Abstract
Extracellular signal regulated kinase 5 (ERK5) is a novel member of the mitogen-activated protein kinase (MAPK) family with a poorly defined physiological function. Since ERK5 and its upstream activator MEK5 are abundant in skeletal muscle we examined a function of the cascade during muscle differentiation. We show that ERK5 is activated upon induction of differentiation in mouse myoblasts and that selective activation of the pathway results in promoter activation of differentiation-specific genes. Moreover, myogenic differentiation is completely blocked when ERK5 expression is inhibited by antisense RNA. Thus, we conclude that the MEK5/ERK5 MAP kinase cascade is critical for early steps of muscle cell differentiation.
INTRODUCTION
Extracellular signal regulated kinase 5 (ERK5), also known as BMK-1 (big MAP kinase-1), is a member of the mitogen-activated protein kinase (MAPK) family and has been shown to be activated by stress stimuli (Abe et al., 1996) as well as by mitogens (Kato et al., 1997, 2000; English et al., 1999; Kamakura et al., 1999). The best-characterized target of ERK5 is the myocyte enhancer factor 2C (MEF2C) (Kato et al., 1997) to which it binds directly (Yang et al., 1998). Members of the MEF2 family of transcription factors were originally described as having a DNA-binding activity recognizing elements on promoters of skeletal muscle-specific genes (Gossett et al., 1989). MEF2 proteins cooperate with myogenic regulatory factors (MRFs), such as MyoD and myogenin, to synergistically activate muscle-specific transcription (Molkentin et al., 1995). One of the features of MRFs is that ectopic expression in fibroblasts or other non-muscle cells leads to initiation of the myogenic program and converts those cells to myogenic derivatives (Choi et al., 1990; Parker et al., 1995). MRFs belong to the superfamily of basic helix–loop–helix (bHLH) proteins. In myoblasts, MRFs form heterodimers with ubiquitously expressed bHLH proteins, known as E-proteins, such as E47, and activate gene transcription by binding to so-called E-box motifs in specific regulatory elements (Lassar et al., 1991).
Both ERK5 and its upstream activator MEK5 are abundantly expressed in heart and skeletal muscle; this and the ability of ERK5 to activate MEF2C upon stimulation suggest a muscle-specific function for the kinase cascade.
Here we show that ERK5 kinase becomes activated upon induction of differentiation in mouse C2C12 skeletal myoblasts, and that activated kinase leads to upregulation of the promoter activity of differentiation-specific genes. Moreover, the process of differentiation in these cells can be inhibited if the expression of the kinase is blocked on the mRNA level using an antisense approach.
RESULTS AND DISCUSSION
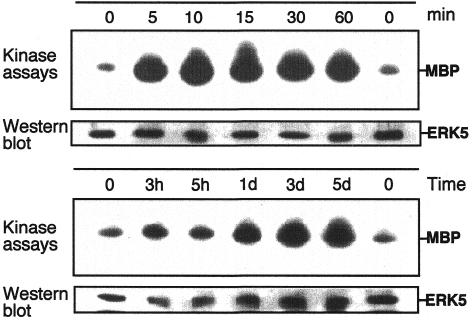
To explore ERK5 involvement in the process of myogenic differentiation we analysed mouse C2C12 skeletal muscle cells, which have been proven to be an excellent model system for defining mechanisms, involved in the ‘decision’ to grow or to differentiate (Yaffe and Saxel, 1977). These cells proliferate in medium containing 10% fetal bovine serum (FBS) and can be induced to differentiate after withdrawal of mitogens by addition of 2% horse serum to the medium (Yaffe and Saxel, 1977). Under such conditions we observed a strong activation of endogenous ERK5 as early as 5 min post-stimulation and persisting up to 60 min (Figure 1). Similar activation kinetics were observed when a transfected flag-tagged version of ERK5 was analysed (data not shown). Interestingly, a sustained upregulation of ERK5 activity was also observed at later stages of differentiation (days 1–5) concomitant with the formation of myotubes. Thus, a regulatory role in both early and later stages of differentiation is suggested. In contrast, in a control, ERK5 is only moderately active in cells cultured in growth medium with 10% FBS (data not shown).
