Abstract
The involvement of phosphatidylinositol 3-kinase (PI3K) in membrane trafficking in mammalian cells has largely come from experiments with wortmannin. This compound inhibits endosome fusion in vitro, possibly by inhibiting the production of phosphatidylinositol (PtdIns)-3-P, which co-regulates EEA1 with Rab5. However, the results from wortmannin inhibition experiments performed in vivo differ significantly. We have recently shown that wortmannin enlarges endosomes containing the epidermal growth factor receptor (EGFR) and enhances the lysosomal degradation of EGFR. In this report, we demonstrate that addition of the PI3K reaction products does not suppress wortmannin-induced enlargement of EGFR-containing endosomes and enhancement of EGFR degradation. Moreover, the effects of wortmannin on the intracellular trafficking of EGFR mimic those of the permanently activated Rab5 mutant, Rab5 Q79L, which stimulates endosome fusion. We also found that an inactive Rab5 mutant, Rab5 S34N, blocks wortmannin-induced endosome enlargement and that wortmannin stimulates the activation of Rab5. We further showed that wortmannin reduced the membrane association of p120 Ras GTPase-activating protein (GAP) and inhibited the interaction between Rab5 and p120 Ras GAP. We conclude that wortmannin alters intracellular trafficking of EGFR by activating Rab5 rather than by inhibiting PI3K.
INTRODUCTION
Attempts to clarify the nature of phosphatidylinositol 3-kinase (PI3K) involvement in membrane trafficking in mammalian cells have been largely based on the use of inhibitors, such as wortmannin, of the catalytic activity of PI3K. Wortmannin blocks the lysosomal degradation of the platelet growth factor receptor (Shpetner et al., 1996) and alters the endocytosis of the transferrin receptor (Martys et al., 1996; Spiro et al., 1996). In addition, wortmannin inhibits endosome fusion in in vitro assays (Jones and Clague, 1995; Li et al., 1995). The combined use of wortmannin and biochemical approaches led to the identification of a direct target for phosphatidylinositol (PtdIns)-3-P, the early-endosomal autoantigen 1 (EEA1), which plays an essential role in endosome fusion (Gaullier et al., 1998; Mills et al., 1998; Patki et al., 1998). Rab5 controls endosome fusion by regulating several effectors, including Rabaptin5 (Stenmark et al., 1995), Rabaptin5β (Gournier et al., 1998) and EEA1 (Christoforidis et al., 1999). It has been proposed that early endosome fusion is mediated by EEA1, which is co-regulated by Rab5 and PI3K (Simonsen et al., 1998).
However, we have recently found that inhibition of PI3K activity by wortmannin or LY294002 does not block the lysosomal targeting and degradation of epidermal growth factor receptor (EGFR). In contrast, EGFR endocytosis is dependent on Rab5 activity. Most interestingly, we have shown that wortmannin enlarges endosomes and enhances the lysosomal degradation of EGFR (Chen and Wang, 2001). Wortmannin treatment also leads to the lysosomal accumulation of resident apical plasma membrane proteins in MDCK cells (Tuma et al., 1999). Previously, it has been reported that wortmannin increases the accumulation of the fluid phase marker Lucifer Yellow, and of transferrin in early endosomes and causes only a small decrease in the rate of transferrin recycling (Martys et al., 1996; Spiro et al., 1996). A pronounced enlargement of endosomes containing these markers is observed after treatment with wortmannin (Shpetner et al., 1996). These results suggest that wortmannin enhances endosome fusion in vivo, and thus are obviously at odds with the results obtained from in vitro endosome fusion experiments. The in vivo effects of wortmannin on intracellular trafficking also differ significantly from the predicted effects of PI3K based on the experiments using fusion proteins of the COOH domain of EEA1. These experiments indicate that both Rab5 and PtdIns-3-P are required to achieve the binding of EEA1 to endosomal membranes (Simonsen et al., 1998). It could therefore be predicted that inhibition of PI3K activity with wortmannin would block endosome fusion.
In this study, we found that the in vivo effects of wortmannin on EGFR endocytosis are not due to PI3K inhibition. Wortmannin does not block the recycling of EGFR; instead, wortmannin regulates EGFR intracellular trafficking by activating Rab5.
