Abstract
The redox process and cellular senescence are involved in a range of essential physiological functions. However, they are also implicated in pathological processes underlying age-related neurodegenerative disorders, including Alzheimer's disease (AD). Elevated levels of reactive oxygen species (ROS) are generated as a result of abnormal accumulation of beta-amyloid peptide (Aβ), tau protein, and heme dyshomeostasis and is further aggravated by mitochondria dysfunction and endoplasmic reticulum (ER) stress. Excessive ROS damages vital cellular components such as proteins, DNA and lipids. Such damage eventually leads to impaired neuronal function and cell death. Heightened oxidative stress can also induce cellular senescence via activation of the senescence-associated secretory phenotype to further exacerbate inflammation and tissue dysfunction. In this review, we focus on how changes in the redox system and cellular senescence contribute to AD and how they are affected by perturbations in heme metabolism and mitochondrial function. While potential therapeutic strategies targeting such changes have received some attention, more research is necessary to bring them into clinical application.
Keywords: Alzheimer's disease, Oxidative stress, Cellular senescence, Heme, Mitochondrial dysfunction, ER stress
Highlights
-
•
Oxidative stress and cellular senescence are linked to Alzheimer's disease.
-
•
Mitochondrial dysfunction, excess heme and ER stress alter ROS levels.
-
•
Antioxidants and senolytic agents are possible therapeutics for Alzheimer's disease.
Abbreviations
- Aβ, Aβ1-42, Aβ40, Aβ42
Amyloid beta peptide
- Ach
Acetylcholine
- AChE
Acetylcholinesterase
- AD
Alzheimer's disease
- ATP
Adenosine triphosphate
- BAX
Bcl-2 Associated X-protein
- Bcl-2
B-cell lymphoma 2
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- D + Q
Dasatinib and quercetin
- ER
Endoplasmic reticulum
- Fe2+
Ferrous iron
- GPX
Glutathione peroxidase
- HEBP1
Heme-binding protein 1
- MAO
Monoamine oxidase
- MB
Methylene blue
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- mtDNA
Mitochondrial DNA
- Na+/K+-ATPase
Sodium–potassium ATPase
- OH8dG
8-hydroxy-2′-deoxyguanosine
- PP2A
Protein phosphatase 2 A
- ROS
Reactive oxygen species
- SA-β-gal
Senescence-associated beta-galactosidase
- SAMD
Senescence-associated mitochondrial dysfunction
- SASP
Senescence-associated secretory phenotype
- SIPS
Stress-induced premature senescence
1. Introduction
It has been estimated that the central nervous system (CNS) consumes approximately 20 % of the body's total energy [1]. Of the various cell types present in the brain, neurons expend much of this energy, where it is used to power both electrical activity as well as synaptic transmission [2,3]. The brain is heavily dependent on glycolysis in the cytoplasm and oxidative phosphorylation in mitochondria to generate sufficient amounts of ATP to sustain its energy needs [4,5]. The high metabolic rate makes it particularly susceptible to oxidative damage, with an estimated 1 to 2 % of oxygen used during respiration being converted to superoxide [1]. Moreover, despite its high iron content, the brain possesses lower amounts of antioxidant enzymes as compared to other organs [6]. These properties further exacerbate the potential to generate excessive reactive oxygen species (ROS) that can contribute to neuronal dysfunction and loss underlying neurodegenerative disorders and aging.
In parallel to oxidative stress, cellular senescence also contributes to neuronal dysfunction and neurodegenerative disorders. Cellular senescence describes the process of irreversible growth arrest that can be triggered by various factors, including DNA damage, telomere dysfunction, mitochondrial dysfunction, activation of oncogenes, aging, as well as oxidative stress [7,8]. Furthermore, oxidative stress is one of the major factors that triggers stress-induced premature senescence (SIPS) and the senescence-associated secretory phenotype (SASP), which releases proinflammatory chemokines, cytokines, and metalloproteinases that have the potential to induce age-related pathology [9,10]. Further, in Alzheimer's disease (AD), studies have unveiled an intricate network of molecular pathways connecting abnormal accumulation of amyloid beta peptides (Aβ40 and Aβ42) and/or hyperphosphorylated tau proteins with redox dyshomeostasis, mitochondrial dysfunction, cellular senescence, synaptic impairment, endoplasmic reticulum (ER) stress, calcium imbalance, inflammatory responses, as well as heme/iron dyshomeostasis [[11], [12], [13], [14], [15]]. Indeed, oxidative stress and cellular senescence could be a prominent early event in the pathogenesis of AD [[16], [17], [18], [19], [20], [21]]. In this review, we outline how redox dyshomeostasis and senescence can contribute to neurodegenerative disorders, and also highlight the intricate relationship between redox reactions, cellular senescence, Aβ and Tau proteins, heme dyshomeostasis, ER stress and mitochondrial dysfunction in the context of AD. Finally, we examine some of the therapeutic interventions that are currently being evaluated or being developed.
2. Redox dyshomeostasis and cellular senescence in AD
2.1. Redox dyshomeostasis
Redox reactions, involving both reduction and oxidation of relevant biomolecules, are important for a range of physiological functions, including ATP generation, cell signaling, antioxidant defense, embryonic development, wound healing, and cancer prevention [[22], [23], [24], [25]]. Conversely, their perturbations have been implicated in pathological events such as oxidative stress, mitochondrial dysfunction, and cancer promotion [[26], [27], [28]]. As aging occurs, altered redox homeostasis can be observed as levels of hydrogen peroxide or nitric oxide increase but additionally, in advanced aging and neurodegenerative disorders (including AD), other highly reactive molecules such as hydroxyl radicals and superoxide cause further damage like synaptic impairment, mitochondrial dysfunction, and cell death [29]. The involvement of oxidative stress in AD is well documented (Fig. 1). Indeed, studies in transgenic Tg2576 mice [30], SK-N-BE neuroblastoma cells, PS1/PS2-deficient, APP deficient and JNK-deficient mouse embryonic fibroblasts [31], and in the brain of AD patients [32] have showed that oxidative stress increases the accumulation of Aβ and the deposition of neurofibrillary tangles. Upregulation of oxidative stress markers such as F2-isoprostanes, malondialdehyde, trans-4-hydroxy-2-nonenal in brain samples and biological fluids (cerebrospinal fluid, plasma and urine samples) obtained from AD and mild cognitive impairment patients have also been documented [[33], [34], [35]].
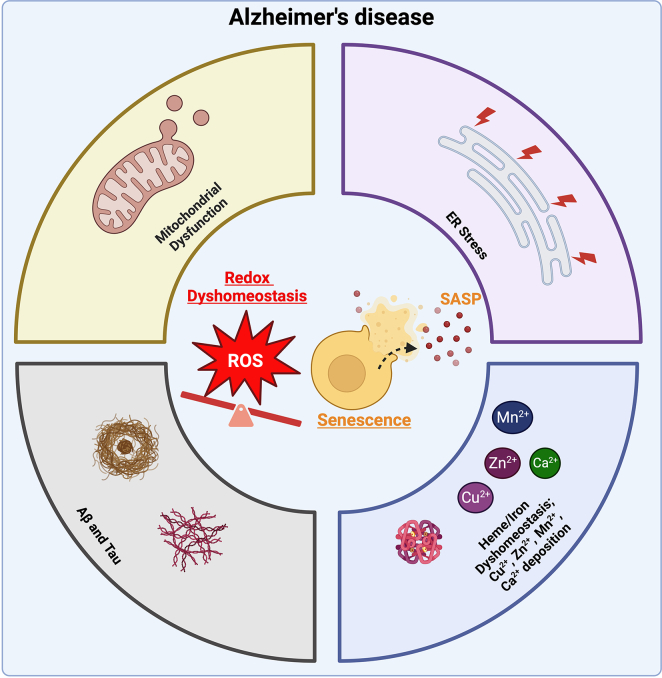
Fig. 1.
