Abstract
Kidney involvement in patients with lupus highly increases morbidity and mortality. In recent years, several reports have emphasized the dissociation between clinical and histological findings and highlighted the role of kidney biopsy as an instrument for diagnosis and follow-up of lupus nephritis. The kidney biopsy at initial diagnosis allows an early diagnosis, assessment of activity and chronicity, and detection of nonimmune complex nephritis. A kidney biopsy repeated months after treatment aids in the detection of persistent histological inflammation, which has been linked to the occurrence of future kidney relapses. A kidney biopsy at a relapse detects histological changes including chronic scarring. Finally, a kidney biopsy in patients with a clinical response undergoing maintenance immunosuppression may aid therapy tapering and/or suspension. The evidence supporting the use of a kidney biopsy in different scenarios across the course of lupus nephritis is heterogeneous, with most reports assessing the value for the diagnosis of a first or relapsing flare. In contrast, less evidence suggests additional therapeutic-modifying information derived from repeat posttreatment biopsies and biopsies to evaluate treatment tapering or suspension. In this clinical case-based review, we examine the role of kidney biopsy as a tool to improve clinical outcomes of patients with lupus nephritis.
Index Words: Biomarkers, histology, kidney biopsy, lupus, lupus nephritis, repeat, systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that affects the kidneys in 30%-60% of patients. Lupus nephritis (LN) can occur at any point during a patient’s life, though it more commonly occurs during the early phase of SLE and tends to have a more aggressive phenotype in men with SLE than in women.1 Kidney involvement in SLE is associated with increased morbidity and mortality, with adjusted mortality rates from 6-26 times higher in patients with LN or kidney failure, respectively.2 The disease prognosis varies according to several parameters, such as age, sex, race, and ethnicity. As such, Black and Hispanic patients are more likely to progress to kidney failure compared to White patients.3 Despite recent advances in drug development, the progression rates of LN have not been substantially modified.4 The advent of new treatment options in LN warrants better use of current diagnostic and follow-up tools.
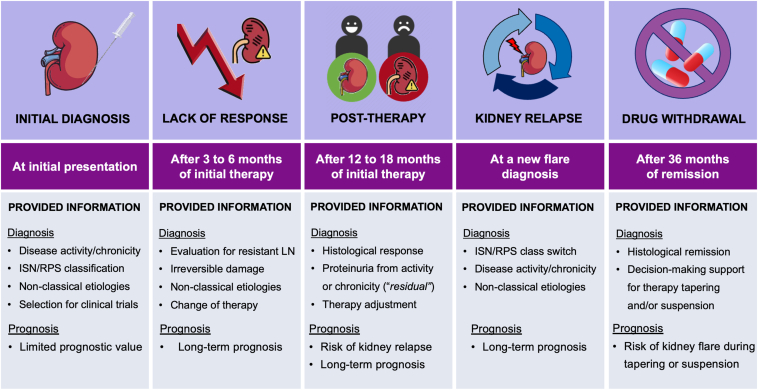
The kidney biopsy remains the gold standard for diagnosing and evaluating both LN inflammatory activity and chronic damage and aids in determining the underlying pathology and individualized treatment approach.5 The LN histological classification has undergone modifications to be more functional and as evidence-based as possible. However, there is still room for improvement as new evidence emerges.6 Besides a role in evaluating kidney disease at the initial phase, during follow-up, a kidney biopsy can be used to assess response to therapy, differentiate proteinuria from inflammation or chronic damage, monitor drug tapering and suspension, and potentially provide insight into LN pathophysiology (Fig 1). The evidence supporting the use of a kidney biopsy in diverse stages of LN is varied with many gaps of knowledge yet to be filled.
Figure 1.
Utility of kidney biopsy in the management of patients with lupus nephritis throughout different clinical scenarios. Abbreviations: ISN/RPS, International Society of Nephrology/Renal Pathology Society; LN, lupus nephritis.
In this evidence-based review, we will discuss the utility of a kidney biopsy for various clinical scenarios across the clinical course of LN. We present a clinical case vignette with complete follow-up, emphasizing the potential utility of the kidney biopsy at each stage of the disease.
Search Strategy
We performed an electronic search of PubMed, Scopus, and EMBASE for publications between 2000 and 2022, in English or Spanish language, using the following MeSH terms or their combinations: “renal biopsy,” “kidney biopsy,” “lupus nephritis,” “systemic lupus erythematosus,” “glomerular disease”. The articles were segregated into those evaluating the kidney biopsy for diagnosis, assessment of response to therapy, kidney flares, or immunosuppression tapering and/or suspension. The review is structured in 4 sections, covering current evidence and evidence gaps for the utility of the kidney biopsy in each of these scenarios.
The Role of Kidney Biopsy at Initial Presentation
Clinical Vignette
A 20-year-old Hispanic woman presents for evaluation of new findings of proteinuria and microscopic hematuria noted on routine urinalysis. She was diagnosed with SLE one year previous, with a history of serositis and musculoskeletal involvement. The current treatment regimen includes prednisone 5 mg daily and hydroxychloroquine 300 mg daily. Her family history is significant for type 2 diabetes. Physical examination is remarkable for a body mass index of 28 kg/m2, blood pressure of 148/92 mm Hg, and mild bilateral peripheral edema. Laboratory investigations demonstrated serum creatinine 0.7 mg/dL, urine protein-creatinine ratio (UPCR) from a 24-hour urine collection of 2,100 mg/g, urinalysis with 8-10 red blood cells (RBC)/hpf, and 10 white blood cells (WBC)/hpf, low C3 and C4, and high anti-dsDNA antibodies. Urine culture did not show any growth. The kidney ultrasound is unremarkable. Would you perform a kidney biopsy?
Kidney Biopsy for Diagnosis of Lupus Nephritis
The initial kidney biopsy may be used to diagnose and classify LN. It also evaluates the degree of active inflammation and chronic kidney damage, which may allow an individualized therapeutic plan and have prognostic implications. There is a broad spectrum of histologic patterns of kidney involvement in LN.7 The most common glomerular pathology in LN is secondary to immune complex deposition. The location of the immune deposits and the percentage of affected glomeruli define the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification.8 Furthermore, other nonimmune complex-dependent pathologies (eg, lupus podocytopathy, collapsing nephropathy, thrombotic microangiopathy, interstitial nephritis) may occur and are not included in the ISN/RPS classification (Table 1).9, 10, 11, 12
Table 1.
Current Suggestions for Lupus Nephritis Classification and Reporting
| Histological lupus nephritis classes definitions | |
|---|---|
| Class I | Minimal mesangial lupus nephritis |
| Class II | Mesangial proliferative lupus nephritis |
| Class III | Focal lupus nephritis |
| Class IV | Diffuse lupus nephritis |
| Class V | Membranous lupus nephritis |
| Class VI | Advanced sclerosing lupus nephritis |
| Activity and chronicity scoring system | |
| Modified NIH activity index (0-24 points) | Summarizes the findings of active lesions:
|
| Modified NIH chronicity index (0-12 points) | Summarizes the findings of chronic lesions:
|
| Additional features to be included in the report | |
| Atypical morphological patterns | Collapsing lupus glomerulopathy |
| Tubulointerstitial lesions | Description of lesions different than those specified in the scoring system |
| Vascular lesions | Including arterial or arteriolar sclerosis, lupus vasculopathy, thrombotic microangiopathy, and vasculitis |
| Electron microscopy findings | Lupus podocytopathy, antimalarial podocyte toxicity, tubuloreticular inclusions |
| Previously used features with questionable usefulness | |
| Specification of “A,” “A/C,” “C” | The scoring system specified above provides more information than the terms “A” (active), “A/C” (active/chronic), and “C” (chronic) |
| Terms “S” and “G” | The clinical importance of distinguishing between “S” (segmental) and “G” (global) has been questioned |
Abbreviation: NIH, National Institutes of Health.
