Abstract
An important unresolved issue related to tyrosine kinase receptor signaling pathways is the lack of specificity of the molecular effectors involved. The specificity of the biological responses that are nevertheless elicited may be explained by differences in activation thresholds, as well as by temporal (transient versus sustained) and topographical aspects of receptor activation. On the basis of recent lessons from endothelial cells, we argue that an additional strategy can be adopted to generate specificity, i.e. tyrosine kinase receptors may form distinct signaling modules with other transmembrane proteins, such as adhesive receptors, to elicit different biological programs in stimulated cells.
Generating specificity: an unsolved problem
Many growth factors transduce their responses by activating tyrosine kinase receptors (TKRs). The requirement for ligand-dependent receptor dimerization to reach threshold levels of tyrosine transphosphorylation, and the assembly of cytosolic multi-molecular complexes composed of adaptor and enzymatic proteins that specifically interact with phosphorylated tyrosine residues in activated TKRs (Hunter, 2000), are well established. However, there is considerable uncertainty about the molecular mechanisms employed by TKRs to generate signaling specificity. For instance, it is unclear how stimulation of the Ras/MAP kinase pathway triggers proliferation versus differentiation of neuroectodermal cells, depending on whether the signal initiates with the Neu or Trk TKR, respectively (Tan and Kim, 1999). Also not fully explained is the fact that, in endothelial cells (ECs), vascular endothelial growth factor (VEGF) receptor (R)-2 engagement supports both survival and motility by binding and activating the same enzyme, i.e. PI 3-kinase (Gerber et al., 1998; Soldi et al., 1999; Dayanir et al., 2001).
Specificity of biological responses may be explained by quantitative considerations, e.g. signal duration and strength. Signal specificity can also stem from qualitative differences in the set of proteins docking to TKR cytoplasmic tails. Moreover, biochemical inputs generated from TKRs can potentially be integrated with those originating from other transmembrane receptors and combined with pre-existing repertoires of transcription factors (Hunter, 2000; Jordan et al., 2000; Simon, 2000).
Hints from blood vessel assembly and VEGFR-2 associations
Vascular development relies upon a co-ordinated network of co-operative interactions between ECs and vascular smooth muscle cells. These are mediated by soluble factors and physical forces (Yancopoulos et al., 2000). In vitro studies, together with mouse models involving genetic disruption and transgenic overexpression, have shown that members of the VEGF family primarily function in ECs and are essential for blood vessel formation, both in the embryo and in the adult organism. Three receptors (VEGFR-1, -2 and -3) have been identified in ECs, but VEGFR-2, working with its cognate ligand VEGF-A, is the most effective in inducing angiogenesis. It does so through a remarkably complex network of signaling proteins (Neufeld et al., 1999; Yancopoulos et al., 2000).
VEGF-A, which exists in four different isoforms, promotes EC proliferation, migration and survival, and also increases vascular permeability. However, the EC response to this ligand differs depending on the physical state of the ECs. VEGF-A induces only very slight tyrosine phosphorylation of VEGFR-2 in confluent ECs, whereas robust tyrosine phosphorylation is observed in sparse cells (Rahimi and Kazlauskas, 1999). Confluent ECs are, however, more effectively protected from apoptosis by VEGF-A, suggesting that VEGF-A signals differently in confluent, compared with sparse cells (Neufeld et al., 1999). These differences may be explained, at least in part, by the fact that VEGFR-2 associates with different transmembrane proteins in each case, forming distinct multimolecular complexes that interact with cytosolic transducers.
A complex that promotes cell survival. One of the complexes in which VEGFR-2 participates also includes VE-cadherin, β-catenin and PI 3-kinase (Carmeliet et al., 1999a). VE-cadherin is a transmembrane protein that mediates endothelial homophilic adhesion and forms clusters at intercellular junctions when cells come into contact with one another. Through its cytoplasmic tail, VE-cadherin binds β-catenin, which in turn interacts with actin (Steinberg and McNutt, 1999). VE-cadherin is not required for EC assembly into the primitive capillary plexus, but is necessary for the subsequent remodeling and stabilization of the vascular tree (Carmeliet et al., 1999a). Interestingly, VE-cadherin–/– ECs cannot respond to survival signals induced by VEGF-A, whereas they can in response to FGF-2, which signals through the FGF receptor (Carmeliet et al., 1999a).
