Abstract
Plants contain a large number of phytochemical components, many of which are known as bioactive compounds and responsible for the expression of various pharmacological activities. The extract of Sonneratia caseolaris fruit collected in Vietnam was investigated for its total phenolic and total flavonoid contents using methanol solvent and different fractions of S. caseolaris fruits (hexane, ethyl acetate, n-butanol, and aqueous). GC–MS analysis was conducted to identify the bioactive chemical constituents occurring in the active extract. Further, the antibacterial activity was tested in vitro on bacterial isolates, namely Escherichia coli, Staphylococcus aureus, and Bacillus subtilis, using the disc diffusion method on tryptic soya agar (TSA) medium. The methanol extract showed high total flavonoid (82.3 ± 0.41 mg QE/g extract) and phenolic (41.0 ± 0.34 mg GAE/g extract) content. GC–MS of the methanol extract and different fractions of S. caseolaris fruits detected 20 compounds, principally fatty alcohols, fatty acids, phenols, lipids, terpenes derivatives, and carboxylic acids derivatives. A 50 mg/ml concentration of methanol extract had the strongest antibacterial activity on E. coli, S. aureus, and B. subtilis. Furthermore, ethyl acetate, aqueous, and n-butanol fractions inhibited S. aureus and B. subtilis the most. The results of the present study suggested that the fruits of S. caseolaris are rich sources of phenolic compounds that can contribute to safe and cost-effective treatments.
Keywords: Antibacterial, GC–MS, flavonoid, phenolic, Sonneratia caseolaris
Introduction
Sonneratia caseolaris is one of the main plants of some mangrove forests, found in less salty areas in the mangroves, usually along tidal channels with slow-flowing water that is deeply muddy, but this species is not found in coral reefs [1]. Currently, many countries have wild S. caseolaris, including Africa, Sri Lanka, Myanmar, Thailand, Vietnam, Cambodia, Philippines, Indonesia, Timor, Hainan Island (China), Northeast Australia, and some countries in Oceania such as Niughnia, New Guinea, Solomon Islands, New Hebrides [2]. In Vietnam, S. caseolaris has grown wild and is grown in coastal mangrove forests from the North to the South where there is a lot of mud and mudflats. In the North, S. caseolaris grows in coastal and estuary forests such as in Hai Phong, Nghe An, and Ha Tinh [3]. In the South, S. caseolaris is a major component of the natural coastal mangroves and grows densely along the canals of the Mekong Delta of Vietnam, the central coastal region [4]. They have great value in forestry production, and coastal protection and support coastal fisheries [5]. In extreme conditions, this is also a plant species with higher biological ecological, and physiological adaptability than other plants in the same ecosystem [6]. In some folk medicine documents, S. caseolaris is indicated as a valuable source of medicinal herbs. Furthermore, its sour-tasting young berry fruits are edible and used as medicine in poultices for relieving sprains [7]. This plant has been discovered to produce protective bioactive phytochemicals, making it a promising source for extracting such compounds [8] such as gallic acid as well as flavonoids such as luteolin and luteolin-7-O-glucoside [9]. It includes the compounds alkaloid, tannin, flavonoid, saponin, phytosterol, and carbohydrate [10]. Extracts of mangrove leaves have shown promise as a potential natural antibiotic source due to their high levels of trace phenolic compounds, including phenolic acids and flavonoid derivatives [11, 12].
Kasote et al. [13] plants have an inherent ability to synthesize antioxidants, primarily in the form of polyphenols, vitamin E, and vitamin C, as a means of safeguarding themselves against UV radiation and pathogens. The most common are phenolic acids, flavonoids, lignans, stilbenes, and tannins [14]. According to da Silva et al. [15] the difference in flavonoid fraction is most likely caused by the different distribution and types of phenolic compounds that are found in different fruit sections and different plant species. In addition, differences in agricultural techniques, soil nutrients, weather, fruit maturity level, and biotic and abiotic factors influence the phenolic content of fruits [16].
In previous research by Koohsari et al. [17], the sensitivity of gram-positive bacteria like B. subtilis and S. aureus and one gram-negative bacteria, E. coli, were selected to study plant extracts. B. subtilis is one of the few genera of bacteria that can survive in the soil, the gastrointestinal tract of ruminants, and the gastrointestinal tract of humans, making it one of the many diverse species of bacillus that can develop endospores, ropiness, sticky and stringy stability, and other characteristics due to the production of long-chain polysaccharides by the organisms [18]. There have been studies on the chemical composition and biological activity of S. caseolaris to confirm its antibacterial, anticancer, and antioxidant properties [19]. Besides that, S. caseolaris extract is considered a potential plant extract for use against pathogenic bacteria. Yompakdee et al. [20] reported that antibacterial activity was found in methanol extract samples from different parts, such as leaves, flowers, and fruit, of the Crabapple Mangrove tree in Thai Lan. The bark tissue of S. caseolaris showed antibacterial activity against B. subtilis and Proteus vulgaris, according to Simlai et al. [19]. In addition, it was found that the methanol extract of S. caseolaris fruit might inhibit the growth of microbes such as E. coli, S. aureus, and Candida albicans [21]. Furthermore, differences in natural conditions, such as temperature and edaphic parameters, between Vietnam and the countries mentioned above may affect chemical constituents, thereby affecting the biological activities of this species. Thus, this study aims to determine the total phenolic, total flavonoid contents and bacterial activity of methanol extract and its fractions of S. caseolaris fruit collected from Ben Tre Province of Vietnam. We also used GC–MS analysis to determine the chemical composition of the methanol extract and its fractions from the fruit of S. caseolaris.
Materials and Methods
Bacterial Species and Chemical Reagents
To determine the antibacterial activity of methanol extract and different fractions of S. caseolaris fruit, bacterial strains such as E. coli (ATCC 25922), S. aureus (ATCC 29247), and B. subtilis (ATCC 6633) were used in this experiment. The following chemicals were used in this study including methanol (XiLong, China), hexane (C6H14; Vietnam), ethyl acetate (C4H8O2, Vietnam), n-Butanol (C4H8O2, China), Amoxicillin (Vietnam), Gallic acid (C7H6O5; China), Folin-Ciocalteu reagent (Germany), Sodium carbonate (Na2CO3; Germany), Quercetin (C15H10O7; Sigma-Aldrich, Singapore), Aluminium chloride (AlCl3; China), Tryptic Soy Agar medium (Sigma-Aldrich, Germany).
