Abstract
Background
Sivelestat, a neutrophil elastase inhibitor, is specifically developed to mitigate the occurrence of acute lung injury (ALI) in individuals who are undergoing cardiovascular surgery. However, its impact on patients who are at a heightened risk of developing ALI after scheduled cardiac surgery has yet to be determined. In order to address this knowledge gap, we undertook a study to assess the efficacy of sivelestat in protecting the lungs of these patients.
Methods
We conducted a prospective cohort study involving 718 patients who were at high risk of developing postoperative acute lung injury (ALI) and underwent scheduled cardiac surgery between April 25th, 2022, and September 7th, 2023. Among them, 52 patients received sivelestat (administered at a dosage of 0.2mg/kg/h for 3 days), while 666 patients served as controls, not receiving sivelestat. The control conditions were the same for all patients, including ventilation strategy, extubating time, and fluid management. Subsequently, a propensity-score matched cohort was established, consisting of 40 patients in both the sivelestat and control groups. The primary outcome measure encompassed a composite of adverse outcomes, including 30-day mortality, ALI, acute respiratory distress syndrome (ARDS), and others. Secondary outcomes assessed included pneumonia, ventricular arrhythmias, mechanical ventilation (MV) time, and more.
Results
After conducting propensity matching in our study, we observed that there were no significant differences in 30-day mortality between the sivelestat and control groups (0% vs 2.5%, P=0.32). However, the use of sivelestat exhibited a significant reduction in the incidence of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) compared to the control group (0% vs 55%, P<0.01), pneumonia (0 vs 37.5%, P<0.01), MV time (median:8 hours, IQR:4–14.8 hours vs median: 15.2 hours, IQR:14–16.3 hours, P<0.01). Compared to the control group, the sivelestat could significantly decrease white cell count (P<0.01), neutrophile percentage (P<0.01) and C-reactive protein (P<0.01) in the period of postoperative 5 days.
Conclusion
The prophylactic administration of sivelestat has shown promising results in reducing the occurrence of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) in patients with a heightened risk of developing these conditions after elective cardiac surgery. Our study findings indicate that sivelestat may provide protective effects by suppressing inflammation triggered by neutrophil activation, thereby safeguarding pulmonary function.
Registration
ChiCTR2200059102, https://www.chictr.org.cn/showproj.html?proj=166643.
Keywords: sivelestat, cardiac surgery, acute lung injury, inflammation
Introduction
Cardiopulmonary bypass (CPB) is a critical life support system used during open-heart surgery. However, it is well acknowledged that CPB-induced systemic inflammatory response syndrome (SIRS) significantly raises the risk of postoperative complications and mortality.1,2 Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are conditions characterized by the accumulation of fluid in the lungs, known as pulmonary edema. These conditions frequently coincide with systemic inflammatory response syndrome (SIRS). Such complications can arise after cardiopulmonary bypass (CPB) and have a significant impact on postoperative outcomes, thereby increasing elevating of morbidity and mortality.3–6 Based on previous research findings, acute lung injury was frequent in the sample of European ICUs, approximately 7.1%; one third of patients with mild ALI, but more than half rapidly evolved to ARDS. The incidence of ARDS after CPB is only 2% to 3%, but its mortality rate is as high as 50%. While the mortality of ARDS remains high, that of mild ALI is twice as low.7,8
The inflammatory reaction triggered by cardiopulmonary bypass (CPB) involves various factors, including complement activation, increased expression of adhesion molecules on leukocytes, and the presence of pro-inflammatory cytokines in the bloodstream.9–14 Neutrophils, a key component of leukocytes, play a crucial role in systemic inflammatory response syndrome (SIRS) by generating superoxide radicals and releasing chemical mediators.14,15 Research has shown that neutrophil activation is a major contributing factor to the development of pulmonary dysfunction following CPB, emphasizing its significance as an initial event in the process.16,17
Sivelestat, a low molecular-weight compound, is a synthetic and specific inhibitor of neutrophil elastase18 approved in China, Japan, and Korea for the improvement of ARDS in patients with SIRS. Extensive research has shown that this inhibitor effectively reduces levels of neutrophil elastase levels and interleukin-6 production, while it preserves neutrophil deformability during extracorporeal circulation.6,19,20 Phase III and IV Japanese studies have shown improvements in pulmonary function, length of intensive care unit stay, and mechanical ventilation.21 The efficacy of sivelestat in the treatment of ALI/ARDS remains controversial in clinical studies. The available evidence suggests that sivelestat may be beneficial in treating ALI/ARDS after cardiac surgery, particularly in cases following cardiopulmonary bypass (CPB).6,14 It is worth noting that emergency cardiovascular surgery typically leads to a higher incidence of acute lung injury (ALI) compared to scheduled cardiac surgery.5,22 Our previous study has provided evidence suggesting that sivelestat holds significant potential in improving postoperative outcomes for patients who have undergone emergency cardiovascular surgery.17 This agent has the potential to hinder the negative effects of SIRS and therefore, may serve as an optimal treatment option for mitigating ALI in patients undergoing cardiovascular surgery.
Due to the high incidence of ALI in cardiac surgery, the administration of sivelestat as a prophylactic measure may enhance postoperative outcomes. However, there is currently a lack of studies investigating the prophylactic administration of sivelestat to high-risk patients vulnerable to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) following cardiac surgery. Therefore, we have designed this study to evaluate the potential impact of prophylactic sivelestat on pulmonary protection in patients deemed to be at a high risk of ALI following scheduled cardiac surgery.