Schematic representation of the various processes and stimuli that trigger neurodegeneration in AD. Redox dyshomeostasis and cellular senescence accompanied by SASP plays a pivotal role in influencing key pathological processes in AD, including mitochondrial dysfunction, ER stress, increased deposition of metal ions (Fe2+, Cu2+, Zn2+, Mn 2+, Ca2+) as well as Aβ and tau. These processes can also directly contribute to the generation of ROS, thereby exacerbating redox dyshomeostasis. Ultimately, these intricately linked pathways collectively culminate in neurodegeneration observed in AD. (Figure created with BioRender.com).
Notably, a reduction in the levels of antioxidant enzymes (including glutathione, glutathione S-transferase, and glutathione peroxidase) correlating with disease severity has been observed in the synaptosomal fractions isolated from both Mild Cognitive Impairment (MCI) and AD patients [36,37]. Moreover, immunohistochemical staining of post-mortem AD brain samples showed increased and decreased levels of glutaredoxin-1 and thioredoxin-1 in neurons, respectively [38]. Thus, disrupted redox homeostasis, characterized by heightened oxidative stress, and decreased antioxidant enzymes, can significantly impact the onset and progression of AD.
2.2. Cellular senescence
Cellular senescence has been shown to play a pivotal role in the progression of AD [20,21]. Increased senescence, with high expression of senescence-associated beta-galactosidase (SA-β-gal), p53 (a mediator of cellular senescence), DNA/telomere damage, and SASP have been observed in various types of brain cells, including astrocytes, microglia, and neurons [7,14,39,40]. Accumulation of senescent cells over time releases SASP and can promote inflammation, oxidative stress, synaptic dysfunction, and neuronal cell death [19,39,40]. Studies have shown that removal of senescent cells can slow down aging processes and pathophysiological changes observed in age-related disease models [41,42]. Importantly, terminally differentiated neurons, which are post-mitotic, can also release SASP [39]. Furthermore, excessive ROS, which can be caused for instance by senescence-associated mitochondrial dysfunction (SAMD), originating from a DNA damage response, also triggers cellular senescence [43,44].
2.3. Crosstalk between oxidative stress and cellular senescence
Studies employing a mouse model of AD (APP/PS1) suggested a link between oxidative stress and cellular senescence, wherein, mice exposed to prolonged oxidative stress exhibited cellular alterations that closely resembled those observed in various senescent states like elevated expression of SA-β-gal, cell cycle arrest, and changes in the shape and structure of cells [45,46]. Zhang et al. demonstrated the upregulation of ROS production and p53 expression in a human astrocyte cell line (U87) upon exposure to Aβ1-40, and this effect was reversed by galantamine pretreatment [47]. Recently, Wang et at. showed that neuronal senescence is triggered by Aβ oligomers, primarily through increased production of ROS in cultured M17 neuronal cells, and Olmesartan mitigates cellular senescence by downregulating p21and p16 through a SIRT1-dependent deacetylation of p53 [48]. In addition, fibroblasts obtained from AD patients showed increased levels of ROS, reduced growth rates, elevated expression of cell cycle regulators like p21, p16, and p53, and exhibited a senescence-like phenotype [49]. Therefore, these findings provide evidence suggesting that oxidative stress associated with cellular senescence plays a pivotal role in mediating pathological processes associated with AD.
3. Aβ, tau and redox dyshomeostasis in AD
3.1. Aβ and oxidative stress
Increased levels of ROS can originate from a number of biological processes, including impaired heme metabolism, mitochondrial dysfunction, ER stress. The ability of pathological species of Aβ and tau to affect these processes and also alter others (including changes in mitochondrial function, disruption in synaptic dysfunction, excitotoxicity, altered calcium homeostasis, and oxidative stress with increased production of ROS-like superoxide, hydrogen peroxide, and hydroxyl radicals) have been extensively documented in recent years [[16], [17], [18],50]. These studies show that excessive Aβ accumulation triggers oxidative stress resulting in lipid peroxidation through the interaction of oxygen-derived free radicals with polyunsaturated fatty acids and subsequent formation of 4-hydroxy-2-nonenal and acrolein (reactive products of lipid peroxidation). This sequence of events disrupts neuronal ion homeostasis including impaired sodium–potassium ATPase (Na+/K+-ATPase) activity, increased calcium concentration, increased susceptibility to glutamate toxicity, and causes structural changes in synaptic membranes, eventually resulting in neuronal cell death [[51], [52], [53]]. Paradis et al. showed that neurons exposed to Aβ1–42 exhibited increased susceptibility to oxidative stress, possibly due to the downregulation of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and increased levels of pro-apoptotic Bcl-2-Associated X-protein (BAX) [54]. Conversely, high levels of ROS can impact the formation of Aβ by elevating the levels of amyloid precursor protein and modifying the activity of key enzymes involved in its processing, like β-secretase and γ-secretase. Consequently, oxidative stress promotes autophagy, leading to intra-lysosomal accumulation of Aβ, and the release of lysosomal enzymes, ultimately resulting in neuronal death [[55], [56], [57], [58], [59]].
3.2. Tau and Oxidative stress
Pathological tau has also been implicated in causing changes in redox status. Cente et al. demonstrated that the presence of a truncated tau protein in a transgenic rat model of tauopathy results in the accumulation of ROS and sensitizes neurons to stress-induced cell death [60]. Conversely, oxidative stress also induces the formation of intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein in neuronal cells via upregulating the activity of tau protein kinase (glycogen synthase kinase-3β) and PP2A-like phosphatase [61,62]. Furthermore, other studies have shown that Aβ triggers the accumulation of hyperphosphorylated tau by activating glycogen synthase kinase-3β and mitogen-activated protein kinase [[63], [64], [65]]. These Aβ- and tau-dependent mechanisms can operate in parallel and reinforce each other, contributing to the pathological changes observed in AD. Nevertheless, the abovementioned findings have mostly been based on preclinical models.
4. The role of heme in causing redox dyshomeostasis in AD
In addition to Aβ and Tau, imbalances in heme and its metabolism have been implicated in redox changes in AD. Heme, a complex of ferrous iron and protoporphyrin IX, is an important iron-containing co-factor that plays a pivotal role in oxygen transport (haemoglobin), electron transfer (mitochondria), storage (myoglobin), and in signal transduction and gene regulation (via the heme response elements) [[66], [67], [68]]. However, an excess of free heme and its cofactor, iron, is detrimental to cells by promoting oxidative stress and lipid peroxidation, eventually resulting in membrane damage and apoptosis. Thus, heme metabolism, its synthesis, transport, utilization and its degradation must be finely balanced [66,69]. Indeed, studies have shown that altered heme homeostasis results in the dysregulation of heme proteins (e.g., heme oxygenase) and affects iron regulation and mitochondrial function in AD [[70], [71], [72]]. Findings also revealed that free heme/iron binds with amyloidogenic and tau proteins leading to the formation of senile plaques and neurofibrillary tangles in AD [[73], [74], [75]].
