Abstract
Estrogen receptors (ERs) orchestrate both transcriptional and non-genomic functions in response to estrogens, xenoestrogens and signals emanating from growth factor signalling pathways. The pleiotropic and tissue-specific effects of estrogens are likely to be mediated by the differential expression of distinct estrogen receptor subtypes (ERα and ERβ) and their coregulators. The recent analysis of transcription complexes associated with estrogen-responsive promoters has revealed unexpected levels of complexity in the dynamics of ER-mediated transcription. Furthermore, a small fraction of ERs also appears to directly interact with components of the cytosolic signalling machinery. Analysis of the interrelationship between these distinct modes of ER action is likely to reveal novel aspects of estrogen signalling that will impact on nuclear receptor biology and human health.
Introduction
Estrogen receptors (ERs) are ligand-activated transcription factors that mediate the pleiotropic effects of the steroid hormone estrogen on the growth, development and maintenance of a diverse range of tissues. Two mammalian ERs (ERα and ERβ) have been identified and exhibit modular structures characteristic of the nuclear receptor superfamily (Figure 1). The activities of a plethora of ER-interacting proteins converge to confer distinct functionalities on ERs, including the activation and repression of transcription, the integration of intracellular signalling pathways and the control of cell cycle progression (Table I). This review focuses on the molecular mechanisms regulating ER-mediated responses to both direct ligand binding and signals emanating from cell surface receptors. For a summary of other aspects of ER biology the reader is directed to reviews by Dechering et al. (2000) and Pettersson and Gustafsson (2001).
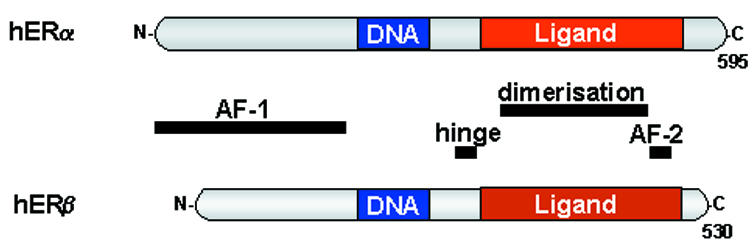
Fig. 1. Functional domains of ERα and ERβ. Both ER subtypes exhibit functional domains characteristic of the nuclear receptor superfamily. These include an agonist-independent transcriptional activation function (AF-1), a conserved DNA-binding domain, a hinge region and a ligand-binding domain which encompasses both an agonist-dependent transcriptional activation function (AF-2) and a dimerization region.
Table I. ER-interacting proteins and their functions.
| Transcriptional regulators | Functions | References |
|---|---|---|
| SRC-1, TIF2 (also known as GRIP-1), AIB1 (also known as ACTR, RAC3, pCIP) | p160 family of coactivatorsMediate the recruitment of protein acetyltransferases, kinases and methyltransferases | Reviewed in Bevan and Parker 1999; McKenna et al., 1999; Westin et al., 2000 |
| p300, CBP, pCAF | Coactivators with intrinsic protein acetyltransferase activity | As above |
| CARM-1 | Coactivator with intrinsic protein methyltransferase activity | Chen et al., 2000a and references therein |
| BRG1 | Component of SWI/SNF ATP-dependent chromatin remodelling complex | DiRenzo et al., 2000 and references therein |
| TRAP220 (also known as PBP, DRIP205) | Component of coactivator complex | Burakov et al., 2000 and references therein |
| RIP140 | CoregulatorMay recruit protein deacetylases | Wei et al., 2000 and references therein |
| RNA-binding DEAD-box proteins p68/p72 | ERα-specific coactivatorBind p160 coactivators and the RNA coactivator SRA | Watanabe et al., 2001 and references therein |
| CIA | Coactivator independent of AF-2 function | Sauvé et al., 2001 |
| TFIIH | General transcription factorLigand-regulated interaction of p62 and XPD subunits directs CDK7 kinase-mediated phosphorylation of ERα | Chen et al., 2000b |
| Cyclin D1 | Cell cycle proteinFacilitates ligand-independent interactions between ER and protein acetyltransferases | Lamb et al., 2000 |
| N-CoR, SMRT | CorepressorsAntagonist-dependent interaction with ERMediate the recruitment of protein deacetylase-containing complexes | Reviewed in Bevan and Parker 1999; McKenna et al., 1999; Westin et al., 2000 |
| MAT-1 | Mediates the recruitment of protein deacetylase-containing complex | Mazumdar et al., 2001 |
| SHP, DAX-1 | Orphan nuclear receptorsCompete for binding of p160 coactivators to ligand-bound ER | Johansson et al., 2000; Zhang et al., 2000 |
| REA | Competes for binding of p160 coactivators to ligand-bound ER | Martini et al., 2000 and references therein |