Correlation Between Clinical and Histological Findings
The clinical presentation of LN is highly heterogeneous, ranging from asymptomatic deposition of immune complexes (“silent LN”) to a rapidly progressive nephritic syndrome.13 Traditionally, specific clinical presentations have been associated with certain histological kidney findings. Nevertheless, several reports have demonstrated the agreement between clinical presentation and histopathologic findings in the kidney biopsy is moderate to poor.14, 15, 16 Hence, patients with low proteinuria and inactive urinary sediment may present with class III, IV, or V LN in kidney biopsy.17 Moreover, nephrotic syndrome, which is usually thought to predict an underlying class V LN in the kidney biopsy, is more frequently found in class IV LN.15
Contrarily, and although not formally studied, the nonimmune complex-mediated kidney involvement may be associated with a more predictable clinical presentation. For example, lupus podocytopathy usually manifests as nephrotic syndrome, while collapsing glomerulopathy and thrombotic microangiopathy commonly present with severe hypertension and impaired kidney function.18, 19, 20 However, due to the low incidence of these lesions, the differentiation from immune complex-mediated LN often requires a kidney biopsy.
Clinical Implications of the Activity and Chronicity Description
The categorization of a kidney biopsy into an ISN/RPS LN class does not necessarily indicate if there is underlying inflammation (“activity”) or exclusively scarring from previous nephritis (“chronicity”). Hence, the 2003 ISN/RPS LN classification added an “A,” “A/C,” or “C” to class III or IV LN to assess for active, active and chronic, or chronic disease, respectively.7 The most recent revision of the ISN/RPS LN classification and clinical practice guidelines suggests a detailed description of each activity and chronicity item in the kidney biopsy reports, which may be integrated into the activity and chronicity indices (Table 1).6,21 The activity and chronicity indices may be more important for decision making in the case of class III/IV LN. The chronicity index in the initial biopsy at presentation has been associated with response to therapy22 and long-term kidney prognosis in several studies.23, 24, 25 Nonetheless, as mentioned below, the prognostic yield of these indices for long-term clinical outcomes is better when evaluated in a repeat kidney biopsy performed after treatment.
Lupus Nephritis With Low Proteinuria
Current guidelines have a proteinuria-centric focus and suggest consideration of a kidney biopsy for patients with persistent UPCR above 500 mg/g, with or without urinary sediment positive for dysmorphic RBCs, RBC or WBC casts.21 Applying this approach to an individual level may lead to a missed opportunity for early recognition of LN in some patients. A significant inflammatory disease may be observed in patients with proteinuria below this threshold and even in patients without any evidence of clinical involvement.16,26, 27, 28, 29 In most of these series, the additional finding of low complement C3 and/or positive anti-dsDNA antibodies was associated with a higher degree of inflammatory activity in the kidney biopsy or a higher risk of progressive disease.26, 27, 28, 29
Conservative Approach of Treating Without an Initial Kidney Biopsy
The rate of major complications of the kidney biopsy procedure varies across centers but approximates 2%-3%.30,31 Several reasonings have been provided to start treatment without a kidney biopsy: (1) many cases of LN that warrant aggressive therapy can be diagnosed clinically; (2) the prognostic value of the initial kidney biopsy to predict response to therapy and long-term outcomes is limited; (3) there may be a lack of agreement in the interpretation among community pathologists; and (4) there is a risk for potential delays in therapy initiation.32, 33, 34 Therefore, some groups advocate for a conservative approach that involves withholding kidney biopsy, and early start of treatment, while reserving kidney biopsy for patients with a lack of response to treatment. This conservative approach seems reasonable for patients at risk for major complications of the kidney biopsy procedure (thrombocytopenia, abnormal coagulation tests, small kidneys), and for centers with low access to or with high rates of complications of the kidney biopsy procedure.35 A small study suggested that the long-term outcomes may not differ with or without the use of a kidney biopsy in patients with clinical data strongly suggesting active LN who were not candidates for a kidney biopsy.36
In conclusion, the initial diagnosis of LN involves several scenarios where the advantages and disadvantages of performing a kidney biopsy must be weighed by the health care team and thoroughly discussed with the patient (Table 2). The decision must consider the individual risks as well as the potential for the kidney biopsy to modify diagnosis, prognosis, and treatment.37, 38, 39, 40, 41, 42, 43, 44, 45
Table 2.
Pros and Cons of a Diagnostic Kidney Biopsy in Patients With Lupus Nephritis
| Pros | Cons |
|---|---|
|
|
Abbreviations: LN, lupus nephritis; SLE, systemic lupus erythematous; TMA, thrombotic microangiopathic.
Clinical Vignette Revisited
The case depicts a patient recently diagnosed with SLE and hypertension, with clinical evidence of disease activity, including low serum complement levels and an active urinalysis suggestive of LN. The pretest probability of finding an active immune complex-mediated LN warranting intense immunosuppressive therapy is high. Hence, from a diagnosis perspective, the utility of a kidney biopsy may be questioned and may not substantially modify the therapeutic decision of increasing immunosuppression. Nevertheless, determining the underlying pathology and location of injury (glomerular, tubulointerstitial, or vascular) and the degree of activity and chronicity may aid in developing an individualized therapeutic plan for the patient. Prompt diagnosis and initiation of appropriate therapy have been associated with improved clinical outcomes.
The Post-therapy Kidney Biopsy: Histological Response, Therapy Adjustment, and Prognosis
Clinical Vignette
The patient underwent a kidney biopsy, which showed ISN/RPS class IV LN with an activity index of 10/24 (endocapillary hypercellularity, karyorrhexis, leukostasis, and interstitial inflammation) and a chronicity index of 2/12. She was started on methylprednisolone pulses followed by oral prednisone 0.5 mg/kg tapered to 5 mg by week 12 and mycophenolate mofetil 2 g/day. By the 12th month of therapy, she presented a partial response with serum creatinine in 0.7 mg/dL and UPCR of 1,000 mg/g. Serum complement C3 improved but remained in the low range, whereas anti-dsDNA antibodies were still positive. Despite the use of reduced-dose glucocorticoids, the patient developed diabetes; thus, metformin and lifestyle recommendations were added to therapy. Would a repeat biopsy provide additional helpful information?
Initial Therapy Response
Response to therapy in LN is classified into complete or partial. Complete remission, usually defined as a preserved kidney function and proteinuria <500 mg/g, has been associated with good long-term prognosis and less than 10% of patients progressing to kidney failure.46 A more recent definition (referred to as primary efficacy renal response in some clinical trials47) includes a protein cutoff below 700 mg/g at 12 months after initiation of immunosuppressive therapy, which has been associated with favorable kidney outcomes.48, 49, 50 In contrast, patients with partial response, usually defined as stable kidney function and a 50% decrease in proteinuria, still have a 50% probability of progressing to kidney failure, either by an increased risk for renal relapses or by persistent kidney inflammatory activity.46,51, 52, 53
Discordance Between Clinical and Histological Remission
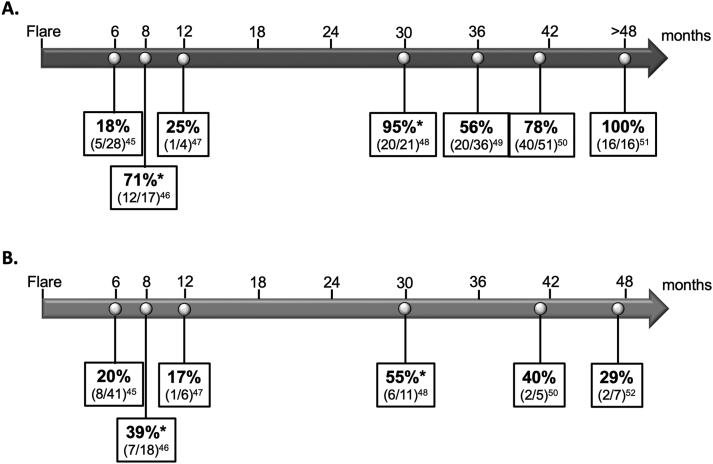
Studies of repeat kidney biopsies performed after the initial therapy have demonstrated a discordance between clinical and histological responses (Fig 2).54, 55, 56, 57, 58, 59, 60, 61 In the studies by Malvar et al54 and Zickert et al,55 where the histological response was defined as an activity index of 0 points by 6 to 8 months after the commencement of the initial immunosuppressive therapy, 18% (5/28) and 71% (12/17) of patients with complete response still had histological evidence of active kidney inflammation, respectively. Histological evidence of ongoing active kidney inflammation was observed in 20% (8/41) and 39% (7/18), respectively, of patients with partial response.54,55 These data raise the question of whether a repeat biopsy is needed to define LN response because the current clinical parameters used to monitor response, especially proteinuria, do not necessarily reflect what is happening at a histological level.