Atamna et al. showed that heme and Aβ can form a complex, initiating heme deficiency and the generation of oxidants like hydrogen peroxide from mitochondria, which ultimately resulted in oxidative stress and cellular toxicity [76]. Using fast kinetics and freeze-quenching techniques, Pal et al. reported that the Aβ-heme complex generates a highly reactive intermediate, compound I. This compound oxidizes neurotransmitters such as serotonin, reducing their levels, thereby causing abnormalities in neurotransmission—an essential pathological event in AD [77]. Furthermore, heme and Aβ can trigger mitochondrial-dependent apoptotic neuronal cell death via heme-binding protein 1 (HEBP1), which is elevated in a subset of patients diagnosed with rapidly progressing forms of AD [78].
Several studies using brain tissue from the APP/PS1 mouse model, human AD tissue, and isolated amyloid plaque cores have also revealed that iron could be chemically reduced in the presence of Aβ, and this process was implicated as a source of excess free radical generation [[79], [80], [81], [82]]. Interestingly, MRI data from AD subjects have shown elevated levels of iron, transferrin, and ferritin, as well as reduced levels of ferroportin-1 in hippocampus, cortex, and basal ganglia. The imbalance originated from an increase in free Fe2+ within cells, ultimately triggering the activation of the ferroptosis pathway, which is a form of cell death that depends on iron [[83], [84], [85], [86]]. In addition to iron, anomalous accumulation of other metal ions such as copper [87], zinc [88], manganese [89], or calcium [90], in various brain regions can potentially contribute to the excessive production of ROS, Aβ and tau proteins, which are closely linked to the development and progression of AD [91,92].
5. Mitochondrial dysfunction, redox changes, and cellular senescence in AD
The mitochondrial electron transport chain is one of the primary intracellular sources of ROS. Ironically, heightened oxidative stress negatively impacts mitochondrial integrity and function [93,94]. In turn, damaged mitochondria, characterized by decreased mitochondrial membrane potential and reduced respiratory capacity, further exacerbates ROS levels and trigger cellular senescence, marked by telomere shortening [[95], [96], [97]]. Using long-term hippocampal neuronal cultures, Dong et al. demonstrated that decrease in mitochondrial membrane potential follows an increase in the proportion of senescent cells (as indicated by SA-β-gal), and is accompanied by increased levels of ROS [98].
Mitochondrial dysfunction with elevated levels of ROS appears to be a prominent and early characteristic of AD. Calkins et al. reported that neurons from an APP transgenic AD mouse model exhibited abnormal mitochondrial structure with reduced membrane potential and increased levels of ROS [99]. Mecocci et al. observed a significant increase in oxidized nucleoside, 8-hydroxy-2′-deoxyguanosine (OH8dG) within mitochondrial DNA (mtDNA) extracted from AD patients compared to controls [100]. Similarly, Wang et al. observed higher levels of multiple oxidized bases in both nuclear and mitochondrial DNA in individuals with AD as compared to control subjects [101]. Collectively, these studies highlight that oxidative stress, senescence, and mitochondria dysfunction work in synergy, reinforcing each other to contribute to AD pathology, with oxidative stress playing a central role. Thus, gaining a comprehensive understanding of these interconnected pathways could assist in identifying additional potential therapeutic approaches for AD.
6. ER and oxidative stress in AD
ER stress is characterised by the accumulation of misfolded or unfolded proteins within the ER, leading to the disturbance of cellular homeostasis. ER and oxidative stress are closely linked, given that protein folding in the ER requires a tightly controlled redox environment and excess ROS generation can severely affect ER homeostasis [102,103]. Excessive and prolonged perturbation of ER stress enhances γ-secretase activity and Aβ secretion, which could eventually lead to the aggregation and accumulation of misfolded/unfolded proteins (e.g., oligomerized Aβ) that can contribute to the progression of AD [[104], [105], [106]]. Increased expression of various ER stress markers, including phospho-PERK phospho-IRE1α, phospho-eIF2α, XBP1, and CHOP, have been documented in post-mortem brain tissues obtained from AD patients [[107], [108], [109]].
Of note, the ER and mitochondria are closely interconnected physically and functionally, at multiple contact sites (ER-mitochondrial contact sites) [110]. These sites regulate a range of cellular functions, including oxidative stress, inflammatory response, metabolite exchange (phospholipids), iron, and calcium homeostasis [[111], [112], [113], [114]]. Area-Gomez et al. observed increased accumulation of presenilin 1 and 2 (presenilins are the catalytic components of the γ-secretase complex) in a subcompartment of the ER-mitochondria associated membranes, thus, corresponds to enhance γ-secretase activity, leading to the accumulation of Aß [115]. Schreiner et al. demonstrated the generation of Aβ at mitochondria-ER contact sites, including mitochondria-associated ER membranes and outer mitochondrial membrane in the brains of C57BL6/J mice [116]. Similarly, studies have showed that Aβ modulates ER-mitochondrial contact sites and alters calcium homeostasis in the human AD brain and different AD mouse models (AppSwe/Lon mice, AppNL −F, and AppNL-G −F) [117,118]. Consequently, accumulation of Aβ could affect the signaling and metabolic pathways at ER-mitochondrial contact sites. Moreover, as mentioned earlier, the aggregation of Aβ leads to an increase production of ROS. Thus, ER stress in AD has the potential to extend to the mitochondria, subsequently amplifying the release of ROS. Therefore, directing therapeutic interventions towards these cellular sources of oxidative stress may provide robust outcomes in the treatment AD.
7. Treatments and interventions targeting redox in AD
There are only a limited number of approved drugs currently available for the treatment of AD. In the United States, FDA-approved drugs targeting disease progression and cognitive symptoms only include anti-amyloid treatments such as Aducanumab and Lecanemab, cholinesterase inhibitors, and glutamate regulators [119]. Although most of these drugs do not directly target oxidative stress, there are efforts to identify and develop strategies targeting components of redox and oxidative stress for clinical treatment of AD. An overview of the therapeutic interventions for AD discussed in this review is presented in Fig. 2.
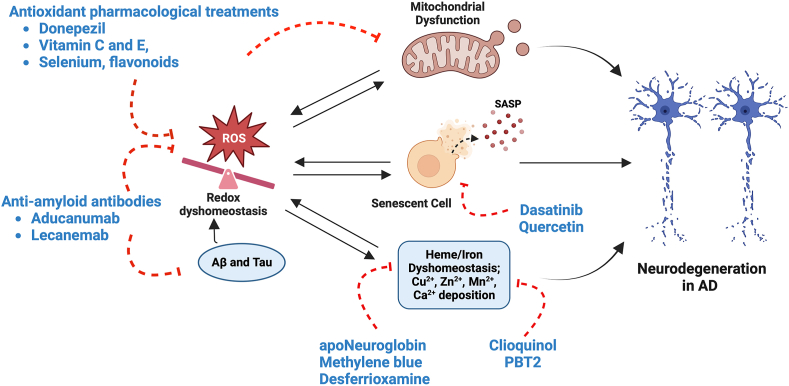
Fig. 2.
An overview of the therapeutic interventions for AD. Redox dyshomeostasis, cellular senescence, mitochondrial dysfunction, and heme dyshomeostasis are major contributors to the pathogenesis of AD. Various current and proposed interventions to mitigate neurodegeneration include antioxidant treatments targeting oxidative stress and mitochondrial dysfunction (such as donepezil, vitamin C and E, selenium, and flavonoids), heme/iron (such as apoNeuroglobin and desferrioxamine), metal ions (clioquinol and PBT2), anti-amyloid antibodies that reduce the accumulation of Aβ and tau proteins (such as Aducanumab and Lecanemab) and senolytic agents (such as Dasatinib and Quercetin) (Figure created withBioRender.com).
