Abstract
The efficient assembly of newly replicated and repaired DNA into chromatin is essential for proper genome function. Based on genetic studies in Saccharomyces cerevisiae, the histone chaperone anti-silencing function 1 (Asf1) has been implicated in the DNA repair response. Here, the human homologs are shown to function synergistically with human CAF-1 to assemble nucleosomes during nucleotide excision repair in vitro. Furthermore, we demonstrate that hAsf1 proteins can interact directly with the p60 subunit of hCAF-1. In contrast to hCAF-1 p60, the nuclear hAsf1 proteins are not significantly associated with chromatin in cells before or after the induction of DNA damage, nor specifically recruited to damaged DNA during repair in a bead-linked DNA assay. A model is proposed in which the synergism between hAsf1 and CAF-1 for nucleosome formation during DNA repair is achieved through a transient physical interaction allowing histone delivery from Asf1 to CAF-1.
INTRODUCTION
The ordered assembly of nucleosomes (the fundamental repeating unit of chromatin) is believed to be mediated by histone chaperones (Kaufman and Almouzni, 2000). The human protein complex CAF-1, composed of the three subunits p150, p60 and p48, is the best characterized histone chaperone by virtue of its unique ability to promote nucleosome assembly specifically onto newly replicated DNA in vitro (Kaufman et al., 1995). CAF-1 also mediates nucleosome assembly coupled to nucleotide excision repair (NER) (Gaillard et al., 1996, 1997) and the repair of single-strand breaks (Moggs et al., 2000).
Recently, a new complex, RCAF, was identified in Drosophila based on its ability to synergize with CAF-1 to assemble nucleosomes onto newly replicated DNA in vitro (Tyler et al., 1999). RCAF consists of the evolutionarily conserved protein anti-silencing function 1 (Asf1) and histones H3 and H4. Asf1 was first identified in Saccharomyces cerevisiae based on its ability to derepress silencing upon overexpression (Le et al., 1997), and Asf1p synergizes with the yeast CAF-1 homolog in nucleosome assembly onto replicated DNA (Sharp et al., 2001). Genetic studies in S. cerevisiae reveal that, whereas cac1 mutants (CAC1 encodes the largest CAF-1 subunit) (Kaufman et al., 1997) are mildly sensitive to UV irradiation, an asf cac1 mutant is highly sensitive compared to either single mutant (Tyler et al., 1999). Asf1 mutants are also sensitive to DNA damaging agents that cause single- and double-strand breaks (Tyler et al., 1999). Furthermore, Asf1p interacts physically and functionally with the DNA damage checkpoint kinase Rad53 (Emili et al., 2001; Hu et al., 2001). These studies suggest a role for Asf1 in the DNA damage response and, potentially, in nucleosome assembly during DNA repair events in yeast.
Although studies in Drosophila and yeast have provided valuable clues to Asf1 function, in mammals the role of Asf1 connected to DNA replication or repair has not been explored. Uniquely, human Asf1 exists in two variant forms, Asf1a and Asf1b (Munakata et al., 2000; Sillje and Nigg, 2001). These variants share 71% homology and, distinct from yeast Asf1p, are phosphorylated in a replication-dependent manner by Tousled-like kinases (Tlks) (Sillje and Nigg, 2001). Given the potential role of Asf1 in maintaining genome integrity in human cells, we examined whether hAsf1 proteins act in nucleosome assembly associated with DNA repair and how synergism between hCAF-1 and hAsf1 for this pathway may be mediated.
