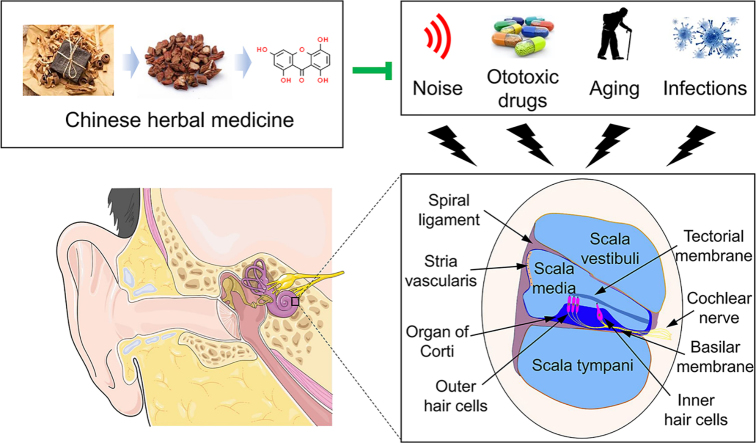
Abstract
According to the World Health Organization's world report on hearing, nearly 2.5 billion people worldwide will suffer from hearing loss by 2050, which may contribute to a severe impact on individual life quality and national economies. Sensorineural hearing loss (SNHL) occurs commonly as a result of noise exposure, aging, and ototoxic drugs, and is pathologically characterized by the impairment of mechanosensory hair cells of the inner ear, which is mainly triggered by reactive oxygen species accumulation, inflammation, and mitochondrial dysfunction. Though recent advances have been made in understanding the ability of cochlear repair and regeneration, there are still no effective therapeutic drugs for SNHL. Chinese herbal medicine which is widely distributed and easily accessible in China has demonstrated a unique curative effect against SNHL with higher safety and lower cost compared with Western medicine. Herein we present trends in research for Chinese herbal medicine for the treatment of SNHL, and elucidate their molecular mechanisms of action, to pave the way for further research and development of novel effective drugs in this field.
KEY WORDS: Sensorineural hearing loss, Chinese herbal medicine, Molecular mechanism, Active ingredients, Prescriptions, Ototoxic drugs, Noise, Hair cells
Graphical abstract
Chinese herbal medicine exhibits remarkable therapeutic effects against sensorineural hearing loss with fewer side effects and lower cost, and may be considered a new strategy for new drug development.
1. Introduction
Hearing loss is one of the most frequent chronic diseases, over 466 million people worldwide (5.5% of the population) have disabling hearing loss. By 2050, it is estimated that one in four people will have hearing problems, and about 2.5 billion people will have varying degrees of hearing loss, of which at least 700 million people will need rehabilitation services (World Health Organization, 2021). Hearing loss not only affects the physical and mental health of individuals, but also wreaks havoc on the national economies. Unemployment rates of people with hearing loss are higher than those with normal hearing in both developed and developing countries. Nowadays there are still no approved effective agents available as yet except devices such as cochlear implants and hearing aids. Therefore, drug therapies for the prevention and treatment of hearing loss must be developed for human use.
Sensorineural hearing loss (SNHL) is the most common sensory deficit and typically occurs due to damage to the cochlear sensory hair cells, stria vascularis, or the synapse with primary auditory neurons in inner ears1, 2, 3. The etiology of SNHL is multifactorial and complicated which may be attributed to ototoxic drugs such as aminoglycoside antibiotics and platinum drugs, exposure to loud noise with prolonged periods, the aging process, or infection4, 5, 6, 7. The underlying mechanisms of SNHL are well investigated in recent years in order to seek novel therapeutic strategies. Currently, steroid therapy has been considered the most widely used treatment for SNHL clinically by immunosuppression and anti-inflammatory action8,9. However the therapeutic effect of steroid therapy remains debatable and there are also lots of side effects such as gastrorrhagia, hypertension, infection, and hepatic dysfunction.
As a treasure of the Chinese nation, Chinese herbal medicine (CHM) has developed for thousands of years. CHM refers to a natural source that has not been processed or is only simply processed and used for disease prevention and medical care under the guidance of traditional Chinese medicine theory. Recently, with the acceleration of the modernization of CHM, the advantages of CHM and its active components in the prevention and treatment of multiple diseases become more obvious. Substantially different from other single small molecule drugs, CHM including formula prescriptions and natural products works by symptomatic treatment through various ways and multiple targets, emphasizing the significance of enhancing the individual's endogenous healing ability from a holistic and natural view10. In addition to being able to treat diseases, CHM also plays a pivotal role in health care and preservation11. Although CHM is confronted with problems such as unclear therapeutic substance basis and mechanism, difficult quality control, registration/policy barriers, and lack of intellectual property rights, its curative effect is remarkable with fewer side effects and lower cost. This review will provide an overview of the prophylaxis and treatment of SNHL with CHM and address new drugs that have potential therapeutic effects.
2. Cause and pathophysiological mechanism of SNHL
2.1. Ototoxic drugs-induced hearing loss
Several drugs can cause cochlear hair cell damage and lead to hearing loss, the most commonly encountered ototoxic drugs are the aminoglycosides and the antineoplastic agent cisplatin. Aminoglycoside antibiotics, including streptomycin, neomycin, gentamicin, kanamycin, and so on, are a kind of glycoside antibiotics formed by connecting amino sugar and amino cyclic alcohol through an oxygen bridge12. They are mainly employed to prevent and treat various systemic infectious diseases in the clinic and can infer acute kidney injury and irreversible hearing loss13. It has been reported that the main mechanism of ototoxicity caused by aminoglycosides is that aminoglycosides enter the mitochondria of vestibular and cochlear hair cells through ion channels or transporters and accumulate, further inhibiting the synthesis of mitochondrial proteins and oxidative phosphorylation of vestibular and cochlear hair cells, resulting in mitochondrial dysfunction, and finally leading to hair cell apoptosis and hearing loss14, 15, 16.
Platinum drugs such as cisplatin, is a chemotherapeutic agent currently prescribed for the treatment of several forms of human cancer. Cisplatin can cause damage to cochlear hair cells and thus lead to hearing loss. Studies have shown that the ototoxicity of cisplatin may be related to the increased reactive oxygen species (ROS) formation and oxidative decompensation in mitochondria17, 18, 19. Permanent ototoxicity induced by cisplatin requires cisplatin to enter cochlear hair cells. Multiple evidence suggests that cisplatin preferentially enters the endolymph in the inner ear through the stria vascularis by copper-like transport-1 and organic cation transporter-220, 21, 22, 23. Then it enters the hair cell cytoplasm and leads to the depletion of glutathione and antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase), resulting in the increase of oxidative stress and promoting apoptotic and necrotic cell death17,24.
2.2. Noise-induced hearing loss
Frequent exposure to excessive recreational, social, and residential noise may affect auditory function. As is reported that acoustic overexposure may increase auditory brainstem response (ABR) threshold shifts, accompanied by loss of cochlear hair cells and synaptic ribbons25, 26, 27. It is currently believed that excessive noise stimulation can lead to the formation of free radicals, ROS, or excitatory toxicity of glutamate, which in turn activates intracellular signaling pathways leading to cell death. ROS generated by noise exposure can also activate nuclear factor-kappa B (NF-κB), cause the production of proinflammatory cytokines, and lead to hearing impairment28. The accumulation of ROS in cells will destroy the cell structure, form damaged organelles and misfolded proteins, activate the occurrence of autophagy, and cause cell death when cells are severely damaged, thus leading to hearing loss29,30. Furthermore, noise-induced Ca2+ overload can also trigger apoptosis and necrotic cell death pathways independently of ROS formation31. Noise exposure activates AMPK through LKB1-mediated pathways to induce hair cell death and synaptic loss32. In addition, extracellular ATP-activated ion channels which are non-selective cation channels expressed in hair cells and squamous epithelial cells of the cochlea can also mediate noise-induced hearing loss33.