Figure 2.
Studies of repeat kidney biopsies in patients with complete (A) or partial (B) clinical response and observed percentages of histological remission. Note. The arrow indicates the months from the initial biopsy when the repeat biopsy was performed in each report. Each box shows the percentage and number of patients/totals that demonstrated histological remission defined as an activity index of zero. ∗Histological response was defined as LN class I, II, III (C) or IV (C) in this study.
Time Course for Histological Remission
It is currently unknown how long it will take for the immune complexes to be eliminated from the kidney tissue with the resolution of these findings in the histopathological evaluation. A recent study suggests histologic lesions have different resolution times: crescents and neutrophil infiltration resolve rapidly in months, while endocapillary hypercellularity and hyaline deposits resolve more gradually.56 Interstitial inflammation and immunofluorescence for immunoglobulins and complement frequently persist for several months.47,55 Few reports in the literature involving inadvertent transplantation of lupus kidneys to recipients without lupus showed that subendothelial immune complexes progressively decreased in intensity but were still detectable by light and immunofluorescence microscopy 6-8 months after kidney transplantation.62, 63, 64, 65 Subendothelial immune complexes disappeared from the kidney biopsies beyond 12 months. Interestingly, subepithelial deposits lasted much longer and were detectable by light microscopy and immunofluorescence microscopy even 3-5 years after transplantation.64,66, 67, 68 Electron microscopy may differentiate “new” from “old” subepithelial immune complex deposits in repeat kidney biopsies. New deposits are dense and found in the subepithelial space, while progressively older deposits migrate through the glomerular basement layers to the subendothelial space.66
Therefore, current evidence suggests that a repeat kidney biopsy performed to evaluate response to therapy may be more useful beyond 12 months after the commencement of the initial therapy. Furthermore, the “persistence” of subepithelial deposits should be interpreted with caution if the biopsy was not evaluated by electron microscopy to differentiate “new” versus “old” deposits.
Implications of Persistent Histologic Activity in Kidney Biopsy
The persistence of histological inflammation in a repeated posttreatment kidney biopsy has been associated with a higher risk of relapses. In a study of protocol biopsies performed after a median of 24 months from the LN flare in incident LN patients, 24% of patients with low proteinuria (below 1g/g) still had activity indices above 3 points (one with 14 points). A higher activity index in the repeat kidney biopsy was associated with higher relapse risk.69 This higher relapse risk associated with a persistently elevated activity index has also been demonstrated in patients beyond 12 months of clinical remission who had kidney biopsies performed before therapy withdrawal (see below).58
It is currently unknown if the intensification of immunosuppression for patients with LN and persistent kidney histological inflammation can modify long-term kidney outcomes. The latter is being evaluated in the ReBIOLUP study (Per-protocol kidney biopsy in incident cases of lupus nephritis, NCT04449991). Alsuwaida et al70 investigated the utility of a repeat kidney biopsy after 12 to 18 months of induction therapy for the management of LN. They noted that after a median of 8.7 years of follow-up, the relative risk for doubling of serum creatinine was 1.68 (95% confidence interval [CI], 1.3-2.2) for patients whose second kidney biopsy had an activity index >2 points, and 1.4 (95% CI, 1.1-1.8) for those with activity index of 1 or 2 points. In this study, 10-year kidney survival was 100% for those with an activity index of 0 in the repeat biopsy, 80% for those with an activity index of 1 or 2, and 44% for those with an activity index >2. While more evidence is necessary, it seems that persistent histological kidney disease activity in LN may require modifications in immunosuppressive therapy.
The repeat post-treatment kidney biopsy has a better association with kidney function prognosis than the initial biopsy in patients with LN.71, 72, 73 Persistent histologic evidence of glomerular and interstitial inflammation, such as glomerular capillary immune complexes and macrophages in tubular lumens, after completing induction therapy has been associated with a higher risk of doubling serum creatinine.71,74
Post-treatment Kidney Biopsy for Nonresponse
Kidney scarring occurs early in LN if therapy is not promptly initiated. Current guidelines suggest performing a kidney biopsy as part of the evaluation of nonresponse to therapy in patients with LN.21 However, kidney biopsies in this context have rarely been reported in previous studies. We assessed a series of 20 biopsies performed for no response (Table 3, personal communication). All 20 patients had a first kidney biopsy performed for diagnosis, showing a proliferative LN in 19 (95%) with a median activity and chronicity indices of 8 points (interquartile range [IQR], 4-14) and 4 points (IQR, 3-6), respectively. After a median 9 months (IQR, 6-12), a second biopsy was performed for no response. In 9 (45%) patients, the repeat biopsy showed an exclusively chronic disease, and immunosuppression was decreased in 10 (50%) cases. An extreme case showed progression from a chronicity index of 0 points to a class VI LN within 10 months.
Table 3.
Post-treatment Kidney Biopsies Performed in 20 Patients With Lupus Nephritis From a Mexican Cohort Due to Nonresponse to Therapy
| Indication for First Biopsy | ISN-RPS LN Class Biopsy 1 | Activity Index | Chronicity Index | Indication for Second Biopsy | Time Interval (months) | ISN-RPS LN Class Biopsy 2 | Activity Index | Chronicity Index | Immunosuppressive Therapy Modification |
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | IV-A + V | 14 | 2 | Nonresponder | 6 | IV-A | 16 | 7 | Intensification |
| Diagnosis | IV-A | 20 | 0 | Nonresponder | 10 | VI | 1 | 12 | Decrease |
| Diagnosis | IV-A + V | 17 | 0 | Nonresponder | 6 | IV-A/C + V | 5 | 9 | Intensification |
| Diagnosis | IV-A + V | 16 | 5 | Nonresponder | 9 | IV-A/C + V | 8 | 8 | Intensification |
| Diagnosis | IV-A/C + V | 9 | 8 | Nonresponder | 7 | IV-A/C + V | 4 | 10 | Decrease |
| Diagnosis | IV-A/C | 12 | 7 | Nonresponder | 7 | IV-C | 1 | 9 | Decrease |
| Diagnosis | IV-A + V | 5 | 3 | Nonresponder | 7 | IV-A/C + V | 7 | 9 | Intensification |
| Diagnosis | III-A/C | 3 | 6 | Nonresponder | 11 | IV-C + V | 2 | 9 | Decrease |
| Diagnosis | V | 1 | 3 | Nonresponder | 8 | III-C + V | 1 | 3 | Change to another agent |
| Diagnosis | IV-A + V | 12 | 6 | Nonresponder | 3 | IV-C + V | 3 | 10 | Decrease |
| Diagnosis | IV-A/C | 7 | 4 | Nonresponder | 12 | IV-C | 1 | 10 | Decrease |
| Diagnosis | III-A + V | 7 | 1 | Nonresponder | 12 | IV-A/C + V | 3 | 10 | Intensification |
| Diagnosis | IV-A/C + V | 5 | 6 | Nonresponder | 8 | IV-A/C + V | 6 | 7 | Intensification |
| Diagnosis | IV-A/C + V | 3 | 6 | Nonresponder | 16 | IV-A/C + V | 6 | 7 | Intensification |
| Diagnosis | IV-A + V | 5 | 3 | Nonresponder | 6 | IV-A + V | 12 | 5 | Intensification |
| Diagnosis | IV-A/C + V | 9 | 7 | Nonresponder | 6 | IV-A/C + TMA | 8 | 10 | No change + total anticoagulation |
| Diagnosis | IV-A + V | 15 | 5 | Nonresponder | 10 | IV-A/C + V | 5 | 10 | Decrease |
| Diagnosis | IV-A + V | 3 | 3 | Nonresponder | 12 | IV-C + V | 1 | 6 | Decrease |
| Diagnosis | IV-A/C + V | 3 | 8 | Nonresponder | 12 | IV-C + V | 1 | 6 | Decrease |
| Diagnosis | IV-A + V | 14 | 3 | Nonresponder | 10 | IV-C + V | 1 | 10 | Decrease |
Notes: Intensification included increasing dose of glucocorticoids and other immunosuppressive agents such as mycophenolate mofetil, cyclophosphamide, calcineurin inhibitors, or B cell-directed therapies.