Fig. 1. ERK5 is activated upon differentiation in C2C12 cells. C2C12 myoblasts were plated at a density of 2.5 × 105 on 10 cm dishes in DMEM/10%FBS. Twenty-four hours later cells were shifted to DMEM/2%HS to induce differentiation. Cell lysates were prepared at different time points as indicated. ERK5 was immunoprecipitated from the lysates using a polyclonal goat anti-ERK5 antiserum. ERK5 activity was measured by an immune complex kinase assay using MBP as a substrate. Kinase assays were performed essentially as described (Ludwig et al., 1996). Equal loading of kinases was assessed by immunoblotting with an ERK5-specific antiserum.
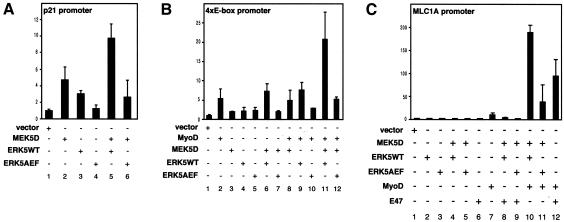
To see whether ERK5 activation has functional consequences during muscle cell differentiation we asked whether overexpression of ERK5 or MEK5 interferes with the promoter transactivation of individual differentiation-specific genes. One of the early markers highly upregulated during muscle development is the cyclin-dependent kinase inhibitor p21/Cip-1 (Walsh and Perlman, 1997). Transient transfection studies on C2C12 cells show that overexpression of either wild-type ERK5 (ERK5WT) or a constitutively active form of the upstream activator MEK5 (MEK5D) results in a moderate transactivation of the p21 promoter (Figure 2A, lanes 2 and 3), which was further potentiated by cotransfection of MEK5D with ERK5WT (Figure 2A, lane 5). This effect was indeed mediated by ERK5 since promoter activity was reduced to basal levels if MEK5D was cotransfected with a kinase-inactive version of ERK5 (ERK5AEF) (Figure 2A, lane 6).
Fig. 2. MEK5 and ERK5 induce the expression of promoters upregulated upon muscle differentiation. A p21 promoter-luciferase construct (A), a 4× E-box-containing promoter construct (B) or a full-length MLC1A promoter luciferase plasmid (C) were cotransfected with expression vectors encoding constitutively active MEK5 (MEK5D), wild-type ERK5 (ERK5WT), a dominant-negative version of ERK5 (ERK5AEF), MyoD or E47, as indicated. Twenty hours after transfection cells were shifted to DMEM/2%HS for another 24 h and then harvested to measure luciferase activity as described. Fold induction is the ratio of luciferase activity in kinase-transfected cells versus cells transfected with the empty vectors.
One feature shared by the p21 promoter and promoter regions of muscle genes is the presence of E-box motifs (el-Deiry et al., 1995; Neville and Rosenthal, 1996). Here myocyte-specific bHLH proteins like MyoD or myogenin bind as heterodimers with ubiquitously expressed bHLH proteins, such as E47, to form transcriptionally active complexes (Lassar et al., 1991). To assess whether MEK5/ERK5 affects E-box-dependent transcription we used a construct with the luciferase gene under the control of a four E-box-containing promoter (4× E-box) (Neufeld et al., 2000). This promoter was moderately transactivated by coexpression of MEK5D and ERK5WT (Figure 2B, lane 6); however, both kinases imparted a strong synergistic effect on the activity of coexpressed MyoD (Figure 2B, lane 11). This transactivation was again abolished if ERK5WT was replaced by kinase-inactive ERK5AEF (Figure 2B, lane 12), indicating the requirement for an active ERK5 for promoter induction. Similar results were obtained for a 10T1/2 fibroblast cell line, which can also be committed to differentiate and exhibit a myogenic phenotype (data not shown).