Materials and Methods
Study Population
This study is a cohort study initiated by the investigators themselves, indicating that they have taken the initiative to design and conduct the research. The project was initiated on April 25th, 2022, and successfully concluded on September 7th, 2023. This study received approval from the ethical committee of Nanjing Drum Tower Hospital (2022-102-01) and was registered in the Chinese Clinical Trial Registry (ChiCTR2200059102). The research involved a cohort of 718 patients who were at high risk of developing postoperative acute lung injury (ALI) and had undergone scheduled cardiac surgery under the care of the Cardiac department at Nanjing Drum Tower Hospital. Prior to their participation, all patients provided their informed consent by signing consent forms.
The following criteria were used for participant selection: adult patients scheduled for on-pump cardiac surgery, aged 18 years or older, with a predicted risk of prolonged ventilation exceeding 3% based on the Society of Thoracic Surgeons Risk models (STS).23,24 The following criteria were considered as exclusion criteria: patients undergoing unscheduled surgery, deep hypothermic circulatory arrest (DHCA), significant hepatorenal dysfunction (Child-Pugh Class B or C, with an estimated glomerular filtration rate <35 mL/min/1.73m2), presence of cardiogenic shock at the end of cardiopulmonary bypass (vasoactive inotropic score >40, cardiac index <2.2L/min·m2, mean arterial pressure <65mmHg),25 fluid overload at the end of cardiopulmonary bypass (inferior vena cava diameter > 21mm),26 abnormal baseline inflammatory indicators (interleukin-6 (IL-6) >10pg/mL, procalcitonin (PCT) >0.5 ng/mL, C-reactive protein (CRP) >10 mg/L),27 diagnosis of inflammatory immune diseases, infectious or tumor diseases, previous treatment with sivelestat, severe allergy or intolerance to sivelestat, and pregnancy.
Medical Intervention
All patients were enrolled within one year, and the control conditions were the same for all patients, including ventilation strategy, extubation time, and fluid management. Anesthesia induction and CBP was standardized. Following the surgical procedure, all patients were transferred to the intensive care unit (ICU) and extubated within 24 hours of the postoperative period. Starting from the first postoperative day (POD1) until the third postoperative day (POD3), After dissolving sivelestat in 0.9% sodium chloride injection, a daily dose of 4.8 mg/kg is diluted in 50 mL of 0.9% sodium chloride injection. The diluted solution is then placed in a sealed package and administered intravenously at a continuous rate of 0.2 mg/kg/h. Blood gas analyses were conducted upon admission to the ICU and repeated every 2 hours following the surgery. These blood gas analyses were used to optimize mechanical ventilation settings with Nellcor Puritan Bennett ventilators from Ireland and Dräger Savina ventilators from Drägerwerk AG & Co. KGaA in Lübeck, Germany. The mechanical ventilation parameters were set as follows: fraction of inspired O2 (FiO2) ranging from 0.4 to 0.9, positive end-expiratory pressure (PEEP) set between 4 and 10cmH2O, with a target PaO2/FiO2 ratio above 300mmHg, PaCO2 maintained between 35 and 45 mm Hg, and arterial lactate level below 2mmol/L, with a pH maintaining between 7.35 and 7.45. Upon admission to the ICU, as well as on the third (POD3) and fifth (POD5) postoperative days, blood samples were collected for the purpose of measuring various markers, including C-reactive protein (CRP), white blood cell (WBC) count, interleukin-6 (IL-6), and procalcitonin (PCT), among others.
Definition and Data Collection
The follow-up period for this study was concluded on September 7th, 2023. In the study cohort, patients were followed up for an average duration of 45.4±7.3 days (median: 40, IQR: 38–50), and all patients successfully completed the follow-up period. The primary outcome measure was a composite of adverse events, including 30-day mortality, extracorporeal membrane oxygenation (ECMO) use, acute lung injury/acute respiratory distress syndrome (ALI/ARDS), continuous renal replacement therapy (CRRT), intra-aortic balloon pump (IABP) support, among others. Secondary outcome measures included pneumonia incidence, occurrence of ventricular arrhythmias, and duration of mechanical ventilation, among others. The detailed variables are shown in tables below.
Statistical Analysis
The statistical analysis was performed using SPSS software, version 24. Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range), while discrete variables were represented as frequencies (n, %). The Student’s t-test was utilized to assess normally distributed continuous variables, while the Mann–Whitney U-test was applied for non-normally distributed continuous variables. Prior to conducting these tests, the Shapiro–Wilk test was used to confirm the normal distribution of the continuous variables. Categorical data were analyzed using either the chi-square test or Fisher’s exact test. Additionally, a repeated measures analysis of variance (ANOVA) was performed to compare differences between the two groups across multiple time points from the day before surgery until POD5.
To minimize bias in our findings, propensity score matching was employed. This approach allowed for a comparison between patients who received sivelestat and a control group with similar risk profiles. The propensity score was derived from a logistic regression model that included variables such as age, gender, left ventricular ejection fraction (LVEF), cardiopulmonary bypass (CPB), hypertension, chronic kidney disease (CKD), and stroke. Propensity score 1-to-1 matching was conducted using the nearest neighbor algorithm without replacement and a caliper set at 0.01. The standardized mean differences (SMD) were assessed before and after matching, with an absolute value of SMD <10% indicating post-match balance. Statistical significance was considered at a p-value less than 0.05. It is important to acknowledge the possibility of bias in our study findings.
