Abstract
The energetic metabolism of photosynthetic organisms is profoundly influenced by state transitions and cyclic electron flow around photosystem I. The former involve a reversible redistribution of the light-harvesting antenna between photosystem I and photosystem II and optimize light energy utilization in photosynthesis whereas the latter process modulates the photosynthetic yield. We have used the wild-type and three mutant strains of the green alga Chlamydomonas reinhardtii—locked in state I (stt7), lacking the photosystem II outer antennae (bf4) or accumulating low amounts of cytochrome b6f complex (A-AUU)—and measured electron flow though the cytochrome b6f complex, oxygen evolution rates and fluorescence emission during state transitions. The results demonstrate that the transition from state 1 to state 2 induces a switch from linear to cyclic electron flow in this alga and reveal a strict cause–effect relationship between the redistribution of antenna complexes during state transitions and the onset of cyclic electron flow.
INTRODUCTION
Originally, state transitions have been described as a short-term chromatic adaptation that allows plants and algae to respond to changes in the spectral quality of light by varying the relative absorption cross-section of photosystem I (PSI) and photosystem II (PSII; for reviews see Allen, 1992; Wollman, 2001). This process is mediated through the transfer of a fraction of the outer PSII antenna complex (LHCII) from PSII to PSI during a state 1 to state 2 transition that optimizes the redistribution of excitation energy between the photosystems. This transition is correlated with the phosphorylation of LHCII (Allen et al., 1981; Horton and Black, 1981; Bennett, 1991), a reversible process, as LHCII is dephosphorylated and reassociates with PSII during a state 2 to state 1 transition (Allen, 1992).
Both the protein kinase and the phosphatase involved in state transitions are bound to the thylakoid membranes. The kinase is activated when the plastoquinone (PQ) pool is reduced (Allen et al., 1981), i.e. when the activity of PSII exceeds that of PSI. The cytochrome b6f complex is involved in state transitions, as shown by their absence in mutants of Chlamydomonas reinhardtii lacking this complex (Wollman and Lemaire, 1988). Recently, it was shown that binding of plastoquinol to the Qo site of the cytochrome b6f complex is required for the activation of the LHCII kinase (Vener et al., 1995; Zito et al., 1999).
In higher plants, only 15–20% of the light-harvesting complex of PSII is transferred to PSI during a state 1 to state 2 transition (Allen, 1992), while in the unicellular green alga C. reinhardtii, the change in relative size of the PSII and PSI antennae is much larger, with 80% of the PSII antenna displaced to PSI in state 2 (Delosme et al., 1996). The same mechanism operates in both cases and the term state transitions has been consequently extended to this alga (Wollman, 2001). In higher plants and C. reinhardtii, a fraction of the cytochrome b6f complex accumulates in the stroma lamellae in state 2 (Vallon et al., 1991). In addition, in C. reinhardtii, it has been shown that the PSII inhibitor 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU) inhibits electron flow through the cytochrome b6f complex in state 1, but not in state 2 (Finazzi et al., 1999). These findings have led to the proposal that in this organism, in state 2, most of the excitation energy is utilized by PSI photochemistry and that cyclic electron transport around PSI prevails over linear electron flow mediated by PSII and PSI. To test this hypothesis, and to elucidate the relationship between the redistribution of LHCII and cytochrome b6f to the stroma lamellae and the switch to cyclic electron flow, we have performed a comparative study of the wild-type and three mutants of C. reinhardtii. One mutant is constitutively locked in state 1 (stt7; Fleischmann et al., 1999), another lacks the LHCII complex (bf4; Olive et al., 1981) and the third mutant accumulates only ∼10–20% of the cytochrome b6f complex (A-AUU; Chen et al., 1995). The results reported here show a strict correlation between the lateral redistribution of LHCII from PSII to PSI in the stroma lamellae and the switch from linear to cyclic electron flow.