RESULTS
Human Asf1 cooperates with CAF-1 in nucleosome assembly coupled to NER
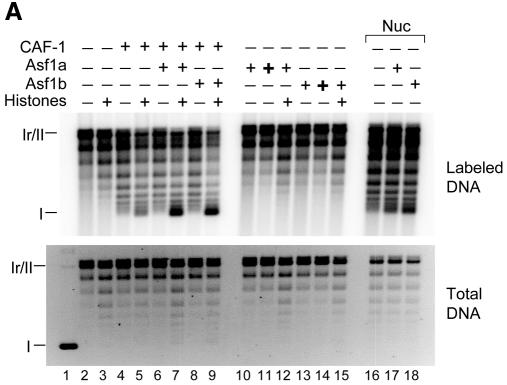
A human cell-free system was used that supports NER on UV-damaged plasmids but is incompetent for nucleosome assembly due to a deficiency in CAF-1 p60 and p150 (Martini et al., 1998). Nucleosome assembly could be promoted by the addition of exogenous hCAF-1. Nucleosome formation was followed by a supercoiling assay in which the accumulation of supercoils is proportional to the number of nucleosomes formed on circular DNA. Incubation of UV-damaged DNA with cytosolic extract alone promoted the repair of UV lesions, as evidenced by the incorporation of radiolabeled nucleotide during the DNA synthesis step of NER in relaxed/nicked circular DNA (form Ir/II), but did not lead to the production of supercoiled molecules (form I) (Figure 1A, lane 2). By titrating recombinant CAF-1 into the reaction (data not shown), a concentration was found that allowed limited accumulation of supercoiled DNA molecules preferentially on repaired material (lane 4, labeled DNA versus total DNA). Under these limiting conditions, the addition of Asf1a or Asf1b together with purified histones led to a synergistic increase in nucleosome assembly specifically on repaired DNA (compare lanes 7 and 9 to 5, 6 and 8). Synergism was also observed when nuclear extract was used as the CAF-1 source. In the absence of CAF-1, however, Asf1 proteins could not promote nucleosome assembly, even at high concentrations or in the presence of purified histones (lanes 10–15). Partial MNase digestion of DNA assembled under similar conditions confirmed that the observed supercoiling was due to the deposition of regularly spaced nucleosomes (Figure 1B). The detection of tri- and tetra-nucleosomal fragments from DNA assembled in the presence of Asf1 and CAF-1, as compared to equivalent concentrations of CAF-1 alone, confirmed the more efficient nucleosome assembly. We conclude that Asf1a and Asf1b can promote nucleosome assembly during NER and that this activity is strictly CAF-1 dependent.
Fig. 1. Human Asf1 acts synergistically with CAF-1 to assemble nucleosomes during NER. (A) UV-damaged DNA substrate was incubated in a human cell-free system alone or complemented with core histones, CAF-1 (16 ng) or nuclear extract (2.5 µg protein). To these reactions, Asf1a or Asf1b (60 ng +, 250 ng +) was added. Lane 1 contains untreated UV-damaged DNA. Radiolabel incorporation due to repair synthesis was visualized by autoradiography (labeled DNA) and total DNA by ethidium bromide staining (total DNA). Relaxed/nicked circular DNA (Ir/II) and supercoiled DNA (I) are indicated. (B) Analysis by MNase digestion of nucleosomal arrays formed on repaired DNA (labeled DNA) in a human cell-free system alone or complemented with histones, CAF-1 (16 ng +, 48 ng +), Asf1a or Asf1b (60 ng) or nuclear extract as control (7.5 µg protein). Three digestion times (45 s and 3 and 6 min) are shown for each reaction. Positions corresponding to mono-, di-, tri- and tetra-nucleosomal DNA are indicated. Nuclear extract produced larger MNase digestion products, suggesting the involvement of additional factors in this extract. (C) Reactions as in (A) but to which Asf1a, Asf1b (60 ng) or nucleoplasmin (60 ng and 120 ng) was added.
We next asked whether synergistic activity with CAF-1 could be exerted by other histone chaperones. Nucleoplasmin, a histone chaperone that promotes histone deposition onto DNA in a manner uncoupled to DNA synthesis (Dilworth et al., 1987), did not further stimulate CAF-1-dependent assembly of repaired DNA into chromatin (Figure 1C). However, nucleoplasmin and hAsf1 proteins functioned with comparable efficiency in a histone chaperone assay to promote histone loading onto DNA (data not shown). We conclude that the observed synergism between Asf1 and CAF-1 reflects a specifically coordinated pathway.