Abbreviations: ISN-RPS, International Society of Nephrology/Renal Pathology Society; LN, lupus nephritis; SLE, systemic lupus erythematosus.
Therefore, in patients with LN without the expected response to therapy, a kidney biopsy may be performed to evaluate the degree of chronic kidney damage, which may influence therapeutic decisions.
Clinical Vignette Revisited
As the case initial diagnostic biopsy demonstrated an active LN with minimal kidney scarring, the aim was to obtain a complete response with immunosuppressive treatment. As described, many patients with LN and a partial response may have underlying active inflammation on histological examination, predisposing them to relapse and/or progressive kidney disease. Therefore, a kidney biopsy would provide valuable information for the management and prognosis of this patient. Once risks and benefits were discussed with the patient, a repeat kidney biopsy was performed after 12 months of treatment. The histological examination revealed an active and chronic class IV LN with an activity index of 4 points and a chronicity index of 4 points. Tacrolimus was added to mycophenolate mofetil, and prednisone was suspended. Over the next 12 months, UPCR decreased to 250 mg/g with a preserved kidney function.
The Role of Kidney Biopsy During Lupus Nephritis Flares: Class Transition and Therapy Adjustment
Clinical Vignette
Two years after complete clinical complete remission, the patient presented with a new rash and arthralgias. Tacrolimus had been suspended one year earlier, and she continued maintenance treatment with mycophenolate mofetil and hydroxychloroquine. Her body mass index is 34 kg/m2, and blood pressure is 138/92 mm Hg. Laboratory investigations revealed serum creatinine 0.8 mg/dL, hemoglobin A1C 8.5%, urinalysis with 2 RBC/hpf, and 2 different morning UPCRs, 880 mg/g and 1,100 mg/g, respectively.
The Kidney Biopsy in Kidney Relapse
An LN relapse may be diagnosed clinically by persistent increases in proteinuria, with or without decreases in kidney function and/or hematuria.21,75 These episodes are usually accompanied by the serological activity of the disease and sometimes with extrarenal SLE activity. Because of a potential LN flare, a repeat kidney biopsy to evaluate the histology may be performed, especially if the result modifies the therapeutic approach.
ISN/RPS LN Class Transitions
Most reports evaluating the kidney biopsy at flares have combined repeat biopsies performed for a suspected LN flare, persistent or worsening proteinuria, or deterioration in kidney function. We analyzed 19 reports including 1,207 patients (Table 4).44,57,69,71,76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 The median interval between biopsies was 3.5 years (IQR, 2.4-4.1). An LN class transition was observed in 52% of repeated biopsies (27%-75%): in a mean of 13% (0%-39%) this involved transition from a “nonproliferative” LN (class II or V) to a “proliferative” LN (class III/IV), and in a mean of 16% (0%-40%), a transition from a “proliferative” LN to a “nonproliferative” LN. In a mean of 60% (32%-86%) and 11%, the first and repeat biopsies showed a “proliferative” and a “nonproliferative” LN, respectively.44,76, 78, 79,81, 82, 83,85,89,92, 93, 94 Hence, a repeat kidney biopsy during a flaring episode might be most valuable for patients with previous nonproliferative LN classes, such as ISN/RPS class II or V LN, given the possibility of immunosuppressive therapy intensification.
Table 4.
Repeat Kidney Biopsy in Patients With Lupus Nephritis Performed for Diverse Indications
| Study | Na | Interval Between Biopsies (y) | Indication | AI Biopsy 1 / AI Biopsy 2 | CI Biopsy 1 / CI Biopsy 2 | Progression to LN Class VI | Nonproliferative and Nonproliferative | Proliferative and Proliferative | Nonproliferative to Proliferative | Proliferative to Nonproliferative | Total LN Class Transitions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esdaile 199376 | 42 | 2.1 (1.8-2.5) | Mixed | 7 / 2 | 2 / 2 | 0 (0) | 5 (12) | 19 (45) | 2 (5) | 16 (38) | 23 (55) |
| Moroni 199977 | 38 | 3.6 (2.1-7.4) | Mixed | 7 / 4 | 1 / 5 | 2 (5) | 1 (3) | 25 (66) | 5 (13) | 5 (13) | 21 (55) |
| Bajaj 200078 | 57 | 4.2 | Mixed | 5.1 / 4.0 | 1.3 / 3.4 | 0 (0) | 0 (0) | 43 (75) | 13 (23) | 1 (2) | 23 (40) |
| Daleboudt 200979 | 49 | 4.1 ± 3.6 | Mixed | 6.2 / 5.3 | 2.6 / 4.2 | 0 (0) | 0 (0) | 42 (86) | 5 (10) | 1 (2) | 24 (49) |
| Lu 201180 | 244 | 3.7 | Mixed | 6.8 / 5.0 | 2.0 / 3.9 | 9 (4) | 27 (11) | 106 (43) | 39 (16) | 62 (25) | 183 (75) |
| Wang 201281 | 50 | NR | Mixed | 5.8 / 4.7 | 1.8 / 3.4 | 1 (2) | 0 (0) | 33 (66) | 8 (16) | 8 (16) | 32 (50) |
| Pagni 201382 | 142 | 4.9 ± 4.9 | Mixed | 4.5 / 3.3 | 1.5 / 3.6 | 0 (0) | 24 (17) | 82 (57) | 18 (13) | 18 (13) | 58 (41) |
| Greloni 201483 | 71 | 3.4 (4.4) | Mixed | NR | 2.9 / 6.6 | 5 (7) | 11 (24) | 34 (48) | 10 (14) | 11 (24) | 39 (55) |
| Alsuwaida 201484 | 11 | 2.0 | Mixed | 3.1 / 5.0 | 2.5 / 5.8 | 0 (0) | 2 (18) | 7 (64) | 2 (18) | 0 (0) | 6 (55) |
| Piñeiro 201657 | 35 | 2.5 (1.1-4.7) | Mixed | 9.9 / 1.3 | 1.6 / 2.5 | 1 (3) | 0 (0) | 20 (57) | 0 (0) | 14 (40) | 26 (74) |
| Kajawo 201785 | 44 | 2.8 ± 1.8 | Mixed | 3.9 / 7.0 | 1.0 / 3.5 | 5 (11) | 9 (20) | 14 (32) | 17 (39) | 4 (9) | 21 (48) |
| Pakozdi 201886 | 71 | 2.7 (1.1-6.7) | Mixed | NR | 3.6 / 5.1 | 3 (4) | 6 (8) | 46 (65) | 12 (17) | 4 (6) | 38 (54) |
| Morales 202187 | 26 | 6.0 ± 4.6 | Mixed | 2 / 1 | 1 / 3 | 0 (0) | 3 (12) | 13 (50) | 8 (31) | 2 (8) | 19 (73) |
| Gatto 202288 | 89 | 6.7 ± 4.9 | Mixed | NR / 4.7 | NR / 4.0 | 0 (0) | 6 (7) | 61 (69) | 10 (11) | 12 (13) | 58 (65) |
| Narvaez 201789 | 54 | 4.0 ± 0.8 | LN flare | 7.9 / 6.6 | 1.1 / 2.2 | 0 (0) | 8 (15) | 33 (61) | 7 (13) | 6 (11) | 25 (46) |
| Tannor 201890 | 96 | 3.0 (1.4-4.9) | LN flare | 7 / 7 | 3 / 4 | 0 (0) | 10 (10) | 74 (77) | 9 (9) | 3 (3) | 26 (27) |
| Alvarado 201491 | 25 | 0.5/3.5 | Protocol | 8.9/4.3/0.9 | 2.8/4.2/4.3 | NR | NR | NR | NR | NR | NR |
| Tannor 201890 | 31 | 0.5 | Protocol | 7 / 2.7 | 2 / 3.7 | 0 (0) | 7 (100) | 17 (55) | 0 (0) | 7 (23) | 13 (42) |
| Parodis 202069 | 42 | 2.0 (1.8-2.2) | Protocol | 8.5 / 3.0 | 1.0 / 2.0 | 0 (0) | 0 (0) | 26 (62) | 0 (0) | 16 (38) | 16 (38) |
Notes: Mixed indications include nonresponse to therapy, persistent proteinuria, suspicion of LN relapse, and deterioration of kidney function. Proliferative LN classes included III, IV, III±V, and IV±V. Nonproliferative LN class included classes I, II, and V.