| Protein kinase signalling pathways/other interactions | Functions | References |
| PI(3)K | PI(3)K kinase activity stimulated by interaction with ligand-bound ERα, but not ERβ | Simoncini et al., 2000 |
| Src | Src tyrosine kinase activity stimulated by ligand-bound ERα, ERβ and AR | Migliaccio et al., 2000; Kousteni et al., 2001 |
| Caveolin-1 | Scaffolding protein/compartmentalization of signal transduction pathway? | Schelegel et al., 1999 |
| MAD2 | Cell cycle spindle checkpoint proteinSpecific for ERβ | Poelzl et al., 2000 |
| Calmodulin, Hsp90 | Modulation of receptor stability/conformation | Li et al., 2001 and references therein |
Chromatin modifications associated with ER-regulated transcription
Access of DNA binding proteins, such as ER, to their recognition elements is restricted by the repressive packaging imposed by chromatin. Transcriptional activation generally requires ATP-dependent chromatin remodelling enzymes in conjunction with histone acetyltransferases (HATs) to alleviate chromatin-mediated repression (Kingston and Narlikar, 1999). Consistent with this notion, ER recruits the SWI/SNF chromatin remodelling complex to estrogen-responsive promoters in a cooperative manner with HATs (DiRenzo et al., 2000; Figure 2A and B).
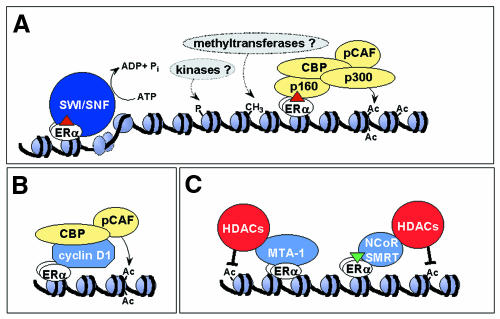
Fig. 2. Chromatin modifying proteins involved in ERα-mediated transcription. (A) In the presence of agonist, ERα recruits an ATP-dependent chromatin remodelling complex (SWI/SNF) and histone modifying enzymes to estrogen-responsive promoters, including the HATs p160, CBP, p300 and pCAF. Additional histone modifying enzymes, including methyltransferases and kinases, are also implicated in ER-mediated transcription. (B) Unliganded-ERα can recruit HATs via cyclin D1. (C) A number of cofactors repress ER-mediated transcription by targeting HDACs to ERα-bound promoters. Although the dynamics of ERβ transcription complexes have not yet been extensively studied, ERβ exhibits distinct interactions to ERα (see text and Figure 4).
Distinct roles for ER coactivators with histone acetyltransferase activity. Transcriptional competence correlates with the acetylation of chromosomal histone proteins at their N-termini, which results in destabilization of protein–DNA contacts and chromatin decompaction (Orphanides and Reinberg, 2000). Thus, it is not surprising that many coactivator proteins required for ER activity are HATs. Members of the p160 coactivator family, including SRC-1 and ACTR, are HATs that interact with the AF-2 domain of agonist-bound ERs through multiple LXXLL amino acid motifs. These coactivators serve as platforms for the recruitment of additional HATs, namely p300, CBP and the p300/CBP-associated factor, pCAF. Unliganded ER can also recruit HATs via cyclin D1 (Lamb et al., 2000 and references therein).
The reason that multiple, seemingly redundant, HATs are recruited to genes during ER-mediated transcription is beginning to become clear. Estrogen treatment of MCF-7 breast cancer cells induces transient hyperacetylation of histone H4 and, to a lesser extent, H3 at the promoters of ERα target genes (Chen et al., 1999). A recent report suggests that ERα-associated HATs are recruited to estrogen-responsive promoters in a sequential manner (Shang et al., 2000). The recruitment of p300 coincides with increased levels of histone acetylation and RNA polymerase II (RNAP II) binding and is followed by the recruitment of CBP and pCAF. Intriguingly, p300 only appears to participate in the first cycle of ER cofactor recruitment and thus may catalyse chromatin modifications that prime the promoter for multiple rounds of transcription. This is consistent with p300 facilitating transcriptional initiation, but not re-initiation, on chromatinized ERα-responsive genes in vitro (Kraus and Kadonaga, 1998) and with the specific interaction of p300 with the initiation-competent (non-phosphorylated) form of RNAP II (Cho et al., 1998). p300 and pCAF exhibit distinct but overlapping specificities for histone acetylation in vitro (Schiltz et al., 1999) and may acetylate distinct histone lysine residues at estrogen-responsive promoters. Furthermore, the specific interaction of pCAF with the elongation-competent form of RNAP II (Cho et al., 1998) may result in the acetylation of chromatin components downstream of the transcription start site (Orphanides and Reinberg, 2000).
