch to features. Adv. Funct. Mater. 2021;31(17) [Google Scholar]
- 4.Lin Rui-Biao, Zhang Zhangjing, Chen Banglin. Achieving high performance metal–organic framework materials through pore engineering. Acc. Chem. Res. 2021;54(17):3362–3376. doi: 10.1021/acs.accounts.1c00328. [DOI] [PubMed] [Google Scholar]
- 5.Hamdalla Taymour A., Aboraia A.M., Darwish A.A.A., Al-Ghamdi S.A., Alfadhli S., Alexander Soldatov. Effect of Mil-88a metal-organic framework coating on the electrochemical properties of LiCoPO4. Ceram. Int. 2023;49(1):1214–1219. [Google Scholar]
- 6.Cohen S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012;112:970–1000. doi: 10.1021/cr200179u. [DOI] [PubMed] [Google Scholar]
- 7.Liao Li-Guo, Duo Ke, Li Guo-Chen, Zhang Sheng, Li Bang-Jing. Cyclodextrin metal-organic framework as a broad-spectrum potential delivery vehicle for the gasotransmitters. Molecules. 2023;28(2):852. doi: 10.3390/molecules28020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y., Wang L., Amer W.A., Yu H., Ji J., Huang L., Shan J., Tong R. Hydrogen storage in metal-organic frameworks. J. Inorg. Organomet. Polym. Mater. 2013;23:270–285. [Google Scholar]
- 9.Lykourinou V., Chen Y., Wang X.S., Meng L., Hoang T., Ming L.J., Musselman R.L., Ma S. Immobilization of MP-11 into a mesoporous metal-organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011;133:10382–10385. doi: 10.1021/ja2038003. [DOI] [PubMed] [Google Scholar]
- 10.Wu M.X., Yang Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017;29 doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 11.Al-Jouhani Saja, Al-Azwari Raghad, Al-Shemari Sara, Al-Anezi Rawan, Syed Khasim, Mohamed S., Al-Ghamdi S.A., Darwish A.A., Hamdalla Taymour A. Journal of Umm Al-Qura University for Applied Sciences; 2023. The Effect of Human Serum Albumin on ZIF-8 Used in Drug Delivery: Structural, Linear and Nonlinear Optical Properties; pp. 1–8. [Google Scholar]
- 12.Shang C., Gautier R., Jiang T., Faulques E., Latouche C., Paris M., Cario L., Bujoli-Doeuff M., Jobic S. A p-type zinc-based metal–organic framework. Inorg. Chem. 2017;56:6208–6213. doi: 10.1021/acs.inorgchem.7b00198. [DOI] [PubMed] [Google Scholar]
- 13.Stassen I., Burtch N., Talin A., Falcaro P., Allendorf M., Ameloot R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017;46:3185–3241. doi: 10.1039/c7cs00122c. [DOI] [PubMed] [Google Scholar]
- 14.Wang Zhaolong, Wang Yaru, Yan Jun, Liu Bin, Chen Yunlin, Tian Yahui. Metal–organic framework-based photonic crystal platforms for gas sensing: a review. Materials Advances. 2022;3(17):6728–6741. [Google Scholar]
- 15.Ashraf G., Chen W., Asif M., Aziz A., Zhong Z.-T., Iftikhar T., Zhao Y.-D. Topical advancements in electrochemical and optical signal amplification for biomolecules detection: a comparison. Mater. Today Chem. 2022;26 [Google Scholar]
- 16.Jia Tao, Gu Yifan, Li Fengting. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: a review. J. Environ. Chem. Eng. 2022 [Google Scholar]
- 17.Wang Shunzhi, Michael McGuirk C., d'Aquino Andrea, Mason Jarad A., Mirkin Chad A. Metal-organic framework nanoparticles. Adv. Mater. 2018;30(37) doi: 10.1002/adma.201800202. [DOI] [PubMed] [Google Scholar]
- 18.Lu Weigang, Wei Zhangwen, Gu Zhi-Yuan, Liu Tian-Fu, Park Jinhee, Park Jihye, Tian Jian, et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014;43(16):5561–5593. doi: 10.1039/c4cs00003j. [DOI] [PubMed] [Google Scholar]