Asf1 interacts with the p60 subunit of CAF-1
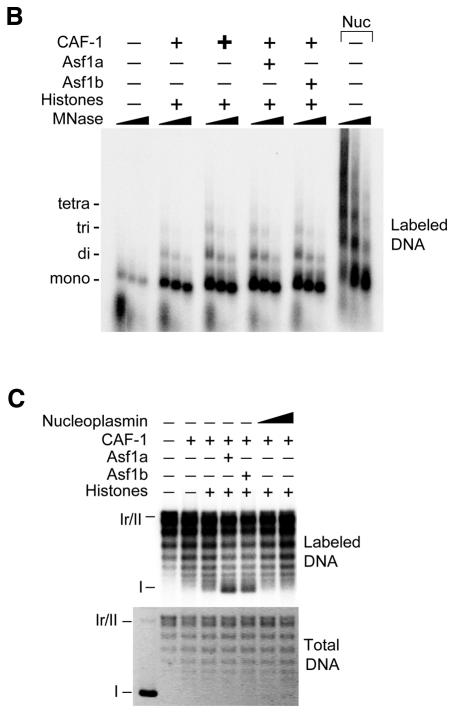
To test potential interactions between hCAF-1 and hAsf1, we performed pull-down assays from human cell-free extract using GST–Asf1 fusion proteins. GST–Asf1a and GST–Asf1b, but not GST alone, efficiently pulled down the largest CAF-1 subunit p150, as well as both the phosphorylated and unphosphorylated forms of p60 (Figure 2A, top). The p48 subunit also interacted specifically with Asf1; however, the fraction bound was relatively small, perhaps reflecting the existance of p48 in other complexes distinct from CAF-1. In a reciprocal experiment, CAF-1 was immunoprecipitated from cell-free extract to which recombinant Asf1a or Asf1b had been added by using an antibody against hp60. Both Asf1a and Asf1b co-immunoprecipitated with native p60, and the interactions were stable to 1 M NaCl (Figure 2A, bottom). These data demonstrate that hAsf1 could interact with endogenous CAF-1 complex in a cell-free extract. To determine which CAF-1 subunit(s) mediated the interaction, we performed pull-down assays containing purified His6–Asf1a or His6–Asf1b and the individual CAF-1 subunits p150, p60 or p48 that were produced by in vitro coupled transcription–translation (IVT). We found that Asf1a and Asf1b interacted with p60, but not with p150 or p48, whereas nickel resin alone showed no interactions, indicating that Asf1 interacts with CAF-1 via the p60 subunit (Figure 2B). We reported recently that hAsf1 is phosphorylated by Tlks (Sillje and Nigg, 2001). Pull-down experiments with Asf1 phosphorylated by Tlk were performed in parallel, but Asf1 phosphorylation had no detectable effect on the interaction in this assay. The interaction between CAF-1 p60 and Asf1 was direct, as shown by pull-down assays using GST–Asf1 and recombinant hp60 (Figure 2C). We conclude that hAsf1 interacts with hCAF-1 via a direct interaction with the p60 subunit independently of their phosphorylation states.
Fig. 2. Asf1a and Asf1b interact directly with CAF-1 p60 independently of their phosphorylation state. (A) Top panel: GST–Asf1a, GST–Asf1b or GST alone was used to pull down native CAF-1 from human cell-free extract; input, bound and unbound fractions of CAF-1 p150, p60 and p48 subunits were detected by western blotting using antibodies against p150, p60 and p48. Bottom panel: anti-p60 immune serum (I) or non-immune serum (N) was used to immunoprecipitate native CAF-1 p60 from human cell-free extract to which purified Asf1 was added; input, unbound, bound and 1 M NaCl-resistant fractions of Asf1 were detected by western blotting using anti-Asf1. (B) Purified His6–Asf1 proteins, either phosphorylated or unphosphorylated, were immobilized on a Ni–NTA resin. Resins were incubated with CAF-1 subunits p60, p150 or p48 that were produced and labeled with [35S]methionine by IVT. Input (In), unbound (U) and bound (B) fractions were visualized by autoradiography. (C) GST–Asf1a, GST–Asf1b or GST alone was used to pull down recombinant CAF-1 p60. Input, bound, unbound and 1 M NaCl-resistant fractions of p60 were detected by western blotting using anti-p60.