Histones are not the only proteins to be acetylated during ER-mediated gene activation. Reversible lysine acetylation is emerging as a key post-translational modification in the regulation of transcription factor activity (Soutoglou et al., 2000 and references therein). It is therefore possible that ER-associated HATs target components of transcription machinery to facilitate architectural rearrangements at estrogen-responsive promoters. Indeed, acetylation of the p160 coactivator ACTR, by either p300 or CBP, disrupts its association with ERα (Chen et al., 1999). Furthermore, the hinge region of ERα is directly acetylated by p300 and experimental substitution of the ERα lysine residues targeted by p300 suggests that these modifications may regulate interactions between the two transcriptional activation domains (Wang et al., 2001).
Other histone post-translational modifications associated with ER-regulated transcription. In addition to acetylation, histones undergo a number of other post-translational modifications (Strahl and Allis, 2000) and it is possible that some of these play a role in ER activation. Interestingly, p160 coactivators possess a protein methyltransferase-interacting domain at their C-termini and the coactivator arginine methyltransferase (CARM-1) functions synergistically with p160 coactivators to enhance ER-mediated transcription (Chen et al., 2000a). It should be noted, however, that CARM-1 predominantly methylates H3 at arginines in vitro, whereas histones are largely methylated at lysines in vivo. Intriguingly, the HAT enzyme CBP associates with a histone methyltransferase activity that is specific for H3 lysine residues (Vandel and Trouche, 2001) as well as with the putative histone H3 kinase pp90rsk (Sassone-Corsi et al., 1999). It will be important to determine whether these histone modifications are utilized during ER-mediated transcription.
Mechanisms of ER-mediated transcriptional repression. Just as HATs acetylate histones to facilitate transcriptional activation, a group of enzymes known as histone deacetylases (HDACs) deacetylate histones to promote transcriptional repression. Not surprisingly, HDACs feature prominently in ER-mediated transcriptional repression (Figure 2C). In the presence of synthetic antagonists such as tamoxifen, ERα recruits the nuclear corepressors NCoR and SMRT to the promoters of estrogen-responsive genes (Shang et al., 2000). These corepressors then recruit HDAC complexes, such as the Sin 3 complex, to repress transcription. The ER cofactor RIP140 also associates with HDACs, suggesting a similar mechanism of transcriptional repression (Wei et al., 2000). ERs can also target HDACs to their promoters in a ligand-independent manner through growth factor-induced expression of metastasis-associated protein 1 (MTA-1), a component of the histone deacetylase and nucleosome remodelling complex (NuRD) (Mazumdar et al., 2001). Although it is clear that HDACs are involved in ER-mediated repression, definition of their precise role in this process awaits a detailed molecular analysis.
An alternative mechanism for ER-mediated transcriptional repression involves factors that compete with the binding of p160 coactivators to agonist-bound ER. These include REA (repressor of estrogen receptor activity; Martini et al., 2000) and the orphan receptors SHP (Johansson et al., 2000) and DAX-1 (Zhang et al., 2000). The potential displacement of ER-bound HATs by components of distinct nuclear receptor signalling pathways (e.g. SHP and DAX-1), together with growth factor-mediated interactions of ERα with components of HDAC-containing complexes (e.g. NuRD), supports a central role for protein acetylation in ensuring a coordinated response by ERs to diverse cellular stimuli.
Mechanisms regulating ER transcription complex dynamics
Cycles of ER-transcription complex formation and transcriptional initiation. In addition to ER binding and targeted chromatin modification, the initiation of transcription at estrogen-responsive promoters requires the recruitment of RNA polymerase II (RNAP II) and the general transcription factors (Orphanides and Reinberg, 2000). A recent study suggests that cycles of ERα-transcription complex binding are coupled to cycles of transcription in the MCF-7 breast cancer cell line (Shang et al., 2000), although it remains to be seen whether this phenomenon occurs on the majority of ER-regulated genes or in other cell types. This regular cycling mechanism may facilitate the continuous sampling of extracellular signals (Shang et al., 2000). Consistent with this notion, agonist-bound ER has been observed to form nuclear matrix-bound foci, within which individual receptors and their associated cofactors can undergo rapid exchange (Stenoien et al., 2001).