7.1. Treatments targeting heme-related dyshomeostasis
As heme dyshomeostasis and the occurrence of heme deficiency can lead to mitochondrial decay observed in AD [120,121], some efforts have been made to identify therapeutic strategies targeting heme (Fig. 2). Studies have indicated that neuroglobins such as apoNeuroglobin can sequester heme from the heme(II)-Aβ complex, which reduces ROS production by heme-Aβ and heme-IAPP complexes. This can, in turn, reduce heme-induced cytotoxicity observed in AD [122]. Although identified, these have yet to be tested clinically. Another drug of interest that could be associated with heme in AD is methylene blue (MB), which has been implicated in senescence [123], and has been suggested as a possible therapeutic [124]. However, there have been reported adverse effects of MB including CNS-related symptoms like confusion, dizziness, and headaches, as well as some increased tone in limbs, sweating, and agitation [125]. Clinical trial findings also found mixed results of MB administration in AD patients, in that the effects of MB could be dependent on dose; moderate doses (138 mg) showed cognitive benefits but there were no effects at higher (228 mg) or lower doses (69 mg) [126].
7.2. Treatments targeting metal dyshomeostasis
Aside from heme dyshomeostasis, metal dyshomeostasis and subsequent elevated ROS activity have been also implicated in AD. Accordingly, improving metal homeostasis could be another possible therapeutic strategy. Specifically, the chelation of metals such as iron, zinc, and copper has been suggested as a treatment option in order to reduce the accumulation of these metals. A clinical study using desferrioxamine (a chelator of free iron) was found to slow down AD progression [127]. Clioquinol, a chelator of zinc and copper that is able to cross the blood-brain barrier, was also introduced as a drug candidate for AD but had conflicting data [128]. The successor to clioquinol, PBT2, was developed as another drug candidate for AD by targeting metal-induced Aβ oligomers. Patients treated with the drug demonstrated improved executive function components on tests as well as a significantly reduced Aβ42 concentration in their cerebrospinal fluids (CSF) [129]. However, metal chelation therapies can come with side effects [130], resulting from the sequestration of healthy functional metal ions and redistribution of other metal ions [131], and hence these therapies are no longer being clinically pursued [132].
7.3. Treatments using acetylcholinesterase inhibitors
Acetylcholinesterase (AChE), the enzyme that degrades acetylcholine (ACh), has been identified as an important target for AD intervention and AChE inhibitors have since been approved for clinical use. Lower levels of ACh have been observed in AD patients [133,134] and AChE has been suggested to play a role in Aβ-aggregation during plaque formation by increasing the neurotoxicity of Aβ aggregates [135]. It has been found that AChE inhibitors can exert therapeutic effects by increasing acetylcholine levels, which can then lead to increased cholinergic neurotransmission and improved cognitive functions in patients [136]. Preclinical findings have also shown that AChE inhibitors may suppress oxidative stress, as treatment using AChE inhibitors mitigated streptozotocin-induced oxidative stress in mice [137]. Moreover, AD patients treated with the AChE inhibitor, donepezil, showed reduced levels of proteins linked to impaired redox homeostasis as compared to untreated patients [138]. Donepezil has been frequently used in FDA-approved clinical treatment of AD and recently, there have been efforts to develop metal directed donepezil, through modifying the drug with copper [139]. Additionally, the development of novel donepezil-related compounds has led to the discovery of compounds that can modulate Aβ misfolding coupled with antioxidant properties [140], as well as those that are multi-functional anti-AD agents that can inhibit both cholinesterase and monoamine oxidase (MAO) activity [141].
7.4. Antioxidant treatments
Other antioxidant treatments for AD - primarily involving Vitamin E, Vitamin C, and selenium - have had mixed results regarding their effectiveness, however. Some findings on Vitamin E have indicated that its combination with Vitamin C could be associated with reduced prevalence of AD [142] and slowing of functional decline [143], whereas other findings suggested only modest reductions in long-term AD risk [144] or no association with AD [145] in participants. Another antioxidant supplement that also has anti-inflammatory properties is selenium, which is a trace element that is found in selenoproteins [146]. One of these proteins, selenoprotein P, has been found to be increased in the CSF of AD patients [147], which made selenium a possible therapeutic target for AD. Nevertheless, although meta-analytical findings suggest that it could alleviate certain AD and MCI symptoms such as glutathione peroxidase (GPX) activity and cognitive deficits, its long-term effects have yet to be ascertained [148]. Altogether, these natural antioxidants that have been approved for clinical trials may come with limitations and results that are not yet conclusive.
Other potential treatments targeting redox mechanisms for AD include redox enzymes such as peroxiredoxins, glutaredoxins, and thioredoxins [149], herbal medicines such as Ginkgo biloba that can increase the activity of antioxidant enzymes like catalase and superoxide dismutase [150,151] as well as induce heme oxygenase-1 (HO-1) resulting in a neuroprotective effect [152], and flavonoids that can have antioxidative neuronal effects [153]. Further efforts to develop treatments targeting redox-related mechanisms are important, given the strong evidence that these mechanisms contribute an important role in the pathophysiology of AD.
7.5. Treatments targeting cellular senescence
In addition to redox-related therapies, interventions targeting senescence, specifically the clearance of senescent cells in AD, by senolytic agents have also been explored. These include dasatinib, an FDA-approved Src/tyrosine kinase inhibitor, and flavonoids such as quercetin and fisetin. Studies have indicated that compared to no senolytic treatment, a combination of dasatinib and quercetin (D + Q) senolytic treatment can help to remove senescent cells and mitigate age-dependent degeneration [154]. Further, in AD, it has been found that D + Q treatment reduced levels of Aβ40 and Aβ42 in the hippocampus and entorhinal cortex of AD mice, as well as alleviated Aβ-plaque associated inflammation and cognitive deficits [155]. Many clinical trials involving senolytic therapeutic interventions are ongoing [156], with a recently completed D + Q trial (NCT04063124) that has focused on AD [157,158]. However, the short- and long-term side effects of senolytic administration in humans remain undetermined [159] and further clinical trials involving senolytics are required.
8. Conclusion and future directions
There is strong evidence that dyshomeostasis and imbalances in redox play a critical role in neurodegenerative diseases like AD. Oxidative stress and senescence-related processes, triggered and/or modulated by heavy metals, primarily heme and iron, together with Aβ and tau proteins, contribute to the pathophysiology of AD. Therapeutic interventions have been approved for use in patients and there are ongoing clinical trials with novel interventions targeting redox based on preclinical evidence. However, the limitations to these still persist. Additional research and clinical trials are required before effective treatment with minimal side effects can be achieved and applied on a wider scale.
CRediT authorship contribution statement
Nicole Yu: Investigation, Visualization, Writing – original draft, Writing – review & editing. Mazhar Pasha: Investigation, Visualization, Writing – original draft, Writing – review & editing. John Jia En Chua: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
This work was supported by a Tier 2 grant from the Singapore Ministry of Education (MOE-Q2 T2EP30122-0006).
Data availability
No data was used for the research described in the article.