The myosin light chain 1A gene (MLC1A) is one of the earliest sarcomeric muscle genes expressed in skeletal muscle (Cox and Buckingham, 1992) and is transcribed during the early stages of differentiation of skeletal muscle cell lines (Cox et al., 1990). The promoter region of the MLC1A gene contains several E-boxes (Catala et al., 1995) and, accordingly, ERK5 coexpression with MEK5D and MyoD results in an enhanced promoter activity in a similar fashion to that observed with the 4× E-box promoter (Figure 2C, lane 10). Interestingly, cotransfection of MEK5D and ERK5WT with E47, another bHLH protein that also plays a role in muscle-specific gene transcription, did not result in any significant promoter transactivation (Figure 2C, lane 8) although E47 readily cooperates with MyoD in this process (Figure 2C, lane 12). This suggests a functional synergism between the MEK5/ERK5 pathway and MyoD in muscle-specific gene transcription.
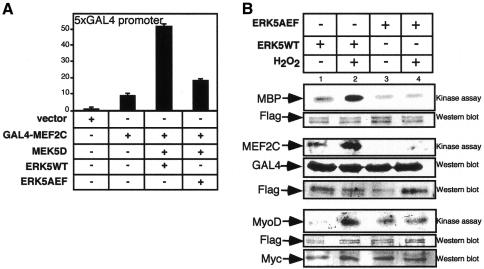
The molecular basis for this synergism might be a cooperation of MyoD with MEF2C, which is a known substrate of ERK5 (Kato et al., 1997) (Figure 3B). To analyse whether ERK5 activation under differentiation conditions also leads to an enhanced transactivation of MEF2C we used a Gal4–MEF2C fusion construct that was strongly activated in the presence of MEK5D and ERK5 (Figure 3A). Interestingly, ERK5 phosphorylates not only MEF2C but also MyoD in vitro. Although there is no in vivo evidence yet it may be the basis of another regulatory level controlled by ERK5. This could explain the strong synergism between ERK5 and MyoD on muscle-specific promoters.
Fig. 3. MEF2C is activated by ERK5 in C2C12 cells under differentiation conditions. (A) C2C12 cells were transfected with a Gal4–MEF2C fusion construct, and transactivation of MEF2C under differentiation conditions induced by coexpressed MEK5D, ERK5WT and ERK5AEF was monitored by luciferase expression from a 5× Gal4 luciferase construct. (B) Flag-ERK5WT and flag-ERK5AEF were immunoprecipitated with an anti-flag antiserum from transfected 293 cells, which were either left untreated or treated with H2O2 (200 µM, 60 min) for activation of the kinase. Immunocomplexes were incubated in a kinase reaction with either MBP (upper panel), Gal4MEF2C (middle panel) or Myc-MyoD (lower panel), which were immunopurified from transfected 293 lysates with anti-Gal4 and anti-Myc antibodies.
In summary, the MEK5/ERK5 pathway appears to enhance myogenic gene transcription by a mechanism dependent on the presence of myogenic activators, such as MyoD and MEF2C.