RESULTS
Wortmannin enlarges EGFR-containing endosomes by a PI3K-independent pathway
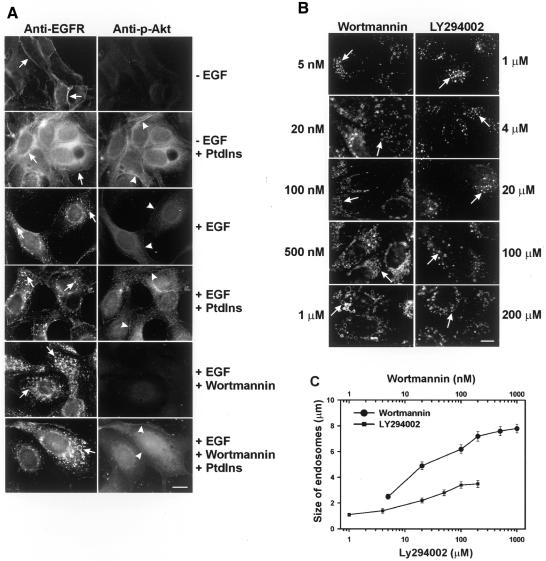
To determine whether the effects of wortmannin on EGFR endocytosis are due to PI3K inhibition, we treated MDCK cells with both wortmannin and a mixture of three PI3K reaction products, PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3, and then examined their effects on the morphology of EGFR-containing endosomes. The addition of PI3K reaction products increased the phosphorylation of Akt, demonstrating the efficacy of the phospholipids. However, there were no effects on the morphology of endosomes and no reversal on wortmannin-induced enlargement of endosomes (Figure 1A).
Fig. 1. Wortmannin enlarges EGFR-containing endosomes by a PI3K-independent pathway. (A) Effects of the addition of PI3K reaction products on wortmannin-induced enlargement of EGFR-containing endosomes. BT20 cells were treated with wortmannin and a mixture of three PI3K reaction products including PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3, and stimulated with EGF for 30 min or not stimulated. EGFR (left panel and arrows) and phospho-Akt (right panel and arrowheads) localization were determined by indirect immunofluorescence. (B) Effects of addition of wortmannin and LY294002 on the size of EGFR-containing endosomes in MDCK cells. Left panel, cells treated with wortmannin. Right panel, cells treated with LY294002. Cells were treated with wortmannin or LY294002 at the indicated concentrations for 15 min and then stimulated with EGF (100 ng/ml) for 30 min. EGFR (arrows) was detected by indirect immunofluorescence. Size bar = 20 µm. (C) Quantification of the results from (B).
Next, we compared effects of two PI3K inhibitors, wortmannin and LY294002, on the endosome morphology of MDCK cells. The dose–response curve showed that the maximum size of endosomes induced by wortmannin at a concentration of 1 µM was 2.4 times as large as that induced by LY294002 at 200 µM (Figure 1B and C). Together, these results suggest that wortmannin-induced endosome enlargement is not due to PI3K inhibition.
Wortmannin enhances the EGF-induced degradation of EGFR by a PI3K-independent pathway
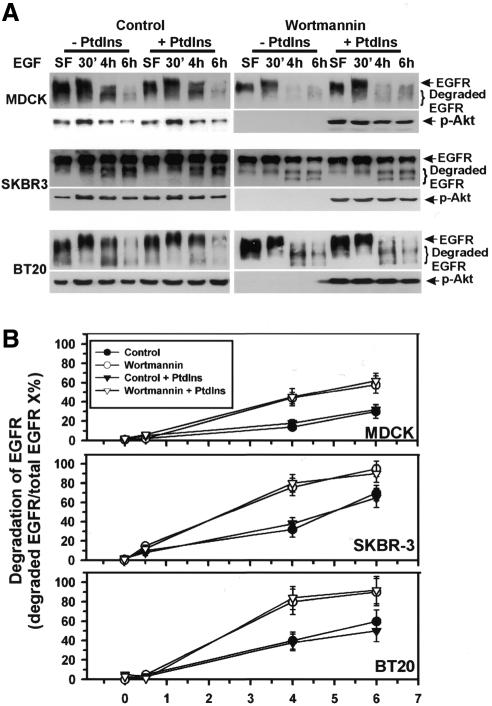
We next examined whether the effects of wortmannin on EGF-induced degradation of EGFR are due to inhibition of PI3K. MDCK, BT20 and SKBR-3 cells were treated with wortmannin, EGF and/or PI3K reaction products for the indicated times. Immunoblotting with anti-EGFR antibodies showed that treatment of cells with PI3K reaction products did not affect EGF-induced degradation of EGFR, nor did it reverse wortmannin-induced enhancement of EGFR degradation (Figure 2). Immunoblotting of the same membrane with anti-phospho-Akt antibodies confirmed that wortmannin treatment abolished Akt phosphorylation, while treatment with PI3K reaction products restored Akt phosphorylation (Figure 2A). As a further control, we showed that PI3K reaction products restored the endosome association of EEA1 (data not shown). We have shown previously that the effect of wortmannin on EGFR synthesis is not significant (Chen and Wang, 2001).