References
- 1.Franco R., Vargas M.R. Redox biology in neurological function, dysfunction, and aging. Antioxidants Redox Signal. 2018;28(18):1583–1586. doi: 10.1089/ars.2018.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Deitmer J.W. Strategies for metabolic exchange between glial cells and neurons. Respir. Physiol. 2001;129(1–2):71–81. doi: 10.1016/s0034-5687(01)00283-3. [DOI] [PubMed] [Google Scholar]
- 4.Davis G.W. Not fade away: mechanisms of neuronal ATP homeostasis. Neuron. 2020;105(4):591–593. doi: 10.1016/j.neuron.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bernhardi R., Eugenín J. Alzheimer's disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxidants Redox Signal. 2012;16(9):974–1031. doi: 10.1089/ars.2011.4082. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Cue C., Rueda N. Cellular senescence in neurodegenerative diseases. Front. Cell. Neurosci. 2020;14:16. doi: 10.3389/fncel.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekaran A., Idelchik M., Melendez J.A. Redox control of senescence and age-related disease. Redox Biol. 2017;11:91–102. doi: 10.1016/j.redox.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinta S.J., Woods G., Rane A., Demaria M., Campisi J., Andersen J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2015;68:3–7. doi: 10.1016/j.exger.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terao R., Ahmed T., Suzumura A., Terasaki H. Oxidative stress-induced cellular senescence in aging retina and age-related macular degeneration. Antioxidants. 2022;11(11) doi: 10.3390/antiox11112189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield D.A., Swomley A.M., Sultana R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxidants Redox Signal. 2013;19(8):823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tu S., Okamoto S., Lipton S.A., Xu H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer's disease. Mol. Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He N., Jin W.L., Lok K.H., Wang Y., Yin M., Wang Z.J. Amyloid-beta(1-42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death Dis. 2013;4(11):e924. doi: 10.1038/cddis.2013.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iliyasu M.O., Musa S.A., Oladele S.B., Iliya A.I. Amyloid-beta aggregation implicates multiple pathways in Alzheimer's disease: understanding the mechanisms. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1081938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8) doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bou Khalil R., Khoury E., Koussa S. Linking multiple pathogenic pathways in Alzheimer's disease. World J. Psychiatr. 2016;6(2):208–214. doi: 10.5498/wjp.v6.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngoi N.Y.L., Liew A.Q.X., Chong S.J.F., Davids M.S., Clement M.-V., Pervaiz S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat R., Crowe E.P., Bitto A., Moh M., Katsetos C.D., Garcia F.U., et al. Astrocyte senescence as a component of Alzheimer's disease. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boccardi V., Pelini L., Ercolani S., Ruggiero C., Mecocci P. From cellular senescence to Alzheimer's disease: the role of telomere shortening. Ageing Res. Rev. 2015;22:1–8. doi: 10.1016/j.arr.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Santolini J., Wootton S.A., Jackson A.A., Feelisch M. The Redox architecture of physiological function. Curr Opin Physiol. 2019;9:34–47. doi: 10.1016/j.cophys.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014;31(6):722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laberge R.M., Awad P., Campisi J., Desprez P.Y. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 2012;5(1):39–44. doi: 10.1007/s12307-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz-Espin D., Canamero M., Maraver A., Gomez-Lopez G., Contreras J., Murillo-Cuesta S., et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155(5):1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Checa J., Aran J.M. Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nousis L., Kanavaros P., Barbouti A. Oxidative stress-induced cellular senescence: is labile iron the connecting link? Antioxidants. 2023;12(6) doi: 10.3390/antiox12061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui H., Kong Y., Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012 doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A., Yegla B., Foster T.C. Redox signaling in neurotransmission and cognition during aging. Antioxidants Redox Signal. 2018;28(18):1724–1745. doi: 10.1089/ars.2017.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apelt J., Bigl M., Wunderlich P., Schliebs R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int. J. Dev. Neurosci. 2004;22(7):475–484. doi: 10.1016/j.ijdevneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Tamagno E., Guglielmotto M., Aragno M., Borghi R., Autelli R., Giliberto L., et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J. Neurochem. 2008;104(3):683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong Y., Zhou W., Fung V., Christensen M.A., Qing H., Sun X., et al. Oxidative stress potentiates BACE1 gene expression and Abeta generation. J. Neural. Transm. 2005;112(3):455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 33.Pratico D., MYL V., Trojanowski J.Q., Rokach J., Fitzgerald G.A. Increased F2-isoprostanes in Alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. Faseb. J. 1998;12(15):1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 34.Reich E.E., Markesbery W.R., Roberts L.J., 2nd, Swift L.L., Morrow J.D., Montine T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer's disease. Am. J. Pathol. 2001;158(1):293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams T.I., Lynn B.C., Markesbery W.R., Lovell M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol. Aging. 2006;27(8):1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Ansari M.A., Scheff S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal P.K., Saharan S., Tripathi M., Murari G. Brain glutathione levels--a novel biomarker for mild cognitive impairment and Alzheimer's disease. Biol. Psychiatr. 2015;78(10):702–710. doi: 10.1016/j.biopsych.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Akterin S., Cowburn R.F., Miranda-Vizuete A., Jimenez A., Bogdanovic N., Winblad B., et al. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13(9):1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 39.Sah E., Krishnamurthy S., Ahmidouch M.Y., Gillispie G.J., Milligan C., Orr M.E. The cellular senescence stress response in post-mitotic brain cells: cell survival at the expense of tissue degeneration. Life. 2021;11(3):229. doi: 10.3390/life11030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaikwad S., Senapati S., Haque M.A., Kayed R. Senescence, brain inflammation, and oligomeric tau drive cognitive decline in Alzheimer’s disease: evidence from clinical and preclinical studies. Alzheimers Dement. 2024;20:709–727. doi: 10.1002/alz.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R.-M. Aging, cellular senescence, and Alzheimer's disease. Int. J. Mol. Sci. 2022;23(4):1989. doi: 10.3390/ijms23041989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pignolo R.J., Passos J.F., Khosla S., Tchkonia T., Kirkland J.L. Reducing senescent cell burden in aging and disease. Trends Mol. Med. 2020;26(7):630–638. doi: 10.1016/j.molmed.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ngoi N.Y., Liew A.Q., Chong S.J.F., Davids M.S., Clement M.V., Pervaiz S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021;22(2):75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meraz-Rios M.A., Franco-Bocanegra D., Toral Rios D., Campos-Pena V. Early onset Alzheimer's disease and oxidative stress. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/375968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suelves N., Saleki S., Ibrahim T., Palomares D., Moonen S., Koper M.J., et al. Senescence-related impairment of autophagy induces toxic intraneuronal amyloid-beta accumulation in a mouse model of amyloid pathology. Acta Neuropathol Commun. 2023;11(1):82. doi: 10.1186/s40478-023-01578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Zhao L., Wu Z., Chen X., Ma T. Galantamine alleviates senescence of U87 cells induced by beta-amyloid through decreasing ROS production. Neurosci. Lett. 2017;653:183–188. doi: 10.1016/j.neulet.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Zheng B., Yang S., Zhou D., Wang J. Olmesartan prevents oligomerized amyloid beta (Abeta)-Induced cellular senescence in neuronal cells. ACS Chem. Neurosci. 2021;12(7):1162–1169. doi: 10.1021/acschemneuro.0c00775. [DOI] [PubMed] [Google Scholar]