The survival response is specific to VEGF-A and its receptor VEGFR-2 since VEGF-C (which binds VEGFR-3 and also VEGFR-2, though less avidly) and placental growth factor (which only binds VEGFR-1) have no effect on EC survival (Carmeliet et al., 1999a). Notably, this response depends on clustering of β-catenin and VE-cadherin, as truncation of the VE-cadherin tail to block binding to β-catenin, or disruption of VE-cadherin clusters through the use of blocking antibodies, prevents VEGF-A survival signaling (Carmeliet et al., 1999a). These findings are consistent with the fact that VEGFR-2 localization is itself dependent on VE-cadherin; whereas it localizes to intercellular junctions in wild-type EC, it does not in cells expressing a truncated form of VE-cadherin (Carmeliet et al., 1999a). These data are further supported by the fact that co-immunoprecipitation experiments with an anti-VEGFR-2 antibody have identified a complex containing VEGFR-2, VE-cadherin, β-catenin and PI 3-kinase in ECs expressing wild-type VE-cadherin, but not in ECs expressing its tail-less form (Carmeliet et al., 1999a). In terms of signaling, the assembly of this complex is instrumental in initiating downstream events including Akt activation, Bcl-2 up-regulation, and p21 and p53 down-regulation (Carmeliet et al., 1999a). Thus, VE-cadherin clusters may act as a scaffold which, through interactions with VEGFR-2 and the effector PI 3-kinase, leads to the generation of survival signals. This mechanism would be operative only when ECs are confluent and VE-cadherin clustered at junctions with VEGFR-2, but would not be active in sparse cells.
A complex involved in substrate adhesion and cell migration. During angiogenesis, ECs adhere to a provisional extracellular matrix (ECM), through αvβ3 integrin. Once engaged with the ECM, this integrin participates in a complex containing VEGFR-2 and PI 3-kinase (Soldi et al., 1999; Borges et al., 2000); its inclusion generates a complex that is distinct from the one discussed above. The impact of the integrin pair on this complex is illustrated by studies performed with an anti-β3 antibody that interferes with αvβ3 clustering but not with cell adhesion to the ECM (Soldi et al., 1999). Not only does the antibody perturb the formation of this module, but it also markedly inhibits VEGFR-2-mediated phosphorylation, PI 3-kinase activity and the proliferation and migration of ECs, events that are all normally triggered by VEGF-A stimulation of the receptor. In contrast, αvβ3 clustering is permissive for VEGFR-2 activation and an optimal response of EC to VEGF-A (Soldi et al., 1999; Byzova et al., 2000).
Formation of the VEGFR-2/αvβ3 integrin complex requires the extracellular domain of the β3 integrin subunit (Borges et al., 2000) and results in an increase in αvβ3 integrin affinity for the extracellular substrate (Byzova et al., 2000). This effect may be mediated by lipid products of PI 3-kinase, since overexpression of PTEN, which counteracts PI 3-kinase activity, inhibits the affinity modulation of αvβ3 integrin that is promoted by VEGF-A (Byzova et al., 2000). Consistent with this, VEGFR-2-mediated EC migration toward VEGF-A depends on PI 3-kinase/Akt activation (Morales-Ruiz et al., 2000). Indeed, PI 3-kinase not only acts as a survival factor (as in the case of the VE-cadherin/VEGFR-2 module), but also regulates cytoskeletal changes necessary for cell migration (Hunter, 2000). Moreover, both VEGFR-2 and αvβ3 activate two known regulators of cell migration, focal adhesion kinase (FAK) and Src (Abedi and Zachary, 1997; Giancotti and Ruoslahti, 1999; He et al., 1999), which work through PI 3-kinase (Giancotti and Ruoslahti, 1999). FAK signaling, either directly or through Src, results in directional and persistent cell migration, as opposed to the random motility induced by MAP kinase activation (Gu et al., 1999). Indeed, activated VEGFR-2 regulates actin reorganization through activation of MAP kinase (Rousseau et al., 2000). Summation of FAK and MAP kinase pathways has been proposed to control the speed and direction of cell migration (Gu et al., 1999); however, the mechanism by which their actions are integrated is unclear. We speculate that the activated VEGFR-2/αvβ3 integrin complex could support the integration of FAK and MAP kinase signaling pathways, finally resulting in the fine-tuning of both the speed and the directionality of EC migration.