Fig. 2. Effects of the addition of PI3K reaction products on wortmannin-induced enhancement of EGFR degradation. (A) MDCK, SKBR-3 and BT20 cells were treated with wortmannin and a mixture of three PI3K reaction products, PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3, and stimulated with EGF for the indicated times. Cell lysates were subjected to immunoblotting with anti-EGFR or anti-phospho-Akt (p-Akt) antibodies. (B) Quantification of the results from (A).
The effects of wortmannin on intracellular trafficking mimic those of Rab5 Q79L
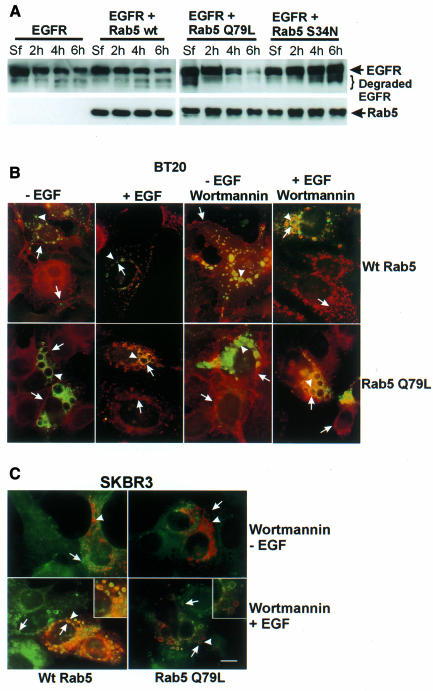
Both wortmannin and Rab5 Q79L result in enlarged endosomes, and wortmannin enhances the degradation of EGFR (Chen and Wang, 2001). To investigate whether Rab5 Q79L enhances EGFR degradation, we transiently transfected 293T cells with plasmids expressing EGFR, wild-type Rab5, mutant Rab5 Q79L and/or S34N, and stimulated the cells with EGF for 30 min, 4 h or 6 h. Immunoblotting showed that after EGF stimulation for 6 h, the EGFR degradation rate was 39.8 ± 5.9% in control cells, transfection of cells with wild-type Rab5 slightly enhanced the degradation of EGFR (50.6 ± 7.2%), and transfection with Rab5 Q79L significantly enhanced the degradation of EGFR (81.2 ± 7.9%); however, transfection with Rab5 S34N blocked the degradation of EGFR (12.5 ± 4.6%) (Figure 3A). These results suggest that the effects of wortmannin on the intracellular trafficking of EGFR mimic those of Rab5 Q79L.
Fig. 3. The effects of wortmannin on intracellular trafficking mimic those of Rab5 Q79L. (A) Regulation of the EGF-induced lysosomal degradation of EGFR by Rab5. 293T cells were transfected with plasmids expressing wild-type Rab5, mutant Rab5 S34N or Q79L for 48 h and then stimulated with EGF for the indicated times. Cell lysates were subjected to immunoblotting analysis with anti-EGFR or anti-Rab5 antibodies. (B and C) The effects of wortmannin and Rab5 on endosome morphology. BT20 (B) and SKBR-3 (C) cells were transfected with wild-type Rab5 or mutant Rab5 Q79L for 48 h. Cells were then treated with wortmannin and stimulated with EGF for 30 min or not stimulated. EGFR and Rab5 were detected by indirect immunofluorescence. Photographs are double exposures with EGFR localization in the red channel (arrows) and Rab5 localization in the green channel (arrowheads) for BT20 cells, and with EGFR localization in the green channel (arrows) and Rab5 localization in the red channel (arrowheads) for SKBR-3 cells. Inset: co-localization of EGFR and Rab5 in endosomes. Size bar = 20 µm.
To confirm that the enlarged endosomes resulting from wortmannin treatment are the same endosomes resulting from the expression of Rab5 Q79L, we transfected BT20 and SKBR-3 cells with Rab5 Q79L or wild-type Rab5. Forty-eight hours after transfection, the cells were treated with wortmannin and EGF. EGFR co-localized with both Rab5 Q79L and wild-type Rab5 in enlarged endosomes (Figure 3B), indicating that the enlarged endosomes induced by Rab5 Q79L are indeed those induced by wortmannin. In addition, we showed that wortmannin did not affect the Rab5 Q79L-induced morphological changes of endosomes.