- 49.Naderi J., Lopez C., Pandey S. Chronically increased oxidative stress in fibroblasts from Alzheimer's disease patients causes early senescence and renders resistance to apoptosis by oxidative stress. Mech. Ageing Dev. 2006;127(1):25–35. doi: 10.1016/j.mad.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Cioffi F., Adam R.H.I., Broersen K. Molecular mechanisms and genetics of oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2019;72(4):981–1017. doi: 10.3233/JAD-190863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark R.J., Lovell M.A., Markesbery W.R., Uchida K., Mattson M.P. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J. Neurochem. 1997;68(1):255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 52.Pocernich C.B., Cardin A.L., Racine C.L., Lauderback C.M., Butterfield D.A. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem. Int. 2001;39(2):141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- 53.Butterfield D.A., Lauderback C.M. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 54.Paradis E., Douillard H., Koutroumanis M., Goodyer C., LeBlanc A. Amyloid beta peptide of Alzheimer's disease downregulates Bcl-2 and upregulates bax expression in human neurons. J. Neurosci. 1996;16(23):7533–7539. doi: 10.1523/JNEUROSCI.16-23-07533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng L., Roberg K., Jerhammar F., Marcusson J., Terman A. Oxidative stress induces intralysosomal accumulation of Alzheimer amyloid beta-protein in cultured neuroblastoma cells. Ann. N. Y. Acad. Sci. 2006;1067:248–251. doi: 10.1196/annals.1354.032. [DOI] [PubMed] [Google Scholar]
- 56.Zheng L., Roberg K., Jerhammar F., Marcusson J., Terman A. Autophagy of amyloid beta-protein in differentiated neuroblastoma cells exposed to oxidative stress. Neurosci. Lett. 2006;394(3):184–189. doi: 10.1016/j.neulet.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 57.Zheng L., Kagedal K., Dehvari N., Benedikz E., Cowburn R., Marcusson J., et al. Oxidative stress induces macroautophagy of amyloid beta-protein and ensuing apoptosis. Free Radic. Biol. Med. 2009;46(3):422–429. doi: 10.1016/j.freeradbiomed.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L., Cedazo-Minguez A., Hallbeck M., Jerhammar F., Marcusson J., Terman A. Intracellular distribution of amyloid beta peptide and its relationship to the lysosomal system. Transl. Neurodegener. 2012;1(1):19. doi: 10.1186/2047-9158-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butterfield D.A., Boyd-Kimball D. Oxidative stress, amyloid-beta peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer's disease. J Alzheimers Dis. 2018;62(3):1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cente M., Filipcik P., Pevalova M., Novak M. Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur. J. Neurosci. 2006;24(4):1085–1090. doi: 10.1111/j.1460-9568.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 61.Mondragon-Rodriguez S., Trillaud-Doppia E., Dudilot A., Bourgeois C., Lauzon M., Leclerc N., et al. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J. Biol. Chem. 2012;287(38):32040–32053. doi: 10.1074/jbc.M112.401240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Y., Xia Y., Yu G., Shu X., Ge H., Zeng K., et al. Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H(2)O(2) J. Neurochem. 2013;126(2):234–242. doi: 10.1111/jnc.12285. [DOI] [PubMed] [Google Scholar]
- 63.Zheng W.H., Bastianetto S., Mennicken F., Ma W., Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115(1):201–211. doi: 10.1016/s0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 64.Takashima A., Honda T., Yasutake K., Michel G., Murayama O., Murayama M., et al. Activation of tau protein kinase I/glycogen synthase kinase-3beta by amyloid beta peptide (25-35) enhances phosphorylation of tau in hippocampal neurons. Neurosci. Res. 1998;31(4):317–323. doi: 10.1016/s0168-0102(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 65.Jin M., Shepardson N., Yang T., Chen G., Walsh D., Selkoe D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. U. S. A. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiabrando D., Vinchi F., Fiorito V., Mercurio S., Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014;5:61. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y., Hon T., Zhang L. Heme initiates changes in the expression of a wide array of genes during the early erythroid differentiation stage. Biochem. Biophys. Res. Commun. 1999;258(1):87–93. doi: 10.1006/bbrc.1999.0586. [DOI] [PubMed] [Google Scholar]
- 68.Mense S.M., Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16(8):681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- 69.Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G.M., Eaton J.W., et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 70.Atamna H. Heme binding to Amyloid-beta peptide: mechanistic role in Alzheimer's disease. J Alzheimers Dis. 2006;10(2–3):255–266. doi: 10.3233/jad-2006-102-310. [DOI] [PubMed] [Google Scholar]
- 71.Schipper H.M., Cisse S., Stopa E.G. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Ann. Neurol. 1995;37(6):758–768. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- 72.Kimpara T., Takeda A., Yamaguchi T., Arai H., Okita N., Takase S., et al. Increased bilirubins and their derivatives in cerebrospinal fluid in Alzheimer's disease. Neurobiol. Aging. 2000;21(4):551–554. doi: 10.1016/s0197-4580(00)00128-7. [DOI] [PubMed] [Google Scholar]
- 73.Chiziane E., Telemann H., Krueger M., Adler J., Arnhold J., Alia A., et al. Free heme and amyloid-beta: a fatal liaison in Alzheimer's disease. J Alzheimers Dis. 2018;61(3):963–984. doi: 10.3233/JAD-170711. [DOI] [PubMed] [Google Scholar]
- 74.Atamna H., Frey W.H., 2nd A role for heme in Alzheimer's disease: heme binds amyloid beta and has altered metabolism. Proc. Natl. Acad. Sci. U. S. A. 2004;101(30):11153–11158. doi: 10.1073/pnas.0404349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith M.A., Harris P.L., Sayre L.M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. U. S. A. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atamna H., Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103(9):3381–3386. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pal I., Nath A.K., Roy M., Seal M., Ghosh C., Dey A., et al. Formation of compound I in heme bound Abeta-peptides relevant to Alzheimer's disease. Chem. Sci. 2019;10(36):8405–8410. doi: 10.1039/c9sc01679a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yagensky O., Kohansal-Nodehi M., Gunaseelan S., Rabe T., Zafar S., Zerr I., et al. Increased expression of heme-binding protein 1 early in Alzheimer's disease is linked to neurotoxicity. Elife. 2019;8 doi: 10.7554/eLife.47498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Everett J., Brooks J., Lermyte F., O'Connor P.B., Sadler P.J., Dobson J., et al. Iron stored in ferritin is chemically reduced in the presence of aggregating Abeta(1-42) Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-67117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Telling N.D., Everett J., Collingwood J.F., Dobson J., van der Laan G., Gallagher J.J., et al. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer's disease. Cell Chem. Biol. 2017;24(10):1205–12015. doi: 10.1016/j.chembiol.2017.07.014. e3. [DOI] [PubMed] [Google Scholar]
- 81.Everett J., Collingwood J.F., Tjendana-Tjhin V., Brooks J., Lermyte F., Plascencia-Villa G., et al. Nanoscale synchrotron X-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer's disease subjects. Nanoscale. 2018;10(25):11782–11796. doi: 10.1039/c7nr06794a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collingwood J.F., Mikhaylova A., Davidson M., Batich C., Streit W.J., Terry J., et al. In situ characterization and mapping of iron compounds in Alzheimer's disease tissue. J Alzheimers Dis. 2005;7(4):267–272. doi: 10.3233/jad-2005-7401. [DOI] [PubMed] [Google Scholar]