- 19.Miles David O., Jiang Dongmei, Burrows Andrew D., Halls Jonathan E., Frank Marken. Conformal transformation of [Co (bdc)(DMF)](Co-MOF-71, bdc= 1, 4-benzenedicarboxylate, DMF= N, N-dimethylformamide) into porous electrochemically active cobalt hydroxide. Electrochem. Commun. 2013;27:9–13. [Google Scholar]
- 20.Arturo Pedro, Rodríguez-Sevilla Erika, Ana Sofía Varela The role of the metal center on charge transport rate in MOF-525: cobalt and nickel porphyrin. Dalton Trans. 2021;50(46):16939–16944. doi: 10.1039/d1dt03435a. [DOI] [PubMed] [Google Scholar]
- 21.Masoomi Mohammad Yaser, Ali Morsali, Dhakshinamoorthy Amarajothi, Garcia Hermenegildo. Mixed‐metal MOFs: unique opportunities in metal-organic framework (MOF) functionality and design. Angew. Chem. 2019;131(43):15330–15347. doi: 10.1002/anie.201902229. [DOI] [PubMed] [Google Scholar]
- 22.You Deng, Shi Hui, Peng Mingming, Yang Liming, Shao Penghui, Yin Kai, Wang Haozhi, Luo Shenglian, Luo Xubiao. Metal-center effect induced efficient charge transfer of metal-organic framework for strengthening Sb (V) capture performance. Nano Res. 2022;15(9):8516–8523. [Google Scholar]
- 23.Abdollahi Bahman, Farshnama Sina, Abbasi Asl Ebrahim, Ahmad Najafidoust, Sarani Mina. Cu (BDC) metal-organic framework (MOF)-based Ag2CrO4 heterostructure with enhanced solar-light degradation of organic dyes. Inorg. Chem. Commun. 2022;138 [Google Scholar]
- 24.Cheng Yikun, Luo Yun, Yang Zheng, Pang Jianxiang, Sun Kaisheng, Hou Juan, Wang Gang, Guo Xuhong, Chen Long. Self-supporting one-dimensional ZnFe-BDC for electrocatalysis oxygen evolution reaction in alkaline and natural seawater. Int. J. Hydrogen Energy. 2022;47:35655–35665. [Google Scholar]
- 25.Syzgantseva Maria A., Ireland Christopher Patrick, Mish Ebrahim Fatmah, Smit Berend, Syzgantseva Olga A. Metal substitution as the method of modifying electronic structure of metal-organic frameworks. J. Am. Chem. Soc. 2019;141(15):6271–6278. doi: 10.1021/jacs.8b13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Hongye, Fu Weichu, Soulmi Nadia, Serre Christian, Steunou Nathalie, Rosso Michel, Henry de Villeneuve Catherine. Growth of Fe-BDC metal-organic frameworks onto functionalized Si (111) surfaces. Chem. Asian J. 2022;17 doi: 10.1002/asia.202200129. [DOI] [PubMed] [Google Scholar]
- 27.Liang Qiannan, Chen Jianmin, Wang Fengliang, Li Yingwei. Transition metal-based metal-organic frameworks for oxygen evolution reaction. Coord. Chem. Rev. 2020;424 [Google Scholar]
- 28.Ma Xiao, Zhao Xue, Sun Jian, Li Dehui, Yang Xiurong. A versatile strategy to fabricate MOFs/carbon material integrations and their derivatives for enhanced electrocatalysis. RSC Adv. 2016;6(10):7728–7735. [Google Scholar]
- 29.Kaur Ramanpreet, Kaur Amandeep, Ahmad Umar, Anderson William A., Kumar Kansal Sushil. Metal-organic framework (MOF) porous octahedral nanocrystals of Cu-BTC: synthesis, properties, and enhanced adsorption properties. Mater. Res. Bull. 2019;109:124–133. [Google Scholar]
- 30.Shete Meera, Kumar Prashant, Bachman Jonathan E., Ma Xiaoli, Smith Zachary P., Xu Wenqian, Andre Mkhoyan K., Long Jeffrey R., Tsapatsis Michael. On the direct synthesis of Cu (BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Membr. Sci. 2018;549:312–320. [Google Scholar]