Effects of wortmannin on the intracellular trafficking of EGFR are dependent on Rab5 activity
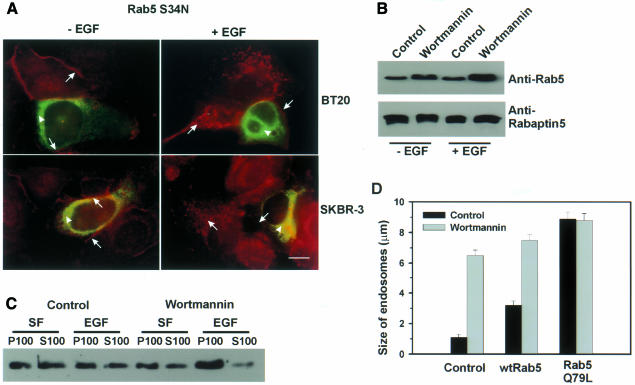
The fact that the effects of wortmannin on the intracellular trafficking of EGFR mimic those of Rab5 Q79L prompted us to propose that wortmannin may exert its effect by activating Rab5. To test this hypothesis, we first determined whether a dominant-negative mutant of Rab5, Rab5 S34N, suppresses the effects of wortmannin on the intracellular trafficking of EGFR. BT20 and SKBR3 cells were transfected with Rab5 S34N and then treated with wortmannin and EGF (Figure 4A). Despite treatment with wortmannin, the endosomes in Rab5 S34N-transfected cells stayed fragmented. This suggests that the enlargement of EGFR-containing endosomes by wortmannin is dependent on functional Rab5.
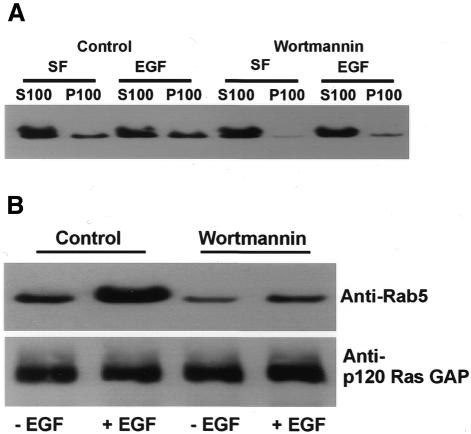
Fig. 4. Wortmannin regulates intracellular trafficking by activating Rab5. (A) Suppression of wortmannin-induced endosome enlargement by Rab5 S34N. BT20 and SKBR-3 cells were transfected with mutant Rab5 S79N for 48 h. The cells were then treated with wortmannin and stimulated with EGF for 30 min or not stimulated. Photographs are double exposures with EGFR localization in the red channel (arrows) and Rab5 localization in the green channel (arrowheads). Size bar = 20 µm. (B) The effects of wortmannin on the association of Rab5 and its effector Rabaptin5. BT20 cells were treated with wortmannin for 15 min or not treated, and then stimulated with EGF for 30 min or not stimulated. Cell lysates were subjected to immunoprecipitation with mouse anti-Rabaptin5 antibody, followed by agarose-conjugated goat anti-mouse IgG. Immunoprecipitates were subjected to immunoblot analysis with mouse anti-Rab5 or anti-Rabaptin5 antibody. (C) The effects of wortmannin on the membrane association of Rab5. BT20 cells were subcellularly fractionated into soluble fraction (S100) and particulate fraction (P100) after treatment with wortmannin (100 nM) and EGF (30 min) as indicated. The subcellular fractions were subjected to immunoblot analysis with anti-Rab5 antibody as described in Methods. (D) The effects of wortmannin and Rab5 on the size of endosomes in BT20 cells. Experiments were performed as described for Figure 3B.
We next examined whether wortmannin induces the activation of Rab5. Since Rabaptin5 only binds to activated Rab5 (Stenmark et al., 1995), co-immunoprecipitation of Rab5 and Rabaptin5 were used to determine the activation state of Rab5. BT20 cells were treated with wortmannin, and cell lysates were subjected to immunoprecipitation with anti-Rabaptin5 antibodies. The immunoprecipitates were then immunoblotted with anti-Rab5 or anti-Rabaptin5 antibodies. In both serum-deprived cells and EGF-treated cells, wortmannin treatment significantly increased the amount of Rab5 that co-immunoprecipitated with Rabaptin5 (Figure 4B). We also examined the effects of wortmannin on membrane association of Rab5. Our results showed that wortmannin treatment significantly increased membrane association of Rab5 in both serum-free and EGF-treated BT20 cells (Figure 4C). These results suggest that wortmannin indeed enhances the function of Rab5 in mediating endosome fusion.
If wortmannin induces endosome enlargement by activating Rab5, expression of wild-type Rab5 and treatment with wortmannin should enlarge endosomes in a synergistic manner. Indeed, our results indicated that expression of wild-type Rab5 and treatment with wortmannin synergically enlarged endosomes. Moreover, no synergism was observed between Rab5 Q79L and wortmannin (Figure 4D).