Abbreviations: AI, activity index; CI, chronicity index; LN, lupus nephritis; NR, not reported.
The number represents the total evaluable repeat kidney biopsies.
Changes in Immunosuppressive Therapy
The utility of kidney biopsy at repeated LN flares can be questioned as its real value for therapeutic decision making has yet to be demonstrated. As such, a repeat kidney biopsy is less likely to modify the preprocedural therapeutic decision in a patient with a clinically evident LN relapse with no significant compromise of kidney function. Unlike LN patients with preserved kidney function, the repeat kidney biopsy in patients with kidney function impairment, with or without a clinical picture suggestive of an LN flare, may be valuable to differentiate active disease from chronic kidney damage. The former would require intensification of immunosuppression, while the latter would only require maximization of kidney protective and antiproteinuric measures without increasing immunosuppression.21 Moreover, a repeat kidney biopsy in LN patients with kidney function impairment may be used for differential diagnosis from other nonimmune complex glomerular diseases.
Clinical Vignette Revisited
The clinical presentation in this patient is very suggestive of systemic and kidney lupus activity without deterioration of kidney function. Without a kidney biopsy, the most probable decision is to increase immunosuppression. A new kidney biopsy has a low probability of modifying this therapeutic decision. After a discussion with the patient, it was decided to withhold kidney biopsy, and considering her comorbidities (eg, diabetes, obesity), she was started on rituximab 1 g in 2 infusions with a 250-mg methylprednisolone pulse before each dose. The patient continued mycophenolate mofetil 2 g daily and hydroxychloroquine with no oral glucocorticoids. After 6 months of observation, her kidney function remained stable, and UPCR decreased below 300 mg/g.
The Kidney Biopsy for Treatment Withdrawal
Clinical Vignette
Three years after obtaining a complete remission, the clinical team intends to progressively withdraw immunosuppression for this patient.
Tapering or Withdrawing Immunosuppression
Determining the optimal duration of maintenance therapy in LN is challenging. Clinical guidelines recommend continuing immunosuppressive therapy for at least one year after achieving persistent complete remission and 3 years of total treatment.21,75 One of the main concerns in determining the optimal time for treatment cessation in LN is the potential discordance between clinical and histological remission. In an observational study, histological remission, defined as an activity index of 0 points on a repeat biopsy performed during the maintenance phase (12 to 18 months after the start of therapy), was an independent predictor for kidney survival. The 10-year kidney survival was 100% for patients with an activity index of 0, 80% for an activity index of 1-2 points, and 44% for an activity index >2 points, regardless of clinical remission.70
De Rosa et al58 further investigated the discordance between clinical and histological remission in patients with LN and clinical remission maintained for more than 12 months and more than 36 months from the start of the initial therapy. Only 20 of 36 (56%) patients with complete clinical remission had histological remission (activity index of 0). Furthermore, over the 24-month follow-up, all patients with an activity index >2 points had an LN flare compared to 13.8% of those with ≤2 points.
A landmark observational study from an Argentinian LN cohort aimed to evaluate further the role of repeat kidney biopsy in guiding the duration of immunosuppression based on the histological activity index.77 Patients who had achieved clinical remission for at least 12 months and over 42 months of total immunosuppressive therapy underwent a repeat per-protocol kidney biopsy. An activity index of 0 resulted in the withdrawal of immunosuppressive therapy, while patients with an activity index of ≥1 continued the same treatment for 24 months. Another kidney biopsy was performed, and immunosuppressive therapy was suspended if the activity index was 0 and continued for any other activity index score. This algorithm was repeated for a median follow-up of 96 months. At the end of the study, only 7 patients relapsed for a flare rate of 1.5 per year, a low percentage considering other reports.95,96 Importantly, in this study no serological or clinical biomarkers predicted the incidence of disease flares.
Thus, in the absence of noninvasive reliable biomarkers, a histologic approach to determining the optimal time of maintenance therapy withdrawal can further augment the current clinical practice. Patients with histologic inactivity might be the best candidates for treatment withdrawal.
Clinical Vignette Revisited
After a discussion with the patient, a repeat biopsy was performed. The histological analysis revealed chronic class IV LN, with no activity and a chronicity index of 5 points, and no evidence of diabetic nephropathy. Immunosuppressive therapy was slowly tapered over the following 6 months. Currently, the patient continues biannual monitoring without new evidence of LN activity.
Conclusions and Perspectives
While the prognosis of LN has improved in the last decades, a substantial number of patients still progress to kidney failure. There has been an intense search for noninvasive biomarkers that allow more accessible and better LN monitoring. These biomarkers must be validated against the kidney biopsy as the gold standard for LN evaluation before entering clinical practice. Meanwhile, the kidney biopsy is the procedure to guide diagnosis, management, and prognosis. Hence, the expanded clinical scenarios in which a kidney biopsy may be helpful in patients with LN should be constantly reassessed based on the most available data. We recommend an individualized patient approach with careful consideration of the potential postprocedural management modifications.