Asf1 sub-nuclear localization is sensitive to Triton extraction
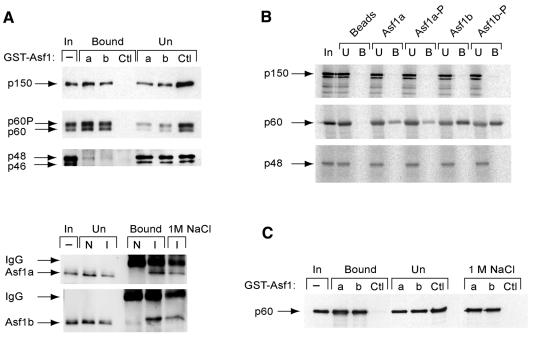
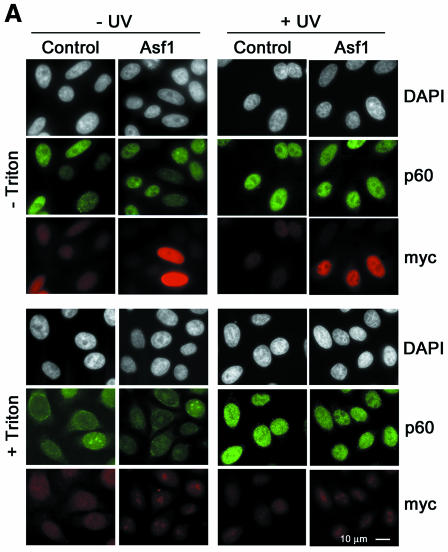
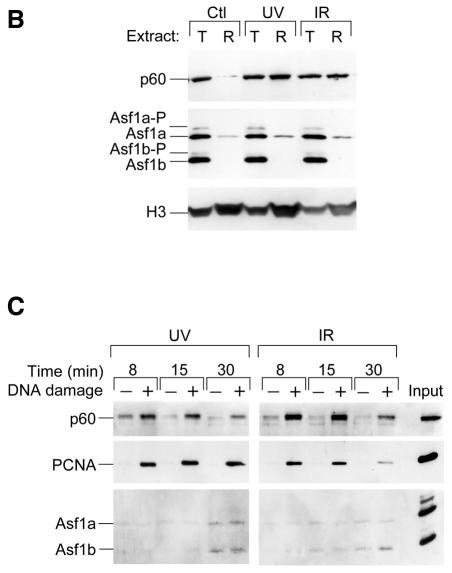
To study the sub-cellular localization of Asf1 before and after UV DNA damage, mycAsf1 proteins were transiently expressed in HeLa cells and compared with native CAF-1 p60 by immunofluorescence. Under these conditions, Asf1a distributed homogeneously throughout the nucleus without major changes upon UV irradiation (Figure 3A, top). Consistent with previous reports (Martini et al., 1998), p60 staining varied in intensity among individual nuclei and increased uniformally after UV irradiation (Figure 3A, top), while Triton extraction before cell fixation revealed an insoluble sub-cellular fraction of p60 that increased strikingly in all nuclei upon UV irradiation (Figure 3A, bottom) and is thought to be associated with repair sites. In contrast, Triton extraction completely removed detectable Asf1a, even at high UV doses (Figure 3A, bottom) and longer incubation times (data not shown). Similar results were found for Asf1b (data not shown). We conclude that mycAsf1 proteins exist in a soluble nuclear fraction that is not tightly associated with chromatin.
Fig. 3. Human Asf1 is not significantly associated with chromatin in human cells before or after exposure to DNA damage, nor specifically recruited to repaired DNA <>in vitro. (A) Sub-cellular localization of ectopically expressed mycAsf1 and native CAF-1 p60 by immunofluorescence. HeLa cells, untransfected or expressing mycAsf1a, were untreated or UV irradiated and, after 30 min, fixed with formaldehyde (− Triton) or Triton extracted before fixation (+ Triton). mycAsf1a and p60 were visualized using anti-myc and anti-p60 antibody. DAPI staining located all nuclei. (B) Western blotting of native Asf1 sub-nuclear fractions in cell-free extracts. HeLa cells were untreated or irradiated with UV or IR and harvested after 2 h. Proteins from whole cells (total, T; 1 × 105 cells) and Triton-extracted cells (resistant, R; 8 × 105 cells) were analyzed by western blotting. CAF-1 p60, Asf1 and histone H3 (as loading control) were detected on the same filter. (C) Assay for recruitment of proteins to damaged DNA in vitro. Bead-linked DNA containing UV- or IR-induced damage, or undamaged control, was incubated in human cell-free extract. Specific proteins bound to DNA were analyzed by western blotting using antibodies against CAF-1 p60, PCNA and Asf1 on the same filter.