Wortmannin stimulates Rab5 activity by blocking the interaction between Rab5 and p120 Ras GAP
One mechanism by which wortmannin stimulates Rab5 activity is to inhibit Rab5 GTPase-activating proteins (GAPs). Several Rab5 GAPs, including Tuberin, p120 Ras GAP and RN-tre, have been identified (Xiao et al., 1997; Liu and Li, 1998; Lanzetti et al., 2000). We have shown that p120 Ras GAP associated with endosomes following EGF stimulation (Wang et al., 1996). To determine whether wortmannin reduces the interaction between p120 Ras GAP and Rab5, we first examined the effects of wortmannin on the membrane association of p120 Ras GAP by immunoblotting. Our results showed that wortmannin reduces the membrane association of p120 Ras GAP in both serum-free and EGF-stimulated BT20 cells (Figure 5A). We further examined the interaction between p120 Ras GAP and Rab5 by co-immunoprecipitation experiments. Our results showed that wortmannin significantly reduced the amount of Rab5 that was co-immunoprecipitated with p120 Ras GAP (Figure 5B). These results suggest that wortmannin may enhance Rab5 activity by inhibiting the interaction between Rab5 and p120 Ras GAP.
Fig. 5. Wortmannin inhibits the interaction between p120 Ras GAP and Rab5. (A) The effects of wortmannin on the membrane association of p120 Ras GAP. BT20 cells were subcellularly fractionated into soluble fraction (S100) and particulate fraction (P100) after treatment with wortmannin (100 nM) and EGF (30 min) as indicated. The subcellular fractions were subjected to immunoblot analysis with anti-p120 Ras GAP antibody as described in Methods. (B) The effects of wortmannin on the association of Rab5 and p120 Ras GAP. BT20 cells were treated with wortmannin for 15 min or not treated, and then stimulated with EGF for 30 min or not stimulated. Cell lysates were subjected to immunoprecipitation with rabbit anti-p120 Ras GAP antibody, followed by protein A–Sepharose. Immunoprecipitates were subjected to immunoblot analysis with mouse anti-Rab5 or anti-Rabaptin5 antibody.
DISCUSSION
In light of the controversy regarding the roles of wortmannin, PI3K and Rab5 in the regulation of endosome fusion and receptor endocytosis, we addressed three specific questions in the present work. First, are the in vivo effects of wortmannin on EGFR endocytosis due to inhibition of PI3K activity? Secondly, does wortmannin regulate EGFR endocytosis by stimulating endosome fusion in a way similar to that of mutant Rab5 Q79L? Thirdly, what is the relationship between wortmannin and Rab5 in regulating EGFR endocytosis?
Our results indicated that the in vivo effects of wortmannin on EGFR endocytosis were not due to inhibition of PI3K. First, suppression of wortmannin-induced PI3K inhibition by addition of PI3K reaction products did not abolish wortmannin-induced enlargement of EGFR-containing endosomes and enhancement of EGFR degradation in lysosomes (Figures 1A and 2). Secondly, another PI3K inhibitor, LY294002, had much less effect on the enlargement of EGFR-containing endosomes (Figure 1B and C). Actually, it has been reported that wortmannin also inhibits the activity of enzymes other than PI3K (Bonser et al., 1991; Cross et al., 1995; Meyers and Cantley, 1997). Our finding further suggests that where a role of PI3K in receptor endocytosis has been assigned purely on the basis of its inhibition by wortmannin, the conclusion may not be correct.
Similar morphological changes in endosomes were observed in wortmannin-treated cells and in Rab5 Q79L-transfected cells (Chen and Wang, 2001). This suggests that wortmannin mimics the function of activated Rab5. Indeed, we showed that EGF-induced degradation of EGFR was similarly enhanced in Rab5 Q79L-transfected cells (Figure 3A) and wortmannin-treated cells (Chen and Wang, 2001). Moreover, we demonstrated that the enlarged endosomes induced by wortmannin were the same endosomes induced by Rab5 Q79L (Figure 3B). Since the effects of Rab5 Q79L on endocytosis have been attributed to enhanced endosome fusion (Stenmark et al., 1994), it is possible that the observed effects of wortmannin on EGFR endocytosis are also caused by enhanced endosome fusion. One argument against this hypothesis is that wortmannin may enlarge endosomes and enhance the degradation of EGFR by blocking recycling of EGFR from endosomes to the plasma membrane. To exclude this possibility, we compared the effects of wortmannin and monensin on EGFR endocytosis. Monensin inhibits the recycling of many receptors, including EGFR (Felder et al., 1990). We showed that in contrast to monensin, wortmannin did not block recycling of EGFR (see Supplementary data, available at EMBO reports Online).