The molecular events that regulate the occupancy and exchange of ER transcription complexes at promoters are beginning to be elucidated. The mediator-like TRAP/DRIP complex, a coactivator of ERα-dependent transcription (Burakov et al., 2000), appears to occupy ERα-bound promoters simultaneously with p160 coactivators (Shang et al., 2000). Competition between TRAP/DRIP and p160 coactivators for binding to nuclear receptors (Treuter et al., 1999) suggests that their co-occupancy at estrogen-responsive promoters may involve cooperative interactions with ERs bound at neighbouring response elements. Dissociation of p160 coactivators from estrogen-responsive promoters is likely to be achieved by p300/CBP-mediated acetylation of residues adjacent to their ER-interacting LXXLL motifs (Chen et al., 1999). The promoter release of both p160 coactivators and ERα is preceeded by phosphorylation of the C-terminal domain of RNAP II, converting the polymerase to an elongation-competent form (Shang et al., 2000). This event is mediated in part by the CDK7 subunit of TFIIH, which also phosphorylates ligand-bound ERα (Chen et al., 2000b), possibly contributing to ERα-transcription complex turnover. The turnover of ERα and its cofactors is also regulated by interactions with components of the ubiquitin proteasome pathway (Lonard et al., 2000 and references therein). Paradoxically, proteasomal activity appears to be required for both ligand-dependent ER transcriptional activation and receptor degradation. Ascertaining the roles of these post-translational modifications in cofactor exchange, promoter clearance and transcriptional attenuation during ER-mediated gene activation will require a detailed analysis of these events in vivo.
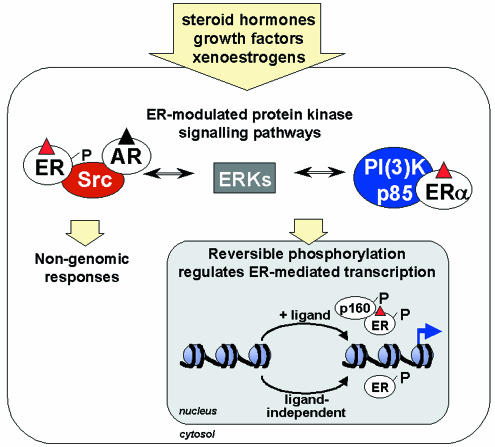
Regulation of ER-mediated transcription by protein kinase signalling pathways. The regulation of estrogen receptor-mediated transcription is not limited to direct ligand binding; ER and ER-associated cofactors are regulated by direct phosphorylation in response to peptide growth factors, PKA-activating agents, neurotransmitters and cyclins (Figure 3). Phosphorylation of the N-terminal activation domain of ERβ by a mitogen activated protein kinase (MAPK) potentiates the ligand-independent recruitment of the p160 coactivator SRC-1 (Tremblay et al., 1999). The p68/72 sub-family of RNA-binding DEAD-box proteins are similarly recruited to the MAPK-phosphorylated AF-1 domain of ERα (Watanabe et al., 2001 and references therein). Protein kinase signalling pathways also potentiate the interaction of the cofactor AIB1 with p300 (de Mora and Brown, 2000). The integration of ER modifications resulting from protein kinase signalling pathways and direct ligand binding is likely to be mediated, at least in part, by coactivators such as p160, which can interact with both ER activation domains simultaneously (Benecke et al., 2000). Yet further levels of regulation could be conferred by additional post-translational modifications. The glycosylation of ER serine and threonine residues by O-linked β-N-acetylglucosamine has recently been reported and the function of this modification may be to block phosphorylation (reviewed in Wells et al., 2001).
Fig. 3. ERs integrate signals through multiple protein kinase cascades. ERs respond to a wide variety of extracellular signals, including steroid hormones, growth factors and xenoestrogens. ER-mediated transcription in the nucleus involves ligand-dependent and ligand-independent mechanisms, both of which are coordinated by a complex pattern of reversible phosphorylation events emanating from cytosolic protein kinases. ERs can also mediate rapid non-genomic responses to steroid hormones.
Molecular basis for tissue-specific differences in ER-mediated transcription
The use of pharmaceutical agents that target ER has revealed the tissue-specific nature of ER responses. It is likely that tissue responsiveness to estrogenic compounds is determined by cell-type specific expression patterns of ER subtypes and their coregulators. The two ER subtypes identified, ERα and ERβ, can mediate distinct functions depending on the nature of bound ligands, post-translational modifications, cofactor interactions and promoter response elements (Figure 4).
