Abstract
Over 50 years ago, standard microbiological methods were established for determining whether bacterial cells were dead or alive. Recently there has been a flurry of reports suggesting that bacteria may exist in an eclipsed state, escaping detection by standard methods. Whether there really is such a state is of more than academic interest, considering the implications for public health. The ensuing debate has been unusually energetic for the normally cultured community of microbiologists.
Introduction
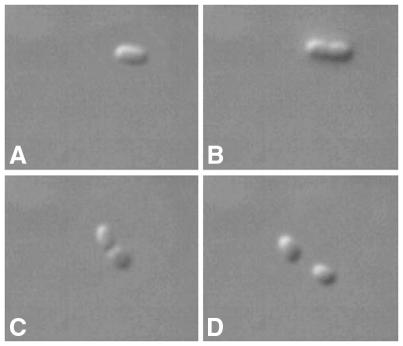
Whether bacterial cells are dead or alive is an important and fascinating question. It is important because the answer is often part of the basis of decisions related to such matters as the safety of food and drinking water, the sterility of pharmaceuticals, and the like. It is fascinating because it is actually quite a challenging question to answer. Consider the microbiologist, contemplating a single bacterial cell under the microscope (Figure 1). It cannot be ascertained by inspection whether the cell is alive or not, only that since it exists it must have been alive at some time! This problem is unique to the microbiologist, since a regular biologist contemplating a mouse in a cage can easily determine whether it is alive or not.
Fig. 1. Cell division of the bacterium Enterobacter aerogenes. A microbiologist contemplating a single cell (A) cannot tell whether it is dead or alive. Confirmation of culturability, namely ability to yield a population discernible to the observer (B and C), is proof that the single cell in (A) was alive. The viability status of the progeny cells (D) in turn cannot be determined unless they exhibit the ability to grow and divide.
Prolonged observation of the single bacterial cell in question may reward the observer with the splendor of cell division (Figure 1). This observation has a limited bearing on the question at hand, however, as it still says nothing about whether the two new cells are alive or not, but only that the single cell originally being observed was alive. Growth and division form the foundation of standard microbiological methods for testing samples for viable bacteria, that is, viability is equated with culturability. Culturability in turn is defined as the ability of a single cell to yield a population discernible by the observer, usually a visible colony on the surface of a nutrient agar plate. Such culture-based techniques have been in use for many decades, have generated a coherent body of information and have a proven track record in protecting public health at relatively low cost.
Recently, it has been proposed that some readily culturable species of bacteria, when subjected to prolonged starvation or other stress, may enter a long-term survival state in which they are not detectable by culturability tests (this review on the question of whether bacterial cells are dead or alive is limited to species of bacteria that are readily culturable in the laboratory). Also, new techniques have been developed which query subcellular activities in bacterial cells and which have been proposed to supplement or even supplant culturability tests. These two developments have raised the questions of whether non-culturable cells of normally readily culturable species of bacteria are really dead, and if not whether they pose any concern given the possibility that they would elude detection by standard culture-based methods.
The ‘viable but non-culturable’ (VBNC) hypothesis
When many species of readily culturable bacteria are subjected to prolonged starvation in a sterile laboratory microcosm, the culturable cell count declines while the total cell count usually remains constant at the initial level. This leads to the accumulation over time (usually several weeks) of substantial numbers of non-culturable cells. The most straightforward explanation of these results is that the non-culturable cells are dead, and indeed this is the basic presumption of culture-based methods (Kell et al., 1998). An alternative explanation has been advanced which assumes that the non-culturable cells are in a ‘viable but non-culturable’ (VBNC) state in which they remain viable but cannot be cultured (Barer et al., 1993; Oliver, 1993, 2000; Kell et al., 1998; Barer and Harwood, 1999).
