Abstract
Dementia affects almost 50 million adults worldwide, and a major cause of death and disability. Hypertension is a leading risk factor for dementia, both Alzheimer disease and Alzheimer disease related dementias. Although this association is well-established, the mechanisms underlying hypertension-induced cognitive decline remain poorly understood. By exploring the mechanisms mediating the detrimental effects of hypertension on the brain, studies have aimed to provide therapeutic insights and strategies on how to protect the brain from the effects of blood pressure elevation. In this review, we focus on the mechanisms contributing to the cerebrovascular adaptions to elevated blood pressure and hypertension-induced microvascular injury. We also assess the cellular mechanisms of neurovascular unit dysfunction, focusing on the premise that cognitive impairment ensues when the dynamic metabolic demands of neurons are not met due to neurovascular uncoupling, and summarize cognitive deficits across various rodent models of hypertension as a resource for investigators. Despite significant advances in antihypertensive therapy, hypertension remains a critical risk factor for cognitive decline, and several questions remain about the development and progression of hypertension induced cognitive impairment.
Keywords: Cerebral small vessel disease, hypertension, cognitive decline, dementia, Alzheimer’s disease, neurovascular unit
INTRODUCTION
Understanding the mechanistic relationship between cognitive decline and vascular health is essential, especially as human life expectancy increases and dementia remains a leading cause of death and disability1. Nearly half the adults in the United States are diagnosed with hypertension2, making it one of the most prevalent vascular risk factors for cognitive impairment and dementia. The association between hypertension and risk of dementia is well established3, yet the mechanisms underlying this association remain under investigation. Studies to date suggest that hypertension not only induces cerebrovascular adaptations that are detrimental to the brain, but it also has a direct impact on the cerebral vasculature and neurovascular unit4. Understanding both the molecular and cellular mechanisms underlying this damage is essential to identify novel therapeutic targets and interventions, and ultimately prevent permanent brain damage and cognitive decline.
This review highlights the impact of hypertension on the neurovasculome5, the entire extracranial and intracranial vasculature and associated cells pertaining to the skull, brain, and meninges, focusing on potential mechanisms of neuronal injury and cognitive decline. A mechanistic discussion of the cerebrovascular adaptations, in both the extracranial and intracranial vasculature, and microvascular injury in response to elevated blood pressure is presented to understand how hypertension alters the brain vasculature. Then, the neurovascular unit (NVU) is examined in detail to explore the effects of hypertension on each specific cell type. Although important and highly relevant, the intersection between hypertension and classical Alzheimer’s disease pathology (amyloid deposition and tau tangles) has been previously discussed4,6, and is thus not included in the present review. Finally, we provide a summary of cognitive deficits in selected rodent models of hypertension and potential associated mechanisms as a tool for future investigation.
CEREBROVASCULAR ADAPTATIONS IN LARGE ARTERIES AND ARTERIOLES
The brain is a major target of end-organ damage in hypertension, and alterations affecting the cerebral vasculature may lead to vascular insufficiency and neuronal dysfunction3. Chronic elevations of blood pressure increase pulsatile stress on the cerebral vasculature, and as a result they will undergo significant adaptation to protect downstream microvessels and maintain adequate cerebral blood flow (CBF) regulation7. The cerebral vasculature will undergo inward remodeling, a rearrangement of the vascular smooth muscle cells (VSMC) that reduces lumen diameter, and vascular hypertrophy, an increase in vascular wall thickness resulting from VSMC proliferation and increased cell volume8. Several studies support a role for angiotensin II (AngII) signaling, a hormone involved in hypertension, as an important contributing mechanism to cerebrovascular remodeling and hypertrophy9. Although oxidative stress alone is not sufficient to induce remodeling10, NADPH oxidase 2 (Nox2)-derived radicals induced by AngII type 1 receptor (AT1R) activation are an important contributing mechanism to cerebrovascular remodeling11. However, other factors may also be important, including altered VSMC mechanotransduction mechanisms12, mineralocorticoid signaling13 or calcium-activated chloride channel function14.