2.3. Presbycusis
Age-related hearing loss, termed presbycusis, is a complex degenerative disease. With the increase of age, hearing decreases symmetrically and progressively, which is characterized by the decline of hearing function, including the increase of hearing threshold and the decrease of frequency34. Presbycusis is the third most common health problem among the elderly, second only to heart disease and arthritis. About two-thirds of people aged 70 or over have hearing impairment35,36. Loss of cochlear hair cells and degeneration of spiral ganglion are the main pathological features of presbycusis37. Compared with the young cochleae, the aging cochleae exhibit severe synapse and nerve fiber loss, significant pro-inflammatory and necrotic reactions, and decreased mitochondria level38. It also has been reported that when presbycusis occurs, the mitochondrial oxidative damage in marginal cells of stria vascularis reduces the generation of adenosine triphosphate, which in turn reduces sodium ions, potassium ions, and the activities of adenosine triphosphate enzymes, resulting in a decrease in endocochlear potential and an increase in hearing threshold39.
2.4. Others
Hereditary hearing loss which results from genetic factors is also devastating. More than 700 thousand babies are born with permanent hearing loss annually in low- and middle-income countries. Hereditary hearing loss can be divided into two types: nonsyndromic hearing loss and syndromic hearing loss. Among these 70% is nonsyndromic hearing loss, and the clinical manifestations are mainly binaural symmetrical hearing loss without other symptoms; Syndromic hearing loss accounts for about 30% of congenital hearing loss with other congenital malformations40. The majority of hereditary hearing loss is related to a few gene mutations, such as GJB2, SLC26A4, OTOF, MYO15A, and TMC141,42. Hearing loss can also result from injuries and infections. Traumatic brain injury which results from a bump, jolt, or penetrating injury to the temporal bone may disrupt the anatomic structures of the inner ear thus leading to hearing loss43. Cytomegalovirus is the most common pathogen that causes congenital infection in neonates. Amounts of studies show that cytomegalovirus is the main cause of hearing loss and nervous system diseases in children44, 45, 46. Mumps is an acute infectious disease caused by the mumps virus, which can cause hearing loss by affecting the cochlea and auditory nerve47. Other viral infections including rubella, measles, and human immunodeficiency virus can also be the cause of congenital or acquired hearing loss.
3. CHM treatment for SNHL
The etiology and pathogenesis of SNHL are complicated, which may be a manifestation of either a single symptom or multiple disease syndromes. In traditional Chinese medicine, hearing loss is closely associated with the dysfunction of other organs such as the kidney, and many herbal species have been used for the nourishment of the kidney and other organs to treat deficiencies in hearing. CHM treatment is a conventional therapy for SNHL and can produce unexpected and unique curative effects.
A great deal of studies have been carried out to find anti-SNHL CHM in the past few decades. Here we overviewed the CHM including active ingredients, single CHM extracts, and CHM prescriptions, that exhibited significant protective effects against hearing loss in preclinical (Table 1) and clinical (Table 2) trials.
Table 1.
Effects of CHM in animal models of SNHL.
| CHM | SNHL Model | Effects | Ref. | ||
|---|---|---|---|---|---|
| Active ingredients of CHM | Triterpenoids | Astragaloside IV | Noise-induced hearing loss in guinea pigs | Reducing iNOS, nitrotyrosine, and active caspase 3 | 48, 49, 50, 51, 52, 53, 54 |
| Ursolic acid | Cisplatin-induced ototoxicity in BALB/c mice | Inhibiting TRPV1-oxidative stress pathway | 55, 56, 57 | ||
| Ginsenoside Rb1 | Gentamicin-induced hearing loss in SD rats; cochlear ischemia-induced hearing loss in Mongolian gerbils | Reducing ROS and inhibiting apoptosis | 58, 59, 60 | ||
| Ginsenoside Rd | Noise-induced hearing loss in guinea pigs | Alleviating apoptosis, oxidative stress, and activating SIRT1/PGC-1α signaling pathway | 61 | ||
| Polyphenols | Rosmarinic acid | Cisplatin-induced ototoxicity in rat explants; noise-induced hearing loss in Wistar rats | Inhibiting apoptosis and reducing ROS; potentiating Nrf2–ARE signaling pathway | 62, 63, 64 | |
| Curcumin | Gentamicin and sodium salicylates-induced ototoxicity in SD rats | Inhibiting oxidative stress, apoptosis, and inflammation | 65, 66, 67, 68, 69, 70 | ||
| Resveratrol | Cisplatin-induced ototoxicity in mice; kanamycin and furosemide-induced ototoxicity in Wistar rats; noise-induced hearing loss in C57BL/6 mice; age-related hearing loss in C57BL/6 mice | Activating PTEN-PI3K-Akt axis; inhibiting oxidative stress, apoptosis and inflammation | 71, 72, 73, 74, 75 | ||
| Epicatechin | Radiation-induced ototoxicity in zebrafish and SD rats; cisplatin-induced ototoxicity in SD rats | Inhibiting oxidative stress and apoptosis | 76,77 | ||
| EGCG | Cisplatin-induced ototoxicity in Wistar rats; amikacin and gentamicin-induced hearing loss in zebrafish | Reducing ROS and inhibiting ERK1/2 and STAT1; inhibiting apoptosis | 78,79 | ||
| Flavonoids | Galangin | Amikacin-induced ototoxicity in ICR mouse cochlear explants | Inhibiting oxidative stress | 80, 81, 82 | |
| Luteolin | Hydrogen peroxide-induced cellular senescence model in HEI-OC1 | Promoting sirtuin 1 expression and inhibiting oxidative stress | 83 | ||
| Hesperetin | Cisplatin-induced ototoxicity in Wistar rats | Increasing antioxidant enzymes and reducing oxidant parameters | 84 | ||
| Puerarin | Noise-induced hearing loss in C57 mice; gentamicin-induced ototoxicity in C57BL/6J mice | Regulating the expression of PKCγ and GABABR; reducing ROS and inhibiting mitochondria-dependent apoptosis | 85, 86, 87 | ||
| Terpenoids | Ginkgolide B | Cisplatin-induced ototoxicity in SD rats | Reducing ROS and inhibiting mitochondrial apoptosis | 88 | |
| Oleuropein | Noise-induced hearing loss in Wistar rats | Reducing ABR hearing threshold shifts and attenuating SGN damage | 89 | ||
| Oridonin | Noise-induced hearing loss in C57 mice; kanamycin-induced hearing loss in Kunming mice | Inhibiting NLRP3-inflammasome activation and caspase-1/GSDMD-related hair cell pyroptosis | 90,91 | ||
| Saccharides | LBP | Cisplatin-induced ototoxicity in SD rats | Reducing ROS and maintaining mitochondrial potential | 92,93 | |
| Extracts of single CHM | Salvia miltiorrhiza | Gentamicin and cisplatin-induced ototoxicity in guinea pigs | Decreasing iNOS and caspase-3 expression | 94, 95, 96 | |
| Ginkgo biloba | Gentamicin-induced ototoxicity in guinea pigs; cisplatin-induced ototoxicity in Wistar rats; noise-induced hearing loss in SD rats | Reducing ROS and NO and inhibiting apoptosis | 97, 98, 99, 100, 101, 102 | ||
| Astragalus membranaceus | Noise-induced hearing loss in guinea pigs | Reducing the change of connexin 26 in stria vascularis | 103,104 | ||
| Rehmannia glutinosa | Cisplatin-induced ototoxicity in HEI-OC1 cells | Increasing activity of antioxidant enzymes; inhibiting lipid peroxidation and scavenging activities of free radicals | 106, 107, 108 | ||
| Curculigo orchioides | Cisplatin and noise-induced hearing loss in ICR mice | Inhibiting lipid peroxidation and scavenging activities against free radicals | 109, 110, 111 | ||
| CHM prescriptions | Erlong Zuoci | Age-related hearing loss in C57 mice; gentamicin-induced ototoxicity in Kunming mice | Inhibiting mitochondrial apoptosis | 112, 113, 114 | |
| Jian Er | Age-related hearing loss in C57 mice | Inhibiting oxidative stress and mitochondrial apoptosis | 119 | ||
Table 2.