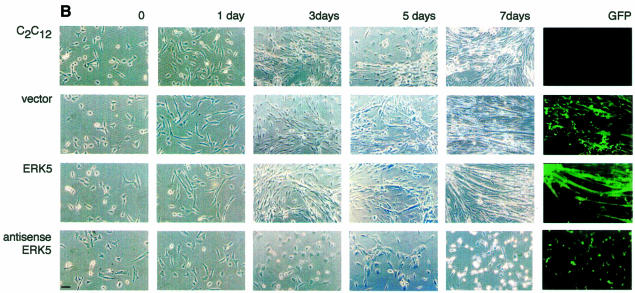
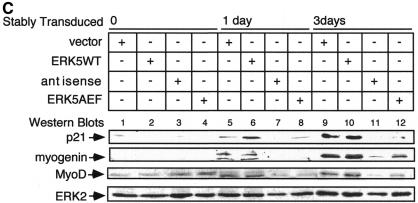
The process of myocyte differentiation is a multicellular phenomenon coincident with cell fusion and formation of myotubes (Walsh and Perlman, 1997). To monitor the function of ERK5 in a pool of differentiating myoblasts we created cell lines overexpressing an extra copy of ERK5WT or ERK5AEF as well as a cell line transcribing an antisense RNA to block the expression of endogenous kinase (Figure 4A). Stable expression in C2C12 cells was achieved by infection with different transducing retroviruses. Transduced cells were identified by expression of green fluorescent protein (GFP) from an internal ribosomal entry site (IRES) element within the same mRNA. After seeding, the different cell lines were allowed to proliferate in growth medium for 24 h and then induced to differentiate. Note that the increase in densities of parental cells and vector, ERK5WT or ERK5-antisense expressing cell lines was not significantly different after 24 h incubation in growth medium, indicating that the proliferative potential is not affected upon positive or negative interference with ERK5 expression (data not shown). However, upon induction of differentiation the typical morphological alterations of differentiating myocytes, e.g. parallel orientation, cell fusion and formation of myotubes (Figure 4B, upper panels), were completely suppressed if ERK5 expression was blocked by an antisense RNA (Figure 4B, lower panel). Accordingly, induced expression of differentiation-specific genes such as MyoD, myogenin or p21 was nearly abolished (Figure 4C). Although the effects on morphology were not that pronounced in the ERK5AEF cell line (data not shown), the expression levels of muscle-specific genes were also strongly reduced (Figure 4C). Impaired expression of these differentiation markers persists throughout the observation period of 7 days (data not shown), indicating that inhibition of ERK5 expression does not simply delay the differentiation process.
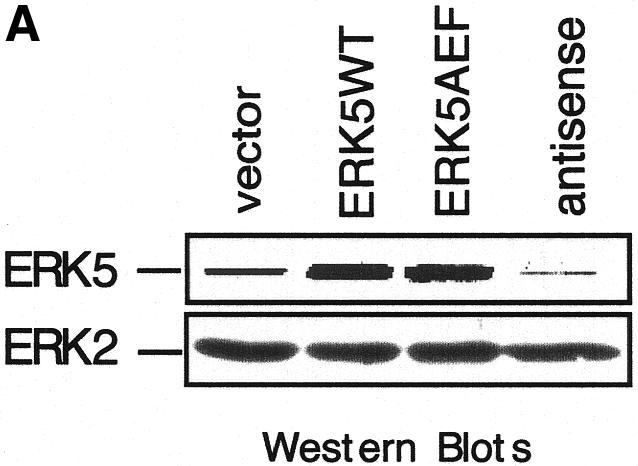
Fig. 4. Block of ERK5 expression results in the inhibition of myogenic differentiation. (A) ERK5 expression in stably transduced C2C12 cell lines harbouring empty vector, ERK5WT, ERK5AEF or antisense-ERK5. (B) Cells were seeded at a density of 2 × 105 cells per 10 cm in DMEM/10%FBS and shifted 24 h later to DMEM/2%HS to induce differentiation. Pictures at indicated time points correspond to distinct stages of differentiation. Expression of GFP (last column) is indicative of successfully transduced cells. Bar, 65 µm. (C) Cells were lysed in TLB after 1 and 3 days post-differentiation induction and protein lysates were separated on SDS–PAGE gels. After subsequent immunoblotting, blots were analysed for p21, MyoD and myogenin with the respective antisera described in Methods. Equal protein load of the different samples was controlled with an anti-ERK2 antiserum.
These experiments show that insufficient levels of ERK5 render C2C12 cells incapable of upregulating differentiation-specific genes such as MyoD, p21/Cip-1 and myogenin, and thereby blocking morphological differentiation (Figure 4B).