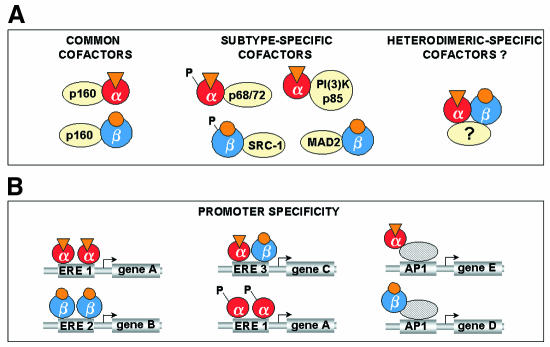
Fig. 4. Distinct ER subtypes may mediate tissue-specific responses to estrogenic compounds. (A) Tissue-specific responses to estrogenic compounds are likely to be mediated by a combination of the differential expression of ER subtypes, the ligand specificity of ER subtypes and ligand-independent signalling pathways. Both ER subtypes integrate signals by recruiting common and/or subtype-specific cofactors. ER heterodimer-specific cofactors may also exist in cells expressing both ER subtypes. (B) ERs can bind to the promoters of estrogen-responsive genes either directly through estrogen response elements (ERE) or indirectly through DNA-bound transcription factors such as AP1. The ligand-independent association of ERs with promoters is mediated, at least in part, via protein kinase cascades in response to a variety of non-steroidal signals. Although specific promoter DNA sequences may favour the binding of each ER subtype, the relative occupancy of estrogen-responsive promoters by ERα and ERβ is not yet clear.
Heterodimerization of ERα and ERβ in cells that possess both receptor subtypes may expand the repertoire of ER activity by combining the transcriptional properties of two distinct partners. ERβ may even inhibit ERα transcription activity (Pettersson and Gustafsson, 2001). Alternative splicing events and/or promoter usage within the genes encoding ERα and ERβ generates additional ER heterodimeric variants that may add further complexity to the biological response to estrogens. A truncated form of ERα (hERα46), lacking the first 173 amino acids, is expressed in breast cancer-derived cell lines resulting in a ligand-inducible receptor which lacks the AF-1 domain (Flouriot et al., 2000). Consequently, hERα46 can act as a competitive inhibitor of full-length ERα, and cell growth-dependent changes in the ratio of hERα46 and full-length ERα implicate ERα/hERα46 heterodimers in the regulation of cellular proliferation. Distinct isoforms of cofactors such as SRC-1 may further diversify the tissue-specific responses of ER-mediated transcription (Kalkhoven et al., 1998).
Activated ERα and ERβ elicit opposite transcriptional responses by interacting with Fos/Jun at an AP1 element (Paech et al., 1997). When bound by an antagonist, ERβ functions as a potent activator in conjunction with transcription factors present at AP1 sites (Paech et al., 1997). Interestingly, ERα and ERβ exhibit ligand-dependent differences in coactivator binding (Routledge et al., 2000). Thus, the nature of the ligand bound by ER subtypes is likely to have profound effects on their functional properties, perhaps leading to the regulation of different downstream genes.
In addition to being responsive to physiological estrogens, both ER subtypes can be activated by a variety of xenoestrogenic compounds including synthetic steroids, pesticides, industrial chemicals and phytoestrogens, leading to potentially adverse health effects in humans and wildlife. The extent to which exposure to these structurally distinct ligands affects different tissues is likely to depend upon their stability, uptake and compartmentalization relative to endogenous ER ligands. Strikingly, ERβ exhibits a higher relative binding affinity for certain phytoestrogens than does ERα (Kuiper et al., 1998), suggesting that ERβ mediates the physiological effects of these compounds and that environmental estrogens will also exhibit tissue-specific effects.