Involvement of another binding partner? In addition to interacting with VE-cadherin or αvβ3 integrin, VEGFR-2 may also complex with neuropilin-1 (Npn-1), a transmembrane protein that is expressed in ECs, but has been better characterized for its involvement in axon guidance. The association between VEGFR-2 and Npn-1 is highly dependent on the ligand isoform: through its unique 44 amino acid stretch encoded by exon 7, VEGF-A165, but not VEGF-A121, triggers the formation of the VEGFR-2/Npn-1 complex. This isoform-specific association may be the molecular mechanism that allows greater stimulation of VEGFR-2 tyrosine kinase activity by VEGF-A165 rather than by VEGF-A121 (Whitaker et al., 2001). Moreover, the 44-amino acid long peptide also confers on VEGF-A165 the ability to bind to cell-surface-associated heparan-sulfate proteoglycans, which interact with VEGFR-2 and positively regulate its VEGF165 binding ability (Neufeld et al., 1999). Hence, interactions between cell surface heparan sulfate proteoglycans and the VEGFR-2 may contribute to allowing maximal VEGF-A165 binding and biological activity.
An emerging concept: the transmembrane signaling module
Previous studies have clearly demonstrated that the spatial localization of TKR signaling is required for efficient and specific biological responses. In Caenorhabditis elegans, interactions between the TKR Let-23 and cytosolic proteins carrying PDZ domains result in restriction of the receptor to the basolateral membrane, a feature that is mandatory for proper receptor activation and vulval differentiation in response to exposure to the ligand Lin-3 (Kaech et al., 1998). Similarly, Ephrin TKRs, which participate in tracing boundaries between distinct neuronal or vascular compartments, are localized to subcellular structures following their interaction with PDZ-containing proteins (Yancopoulos et al., 2000). Such topological specificity of signaling depending on the interaction of TKRs with PDZ proteins fits well with the concept of a module, a protein complex with a high level of biological organization that enables it to exceed the activities of single components (Hartwell et al., 1999).
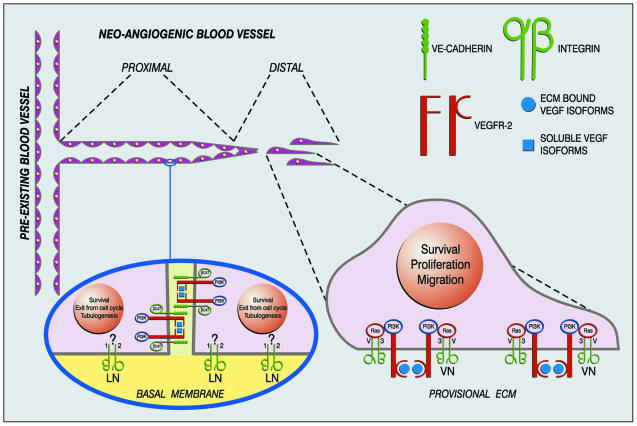
Importantly, modules may make it possible to understand how the same TKR may elicit different biological responses by activating the same intracellular pathways. Moreover, the fact that VEGFR-2 can associate with different transmembrane proteins within a single cell type, and can thereby elicit different biological responses, takes the concept beyond a static view of the term module. It suggests, instead, a dynamic scenario where partners can be exchanged. We speculate that at any given time VEGFR-2 may give rise to two distinct signaling modules by interacting with either VE-cadherin or αvβ3 integrin. Thus, the same molecules would direct TKR signaling toward distinct biological responses depending on the adhesive state of the ECs. A working hypothesis is depicted in Figure 1. At the distal tip of new-forming blood vessels, where cell–ECM interactions predominate, the VEGFR-2/αvβ3 signaling module would promote EC proliferation, migration and survival, while in a more proximal region, where cell–cell contacts are more numerous and stable, the VEGFR-2-VE-cadherin signaling module would promote maturation of EC tubular structures, as well as their stabilization, and survival. The presence of Npn-1 in either of these better-established modules (Whitaker et al., 2001) has not yet been investigated, but could contribute to further differentiate cellular responses to VEGF-A.