Plant Material and Preparation of Extract
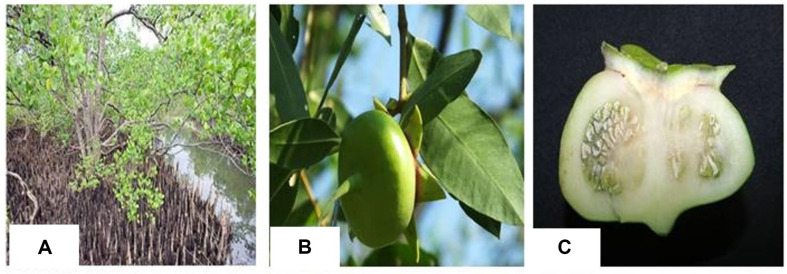
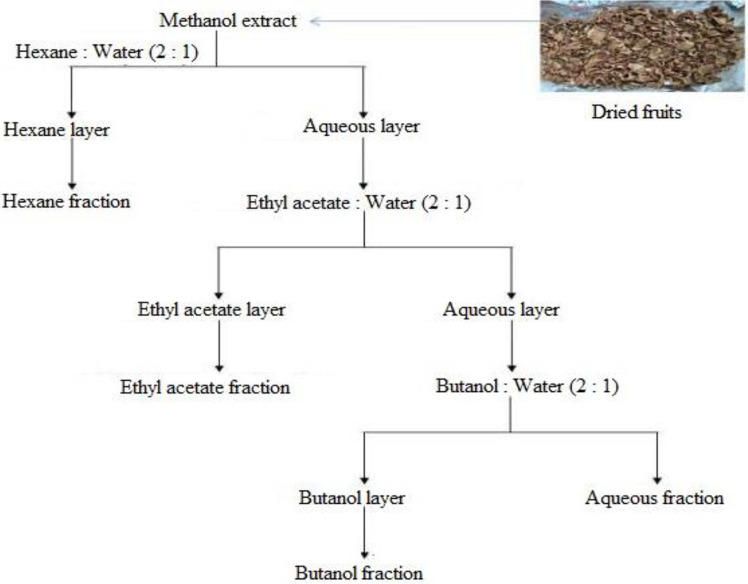
The selected mangrove apple (S. caseolaris) fruits were collected at the bank of Ben Tre River, Ben Tre province in September 2019 (Fig. 1). Fruits were transported to the laboratory, washed, cut into slices, and dried in the drying oven at 55°C for three days. The dried fruits were ground into a fine powder. The powder of fruits was stored in a tightly closed bag and extracted by maceration. The powder (100 g) was shaken in a glass bottle containing a total of 1000 ml methanol solvent for 3 days (27–29°C). The plant extracts were filtered with a vacuum filtration apparatus and then the solvent was removed using a Rotary evaporator (SB-350-EYALA, Japan) at 30°C. The weight volume of dried material was recorded before storage at 4 – 6C until fraction. To separate polar and non-polar organic compounds in the methanol extracts of the sample, we used four solvents, including hexane, ethyl acetate, n-butanol, and distilled water as shown in Fig. 2. 200 ml of various solvents, including hexane, ethyl acetate, n-butanol, and distilled water, were dissolved in the dried extract (v/v: 2:1), yielding separate fractions at the end of the operation. All fractions were evaporated to dryness in a rotary evaporator under a vacuum at 30°C before being redissolved in methanol, hexane, ethyl acetate, n-butanol, and distilled water for further analysis.
Fig. 1. Sonneratia caseolaris in Ben Tre province.
(A) Sonneratia caseolaris tree (B) fruit and (C) fruit in cross-section.
Fig. 2. The procedure for collecting methanol extract and 4 fractions of Sonneratia caseolaris fruits.
Estimation of Total Phenolic Content
The total phenolic content of five fractions was determined by the Folin–Ciocalteu reagent [22] with slight modifications. In summary, the reaction mixture contained 1 ml of the fraction (1 mg/ml) or standard gallic acid solution (20, 40, 60, 80, and 100 g/ml) was mixed with 2.5 ml of Folin–Ciocalteu 10%, shaken well and held for 5 min and then 2 ml of Na2CO3 (7.5 %) was added. The mixture was kept at room temperature for 60 min. The absorbance at 765 nm was read against a blank sample. Using a spectrophotometer (HACH DR/4000U, USA), the total phenolic content was determined based on a gallic acid calibration curve. The results were expressed in terms of gallic acid equivalents (mg of GAE/g extract). The samples were analysed in triplicate.
Estimation of Total Flavonoid Content
The total flavonoid content of the five fractions was analysed according to Abdeslam et al. [23], with some modifications. Briefly, the reaction mixture containing 2 ml of the fraction (1 mg/ml in methanol) was mixed with 2ml of 2 % AlCl3 in methanol. After keeping it at room temperature for 40 min, the absorbance against a blank was read at 415 nm. The total flavonoid content was determined using a standard curve with quercetin (QE) as the standard (20, 40, 60, 80, and 100 g/ml). Total flavonoid contents were expressed as quercetin equivalent (mg QE/g extract). The samples were analysed in triplicate.