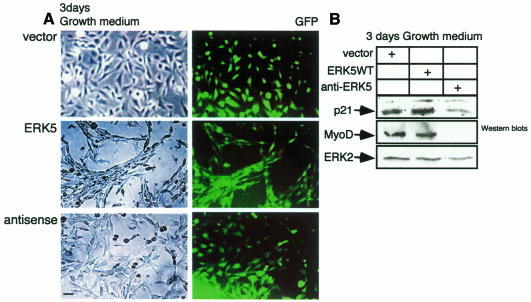
To examine whether ERK5 is not only required but also sufficient to induce differentiation we cultured early passages of the different cell lines for 3 days in growth medium. Interestingly, under these conditions the morphology of ERK5WT overexpressing cells resembles the phenotypic changes observed in early stages of the myocyte differentiation process (Figure 5A). Expression of MyoD is not altered (Figure 5B) and myogenin is not induced under these conditions; however, expression of p21 is upregulated (Figure 5B). This may give rise to the induction of a differentiation-prone phenotype. These data demonstrate that ERK5WT overexpression is sufficient for the commitment of C2C12 cells to differentiate.
Fig. 5. ERK5WT overexpression is sufficient for the commitment of muscle cells to differentiate. (A) The C2C12 cell lines indicated were grown for 3 days in DMEM/10% FBS and were then examined morphologically. (B) Cells shown in (A) were lysed and cell lysates were subjected to SDS–PAGE. After blotting, proteins were detected with antibodies against p21, MyoD, myogenin (not shown) and ERK2 as a loading control.
The MEK5/ERK5 pathway was previously shown to be involved in the stress and mitogenic responses of cells (Abe et al., 1996; Kato et al., 1997, 2000; English et al., 1999). Our studies demonstrate for the first time a biological role for ERK5 in the process of muscle cell differentiation. Thus, the MEK5/ERK5 cascade in muscle cells may resemble the classical mitogenic MAP kinase cascade, which has similarly been shown to be important for both proliferation and differentiation in certain cell types (Qui and Green, 1992; Marshall, 1995).
METHODS
Cell culture, DNAs and transfection procedures. The mouse embryonic muscle cell line C2C12, the Phoenix™ ECO virus packaging cell line, HEK 293 cells and stably transduced C2C12 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) FBS, at 37°C in humidified air with 6% CO2. To induce differentiation DMEM/10%FBS was replaced by DMEM supplemented with 2% (v/v) horse serum (DMEM/2%HS). The MyoD expression plasmid was kindly provided by R. Rupp, Tübingen, Germany (Dias et al., 1992). The p21 promoter construct (Datto et al., 1995), the 4× E-box-containing promoter-luciferase construct (Neufeld et al., 2000) and an expression vector for E47 (Neufeld et al., 2000) have been previously described. The full-length MLC1A-promoter construct (Catala et al., 1995) was used with kind permission of M. Buckingham, Paris, France. pCDNA3 vectors expressing wild-type ERK5 (ERK5WT), constitutively active MEK5 (MEK5D) and kinase-inactive ERK5 (ERK5AEF), as well as a Gal4-MEF2C fusion construct and a 5× Gal4 luc vector, have been described previously (Kato et al., 1997). For generation of ERK5WT-sense, ERK5AEF and ERK5-antisense expressing cell lines, the corresponding inserts were digested out of the pCDNA3 vector with HindIII and XbaI and subcloned into the pCMV5 vector using the same restriction sites. The inserts were digested with BamHI and religated to the pCFG5 IEGZ retroviral vector, for expression of both the gene of interest from the retroviral long-terminal repeat (LTR) and the GFP from an IRES. Cells were transfected by a standard calcium phosphate coprecipitation method according to a modified Stratagene protocol. Generation of stably transduced cell lines was previously described (Pear et al., 1993). The lethal dose of zeocin (Invitrogen) in C2C12 cells based on our own tests was estimated at 400 µg/ml. One day post-infection the cells were incubated in the presence of the antibiotic to maintain positive selection. The drug-resistant clones were further selected on the basis of expression of GFP. Stably transduced cell lines were cultured in the presence of 400 µg/ml zeocin after every two to three cell cycles to maintain the transduced constructs.