Endogenous Asf1 could be analyzed in extracts made from HeLa cells before or after UV damage, and total cell extracts were compared with extracts made from Triton-extracted cells. Consistent with previous reports (Martini et al., 1998), an insoluble p60 fraction markedly increased after UV irradiation, whereas total p60 was unchanged. For Asf1, a pattern of phosphorylated and unphosphorylated forms was found in total cell extracts (Figure 3B) (Sillje and Nigg, 2001) that did not change afer UV irradiation. A Triton-resistant fraction of endogenous Asf1a was consistently found by western blotting, but this insoluble fraction did not change significantly after UV irradiation (Figure 3B), neither at high UV doses nor at earlier or later times (data not shown). Given that an asf1 mutant in yeast displays sensitivity to agents that cause DNA double-strand breaks (Tyler et al., 1999), we also examined lysates from cells exposed to ionizing radiation (IR), which produces single- and double-strand DNA breaks. Notably, the Triton-resistant p60 fraction increased in response to IR (Figure 3B). In contrast, no change was found in the expression or phosphorylation of Asf1, nor in the Asf1a Triton-resistant fraction. Taken together, we conclude from these results that the majority of hAsf1 proteins are present in a soluble fraction and that this distribution is not changed substantially after UV or IR exposure.
Asf1 is not specifically recruited to repaired DNA in vitro
To test whether Asf1 is recruited to damaged DNA during DNA repair in vitro, linearized DNA substrate linked to paramagnetic beads and containing either no damage, UV-induced damage or IR-induced damage was incubated in a human cell-free system (Moggs et al., 2000). Bound proteins were eluted over time and analyzed by western blotting. In this assay, PCNA and CAF-1 p60 were immediately recruited specifically to DNA containing UV lesions and were associated with DNA over 30 min (Figure 3C). In contrast, Asf1a and Asf1b bound weakly and non-specifically at all times examined. We conclude that PCNA and CAF-1, but not Asf1, are specifically associated with damaged DNA during NER. When bead-linked DNA containing IR-induced DNA damage was incubated with extract, PCNA and CAF-1 p60 were recruited rapidly and specifically but dissociated slowly over 30 min (Figure 3C). Again, Asf1a and Asf1b bound weakly and non-specifically, indicating they were not recruited to sites of single- and double-strand break repair. We conclude that, although hAsf1 proteins synergize with hCAF-1 in nucleosome assembly coupled to DNA repair, they are not recruited directly to damaged DNA during this function.
DISCUSSION
In yeast, defects in Asf1p significantly increase the sensitivity of cac-1 mutants to UV irradiation, and asf1 mutants alone are hypersensitive to a range of DNA damaging agents (Tyler et al., 1999). Although the viability of an Asf1Cac1 strain illustrates that both proteins are dispensable for DNA replication, these studies also suggested, importantly, that each protein plays a role in NER, presumably through chromatin assembly (Tyler et al., 1999). In this study of hAsf1, we have provided direct biochemical evidence that hAsf1 and hCAF-1 can act together to assemble nucleosomes during NER.
In our assays, Asf1a and Asf1b functioned synergistically with CAF-1 to assemble nucleosomes during NER. Given that nucleoplasmin failed to amplify CAF-1 activity, this phenomenon appears to be highly coordinated and specific for Asf1 and CAF-1. The corresponding proteins in Drosophila (Tyler et al., 1999) and S. cerevisiae (Sharp et al., 2001) display synergistic activity in nucleosome assembly coupled to simian virus 40 (SV40) replication. Thus, our results demonstrate that synergism between these proteins is (i) highly conserved throughout evolution and (ii) active in nucleosome assembly coordinated to two different DNA transactions—replication and repair. We infer, therefore, that this mechanism serves an important function. It remains unclear why two Asf1 variants exist in humans, but we note that a Triton-resistant Asf1a fraction found by western blotting is the first hint of a distinction between the two proteins. Yeast Asf1p contains a C-terminal acidic stretch that is absent from Drosophila and human Asf1; given the lack of a yeast Tlk ortholog, we suggested that this acidic region provides the structural equivalent to that produced by Tlk phosphorylation of hAsf1 (Sillje and Nigg, 2001). To date, however, a functional role for Asf1 phosphorylation in a repair-coupled nucleosome assembly has been difficult to assess in our experimental system.