Gas Chromatography-Mass Spectrometry (GC–MS) Analysis of the Fractions
GC–MS analysis of the five fractions was carried out using a Perkin-Elmer GC Clarus 500 system gas chromatograph interfaced with a mass spectrometer (JMS-T100 GCV, Jeol Ltd., Japan) equipped with a DB-5MS column (30 mm × 250 μm × 0.25 μm; Agilent, USA) as described by Jenecius et al. [24] with some modifications. 100 mg of each fraction was diluted with 1 ml of MeOH, filtered with a 0.45 μm filter, and then 1 μl was injected into a GC–MS. An electron ionization system with an ionizing energy of 70 eV was used for GC–MS detection. Helium gas (99.999 %) was used as the carrier gas at a flow rate of 1 ml/min and an injection volume of 0.5 μl was employed (ratio of 10:1). The oven temperature program was as follows: 50°C (for 5 min), with an increase of 5°C/min, to 200°C, then 10°C/min to 280°C, ending with isothermal at 280°C. Analysis of the mass spectrum from GC–MS was processed using the database of the National Institute of Standards and Technology (NIST). After obtaining the spectrum of the unknown component, it was compared to the spectra of known components that were archived in the NIST library.
Antibacterial Activity Test
The disc diffusion method for antibacterial activity was applied according to Razmavar et al. [25] with some modifications. 100 ml of each bacterial suspension was uniformly spread on the tryptone soya agar medium in a Petri dish. The five fractions were diluted to concentrations of 10, 30, and 50mg/ml. Three sterile paper discs with a diameter of 6 mm are placed on the surface of each agar plate and then impregnated with 30 ml of diluted fractions. The positive and negative controls were amoxicillin and methanol, respectively. Plates were incubated for 24 h at room temperature (25 – 30°C). Antibacterial activity was assessed by measuring the inhibition zone diameter around the discs. The test was performed three times. If the inhibition zone is ³ 15 mm in size, the inhibitory response is categorised as strong (+++). The inhibitory response is categorised as medium (++) for an inhibition zone of 10–15 mm in size, weak (+) for 9 mm, and no resistance (-) for 0–6 mm.
Data Analysis
The experimental data were expressed as mean ± standard deviation (SD). The statistical significance of the means inhibition zone data of the fractions for each bacterium was performed with a two-way ANOVA followed by Tukey's post hoc multiple comparison tests to determine the significant differences at p < 0.05. Statistical analyses used the statistical analysis software (SAS) (version 8.2).
Results
Total Phenolic and Flavonoid Contents
The total phenolic content in the methanol extract and different fractions of S. caseolaris estimated by the Folin–Ciocalteu method using gallic acid as the standard is shown in Table 1. The total phenolic content ranged from 59.6 to 82.7 mg GAE/g extract. The highest total phenolic content was found in the butanol and methanol fractions with 82.7 ± 0.81 mg GAE/g extract and 82.3 ± 0.41 mg GAE/g extract, respectively. The total phenolic content of the hexane extract from the fruit was significantly lower (p < 0.05) when compared with the other fractions. The content of total flavonoids ranged from 1.81 ± 0.24 mg QE/g extract for aqueous to 41.0 ± 0.34 mg QE/g extract for methanol extract (Table 1). The highest total flavonoid content of S. caseolaris fruits was found in the methanol extract.
Table 1.
Total phenolic and flavonoid content of Sonneratia caseolaris fruit fractions.
| Fractions | Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/g) |
|---|---|---|
| Methanol | 82.27 ± 0.41a | 40.95 ± 0.34a |
| Hexane | 59.58 ± 2.70d | 16.54 ± 0.44c |
| Ethyl acetate | 77.67 ± 0.32b | 26.28 ± 0.93b |
| n-Butanol | 82.67 ± 0.81a | 9.13 ± 0.34d |
| Aqueous | 70.26 ± 0.35c | 1.81 ± 0.24e |
Mean ± Standard deviation; in the same column, means with the same letter were not significantly different (p < 0.05).
Chemical Profiles Identified by GC–MS
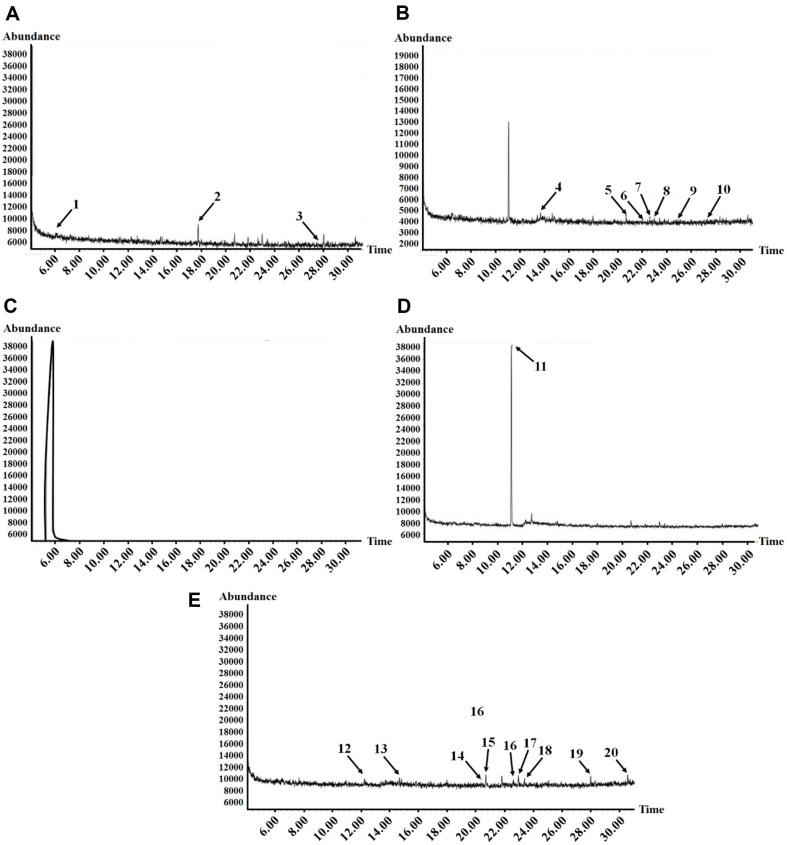
The chemical components in the methanol extract and different fractions of S. caseolaris fruit were determined using GC–MS analysis (Table 2, Fig. 3).
Table 2.