Fig. 1. A working hypothesis for endothelial transmembrane signaling modules during sprouting angiogenesis. When a neo-angiogenic sprout buds from a pre-existing blood vessel, ECs localized at the tip of the sprout proliferate and migrate, whereas those more proximal to the pre-existing blood vessel exit from the cell cycle and take the shape of stable and functional capillaries. In the basal membrane domain integrins mediate adhesion of EC to the ECM. In the lateral membrane domain cadherins mediate cell–cell interactions. At the distal tip cell–cell contacts are absent or transient, whereas in the proximal section of the neo-vessel, firm and stable intercellular contacts are mandatory for a proper tubuligenesis. At the distal tip, within the VEGFR-2-αvβ3 signaling module, adhesive interaction of vitronectin with αvβ3 allows VEGFR-2 interactions with ECM-bound VEGF isoforms switching on a proliferative, migratory and survival program. In the proximal vessel portion, in the context of the VEGFR-2-VE-cadherin signaling module, homophilic interactions between VE-cadherin permit VEGFR-2 binding to soluble VEGF isoforms, which induces maturation, remodeling and long-term survival of stabilized EC tubes. In addition, whereas adhesion to vitronectin through the Shc-linked integrin αvβ3 promotes proliferation, adhesion to laminin of basal lamina through the non-Shc-linked α2β1 integrin promotes exit from the cell cycle and co-operates with VE-cadherin to the formation of endothelial tubular structures (Languino et al., 1989).
The modular signaling outlined here does not seem to be restricted to only VEGFR-2. αvβ3 integrin is able to interact with the insulin receptor and also PDGFR. As a consequence of such interactions, both growth and motility responses to insulin and PDGF-BB are greatly amplified following engagement in cell–ECM interactions. These findings further support the importance of αvβ3-containing signaling modules, not only for angiogenesis, but also for tissue regeneration and tumor metastasis (Vuori and Ruoslahti, 1994; Schneller et al., 1997; Woodard et al., 1998; Borges et al., 2000). Another example of a module that leads to sustained proliferation exists in cells infected by papillomavirus. The viral E5 gene encodes a transmembrane protein that, upon cell infection, dimerizes and specifically binds PDGFR, forming a stable complex. This complex is able to activate the receptor’s catalytic activity and to sustain ligand-independent cell proliferation (Lai et al., 1998). Finally, although the existence of signaling modules involving the TKR c-Kit has not yet been demonstrated, the activation of this receptor in hematopoietic cells has been shown to result in distinct biological outcomes—apoptosis versus proliferation—depending on whether it interacts with α4β1 integrin or α5β1 integrin, respectively (Kapur et al., 2001).
How do VEGFR-2 transmembrane signaling modules work?
A crucial question about modules is what factors determine with which module a receptor like VEGFR-2 might become associates under a particular set of conditions. One possibility relates to the ability of the extracellular domain of VEGFR-2 to fold into different tertiary structures, which could favor its interaction with specific VEGF-A isoforms depending on the adhesion molecule involved. Furthermore, VE-cadherin and αvβ3 are localized differently in the plasma membrane and, consequently, react only with the nearest VEGF-A isoforms. For instance, VEGF-A165, VEGF-A189 and VEGF-A206 are anchored to ECM proteoglycans and are indeed located in close proximity to the signaling module carrying αvβ3 integrin. On the other hand, soluble VEGF isoforms, such as VEGF-A121, could be more accessible to VEGFR-2 complexed with VE-cadherin at cell–cell contact sites. This hypothesis is based on recent evidence that different VEGF-A isoforms play distinct roles in angiogenesis (Carmeliet et al., 1999b; Grunstein et al., 2000).
A second issue concerns the change in cell–cell adhesive states of ECs that occurs over the course of differentiation of the tissue (i.e. continuity versus discontinuity of the monolayer), which might favor the transfer of VEGFR-2 from αvβ3 integrin to VE-cadherin. Recently, it has been shown that activated αvβ3 integrin localizes to the lamellipodial edge of a single EC to promote directed motility (Kiosses et al., 2001). Intriguingly, VEGF-A stimulation has been observed to cause an enrichment of VEGFR-2 at lamellipodia, where the receptor colocalizes with αvβ3 integrin (S. Mitola and F. Bussolino, unpublished observations). This type of localization of VEGFR-2 to the EC leading edge could amplify motogenic and mitogenic signals generated by αvβ3 integrin interaction with provisional ECM (Giancotti and Ruoslahti, 1999). Alternatively, stimulation-triggered localization of the receptor to sites of cell–cell contact might amplify quiescence and differentiation signals induced by VE-cadherin-based adherens junctions (Steinberg and McNutt, 1999).