Effects of CHM against SNHL in clinical trials.
| CHM | Research type | Sample | Intervention | control | Observation period | Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Radix Astragali | Cohort study | 92 | Radix Astragali was administrated intravenously at the dosage of 0.5 mL/kg/day for 10 continuous days | Non-RA treatment | 12 weeks | Pure-tone audiometric threshold change | 105 |
| Erlong Zuoci | Prospective randomized controlled study | 60 | Erlong Zuoci were orally administrated 6 g Bid combined with sound therapy for 3 months | Sound therapy for 3 months | 3 months | Pure-tone audiometric threshold change, inflammatory factors | 115 |
| Wu Ling San | One-arm observational study | 86 | Supplemented Wu Ling San decoction was orally administrated 1–2 doses/day | Null | Commonly 2 weeks | Symptoms | 116,117 |
| Retrospective observational study | 178 | Wu Ling San was orally administrated 7.5 g/day | Steroid, diuretic, drug combinations | 7 days | Pure-tone audiometric threshold change | 118 | |
| Jian Er | Prospective randomized controlled study | 80 | Jian Er decoction was orally administrated 1 dose/day | Masking method | 20–30 days | Symptoms | 120 |
| Gushen Pian | Prospective randomized controlled study | 120 | Gushen Pian was orally administrated 5 tablets, Tid | Placebo | 4 weeks | Pure-tone audiometric threshold change, symptoms, Safety evaluation | 121,122 |
3.1. Active ingredients of CHM
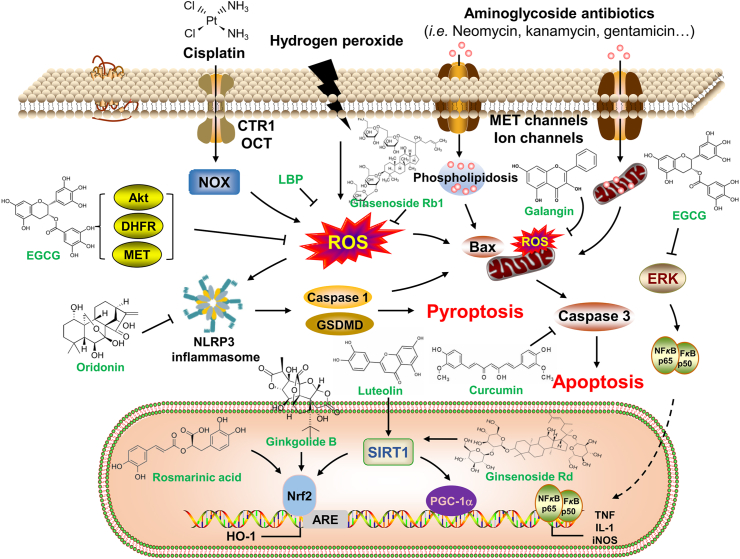
The major active components are the pharmacodynamic material basis of CHM. The identified anti-SNHL active ingredients which showed anti-inflammatory, anti-oxidative, anti-apoptotic, and neuroprotective effects could be generally divided into triterpenoids, polyphenols, flavonoids, terpenoids, and saccharides (Fig. 1).
Figure 1.
Molecular mechanisms of active ingredients of CHM for the treatment of SNHL.
3.1.1. Triterpenoids
Triterpenoids are extensively distributed in nature, some of them exist in plants in free form, called triterpenoid sapogenins. Some exist in the form of glycosides by combining with sugars, called triterpenoid saponins. Triterpenoids have a wide range of physiological properties, such as hypoglycaemic48, immunoregulatory49, anti-cancer50, and anti-inflammatory51 activities. Triterpenoid saponins have been highlighted for their neuroprotective effects52. Astragaloside IV, which is the major active component of the Chinese herb A. membranaceus, shows a significant protective effect against noise-induced cochlear damage. By oral administration of astragaloside IV, increased levels of iNOS and nitrotyrosine and apoptotic marker active caspase 3 induced by impulse noise were effectively reduced53,54. Ursolic acid is a triterpene compound derived from C. officinalis with significant antioxidant effects55. It has been reported that ursolic acid treatment attenuates cisplatin-induced ototoxicity through inhibiting the activation of the TRPV1-oxidative stress pathway56. And pre-treatment with ursolic acid (0.05–2 μg/mL) could effectively protect against hydrogen peroxide-induced auditory House Ear Institute-Organ of Corti 1 (HEI-OC1) cell damage and reverse the decrease of catalase and glutathione peroxidase activities57. Ginsenoside is the most important active ingredient in P. ginseng and exerts strong antioxidant, anti-tumor, anti-aging, and neuroprotective effects58. Studies showed that ginsenoside Rb1 could protect against gentamicin-induced vestibular/hearing dysfunction by reducing reactive oxygen species production and inhibiting apoptotic pathways59, and attenuated cochlear spiral ganglion cell injury caused by ischemic insult60. Ginsenoside Rd, another ginsenoside compound, also exhibits otoprotective effects against noise-induced hearing loss mainly by activating the SIRT1/PGC-1α signaling pathway61.
3.1.2. Polyphenols
Polyphenols refer to a general term of naturally occurring compounds with phenolic hydroxyl structures, which exhibit potent antioxidant properties and can protect against various chronic diseases induced by oxidative stress. Rosmarinic acid, which is a polyphenol and a water-soluble component isolated from the Chinese herb S. miltiorrhiza, inhibits cisplatin-induced apoptosis of auditory cells by targeting caspase 1 and suppressing the activation of downstream signal molecules such as BAX/BCL-2/caspase 3, NF-κB and cytochrome c62. In noise-induced hearing loss, rosmarinic acid significantly decreased cochlear oxidative stress and ABR threshold after noise exposure by potentiating NRF2–ARE signaling pathway63,64. Curcumin is the most known and major biologically active constituent of C. longa. A large number of studies have been conducted to determine its anti-inflammatory, anti-oxidant, anti-fungal, anti-cancer, and other activities65, 66, 67, 68, 69. The otoprotective effects of curcumin have been evaluated in models of drug- and noise-induced ototoxicity. Curcumin could alleviate gentamicin- and sodium salicylates-induced reduction of glutathione and catalase activity, and increased expression of caspase-3 and NF-κB, thus promoting hearing recovery70. Resveratrol is a natural polyphenol abundant in a wide range of plants. Amounts of research have demonstrated the protective effects of resveratrol on auditory dysfunction induced by drugs, noise, and aging71, 72, 73, 74, 75. The mechanisms of the otoprotective effects of resveratrol may be related to the attenuation of oxidative stress, inflammation, and apoptosis. Epicatechin and epigallocatechin-3-gallate (EGCG) are the major active compounds in green tea polyphenols from C. sinensis. The otoprotective effects of epicatechin have been demonstrated in vivo and in vitro with its anti-oxidative and anti-apoptotic effects76,77. EGCG has been shown to ameliorate cisplatin-induced hair cell and ribbon synapse loss through inhibition of ERK1/2 and STAT1 activation78. Other mechanisms of otoprotective effects of EGCG are associated with the reduction of aminoglycosides-induced ROS accumulation and hair cell apoptosis by targeting AKT1, DHFR, and MET79.
3.1.3. Flavonoids
Flavonoids, which widely exist in nature and belong to the secondary metabolites of plants, are a class of polyphenolic compounds with a basic structural unit of 2-phenylchromone. Flavonoids can be found almost in all foods of vegetable origin and adequate evidence has demonstrated that flavonoids exhibit strong anti-oxidative, anti-inflammatory, and neuroprotective properties80,81. Galangin, the most abundant flavonoid found in the rhizomes of A. officinarum, has been proven to exert protective effects on aminoglycoside-induced ototoxicity82. The otoprotective effects were attributed to the improvement of hair cell damage and inhibition of mitochondrial ROS production. Luteolin is a natural flavonoid abundant in chamomile. It has been reported that luteolin significantly attenuates hydrogen peroxide-induced auditory HEI-OC1 cellular senescence by promoting SIRT1 expression. The protective effect of luteolin was abolished when SIRT1 was downregulated, which indicated that SIRT1 might be a potential target of luteolin83. Hesperetin is a flavonoid derived from C. aurantium that acts as an antioxidant. In a cisplatin-induced ototoxicity rat model, it prevented hearing loss at frequencies 8.4, 9.6, and 9.96, and ameliorated cisplatin-induced injury in the organ of corti, spiral ganglion, and stria vascularis84. P. lobata is traditionally used in China for the treatment of various health ailments85. Puerarin is a major isoflavonoid extracted from P. lobata and has been investigated for the prevention of hearing loss. Puerarin treatment significantly alleviated the auditory functions in noise-induced hearing loss mice, which might be related to the regulation of the expression of PKCγ and GABABR86. Puerarin administration also protected against gentamicin-induced cochlear hair cell apoptosis by inhibiting the mitochondria-dependent apoptosis pathway87.