Non-genomic modes of estrogen receptor action
The rapid response of a variety of cell-types to estrogens suggest that ERs perform transcription-independent functions in the cytosol and recent data have revealed direct interactions between estrogen receptors and components of the cytosolic signalling machinery (Table I and Figure 3). Estrogen-bound ERα associates with the regulatory subunit of phosphatidylinositol-3-OH kinase, resulting in the activation of the AKT serine/threonine kinase and endothelial nitric oxide synthase in endothelial cells (Simoncini et al., 2000). Interestingly, AKT can directly phosphorylate ERα, resulting in enhanced ligand-independent transcription of estrogen-responsive genes (Campbell et al., 2001). This finding suggests that components of the same intracellular signaling pathways can regulate both the transcriptional and non-genomic functions of ERs. Both ERs and AR have been observed to coimmunoprecipitate with Src in a steroid hormone-dependent manner (Migliaccio et al., 2000) and signalling via a ligand-bound ternary AR/ERβ/Src complex is thought to promote cell proliferation in prostate cancer cells. The association of ER and AR with the Src/Shc/ERK signalling pathway has recently been demonstrated to result in an anti-apoptotic affect that can be dissociated from ER transcriptional activity (Kousteni et al., 2001). Surprisingly, and in contrast to the highly specific transcriptional effects of ER and AR in the nucleus, both receptors function similarly in this anti-apoptotic response to either estrogens or androgens, suggesting a distinct conformation of their ligand-binding domains. The potential existence of cytosolic ERs with altered or reduced specificity for binding to estrogenic compounds has profound implications for the development of therapeutic ligands and for assessing the potentially harmful effects of xenoestrogens.
Summary and perspectives
Far from being simple ligand activated transcription factors, ERs integrate multiple signals from both ligands and intracellular signalling pathways to perform their functions in the nucleus and cytosol. The differential and spatio-temporal expression of ER subtypes and their cofactors is likely to dictate the physiological effects of estrogen. Understanding which subsets of cofactors modulate particular estrogen-dependent physiological processes should allow the development of more selective estrogen receptor modulators (SERMs) as therapeutics for reproductive function, disorders of bone metabolism, cardiovascular disease and endocrine diseases (McKenna and O’Malley, 2000).
There is also considerable scientific and public interest in the potentially harmful effects of exposure of humans and wildlife to estrogenic compounds present in the environment. It is important that we gain a detailed molecular understanding of the ways in which these compounds mediate their harmful effects so that potential hazards can be properly assessed. ERs clearly have a complex mode of action which is not captured by many of the current screens for estrogen action, thus creating a need for more sophisticated assays. Genomic methods, including gene expression profiling, are being used to identify patterns of gene expression associated with estrogenicity (Pennie and Brooks, 2000). When combined with cell lines expressing specific ER subtypes, this approach should give a genome-wide view of the transcriptional targets of different estrogen receptor subtypes, as well as revealing novel targets for ER action and cross-talk between distinct intracellular signalling pathways. Non-genomic modes of ER action, together with the regulation of ER-mediated transcription by multiple posttranslational modifications, may be dissected using proteomic technology. The biochemical definition of how specific subsets of ER-interacting proteins generate cell-type and tissue-specific effects of estrogen represents a major challenge for the future.
Acknowledgments
Acknowledgements
We would like to thank M. Parker, W.D. Pennie, I. Kimber, J. Ashby and members of our laboratory for critical comments on this manuscript. We are also grateful to F. Auricchio for communicating data prior to publication. We apologize to all authors whose work could not be cited due to space limitations.
References
- Benecke A., Chambon, P. and Gronemeyer, H. (2000) Synergy between estrogen receptor α activation functions AF1 and AF2 mediated by transcription intermediary factor TIF2. EMBO Rep., 1, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan C. and Parker, M. (1999) The role of coactivators in steroid hormone action. Exp. Cell Res., 253, 349–356. [DOI] [PubMed] [Google Scholar]
- Burakov D., Wong, C.W., Rachez, C., Cheskis, B.J. and Freedman, L.P. (2000) Functional interactions between the estrogen receptor and DRIP205, a subunit of the heteromeric DRIP coactivator complex. J. Biol. Chem., 275, 20928–20934. [DOI] [PubMed] [Google Scholar]
- Campbell R.A., Bhat-Nakshatri, P., Patel, N.M., Constantinidou, D., Ali, S. and Nakshatri, H. (2001) PI3 kinase/AKT-mediated activation of estrogen receptor α: a new model for anti-estrogen resistance. J. Biol. Chem., 276, 9817–9824. [DOI] [PubMed] [Google Scholar]