- 31.Nafee Sherif S., Hamdalla Taymour A., Darwish A.A.A. Studies of the morphology and optical properties of nano erbium oxide embedded in PMMA matrix. Opt Laser. Technol. 2020;129 [Google Scholar]
- 32.Madhu Ragunath, Karmakar Arun, Krishnendu Bera ab, Nagappan Sreenivasan, Dhandapani Hariharan N., De Aditi, Roy Suprobhat Singha, Kundu Subrata. Recent developments in transition metal-basedMOFs for electrocatalytic water splitting emphasizing fundamental and structural aspects. Mater. Chem. Front. 2023;7:2120. [Google Scholar]
- 33.Hamdalla Taymour A., Seleim Seleim M., Mohamed Rabah Hanem A., Darwish A.A.A., Hanafy T.A., Mahmoud Mohamed E. Synthesis, characterization and optical properties of nanosized lanthanum (III) complexes thin film with aryl-azo-pyrogallol derivatives. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020;238 doi: 10.1016/j.saa.2020.118448. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ghamdi S.A., Darwish A.A.A., Hamdalla Taymour A., Alzahrani Ahmed Obaid M., Syed Khasim, Qashou Saleem I., Abd El-Rahman K.F. Preparation of TlInSe2 thin films using substrate temperature: characterization, optical and electrical properties. Opt. Mater. 2022;129 [Google Scholar]
- 35.Wemple S.H., DiDomenico M., Jr. Theory of the elasto-optic effect in nonmetallic crystals. Phys. Rev. B. 1970;1(1):193. [Google Scholar]
- 36.Rashad M., Abd-Elnaiem Alaa M., Hanafy T.A., Shaalan N.M., Shamekh A.M.A. Optical properties of functional Al2O3 nano-filler in eco-friendly PVA polymer for flexible optoelectronic devices. Opt. Mater. 2023;141 [Google Scholar]
- 37.Abd-Elnaiem, Alaa M., Hamdalla Taymour A., Seleim Seleim M., Hanafy T.A., Aljohani Meshari, Rashad M. Influence of incorporation of gallium oxide nanoparticles on the structural and optical properties of polyvinyl alcohol polymer. J. Inorg. Organomet. Polym. Mater. 2021;31(10):4141–4149. [Google Scholar]
- 38.Musho Terence, Li Jiangtan, Wu Nianqiang. Band gap modulation of functionalized metal–organic frameworks. Phys. Chem. Chem. Phys. 2014;16(43):23646–23653. doi: 10.1039/c4cp03110e. [DOI] [PubMed] [Google Scholar]
- 39.Lin Chi-Kai, Zhao Dan, Gao Wen-Yang, Yang Zhenzhen, Ye Jingyun, Xu Tao, Ge Qingfeng, Ma Shengqian, Liu Di-Jia. Tunability of band gaps in metal-organic frameworks. Inorg. Chem. 2012;51(16):9039–9044. doi: 10.1021/ic301189m. [DOI] [PubMed] [Google Scholar]
- 40.Al-Ghamdi S.A., Hamdalla Taymour A., El-Zaidia E.F.M., Alzahrani Ahmed Obaid M., Alghamdi Nawal, Syed Khasim, Yahia I.S., Darwish A.A.A. Structural, electronic, and optoelectronic characteristics of GaClPc/n-Si heterojunction for photodiode device. Mater. Sci. Semicond. Process. 2022;147 [Google Scholar]
- 41.Syed Khasim, Pasha Apsar, Lakshmi Mohana, Chellasamy Paneerselvam, Kadarkarai Murugan, Darwish A.A.A., Hamdalla Taymour A., Al-Ghamdi S.A., Alfadhli S. Post treated PEDOT-PSS films with excellent conductivity and optical properties as multifunctional flexible electrodes for possible optoelectronic and energy storage applications. Opt. Mater. 2022;125 [Google Scholar]
- 42.Syed Khasim, Dastager Syed G., Alahmdi Mohammed Issa, Hamdalla Taymour A., Panneerselvam Chellasamy, Makandar Mohammad Basha. Novel Biogenic Synthesis of Pd/TiO@BC as an electrocatalytic and possible energy storage materials. Ceram. Int. 2023;49:15874–15883. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Authors confirm that the datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.