Components detected in fractions of Sonneratia caseolaris fruit by GC-MS.
| Fractions | No. | Retention time | Name of the putative compound | Peak area | Chemical class | Mass (g/mol) |
|---|---|---|---|---|---|---|
| Methanol | 1 | 5.097 | 13-Heptadecyn-1-ol | 74.01 | Long-chain fatty alcohol | 252.44 |
| 2 | 17.707 | Estragole | 586.26 | Phenol | 148.20 | |
| 3 | 27.998 | 2-Hexadecanol | 1225.51 | Fatty alcohol | 242.44 | |
| Hexane | 4 | 13.697 | 1-Octanol | 4731.81 | Fatty alcohol | 130.23 |
| 5 | 20.666 | Myristynoyl pantetheine | 704.36 | Carboxylic acid | 554.72 | |
| 6 | 21.836 | Triacetin | 2635.65 | Glycerolipids | 218.20 | |
| 7 | 22.672 | 2-Myristynoyl pantetheine | 587.09 | Carboxylic acid | 554.72 | |
| 8 | 22.991 | Cubedol | 658.25 | Prenol lipid | 222.37 | |
| 9 | 25.127 | Cyclobarbital | 449.96 | Barbituric acid | 236.27 | |
| 10 | 27.618 | β-curcumene | 3006.88 | Sesquiterpene | 204.35 | |
| Ethyl acetate | 11 | 11.095 | Butanoic acid | 42605.00 | Fatty acid | 88.106 |
| n-Butanol | 11 | 11.103 | Butanoic acid | 11158.00 | Fatty acid | 88.106 |
| Aqueous | 12 | 12.226 | α-Santonin | 151.42 | Terpene | 246.30 |
| 13 | 14.662 | 2-Myristynoyl pantetheine | 286.68 | Carboxylic acid | 554.72 | |
| 14 | 20.713 | Tridecanedial | 500.33 | Volatile oil | 212.33 | |
| 15 | 21.789 | Falcarinol | 535.55 | Fatty alcohol | 244.37 | |
| 16 | 22.627 | Prednisone | 33.56 | Steroid | 358.43 | |
| 17 | 22.967 | Safrole | 342.14 | Colorless oil | 162.19 | |
| 18 | 23.394 | tert-Hexadecanethiol | 236.03 | Colorless liquid | 258.51 | |
| 19 | 27.998 | Rhodopin | 766.47 | Carotenoid | 554.89 | |
| 20 | 32.744 | Geldanamycin | 138.80 | Phenol | 560.64 |
Fig. 3. GC-MS total ion chromatogram of S.caseolaris fruits methanolic extract and fractions.
Peak identification: (A-Methanol extract: 1, 13-Heptadecyn-1-ol; 2, Estragole; 3, 2-Hexadecanol), (B-Hexane fraction: 4, 1-Octanol; 5, Myristynoyl pantetheine; 6, Triacetin; 7, 2-Myristynoyl pantetheine; 8, Cubedol; (9) Cyclobarbital; (10) β-curcumene); (CEthyl acetate: (11) Butanoic acid); (D-n-Butanol: (11) Butanoic acid); (E-Aqueous: (12) α-Santonin; (13) 2-Myristynoyl pantetheine; (14) Tridecanedial; (15) Falcarinol; (16) Prednisone; (17) Safrole; (18) tert-Hexadecanethiol; (19) Rhodopin; (20) Geldanamycin).
GC–MS analysis of S. caseolaris extract showed total components in methanol extract, hexane, ethyl acetate, n-butanol, and aqueous fractions were 3, 7, 1,1 and 9 respectively (Table 2). Twenty compounds were detected by using GC–MS, principally belonging to fatty alcohols, fatty acids, phenols, lipids, terpenes derivatives, and carboxylic acid derivatives. The aqueous fraction was found to contain the highest number of components followed by the hexane fraction. The same component by only butanoic acid was detected in ethyl acetate and butanol fractions (Table 2). The solvents that brought the best results to extract phytochemicals in S. caseolaris fruits were hexane and aqueous, fractions while methanol, ethyl acetate, and butanol showed the least efficacy.
Antibacterial Activity Test
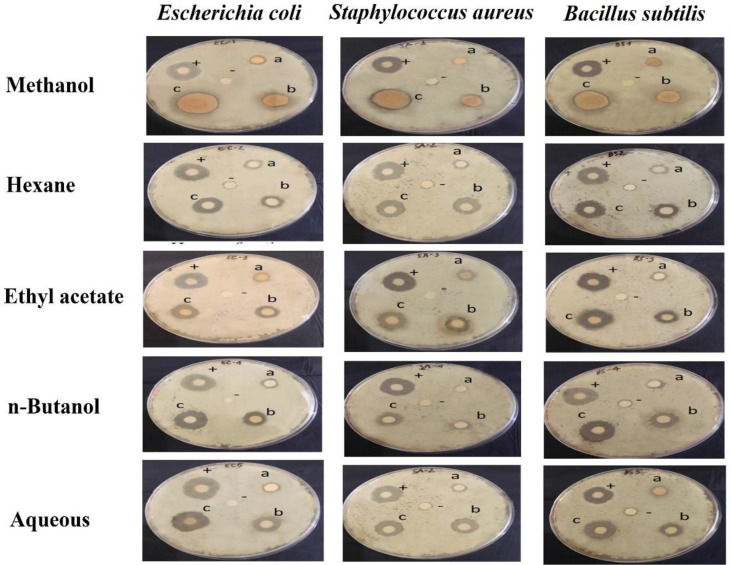
The antibacterial activity of the methanolic extract and different fractions against E. coli, S. aureus and B. subtilis are shown in Table 3 and Fig. 4. The results showed that the methanol extract and different fractions of 10 mg/ml, 30 mg/ml, and 50 mg/ml all had inhibitory effects on E. coli, S. aureus and B. subtilis at different levels. Most concentrations tested 50 mg/ml showed the strongest antibacterial capacity with the largest zone of inhibition. When the bacterial activity of individual fractions of fruits of S. caseolaris was measured the methanol extract exhibited the strongest inhibitory activity against E. coli with a mean zone of inhibition of 14.23 mm in diameter, ethyl acetate and aqueous fractions exhibited the strongest inhibitory activity against S. aureus (11.56 and 11.77mm in diameter, respectively), and n-butanol fraction inhibited the growth of B. subtilis with the largest zone of inhibition (13.33 mm in diameter). These results indicate that the fruits of S. caseolaris have antibacterial activity against E. coli, S. aureus, and B. subtilis and that the extraction of fruits with methanol, ethyl acetate, n-butanol and aqueous solvent might be utilised in the treatment of infectious diseases caused by resistant microbes.