The VBNC hypothesis has frequently generated sharp debate. Proponents often warn about the alleged inadequacies of standard methods, and the presumed threat to public health posed by bacteria in the VBNC state. Skeptics note that standard methods have proven track records, and that there is no evidence that the public health is at risk from VBNC bacteria or that the VBNC hypothesis clarifies any unexplained phenomena.
Indirect, non-culture-based methods for measuring viability
Most assertions that non-culturable cells are alive are based on labeling methods that query cells indirectly, using techniques that are intended to report cell viability without any direct indication that they are capable of growth and division. The assumption is that such labeling techniques alone can give a reliable indication of cell viability. These indirect methods include the use of nucleic acid stains, redox indicators, membrane potential probes and the use of flow cytometry and reporter gene systems, and have recently been reviewed in depth (Barer and Harwood, 1999).
In a growing population of cells, the total cell count is essentially equal to the culturable plate count; that is, essentially all of the cells in the population are alive as determined by a direct culturability test. When such unquestionably live cells are subjected to one of the indirect labeling techniques, the outcome is taken as an indication of how the technique labels live cells. If a similar labeling outcome is obtained with some or all of the cells in a sample of non-culturable cells, it is argued that those non-culturable cells are alive. A shortcoming of such methods, as Barer and Harwood (1999) point out, is that a labeling property associated with living cells does not by itself support the assumption that the method is capable of detecting viability in non-culturable cells. To say otherwise is to make an argument such as ‘living cells stain purple, therefore all purple-staining cells are alive’. In fact, there have been reports that some of the new viability markers score dead cells as alive (Josephson et al., 1993; Ericsson et al., 2000; Villarino et al., 2000).
New techniques can be seductive, and one must maintain a critical view of reports that rely entirely on indirect viability methods in the absence of a direct demonstration of culturability. However, if one accepts that these indirect methods are accurate, then one could conclude that there have been many reports that non-culturable cells are alive (reviewed in Barer et al., 1993; Oliver, 1993, 2000; Kell et al., 1998; Barer and Harwood, 1999).
On the other hand, insistence that these new techniques be supplemented with standard culture-based viability assays highlights a conundrum of the VBNC hypothesis: if the cells are non-culturable, how can a culturability test be applied? Recognizing this, there has been a long search for techniques to restore culturability to non-culturable cells.
‘Resuscitation’, the keystone of the VBNC hypothesis
Given that it is conventional microbiological wisdom to equate viability with culturability, it has widely been recognized that confirmation of the VBNC hypothesis would ultimately require recovery of culturable cells from a population of non-culturable cells. There have been numerous reports of the appearance of culturable cells after the addition of nutrients to non-culturable cells, in a process termed ‘resuscitation’. However, critical reviews have attributed these reports to the presence of a low level of residual culturable cells that simply grow and divide in response to the added nutrients (Barer et al., 1993; Kell et al., 1998; Barer and Harwood, 1999).
Nutrient addition experiments performed with populations of only non-culturable cells have not resulted in resuscitation (Bogosian et al., 1996, 1998). It has been suggested that the presence of culturable cells may be required for the production of a factor that triggers resuscitation in non-culturable cells (Votyakova et al., 1994). Such an idea necessarily cannot be evaluated with pure cultures; it is not sufficient to simply mix culturable and non-culturable cells of the same strain since it would not be possible to determine, after nutrient addition and incubation, whether the additional culturable cells were the product of growth of the culturable cells or resuscitation of the non-culturable cells. Instead, a ‘mixed culture recovery’ (MCR) method was developed expressly to address this question (Bogosian et al., 1998). This method employs easily distinguishable strains of the test bacterium, most preferably differing in only one genetic marker. In the MCR test, a small inoculum of culturable cells of one strain is added to larger populations of non-culturable cells of the other strain (Figure 2). After the addition of nutrient and a period of incubation, the resulting culture is plated on an agar medium on which the two strains form colonies that can be visually discriminated. Repeated application of the MCR test to several species of bacteria has demonstrated that the only response to added nutrient was the growth of the culturable cells, with no effect on non-culturable cells.