3.1.4. Terpenoids
Terpenoids, a kind of natural hydrocarbons that widely exist in plants, have important physiological activities and are important sources for studying natural products and developing new drugs. Ginkgolide B is a major terpenoid component of G. biloba extract. It has been shown that it can activate the AKT signaling pathway, reduce NOX2 expression, and enhance the NRF2–HO-1 antioxidant pathway, furthermore reducing ROS generation, and inhibiting cisplatin-induced mitochondrial apoptosis in vivo and in vitro88. Oleuropein is a non-toxic secoiridoid glycoside compound isolated from the leaves of olive trees. It has been reported that oleuropein has a partial protective effect against noise-induced hearing loss89. The noise-induced elevated ABR thresholds were decreased in the oleuropein group and the damage of spiral ganglion cells was attenuated. Oridonin is a bioactive ent-kaurane diterpenoid isolated from R. rubescens of the Labtea family. Treatment with oridonin inhibited the activation of inflammasome in mouse cochleae, protecting against noise-induced hearing loss90. And oridonin was also shown to attenuate kanamycin-induced hearing loss by inhibiting NLRP3-inflammasome activation and caspase-1/GSDMD-related hair cell pyroptosis91.
3.1.5. Saccharides
Saccharides is a general term for polyhydroxy aldehydes or ketones and their derivatives and polymers. Saccharides can be divided into monosaccharides (including glucose, fructose, galactose, etc.), disaccharides (including sucrose, maltose, lactose, etc.), and polysaccharides (including starch, liver glycogen, muscle glycogen, etc.) according to their different structures. Saccharides are the most abundant macromolecules that play a key role in biological processes. L. barbarum is a traditional Chinese herbal medicine used as a medicinal and functional food due to its significant beneficial effects. L. barbarum polysaccharides (LBP) are the major constituents extracted from L. barbarum and have been shown to exert anti-oxidative, immunoregulatory, anti-aging, and neuroprotective properties92. It has been reported that LBP pretreatment attenuates cisplatin-induced cochlear hair cell loss. The protective effects of LBP may be attributed to the inhibition of ROS production and maintenance of mitochondrial membrane potential93.
3.2. Extracts of single CHM
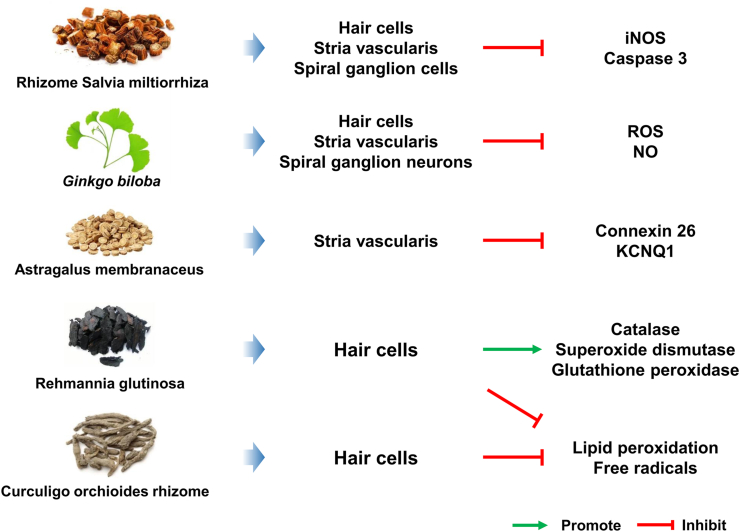
Single CHM extract is a kind of CHM product that is obtained from the extraction, separation, and processing of CHM with advanced technology and has a relatively clear material basis and strict quality standard. Numerous scientific studies of the extracts of single CHM have demonstrated the in vitro and in vivo protective effects on SNHL through different target cells and different mechanisms of action (Fig. 2).
Figure 2.
Illustration of the protective mechanisms of single extracts of CHM for SNHL.
3.2.1. Salvia miltiorrhiza
Rhizome S. miltiorrhiza, which is derived from the root of S. miltiorrhizae Bunge, has been extensively applied for various diseases for many years and exerts antioxidative, neuroprotective, anti-inflammatory, and antineoplastic activities94. It showed that pretreatment with S. miltiorrhiza extracts alleviated gentamicin-induced hearing loss by decreasing iNOS and caspase-3 expression, further reducing apoptotic cells95. In cisplatin-induced ototoxicity in guinea pigs, S. miltiorrhiza attenuated cisplatin-induced significant elevation of ABR threshold and loss of cochlear hair cells. S. miltiorrhiza also showed protective effects against cisplatin-induced stria vascularis and spiral ganglion cell injury96.
3.2.2. Ginkgo biloba
As a “living fossil”, G. biloba is known as a valuable herb and the extracts of G. biloba leaves have been used for medicinal purposes for more than 2000 years in China97. G. biloba leaves have been among the most widely used CHM for centuries and applied for the treatment of Alzheimer's disease, stroke, cerebrovascular disability, depression, and vascular dementia98,99. By establishing in vivo animal models and in vitro explant cultures, standardized G. biloba leaf extract (EGb761) exhibited a protective effect on gentamicin-induced ototoxicity. EGb761 could significantly decrease gentamicin-induced ROS and NO production and improve auditory function by inhibiting apoptosis of cochlear hair cells100. It was reported that G. biloba could relieve cisplatin-triggered damage of the Corti organ, spiral ganglion neurons, and striae vascularis101. In noise-induced hearing loss, EGb761 could also decrease noise-induced elevated distortion product otoacoustic emission and ABR threshold via its antioxidant activity102.
3.2.3. Astragalus membranaceus
A. membranaceus is one of the most significant tonic herbs in CHM and is classified as the qi-invigorating drug in “Shen Nong Ben Cao Jing” which is the first book recording CHM in China. It is demonstrated that A. membranaceus has prominent immunomodulating, anti-inflammatory, anti-hyperglycemic, anti-oxidant, and anti-viral effects103. It has been reported that A. membranaceus could obviously attenuate the ABR deficits and stria vascularis damage with the reduction of the expression of connexin 26 and KCNQ1 in stria vascularis after acoustic trauma104. In a clinical trial, intravenously administration of A. membranaceus in patients with hearing impairment had a better recovery than non-A. membranaceus groups105.
3.2.4. Rehmannia glutinosa
R. glutinosa belongs to the family of Scrophulariaceae and has multiple pharmacological actions on different systems of the human body such as the immune, nervous, endocrine, and blood system106. In vitro study showed that the ethanol extract of steamed roots of R. glutinosa (SRG) could increase the activity of antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase in auditory HEI-OC1 cells, which indicated that SRG had significant anti-oxidant effects107. Further research suggested that SRG protected against cisplatin-induced HEI-OC1 cell injury by suppressing lipid peroxidation and scavenging activities of free radicals108. However, there is still limited evidence about the otoprotective effects of R. glutinosa and the underlying mechanism of action needs further investigation.
3.2.5. Curculigo orchioides
C. orchioides Gaertn which is widely distributed in China has been considered to have anti-aging, anti-oxidant, anti-cancer, and anti-diabetic activities109. The effect of ethanol extract of C. orchioides rhizome was evaluated in cisplatin-induced auditory damage in vivo and in vitro. The result showed that the extract of C. orchioides rhizome could attenuate cisplatin-induced cochlear and peripheral auditory function impairments by inhibiting lipid peroxidation and scavenging free radicals110. It is also demonstrated that oral administration of the extract of C. orchioides reduces noise exposure-induced ABR threshold shifts and cochlear function deficits111. These results indicate that C. orchioides may be used as a potential therapeutic natural product for SNHL, but the deep molecular mechanisms need further exploration.
3.3. CHM prescriptions
3.3.1. Erlong Zuoci
Erlong Zuoci Wan (ELZC) which consists of Radix Rehmanniae Preparata, Fructus Corni, Rhizoma Dioscoreae, Poria cocos, Rhizoma Alismatis, Cortex moutan, Radix Bupleuri and Magnetitum, is a typical kidney-tonifying CHM prescription available for the treatment of hearing loss. A preclinical study showed that ELZC could decrease the rise of ABR threshold in age-related hearing loss mice. The effects were attributed to attenuating spiral ganglion cell damage by inhibiting the expression level of P53 and BAK112. By a combination of network pharmacology with experimental validation, the possible molecular mechanisms underlying ELZC were investigated. The results indicated that the protective effects of ELZC against age-related hearing loss were linked to cellular senescence, inflammatory response, and synaptic connections, and the potential targets and regulatory signal pathways might be associated with AKT, ERK, JNK/STAT3 pathways113. Studies also demonstrated that EGZC protected against gentamicin-induced cochlear hair cell injury. Its disassembled prescriptions, Liuwei Dihuang was the main component for the otoprotective effects114. A recent clinical trial showed that age-related hearing loss patients treated with ELZC exhibited a better hearing recovery compared with the control group, and there was no serious adverse event after ELZC treatment115.