Article Information
Authors’ Full Names and Academic Degrees
Sonia Rodriguez-Ramirez, MD, MSc, Nasim Wiegley, MD, and Juan Manuel Mejia-Vilet, MD, PhD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received March 16, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form November 12, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Almaani S., Meara A., Rovin B.H. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yap D.Y.H., Tang C.S.O., Ma M.K.M., Lam M.F., Chan T.M. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27(8):3248–3254. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 3.Contreras G., Lenz O., Pardo V., et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69(10):1846–1851. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 4.Tektonidou M.G., Dasgupta A., Ward M.M. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol. 2016;68(6):1432–1441. doi: 10.1002/art.39594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayoub I., Cassol C., Almaani S., Rovin B., Parikh S.V. The kidney biopsy in systemic lupus erythematosus: a view of the past and a vision of the future. Adv Chronic Kidney Dis. 2019;26(5):360–368. doi: 10.1053/j.ackd.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Bajema I.M., Wilhelmus S., Alpers C.E., et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Anders H.J., Weening J.J. Kidney disease in lupus is not always ‘lupus nephritis.’. Arthritis Res Ther. 2013;15(2):108. doi: 10.1186/ar4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weening J.J., D’Agati V.D., Schwartz M.M., et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 9.Salvatore S.P., Barisoni L.M.C., Herzenberg A.M., Chander P.N., Nickeleit V., Seshan S.V. Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol. 2012;7(6):914–925. doi: 10.2215/CJN.11751111. [DOI] [PubMed] [Google Scholar]
- 10.Clark M.R., Trotter K., Chang A. The pathogenesis and therapeutic implications of tubulointerstitial inflammation in human lupus nephritis. Semin Nephrol. 2015;35(5):455–464. doi: 10.1016/j.semnephrol.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mejía-Vilet J.M., Córdova-Sánchez B.M., Uribe-Uribe N.O., Correa-Rotter R., Morales-Buenrostro L.E. Prognostic significance of renal vascular pathology in lupus nephritis. Lupus. 2017;26(10):1042–1050. doi: 10.1177/0961203317692419. [DOI] [PubMed] [Google Scholar]
- 12.Bomback A.S., Markowitz G.S. Lupus podocytopathy: a distinct entity. Clin J Am Soc Nephrol. 2016;11(4):547–548. doi: 10.2215/CJN.01880216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mejia-Vilet J.M., Rovin B.H. In: Dubois’ Systemic Lupus Erythematosus and Related Syndromes. 9th ed. Wallace D.J., Hannahs Hahn B., editors. Elsevier; 2019. Chapter 59. Epidemiology and Management of Lupus Nephritis; pp. 727–744. [Google Scholar]
- 14.Mavragani C.P., Fragoulis G.E., Somarakis G., Drosos A., Tzioufas A.G., Moutsopoulos H.M. Clinical and laboratory predictors of distinct histopathogical features of lupus nephritis. Medicine (Baltimore) 2015;94(21):e829. doi: 10.1097/MD.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulgeri C., Carpio J.D., Ardiles L. Lesiones renales en el lupus eritematoso diseminado: ausencia de relación entre datos clínicos e histológicos [Kidney injury in systemic lupus erythematosus: lack of correlation between clinical and histological data] Nefrología. 2018;38(4):386–393. doi: 10.1016/j.nefro.2017.11.016. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 16.Silaide de Araújo Júnior A., Sato E.I., Silva de Souza A.W., et al. Development of an instrument to predict proliferative histological class in lupus nephritis based on clinical and laboratory data. Lupus. 2023;32(2):216–224. doi: 10.1177/09612033221143933. [DOI] [PubMed] [Google Scholar]
- 17.Carlucci P.M., Li J., Fava A., et al. High incidence of proliferative and membranous nephritis in SLE patients with low proteinuria in the Accelerating Medicines Partnership. Rheumatology (Oxford) 2022;61(11):4335–4343. doi: 10.1093/rheumatology/keac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W., Chen Y., Wang S., et al. Clinical–morphological features and outcomes of lupus podocytopathy. Clin J Am Soc Nephrol. 2016;11(4):585–592. doi: 10.2215/CJN.06720615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasr R., Johns C., Gertner E. Collapsing glomerulopathy in collagen vascular-like disease. Lupus. 2014;23(1):75–80. doi: 10.1177/0961203313513509. [DOI] [PubMed] [Google Scholar]
- 20.Barber C., Herzenberg A., Aghdassi E., et al. Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clin J Am Soc Nephrol. 2012;7(5):757–764. doi: 10.2215/CJN.02870311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovin B.H., Adler S.G., Barratt J., et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Helget L.N., Dillon D.J., Wolf B., et al. Development of a lupus nephritis suboptimal response prediction tool using renal histopathological and clinical laboratory variables at the time of diagnosis. Lupus Sci Med. 2021;8(1) doi: 10.1136/lupus-2021-000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broder A., Mowrey W.B., Khan H.N., et al. Tubulointerstitial damage predicts end stage renal disease in lupus nephritis with preserved to moderately impaired renal function: A retrospective cohort study. Semin Arthritis Rheum. 2018;47(4):545–551. doi: 10.1016/j.semarthrit.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Londoño Jimenez A., Mowrey W.B., Putterman C., Buyon J., Goilav B., Broder A. Brief report: tubulointerstitial damage in lupus nephritis: a comparison of the factors associated with tubulointerstitial inflammation and renal scarring. Arthritis Rheumatol. 2018;70(11):1801–1806. doi: 10.1002/art.40575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa S., Toyama T., Iwata Y., et al. The relationship between the modified National Institute of Health activity and chronicity scoring system, and the long-term prognosis for lupus nephritis: a retrospective single-center study. Lupus. 2021;30(11):1739–1746. doi: 10.1177/09612033211034234. [DOI] [PubMed] [Google Scholar]
- 26.Wakasugi D., Gono T., Kawaguchi Y., et al. Frequency of class III and IV nephritis in systemic lupus erythematosus without clinical renal involvement: an analysis of predictive measures. J Rheumatol. 2012;39(1):79–85. doi: 10.3899/jrheum.110532. [DOI] [PubMed] [Google Scholar]
- 27.Chedid A., Rossi G.M., Peyronel F., et al. Low-level proteinuria in systemic lupus erythematosus. Kidney Int Rep. 2020;5(12):2333–2340. doi: 10.1016/j.ekir.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yargucu Zihni F., Karabulut G., Oksel F. Retrospective review of the clinical and laboratory data in silent lupus nephritis. Int Urol Nephrol. 2022;54(8):1933–1938. doi: 10.1007/s11255-021-03066-4. [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Spielman A., Ginsberg M., et al. Short- and long-term progression of kidney involvement in systemic lupus erythematosus patients with low-grade proteinuria. Clin J Am Soc Nephrol. 2022;17(8):1150–1158. doi: 10.2215/CJN.01280122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y.S., Sun I.T., Wang H.K., et al. Risk of complications of ultrasound-guided renal biopsy for adult and pediatric patients with systemic lupus erythematosus. Lupus. 2018;27(5):828–836. doi: 10.1177/0961203317751048. [DOI] [PubMed] [Google Scholar]
- 31.Mejia-Vilet J.M., Márquez-Martínez M.A., Cordova-Sanchez B.M., Ibargüengoitia M.C., Correa-Rotter R., Morales-Buenrostro L.E. Simple risk score for prediction of haemorrhagic complications after a percutaneous renal biopsy. Nephrology (Carlton) 2018;23(6):523–529. doi: 10.1111/nep.13055. [DOI] [PubMed] [Google Scholar]