RESULTS
Analysis of the stt7 mutant deficient in state transition
Electron transfer under state 1 and state 2 conditions. In a previous study, the stt7 mutant was shown to be deficient in state transitions and locked in state 1, while accumulating normal levels of the cytochrome b6f complex and maintaining the functional connection between the photosystems and the cytochrome b6f complex (Fleischmann et al., 1999). Its phenotype has been attributed to a defect in the signal transduction pathway from the cytochrome b6f complex to the LHCII kinase, or of the kinase itself. This strain, therefore, is appropriate to test the hypothesis that a transition from state 1 to state 2 induces a switch from linear to cyclic electron flow. Indeed, within this framework, no switch is expected in the stt7 mutant.
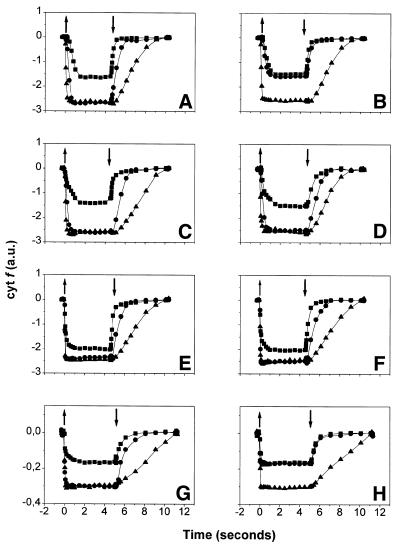
The rate of cytochrome f reduction measured under continuous illumination is an appropriate parameter to test the connectivity between the cytochrome b6f complex and both PSII and PSI because it depends on the concentration of photogenerated plastoquinol (PQH2) and oxidized plastocyanin. This holds not only for the wild-type but also for any mutant strain, provided the mutation does not alter the intrinsic kinetic properties of the cytochrome b6f complex. We have verified this with the stt7 mutant by measuring the kinetics of cytochrome f redox changes after a single turnover flash (for a review see Joliot et al., 1998). A t1/2 of ∼2 ms was found for the reduction of cytochrome f (data not shown), which is identical to that of the wild type (Finazzi et al., 1997). Because the intrinsic electron transfer properties of the cytochrome b6f complex in the mutant and wild-type strains are similar, we measured the kinetics of cytochrome f redox changes under continuous illumination in the stt7 strain to test the strength of the correlation between the transition to state 2 and the switch from linear to cyclic electron transfer. The results obtained with stt7 are shown in Figure 1C and D, and those from the wild type, already established previously (Finazzi et al., 1999), are presented in A and B for comparison. In both strains, a similar behaviour was observed under state 1-promoting conditions (Figure 1A and C); switching the actinic light on resulted in the oxidation of cytochrome f (squares), which rapidly reached a plateau level. After the light was switched off, cytochrome f reduction was observed and the absorption signal returned to its initial value. The oxidation yield was increased by addition of DCMU (circles), a PSII inhibitor (Bennoun, 1970) or of 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB) (triangles), an inhibitor of cytochrome f reduction by PQH2 (reviewed in Frank and Trebst, 1995). As expected for the linear electron flow, this indicates that cytochrome f reduction is inhibited with a similar efficiency by blocking either PQ reduction by PSII or PQH2 oxidation by the b6f complex. Under state 2 conditions, however, the oxidation yield of cytochrome f was no longer increased by DCMU in the wild-type strain (Figure 1B, circles), but it was still increased by DBMIB (Figure 1B, triangles). This suggests the existence of another source of reducing equivalent other than PSII, which, as discussed below, we ascribe to cyclic electron flow (see Discussion). This alternative path for PQ reduction was not observed in the stt7 mutant (Figure 1D), where the yield of cytochrome f oxidation was similar in the presence of DCMU and DBMIB (compare circles with triangles). Therefore, we conclude that PSII is the major source of reducing equivalents in stt7 even under state 2-promoting conditions.
Fig. 1. Cytochrome f redox changes in continuous light as measured by absorbance changes. (A, C, E and G) State 1. (B, D, F and H) State 2. (A and B) wild type; (C and D) stt7; (E and F) bf4; (G and H) A-AUU strain. Symbols: squares, no inhibitors; circles, DCMU 10 µM; triangles, DBMIB 2 µM. Other conditions are described in Methods. Upward and downward arrows indicate the switch on and off of the actinic light, respectively. A decrease of absorbance corresponds to oxidation of cytochrome f. Traces were normalized on the amplitude of a PSI-driven charge separation signal measured at 515 nm.