- 83.Masaldan S., Bush A.I., Devos D., Rolland A.S., Moreau C. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019;133:221–233. doi: 10.1016/j.freeradbiomed.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 84.Bao W.D., Pang P., Zhou X.T., Hu F., Xiong W., Chen K., et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28(5):1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghadery C., Pirpamer L., Hofer E., Langkammer C., Petrovic K., Loitfelder M., et al. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol. Aging. 2015;36(2):925–932. doi: 10.1016/j.neurobiolaging.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 86.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: evidence of ferroptosis. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matheou C.J., Younan N.D., Viles J.H. Cu(2)(+) accentuates distinct misfolding of Abeta(1)(-)(4)(0) and Abeta(1)(-)(4)(2) peptides, and potentiates membrane disruption. Biochem. J. 2015;466(2):233–242. doi: 10.1042/BJ20141168. [DOI] [PubMed] [Google Scholar]
- 88.Miller Y., Ma B., Nussinov R. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. U. S. A. 2010;107(21):9490–9495. doi: 10.1073/pnas.0913114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tong Y., Yang H., Tian X., Wang H., Zhou T., Zhang S., et al. High manganese, a risk for Alzheimer's disease: high manganese induces amyloid-beta related cognitive impairment. J Alzheimers Dis. 2014;42(3):865–878. doi: 10.3233/JAD-140534. [DOI] [PubMed] [Google Scholar]
- 90.Kuchibhotla K.V., Goldman S.T., Lattarulo C.R., Wu H.Y., Hyman B.T., Bacskai B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59(2):214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.James S.A., Churches Q.I., de Jonge M.D., Birchall I.E., Streltsov V., McColl G., et al. Iron, copper, and zinc concentration in abeta plaques in the APP/PS1 mouse model of Alzheimer's disease correlates with metal levels in the surrounding neuropil. ACS Chem. Neurosci. 2017;8(3):629–637. doi: 10.1021/acschemneuro.6b00362. [DOI] [PubMed] [Google Scholar]
- 92.Wang L., Yin Y.L., Liu X.Z., Shen P., Zheng Y.G., Lan X.R., et al. Current understanding of metal ions in the pathogenesis of Alzheimer's disease. Transl. Neurodegener. 2020;9:10. doi: 10.1186/s40035-020-00189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Almeida A., de Oliveira J., da Silva Pontes L.V., de Souza Junior J.F., Goncalves T.A.F., Dantas S.H., et al. ROS: basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/1225578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giorgi C., Marchi S., Simoes I.C.M., Ren Z., Morciano G., Perrone M., et al. Mitochondria and reactive oxygen species in aging and age-related diseases. Int Rev Cell Mol Biol. 2018;340:209–344. doi: 10.1016/bs.ircmb.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Passos J.F., Saretzki G., Ahmed S., Nelson G., Richter T., Peters H., et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5(5):e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J., et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010;6:347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miwa S., Kashyap S., Chini E., von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Invest. 2022;132(13) doi: 10.1172/JCI158447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong W., Cheng S., Huang F., Fan W., Chen Y., Shi H., et al. Mitochondrial dysfunction in long-term neuronal cultures mimics changes with aging. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2011;17(4):BR91–B96. doi: 10.12659/MSM.881706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calkins M.J., Manczak M., Mao P., Shirendeb U., Reddy P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mecocci P., Beal M.F., Cecchetti R., Polidori M.C., Cherubini A., Chionne F., et al. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Mol. Chem. Neuropathol. 1997;31(1):53–64. doi: 10.1007/BF02815160. [DOI] [PubMed] [Google Scholar]
- 101.Wang J., Xiong S., Xie C., Markesbery W.R., Lovell M.A. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer's disease. J. Neurochem. 2005;93(4):953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- 102.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants Redox Signal. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhandary B., Marahatta A., Kim H.R., Chae H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012;14(1):434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiti F., Dobson C.M. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 105.Ajoolabady A., Lindholm D., Ren J., Pratico D. ER stress and UPR in Alzheimer's disease: mechanisms, pathogenesis, treatments. Cell Death Dis. 2022;13(8):706. doi: 10.1038/s41419-022-05153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ohta K., Mizuno A., Li S., Itoh M., Ueda M., Ohta E., et al. Endoplasmic reticulum stress enhances gamma-secretase activity. Biochem. Biophys. Res. Commun. 2011;416(3–4):362–366. doi: 10.1016/j.bbrc.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 107.Unterberger U., Hoftberger R., Gelpi E., Flicker H., Budka H., Voigtlander T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 2006;65(4):348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 108.Hoozemans J.J., van Haastert E.S., Nijholt D.A., Rozemuller A.J., Eikelenboom P., Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 2009;174(4):1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee J.H., Won S.M., Suh J., Son S.J., Moon G.J., Park U.J., et al. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice. Exp. Mol. Med. 2010;42(5):386–394. doi: 10.3858/emm.2010.42.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilson E.L., Metzakopian E. ER-mitochondria contact sites in neurodegeneration: genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ. 2021;28(6):1804–1821. doi: 10.1038/s41418-020-00705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinvalet D. The role of the mitochondria and the endoplasmic reticulum contact sites in the development of the immune responses. Cell Death Dis. 2018;9(3):336. doi: 10.1038/s41419-017-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tubbs E., Rieusset J. Metabolic signaling functions of ER-mitochondria contact sites: role in metabolic diseases. J. Mol. Endocrinol. 2017;58(2):R87–R106. doi: 10.1530/JME-16-0189. [DOI] [PubMed] [Google Scholar]
- 113.Krols M., Bultynck G., Janssens S. ER-Mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J. Cell Biol. 2016;214(4):367–370. doi: 10.1083/jcb.201607124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu L., Wang X., Tong C. Endoplasmic reticulum-mitochondria contact sites and neurodegeneration. Front. Cell Dev. Biol. 2020;8:428. doi: 10.3389/fcell.2020.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Area-Gomez E., de Groof A.J., Boldogh I., Bird T.D., Gibson G.E., Koehler C.M., et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 2009;175(5):1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schreiner B., Hedskog L., Wiehager B., Ankarcrona M. Amyloid-beta peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J Alzheimers Dis. 2015;43(2):369–374. doi: 10.3233/JAD-132543. [DOI] [PubMed] [Google Scholar]
- 117.Hedskog L., Pinho C.M., Filadi R., Ronnback A., Hertwig L., Wiehager B., et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models. Proc. Natl. Acad. Sci. U. S. A. 2013;110(19):7916–7921. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leal N.S., Dentoni G., Schreiner B., Naia L., Piras A., Graff C., et al. Amyloid beta-peptide increases mitochondria-endoplasmic reticulum contact altering mitochondrial function and autophagosome formation in Alzheimer's disease-related models. Cells. 2020;9(12) doi: 10.3390/cells9122552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alzheimer's A. 2023. FDA-approved Treatments for Alzheimer's. [Google Scholar]
- 120.Duvigneau J.C., Esterbauer H., Kozlov A.V. Role of heme oxygenase as a modulator of heme-mediated pathways. Antioxidants. 2019;8(10):475. doi: 10.3390/antiox8100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vidal C., Zhang L. An analysis of the neurological and molecular alterations underlying the pathogenesis of Alzheimer's disease. Cells. 2021;10(3) doi: 10.3390/cells10030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seal M., Uppal S., Kundu S., Dey S.G. Interaction of apoNeuroglobin with heme-Abeta complexes relevant to Alzheimer's disease. J. Biol. Inorg. Chem. 2015;20(3):563–574. doi: 10.1007/s00775-015-1241-y. [DOI] [PubMed] [Google Scholar]