3.3.2. Wu Ling San
Wu Ling San (WLS) is a CHM formula that was first recorded in “Shang Han Lun” written by Zhongjing Zhang in the Han dynasty. It is made up of five kinds of Chinese herbs: Poria cocos, Rhizoma Alismatis, P. umbellatus, C. ramulus, and Rhizoma Atractylodis Macrocephalae. WLS is commonly used for the treatment of urinary difficulties due to its diuretic properties116. WLS has been shown to be effective for Ménière's disease117. It was also reported that steroid-WLS combination therapy was more effective than the diuretic alone, WLS alone, or steroid-diuretic combination, indicating that WLS played a critical role in the treatment of acute low-tone sensorineural hearing loss118.
3.3.3. Jian Er
The CHM prescription Jian Er preparation (JEP) is composed of Radix astragali, Radix puerariae, Radix salviae miltiorrhizae, and Rhizoma drynariae. JEP exhibits multifunctional properties such as reduction of blood lipids, prevention of arteriosclerosis, improvement of microcirculation, and reduction of oxidative stress. In the age-related hearing loss model, JEP was shown to decrease the cochlear and auditory cortex malondialdehyde content, lessen mitochondrial DNA damage, and reduce the expression of apoptosis-related protein caspase 3 in cochlear cells, thus protecting against presbycusis119. JEP which has been used for the treatment of patients with SNHL also has a good clinical ability to protect hearing in humans120.
3.3.4. Gushen Pian
Gushen Pian (GSP) which includes Rhizoma Drynariae, Calcined Ci Shi, S. miltiorrhiza, and G. glabra, is a pure prescription of CHM. With the function of nourishing kidney, tonifying spleen, resolving phlegm, eliminating dampness, and removing blood stasis121, it is effective in the treatment of SNHL and tinnitus due to splenonephric hypofunction and phlegm-accumulation stasis. It has been reported that the GSP has significant therapeutic effects on SNHL patients according to a Phase II clinical trial. It can also attenuate dizziness, insomnia, and fatigue without any adverse effects and toxicity122. However, the mechanism of action of GSP on auditory protection should be further investigated.
4. Conclusions
There is a very long history of CHM for the treatment of hearing loss in China. In recent years, CHM has made considerable progress in the prevention and treatment of SNHL, which provides adequate experimental evidence for the clinical application of CHM. However, there are still many problems required to be further investigated. For example, the technical indicators are limited to the exploration of morphology and auditory function. Repairment or regeneration of cochlear sensory hair cells are the major biological approaches for restoring hearing function in SNHL, which should also be paid more attention to in the mechanism research of CHM. In this review, the potential molecular mechanisms of CHM for its protection of SNHL we elucidate involve the alleviation of ROS production, inflammation, apoptosis, and pyroptosis in different structures of the auditory system, but there is still limited evidence in the specific pharmacological mechanism of some of the CHM herbs or isolated ingredients.
Nowadays advanced chemical analyses are employed for the separation of various bioactive molecules from the herbs. After being synthetically modified the bioactive compounds exert stronger potentials for the treatment of SNHL and are likely to be further investigated as promising agents. With the development of experimental technologies such as metabolomics, transcriptomics, and proteomics, integrative platforms are set up to give a comprehensive interpretation of CHM mechanisms from a modern biological and medical view. Therefore, it is urgent to find safe and effective therapies via the development of CHM with novel mechanisms of action, and extensive clinical trials will be required to find the most effective treatments. With the development of new technologies and methods, we expect to see exciting breakthroughs in the near future.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (Nos. 2021YFA1101300, 2021YFA1101800, and 2020YFA0112503), Strategic Priority Research Program of the Chinese Academy of Science (XDA16010303, China), National Natural Science Foundation of China (Nos. 82101228, 82030029, 81970882, and 92149304), Science and Technology Department of Sichuan Province (No. 2021YFS0371, China), Shenzhen Science and Technology Program (JCYJ20190814093401920 and JCYJ20210324125608022, China), and Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (No. SKLGE-2104, China).
Author contributions
Yunhao Wu, Wenyong Chen, and Renjie Chai conceived the manuscript. Yunhao Wu, and Jingwen Zhang wrote the manuscript. Qiuping Liu and Zhuang Miao gave advice and suggestions.
Conflicts of interest
The authors declare no competing financial interests.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yunhao Wu, Email: yunhaowu0730@outlook.com.
Renjie Chai, Email: renjiec@seu.edu.cn.
Wenyong Chen, Email: wenyongch@163.com.
References
- 1.Vlajkovic S.M., Thorne P.R. Molecular mechanisms of sensorineural hearing loss and development of inner ear therapeutics. Int J Mol Sci. 2021;22:5647. doi: 10.3390/ijms22115647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kujawa S.G., Liberman M.C. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang T.W., Iyer A.A., Manalo J.M., Woo J., Bosquez Huerta N.A., McGovern M.M., et al. Glial-specific deletion of Med12 results in rapid hearing loss via degradation of the stria vascularis. J Neurosci. 2021;41:7171–7181. doi: 10.1523/JNEUROSCI.0070-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J., Chai R., Li H., Sun S. Protection of hair cells from ototoxic drug-induced hearing loss. Adv Exp Med Biol. 2019;1130:17–36. doi: 10.1007/978-981-13-6123-4_2. [DOI] [PubMed] [Google Scholar]
- 5.Moore B.C.J. The effect of exposure to noise during military service on the subsequent progression of hearing loss. Int J Environ Res Public Health. 2021;18:2436. doi: 10.3390/ijerph18052436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X.Z., Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- 7.Foulon I., De Brucker Y., Buyl R., Lichtert E., Verbruggen K., Piérard D., et al. Hearing loss with congenital cytomegalovirus infection. Pediatrics. 2019;144 doi: 10.1542/peds.2018-3095. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura T., Okayasu T., Hosoi H., Kitahara T. Long-term (16–26 years) follow-up outcome of steroid therapy in refractory autoimmune sensorineural hearing loss. J Autoimmun. 2021;121 doi: 10.1016/j.jaut.2021.102664. [DOI] [PubMed] [Google Scholar]
- 9.Filipo R., Attanasio G., Russo F.Y., Viccaro M., Mancini P., Covelli E. Intratympanic steroid therapy in moderate sudden hearing loss: a randomized, triple-blind, placebo-controlled trial. Laryngoscope. 2013;123:774–778. doi: 10.1002/lary.23678. [DOI] [PubMed] [Google Scholar]
- 10.Li S., Wu Z., Le W. Traditional Chinese medicine for dementia. Alzheimers Dement. 2021;17:1066–1071. doi: 10.1002/alz.12258. [DOI] [PubMed] [Google Scholar]
- 11.Gu S., Pei J. Innovating Chinese herbal medicine: from traditional health practice to scientific drug discovery. Front Pharmacol. 2017;8:381. doi: 10.3389/fphar.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker B., Cooper M.A. Aminoglycoside antibiotics in the 21st century. ACS Chem Biol. 2013;8:105–115. doi: 10.1021/cb3005116. [DOI] [PubMed] [Google Scholar]