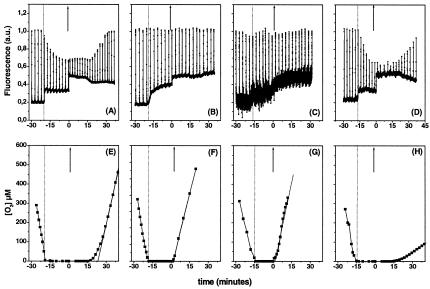
Oxygen evolution and fluorescence changes under state 1 and state 2. The oxygen evolution capacity may also be taken as a good indicator of the functional connection between PSII and PSI. In the wild type, it has already been observed that no light-induced oxygen evolution is observed in state 2, although electron transfer through the cytochrome b6f complex still occurs (Finazzi et al., 1999). The same measurements were performed with the stt7 mutant (Figure 2E) and compared with those obtained with the wild type (Figure 2F). In both strains, incubation in the dark induced oxygen consumption until anaerobiosis was attained. At this stage (Figure 2A and B, lower thick line), the dark fluorescence yield (Fo) increased in both strains, indicating that the PQ pool was reduced. A slow rise phase was observed in the mutant, suggesting that some PSII centres equilibrate slowly with the PQ pool. The same phenomenon was not observed in the wild type, probably because the fluorescence decline caused by the transition to state 2 compensated for this increase in fluorescence. Whereas the maximal fluorescence level (Fm) decreased in the wild type (Figure 2A, spikes), indicating the transition from state 1 to state 2, it remained constant in the stt7 strain (Figure 2B), in agreement with the lack of state transitions in this mutant. When the light was switched on (at time t = 0), recovery of oxygen evolution in the stt7 cells occurred much faster (∼1 min, Figure 2F) than in the wild type (∼20 min, Figure 2E), in which the recovery of oxygen evolution occurred concomitantly with the increase in the fluorescence yield to the state 1 level. The delay was determined by tracing the intercept between the steady-state rate of oxygen evolution and the abscissa axis (Figure 2).
Fig. 2. Fluorescence emission (A–D), oxygen consumption and evolution (E–H) in C. reinhardtii wild-type (A and E), stt7 (B and F), bf4 (C and G) and A-AUU (D and H) cells during state transition. Cells were incubated in the dark. At 3 min intervals, a saturating light pulse was given to measure Fm. Fo corresponds to the lower trace in (A)–(D). The upward arrow indicates the switch on of the actinic light at time 0. Note that fluorescence emission was multiplied by a factor of 4 in (C).
Analysis of the bf4 mutant deficient in the LHCII antenna
This strain lacked most of the PSII chlorophyll binding proteins (Olive et al., 1981), but accumulated wild-type amounts of PSII, PSI and the cytochrome b6f complex (F.-A. Wollman, personal communication). As expected, the intrinsic activity of the cytochrome b6f complex, estimated by the kinetics of cytochrome f redox changes after a single turnover flash (for a review see Joliot et al., 1998), was identical to that of the wild type (∼2 ms); the degree of oxidation of the cytochrome b6f complex in the presence of DCMU or DBMIB was also the same as that for the wild type (compare A with E in Figure 1). Under both state 1 and state 2 promoting conditions, the oxidation yield of cytochrome f was increased by DCMU addition under continuous illumination, as in the case of the stt7 mutant (Figure 1E and F). We note, however, that cytochrome f was more oxidized during illumination than in the wild type, in agreement with the fact that excitation energy is preferentially absorbed by PSI in the absence of LHCII.
No quenching of fluorescence was observed in the bf4 mutant (Figure 2C) under conditions promoting the transition to state 2 in the wild type. This is consistent with previous data, which indicate no redox-induced protein phosphorylation in the thylakoids of this strain (de Vitry and Wollman, 1988). The overall fluorescence emission was diminished, however, due to the reduced chlorophyll content of the strain (Figure 2C). In bf4 cells, the lag in oxygen evolution measured after a dark anaerobic incubation was identical to that observed in the stt7 mutant, provided that a saturating illumination was used (Figure 2G).