Table 3.
The diameter of the zone (mm) of inhibition against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis by the methanol extract and different fractions from Sonneratia caseolaris fruit at different concentrations.
| Bacterial strains | Fractions | Concentrations | Mean of Concentration | |||||
|---|---|---|---|---|---|---|---|---|
| 10 mg/ml | Level | 30 mg/ml | Level | 50 mg/ml | Level | |||
| Escherichia coli | Methanol | 7.67 ± 0.58f | + | 14.00 ± 0.00c | ++ | 21.00 ± 1.00a | +++ | 14.22 A |
| Hexane | 7.33 ± 0.58f | + | 11.67 ± 0.58de | ++ | 14.67 ± 0.58c | ++ | 11.22 C | |
| Ethyl acetate | 7.33 ± 0.58f | + | 11.33 ± 0.58e | ++ | 13.67 ± 0.58cd | ++ | 10.78 C | |
| n-Butanol | 7.00 ± 0.00f | + | 14.33 ± 0.58c | ++ | 17.67 ± 0.47b | +++ | 12.99 B | |
| Aqueous | 7.33 ± 0.58f | + | 14.67 ± 0.58c | ++ | 17.33 ± 1.53b | +++ | 13.11 B | |
| Mean of Concentration | 7.33 C | 13.20 B | 16.86 A | |||||
| Staphylococcus aureus | Methanol | 0.00f | - | 11.67 ± 0.58d | ++ | 21.00 ± 1.00a | +++ | 10.890 AB |
| Hexane | 7.33 ± 0.58e | + | 11.67 ± 0.58d | ++ | 12.67 ± 0.58cd | ++ | 10.5567 B | |
| Ethyl acetate | 7.67 ± 0.58e | + | 12.33 ± 1.15d | ++ | 14.67 ± 0.58bc | ++ | 11.5567 A | |
| n-Butanol | 0.00f | - | 8.33 ± 0.58e | + | 11.33 ± 1.15d | ++ | 6.5533 C | |
| Aqueous | 7.33 ± 0.58e | + | 11.67 ± 0.58d | ++ | 16.33 ± 0.58b | +++ | 11.7767 A | |
| Mean of Concentration | 4.47 C | 11.13 B | 15.20 A | |||||
| Bacillus subtilis | Methanol | 7.00 ± 0.00f | + | 11.33 ± 0.58de | ++ | 18.67 ± 0.58a | +++ | 12.3333 B |
| Hexane | 7.67 ± 0.58f | + | 10.33 ± 0.58e | ++ | 16.33 ± 0.58b | +++ | 11.4433 C | |
| Ethyl acetate | 7.33 ± 0.58f | + | 12.33 ± 0.58cd | ++ | 17.33 ± 0.58ab | +++ | 12.3300 B | |
| n-Butanol | 8.33 ± 0.58f | + | 13.33 ± 1.52c | ++ | 18.33 ± 0.58a | +++ | 13.3300 A | |
| Aqueous | 0.00g | - | 12.33 ± 0.58cd | ++ | 16.67 ± 0.58b | +++ | 9.6667 D | |
| Mean of Concentration | 6.07 C | 11.93 B | 17.47 A | |||||
| P (Fractions) | < 0.05 | |||||||
| P (Concentration) | < 0.05 | |||||||
| P (Fractions * Concentration) | < 0.05 | |||||||
Mean ± SD; in the same row, means with the same letter were not significantly different (P < 0.05). Levels as (+++): Strong; (++): Moderate; (+): Weak; (−): Negative
Fig. 4. Test of Escherichia coli, Staphylococcus aureus, and Bacillus subtilis resistance activity of methanol extract and 4 fractions of Sonneratia caseolaris fruits.
(+) positive control amoxicillin 50 mg/ml; (-): negative control methanol; (a): 10 mg/ml; (b): 30 mg/ml; (c): 50 mg/ml.
Discussion
Plants contain a large number of phytochemical components, many of which are known as bioactive compounds and responsible for the expression of various pharmacological activities [26]. There was higher than those found in S. ovata fruit with 22.5 mg GAE/g and Cashew apple fruit with 53 mg GAE/g, according to Wetwitayaklung et al. [27] and Silva et al. [28] respectively. S. apetala, a plant of the same genus was examined for leaves, stem bark and roots and extracts were shown to have phenolics 47.5 ± 2.22 mg GAE/g, 42.7 ± 2.75 mg GAE/g, and 42.8 ± 1.67 mg GAE/g, respectively, according to Banerjee et al. [29]. In this study, methanol, the most polar extract, was found to contain the highest content of total phenolic (82.3 mg/g) and flavonoid (41.0 mg/g) as compared to other fractions (Table 2). Previous studies have demonstrated that phenolic compounds have shown potential biological activities such as antioxidant, antidiabetic, hepatoprotective, anti-inflammatory, antimicrobial, and anticancer [30, 31]. In this study, the total flavonoid content of methanol extract for S. caseolaris fruit was higher than that of Hossain and Rahman [32] and Liu et al. [33] who reported those in Bangladeshi pineapple fruit and mangrove plants (S. apetala Buch) extracts. Another study reported that two flavonoids, namely luteolin and luteolin 7-O-βglucoside were found in S. caseolaris fruit, which explains the antioxidant activity of the fruit [34]. The fruits of S. caseolaris were rich in phenolics and it can be proposed that the biological activity of this species could be due to the presence of flavonoids and other phenolics. Methanol was found to facilitate the extraction of more phytochemical compounds due to being more polar [35].