- 13.Jospe-Kaufman M., Siomin L., Fridman M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorg Med Chem Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kros C.J., Steyger P.S. Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med. 2019;9 doi: 10.1101/cshperspect.a033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenyon E.J., Kirkwood N.K., Kitcher S.R., O'Reilly M., Derudas M., Cantillon D.M., et al. Identification of ion-channel modulators that protect against aminoglycoside-induced hair cell death. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie J., Talaska A.E., Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011;281:28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheth S., Mukherjea D., Rybak L.P., Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci. 2017;11:338. doi: 10.3389/fncel.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.S., Schrader A., Warchol M., Sheets L. Cisplatin exposure acutely disrupts mitochondrial bioenergetics in the zebrafish lateral-line organ. Hear Res. 2022;426 doi: 10.1016/j.heares.2022.108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y., Zheng Z., Liu C., Li W., Zhao L., Nie G., et al. Inhibiting DNA methylation alleviates cisplatin-induced hearing loss by decreasing oxidative stress-induced mitochondria-dependent apoptosis via the LRP1–PI3K/AKT pathway. Acta Pharm Sin B. 2022;12:1305–1321. doi: 10.1016/j.apsb.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breglio A.M., Rusheen A.E., Shide E.D., Fernandez K.A., Spielbauer K.K., McLachlin K.M., et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654. doi: 10.1038/s41467-017-01837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu Y.H., Sibrian-Vazquez M., Escobedo J.O., Phillips A.R., Dickey D.T., Wang Q., et al. Systemic delivery and biodistribution of cisplatin in vivo. Mol Pharm. 2016;13:2677–2682. doi: 10.1021/acs.molpharmaceut.6b00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciarimboli G., Deuster D., Knief A., Sperling M., Holtkamp M., Edemir B., et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waissbluth S., Daniel S.J. Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hear Res. 2013;299:37–45. doi: 10.1016/j.heares.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.J., Ho Hur J., Park C., Kim H.J., Oh G.S., Lee J.N., et al. Bucillamine prevents cisplatin-induced ototoxicity through induction of glutathione and antioxidant genes. Exp Mol Med. 2015;47:e142. doi: 10.1038/emm.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberman M.C., Kujawa S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waqas M., Gao S., Iram-Us-Salam, Ali M.K., Ma Y., Li W. Inner ear hair cell protection in mammals against the noise-induced cochlear damage. Neural Plast. 2018;2018 doi: 10.1155/2018/3170801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurabi A., Keithley E.M., Housley G.D., Ryan A.F., Wong A.C. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–137. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto H., Omelchenko I., Shi X., Nuttall A.L. The influence of NF-kappaB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J Neurosci Res. 2009;87:1832–1840. doi: 10.1002/jnr.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicotera T.M., Hu B.H., Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–477. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anttonen T., Herranen A., Virkkala J., Kirjavainen A., Elomaa P., Laos M., et al. c-Jun N-terminal phosphorylation: biomarker for cellular stress rather than cell death in the injured cochlea. eNeuro. 2016;3 doi: 10.1523/ENEURO.0047-16.2016. ENEURO.0047-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le T.N., Straatman L.V., Lea J., Westerberg B. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg. 2017;46:41. doi: 10.1186/s40463-017-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill K., Yuan H., Wang X., Sha S.H. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. J Neurosci. 2016;36:7497–7510. doi: 10.1523/JNEUROSCI.0782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Housley G.D., Morton-Jones R., Vlajkovic S.M., Telang R.S., Paramananthasivam V., Tadros S.F., et al. ATP-gated ion channels mediate adaptation to elevated sound levels. Proc Natl Acad Sci U S A. 2013;110:7494–7499. doi: 10.1073/pnas.1222295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Revuelta M., Santaolalla F., Arteaga O., Alvarez A., Sánchez-Del-Rey A., Hilario E. Recent advances in cochlear hair cell regeneration-A promising opportunity for the treatment of age-related hearing loss. Ageing Res Rev. 2017;36:149–155. doi: 10.1016/j.arr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhan W., Cruickshanks K.J., Klein B.E., Klein R., Huang G.H., Pankow J.S., et al. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meister H., Rählmann S., Walger M., Margolf-Hackl S., Kießling J. Hearing aid fitting in older persons with hearing impairment: the influence of cognitive function, age, and hearing loss on hearing aid benefit. Clin Interv Aging. 2015;10:435–443. doi: 10.2147/CIA.S77096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sha S.H., Kanicki A., Dootz G., Talaska A.E., Halsey K., Dolan D., et al. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyu A.R., Kim T.H., Park S.J., Shin S.A., Jeong S.H., Yu Y., et al. Mitochondrial damage and necroptosis in aging cochlea. Int J Mol Sci. 2020;21:2505. doi: 10.3390/ijms21072505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowl M.R., Dawson S.J. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9 doi: 10.1101/cshperspect.a033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bayazit Y.A., Yilmaz M. An overview of hereditary hearing loss. ORL J Otorhinolaryngol Relat Spec. 2006;68:57–63. doi: 10.1159/000091090. [DOI] [PubMed] [Google Scholar]
- 41.Mishra S., Pandey H., Srivastava P., Mandal K., Phadke S.R. Connexin 26 (GJB2) mutations associated with non-syndromic hearing loss (NSHL) Indian J Pediatr. 2018;85:1061–1066. doi: 10.1007/s12098-018-2654-8. [DOI] [PubMed] [Google Scholar]
- 42.Hilgert N., Smith R.J.H., Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics?. Mutat Res. 2009;681:189–196. doi: 10.1016/j.mrrev.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roup C.M., Ross C., Whitelaw G. Hearing difficulties as a result of traumatic brain injury. J Am Acad Audiol. 2020;31:137–146. doi: 10.3766/jaaa.18084. [DOI] [PubMed] [Google Scholar]
- 44.Kabani N., Ross S.A. Congenital cytomegalovirus infection. J Infect Dis. 2020;221:S9–S14. doi: 10.1093/infdis/jiz446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andronaco D.W. Congenital cytomegalovirus and hearing loss. J Obstet Gynecol Neonatal Nurs. 2020;49:293–304. doi: 10.1016/j.jogn.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Vos B., Noll D., Whittingham J., Pigeon M., Bagatto M., Fitzpatrick E.M. Cytomegalovirus—a risk factor for childhood hearing loss: a systematic review. Ear Hear. 2021;42:1447–1461. doi: 10.1097/AUD.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 47.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014;18 doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang R., Xu S., Zhang X., Zheng X., Liu Y., Jiang C., et al. Cyclocarya paliurus triterpenoids attenuate glomerular endothelial injury in the diabetic rats via ROCK pathway. J Ethnopharmacol. 2022;291 doi: 10.1016/j.jep.2022.115127. [DOI] [PubMed] [Google Scholar]
- 49.Qian Y., Zheng Y., Jin J., Wu X., Xu K., Dai M., et al. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv Mater. 2022;34 doi: 10.1002/adma.202200521. [DOI] [PubMed] [Google Scholar]
- 50.Chen L., Liu Y., Li Y., Yin W., Cheng Y. Anti-cancer effect of sesquiterpene and triterpenoids from agarwood of Aquilaria sinensis. Molecules. 2022;27:5350. doi: 10.3390/molecules27165350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su H.G., Peng X.R., Shi Q.Q., Huang Y.J., Zhou L., Qiu M.H. Lanostane triterpenoids with anti-inflammatory activities from Ganoderma lucidum. Phytochemistry. 2020;173 doi: 10.1016/j.phytochem.2019.112256. [DOI] [PubMed] [Google Scholar]
- 52.Cho H.M., Ha T.K., Doan T.P., Dhodary B., An J.P., Lee B.W., et al. Neuroprotective effects of triterpenoids from Camellia japonica against amyloid β-induced neuronal damage. J Nat Prod. 2020;83:2076–2086. doi: 10.1021/acs.jnatprod.9b00964. [DOI] [PubMed] [Google Scholar]
- 53.Xiong M., He Q., Lai H., Wang J. Astragaloside IV inhibits apoptotic cell death in the guinea pig cochlea exposed to impulse noise. Acta Otolaryngol. 2012;132:467–474. doi: 10.3109/00016489.2011.643457. [DOI] [PubMed] [Google Scholar]
- 54.Xiong M., Lai H., He Q., Wang J. Astragaloside IV attenuates impulse noise-induced trauma in guinea pig. Acta Otolaryngol. 2011;131:809–816. doi: 10.3109/00016489.2011.568524. [DOI] [PubMed] [Google Scholar]