Analysis of the A-AUU mutant accumulating low amounts of the cytochrome b6f complex
This strain contained reduced amounts of the cytochrome b6f complex (∼10–20%) because of a mutation in the initiation codon of petA that reduces the efficiency of cytochrome f mRNA translation (Chen et al., 1995). However, A-AUU cells accumulated normal levels of the LHCII, PSII and PSI proteins. The cytochrome b6f activity of this mutant was lower than that of the wild type under saturating single turnover flash illumination, but this activity increased when the light intensity was reduced (data not shown). This is consistent with the fact that PSI is present in excess relative to the b6f complex and thus a single turnover of this complex can only be observed under low light conditions. This phenotype is typical of mutants with reduced amounts of cytochrome b6f complex (de Vitry et al., 1999) and indicates the occurrence of multiple turnovers of this complex required to re-reduce all the oxidized plastocyanin generated by PSI.
Under continuous light, the overall amount of oxidized cytochrome f was diminished to ∼12%, in agreement with the poor accumulation of the b6f complex (compare G with A in Figure 1). The A-AUU strain was able to perform state transitions (Figure 2D) and its cytochrome f turnover was insensitive to DCMU under state 2-promoting conditions (Figure 1H), as observed for the wild type. Oxygen evolution in state 2-adapted samples could only be observed after several minutes of illumination (Figure 2H). Its extent was reduced to ∼15% of the wild-type value.
DISCUSSION
Involvement of state transitions in the switch between linear and cyclic electron flow in C. reinhardtii
The results reported here provide strong evidence for the implication of state transitions in the switch from linear to cyclic electron flow in the photosynthetic electron transfer chains of C. reinhardtii (Figure 3). Indeed, incubation of the algae under conditions promoting state 2 (anaerobiosis or aerobiosis plus FCCP) generates a source of electrons for cytochrome b6f that is different from PSII and is only active when transition from state 1 to state 2 does occur (Figure 1). As discussed below, the source is likely to be PSI itself, i.e. cyclic electron flow, rather than the overall cell metabolism. Moreover, the reactivation of linear electron flow between PSII and PSI requires the switch from state 2 to state 1, as indicated by the concomitant increase of the fluorescence yield and the recovery of oxygen evolution activity in the wild-type and A-AUU strains (Figure 2). This results in a ‘lag’ of several minutes in oxygen evolution (Figure 2E and H), which is absent in the strains where no lateral migration of LHCII occurs (stt7 and bf4; Figure 2F and G).
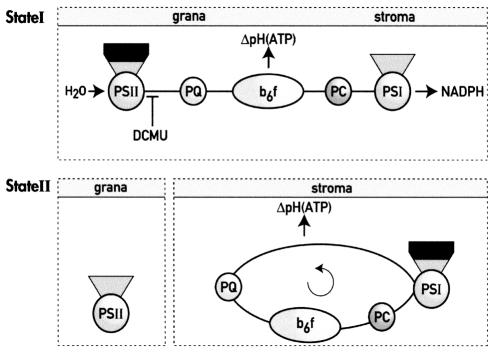
Fig. 3. State 1 and state 2 correspond to two different modes of electron transfer in C. reinhardtii. In state 1, PSII and PSI are functionally connected through the linear electron transfer chain, which generates NADPH and ATP via a pH gradient across the thylakoid membrane. Upon transition from state 1 to state 2, redistribution of LHCII (drawn in black) from PSII to PSI and migration of a fraction of the cytochrome b6f complex from the grana to the stroma thylakoid region induce cyclic electron flow around PSI at the expense of linear electron flow thus generating solely ATP. PC, plastocyanin. The site of action of DCMU is indicated. Grana and stroma refer to the thylakoid grana (PSII membrane domains) and stroma lamellae (PSI membrane domains).