Immune complex kinase assays, antibodies and western blot analysis. C2C12 cells were lysed in Triton-lysis-buffer (TLB) [20 mM Tris pH 7.4, 50 mM sodium β-glycerophosphate, 20 mM sodium pyrophosphate, 137 mM NaCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 2 mM EDTA, and an inhibitor mix containing 1 mM Pefabloc, 1 mM sodium orthovanadate, 5 mM benzamidine, 5 µg/ml aprotinin, 5 µg/ml leupeptin] on ice for 10 min. Cell debris was removed by centrifugation. For the precipitation of endogenous ERK5 or transfected flag ERK5 from precleared TLB-lysates (500 µg of total protein), 10 µg of polyclonal goat ERK5 antiserum (C-20, Santa Cruz Biotechnology) or an anti-flag monoclonal antibody (Kodak) were used, respectively. The immune complexes were precipitated with 25 µl of protein G–agarose beads and washed twice with TLB and with the kinase buffer (10 mM MgCl2, 25 mM β-glycerophosphate, 25 mM HEPES pH 7.5, 5 mM benzamidine, 0.5 mM dithiothreitol, 1 mM sodium vanadate). In vitro kinase assays were performed with myelin basic protein (MBP) (1 µg) or immunopurified Gal4-MEF2C and Myc-MyoD as substrates, incubating for 30 min in kinase buffer supplemented with 5 µCi of [γ-32P]ATP (Amersham) and 0.1 mM ATP at 30°C. Proteins were separated by SDS–PAGE and the corresponding bands detected by autoradiography. Endogenous ERK5, myogenin, MyoD, p21 and ERK2 in C2C12 cells were detected by immunoblotting, using respectively, a polyclonal goat anti-ERK5 antiserum (C-20) antibody and polyclonal rabbit anti-myogenin (M-225), anti MyoD (C-20), anti-p21 (C-19) and anti-ERK2 (C-14) antibodies, all from Santa Cruz Biotechnology.
Reporter gene assays. Thirty-six hours post-transfection, C2C12 luciferase assays were performed essentially as described (Neufeld et al., 2000). The luciferase activities were normalized to the β-galactosidase activity of cotransfected β-Gal vector. The β-galactosidase assay was performed with 20 µl of precleared cell lysate according to a standard protocol. Mean values and standard deviations from two independent experiments performed in triplicate are shown.
Acknowledgments
ACKNOWLEDGEMENTS
We thank R. Cassada, S.M. Feller, J. Troppmair and T. Wirth for critical reading of the manuscript and helpful suggestions. This work was supported by the Deutsche Forschungsgemeinschaft, Grant Lu477/7-1 to S.L.
REFERENCES
- Abe J., Kusuhara, M., Ulevitch, R.J., Berk, B.C. and Lee, J.D. (1996) Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J. Biol. Chem., 271, 16586–16590. [DOI] [PubMed] [Google Scholar]
- Catala F., Wanner, R., Barton, P., Cohen, A., Wright, W. and Buckingham, M. (1995) A skeletal muscle-specific enhancer regulated by factors binding to E and CArG boxes is present in the promoter of the mouse myosin light-chain 1A gene. Mol. Cell. Biol., 15, 4585–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Costa, M.L., Mermelstein, C.S., Chagas, C., Holtzer, S. and Holtzer, H. (1990) MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc. Natl Acad. Sci. USA, 87, 7988–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.D. and Buckingham, M.E. (1992) Actin and myosin genes are transcriptionally regulated during mouse skeletal muscle development. Dev. Biol., 149, 228–234. [DOI] [PubMed] [Google Scholar]
- Cox R.D., Garner, I. and Buckingham, M.E. (1990) Transcriptional regulation of actin and myosin genes during differentiation of a mouse muscle cell line. Differentiation, 43, 183–191. [DOI] [PubMed] [Google Scholar]
- Datto M.B., Yu, Y. and Wang, X.F. (1995) Functional analysis of the transforming growth factor β responsive elements in the WAF1/Cip1/p21 promoter. J. Biol. Chem., 270, 28623–28628. [DOI] [PubMed] [Google Scholar]