GC–MS is one of the most exact methods to identify secondary metabolites in plant extracts with the help of the NIST library. The current result of the GC–MS analysis of S. caseolaris extract showed the presence of several important chemical compounds like fatty alcohols, fatty acids, phenols, lipids, terpenes derivatives, and carboxylic acids derivatives. A study by Bandaranayake [36] reported that chemical compounds such as phenols, terpenes, and carboxylic acid derivatives found in mangroves have been used and are in demand in industry and modern medicine. McGaw and Staden [37] noted that fatty acids are important constituents of plants and are commonly known to possess antimicrobial activities. In this study, a total of chemical compounds belonging to fatty alcohols, fatty acids, phenols, lipids, terpenes derivatives, and carboxylic.
The extract concentration for antibacterial assay in the present study was determined as 10, 30 and 50 mg/ml. Saif [38] in a study evaluated the antimicrobial activity of methanol extract from Elaeophorbia drupifera (Thonn.) Stapf. (Euphorbiaceae) at a concentration of 50 mg/ml against S. aureus. In the extract concentration of 50 mg/ml that was applied in the Yavuz et al. [49] study, antibacterial activity was observed against the E. coli using the methanol extract of some plant species belonging to the Lamiaceae family (Stachys annua, Scutellaria salviifolia, and Nepeta nuda). Similar to the results of our study evaluated the antimicrobial activity of methanol extract and different fractions of leaf basil against E. coli set as 10, 30, and 50 mg/ml [40]. The methanol extract and different fractions of S caseolaris fruits had relatively high antibacterial activity against E. coli, S. aureus, and B. subtilis. The antibacterial effects of the methanolic fruit extracts of S. caseolaris against S. aureus, E. coli, and C. albicans were reported by Ahmad et al. [21]. In this study, compared with S. caseolaris fractions of the same concentration, there was not much difference, and all fractions gave very weak levels shown by the zone of inhibition value (< 8 mm). Also, differences in the ability of Mangrove fruit extracts to inhibit or kill the growth of microbes may be caused by sensitivity to antimicrobial compounds contained in extracts, wherein the constituent is more sensitive to the yeast and the gram-negative bacteria compared to the gram-positive bacteria. In the previous study, Simlai et al.[19] also reported that the methanol and water extracts from the bark tissue of S. caseolaris exhibited antibacterial activity against B. subtilis and E. coli with 18.3 ± 0.76 mm and 15.8 ± 0.29 mm, respectively. The antibacterial activity of Sonneratia was assessed by three different agar-based assays with methanol extract from seeds and gallic acid for testing by Jongjan et al. [41]. They showed that methanol extract was able to inhibit S. aureus and C. albicans but did not inhibit E. coli while gallic acid only showed activity against S. aureus. The study by da Costa et al. [42] showed the methanolic extract of the bark of S. caseolaris has been found to possess the highest activity against B. subtilis. In this research, among solvents used to extract, the best activity was methanol at 50 mg/ml which exhibited antibacterial activity against E. coli, S. aureus, and B. subtilis. Additionally, ethyl acetate, aqueous and n-butanol fractions showed the strongest inhibitory activity with S. aureus and B. subtilis. Therefore, conducting extensive research is necessary for the isolation, purification, and standardization of the active antibacterial components in S. caseolaris fruits depending on the GC–MS results of each segment.
The results of GC–MS and preliminary photochemical testing indicated that S. caseolaris fruits contained numerous bioactive phytoconstituents belonging to fatty alcohols, fatty acids, phenols, lipids, terpenes derivatives, and carboxylic acid derivatives that may be responsible for antibacterial activity. 1- Dodecanol compound belongs to fatty alcohol with a long chain registered for S. aureus with the highest antibacterial activity by Togashi et al.[43]. According to Marwa et al. [44], 2-Hexadecanol, which was discovered through GC–MS analysis of Paecilomyces lilacinus acetone extract, exhibited antimicrobial and antibacterial activity. There have been several reports on the antibacterial activities of estragole against the S. aureus 1199B strain [45, 46], S. aureus RN4220 and S. aureus RN4220 [47]. Minqing et al. [48] isolated twenty-four compounds from Chinese S. caseolaris stem and twigs, but none of the compounds showed significant antibacterial activity against S. aureus. In the present study, three compounds (13-Heptadecyn-1-ol, Estragole and 2-Hexadecanol) were identified in GC–MS analysis of the methanol extract of the fruit S. caseolaris which was evaluated as the highest antibacterial activity. There have been several reports on the antibacterial activities of long-chain fatty alcohols [49-51]. In this study, we found a 13-Heptadecyl-1-ol compound of long-chain fatty alcohol in the methanol extract of the fruit S. caseolaris that showed potent antibacterial activity using GC–MS. This study provides the basis for further extensive research in exploring the possibility of new naturally biologically active compounds with antibacterial activity.
Acknowledgments
The authors would also express gratitude toward Van Lang University (VLU) for its great support with both research funding and experimental facility. Some experiments were carried out in the laboratory of the School of Engineering and Technology (VLSET) - at Van Lang University (VLU). This work was carried out under the support of the Queensland Alliance for Agriculture and Food Innovation (Crop Sciences Lab) at The University of Queensland, St Lucia, QLD, Australia. On the other hand, the High Agricultural Technology Research Institute for Mekong Delta, Vietnam (HATRI) provides important efforts in collecting and examining several samples.