- Chen D., Huang, S.M. and Stallcup, M.R. (2000a) Synergistic, p160 coactivator-dependent enhancement of estrogen receptor function by CARM1 and p300. J. Biol. Chem., 275, 40810–40816. [DOI] [PubMed] [Google Scholar]
- Chen D., Riedl, T., Washbrook, E., Pace, P.E., Coombes, C., Egly, J.-M. and Ali, S. (2000b) Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol. Cell, 6, 127–137. [PubMed] [Google Scholar]
- Chen H., Lin, R.J., Xie, W., Wilpitz, D. and Evans, R.M. (1999) Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell, 98, 675–686. [DOI] [PubMed] [Google Scholar]
- Cho H., Orphanides, G., Sun, X., Yang, X.J., Ogryzko, V., Lees, E., Nakatani, Y. and Reinberg, D. (1998) A human RNA polymerase II complex containing factors that modify chromatin structure. Mol. Cell. Biol., 18, 5355–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mora J.F. and Brown, M. (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol., 20, 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechering K., Boersma, C. and Mosselman, S. (2000) Estrogen receptors α and β: Two receptors of a kind? Curr. Med. Chem., 7, 561–576. [DOI] [PubMed] [Google Scholar]
- DiRenzo J., Shang, Y., Phelan, M., Sif, S., Myers, M., Kingston, R. and Brown, M. (2000) BRG-1 Is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol. Cell. Biol., 20, 7541–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouriot G., Brand, H., Denger, S., Metivier, R., Kos, M., Reid, G., Sonntag-Buck, V. and Gannon, F. (2000) Identification of a new isoform of the human estrogen receptor-α (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J., 19, 4688–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L., Bavner, A., Thomsen, J.S., Farnegardh, M. and Gustafsson, J.-A. (2000) The orphan nuclear receptor SHP utilizes conserved LXXLL motifs for interactions with ligand-activated estrogen receptor. Mol. Cell. Biol., 20, 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoven E., Valentine, J.E., Heery, D.M. and Parker, M.G. (1998) Isoforms of steroid receptor coactivator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J., 17, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R.E. and Narlikar, G.J. (1999) ATP-dependent remodelling and acetylation as regulators of chromatin fluidity. Genes Dev., 13, 2339–2352. [DOI] [PubMed] [Google Scholar]
- Kousteni S. et al. (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell, 104, 719–730. [PubMed] [Google Scholar]
- Kraus W.L. and Kadonaga, J.T. (1998) p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev., 12, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G.J.M., Lemmen, J.G., Carlsson, B., Corton, J.C., Safe, S.H., van der Saag, P.T., van der Burg, B. and Gustafsson, J.-A. (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinol., 139, 4252–4263. [DOI] [PubMed] [Google Scholar]
- Lamb J., Ladha, M.H., McMahon, C., Sutherland, R.L. and Ewen, M.E. (2000) Regulation of the functional interaction between cyclin D1 and estrogen receptor. Mol. Cell. Biol., 20, 8667–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Joyal, J.L. and Sacks, D.B. (2001) Calmodulin enhances the stability of the estrogen receptor. J. Biol. Chem., 276, 17354–17360. [DOI] [PubMed] [Google Scholar]
- Lonard D.M., Nawaz, Z., Smith, C.L. and O’Malley, B.W. (2000) The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol. Cell, 5, 939–948. [DOI] [PubMed] [Google Scholar]
- Martini P.G.V., Delage-Mourroux, R., Kraichely, D.M. and Katzenellenbogen, B.S. (2000) Prothymosin α selectively enhances estrogen receptor transcriptional activity by interacting with a repressor of estrogen receptor activity. Mol. Cell. Biol., 20, 6224–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar A., Wang, R.-A., Mishra, S.K., Adam, L., Bagheri-Yarmand, R., Mandal, M., Vadlamudi, R.K. and Kumar, R. (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biol., 3, 30–37. [DOI] [PubMed] [Google Scholar]
- McKenna N.J. and O’Malley, B.W. (2000) An issue of tissues: divining the split personalities of selective estrogen receptor modulators. Nature Med., 6, 960–962. [DOI] [PubMed] [Google Scholar]
- McKenna N.J., Lanz, R.B. and O’Malley, B.W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev., 20, 321–344. [DOI] [PubMed] [Google Scholar]
- Migliaccio A. et al. (2000) Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J., 19, 5406–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G. and Reinberg, D. (2000) RNA polymerase II elongation through chromatin. Nature, 407, 471–475. [DOI] [PubMed] [Google Scholar]
- Paech K., Webb, P., Kuiper, G.G.J.M., Nilsson, S., Gustafsson, J.-A., Kushner, P.J. and Scanlan, T.S. (1997) Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science, 277, 1508–1510. [DOI] [PubMed] [Google Scholar]
- Pennie W.D. and Brooks, A.N. (2000) Toxicogenomics: DNA microarrays in toxicity testing and mechanistic studies. In Balls, M., van Zeller, A.M. and Halder, M.E. (eds), Progress in the Reduction, Refinement and Replacement of Animal Experimentation, pp. 185–192. Elsevier Science B.V.