Hypertension is also a common co-morbidity with the reduced distensibility, and compliance of vasculature known as arterial stiffening15. Although this process can occur throughout the body, it is particularly detrimental in the aorta and carotid arteries which are essential to delivery of oxygen and nutrients to the brain15. Elevated arterial pressure causes mechanical stress on the vasculature, resulting in the elastin fragmentation, collagen deposition, and subsequent increase in vascular stiffness9. Thus, arterial stiffening hinders the process of healthy continuous blood flow by exposing the smaller, less compliant cerebral capillaries to high velocity pulse waves of blood16. Dynamic cytoskeletal and cell-matrix interactions which regulate VSMC function and elasticity may also play a role in arterial stiffening, and interestingly these alterations are suggested to be AT1R-dependent17. Importantly, human studies have associated aortic stiffening and resulting increased pulse wave velocity with cognitive impairment and neurodegenerative biomarkers18. Research on the mechanisms behind these associations is limited, but animal studies suggest that the reduced compliance of large arteries reduce resting CBF16, disrupt the blood-brain barrier (BBB)16, and induce marked cerebral gliosis mediated by oxidative stress19. However, the molecular mechanisms of neuronal dysfunction remain to be uncovered.
Lastly, hypertension is also associated with atherosclerosis, and particularly atherosclerosis in the Circle of Willis, which results in cerebral hypoperfusion and may be an indirect way hypertension contributes to AD20. Of note, these adaptations in large arteries and arterioles have significant effects on the downstream vasculature and thus make relevant contributions to cognitive decline.
MICROVASCULAR INJURY
Cerebral small vessel disease (CSVD) is a multifaceted vascular disease that impacts arterioles, capillaries, and small veins supplying deep structures and white matter of the brain, and represents the most prevalent vascular risk factor for dementia21. CSVD is typically characterized in brain MRIs by white matter lesions, lacunar infarcts, enlarged perivascular spaces, and cerebral microbleeds (Fig. 1)21. White matter lesions are densities identified on FLAIR MRIs that are characterized by decreased vascular density, vessel wall thickening, increased vessel tortuosity, and plasma proteins21. These lesions typically result in demyelination of neurons, gliosis, and loss of fibers and oligodendrocytes21. There are several hypotheses on the etiology of white matter lesions, including flow-dependent mechanisms such as ischemia, vasculopathy, reduced white matter perfusion, and flow-independent mechanisms such as loss of pericyte coverage and BBB breakdown21,22, inflammation, and activation of endothelial cells within capillaries21. Hypertension, which is an important risk factor for white matter lesions21, causes increases in blood vessel fibrosis and altering of the structural integrity of arterioles and capillaries9. Lacunes are also an MRI-defined feature of CSVD highly associated with hypertension and are characterized as small deep brain infarcts caused by occlusions of branches of large cerebral arteries, including the middle cerebral artery and the Circle of Willis23. These infarcts typically appear in the deep white matter and contain necrotic waste and have evidence of gliosis, myelin loss, and neuronal loss23. Enlarged perivascular spaces are also a neuroimaging marker of CSVD. Perivascular spaces are cerebrospinal fluid (CSF) filled spaces surrounding the penetrating arterioles and are important for drainage of interstitial fluids24. While the primary cause of enlarged perivascular spaces is not known, both animal and human studies suggest inflammation may play an important role24.
Figure 1. Cerebral small vessel disease manifestations in hypertension.