Results
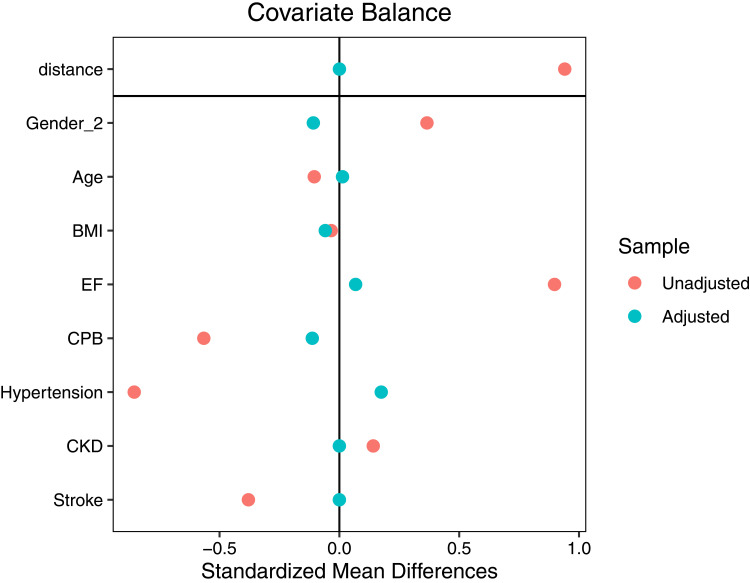
After performing propensity matching, Our study included a total of 80 patients, with 40 patients assigned to the sivelestat group and 40 patients allocated to the control group. The SMD of age, gender, LVEF, CPB, hypertension, CKD, and stroke were displayed in Figure 1. The absolute values of these variables’ SMDs were less than 10%. Therefore, these variables were found to be well-balanced.
Figure 1.
Gender_2: female.
Abbreviations: BMI, Body Mass Index; EF, Left ventricular ejection fraction; CPB, Cardiopulmonary bypass; CKD, Chronic kidney disease.
In the matched cohort, there were no differences in baseline and demographic variables between the two groups, as shown in Table 1. Adverse events, including 30-day mortality (n=4), ECMO (n=4), CRRT (n=7), IABP (n=2), ALI/ARDS (n=37), and stroke (n=1), occurred in 46 patients (6.41%) before propensity matching. In the pre-matched cohort, apart from ALI/ARDS (P<0.01), no significant differences were observed between the two groups for other events (P>0.05).
Table 1.
Characteristics in Pre- and After- Propensity Matching
| Variables | Pre-Propensity Matched | SMD | After-Propensity Matched | SMD | ||||
|---|---|---|---|---|---|---|---|---|
| Sivelestat (N=52) | Control (n=666) | P value | Sivelestat (n=40) | Control (n=40) | P value | |||
| Age (year) | 70.0 (60.0–77.0) | 60.0 (49.0–66.8) | 0.44 | 0.12 | 60.0 (50.0–66.7) | 56.5 (40.0–76.0) | 0.98 | 0.05 |
| Gender (n, %)* | 23,44.2% | 425,63.8% | <0.01 | 0.40 | 20,48.8% | 21,51.2% | 0.82 | 0.05 |
| BMI (kg/m2) | 23.6 (21.9–26.0) | 23.9 (22.4–26.0) | 0.56 | 0.08 | 23.8 (22.4–26.6) | 24.3 (23.0–26.2) | 0.62 | 0.18 |
| LVEF (%) | 58.0 (55.0–60.0) | 61.8 (53.6–65.2) | <0.01 | 0.84 | 59.8 (53.0–63.3) | 58.5 (55.0–60.0) | 0.55 | 0.06 |
| ePASP (mmHg) | 35.0 (28.0–45.0) | 38.7 (32.0–40.7) | 0.56 | 0.06 | 35.0 (28.0–44.5) | 36.0 (33.7–37.0) | 0.82 | 0.02 |
| EuroSCORE II (%) | 2.00 (1.2–3.4) | 1.3 (1.3–3.3) | 0.81 | 0.05 | 2.0 (1.2–3.4) | 1.33 (1.33–3.33) | 0.44 | 0.15 |
| STS-prolonged ventilation (%) | 7.5 (5.9–9.9) | 6.8 (4.4–11.5) | 0.11 | 0.01 | 7.0 (5.6–9.8) | 7.5 (6.1–11.0) | 0.49 | 0.28 |
| History (n, %) | ||||||||
| Diabetes | 4,7.7% | 81,12.2% | 0.34 | 0.15 | 4,10% | 4,10% | – | <0.01 |
| Hypertension | 16,30.8% | 473,71.1% | <0.01 | 0.88 | 15,37.5% | 15,37.5% | – | <0.01 |
| CKD | 1,1.9% | 0 | <0.01 | 0.20 | 0 | 0 | – | <0.01 |
| CLD | 0 | 0 | – | – | 0 | 0 | – | – |
| Immune diseases | 0 | 6,0.9% | 0.49 | 0.14 | 0 | 0 | – | – |
| Marfan syndrome | 0 | 2,0.3% | 0.69 | 0.08 | 0 | 0 | – | – |
| Stroke | 0 | 80,12.0% | <0.01 | 0.52 | 0 | 1,2.5% | 0.32 | 0.22 |
| Cancer | 1,1.9% | 6,0.9% | 0.47 | 0.09 | 1,2.5% | 0 | 0.32 | 0.22 |
| Smoking | 7,13.5% | 147,22.1% | 0.14 | 0.23 | 5,12.5% | 6,15.0% | 0.75 | 0.14 |
| Drinking | 6,11.5% | 105,15.8% | 0.42 | 0.12 | 4,10% | 4,10% | – | 0.07 |
| PCS | 6,11.5% | 73,11.0% | 0.90 | 0.02 | 5.12.5% | 5,12.5% | – | <0.01 |
| Previous PCI | 0 | 11,1.7% | 0.35 | 0.18 | 0 | 1,2.5% | 0.32 | 0.22 |
| β-blocker | 7,13.5% | 36,5.4% | 0.02 | 0.28 | 6,15% | 7,17.5% | 0.76 | 0.07 |
| ACEi/ARB | 4,7.7% | 124,18.6% | 0.04 | 0.33 | 3,7.5% | 5,12.5% | 0.46 | 0.17 |
| CCB | 14,26.9% | 159,23.9% | 0.62 | 0.07 | 11,27.5% | 9,22.5% | 0.61 | 0.12 |
| Diuretic | 44,84.6% | 44,6.6% | <0.01 | 2.52 | 33,82.5% | 29,72.5% | 0.29 | 0.24 |
| Clopidogrel | 4,7.7% | 11,1.7% | 0.03 | 0.30 | 3,7.5% | 1,2.5% | 0.31 | 0.23 |
| Ticagrelor | 0 | 0 | – | – | 0 | 0 | – | – |
| TCO (n,%) | 0.38 | 0.53 | 0.82 | 0.58 | ||||
| VR | 9,17.3% | 62,9.3% | 7,17.5% | 4,10% | ||||
| VR+VP | 11,21.2% | 176,26.5% | 10,25% | 16,40% | ||||
| VP | 27,51.9% | 353,53.1% | 19,47.5% | 13,32.5% | ||||
| CABG | 5,9.6% | 24,3.6% | 4,10% | 4,10% | ||||
| CABG+VR/P | 0 | 50,7.5% | 0 | 3,7.5% | ||||
| CPB (min) | 135.0 (113.8–176.2) | 173.0 (151.0–210.0) | <0.01 | 0.51 | 139.5 (121.8–188.2) | 159.0 (123.0–192.2) | 0.73 | 0.09 |
| Op- time (min) | 256.8 (203.3–324.1) | 270.0 (225.0–347.5) | 0.16 | 0.10 | 367.5 (228.8–350.0) | 304.7 (250.9–373.2) | 0.19 | 0.19 |
Notes: *Male; CLD: include chronic obstructive pulmonary disease, chronic interstitial lung diseases, chronic pneumonia.