Asf1 was entirely dependent upon CAF-1 to promote nucleosome assembly during NER. Asf1 could function independently only at high protein concentrations, facilitating histone loading onto DNA independently of repair to produce core particles (data not shown; Munakata et al., 2000). A similar requirement for CAF-1 was demonstrated using yeast Asf1p and Cac-1 in a chromatin assembly assay coupled to SV40 replication (Sharp et al., 2001). We conclude that Asf1 lacks the capacity to promote nucleosome assembly directly on DNA during replication and NER, instead requiring a partner protein to perform this role. In yeast, the hypersensitivity of asf1, but not cac1, mutants to DNA double-strand break inducers suggests that Asf1 is involved in a distinct assembly pathway associated with double-strand break repair (Tyler et al., 1999). It is possible that, in this context, Asf1 acts alone to promote nucleosome assembly or, alternatively, cooperates with another, as yet unidentified, protein.
Our discovery of a direct interaction between hAsf1 and hCAF-1 through the p60 subunit provides a physical mechanism by which synergism between these proteins in nucleosome assembly can be mediated. This interaction appears to be evolutionarily conserved, as Drosophila Asf1 was recently shown to interact with only one of apparently two distinct forms of dCAF-1 that differ in the size of the middle subunit (Tyler et al., 2001). In contrast to CAF-1 p60, we found that Asf1 was not significantly associated with chromatin in cells after UV or IR irradiation, nor specifically recruited to damaged DNA during repair. These results predict that Asf1 interacts with CAF-1 in the context of their functional synergism at a step that occurs away from DNA, before the recruitment of CAF-1 to DNA. Alternatively, Asf1 may interact transiently, such that it cannot be found tightly associated with chromatin. Phosphorylation of hAsf1 did not affect its interaction with CAF-1, indicating that Tlk does not regulate Asf1 activity at this level. In yeast, Rad53 associates with Asf1p and may thereby regulate Asf1p activity (Emili et al., 2001; Hu et al., 2001). It will be important to determine whether the human Rad53 homolog, Chk2, or other protein interactors of Asf1 or CAF-1 modulate their interaction.
Speculation
Based on the results presented here, we propose that hAsf1 interacts directly with CAF-1 via its p60 subunit to facilitate the delivery of histones H3 and H4 from Asf1 to CAF-1. By continually supplying CAF-1 with new histones, either near or at sites of DNA replication and repair, Asf1 could facilitate a limited number of CAF-1 molecules to assemble nucleosomes successively and rapidly, thereby imparting efficiency to the process. Furthermore, the specific delivery of histones to CAF-1 by Asf1 could provide a means to monitor histone usage in chromatin assembly and, in this way, integrate information related to progress in S phase and DNA damage.
METHODS
DNA templates
PBluescript (pBS) was irradiated with 500 J/m2 UV-C (254 nm) (Gaillard et al., 1996). For Asf1 expression plasmids, PCR was used to make N-terminal fusions in pBS–myc and fusions cloned into pRcCMV (Invitrogen).
Analysis of repair and chromatin assembly in vitro
HeLa cytosolic extract was complemented with CAF-1 or nuclear extract to promote chromatin assembly and DNA repair (Martini et al., 1998). This mixture was incubated with UV-damaged pBS, His6–hAsf1 and/or core histones [120 ng (H3–H4)2 tetramers and H2A–H2B dimers purified from chicken erythrocytes (Simon and Felsenfeld, 1979)] for 3 h at 37°C. Nucleosome assembly was followed by supercoiling or MNase digestion (Gaillard et al., 1996).
Purified proteins
His6-tagged and GST-tagged hAsf1a/b and mycTlk1 were produced as described previously (Sillje et al., 1999; Sillje and Nigg, 2001). CAF-1 (Verreault et al., 1996) and nucleoplasmin were generous gifts from A. Verreault (ICRF, South Mimms, Hertfordshire, UK) and B. Edde (CRBM, Montpellier, France). Bacterially expressed His6–hp60 (Marheineke and Krude, 1998) was purified under native conditions on TALON Affinity Resin (Clontech). CAF-1 subunits were produced using TnT-coupled reticulocyte lysate system (Promega).