In principle, the reducing equivalents that prevent the complete oxidation of cytochrome f in the wild type in state 2 (even in the presence of DCMU) might reflect the reducing pressure that develops under anaerobic conditions (Bennoun, 1982) rather than cyclic electron flow through PSI. This possibility could not be ruled out in a previous study (Finazzi et al., 1999). On the contrary, the present results clearly argue against this possibility; in the stt7 and bf4 mutants, the reducing pressure fails to prevent the full oxidation of cytochrome f when PSII is inhibited by DCMU (Figure 1D and F, circles). However, in both strains, a reducing pressure develops under anaerobic conditions as shown by the reduction of the PQ pool in darkness, witnessed by the increase in Fo (Figure 2B and C). Therefore, we conclude that in C. reinhardtii, a strict cause–effect relationship exists between state transitions and the switch between linear and cyclic electron flow.
Mechanism of regulation of linear and cyclic electron transport
Upon state transitions, two major complexes undergo a lateral redistribution; both the cytochrome b6f complex and LHCII reversibly migrate to the stroma domains in state 2. Therefore, it is tempting to propose that their rearrangement accounts for the switch from cyclic to linear electron flow.
The accumulation of LHCII in the stroma membranes in state 2 might explain the switch. Indeed, the fraction of LHCII moving from the PSII-enriched grana region to the stroma lamellae (∼80%; Delosme et al., 1996) is compatible with the extensive transition to cyclic electron flow. In addition, during a state 2 to state 1 transition, the recovery of linear electron flow in the wild-type and A-AUU strains (witnessed by the recovery of oxygen evolution; Figure 2E and H) is accompanied by an increase in Fm, i.e. by the migration of LHCII from PSI to PSII (Figure 2A and D). In the stt7 mutant, in which no LHCII redistribution occurs, recovery of oxygen evolution is much faster than in the wild type (Figure 2F). In the absence of LHCII (bf4), the electron flow is linear and no switch to cyclic electron transfer is observed upon transition to anaerobic conditions (Figures 1 and 2). In contrast, it is difficult to explain the switch in electron flow observed in state 2 only by the redistribution of the cytochrome b6f complex. Even if the average distance between the cytochrome b6f complex and PSII is increased with respect to state 1, because of the accumulation of the cytochrome b6f complex in the stroma lamellae (Vallon et al., 1991), the lateral redistribution of the cytochrome b6f complex is too small (15–20% of the total; Vallon et al., 1991) to account for the complete switch to cyclic electron transport observed in the wild type in state 2 (Figure 1B). Moreover, although the cytochrome b6f:PSII ratio in the grana of the A-AUU strain in state 1 is lower than that of the wild type in state 2, the electron flow is linear until transition to state 2 is induced (Figure 1G and H).
Taken together, these results suggest that the presence of LHCII is required for the switch to cyclic electron flow under state 2 conditions and that its association to PSI (rather than its dissociation from PSII) is the cause of this phenomenon. It has been proposed previously (Lavergne and Joliot, 1991) that the PQH2 diffusion is restricted to relatively small domains in the membranes, which are generated by the dense packing of proteins, mainly LHCII, in the thylakoid membranes. Within the same domain, PQH2 connects PSII and the cytochrome b6f complex in a time range (ms) compatible with its physiological function, while its diffusion between different domains is a comparatively slow process (in the second time range). In line with this hypothesis, it is tempting to propose that during a state 1 to state 2 transition, the association of LHCII to PSI modifies the dynamics of PQ diffusion in the thylakoid membranes by changing the structure and protein composition of the domains (Figure 3). This would slow down the rate of PQ-mediated electron transfer between PSII and the cytochrome b6f complex, while favouring the onset of cyclic flow around PSI.
Consistently with this hypothesis, the steady-state fluorescence level measured in the wild type and A-AUU mutant (Figure 2A and D) is higher than that of the stt7 or bf4 mutants (Figure 2B and C), while the cytochrome f turnover rate is very similar in all strains (Figure 1). This indicates that in state 2, the PQ pool present in the grana (i.e. linked to PSII but functionally disconnected from the b6f complex) is much more reduced than the one present in the stroma, which is connected to the cytochrome complex.