Fig. 2. The mixed culture recovery (MCR) test. The flask at the top contains non-culturable cells (blue circles) of the test bacterium, suspended in a nutrient medium appropriate for their growth. A smaller number of culturable cells (orange circles) are introduced, and the cultures allowed to grow. If the culturable cells resuscitate the non-culturable cells, both cell types increase in number (lower right flask). If growth is limited to the culturable cells, only that cell type increases in number (lower left flask). The key to the MCR test is that the two cell types are easily discriminated.
One response to such results has been the suggestion that something in the added nutrients may inhibit the recovery of culturable cells from non-culturable cells (Whitesides and Oliver, 1997). Experimental support for such an idea requires the establishment of other resuscitation methods. Whitesides and Oliver (1997) reported that non-culturable cells could be resuscitated by a temperature upshift.
Since the Whitesides and Oliver paper is regarded as the most convincing evidence to date in support of the VBNC hypothesis, it warrants closer examination. These workers employed the bacterium Vibrio vulnificus, an organism which has become the paradigm of the VBNC hypothesis (Oliver, 2000). The optimal temperature for growth of V. vulnificus is 37°C, and the bacterium rapidly becomes non-culturable at temperatures below 5°C. Non-culturable cells of V. vulnificus, obtained by starvation in flasks of cold sterile seawater, yielded culturable cells when warmed to room temperature. The authors concluded that they had for the first time observed ‘true resuscitation’. This was a justifiable interpretation of the data presented.
The Whitesides and Oliver report was extended in a study designed to replicate the experimental conditions employed (Bogosian et al., 2000). It was shown that the decline in the culturable cell count of V. vulnificus was due to the death of the cells. The loss of viability was gradual, and the cells passed through an injured state as they died. In this injured state, the cells were sensitive to low levels of hydrogen peroxide naturally present in the rich agar plate count medium. The warming step permitted the injured cells to grow on nutrients provided by the dead cells and concomitantly regain hydrogen peroxide resistance, giving the illusion of resuscitation. The injured state was transient, and extending the study by only a few more days yielded suspensions of cells that were all dead and unresponsive to a temperature upshift.
The need to distinguish between injured cells and VBNC cells
Others have noted that the recoverability of cells was a transient property, and that such cells were sensitive to hydrogen peroxide (Mizunoe et al., 1999, 2000). These workers argued that their results supported the VBNC hypothesis in that the cells were viable but non-culturable on rich agar plates. On this basis, bacteria could be described as VBNC on any medium which does not support their growth. Furthermore, such arguments fail to distinguish the proposed VBNC state from long-established concepts of injury in bacteria.
Injury in bacteria is defined as an increased sensitivity to components of growth media that are not normally inhibitory (Ray, 1989; Mackey, 2000). The injured state is transient, resulting from cumulative cellular damage, and can be reversed under appropriate conditions to enable the injured cell to resume growth. Death in bacteria is defined as the point where the extent of injury is beyond the ability of a cell to resume growth.
The VBNC hypothesis, by contrast, postulates a specific program of differentiation into a long-term survival state. This is distinctly different from degeneration into a short-term injured state followed by further degeneration to death. Furthermore, resuscitation from the VBNC state is defined as the conversion of non-culturable cells into culturable cells without any change in cell numbers due to regrowth (Figure 3).