We further demonstrated that wortmannin enlarged endosomes and enhanced the degradation of EGFR by activating Rab5. Our conclusion was supported by the following evidence. First, the effects of wortmannin were dependent on Rab5 activity. Expression of the dominant-negative mutant Rab5 S34N blocked wortmannin-induced enlargement of endosomes (Figure 4A). Secondly, wortmannin increased Rab5 activity. As shown by co-immunoprecipitation experiments, wortmannin treatment increased the association of Rab5 with its effector Rabaptin5 (Figure 4B). Given that only the activated Rab5 binds to Rabaptin5 (Stenmark et al., 1995), our results indicate that wortmannin stimulates Rab5 activation. Thirdly, wortmannin enhances the membrane association of Rab5 (Figure 4C). Finally, while expression of wild-type Rab5 and wortmannin treatment synergistically enlarged endosomes, no synergism was observed between Rab5 Q79L and wortmannin (Figure 4D).
Wortmannin may activate Rab5 by various mechanisms. Wortmannin may promote the interaction between Rab5 and its guanine nucleotide exchange factors (GEFs) such as Rabex-5, enhance the function of Rab5-GDI (guanine-nucleotide dissociation inhibitor) that targets Rab5 to endosome, and/or inhibit the interaction between Rab5 and its GAPs. Several Rab5 GAPs, including Tuberin, p120 Ras GAP and RN-tre, have been identified (Xiao et al., 1997; Liu and Li, 1998; Lanzetti et al., 2000), and the last two GAPs form a complex with activated EGFR (Wang et al., 1996; Lanzetti et al., 2000). We showed that wortmannin reduced the membrane association of p120 Ras GAP in both serum-free and EGF-stimulated BT20 cells (Figure 5A), and that wortmannin significantly reduced the amount of Rab5 that was co-immunoprecipitated with p120 Ras GAP (Figure 5B). These results suggest that wortmannin may enhance Rab5 activity by inhibiting the interaction between Rab5 and one of its GAPs, p120 Ras GAP. However, we do not exclude the possibility that wortmannin may also affect the interactions between Rab5 and its GEFs and GDIs.
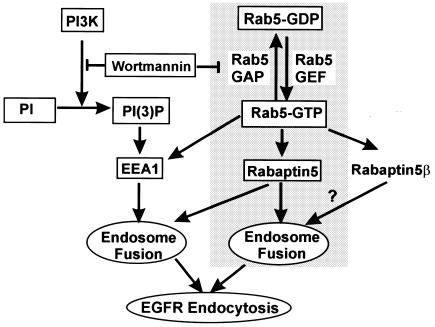
Based on our current and previous results (Chen and Wang, 2001), and results from other laboratories (Simonsen et al., 1998), we propose a model to describe how wortmannin and Rab5 regulate endosome fusion and EGFR endocytosis (Figure 6). In this model, wortmannin inhibits PI3K activity and subsequent endosome association of EEA1, which reduces endosome fusion, especially in vitro. On the other hand, wortmannin also promotes interaction between Rab5 and Rabaptin5, partially by preventing the interaction between Rab5 and p120 Ras GAP, which enhances endosome fusion and EGFR endocytosis. The net effects of wortmannin on EGFR endocytosis in vivo are to enhance the endosome fusion and EGFR endocytosis.
Fig. 6. A schematic model that shows the regulation of endosome fusion and EGFR endocytosis by wortmannin and Rab5.
METHODS
Antibodies and chemicals.
Sheep anti-EGFR antibodies were purchased from Upstate Technology (Lake Placid, NY). Rabbit anti-EGFR and mouse anti-p120 Ras GAP antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antibodies to Rab5 and Rabaptin5 were from BD PharMingen Research (San Jose, CA). Rabbit anti-phospho-Akt antibodies were from New England Biolabs. PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3 were from Matreya. FITC- and rhodamine-conjugated secondary antibodies were from Jackson Immunology. Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Bio-Rad. Unless otherwise specified, all chemicals were from Sigma.
Wortmannin, LY294002 and monensin treatment.
Cells were treated with wortmannin (100 nM unless indicated otherwise), LY294002 (as indicated) or monensin (100 µM) for 15 min at 37°C. Cells were then treated with EGF (100 ng/ml) for the indicated times in the continuous presence of wortmannin, LY294002 or monensin, or without EGF stimulation. For treatments longer than 2 h, medium was replaced with fresh medium containing the same concentrations of wortmannin, LY294002 or monensin.
Transient transfection.