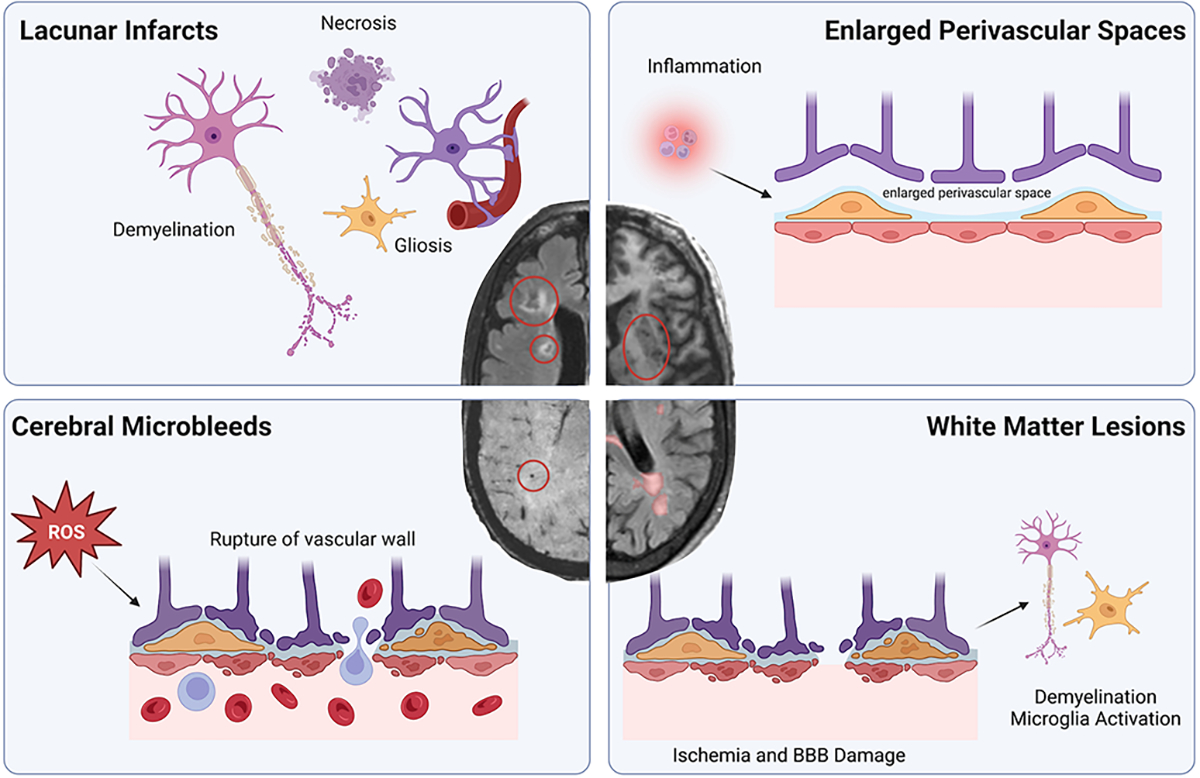
White matter lesions are visualized as white densities on FLAIR MRIs, and may result from ischemia and breakdown of the blood-brain barrier (BBB) leading to loss of oligodendrocytes, demyelination of neurons, and microglia activation. Lacunar infarcts are found on FLAIR MRIs and contain necrotic waste and have evidence of gliosis, myelin loss, and neuronal loss. Enlarged perivascular spaces are CSF filled spaces that can be seen on T2-weighted MRIs. Inflammation results in the enlargement of these spaces. Cerebral microbleeds, visualized as small circular depositions of blood in SWI MRIs. Studies suggest increased oxidative stress leads to weakening of the microvessels allowing them to rupture in response to the increased pressure. FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; BBB, blood brain barrier; CSF, cerebral spinal fluid; ROS, reactive oxygen species, SWI, susceptibility weighted imaging.
Hypertension is also a major risk factor for cerebral microbleeds25,26. Although the mechanisms leading to cerebral microbleeds are still under investigation, their pathogenesis is thought to be initiated by oxidative stress and inflammation weakening the structural integrity of the microvessels and thus allowing them to rupture25. Furthermore, hypertension-induced microbleeds are also worsened by aging27 and amyloid pathology28. Cerebral microbleeds are associated with worse cognitive function and the mechanisms may involve local brain injury and long-lasting inflammation29.