Abbreviations: SMD, standardized mean difference; BMI, Body mass index; LVEF, Left ventricular ejection fraction; ePASP, estimated pulmonary artery systolic pressure by echo; CKD, Chronic kidney disease; CLD, Chronic lung disease; PCS, Previous cardiac surgery; PCI, Percutaneous Coronary Intervention; ACEi, Angiotensin-converting enzyme inhibitors; ARB, Angiotensin receptor blockers; CCB, Calcium channel blocker; TCO, Type of cardiac operation; VR, Valvular replacement; VP, Valvular plasty; CABG, Coronary artery bypass grafting; CPB, Cardiopulmonary bypass; Op-time, operation time.
Table 2 presents the postoperative outcomes for patients after propensity matching. Following propensity matching, there were no significant differences observed between the two groups in terms of primary outcomes, including 30-day mortality (P=0.32), ECMO (P=0.32), IABP (P=1.00), and re-operation (P=0.32). After propensity matching, differences between two groups were still observed in the ALI/ARDS.
Table 2.
Postoperative Outcomes in Propensity-Matched Patients
| Variable | Sivelestat (n=40) | Control (n=40) | P value |
|---|---|---|---|
| Primary outcomes (n, %) | |||
| 30-day mortality | 0 | 1,2.5% | 0.32 |
| ALI/ARDS | 0 | 22,55% | <0.01* |
| Cerebral vascular events | 0 | 1,2.5% | 0.32 |
| ECMO use | 0 | 1,2.5% | 0.32 |
| IABP use | 0 | 0 | – |
| CRRT use | 0 | 1,2.5% | 0.32 |
| Re-operation | 0 | 1,2.5% | 0.32 |
| Secondary outcomes (n, %) | |||
| Pneumonia | 0 | 15,37.5% | <0.01* |
| Re-intubation | 0 | 2,5% | 0.16 |
| Ventricular fibrillation | 0 | 3,7.5% | 0.08 |
| High arterial lactic > 48 hours | 0 | 8,20% | <0.01* |
| New-onset atrial fibrillation | 0 | 2,5% | 0.16 |
| Length of ICU stay (days) | 3.0 (2.2–4.0) | 5(2.0–70) | 0.05 |
| MV time (hours) | 8.0 (4.0–14.8) | 15.2 (14.0–16.4) | <0.01 |
Notes: The bold font indicates two outcome measures; *P<0.0001.
Abbreviations: ALI/ARDS, acute lung injury/acute respiratory distress syndrome; ECMO, Extracorporeal membrane oxygenation; IABP, Intra-aortic balloon pump; CRRT, Continuous renal replacement therapy; MV, Mechanical ventilation.
In the propensity-matched cohort, the analysis of secondary outcomes demonstrated significant differences between the sivelestat and control groups. Specifically, the sivelestat group exhibited lower incidence of pneumonia (P<0.01), shorter mechanical ventilation time (P<0.01), and a reduced duration of high arterial lactate (>2mmol/L) for more than 48 hours (P<0.01). Additionally, there was a trend towards a reduction in ventricular fibrillation (P=0.08) and a decrease in ICU length of stay (P=0.05) in both groups, suggesting potential benefits associated with sivelestat administration.