- 55.Habtemariam S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/8512048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Y., Xu T., Tian Y., Ma T., Qu D., Wang Y., et al. Ursolic acid protects against cisplatin-induced ototoxicity by inhibiting oxidative stress and TRPV1-mediated Ca2+-signaling. Int J Mol Med. 2020;46:806–816. doi: 10.3892/ijmm.2020.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H.H., Hur J.M., Seo S.J., Moon H.D., Kim H.J., Park R.K., et al. Protective effect of ursolic acid from Cornus officinalis on the hydrogen peroxide-induced damage of HEI-OC1 auditory cells. Am J Chin Med. 2009;37:735–746. doi: 10.1142/S0192415X0900720X. [DOI] [PubMed] [Google Scholar]
- 58.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian C.J., Kim S.W., Kim Y.J., Lim H.J., Park R., So H.S., et al. Red ginseng protects against gentamicin-induced balance dysfunction and hearing loss in rats through antiapoptotic functions of ginsenoside Rb1. Food Chem Toxicol. 2013;60:369–376. doi: 10.1016/j.fct.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 60.Fujita K., Hakuba N., Hata R., Morizane I., Yoshida T., Shudou M., et al. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113–117. doi: 10.1016/j.neulet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Chen X.M., Ji S.F., Liu Y.H., Xue X.M., Xu J., Gu Z.H., et al. Ginsenoside Rd ameliorates auditory cortex injury associated with military aviation noise-induced hearing loss by activating SIRT1/PGC-1α signaling pathway. Front Physiol. 2020;11:788. doi: 10.3389/fphys.2020.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeong H.J., Choi Y., Kim M.H., Kang I.C., Lee J.H., Park C., et al. Rosmarinic acid, active component of Dansam-Eum attenuates ototoxicity of cochlear hair cells through blockage of caspase-1 activity. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fetoni A.R., Paciello F., Rolesi R., Eramo S.L., Mancuso C., Troiani D., et al. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic Biol Med. 2015;85:269–281. doi: 10.1016/j.freeradbiomed.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Fetoni A.R., Eramo S.L.M., Di Pino A., Rolesi R., Paciello F., Grassi C., et al. The antioxidant effect of rosmarinic acid by different delivery routes in the animal model of noise-induced hearing loss. Otol Neurotol. 2018;39:378–386. doi: 10.1097/MAO.0000000000001700. [DOI] [PubMed] [Google Scholar]
- 65.White C.M., Pasupuleti V., Roman Y.M., Li Y., Hernandez A.V. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;146 doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
- 66.Samarghandian S., Azimi-Nezhad M., Farkhondeh T., Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 67.Xue B., Zhang Y., Xu M., Wang C., Huang J., Zhang H., et al. Curcumin-silk fibroin nanoparticles for enhanced anti-candida albicans activity in vitro and in vivo. J Biomed Nanotechnol. 2019;15:769–778. doi: 10.1166/jbn.2019.2722. [DOI] [PubMed] [Google Scholar]
- 68.Elbadawy M., Hayashi K., Ayame H., Ishihara Y., Abugomaa A., Shibutani M., et al. Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112043. [DOI] [PubMed] [Google Scholar]
- 69.Yan Z., Dai Y., Fu H., Zheng Y., Bao D., Yin Y., et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol. 2018;60:261–271. doi: 10.1530/JME-17-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abd-Elhakim Y.M., Abdel-Motal S.M., Malhat S.M., Mostafa H.I., Ibrahim W.M., Beheiry R.R., et al. Curcumin attenuates gentamicin and sodium salicylate ototoxic effects by modulating the nuclear factor-kappaB and apoptotic pathways in rats. Environ Sci Pollut Res Int. 2022;29:89954–89968. doi: 10.1007/s11356-022-21932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., Wu H., Zhang F., Yang J., He J. Resveratrol upregulates miR-455-5p to antagonize cisplatin ototoxicity via modulating the PTEN–PI3K–AKT axis. Biochem Cell Biol. 2021;99:385–395. doi: 10.1139/bcb-2020-0459. [DOI] [PubMed] [Google Scholar]
- 72.Lee C.H., Kim K.W., Lee S.M., Kim S.Y. Dose-dependent effects of resveratrol on cisplatin-induced hearing loss. Int J Mol Sci. 2020;22:113. doi: 10.3390/ijms22010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.García-Alcántara F., Murillo-Cuesta S., Pulido S., Bermúdez-Muñoz J.M., Martínez-Vega R., Milo M., et al. The expression of oxidative stress response genes is modulated by a combination of resveratrol and N-acetylcysteine to ameliorate ototoxicity in the rat cochlea. Hear Res. 2018;358:10–21. doi: 10.1016/j.heares.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Xiong H., Ou Y., Xu Y., Huang Q., Pang J., Lai L., et al. Resveratrol promotes recovery of hearing following intense noise exposure by enhancing cochlear SIRT1 activity. Audiol Neurootol. 2017;22:303–310. doi: 10.1159/000485312. [DOI] [PubMed] [Google Scholar]
- 75.Muderris T., Yar Sağlam A.S., Unsal D., Mülazimoğlu S., Sevil E., Kayhan H. Efficiency of resveratrol in the prevention and treatment of age-related hearing loss. Exp Ther Med. 2022;23:40. doi: 10.3892/etm.2021.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pyun J.H., Kang S.U., Hwang H.S., Oh Y.T., Kang S.H., Lim Y.A., et al. Epicatechin inhibits radiation-induced auditory cell death by suppression of reactive oxygen species generation. Neuroscience. 2011;199:410–420. doi: 10.1016/j.neuroscience.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Lee J.S., Kang S.U., Hwang H.S., Pyun J.H., Choung Y.H., Kim C.H. Epicatechin protects the auditory organ by attenuating cisplatin-induced ototoxicity through inhibition of ERK. Toxicol Lett. 2010;199:308–316. doi: 10.1016/j.toxlet.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 78.Borse V., Al Aameri R.F.H., Sheehan K., Sheth S., Kaur T., Mukherjea D., et al. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zong Y., Chen F., Li S., Zhang H. (–)-Epigallocatechin-3-gallate (EGCG) prevents aminoglycosides-induced ototoxicity via anti-oxidative and anti-apoptotic pathways. Int J Pediatr Otorhinolaryngol. 2021;150 doi: 10.1016/j.ijporl.2021.110920. [DOI] [PubMed] [Google Scholar]
- 80.Wen K., Fang X., Yang J., Yao Y., Nandakumar K.S., Salem M.L., et al. Recent research on flavonoids and their biomedical applications. Curr Med Chem. 2021;28:1042–1066. doi: 10.2174/0929867327666200713184138. [DOI] [PubMed] [Google Scholar]
- 81.Spencer J.P. Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc. 2008;67:238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 82.Kim Y.R., Kim M.A., Cho H.J., Oh S.K., Lee I.K., Kim U.K., et al. Galangin prevents aminoglycoside-induced ototoxicity by decreasing mitochondrial production of reactive oxygen species in mouse cochlear cultures. Toxicol Lett. 2016;245:78–85. doi: 10.1016/j.toxlet.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Zhu R.Z., Li B.S., Gao S.S., Seo J.H., Choi B.M. Luteolin inhibits H2O2-induced cellular senescence via modulation of SIRT1 and p53. Korean J Physiol Pharmacol. 2021;25:297–305. doi: 10.4196/kjpp.2021.25.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kara M., Türkön H., Karaca T., Güçlü O., Uysal S., Türkyılmaz M., et al. Evaluation of the protective effects of hesperetin against cisplatin-induced ototoxicity in a rat animal model. Int J Pediatr Otorhinolaryngol. 2016;85:12–18. doi: 10.1016/j.ijporl.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z., Lam T.N., Zuo Z. Radix Puerariae: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2013;53:787–811. doi: 10.1002/jcph.96. [DOI] [PubMed] [Google Scholar]
- 86.Qu J., Liao Y.H., Kou Z.Z., Wei Y.Y., Huang J., Chen J., et al. Puerarin alleviates noise-induced hearing loss via affecting PKCγ and GABAB receptor expression. J Neurol Sci. 2015;349:110–116. doi: 10.1016/j.jns.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 87.Niu P., Sun Y., Wang S., Li G., Tang X., Sun J., et al. Puerarin alleviates the ototoxicity of gentamicin by inhibiting the mitochondria-dependent apoptosis pathway. Mol Med Rep. 2021;24:851. doi: 10.3892/mmr.2021.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma W., Hu J., Cheng Y., Wang J., Zhang X., Xu M. Ginkgolide B protects against cisplatin-induced ototoxicity: enhancement of Akt–Nrf2–HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother Pharmacol. 2015;75:949–959. doi: 10.1007/s00280-015-2716-9. [DOI] [PubMed] [Google Scholar]