A third hypothesis suggests that the different behavior exhibited by VEGFR-2 stimulated cells could be caused by differences in intracellular signals. Both modules are able to activate PI 3-kinase, but most studies have ignored the subtype of enzyme involved in each case. In this light, it has been recently reported that, whereas p110α stimulates proliferation in macrophages, the p110β and p110δ isoforms induce migration (Vanhaesebroeck et al., 1999). It has also been found that only certain isoforms of the regulatory subunit p85 can modulate cellular response to insulin. It may be that the different p110/p85 isoforms mediate these diverse effects by interacting with distinct effector proteins, such as those of the functionally diverse Ras and Rho GTPase families (Vanhaesebroeck et al., 1999; Ueki et al., 2000). Thus, different scaffolding proteins may allow the interaction of the same signaling pathway with different effector molecules, depending on the signaling module in which the TKR is engaged.
Perspectives
Substantial evidence suggests that signaling is a compartmentalized event (Hunter, 2000). Here we propose the concept of dynamic signaling modules as an attempt to pass from a view of science analyzing single elements, to one where different molecules interact to make a function possible. Within any such module, a given TKR could interact with a particular set of transmembrane proteins located in the cell surface domain in which it finds itself to elicit a distinct cellular phenomenon. If this is true, it will be important to establish how the formation and disruption of signaling modules is regulated, and whether their composition can vary in different cells or under changing conditions. Moreover, it will have to be determined whether such a system is peculiar to TKRs or is also common to other types of receptors.
The modules described in ECs may represent a general strategy that contributes to the relative complexity of some species, including humans, compared to other organisms, for example plants and worms. Rather than using a larger number of genes to achieve a greater level of biological complexity (International Human Genome Mapping Consortium, 2001), higher organisms have evolved more intricate biochemical architectures, which are built from a relatively limited number of proteins.
Acknowledgments
Acknowledgements
This study was supported by A.I.R.C., I.S.S. (AIDS Projects 40B.19 and 30B.9), M.U.R.S.T. (60% and PRIN: 1998–2000, 1999), Human Frontier, The European Community and CNR (P.F. Biotectonologie).
REFERENCES
- Abedi H. and Zachary, I. (1997) Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem., 272, 15442–15451. [DOI] [PubMed] [Google Scholar]
- Borges E., Jan, Y. and Ruoslahti, E. (2000) PDGF-receptor-β and VEGF-receptor-2 bind to the β3 integrin through its extracellular domain. J. Biol. Chem., 275, 39867–39873. [DOI] [PubMed] [Google Scholar]
- Byzova T.V., Goldman, C.K., Pampori, N., Thomas, K.A., Bett, A., Shatti, S.J. and Plow, E.F. (2000) A mechanism for modulation of cellular response to VEGF: activation of integrins. Mol. Cell, 6, 851–860. [PubMed] [Google Scholar]
- Carmeliet P. et al. (1999a) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell, 98, 147–157. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. (1999b) Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nature Med., 5, 495–502. [DOI] [PubMed] [Google Scholar]
- Dayanir V., Meyer, R.D., Lashkari, K. and Rahimi, N. (2001) Identification of tyrosine residues in vascular endothelia growth factor 2/Flk-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J. Biol. Chem., 276, 17686–17692. [DOI] [PubMed] [Google Scholar]
- Gerber H.P., McMurtrey, A., Kowalski, J., Yan, M., Keyt, B.A., Dixit, V. and Ferrara, N. (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem., 273, 30336–30343. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti, E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Grunstein J., Masbad, J.J., Hickey, R., Giordano, F. and Johnson, R.S. (2000) Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol. Cell. Biol., 20, 7282–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Tamura, M., Pankov, R., Danen, E.H., Takino, T., Matsumoto, K. and Yamada, K.M. (1999) Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J. Cell. Biol., 146, 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L.H., Hopfield, J.J., Leibler, S. and Murray, A.W. (1999) From molecular to modular cell biology. Nature, 402, C47–C52. [DOI] [PubMed] [Google Scholar]