- 32.Wernick R.M., Smith D.L., Houghton D.C., et al. Reliability of histologic scoring for lupus nephritis: a community-based evaluation. Ann Intern Med. 1993;119(8):805–811. doi: 10.7326/0003-4819-119-8-199310150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Esdaile J.M., Joseph L., MacKenzie T., Kashgarian M., Hayslett J.P. The benefit of early treatment with immunosuppressive agents in lupus nephritis. J Rheumatol. 1994;21(11):2046–2051. [PubMed] [Google Scholar]
- 34.Fiehn C., Hajjar Y., Mueller K., Waldherr R., Ho A.D., Andrassy K. Improved clinical outcome of lupus nephritis during the past decade: importance of early diagnosis and treatment. Ann Rheum Dis. 2003;62(5):435–439. doi: 10.1136/ard.62.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oates J. Renal biopsy at the onset of clinical lupus nephritis: can it yield useful information? J Rheumatol. 2007;34(2):256–258. [PMC free article] [PubMed] [Google Scholar]
- 36.Jakez-Ocampo J., Arreola-Zavala R., Richaud-Patin Y., Romero-Díaz J., Llorente L. Lupus nephritis outcome with and without renal biopsy: a 5-year comparative study. J Clin Rheumatol. 2004;10(6):289–294. doi: 10.1097/01.rhu.0000147046.78645.60. [DOI] [PubMed] [Google Scholar]
- 37.Mok C.C., Cheung T.T., Lo W.H. Minimal mesangial lupus nephritis: a systematic review. Scand J Rheumatol. 2010;39(3):181–189. doi: 10.3109/03009740903456300. [DOI] [PubMed] [Google Scholar]
- 38.Baranowska-Daca E., Choi Y.J., Barrios R., Nassar G., Suki W.N., Truong L.D. Nonlupus nephritides in patients with systemic lupus erythematosus: a comprehensive clinicopathologic study and review of the literature. Hum Pathol. 2001;32(10):1125–1135. doi: 10.1053/hupa.2001.28227. [DOI] [PubMed] [Google Scholar]
- 39.Hebert L.A., Sharma H.M., Sedmak D.D., Bay W.H. Unexpected renal biopsy findings in a febrile systemic lupus erythematosus patient with worsening renal function and heavy proteinuria. Am J Kidney Dis. 1989;13(6):504–507. doi: 10.1016/S0272-6386(89)80010-1. [DOI] [PubMed] [Google Scholar]
- 40.Tektonidou M.G., Sotsiou F., Nakopoulou L., Vlachoyiannopoulos P.G., Moutsopoulos H.M. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 2004;50(8):2569–2579. doi: 10.1002/art.20433. [DOI] [PubMed] [Google Scholar]
- 41.Tektonidou M.G. Renal involvement in the antiphospholipid syndrome (APS)-APS nephropathy. Clin Rev Allergy Immunol. 2009;36(2-3):131–140. doi: 10.1007/s12016-008-8112-z. [DOI] [PubMed] [Google Scholar]
- 42.Hill G.S., Nochy D. Antiphospholipid syndrome in systemic lupus erythematosus. J Am Soc Nephrol. 2007;18(9):2461–2464. doi: 10.1681/ASN.2007030257. [DOI] [PubMed] [Google Scholar]
- 43.Kraft S.W., Schwartz M.M., Korbet S.M., Lewis E.J. Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol. 2005;16(1):175–179. doi: 10.1681/ASN.2004050350. [DOI] [PubMed] [Google Scholar]
- 44.Rovin B.H., Parikh S.V., Alvarado A. The kidney biopsy in lupus nephritis: is it still relevant? Rheum Dis Clin North Am. 2014;40(3):537–552. doi: 10.1016/j.rdc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rovin B.H. Glomerular disease: lupus nephritis treatment: are we beyond cyclophosphamide? Nat Rev Nephrol. 2009;5(9):492–494. doi: 10.1038/nrneph.2009.130. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y.E., Korbet S.M., Katz R.S., Schwartz M.M., Lewis E.J., Collaborative Study Group Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3(1):46–53. doi: 10.2215/CJN.03280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furie R., Rovin B.H., Houssiau F., et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383(12):1117–1128. doi: 10.1056/NEJMoa2001180. [DOI] [PubMed] [Google Scholar]
- 48.Tamirou F., Lauwerys B.R., Dall’Era M., et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med. 2015;2(1) doi: 10.1136/lupus-2015-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dall’Era M., Cisternas M.G., Smilek D.E., et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the euro-lupus nephritis cohort. Arthritis Rheumatol. 2015;67(5):1305–1313. doi: 10.1002/art.39026. [DOI] [PubMed] [Google Scholar]
- 50.Ugolini-Lopes M.R., Seguro L.P.C., Castro M.X.F., et al. Early proteinuria response: a valid real-life situation predictor of long-term lupus renal outcome in an ethnically diverse group with severe biopsy-proven nephritis? Lupus Sci Med. 2017;4(1) doi: 10.1136/lupus-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichinose K., Kitamura M., Sato S., et al. Complete renal response at 12 months after induction therapy is associated with renal relapse-free rate in lupus nephritis: a single-center, retrospective cohort study. Lupus. 2019;28(4):501–509. doi: 10.1177/0961203319829827. [DOI] [PubMed] [Google Scholar]
- 52.Pirson V., Enfrein A., Houssiau F.A., Tamirou F. Absence of renal remission portends poor long-term kidney outcome in lupus nephritis. Lupus Sci Med. 2021;8(1) doi: 10.1136/lupus-2021-000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zavala-Miranda M.F., Perez-Arias A.A., Márquez-Macedo S.E., et al. Characteristics and outcomes of a Hispanic lupus nephritis cohort from Mexico. Rheumatology (Oxford) 2023;62(3):1136–1144. doi: 10.1093/rheumatology/keac407. [DOI] [PubMed] [Google Scholar]
- 54.Malvar A., Pirruccio P., Alberton V., et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. 2017;32(8):1338–1344. doi: 10.1093/ndt/gfv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zickert A., Sundelin B., Svenungsson E., Gunnarsson I. Role of early repeated renal biopsies in lupus nephritis. Lupus Sci Med. 2014;1(1) doi: 10.1136/lupus-2014-000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malvar A., Alberton V., Lococo B., et al. Remission of lupus nephritis: the trajectory of histological response in successfully treated patients. Lupus Sci Med. 2023;10(1) doi: 10.1136/lupus-2023-000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mejia-Vilet J.M., Zhang X.L., Cruz C., et al. Urinary soluble CD163: a novel noninvasive biomarker of activity for lupus nephritis. J Am Soc Nephrol. 2020;31(6):1335–1347. doi: 10.1681/ASN.2019121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piñeiro G.J., Arrizabalaga P., Solé M., Abellana R.M., Espinosa G., Cervera R. Repeated renal biopsy - a predictive tool to assess the probability of renal flare in lupus nephritis. Am J Nephrol. 2016;44(6):439–446. doi: 10.1159/000452229. [DOI] [PubMed] [Google Scholar]
- 59.De Rosa M., Azzato F., Toblli J.E., et al. A prospective observational cohort study highlights kidney biopsy findings of lupus nephritis patients in remission who flare following withdrawal of maintenance therapy. Kidney Int. 2018;94(4):788–794. doi: 10.1016/j.kint.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Lledó-Ibáñez G.M., Xipell M., Ferreira M., et al. Kidney biopsy in lupus nephritis after achieving clinical renal remission: paving the way for renal outcome assessment. Clin Kidney J. 2022;15(11):2081–2088. doi: 10.1093/ckj/sfac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das U., Patel R., Guditi S., Taduri G. Correlation between the clinical remission and histological remission in repeat biopsy findings of quiescent proliferative lupus nephritis. Lupus. 2021;30(6):876–883. doi: 10.1177/0961203321995251. [DOI] [PubMed] [Google Scholar]
- 62.Lipkowitz G.S., Madden R.L., Kurbanov A., et al. Transplantation and 2-year follow-up of kidneys procured from a cadaver donor with a history of lupus nephritis. Transplantation. 2000;69(6):1221–1224. doi: 10.1097/00007890-200003270-00030. [DOI] [PubMed] [Google Scholar]
- 63.Schwartzman M.S., Zhang P.L., Potdar S., et al. Transplantation and 6-month follow-up of renal transplantation from a donor with systemic lupus erythematosus and lupus nephritis. Am J Transplant. 2005;5(7):1772–1776. doi: 10.1111/j.1600-6143.2005.00922.x. [DOI] [PubMed] [Google Scholar]