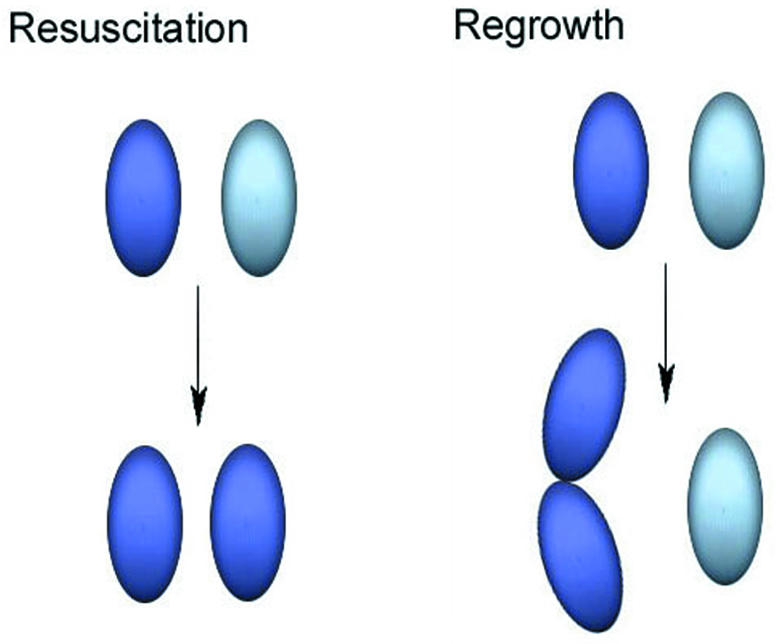
Fig. 3. Regrowth versus resuscitation. A mixture of culturable (dark blue) and non-culturable (light blue) cells are subjected to a proposed resuscitation technique. If the only response is the growth of the culturable cells, then regrowth has occurred (right). If there is conversion of non-culturable cells into culturable cells without any change in cell numbers due to regrowth (left), then true resuscitation has occurred.
These distinctions between injury and the VBNC state, and between regrowth and resuscitation, must be maintained in order for the VBNC hypothesis to retain any unique identity. Otherwise, the debate is reduced to semantic quibbling.
VBNC bacteria in the real world
There have been very few attempts to study non-culturable cells (of readily culturable species of bacteria) in natural systems. Studies employing non-sterile soil or river water have indicated that non-culturable cells of bacteria do not persist, and are apparently consumed by the indigenous microbes (Bogosian et al., 1996; Mascher et al., 2000). Such results suggest that the conversion from the culturable state to the non-culturable state would be an unlikely survival strategy in the real world.
Another important question is whether pathogens can exist in the VBNC state, hidden from detection and yet retaining infectivity. In studies with non-culturable cells of Campylobacter jejuni, infectivity was reported in one case (Jones et al., 1991) but not in two others (Medema et al., 1992; Hald et al., 2001). Non-culturable cells of Francisella tularensis were unable to infect mice (Forsman et al., 2000). Others have reported infectivity of non-culturable cells of Vibrio cholerae (Colwell et al., 1996) and V. vulnificus (Oliver and Bockian, 1995), but in both of these cases infectivity was exhibited for only a short time after the cells had become non-culturable, but not later. This pattern suggests that injured culturable cells were present in the ‘young’ non-culturable cell samples and were able to cause infection, but were not in the ‘older’ non-culturable cell samples. All of these studies had shortcomings in their design, including no statistical definition of the precision of the infectivity assays, and lack of adherence to widely used and standardized test procedures. What is required to overcome the statistical limitations of the assays are multiple experiments with adequate numbers of test animals inoculated with samples from a dilution series of a mixture of very low numbers of culturable bacteria and very large numbers of non-culturable bacteria. In the only publication we are aware of with a sufficiently rigorous approach, non-culturable cells of Salmonella typhimurium were unable to infect mice (Smith et al., 1999).
It remains to be established whether non-culturable cells of pathogenic bacteria pose any risk to human health. Standard culture-based microbiological methods have, over the course of many decades, generated a coherent body of knowledge, and have proven sufficiently effective to protect the public health. Otherwise, developed nations would be awash in unexplained outbreaks of disease from drinking water and the like, but such is not the case. A danger of promoting the VBNC theory without more experimental support is that this may tempt developing nations into investing in expensive new technologies when they might actually be best served by less expensive standard microbiological methods.