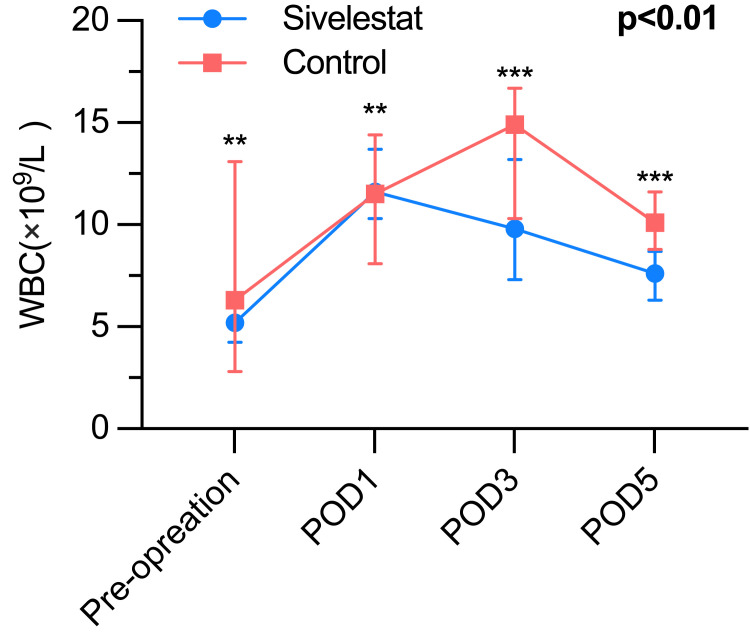
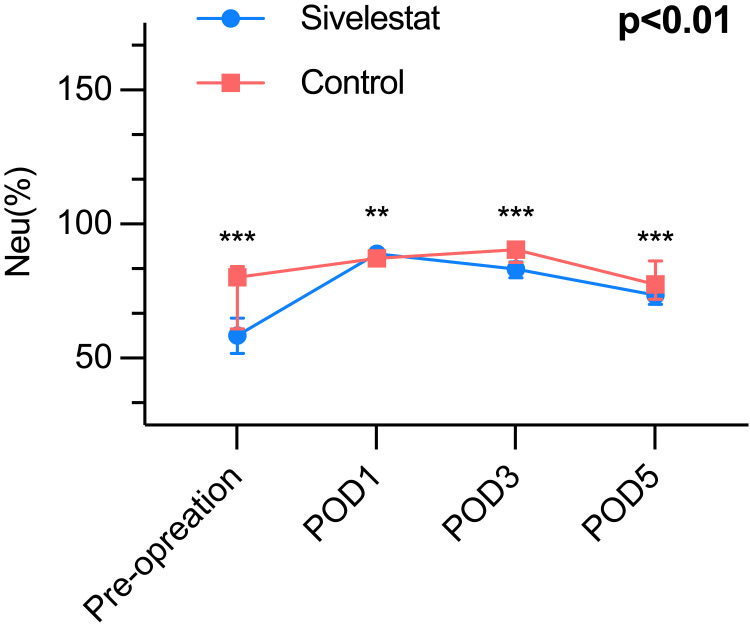
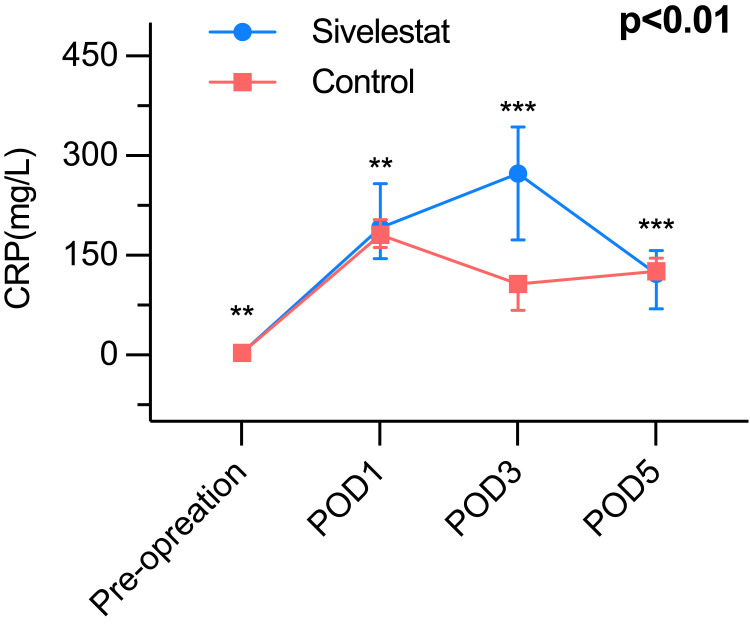
It presents compelling evidence that the sivelestat group experienced a noteworthy increase in the lowest oxygen index (PaO2/FiO2) throughout the period from postoperative day 1 to postoperative day 5, when compared to the control group (Table 3). The inflammatory biomarkers of the postoperative period, such as WBC count (Figure 2), neutrophil (Figure 3), and CRP (Figure 4) were greatly different (P<0.01) between the sivelestat and control group from pre-operation until POD5. Notably, no differences were observed in white blood cell (WBC) count, neutrophil levels, and C-reactive protein (CRP) levels between the two groups before the operation or on postoperative day 1 (POD1). However, the sivelestat group displayed significantly lower levels of interleukin-6 (IL-6) (P<0.01) and procalcitonin (PCT) (P<0.01) on both POD3 and POD5 compared to the control group. Nevertheless, there were no significant differences in IL-6 and PCT levels between the two groups throughout the pre-operation period until POD5.
Table 3.