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Yompakdee C, Thunyaharn S, Phaechamud T. Bactericidal activity of methanol extracts of crabapple mangrove tree (Sonneratia caseolaris Linn.) against multi-drug resistant pathogens. Indian J. Pharm. Sci. 2012;74:230–236. doi: 10.4103/0250-474X.106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdicken K. A guide to monitoring carbon storage in forestry and agroforestry projects. 1997. [Google Scholar]
- 3.Binh NN. Scientific report on "Characteristics of mangrove soil (salic fluvisols) under different coastal mangrove vegetation in Viet Nam", National research project for period of 2000-2002. Forest Science Institute of Vietnam; 2001. [Google Scholar]
- 4.Minh TP, Jacques P. Status and changes of mangrove forest in Mekong Delta: case study in Tra Vinh, Vietnam. Estuar. Coast Shelf Sci. 2007;71:98–109. doi: 10.1016/j.ecss.2006.08.007. [DOI] [Google Scholar]
- 5.Kamaruzaman J. Malaysian Mangrove forests and their significance to the Coastal Marine environment. Pol. J. Environ. Stud. 2013;22:979–1005. [Google Scholar]
- 6.Kandasamy K, Bingham B. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001;40:81–251. doi: 10.1016/S0065-2881(01)40003-4. [DOI] [Google Scholar]
- 7.Sahoo P, Dash RK. Economic growth in South Asia: Role of infrastructure. J. Int. Trade Econ. Dev. 2012;21:217–252. doi: 10.1080/09638191003596994. [DOI] [Google Scholar]
- 8.Amsyir J, Batubara RR, Jogja EG, Batubara I, Audah KA, Nunuk KN. Introduction of bioprospecting opportunities for Indonesian Mangrove Species; Proceedings of the International Biotechnology Conference on Estate Crops; Jakarta, Indonesia. 18-20 October; 2017. pp. 1–5. [Google Scholar]
- 9.Wetwitayaklung P, Limmatvapirat C, Phaechamud T. Antioxidant and anti-cholinesterase activities in various parts of Sonneratia caseolaris (L.) Indian J. Pharm. Sci. 2013;75:649. [PMC free article] [PubMed] [Google Scholar]
- 10.Jana H, Mondal KC, Pati BR, Mitra A. Evaluation of anti-infective potential of fruits of common mangrove tree Sonneratia apetalaagainst some selected pathogenic fungi and bacteria. Int. J. Herb. Med. 2015;3:34–37. [Google Scholar]
- 11.Howlder MSI, Dey SK, Hira A, Ahmed A. Evaluation of antinociceptive and antioxidant properties of the ethanolic extract of Sonneratia caseolaris leaves. Der. Pharmacia. Sinica. 2012;3:536–541. [Google Scholar]
- 12.Mouafi FE, Abdel-aziz SM, Bashir AA, Fyiad AA. Phytochemical analysis and antimicrobial activity of mangrove leaves (Avicenna Marina and Rhizophora Stylosa) against some pathogens. World Appl. Sci. J. 2014;29:547–554. [Google Scholar]
- 13.Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 15.da Silva LMR, de Figueiredo EAT, Ricardo NMPS, Vieira IGP, de Figueiredo RW, Brasil IM, et al. Quantification of bioactive compounds in pulps and by products of tropical fruits from Brazil. Food Chem. 2014;143:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Mokhtar SI, Pheen CL, Lee EV, Abd Aziz NA. Total Phenolic contents, antioxidant activities and organic acids composition of three selected fruit extracts at different maturity stages. J. Sustain. Trop. Agric. Res. 2014;2:40–46. doi: 10.47253/jtrss.v2i2.491. [DOI] [Google Scholar]
- 17.Koohsari H, Ghaemi EA, Sheshpoli MS, Jahedi M, Zahiri M. The investigation of antibacterial activity of selected native plants from North of Iran. J. Med. Life. 2015;8:38–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Michael T, Jonh MM. Brock biology of microorganisms. Int. J. Microbiol. 2005;8:149–150. [Google Scholar]
- 19.Simlai A, Rai A, Mishra S, Mukherjee K, Roy M. Antimicrobial and antioxidative activities in the bark extracts of Sonneratia caseolaris, a mangrove plant. EXCLI J. 2014;13:997–1010. [PMC free article] [PubMed] [Google Scholar]
- 20.Yompakdee C, Thunyaharn S, Phaechamud T. Bactericidal activity of methanol extracts of crabapple mangrove tree (Sonneratia caseolaris Linn.) against multi-drug resistant pathogens. Indian J. Pharm. Sci. 2012;74:230–236. doi: 10.4103/0250-474X.106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad I, Ambarwati NSS, Lukman A, Masruhim MA, Rijai L, Mun'im A. In vitro antimicrobial activity evaluation of mangrove fruit (Sonneratia caseolaris L.) extract. Pharmacogn. J. 2018;10:598–601. doi: 10.5530/pj.2018.3.98. [DOI] [Google Scholar]
- 22.Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J. Physiol. 2011;3:10–14. [Google Scholar]
- 23.Abdeslam ET, Hajiba F, Meryem M, Abdelhakim B, Nadia D, Hassane A, et al. Screening of antioxidant, antibacterial and antileishmanial activities of Salvia officinalis L. extracts from Morocco. Br. Microbiol. Res. J. 2016;16:1–10. doi: 10.9734/BMRJ/2016/28307. [DOI] [Google Scholar]
- 24.Jenecius AA, Uthayakumari F, Mohan VR. GC-MS determination of bioactive components of Sauropus bacciformis blume (Euphorbiaceae) J. Curr. Chem. Pharm. Sci. 2012;2:347–358. [Google Scholar]
- 25.Razmavar S, Abdulla MA, Ismail SB, Hassandarvish P. Antibacterial activity of leaf extracts of Baeckea frutescens against methicillin-resistant Staphylococcus aureus. BioMed Res. Int. 2014;2014:521287. doi: 10.1155/2014/521287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Rahman TMA, Hegazy AK, Sayed AM, Kabiel HF, ElAlfy T, El-Komy SM. Study on combined antimicrobial activity of some biologically active constituents from wild Moringa peregrina Forssk. J. Yeast Fungal Res. 2010;1:15–24. [Google Scholar]