Conclusions
The only validated operational test of bacterial viability is propagation in culture. For readily culturable species of bacteria, it is expected that VBNC cells would be capable of regaining culturability. This long-sought grail of ‘true resuscitation’ remains elusive, if one maintains that it would be demonstrated only by recovery under conditions that exclude the possibility of regrowth. If it is shown that normally culturable species of bacteria are capable of differentiating into a long-term VBNC survival state, then presumably such differentiation would be genetically programmed. The next experimental test would be to demonstrate that expression of genes within the putative ‘VBNC regulon’ would cause cells to enter the VBNC state.
The modern VBNC debate has its origins in papers from the early 1980s, but the idea and the debate are much older. For example, in the early 1950s it was reported that apparently dead cells of Escherichia coli could be ‘reactivated’ (Heinmets et al., 1954), a finding that was subsequently shown by several other research groups to be due to the presence of residual culturable cells in the non-culturable cell suspensions (Garvie, 1955; Chambers et al., 1957; Hurwitz et al., 1957). The concepts behind such VBNC-like hypotheses have often been ‘resuscitated’ by microbiologists who wanted to challenge established principles. While VBNC concepts have stimulated much interesting work, the results have yet to clear the high hurdle of practical success set by the proven accomplishments of established microbiological principles.

Edward V. Bourneuf & Gregg Bogosian
Acknowledgments
Acknowledgements
We thank Edwin Geldreich for pointing us to the early VBNC literature. We thank Michael Barer, Gary Bond, Rich Erlich, Patricia Morris, Julia O’Neil and Ronald Somerville for critically reviewing the manuscript.
References
- Barer M.R. and Harwood, C.R. (1999) Bacterial viability and culturability. Adv. Microb. Physiol., 41, 93–137. [DOI] [PubMed] [Google Scholar]
- Barer M.R., Gribbon, L.T., Harwood, C.R. and Nwoguh, C.E. (1993) The viable but non-culturable hypothesis and medical bacteriology. Rev. Med. Microbiol., 4, 183–191. [Google Scholar]
- Bogosian G., Sammons, L.E., Morris, P.J.L., O’Neil, J.P., Heitkamp, M.A. and Weber, D.B. (1996) Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl. Environ. Microbiol., 62, 4114–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogosian G., Morris, P.J.L. and O’Neil, J.P. (1998) A mixed culture recovery method indicates that enteric bacteria do not enter the viable but nonculturable state. Appl. Environ. Microbiol., 64, 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogosian G., Aardema, N.D., Bourneuf, E.V., Morris, P.J.L. and O’Neil, J.P. (2000) Recovery of hydrogen peroxide-sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J. Bacteriol., 182, 5070–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.W., Tabak, H.H. and Kabler, P.W. (1957) Effect of Krebs cycle metabolites on the viability of Escherichia coli treated with heat and chlorine. J. Bacteriol., 73, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R.R., Brayton, P., Herrington, D., Tall, B., Huq, A. and Levine, M.M. (1996) Viable but non-culturable Vibrio cholerae 01 revert to a culturable state in the human intestine. World J. Microbiol. Biotechnol., 12, 28–31. [DOI] [PubMed] [Google Scholar]
- Ericsson M., Hanstorp, D., Hagberg, P., Enger, J. and Nystrom, T. (2000) Sorting out bacterial viability with optical tweezers. J. Bacteriol., 182, 5551–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman M., Henningson, E.W., Larsson, E., Johansson, T. and Sandstrom, G. (2000) Francisella tularensis does not manifest virulence in viable but non-culturable state. FEMS Microbiol. Ecol., 31, 217–224. [DOI] [PubMed] [Google Scholar]