PaO2/FiO2 and Biomarkers of Inflammation Within Postoperative 5 Days
| Variable | Sivelestat (n=40) | Control (n=40) | P value |
|---|---|---|---|
| Lowest PaO2/FiO2 during the period of POD5 (mmHg) | 272.1 (258.4–319.0) | 111.5 (84.5–183.5) | <0.01* |
| White cell count (×109/L) | <0.01* | ||
| Pre-operation | 5.2 (4.5–6.4) | 6.3 (2.8–13.1) | 0.60 |
| POD 1 | 11.6 (10.3–13.7) | 11.5 (8.1–14.4) | 0.38 |
| POD 3 | 9.8 (7.3–13.2) | 14.9 (10.3–16.7) | <0.01* |
| POD 5 | 7.6 (6.3–8.7) | 10.1 (8.8–11.6) | <0.01* |
| Neutrophil (%) | <0.01 | ||
| Pre-operation | 58.5 (51.7–65.0) | 80.2 (61.0–84.1) | <0.01 |
| POD 1 | 89.0 (86.2–90.1) | 87.2 (86.1–89.5) | 0.86 |
| POD 3 | 83.2 (80.0–85.9) | 90.4 (85.8–91.3) | <0.01 |
| POD 5 | 73.5 (70.0–78.6) | 77.5 (72.0–86.3) | <0.01 |
| C-reactive protein (mg/L) | <0.01 | ||
| Pre-operation | 3.1 (0.8–4.3) | 3.5 (2.7–4.1) | 0.42 |
| POD 1 | 191.4 (145.2–257.6) | 181.0 (162.0–204.0) | 0.41 |
| POD 3 | 272.9 (173.6–343.3) | 107.0 (67.0–116.0) | <0.01* |
| POD 5 | 122.3 (69.3–157.2) | 125.8 (119.0–146.2) | <0.01 |
| Interleukin-6 (pg/mL) | 0.546 | ||
| Pre-operation | 2.8 (2.2–4.6) | 3.4 (2.2–4.2) | 0.63 |
| POD 1 | 183.1 (113.6–387.7) | 256.0 (183.0–301.0) | 0.34 |
| POD 3 | 46.2 (36.4–57.6) | 72.0 (64.0–89.0) | <0.01* |
| POD 5 | 15.15 (11.6–28.8) | 28.0 (24.0–31.0) | <0.01* |
| Procalcitonin (ng/mL) | 0.584 | ||
| Pre-operation | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.47 |
| POD 1 | 8.7 (1.6–18.1) | 12.0 (4.0–15.0) | 0.11 |
| POD 3 | 1.7 (0.9–5.6) | 5.0 (5.0–8.0) | <0.01* |
| POD 5 | 0.6 (0.1–3.7) | 1.0 (0.6–3.0) | <0.01 |
Note: *P<0.0001.
Abbreviations: POD1, The first postoperative day; POD3, The third postoperative day; POD5, The fifth postoperative day.
Figure 2.
The White blood cell, P<0.01; ***P< 0.01, **P> 0.01.
Figure 3.
The Neutrophils, P<0.01; ***P< 0.01, **P> 0.01.
Figure 4.
The C reactive protein, P<0.01; ***P< 0.01, **P> 0.01.
Discussion
Postoperative acute lung injury (ALI), known for its significant impact on morbidity and mortality, is a severe inflammatory reaction triggered by cardiopulmonary bypass (CPB).3–5 Neutrophil activation plays a key role in initiating this process.16 Imbalances in neutrophil elastase and its endo genous protease inhibitors have been implicated in lung tissue destruction.16 Sivelestat, a neutrophil elastase inhibitor, has shown promise in preventing ALI during planned cardiac surgery.6,14 However, the effectiveness of sivelestat in high-risk patients undergoing scheduled cardiac surgery, who are susceptible to ALI, remains unknown. Our study revealed that prophylactic administration of sivelestat may reduce the incidence of postoperative ALI in high-risk patients undergoing scheduled cardiac surgery, particularly in those at risk of acute kidney injury (AKI). These findings suggest that sivelestat has the potential to protect pulmonary function by mitigating CPB-induced inflammatory damage.
Activated neutrophils play a crucial role in the pathogenesis of ALI/ARDS following open heart surgery by adhering to pulmonary vascular endothelial cells through adhesion molecule expression, leading to endothelial cell damage.5,9–11 Additionally, activated neutrophils contribute to the production of elastase, superoxide, and cytokines, exacerbating the inflammatory response.5,9–11,13 The consequences of these processes include increased pulmonary capillary permeability and interstitial edema in the lungs.15,16 Ultimately, ALI/ARDS are severe complications that can arise following open heart surgery. They have the potential to result in mortality rates of 15% to 28% in individuals receiving CPB.3–6 An agent that can inhibit neutrophils or neutrophil elastase could be extremely beneficial in preventing adverse outcomes associated with ALI. Previous studies have shown that sivelestat, a neutrophil elastase inhibitor, can significantly enhance postoperative outcomes in patients undergoing cardiopulmonary bypass (CPB).6,14,18,22 However, these studies primarily focused on patients with congenital heart defects6,14 or had small sample sizes, involving only 14 patients.22 Given the potential for severe ALI-related outcomes in patients at high risk of postoperative ALI, it is crucial to investigate the prophylactic administration of sivelestat in this population. It is indeed surprising that no studies have investigated the use of sivelestat as a prophylactic measure in high-risk patients for ALI following cardiac surgery. Previous studies have demonstrated the usefulness of prolonged ventilation (>3% STS) for predicting postoperative ALI.28–30 Therefore, in this study, we utilized STS-prolonged ventilation to ascertain “the high risk of ALI”. We prophylactically administered sivelestat to patients who transferred to the ICU, regardless of whether they developed ALI, based on their classification as a “high-risk cohort”. Meanwhile, we found that among the 718 patients before matching, The incidence rate of ALI/ARDS in the experimental group is 8%, and the control group is 59%, resulting in an overall incidence rate of 55.1%. However, the incidence rate of acute lung injury after cardiac surgery is generally estimated to be around 30–50%.28 Therefore, using this criterion to define high-risk patients has a certain degree of indicative significance. Our study discovered that sivelestat has the potential to decrease the prevalence of ALI. It has bridged the gap for “high-risk” patients. Regarding the selection of exclusion criteria, our center published an article < The neutrophil elastase inhibitor, sivelestat, attenuates acute lung injury in patients with cardiopulmonary bypass > in 2022,17 which is aimed to evaluate the effect of sivelestat on pulmonary protection in patients with ALI after emergent cardiovascular surgery. Therefore, based on the above study, the main subjects of our study are patients undergoing scheduled cardiac surgery. And due to the various inflammatory reactions may caused by DHCA and fluid overload, which renders our study non-specific.31 Therefore, we have included them in our exclusion criteria.