Protein interactions
For pull-down assays, 2 µg GST–Asf1 or GST bound to glutathione-Sepharose 4B resin (Pharmacia-Amersham) was incubated with 30 µl nuclear extract or 500 ng His6–p60 in 300 µl binding buffer [50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.05% NP40, 1 mM DTT, 0.1 mM PMSF, 1% BSA, 10 µg/ml leupeptin and pepstatin (PIs)] for 1 h at 4°C. For co-immunoprecipitation, cytosolic (300 µg protein) and nuclear (50 µg protein) extracts were incubated with 150 ng His6–hAsf1 and anti-p60 antibody or non-immune serum in 300 µl LS Buffer (20 mM HEPES pH 7.8, 100 mM NaCl, 5 mM potassium acetate, 0.5 mM MgCl2, 0.5 mM DTT, PIs) for 1 h at 4°C. Protein-A agarose beads (Boehringer Mannheim) were added and incubated for 1 h at 4°C. For pull-down assays with CAF-1 subunits, His6–hAsf1 proteins, phosphorylated or not by mycTlk (Sillje et al., 1999), were coupled to Ni–NTA resin (Qiagen) in buffer B (50 mM Tris pH 7.5, 10 mM MgCl2, 5 mM CaCl2, 150 mM NaCl, 0.5% NP40, 1% BSA, PIs) for 30 min at 4°C, washed, and equal volumes IVT and resin incubated 45 min at 4°C. All resins were washed in reaction buffer that, where noted, contained 1 M NaCl.
Assay for proteins recruited during DNA repair
Linearized pUC19 coupled to Dynabeads M-280 (Dynal SA) was irradiated at 5 J/cm2 UV-C (254 nm) (Moggs et al., 2000) or 150 Gy (4.4 Gy/min) IR from a 137Cs source in an IBL 637 irradiator (CIS Biointernational). Reactions containing bead-linked DNA and cytosolic and nuclear extract were as described previously (Moggs et al., 2000).
Cell extractions
Asynchronous HeLa cells were irradiated with 30 J/m2 UV-C (254 nm) (Martini et al., 1998) or 10 Gy (1.8 Gy/min) IR. Extractions were performed after 2 h on cells untreated or treated with 0.5% Triton X-100 (Martini et al., 1998).
Cell transfections and immunofluorescence
HeLa cells were transfected with CMV-mycAsf1 using Effectene (Qiagen), incubated for 36 h and untreated or irradiated with 3000 J/m2 UV-C (254 nm). After 30 min, cells were fixed with 2% paraformaldehyde or extracted with 0.5% Triton X-100 before fixation, permeabilized and incubated with 9E10 monoclonal anti-myc and Ab1 anti-p60 antibodies in blocking buffer (Martini et al., 1998). Primary antibodies were visualized by fluorescein- or Texas-Red-conjugated secondary antibody (Jackson Immunoresearch Laboratories) and an epifluorescence microscope (Leica) equipped with a chilled charge-coupled device camera (Hamamatsu Photonics).
Antibodies
Rabbit polyclonal antibodies included Ab1 against CAF-1 p60 (Marheineke and Krude, 1998), 573 against CAF-1 p150 (Quivy et al., 2001), RbAp46/RbBp48 against CAF-1 p48 (A. Verreault), FL-261 against PCNA (Santa Cruz), anti-hAsf1 (Sillje and Nigg, 2001) and C-16 goat polyclonal for histone H3 (Santa Cruz). Primary antibodies were detected using horseradish-peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) and a Supersignal detection kit (Pierce).
Supplementary data
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank A. Verreault, C. Keller and B. Edde for the generous gifts of hCAF-1, pET23a-hp60 and nucleoplasmin, and P. Ridgway for critical comments. J.A.M. was supported by a Chateaubriand fellowship and a grant from Fonds de Recherche Aventis (ex-Hoechst Marion Roussel) 98ONC038, and D.B.K. was supported by EEC-RTN (to G.A.). The team of G.A. is supported by La Ligue Contre le Cancer, Euratom, EU-RTN and LRC of CEA.