- Garvie E.I. (1955) The growth of Escherichia coli in buffer substrate and distilled water. J. Bacteriol., 69, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Knudsen, K. and Madsen, M. (2001) Study of the infectivity of saline-stored Campylobacter jejuni for day-old chicks. Appl. Environ. Microbiol., 67, 2388–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinmets F., Taylor, W.W. and Lehman, J.J. (1954) The use of metabolites in the restoration of the viability of heat and chemically inactivated Escherichia coli. J. Bacteriol., 67, 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Rosano, C.L. and Blattberg, B. (1957) A test of the validity of reactivation of bacteria. J. Bacteriol., 73, 743–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.M., Sutcliffe, E.M. and Curry, A. (1991) Recovery of viable but non-culturable Campylobacter jejuni. J. Gen. Microbiol., 137, 2477–2482. [DOI] [PubMed] [Google Scholar]
- Josephson K.L., Gerba, C.P. and Pepper, I.L. (1993) Polymerase chain reaction detection of nonviable bacterial pathogens. Appl. Environ. Microbiol., 59, 3513–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D.B., Kaprelyants, A.S., Weichart, D.H., Harwood, C.R. and Barer, M.R. (1998) Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek, 73, 169–187. [DOI] [PubMed] [Google Scholar]
- Mackey B.M. (2000) Injured bacteria. In Lund, B.M., Baird-Parker, A. and Gould, G.M. (eds), The Microbiological Safety and Quality of Food. Aspen Publishers, Inc., Gaithersburg, MD, pp. 315–341.
- Mascher F., Hase, C., Moenne-Loccoz, Y. and Defago, G. (2000) The viable-but-nonculturable state induced by abiotic stress in the biocontrol agent Pseudomonas fluorescens CHA0 does not promote persistence in soil. Appl. Environ. Microbiol., 66, 1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G.J., Schets, F.M., van de Giessen, A.W. and Havelar, A.H. (1992) Lack of colonisation of 1 day old chicks by viable, non-culturable Campylobacter jejuni. J. Appl. Bacteriol., 72, 512–516. [DOI] [PubMed] [Google Scholar]
- Mizunoe Y., Wai, S.N., Takade, A. and Yoshida, S. (1999) Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch. Microbiol., 172, 63–67. [DOI] [PubMed] [Google Scholar]
- Mizunoe Y., Wai, S.N., Ishikawa, T., Takade, A. and Yoshida, S. (2000) Resuscitation of viable but nonculturable cells of Vibrio parahemolyticus induced at low temperture under starvation. FEMS Microbiol. Lett., 186, 115–120. [DOI] [PubMed] [Google Scholar]
- Oliver J.D. (1993) Formation of viable but nonculturable cells. In Kjelleberg, S. (ed.), Starvation in Bacteria. Plenum Press, New York, NY, pp. 239–272.
- Oliver J.D. (2000) The public health significance of viable but nonculturable bacteria. In Colwell, R.R. and Grimes, D.J. (eds), Nonculturable Microorganisms in the Environment. ASM Press, Washington, DC, pp. 277–300.
- Oliver J.D. and Bockian, R. (1995) In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol., 61, 2620–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. (1989) Injured Index and Pathogenic Bacteria. CRC Press, Boca Raton, FL.
- Smith R.J., Newton, A.T., Proctor, G.P., Harwood, C.R. and Barer, M.R. (1999) Active but nonculturable cells of Salmonella typhimurium are not infective in BALB/c mice. Abstracts of the 99th General Meeting of the American Society for Microbiology, abstract Q-322, p. 595.
- Villarino A., Bouvet, O.M.M., Regnault, B., Martin-Delautre, S. and Grimont, P.A.D. (2000) Exploring the frontier between life and death in Escherichia coli: evaluation of different viability markers in live and heat- or UV-killed cells. Res. Microbiol., 151, 755–768. [DOI] [PubMed] [Google Scholar]
- Votyakova T.V., Kaprelyants, A.S. and Kell, D.B. (1994) Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl. Environ. Microbiol., 60, 3284–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides M.D. and Oliver, J.D. (1997) Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl. Environ. Microbiol., 63, 1002–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]