The PaO2/FiO2 ratio is a well-established parameter used to assess respiratory function, as cited in reference.32 For the definition of moderate ALI/ARDS, a PaO2/FiO2 ratio of less than or equal to 200mmHg and greater than 100mmHg was employed. The severe form of ALI/ARDS was characterized by a PaO2/FiO2 ratio of less than or equal to 100mmHg.32 In our study, the median lowest PaO2/FiO2 is 272.1 (258.4–319.0) mmHg in the sivelestat group, and the median lowest PaO2/FiO2 is 111.5 (84.5–183.5) mmHg. The results revealed a significant improvement in the PaO2/FiO2 ratio in the sivelestat group compared to the control group (P<0.01). Additionally, the incidence of ALI/ARDS was lower in the sivelestat group compared to the control group, as indicated in Table 2. These findings align with the conclusions reported in previous literature.32 Furthermore, to ensure the highest level of homogeneity between the two groups, we employed the propensity score matching technique. From Table 1, it is evident that the propensity matching resulted in good balance between the groups (SMD<0.05).
It enhances the credibility of our conclusion. Our study demonstrated that the administration of sivelestat resulted in decreased levels of inflammatory biomarkers, including white blood cell count (WBC), neutrophils (Neu), and C-reactive protein (CRP), in the sivelestat group. These findings further support the conclusion that anti-neutrophil therapies have the potential to mitigate the severity of ALI. Hence, Sivelestat, as an anti-inflammatory drug, holds promise in potentially yielding beneficial effects on organ protection in patients at a high risk of ALI undergoing cardiac surgery. Our study provided additional evidence in support of this hypothesis. However, as seen from Table 2, most of the primary outcomes in the experimental group are reported as zero. This is because we excluded some data after propensity matching. Therefore, further confirmation through a larger sample randomized controlled trial is necessary to evaluate the impact of Sivelestat on clinical endpoints.
In conclusion, our study suggests that the prophylactic administration of sivelestat may have a positive impact on postoperative outcomes for patients at high risk of ALI after scheduled cardiac surgery. These findings indicate that sivelestat has the potential to protect pulmonary function from inflammatory damage resulting from CPB.
Study Limitation
Our study design is limited to a single center, which inherently carries disadvantages associated with an observational study, making it susceptible to bias. While we attempted to mitigate bias through the use of propensity score matching, it is important to note that unobservable factors that influence treatment assignment and outcomes may remain unaccounted for. This could introduce potential statistical flaws and hidden biases. Additionally, the exclusion of a substantial number of patients during the analysis may lead to increased statistical errors. Therefore, the prognostic value of sivelestat should be further evaluated in future studies. Furthermore, there is a lack of published clinical trials reporting the effects of sivelestat on gastrointestinal function. Although our study did not observe any gastrointestinal disturbances, confirmation through prospective clinical trials is necessary. The postoperative respiratory status is significantly affected by pre-existing lung diseases such as chronic obstructive pulmonary disease or interstitial pneumonia, and preoperative lung function. However, due to the invasive nature of blood gas analysis, which is not a routine preoperative test at our institution, baseline lung function data were not available for this study. This could potentially introduce bias to the results to some extent.
It is important to acknowledge these limitations in our assessment.
Funding Statement
The clinical medical center of Nanjing cardiovascular diseases (CZLB506-2020); National Natural Science Foundation of China (82300459, 82241212, 82270346); The healthy science and technological development foundation of Nanjing (YKK22096), and Clinical Trial Projects of the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2023-LCYJ-MS-26).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Statement
Ethical approval for this retrospective observational study was obtained from the Medical Ethics Committee of Nanjing Drum Tower Hospital, Nanjing University School of Medicine (2022-102-01), and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Yu-Xian Tang, Zhi-Wei Fan and Jing Li are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Ann Thorac Surg. 1997;63(1):277–284. doi: 10.1016/s0003-4975(96)01061-2 [DOI] [PubMed] [Google Scholar]
- 2.Miller BE, Levy JH. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11(3):355–366. doi: 10.1016/s1053-0770(97)90106-3 [DOI] [PubMed] [Google Scholar]
- 3.Journois D, Israel-Biet D, Pouard P, et al. High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology. 1996;85(5):965–976. doi: 10.1097/00000542-199611000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119(3):884–888. doi: 10.1378/chest.119.3.884 [DOI] [PubMed] [Google Scholar]
- 5.Asimakopoulos G, Smith PL, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68(3):1107–1115. doi: 10.1016/s0003-4975(99)00781-x [DOI] [PubMed] [Google Scholar]
- 6.Toyama S, Hatori F, Shimizu A, Takagi T. A neutrophil elastase inhibitor, sivelestat, improved respiratory and cardiac function in pediatric cardiovascular surgery with cardiopulmonary bypass. J Anesth. 2008;22(4):341–346. doi: 10.1007/s00540-008-0645-z [DOI] [PubMed] [Google Scholar]
- 7.Kiessling AH, Guo FW, Gokdemir Y, et al. The influence of selective pulmonary perfusion on the inflammatory response and clinical outcome of patients with chronic obstructive pulmonary disease undergoing cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2014;18(6):732–739. doi: 10.1093/icvts/ivu062 [DOI] [PubMed] [Google Scholar]