BT20, SKBR-3 and 293T cells were transiently transfected by calcium phosphate precipitation with vectors encoding wild-type Rab5 (wtRab5), mutant Rab5 Q79L, Rab5 S34N and/or EGFR. While BT20 and SKBR-3 cells were used for indirect immunofluorescence after transfection for 48 h, 293T cells were used for immunoblotting after transfection for 24 h.
Treatment of cells with phospholipids.
To allow phospholipids to reach their targets in the cells, the methods established by several laboratories were adapted (Jones et al., 1999). Briefly, synthetic DiC16 PtdIns-3-P, DiC16 PtdIns-3,4-P2, DiC16 PtdIns-3,4,5-P3 (Matreya), phosphocholin and phosphoinositol (Sigma) were mixed at a ratio of 1:1:1:100:100 and dried under N2, re-suspended in 10 mM HEPES pH 7.4 containing 1 mM EDTA and sonicated. Mixed vesicles containing these phospholipids were then diluted 100 times with medium to a final concentration of 10 µM for PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3. Cells were treated with Saponin in phosphate-buffered saline (0.02mg/ml) for 10 min and then incubated with medium containing wortmannin (100 nM) and the mixture of PtdIns-3-P, PtdIns-3,4-P2 and PtdIns-3,4,5-P3 for 15 min. EGF (100 ng/ml) was added to the medium and cells were incubated at 37°C for the indicated times. The cells were processed for indirect immunofluorescence or immunoblotting.
Indirect immunofluorescence.
Indirect immunofluorescence was carried out as described previously (Wang et al., 1996). To detect EGFR alone, the primary antibody was sheep anti-EGFR antibody, and the secondary antibody was rhodamine-labeled donkey anti-sheep IgG. For BT20 and SKBR-3 cells transfected with wild-type or mutant Rab5, the primary antibodies were sheep anti-EGFR and mouse anti-Rab5 antibodies. For BT20 cells treated with wortmannin and the PI3K reaction products, the primary antibodies were sheep anti-EGFR and rabbit anti-phospho-Akt (p-Akt) antibodies. For double indirect immunofluorescence of cells treated with monensin, the primary antibodies were sheep anti-EGFR and mouse anti-phosphotyrosine antibodies. Color photographs were taken with a digital camera by superimposing the monochrome graphs of two channels. To measure the size of endosomes, 10 photographs of representative areas were taken and 50 endosomes in each photograph were measured. Thus, each data point is the mean of 500 endosomes.
Immunoprecipitation.
Immunoprecipitation experiments were carried out as described previously (Wang et al., 1996). Following treatment with wortmannin (100 nM) and/or EGF (100 ng/ml) for 15 min at 37°C, BT20 cells were lysed with immunoprecipitation buffer. After centrifugation to remove debris, the supernatants containing 1 mg of total protein were incubated with 1 µg of mouse anti-Rabadaptin5 or rabbit anti-p120 Ras GAP antibodies (Wang et al., 1996), followed by goat anti-mouse IgG conjugated to agarose or protein A–Sepharose. For control experiments, mouse anti-Rabadaptin5 antibodies were substituted with normal mouse IgG1, and rabbit anti-GAP antibodies were substituted with pre-immune serum (no Rabadaptin5, p120 Ras GAP and Rab5 were precipitated in controls).
Immunoblotting.
Immunoblotting was performed as described previously (Wang et al., 1996). For the EGFR degradation assay, cells were lysed with M-PER™ Mammalian Protein Extraction Reagent (Pierce Chemical) in the presence of 0.5 mM Na3VO4, 0.02% NaN3, 0.1 mM AEBSF, 10 µg/ml aprotinin, 1 µM pepstatin A. Aliquots containing 20 µg of protein from each sample were used for electrophoresis. For the detection of Rab5, Rabaptin5 and p120 Ras GAP in the anti-Rabaptin5 and anti-p120 Ras GAP immunoprecipitates, 10% of the immunoprecipitate was analyzed. EGFR, phospho-Akt, Rab5, Rabaptin5 and p120 Ras GAP were detected with polyclonal rabbit anti-EGFR, anti-phospho-Akt, monoclonal mouse anti-Rab5, anti-Rabaptin5 or anti-p120 Ras GAP antibodies, respectively, followed by HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG. Protein bands were detected by enhanced chemiluminescence (Pierce Chemical) and quantitated with a FluorChem digital imaging system (Alpha Innotech Corporation).
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. Zerial for Rab5 reagents and R. Rachubinski and D. Brindley for comments on the manuscript. This work was supported in part by a grant from the Canadian Institutes of Health Research (CIHR) and the Alberta Heritage Foundation for Medical Research. Z.W. is a CIHR Scholar and an Alberta Heritage Foundation for Medical Research Scholar.