- He H., Venema, V.J., Gu, X., Venema, R.C., Marrero, M.B. and Caldwell, R.B. (1999) Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem., 274, 25130–25135. [DOI] [PubMed] [Google Scholar]
- Hunter T. (2000) Signaling-2000 and beyond. Cell, 100, 113–127. [DOI] [PubMed] [Google Scholar]
- International Human Genome Mapping Consortium (2001) Initial sequencing and analysis of the human genome. Nature, 409, 860–921. [DOI] [PubMed] [Google Scholar]
- Jordan J.D., Landau, E.M. and Iyengar, R. (2000) Signaling networks: the origins of cellular multitasking. Cell, 193, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., Whitfield, C.W. and Kim, S.K. (1998) The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell, 94, 762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R., Cooper, R., Zhang, L. and Williams, D.A. (2001) Cross-talk between α4β1/α5β1 and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood, 97, 1975–1981. [DOI] [PubMed] [Google Scholar]
- Kiosses W.B., Shattil, S.J., Pampori, N. and Schwartz, M.A. (2001) Rac recruits high-affinity integrin αvβ3 to lamellipodia in endothelial cell migration. Nature Cell. Biol., 3, 316–320. [DOI] [PubMed] [Google Scholar]
- Lai C., Henningson, C. and DiMaio, D. (1998) Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor β receptor. Proc. Natl Acad. Sci. USA, 95, 15241–15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino L.R., Gehlsen, K.R., Wayner, E., Carter, W.G., Engvall, E. and Ruoslahti, E. (1989) Endothelial cells use α2β1 integrin as a laminin receptor. J. Cell. Biol., 109, 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ruiz M., Fulton, D., Sowa, G., Languino, L.R., Fujio, Y., Walsh, K. and Sessa, W.C. (2000) Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res., 86, 892–896. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Cohen, T., Gengrinovitch, S. and Poltorak, Z. (1999) Vascular endothelial growth factor (VEGF) and its receptor. FASEB J., 13, 9–22. [PubMed] [Google Scholar]
- Rahimi N. and Kazlauskas, A. (1999) A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells. Mol. Biol. Cell, 10, 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S., Houle, F., Kotanides, H., Witte, L., Waltenberger, J., Landry, J. and Huot, J. (2000) Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J. Biol. Chem., 275, 10661–10672. [DOI] [PubMed] [Google Scholar]
- Schneller M., Vuori, K. and Ruoslahti, E. (1997) αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J., 16, 5600–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M.A. (2000) Receptor tyrosine kinases: specific outcomes from general signals. Cell, 103, 13–15. [DOI] [PubMed] [Google Scholar]
- Soldi R., Mitola, S., Strasly, S., Defilippi, P., Tarone, G. and Bussolino, F. (1999) Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J., 18, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M.S. and McNutt, P.M. (1999) Cadherins and their connections: adhesion junctions have broader functions. Curr. Opin. Cell Biol., 11, 554–560. [DOI] [PubMed] [Google Scholar]
- Tan B.O. and Kim, S.K. (1999) Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet., 15, 145–149. [DOI] [PubMed] [Google Scholar]
- Ueki K., Algenstaedt, P., Mauvais-Jarvis, F. and Kahn, C.R. (2000) Positive and negative regulation of phosphoinositide 3-kinase-dependent signaling pathways by three different gene products of the p85α regulatory subunit. Mol. Cell. Biol., 20, 8035–8046.11027274 [Google Scholar]
- Vanhaesebroeck B., Jones, G.E., Allen, W.E., Zicha, D., Hoosmand-Rad, R., Sawyer, C., Wells, C., Waterfield, M.D. and Ridley, A.J. (1999) Distinct PI(3)Ks mediate mitogenic signaling and cell migration in macrophages. Nature Cell Biol., 1, 69–71. [DOI] [PubMed] [Google Scholar]
- Vuori K. and Ruoslahti, E. (1994) Association of insulin receptor substrate-1 with integrins. Science, 266, 1576–1578. [DOI] [PubMed] [Google Scholar]
- Whitaker G.B., Limberg, B.J. and Rosenbaum, J.S. (2001) VEGFR-2 and neuropilin-1 form a receptor complex that is responsible for the differential signaling potency of VEGF165 and VEGF121. J. Biol. Chem., 276, 25520–25531. [DOI] [PubMed] [Google Scholar]
- Woodard A.S., Garcia-Cardena, G., Leong, M., Madri, J.A., Sessa, C.W. and Languino, R.L. (1998) The synergistic activity of αvβ3 integrin and PDGF receptor increases cell migration. J. Cell Sci., 111, 469–478. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G.D., Davis, S., Gale, N.W., Rudge, J.S., Wiegand, S.J. and Holash, J. (2000) Vascular-specific growth factors and blood vessel formation. Nature, 407, 242–248. [DOI] [PubMed] [Google Scholar]