Microvascular rarefaction refers to the reduction of arteriolar and capillary density. Functional rarefaction refers to reversible constriction and reduction of capillaries, while structural rarefaction is an irreversible loss of arterioles and capillaries30. It is hypothesized that long-term, functional rarefaction precedes the permanent structural changes30. Indeed, arteriolar and capillary rarefaction is observed in many animal models of hypertension31 and in hypertensive patients, specifically at the earlier stages32. The mechanisms underlying cerebral microvascular rarefaction in hypertension are complex and remain under investigation but have been suggested to be a consequence of the transmission of increased pressure to the brain microvessels6, and particularly pre-capillary arterioles and post-capillary venules are sensitive to the increased resistance in hypertension. Endothelial cell dysfunction may directly contribute to rarefaction of cerebral vessels6, but also indirectly by reducing cross-talk between endothelial cells and pericytes thus inducing loss of pericytes and ultimately reduction in capillary density33. This may lead to the formation of string vessels, or collapsed remnants of basement membrane that have no endothelial cells34. Interestingly, venular rarefaction has been reported in the retinal vasculature during malignant hypertension35. Additional supporting evidence of the multifactorial pathogenesis come from studies indicating that hypertension-induced cerebral microvessel rarefaction is exacerbated by aging31, but may be rescued by exercise36 and reducing lipid levels37. Of note, the CRUCIAL study (evaluation of microvascular rarefaction in vascular cognitive impairment and heart failure) is expected to report first results in 2025 and is planning to develop novel non-invasive measures for detection of cerebral microvascular rarefaction38, which will contribute to furthering our understanding of hypertension-induced microvascular rarefaction and its contribution to cognitive impairment.
NEUROVASCULAR UNIT DYSFUNCTION
The brain has a high and dynamic metabolic demand that requires tight regulation of CBF throughout the system. Brain, vascular, and perivascular cells work together as a unit to maintain the homeostatic environment of the brain. Neurovascular coupling is known as the ability of the cerebral vasculature to increase local CBF to regions with enhanced neuronal activity and is essential to the maintenance of proper neuronal function39. Importantly, the bidirectional communication between the vasculature and neurons is an important physiological mechanism that may be impaired in dementia. The complex nature of the NVU in health has been previously reviewed39, thus in this section we will focus on the hypertension-induced effects on the NVU (Fig. 2) and their potential association with cognitive impairment. This is based on the hypothesis that cognitive impairment occurs when the metabolic demands of neurons are not met due to neurovascular uncoupling40,41. Neurovascular uncoupling in hypertension most commonly presents as a reduction or absence of vasodilation in response to neuronal activity, although in acute brain injury can also present as vasoconstriction leading to a reduction in local CBF in response to neural activity (inverse neurovascular coupling)42. Interestingly, a recent study described a prolonged inverse hemodynamic response in stroke-prone salt-sensitive spontaneously hypertensive rats43. In this review, we will discuss all cells involved with cerebral arterioles, capillaries, and venules as members of the NVU.
Figure 2. Hypertension impairs the function of the neurovascular unit.