- 89.Kümüş Ö., Olgun Y., Mungan Durankaya S., Aktaş S., Kirkim G., Sütay S. Oleuropein effect on noise-induced hearing loss. J Int Adv Otol. 2022;18:118–124. doi: 10.5152/iao.2022.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li M., Zhang Y., Qiu S., Zhuang W., Jiang W., Wang C., et al. Oridonin ameliorates noise-induced hearing loss by blocking NLRP3–NEK7 mediated inflammasome activation. Int Immunopharmacol. 2021;95 doi: 10.1016/j.intimp.2021.107576. [DOI] [PubMed] [Google Scholar]
- 91.Wu L., Chen M., Li M., Wang Y., Li Y., Zheng L., et al. Oridonin alleviates kanamycin-related hearing loss by inhibiting NLRP3/caspase-1/gasdermin D-induced inflammasome activation and hair cell pyroptosis. Mol Immunol. 2022;149:66–76. doi: 10.1016/j.molimm.2022.06.006. [DOI] [PubMed] [Google Scholar]
- 92.Tian X., Liang T., Liu Y., Ding G., Zhang F., Ma Z. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: a review. Biomolecules. 2019;9:389. doi: 10.3390/biom9090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Q., Li Y., Hu L., Wang D. Lycium barbarum polysaccharides attenuate cisplatin-induced hair cell loss in rat cochlear organotypic cultures. Int J Mol Sci. 2011;12:8982–8992. doi: 10.3390/ijms12128982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su C.Y., Ming Q.L., Rahman K., Han T., Qin L.P. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med. 2015;13:163–182. doi: 10.1016/S1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 95.Shi L., An Y., Wang A., Gao Q., Yang Y. The protective effect of Salvia miltiorrhiza on gentamicin-induced ototoxicity. Am J Otolaryngol. 2014;35:171–179. doi: 10.1016/j.amjoto.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 96.Xu O., Liu Y., Li X., Yang Y., Zhang Z., Wang N., et al. Protective effects of Salvia miltiorrhiza against cisplatin-induced ototoxicity in guinea pigs. Am J Otolaryngol. 2011;32:228–234. doi: 10.1016/j.amjoto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 97.Singh B., Kaur P., Gopichand, Singh R.D., Ahuja P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79:401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Eisvand F., Razavi B.M., Hosseinzadeh H. The effects of Ginkgo biloba on metabolic syndrome: a review. Phytother Res. 2020;34:1798–1811. doi: 10.1002/ptr.6646. [DOI] [PubMed] [Google Scholar]
- 99.Moreira J., Machado M., Dias-Teixeira M., Ferraz R., Delerue-Matos C., Grosso C. The neuroprotective effect of traditional Chinese medicinal plants—a critical review. Acta Pharm Sin B. 2023;13:3208–3237. doi: 10.1016/j.apsb.2023.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang T.H., Young Y.H., Liu S.H. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J Nutr Biochem. 2011;22:886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Esen E., Özdoğan F., Gürgen S.G., Özel H.E., Başer S., Genç S., et al. Ginkgo biloba and lycopene are effective on cisplatin induced ototoxicity?. J Int Adv Otol. 2018;14:22–26. doi: 10.5152/iao.2017.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sjostrand A.P., Dogan R., Kocyigit A., Karatas E., Budak B.B., Ozturan O. Therapeutic efficacy of Ginkgo biloba for early-period noise-induced hearing loss: an experimental animal study. Am J Otolaryngol. 2016;37:416–424. doi: 10.1016/j.amjoto.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Fu J., Wang Z., Huang L., Zheng S., Wang D., Chen S., et al. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 104.Xiong M., Zhu Y., Lai H., Fu X., Deng W., Yang C., et al. Radix astragali inhibits the down-regulation of connexin 26 in the stria vascularis of the guinea pig cochlea after acoustic trauma. Eur Arch Otorhinolaryngol. 2015;272:2153–2160. doi: 10.1007/s00405-014-3093-4. [DOI] [PubMed] [Google Scholar]
- 105.Xiong M., He Q., Lai H., Huang W., Wang L., Yang C. Radix astragali injection enhances recovery from sudden deafness. Am J Otolaryngol. 2012;33:523–527. doi: 10.1016/j.amjoto.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 106.Zhang R.X., Li M.X., Jia Z.P. Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117:199–214. doi: 10.1016/j.jep.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 107.Yu H.H., Kim Y.H., Jung S.Y., Shin M.K., Park R.K., So H.S., et al. Rehmannia glutinosa activates intracellular antioxidant enzyme systems in mouse auditory cells. Am J Chin Med. 2006;34:1083–1093. doi: 10.1142/S0192415X06004545. [DOI] [PubMed] [Google Scholar]
- 108.Yu H.H., Seo S.J., Kim Y.H., Lee H.Y., Park R.K., So H.S., et al. Protective effect of Rehmannia glutinosa on the cisplatin-induced damage of HEI-OC1 auditory cells through scavenging free radicals. J Ethnopharmacol. 2006;107:383–388. doi: 10.1016/j.jep.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 109.Chauhan N.S., Sharma V., Thakur M., Dixit V.K. Curculigo orchioides: the black gold with numerous health benefits. J Chin Integ Med. 2010;8:613–623. doi: 10.3736/jcim20100703. [DOI] [PubMed] [Google Scholar]
- 110.Kang T.H., Hong B.N., Jung S.Y., Lee J.H., So H.S., Park R., et al. Curculigo orchioides protects cisplatin-induced cell damage. Am J Chin Med. 2013;41:425–441. doi: 10.1142/S0192415X13500316. [DOI] [PubMed] [Google Scholar]
- 111.Hong B.N., You Y.O., Kang T.H. Curculigo orchioides, natural compounds for the treatment of noise-induced hearing loss in mice. Arch Pharm Res. 2011;34:653–659. doi: 10.1007/s12272-011-0416-5. [DOI] [PubMed] [Google Scholar]
- 112.Dong Y., Guo C.R., Ding Y., Zhang Y., Song H.Y., Peng Y.T., et al. Effects of Erlong Zuoci decoction on the age-related hearing loss in C57BL/6J mice. J Ethnopharmacol. 2016;181:59–65. doi: 10.1016/j.jep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 113.Liu Q., Li N., Yang Y., Yan X., Dong Y., Peng Y., et al. Prediction of the molecular mechanisms underlying Erlong Zuoci treatment of age-related hearing loss via network pharmacology-based analyses combined with experimental validation. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.719267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong Y., Cao B.Y., Wang J., Ding D.L., Han Z.F., Shi J.R. Effects of Erlong Zuoci pill and its disassembled prescriptions on gentamicin-induced ototoxicity model in vitro. Chin J Integr Med. 2010;16:258–263. doi: 10.1007/s11655-010-0258-x. [DOI] [PubMed] [Google Scholar]
- 115.Zou L.Y. Clinical study on Erlong Zuoci pills for presbycusis. J New Chin Med. 2020;52:25–27. [Google Scholar]
- 116.Tsai C.H., Chen Y.C., Chen L.D., Pan T.C., Ho C.Y., Lai M.T., et al. A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res. 2008;36:17–24. doi: 10.1007/s00240-007-0122-4. [DOI] [PubMed] [Google Scholar]
- 117.Ye K.F. Treating 86 cases of Ménière’s syndrome with Wu Ling San. J New TCM. 1999;31:43–44. [Google Scholar]
- 118.Okada K., Ishimoto S., Fujimaki Y., Yamasoba T. Trial of Chinese medicine Wu-Ling-San for acute low-tone hearing loss. ORL J Otorhinolaryngol Relat Spec. 2012;74:158–163. doi: 10.1159/000337819. [DOI] [PubMed] [Google Scholar]
- 119.Xuan Y., Ding D., Xuan W., Huang L., Tang J., Wei Y., et al. A traditional Chinese medicine compound (Jian Er) for presbycusis in a mouse model: reduction of apoptosis and protection of cochlear sensorineural cells and hearing. Int J Herb Med. 2018;6:127–135. [PMC free article] [PubMed] [Google Scholar]
- 120.Xuan W., Lan Y., Wang C. Clinical observation on treatment of 40 senilis of high frequency tinnitus with combination of Chinese Drug and masking method. Chin J Otorhinolaryngol Integ Med. 2001;9:131–133. [Google Scholar]
- 121.Wang H., Yuan H., Guo Y. Clinical observation of treating the lung diseases accompanied by syndrome of blood stasis due to Qi deficiency with Shenyuan Pill. J Chin Med. 2003;18:530–532. [Google Scholar]
- 122.Zhai S., Fang Y., Yang W., Gu R., Han D., Yang S. Clinical investigation on the beneficial effects of the Chinese medicinal herb Gushen Pian on sensorineural deafness and tinnitus. Cell Biochem Biophys. 2013;67:785–793. doi: 10.1007/s12013-013-9536-5. [DOI] [PubMed] [Google Scholar]