METHODS
Strains and culture conditions. Chlamydomonas reinhardtii wild-type (from strain 137 C), stt7, bf4 and A-AUU cells were grown at 24°C in acetate-supplemented medium (Harris, 1989) under ∼60 µE/m2/s of continuous white light. The cells were harvested during exponential growth (∼2 × 106 cells/ml) and resuspended in minimal medium (Harris, 1989). Chlorophyll concentration was determined by measuring the absorbance of the cell culture at 680 nm, on the basis of a calibration curve constructed after extraction of the chlorophyll with 80% acetone (Finazzi et al., 1999).
Oxygen evolution and uptake and fluorescence measurements. Photosynthesis and respiration were measured as oxygen exchanges using a Clark-type electrode in a home-made cell at 25°C. Illumination was provided by a halogen lamp. The light was filtered through a heat filter and its intensity was 200 µE/m2/s. Chlorophyll concentration was 20 µg/ml. Fluorescence emission was measured in the same chamber used for oxygen measurements using a PAM fluorimeter (Walz, Germany; Schreiber et al., 1986). Maximal fluorescence was induced by a saturating pulse of white light (1 s, 2200 µE/m2/s)
Spectroscopic measurements. Algae were resuspended in the same minimal medium, with the addition of 20% Ficoll (w/v) to prevent cell sedimentation. Chlorophyll concentration was ∼70 µg/Chl/ml, with the exception of the bf4 mutant, which was adjusted to the same number of cells as the wild type. Spectroscopic measurements were performed at room temperature, using a home-made spectrophotometer (Joliot et al., 1980, 1998). Actinic light was provided either by a xenon lamp filtered through a Schott filter (RG 695) or by an array of red LEDs placed on both sides of the measuring cuvette. In this case, light intensity was 1700 µE/m2/s (70–80 photons per photosystem per second). Light-induced absorption changes were measured as absorption of flash monochromatic light at discrete times. Cytochrome f redox changes were calculated as the difference between the absorption at 554 nm and a baseline drawn between 545 and 573 nm (Finazzi et al., 1997), and corrected for the contribution of the electrochromic signal (5% of the signal observed at 515 nm; Finazzi et al., 1999). State 1 was obtained through dark incubation of the cells under strong agitation, whereas state 2 was induced through anaerobiosis (Wollman and Delepelaire, 1984) or by addition of FCCP to aerobically grown cells (Bulté et al., 1990). Both methods gave identical results in the present work (see Finazzi et al., 1999).
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. Goldschmidt-Clermont and F.-A. Wollman for helpful comments. This work was supported by the C.N.R.S., the C.N.R. Target Project on Biotechnology and by the MURST Special Project ‘Fotosintesi: stress e protezione’.
REFERENCES
- Allen J.F. (1992) Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta, 1098, 275–335. [DOI] [PubMed] [Google Scholar]
- Allen J.F., Bennett, J., Steinback, K.E. and Arntzen, C.J. (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature, 291, 25–29. [Google Scholar]
- Bennett J. (1991) Protein phosphorylation in green plant chloroplast. Annu. Rev. Plant Physiol. Plant Mol. Biol., 42, 281–311. [Google Scholar]
- Bennoun P. (1970) Reoxidation of the fluorescence quencher ‘Q’ in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Biochim. Biophys. Acta, 216, 357–363. [DOI] [PubMed] [Google Scholar]
- Bennoun P. (1982) Evidence for a respiratory chain in chloroplast. Proc. Natl Acad. Sci. USA, 79, 4352–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulté L., Gans, P., Rebéillé, F. and Wollman, F.A. (1990) ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 1020, 72–80. [Google Scholar]
- Chen X., Kindle, K.L. and Stern, D.B. (1995) The initiation codon determines the efficiency but not the site of translation initiation in Chlamydomonas chloroplasts. Plant Cell, 7, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delosme R., Olive, J. and Wollman, F.A. (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta, 1273, 150–158. [Google Scholar]