- Pettersson K. and Gustafsson, J.-A. (2001) Role of estrogen receptor β in estrogen action. Annu. Rev. Physiol., 63, 165–192. [DOI] [PubMed] [Google Scholar]
- Poelzl G., Kasai, Y., Mochizuki, N., Shaul, P.W., Brown, M. and Mendelsohn, M.E. (2000) Specific association of estrogen receptor β with the cell cycle spindle assembly checkpoint protein, MAD2. Proc. Natl Acad. Sci. USA, 97, 2836–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge E.J., White, R., Parker, M.G. and Sumpter, J.P. (2000) Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) α and ER β. J. Biol. Chem., 275, 35986–35993. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Mizzen, C.A., Cheung, P., Crosio, C., Monaco, L., Jacqout, S., Hanauer, A. and Allis, C.D. (1999) Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science, 285, 886–891. [DOI] [PubMed] [Google Scholar]
- Sauvé F., McBroom, L.D.B., Gallant, J., Moriatis, A.N., Labrie, F. and Giguère, V. (2001) CIA, a novel estrogen receptor coactivator with a bifunctional nuclear receptor interacting determinant. Mol. Cell. Biol. 21, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegel A., Wang, C. Katzenellenbogen, B.S., Pestell, R.G. and Lisanti, M.P. (1999) Caveolin-1 potentiates estrogen receptor α (ERα) signaling. Caveolin-1 drives ligand-independent nuclear translocation and activation of ERα. J. Biol. Chem., 274, 33551–33556. [DOI] [PubMed] [Google Scholar]
- Schiltz R.L., Mizzen, C.A., vassilev, A., Cook, R.G., Allis, C.D. and Nakatani, Y. (1999) Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and pCAF within nucleosomal substrates. J. Biol. Chem., 274, 1189–1192. [DOI] [PubMed] [Google Scholar]
- Shang Y., Hu, X., DiRenzo, J., Lazar, M.A. and Brown, M. (2000) Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell, 103, 843–852. [DOI] [PubMed] [Google Scholar]
- Simoncini T., Hafezi-Moghadam, A., Brazil, D.P., Ley, K., Chin, W.W. and Liao, J.K. (2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature, 407, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutoglou E., Katrakili, N. and Talianidis, I. (2000) Acetylation regulates transcription factor activity at multiple levels. Mol. Cell, 5, 745–751. [DOI] [PubMed] [Google Scholar]
- Stenoien D.L., Patel, K., Mancini, M.G., Dutertre, M., Smith, C.L., O’Malley, B.W. and Mancini, M.A. (2001) FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nature Cell Biol., 3, 15–23. [DOI] [PubMed] [Google Scholar]
- Strahl B.D. and Allis, C.D. (2000) The language of covalent histone modifications. Nature, 403, 41–45. [DOI] [PubMed] [Google Scholar]
- Tremblay A., Tremblay, G.B., Labrie, F. and Giguere, V. (1999) Ligand-independent recruitment of Src-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol. Cell, 3, 513–519. [DOI] [PubMed] [Google Scholar]
- Treuter E. et al. (1999) Competition between thyroid hormone receptor-associated protein (TRAP) 220 and transcriptional intermediary factor (TIF) 2 for binding to nuclear receptors. J. Biol. Chem., 274, 6667–6677. [DOI] [PubMed] [Google Scholar]
- Vandel L. and Trouche, D. (2001) Physical association between the histone acetyl transferase CBP and a histone methyl transferase. EMBO Rep., 2, 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. (2001) Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J. Biol. Chem., 276, 18375–18383. [DOI] [PubMed] [Google Scholar]
- Watanabe M. et al. (2001) A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor α coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J., 20, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wei L.-N., Hu, X., Chandra, D., Seto, E. and Farooqui, M. (2000) Receptor interacting protein 140 (RIP140) directly recruits histone deacetylases for gene silencing. J. Biol. Chem., 275, 40782–40787. [DOI] [PubMed] [Google Scholar]
- Wells L., Vosseller, K. and Hart, G.W. (2001) Glycosylation of nucleocytoplasmic Proteins: signal transduction and O-GlcNAc. Science, 291, 2376–2378. [DOI] [PubMed] [Google Scholar]
- Westin S., Rosenfeld, M.G. and Glass, C.K. (2000) Nuclear receptor coactivators. Adv. Pharmacol., 47, 89–112. [DOI] [PubMed] [Google Scholar]
- Zhang H., Thomsen, J.S., Johansson, L., Gustafsson, J.-A. and Treuter, E. (2000) DAX-1 functions as LXXLL-containing corepressor for activated estrogen receptors. J. Biol. Chem., 275, 39855–39859. [DOI] [PubMed] [Google Scholar]