- 27.Wetwitayaklung P, Limmatvapirat C, Phaechamud T. Antioxidant and anticholinesterase activities in various parts of Sonneratia caseolaris (L.) Int. J. Pharm. 2013;75:649–656. [PMC free article] [PubMed] [Google Scholar]
- 28.Silva EP, Siqueira HH, Lago RC, Rosell CM, Boas EVBV. Developing fruit-based nutritious snack bars. J. Sci. Food Agric. 2014;94:52–56. doi: 10.1002/jsfa.6282. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee D, Chakrabarti S, Hazra AK. Antioxidant activity and total phenolics of some mangroves in Sundarbans. Afr. J. Biotechnol. 2008;7:805–810. [Google Scholar]
- 30.Shashank K, Abhay KP. Chemistry and biological activities of flavonoids: an overview. Scientific WorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulaiman SF, Sajak AAB, Ooi KL, Supriatno SEM. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011;24:506–515. doi: 10.1016/j.jfca.2011.01.020. [DOI] [Google Scholar]
- 32.Hossain MA, Rahman SMM. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Int. Food Res. J. 2011;44:672–676. doi: 10.1016/j.foodres.2010.11.036. [DOI] [Google Scholar]
- 33.Liu J, Luo D, Wu Y, Gao C, Lin G, Chen J, et al. The protective effect of Sonneratia apetala fruit extract on acetaminopheninduced liver injury in Mice. Evid. Based Complement. Alternat. Med. 2019;2019:6919834. doi: 10.1155/2019/6919834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadhu SK, Ahmed F, Ohtsuki T, Ishibashi M. Flavonoids from Sonneratia caseolaris. J. Nat. Med. 2006;60:264–265. doi: 10.1007/s11418-006-0029-3. [DOI] [PubMed] [Google Scholar]
- 35.Hasbay İ, Çetin H, Yener E, Bayindirli A. Subcritical (carbon dioxide + ethanol) extraction of polyphenols from apple and peach pomaces, and determination of the antioxidant activities of the extracts. J. Supercrit. Fluids. 2007;43:55–63. doi: 10.1016/j.supflu.2007.04.012. [DOI] [Google Scholar]
- 36.Bandaranayake WM. Bioactivities, bioactive compounds, and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002;10:421–452. doi: 10.1023/A:1021397624349. [DOI] [Google Scholar]
- 37.McGaw LJ, Jäger AK, Staden J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002;68:417–423. doi: 10.1016/S0254-6299(15)30367-7. [DOI] [Google Scholar]
- 38.Saif MMS, Al-Fakih AA, Hassan MAM. Antibacterial activity of selected plant (Aqueous and methanolic) extracts against some pathogenic bacteria. J. Pharmacogn. Phytochem. 2017;6:1929–1935. [Google Scholar]
- 39.Yavuz C, Kilic DD, Ayar A, Yildirim T. Antibacterial effects of methanol extracts of some plant species belonging to Lamiaceae family. Int. J. Second. Metab. 2017;4:429–433. doi: 10.21448/ijsm.376691. [DOI] [Google Scholar]
- 40.Ha PTT, Linh DTN, Dat MTT, Trang PTT. Inhibitory in vitro effects of Basil (Ocimum basilicum) leaf extracts on cholesterol esterase activity and the growth of Escherichia coli. J. Food Process. Preserv. 2021;45:e16105. doi: 10.1111/jfpp.16105. [DOI] [Google Scholar]
- 41.Jongjan M, Thawatchai P, Chantana W. Antimicrobial studies of Sonneratia caseolaris using different agar diffusion. J. Pharm. Biol. Chem. Sci. 2012;3:404–410. [Google Scholar]
- 42.da Costa RHS, Rocha JE, de Freitas TS, Pereira RLS, Junior FNP, de Oliveira MRC, et al. Evaluation of antibacterial activity and reversal of the NorA and MepA efflux pump of estragole against Staphylococcus aureus bacteria. Arch. Microbiol. 2021;203:3551–3555. doi: 10.1007/s00203-021-02347-x. [DOI] [PubMed] [Google Scholar]
- 43.Togashi N, Shiraishi A, Nishizaka M, Matsuoka K, Endo K, Hamashima H, et al. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules. 2007;12:139–148. doi: 10.3390/12020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marwa TA, Abdel-Wareth, Mosad AG, Mohamed SA, Ali ME. Snailicidal, antimicrobial, antioxidant, and anticancer activities of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus fungal extracts. Egypt. J. Aquat. Biol. Fish. 2019;23:195–212. doi: 10.21608/ejabf.2019.30550. [DOI] [Google Scholar]
- 45.Coêlho M, Ferreira J, Siqueira-Júnior J, Kaatz G, Barreto H, Cavalcante A. Inhibition of the NorA multidrug transporter by oxygenated monoterpenes. Microb. Pathog. 2016;99:173–177. doi: 10.1016/j.micpath.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Muniz DF, Dos Santos Barbosa CR, de Menezes IRA, de Sousa EO, Pereira RLS, Júnior JTC, et al. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021;337:127776. doi: 10.1016/j.foodchem.2020.127776. [DOI] [PubMed] [Google Scholar]
- 47.Bezerra AH, Bezerra SR, Macêdo NS, de Sousa Silveira Z, Dos Santos Barbosa CR, de Freitas TS, et al. Effect of estragole over the RN4220 Staphylococcus aureus strain and its toxicity in Drosophila melanogaster. Life Sci. 2020;264:118675. doi: 10.1016/j.lfs.2020.118675. [DOI] [PubMed] [Google Scholar]
- 48.Minqing T, Haofu D, Xiaoming L, Bingui W. Chemical constituents of marine medicinal mangrove plant Sonneratia caseolaris. Chin. J. Oceanol. Limnol. 2009;27:288–296. doi: 10.1007/s00343-009-9138-7. [DOI] [Google Scholar]
- 49.Kubo I, Muroi H, Kubo A. Antibacterial activity of long-chain alcohol against Streptococcus mutans. J. Agric. Food Chem. 1993;41:2447–2450. doi: 10.1021/jf00036a045. [DOI] [Google Scholar]
- 50.Tanaka Y, Fukuda S, Kikuzaki H, Nakatani N. Antibacterial activity of aliphatic long-chain compounds against upper-airway respiratory tract bacteria. ITE Lett. Batter. New Technol. Med. 2002;1:777–780. [Google Scholar]
- 51.Kabelitz N, Santos PM, Heipieper HJ. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 2003;220:223–227. doi: 10.1016/S0378-1097(03)00103-4. [DOI] [PubMed] [Google Scholar]