- de Vitry C. and Wollman, F.A. (1988) Changes in phosphorylation of thylakoid membrane proteins in light-harvesting complex mutants from Chlamydomonas reinhardtii.Biochim. Biophys. Acta, 933, 444–449. [Google Scholar]
- de Vitry C., Finazzi, G., Baymann, F. and Kallas, T. (1999) Analysis of the nucleus-encoded and chloroplast-targeted rieske protein by classic and site-directed mutagenesis of Chlamydomonas. Plant Cell, 11, 2031–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi G., Büschlen, S., de Vitry, C., Rappaport, F., Joliot, P. and Wollman, F.A. (1997) Function-directed mutagenesis of the cytochrome b6f complex in Chlamydomonas reinhardtii—involvement of the cd loop of cytochrome b6 in quinol binding to the Qo site. Biochemistry, 36, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Finazzi G., Furia, A., Barbagallo, R.P. and Forti, G. (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii.Biochim. Biophys. Acta, 1413, 117–129. [DOI] [PubMed] [Google Scholar]
- Fleischmann M.M., Ravanel, S., Delosme, R., Olive, J., Zito, F., Wollman, F.A. and Rochaix, J.D. (1999) Isolation and characterization of photoautotrophic mutants of Chlamydomonas reinhardtii deficient in state transition. J. Biol. Chem., 274, 30987–30994. [DOI] [PubMed] [Google Scholar]
- Frank K. and Trebst, A. (1995) Quinone binding sites on cytochrome b/c complexes. Photochem. Photobiol., 61, 2–9. [DOI] [PubMed] [Google Scholar]
- Harris E. (1989) The Chlamydomonas Sourcebook. Academic Press, San Diego, CA.
- Horton P. and Black, M.T. (1981) Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5′-triphosphate. Biochim. Biophys. Acta, 635, 53–62. [DOI] [PubMed] [Google Scholar]
- Joliot P., Béal, D. and Frilley, B. (1980) Une nouvelle méthode spectrophotométrique destinée à l’étude des réactions photosynthétiques. J. Chim. Phys., 77, 209–216. [Google Scholar]
- Joliot P., Béal, D. and Delosme, R. (1998) In vivo measurements of photosynthetic activity: methods. In Rochaix, J.-D., Goldschmidt-Clermont, M. and Merchant, S. (eds), The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic, Dordrecht, The Netherlands, pp. 433–449. [Google Scholar]
- Lavergne J. and Joliot, P. (1991) Restricted diffusion in photosynthetic membranes. Trends Biochem. Sci., 16, 129–134. [DOI] [PubMed] [Google Scholar]
- Olive J., Wollman, F.A., Bennoun, P. and Recouvreur, M. (1981) Ultrastructure of thylakoid membranes in C. reinhardtii: evidence for variations in the partition coefficient of the light-harvesting complex-containing particles upon membrane fracture. Arch. Biochem. Biophys., 208, 456–467. [DOI] [PubMed] [Google Scholar]
- Schreiber U., Schliwa, U. and Bilger, W. (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res., 10, 51–62. [DOI] [PubMed] [Google Scholar]
- Vallon O., Bulté, L., Dainese, P., Olive, J., Bassi, R. and Wollman, F.A. (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc. Natl Acad. Sci. USA, 88, 8262–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener A.V., Van Kan, P.J., Gal, A., Andersson, B. and Ohad, I. (1995) Activation/deactivation cycle of redox-controlled thylakoid protein phosphorylation. Role of plastoquinol bound to the reduced cytochrome bf complex. J. Biol. Chem., 270, 25225–25232. [DOI] [PubMed] [Google Scholar]
- Wollman F.A. (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J., 20, 3623–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.A. and Delepelaire, P. (1984) Correlation between changes in light energy distribution and changes in thylakoid membrane polypeptide phosphorylation in Chlamydomonas reinhardtii. J. Cell Biol., 98, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.A. and Lemaire, C. (1988) Studies on kinase-controlled state transitions in photosystem II and b6f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim. Biophys. Acta, 85, 85–94. [Google Scholar]
- Zito F., Finazzi, G., Delosme, R., Nitschke, W., Picot, D. and Wollman, F.A. (1999) The Qo site of cytochrome b6f complexes controls the activation of the LHCII-kinase. EMBO J., 18, 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]