- 8.Ranucci M, Ballotta A, La Rovere MT, Castelvecchio S, Frati G; Surgical, Clinical Outcome Research G. Postoperative hypoxia and length of intensive care unit stay after cardiac surgery: the underweight paradox? PLoS One. 2014;9(4):e93992. doi: 10.1371/journal.pone.0093992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86(6):845–857. doi: 10.1016/S0022-5223(19)39061-0 [DOI] [PubMed] [Google Scholar]
- 10.Tamiya T, Yamasaki M, Maeo Y, Yamashiro T, Ogoshi S, Fujimoto S. Complement activation in cardiopulmonary bypass, with special reference to anaphylatoxin production in membrane and bubble oxygenators. Ann Thorac Surg. 1988;46(1):47–57. doi: 10.1016/s0003-4975(10)65851-1 [DOI] [PubMed] [Google Scholar]
- 11.Dreyer WJ, Michael LH, Millman EE, Berens KL. Neutrophil activation and adhesion molecule expression in a canine model of open heart surgery with cardiopulmonary bypass. Cardiovasc Res. 1995;29(6):775–781. doi: 10.1016/S0008-6363(96)88612-3 [DOI] [PubMed] [Google Scholar]
- 12.Diegeler A, Doll N, Rauch T, et al. Humoral immune response during coronary artery bypass grafting: a comparison of limited approach, ”off-pump” technique, and conventional cardiopulmonary bypass. Circulation. 2000;102(19 Suppl 3):III95–III100. doi: 10.1161/01.cir.102.suppl_3.iii-95 [DOI] [PubMed] [Google Scholar]
- 13.Dreyer WJ, Phillips SC, Lindsey ML, et al. Interleukin 6 induction in the canine myocardium after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2000;120(2):256–263. doi: 10.1067/mtc.2000.108168 [DOI] [PubMed] [Google Scholar]
- 14.Nomura N, Asano M, Saito T, Nakayama T, Mishima A. Sivelestat attenuates lung injury in surgery for congenital heart disease with pulmonary hypertension. Ann Thorac Surg. 2013;96(6):2184–2191. doi: 10.1016/j.athoracsur.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 15.Ohwada S, Tomizawa N, Takahashi T, et al. Effects of a specific neutrophil elastase inhibitor (ONO-5046 Na) and neutrophil depletion using a G-1 column on lung reperfusion injury in dogs. Transplant Proc. 1996;28(3):1826–1827. [PubMed] [Google Scholar]
- 16.Ng CS, Wan S, Yim AP, Arifi AA. Pulmonary dysfunction after cardiac surgery. Chest. 2002;121(4):1269–1277. doi: 10.1378/chest.121.4.1269 [DOI] [PubMed] [Google Scholar]
- 17.Pan T, Tuoerxun T, Chen X, et al. The neutrophil elastase inhibitor, sivelestat, attenuates acute lung injury in patients with cardiopulmonary bypass. Front Immunol. 2023;14:1082830. doi: 10.3389/fimmu.2023.1082830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morimoto N, Morimoto K, Morimoto Y, et al. Sivelestat attenuates postoperative pulmonary dysfunction after total arch replacement under deep hypothermia. Eur J Cardiothorac Surg. 2008;34(4):798–804. doi: 10.1016/j.ejcts.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki K, Hiramatsu Y, Homma S, Sato S, Shigeta O, Sakakibara Y. Sivelestat reduces inflammatory mediators and preserves neutrophil deformability during simulated extracorporeal circulation. Ann Thorac Surg. 2005;80(2):611–617. doi: 10.1016/j.athoracsur.2005.02.038 [DOI] [PubMed] [Google Scholar]
- 20.Goto Y, Hiramatsu Y, Ageyama N, et al. Rolipram plus Sivelestat inhibits bone marrow-derived leukocytic lung recruitment after cardiopulmonary bypass in a primate model. J Artif Organs. 2019;22(1):44–52. doi: 10.1007/s10047-018-1071-0 [DOI] [PubMed] [Google Scholar]
- 21.Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag. 2014;10:621–629. doi: 10.2147/TCRM.S65066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryugo M, Sawa Y, Takano H, et al. Effect of a polymorphonuclear elastase inhibitor (sivelestat sodium) on acute lung injury after cardiopulmonary bypass: findings of a double-blind randomized study. Surg Today. 2006;36(4):321–326. doi: 10.1007/s00595-005-3160-y [DOI] [PubMed] [Google Scholar]
- 23.Kuplay H, Bayer Erdogan S, Bastopcu M, Karpuzoglu E, Er H. Performance of the EuroSCORE II and the STS score for cardiac surgery in octogenarians. Turk Gogus Kalp Damar Cerrahisi Derg. 2021;29(2):174–182. doi: 10.5606/tgkdc.dergisi.2021.21403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056 [DOI] [PubMed] [Google Scholar]
- 25.Pan T, Long GF, Chen C, et al. Heparin-binding protein measurement improves the prediction of myocardial injury-related cardiogenic shock. BMC Cardiovasc Disord. 2020;20(1):124. doi: 10.1186/s12872-020-01406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellicori P, Platz E, Dauw J, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail. 2021;23(5):703–712. doi: 10.1002/ejhf.2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Han X, Pan T, et al. Evaluation of low-dose colchicine in patients with cardiopulmonary bypass: study protocol for a randomised controlled trial. BMJ Open. 2022;12(2):e050577. doi: 10.1136/bmjopen-2021-050577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JP, Shahian DM, Badhwar V, et al. The society of thoracic surgeons 2021 adult cardiac surgery risk models for multiple valve operations. Ann Thorac Surg. 2022;113(2):511–518. doi: 10.1016/j.athoracsur.2021.03.089 [DOI] [PubMed] [Google Scholar]
- 29.Orfanoudaki A, Giannoutsou A, Hashim S, Bertsimas D, Hagberg RC. Machine learning models for mitral valve replacement: a comparative analysis with the society of thoracic surgeons risk score. J Card Surg. 2022;37(1):18–28. doi: 10.1111/jocs.16072 [DOI] [PubMed] [Google Scholar]
- 30.Schrutka L, Rohmann F, Binder C, et al. Discriminatory power of scoring systems for outcome prediction in patients with extracorporeal membrane oxygenation following cardiovascular surgery. Eur J Cardiothorac Surg. 2019;56(3):534–540. doi: 10.1093/ejcts/ezz040 [DOI] [PubMed] [Google Scholar]
- 31.Malbrain M, Langer T, Annane D, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10(1):64. doi: 10.1186/s13613-020-00679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]