REFERENCES
- Bonser R.W., Thompson, N.T., Randall, R.W., Tateson, J.E., Spacey, G.D., Hodson, H.F. and Garland, L.G. (1991) Demethoxyviridin and wortmannin block phospholipase C and D activation in the human neutrophil. Br. J. Pharmacol., 103, 1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. and Wang, Z. (2001) Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep., 2, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S., McBride, H.M., Burgoyne, R.D. and Zerial, M. (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Cross M.J., Stewart, A., Hodgkin, M.N., Kerr, D.J. and Wakelam, M.J. (1995) Wortmannin and its structural analogue demethoxyviridin inhibit stimulated phospholipase A2 activity in Swiss 3T3 cells. Wortmannin is not a specific inhibitor of phosphatidylinositol 3-kinase. J. Biol. Chem., 270, 25352–25355. [DOI] [PubMed] [Google Scholar]
- Felder S., Miller, K., Moehren, G., Ullrich, A., Schlessinger, J. and Hopkins, C.R. (1990) Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell, 61, 623–634. [DOI] [PubMed] [Google Scholar]
- Gaullier J.M., Simonsen, A., D’Arrigo, A., Bremnes, B., Stenmark, H. and Aasland, R. (1998) FYVE fingers bind PtdIns3P. Nature, 394, 432–433. [DOI] [PubMed] [Google Scholar]
- Gournier H., Stenmark, H., Rybin, V., Lippe, R. and Zerial, M. (1998) Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO J., 17, 1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.T. and Clague, M.J. (1995) Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem. J., 311, 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.M., Klinghoffer, R., Prestwich, G.D., Toker, A. and Kazlauskas, A. (1999) PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr. Biol., 9, 512–521. [DOI] [PubMed] [Google Scholar]
- Lanzetti L., Rybin, V., Malabarba, M.G., Christoforidis, S., Scita, G., Zerial, M. and Di Fiore, P.P. (2000) The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature, 408, 374–377. [DOI] [PubMed] [Google Scholar]
- Li G., D’Souza-Schorey, C., Barbieri, M.A., Roberts, R.L., Klippel, A., Williams, L.T. and Stahl, P.D. (1995) Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl Acad. Sci. USA, 92, 10207–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K. and Li, G. (1998) Catalytic domain of the p120 Ras GAP binds to RAb5 and stimulates its GTPase activity. J. Biol. Chem., 273, 10087–10090. [DOI] [PubMed] [Google Scholar]
- Martys J.L., Wjasow, C., Gangi, D.M., Kielian, M.C., McGraw, T.E. and Backer, J.M. (1996) Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. Differential effects on endocytosis and lysosomal sorting. J. Biol. Chem., 271, 10953–10962. [DOI] [PubMed] [Google Scholar]
- Meyers R. and Cantley, L.C. (1997) Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J. Biol. Chem., 272, 4384–4390. [DOI] [PubMed] [Google Scholar]
- Mills I.G., Jones, A.T. and Clague, M.J. (1998) Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr. Biol., 8, 881–884. [DOI] [PubMed] [Google Scholar]
- Patki V., Lawe, D.C., Corvera, S., Virbasius, J.V. and Chawla, A. (1998) A functional PtdIns3P-binding motif. Nature, 394, 433–434. [DOI] [PubMed] [Google Scholar]
- Shpetner H., Joly, M., Hartley, D. and Corvera, S. (1996) Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol., 132, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A. et al. (1998) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Spiro D.J., Boll, W., Kirchhausen, T. and Wessling-Resnick, M. (1996) Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol. Biol. Cell, 7, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Parton, R.G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J. and Zerial, M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J., 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H., Vitale, G., Ullrich, O. and Zerial, M. (1995) Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell, 83, 423–432. [DOI] [PubMed] [Google Scholar]
- Tuma P.L., Finnegan, C.M., Yi, J.H. and Hubbard, A.L. (1999) Evidence for apical endocytosis in polarized hepatic cells: phosphoinositide 3-kinase inhibitors lead to the lysosomal accumulation of resident apical plasma membrane proteins. J. Cell Biol., 145, 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Tung, P.S. and Moran, M.F. (1996) Association of p120 ras GAP with endocytic components and colocalization with epidermal growth factor (EGF) receptor in response to EGF stimulation. Cell Growth Differ., 7, 123–133. [PubMed] [Google Scholar]
- Xiao G.H., Shoarinejad, F., Jin, F., Golemis, E.A. and Yeung, R.S. (1997) The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem., 272, 6097–6100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.