- Dias P., Parham, D.M., Shapiro, D.N., Tapscott, S.J. and Houghton, P.J. (1992) Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res., 52, 6431–6439. [PubMed] [Google Scholar]
- el-Deiry W.S. et al. (1995) Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res., 55, 2910–2919. [PubMed] [Google Scholar]
- English J.M., Pearson, G., Hockenberry, T., Shivakumar, L., White, M.A. and Cobb, M.H. (1999) Contribution of the ERK5/MEK5 pathway to Ras/Raf signaling and growth control. J. Biol. Chem., 274, 31588–31592. [DOI] [PubMed] [Google Scholar]
- Gossett L.A., Kelvin, D.J., Sternberg, E.A. and Olson, E.N. (1989) A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol. Cell. Biol., 9, 5022–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura S., Moriguchi, T. and Nishida, E. (1999) Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J. Biol. Chem., 274, 26563–26571. [DOI] [PubMed] [Google Scholar]
- Kato Y., Kravchenko, V.V., Tapping, R.I., Han, J., Ulevitch, R.J. and Lee, J.D. (1997) BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J., 16, 7054–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Chao, T.H., Hayashi, M., Tapping, R.I. and Lee, J.D. (2000) Role of BMK1 in regulation of growth factor-induced cellular responses. Immunol. Res., 21, 233–237. [DOI] [PubMed] [Google Scholar]
- Lassar A.B., Davis, R.L., Wright, W.E., Kadesch, T., Murre, C., Voronova, A., Baltimore, D. and Weintraub, H. (1991) Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell, 66, 305–315. [DOI] [PubMed] [Google Scholar]
- Ludwig S., Engel, K., Hoffmeyer, A., Sithanandam, G., Neufeld, B., Palm, D., Gaestel, M. and Rapp, U.R. (1996) 3pK, a novel mitogen-activated protein (MAP) kinase-activated protein kinase, is targeted by three MAP kinase pathways. Mol. Cell. Biol., 16, 6687–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Black, B.L., Martin, J.F. and Olson, E.N. (1995) Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell, 83, 1125–1136. [DOI] [PubMed] [Google Scholar]
- Neufeld B., Grosse-Wilde, A., Hoffmeyer, A., Jordan, B.W., Chen, P., Dinev, D., Ludwig, S. and Rapp, U.R. (2000) Serine/Threonine kinases 3pK and MAPK-activated protein kinase 2 interact with the basic helix–loop–helix transcription factor E47 and repress its transcriptional activity. J. Biol. Chem., 275, 20239–20242. [DOI] [PubMed] [Google Scholar]
- Neville C. and Rosenthal, N. (1996) Transcriptional regulation of skeletal myogenesis. In Goodbourn, S. (ed.), Eukaryotic Gene Transcription. IRL Press, Oxford, UK, Vol. 12, pp. 192–233.
- Parker S.B., Eichele, G., Zhang, P., Rawls, A., Sands, A.T., Bradley, A., Olson, E.N., Harper, J.W. and Elledge, S.J. (1995) p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science, 267, 1024–1027. [DOI] [PubMed] [Google Scholar]
- Pear W.S., Nolan, G.P., Scott, M.L. and Baltimore, D. (1993) Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl Acad. Sci. USA, 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui M.S. and Green, S.H. (1992) PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron, 9, 705–717. [DOI] [PubMed] [Google Scholar]
- Walsh K. and Perlman, H. (1997) Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev., 7, 597–602. [DOI] [PubMed] [Google Scholar]
- Yaffe D. and Saxel, O. (1977) A myogenic cell line with altered serum requirements for differentiation. Differentiation, 7, 159–166. [DOI] [PubMed] [Google Scholar]
- Yang C.C., Ornatsky, O.I., McDermott, J.C., Cruz, T.F. and Prody, C.A. (1998) Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res., 26, 4771–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]