- 123.Atamna H., Nguyen A., Schultz C., Boyle K., Newberry J., Kato H., et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. Faseb. J. 2008;22(3):703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- 124.Pramanik D., Ghosh C., Mukherjee S., Dey S.G. Interaction of amyloid β peptides with redox active heme cofactor: relevance to Alzheimer's disease. Coord. Chem. Rev. 2013;257(1):81–92. [Google Scholar]
- 125.Martindale S.J., Stedeford J.C. Neurological sequelae following methylene blue injection for parathyroidectomy. Anaesthesia. 2003;58(10):1041–1042. doi: 10.1046/j.1365-2044.2003.03415_23.x. [DOI] [PubMed] [Google Scholar]
- 126.Wischik C.M., Staff R.T., Wischik D.J., Bentham P., Murray A.D., Storey J.M.D., et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer's disease. J. Alzheim. Dis.: JAD. 2015;44(2):705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 127.McLachlan D. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet. 1991;337(8753):1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 128.Grossi C., Francese S., Casini A., Rosi M.C., Luccarini I., Fiorentini A., et al. Clioquinol decreases amyloid-β burden and reduces working memory impairment in a transgenic mouse model of Alzheimer's disease. JAD. 2009;17(2):423–440. doi: 10.3233/JAD-2009-1063. [DOI] [PubMed] [Google Scholar]
- 129.Lannfelt L., Blennow K., Zetterberg H., Batsman S., Ames D., Harrison J., et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7(9):779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 130.Flora S.J.S., Pachauri V. Chelation in metal intoxication. Int. J. Environ. Res. Publ. Health. 2010;7(7):2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fasae K.D., Abolaji A.O., Faloye T.R., Odunsi A.Y., Oyetayo B.O., Enya J.I., et al. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer's disease: limitations, and current and future perspectives. J. Trace Elem. Med. Biol. 2021;67 doi: 10.1016/j.jtemb.2021.126779. [DOI] [PubMed] [Google Scholar]
- 132.Pal I., Dey S.G. The role of heme and copper in Alzheimer's disease and type 2 diabetes mellitus. JACS Au. 2023;3(3):657–681. doi: 10.1021/jacsau.2c00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whitehouse P.J., Martino A.M., Marcus K.A., Zweig R.M., Singer H.S., Price D.L., et al. Reductions in acetylcholine and nicotine binding in several degenerative diseases. Arch. Neurol. 1988;45(7):722–724. doi: 10.1001/archneur.1988.00520310028012. [DOI] [PubMed] [Google Scholar]
- 134.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 135.Carvajal F.J., Inestrosa N.C. Interactions of AChE with Aβ aggregates in Alzheimer's brain: therapeutic relevance of IDN 5706. Front. Mol. Neurosci. 2011;4:19. doi: 10.3389/fnmol.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vecchio I., Sorrentino L., Paoletti A., Marra R., Arbitrio M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer's disease. J. Cent. Nerv. Syst. Dis. 2021;13 doi: 10.1177/11795735211029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saxena G., Singh S.P., Agrawal R., Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur. J. Pharmacol. 2008;581(3):283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 138.Atukeren P., Cengiz M., Yavuzer H., Gelisgen R., Altunoglu E., Oner S., et al. The efficacy of donepezil administration on acetylcholinesterase activity and altered redox homeostasis in Alzheimer's disease. Biomed. Pharmacother. 2017;90:786–795. doi: 10.1016/j.biopha.2017.03.101. [DOI] [PubMed] [Google Scholar]
- 139.Junaid M., Islam N., Hossain M.K., Ullah M.O., Halim M.A. Metal based donepezil analogues designed to inhibit human acetylcholinesterase for Alzheimer's disease. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0211935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu M.-Y., Esteban G., Brogi S., Shionoya M., Wang L., Campiani G., et al. Donepezil-like multifunctional agents: design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016;121:864–879. doi: 10.1016/j.ejmech.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Li F., Wang Z.-M., Wu J.-J., Wang J., Xie S.-S., Lan J.-S., et al. Synthesis and pharmacological evaluation of donepezil-based agents as new cholinesterase/monoamine oxidase inhibitors for the potential application against Alzheimer's disease. J. Enzym. Inhib. Med. Chem. 2016;31(sup3):41–53. doi: 10.1080/14756366.2016.1201814. [DOI] [PubMed] [Google Scholar]
- 142.Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T., et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch. Neurol. 2004;61(1):82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 143.Dysken M.W., Sano M., Asthana S., Vertrees J.E., Pallaki M., Llorente M., et al. Effect of vitamin E and memantine on functional decline in alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Devore E.E., Grodstein F., van Rooij F.J.A., Hofman A., Stampfer M.J., Witteman J.C.M., et al. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010;67(7):819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gray S.L., Anderson M.L., Crane P.K., Breitner J.C.S., McCormick W., Bowen J.D., et al. Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults. J. Am. Geriatr. Soc. 2008;56(2):291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 146.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 2014;94(3):739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rueli R.H.L.H., Parubrub A.C., Dewing A.S.T., Hashimoto A.C., Bellinger M.T., Weeber E.J., et al. Increased selenoprotein P in choroid plexus and cerebrospinal fluid in Alzheimer's disease brain. JAD. 2015;44(2):379–383. doi: 10.3233/JAD-141755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pereira M.E., Souza J.V., Galiciolli M.E.A., Sare F., Vieira G.S., Kruk I.L., et al. Effects of selenium supplementation in patients with mild cognitive impairment or Alzheimer's disease: a systematic review and meta-analysis. Nutrients. 2022;14(15):3205. doi: 10.3390/nu14153205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jia J., Zeng X., Xu G., Wang Z. The potential roles of redox enzymes in Alzheimer's disease: focus on thioredoxin. ASN Neuro. 2021;13 doi: 10.1177/1759091421994351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bridi R., Crossetti F.P., Steffen V.M., Henriques A.T. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res.: PT. 2001;15(5):449–451. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 151.Shi C., Liu J., Wu F., Yew D. Ginkgo biloba extract in Alzheimer's disease: from action mechanisms to medical practice. Int. J. Mol. Sci. 2010;11(1):107–123. doi: 10.3390/ijms11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saleem S., Zhuang H., Biswal S., Christen Y., Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39(12):3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bakhtiari M., Panahi Y., Ameli J., Darvishi B. Protective effects of flavonoids against Alzheimer's disease-related neural dysfunctions. Biomed. Pharmacother. 2017;93:218–229. doi: 10.1016/j.biopha.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 154.Novais E.J., Tran V.A., Johnston S.N., Darris K.R., Roupas A.J., Sessions G.A., et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021;12(1):5213. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang P., Kishimoto Y., Grammatikakis I., Gottimukkala K., Cutler R.G., Zhang S., et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat. Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaib S., Tchkonia T., Kirkland J.L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 2022;28(8):1556–1568. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020;288(5):518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Banerjee P., Kotla S., Reddy Velatooru L., Abe R.J., Davis E.A., Cooke J.P., et al. Senescence-associated secretory phenotype as a hinge between cardiovascular diseases and cancer. Frontiers in Cardiovascular Medicine. 2021;8 doi: 10.3389/fcvm.2021.763930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Raffaele M., Vinciguerra M. The costs and benefits of senotherapeutics for human health. The Lancet Healthy Longevity. 2022;3(1):e67–e77. doi: 10.1016/S2666-7568(21)00300-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.