REFERENCES
- Dilworth S.M., Black, S.J. and Laskey, R.A. (1987) Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell, 51, 1009–1018. [DOI] [PubMed] [Google Scholar]
- Emili A., Schieltz, D.M., Yates, J.R. III and Hartwell, L.H. (2001) Dynamic interaction of DNA damage checkpoint protein Rad53 with chromatin assembly factor Asf1. Mol. Cell, 7, 13–20. [DOI] [PubMed] [Google Scholar]
- Gaillard P.H., Martini, E.M., Kaufman, P.D., Stillman, B., Moustacchi, E. and Almouzni, G. (1996) Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell, 86, 887–896. [DOI] [PubMed] [Google Scholar]
- Gaillard P.H., Moggs, J.G., Roche, D.M., Quivy, J.P., Becker, P.B., Wood, R.D. and Almouzni, G. (1997) Initiation and bidirectional propagation of chromatin assembly from a target site for nucleotide excision repair. EMBO J., 16, 6281–6289 [Erratum, EMBO J., 16, 6613]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Alcasabas, A.A. and Elledge, S.J. (2001) Asf1 links Rad53 to control of chromatin assembly. Genes Dev., 15, 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P.D. and Almouzni, G. (2000) DNA replication, nucleotide excision repair and nucleosome assembly. In Elgin, S. and Workman, J. (eds), Chromatin Structure and Gene Expression. Oxford University Press, Oxford, UK, pp. 24–48.
- Kaufman P.D., Kobayashi, R., Kessler, N. and Stillman, B. (1995) The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell, 81, 1105–1114. [DOI] [PubMed] [Google Scholar]
- Kaufman P.D., Kobayashi, R. and Stillman, B. (1997) Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev., 11, 345–357. [DOI] [PubMed] [Google Scholar]
- Le S., Davis, C., Konopka, J.B. and Sternglanz, R. (1997) Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast, 13, 1029–1042. [DOI] [PubMed] [Google Scholar]
- Marheineke K. and Krude, T. (1998) Nucleosome assembly activity and intracellular localization of human CAF-1 changes during the cell division cycle. J. Biol. Chem., 273, 15279–15286. [DOI] [PubMed] [Google Scholar]
- Martini E., Roche, D.M., Marheineke, K., Verreault, A. and Almouzni, G. (1998) Recruitment of phosphorylated chromatin assembly factor 1 to chromatin after UV irradiation of human cells. J. Cell Biol., 143, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moggs J.G., Grandi, P., Quivy, J.P., Jonsson, Z.O., Hubscher, U., Becker, P.B. and Almouzni, G. (2000) A CAF-1–PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol., 20, 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata T., Adachi, N., Yokoyama, N., Kuzuhara, T. and Horikoshi, M. (2000) A human homologue of yeast anti-silencing factor has histone chaperone activity. Genes Cells, 5, 221–233. [DOI] [PubMed] [Google Scholar]
- Quivy J.P., Grandi, P. and Almouzni, G. (2001) Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J., 20, 2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J.A., Fouts, E.T., Krawitz, D.C. and Kaufman, P.D. (2001) Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr. Biol., 11, 463–473. [DOI] [PubMed] [Google Scholar]
- Sillje H.H. and Nigg, E.A. (2001) Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr. Biol., 11, 1068–1073. [DOI] [PubMed] [Google Scholar]
- Sillje H.H., Takahashi, K., Tanaka, K., Van Houwe, G. and Nigg, E.A. (1999) Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J., 18, 5691–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R.H. and Felsenfeld, G. (1979) A new procedure for purifying histone pairs H2A + H2B and H3 + H4 from chromatin using hydroxylapatite. Nucleic Acids Res., 6, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J.K., Adams, C.R., Chen, S.R., Kobayashi, R., Kamakaka, R.T. and Kadonaga, J.T. (1999) The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature, 402, 555–560. [DOI] [PubMed] [Google Scholar]
- Tyler J.K., Collins, K.A., Prasad-Sinha, J., Amiott, E., Bulger, M., Harte, P.J., Kobayashi, R. and Kadonaga, J.T. (2001) Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol. Cell. Biol., 21, 6574–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreault A., Kaufman, P.D., Kobayashi, R. and Stillman, B. (1996) Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell, 87, 95–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.