- 64.Magoon S., Zhou E., Pullman J., Greenstein S.M., Glicklich D.G. Successful transplantation of a donor kidney with diffuse proliferative lupus nephritis and crescents--a case report. Nephrol Dial Transplant. 2010;25(12):4109–4113. doi: 10.1093/ndt/gfq517. [DOI] [PubMed] [Google Scholar]
- 65.McRae M., Rousseau-Gagnon M., Philibert D., et al. The interpretation of repeat renal biopsies in patients with lupus nephritis. Rheumatology (Oxford) 2014;53(6):1151–1152. doi: 10.1093/rheumatology/ket420. [DOI] [PubMed] [Google Scholar]
- 66.Zickert A., Lannfelt K., Schmidt Mende J., Sundelin B., Gunnarsson I. Resorption of immune deposits in membranous lupus nephritis following rituximab vs conventional immunosuppressive treatment. Rheumatology (Oxford) 2021;60(7):3443–3450. doi: 10.1093/rheumatology/keaa788. [DOI] [PubMed] [Google Scholar]
- 67.Qin H.Z., Zhang M.C., Le W.B., et al. Combined assessment of phospholipase A2 receptor autoantibodies and glomerular deposits in membranous nephropathy. J Am Soc Nephrol. 2016;27(10):3195–3203. doi: 10.1681/ASN.2015080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoshida M., Hirashio S., Doi T., Masuda Y., Shimizu A., Masaki T. Low-vacuum scanning electron microscopy to assess histopathological resolution of class V lupus nephritis: a case report. Case Rep Nephrol Dial. 2021;11(1):36–47. doi: 10.1159/000509470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parodis I., Adamichou C., Aydin S., et al. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology (Oxford) 2020;59(11):3424–3434. doi: 10.1093/rheumatology/keaa129. [DOI] [PubMed] [Google Scholar]
- 70.Alsuwaida A., Husain S., Alghonaim M., et al. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27(4):1472–1478. doi: 10.1093/ndt/gfr517. [DOI] [PubMed] [Google Scholar]
- 71.Hill G.S., Delahousse M., Nochy D., et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59(1):304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 72.Parikh S.V., Alvarado A., Malvar A., Rovin B.H. The kidney biopsy in lupus nephritis: past, present, and future. Semin Nephrol. 2015;35(5):465–477. doi: 10.1016/j.semnephrol.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Parodis I., Tamirou F., Houssiau F.A. Treat-to-target in lupus nephritis. What is the role of the repeat kidney biopsy? Arch Immunol Ther Exp (Warsz) 2022;70(1):8. doi: 10.1007/s00005-022-00646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill G.S., Delahousse M., Nochy D., et al. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int. 2000;58(3):1160–1173. doi: 10.1046/j.1523-1755.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 75.Fanouriakis A., Kostopoulou M., Cheema K., et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713–723. doi: 10.1136/annrheumdis-2020-216924. [DOI] [PubMed] [Google Scholar]
- 76.Esdaile J.M., Joseph L., MacKenzie T., Kashgarian M., Hayslett J.P. The pathogenesis and prognosis of lupus nephritis: information from repeat renal biopsy. Semin Arthritis Rheum. 1993;23(2):135–148. doi: 10.1016/S0049-0172(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 77.Moroni G., Pasquali S., Quaglini S., et al. Clinical and prognostic value of serial renal biopsies in lupus nephritis. Am J Kidney Dis. 1999;34(3):530–539. doi: 10.1016/S0272-6386(99)70082-X. [DOI] [PubMed] [Google Scholar]
- 78.Bajaj S., Albert L., Gladman D.D., Urowitz M.B., Hallett D.C., Ritchie S. Serial renal biopsy in systemic lupus erythematosus. J Rheumatol. 2000;27(12):2822–2826. [PubMed] [Google Scholar]
- 79.Daleboudt G.M.N., Bajema I.M., Goemaere N.N.T., van Laar J.M., Bruijn J.A., Berger S.P. The clinical relevance of a repeat biopsy in lupus nephritis flares. Nephrol Dial Transplant. 2009;24(12):3712–3717. doi: 10.1093/ndt/gfp359. [DOI] [PubMed] [Google Scholar]
- 80.Lu J., Tam L.S., Lai F.M.M., et al. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. Am J Nephrol. 2011;34(3):220–225. doi: 10.1159/000330356. [DOI] [PubMed] [Google Scholar]
- 81.Wang G.B., Xu Z.J., Liu H.F., Zhou Q.G., Zhou Z.M., Jia N. Changes in pathological pattern and treatment regimens based on repeat renal biopsy in lupus nephritis. Chin Med J (Engl) 2012;125(16):2890–2894. [PubMed] [Google Scholar]
- 82.Pagni F., Galimberti S., Goffredo P., et al. The value of repeat biopsy in the management of lupus nephritis: an international multicentre study in a large cohort of patients. Nephrol Dial Transplant. 2013;28(12):3014–3023. doi: 10.1093/ndt/gft272. [DOI] [PubMed] [Google Scholar]
- 83.Greloni G., Scolnik M., Marin J., et al. Value of repeat biopsy in lupus nephritis flares. Lupus Sci Med. 2014;1(1) doi: 10.1136/lupus-2013-000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alsuwaida A.O. The clinical significance of serial kidney biopsies in lupus nephritis. Mod Rheumatol. 2014;24(3):453–456. doi: 10.3109/14397595.2013.844293. [DOI] [PubMed] [Google Scholar]
- 85.Kajawo S., Botha F.C.J., Okpechi I.G. Clinico-pathological features of repeat renal biopsies in patients with lupus nephritis at Groote Schuur Hospital, Cape Town. Lupus. 2017;26(12):1339–1346. doi: 10.1177/0961203317695466. [DOI] [PubMed] [Google Scholar]
- 86.Pakozdi A., Pyne D., Sheaff M., Rajakariar R. Utility of a repeat renal biopsy in lupus nephritis: a single centre experience. Nephrol Dial Transplant. 2018;33(3):507–513. doi: 10.1093/ndt/gfx019. [DOI] [PubMed] [Google Scholar]
- 87.Morales E., Trujillo H., Bada T., et al. What is the value of repeat kidney biopsies in patients with lupus nephritis? Lupus. 2021;30(1):25–34. doi: 10.1177/0961203320965703. [DOI] [PubMed] [Google Scholar]
- 88.Gatto M., Radice F., Saccon F., et al. Clinical and histological findings at second but not at first kidney biopsy predict end-stage kidney disease in a large multicentric cohort of patients with active lupus nephritis. Lupus Sci Med. 2022;9(1) doi: 10.1136/lupus-2022-000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narváez J., Ricse M., Gomà M., et al. The value of repeat biopsy in lupus nephritis flares. Medicine (Baltimore) 2017;96(24) doi: 10.1097/MD.0000000000007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tannor E.K., Bates W.D., Moosa M.R. The clinical relevance of repeat renal biopsies in the management of lupus nephritis: a South African experience. Lupus. 2018;27(4):525–535. doi: 10.1177/0961203317726864. [DOI] [PubMed] [Google Scholar]
- 91.Alvarado A.S., Malvar A., Lococo B., et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus. 2014;23(8):840–847. doi: 10.1177/0961203313518625. [DOI] [PubMed] [Google Scholar]
- 92.Gupta K., Bharati J., Anakutti H., et al. Contribution of clinically indicated repeat renal biopsy in Indian patients with lupus nephritis. Indian J Nephrol. 2020;30(6):377–381. doi: 10.4103/ijn.IJN_166_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee H.S., Mujais S.K., Kasinath B.S., Spargo B.H., Katz A.I. Course of renal pathology in patients with systemic lupus erythematosus. Am J Med. 1984;77(4):612–620. doi: 10.1016/0002-9343(84)90350-4. [DOI] [PubMed] [Google Scholar]
- 94.Fava A., Fenaroli P., Rosenberg A., et al. History of proliferative glomerulonephritis predicts end stage kidney disease in pure membranous lupus nephritis. Rheumatology (Oxford) 2022;61(6):2483–2493. doi: 10.1093/rheumatology/keab775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malvar A., Alberton V., Lococo B., et al. Kidney biopsy–based management of maintenance immunosuppression is safe and may ameliorate flare rate in lupus nephritis. Kidney Int. 2020;97(1):156–162. doi: 10.1016/j.kint.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 96.Mosca M., Tani C., Aringer M. Withdrawal of therapy in non-renal systemic lupus erythematosus: is this an achievable goal? Clin Exp Rheumatol. 2013;31(4 suppl 78):S71–S74. [PubMed] [Google Scholar]