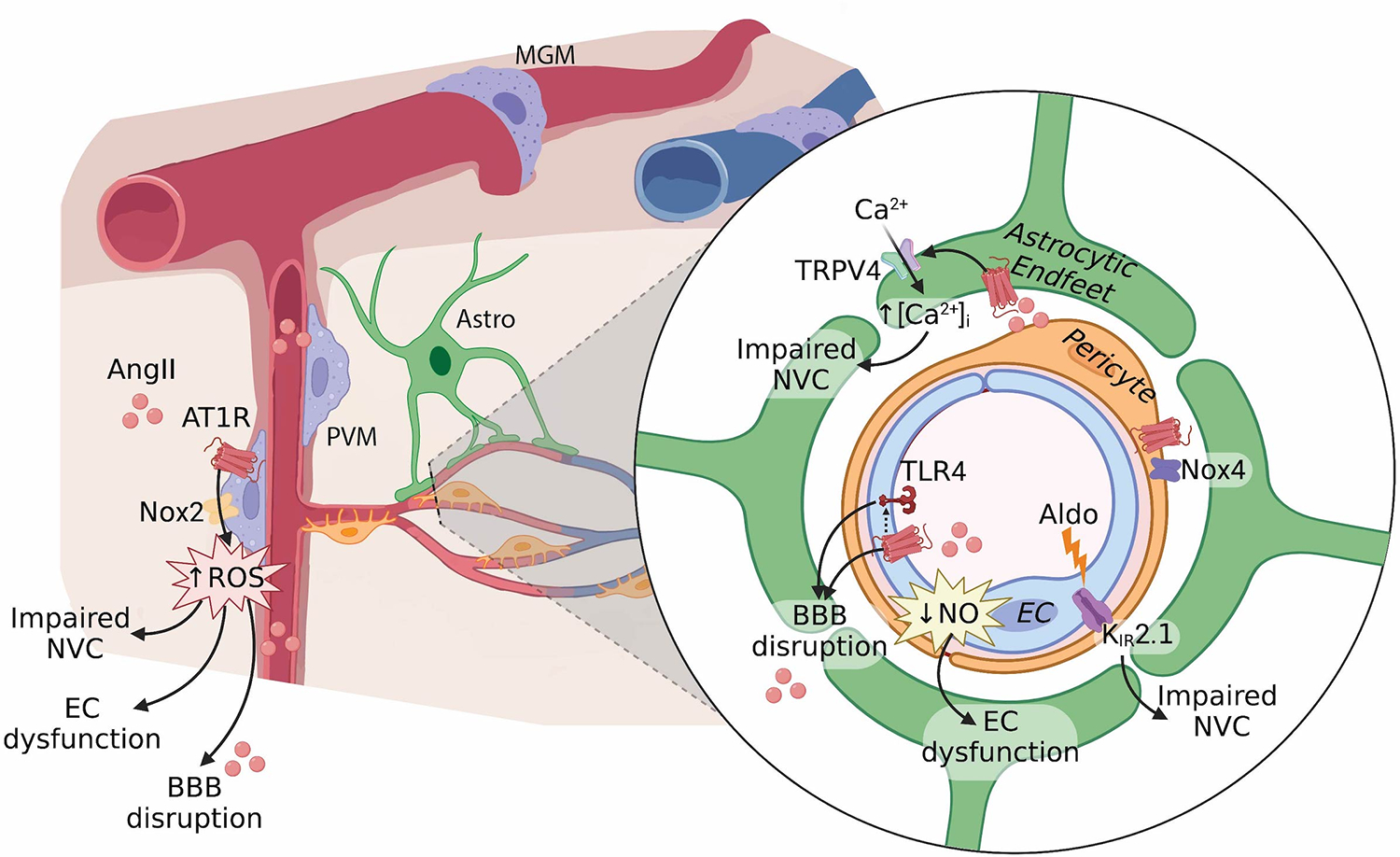
Hypertension affects all cell-types of the neurovascular unit via various mechanisms resulting in endothelial cell (EC) dysfunction, BBB disruption, and impaired neurovascular coupling (NVC). Endothelial dysfunction may result from reduced nitric oxide (NO) bioavailability, resulting from impaired eNOS function and increased ROS production by perivascular macrophages (PVM). Angiotensin II (AngII) type 1 receptor (AT1R) activation in both EC and PVM, and a potential interaction with toll-like receptor 4 (TLR4) in EC, contribute to disruption of the blood-brain barrier (BBB). Neurovascular coupling is impaired by PVM-derived ROS, aldosterone (Aldo)-induced damage of KIR2.1 channels and endothelial hyperpolarization, as well as altered calcium signaling in astrocytic endfeet. Pericytes express Nox4 which is upregulated by AngII and may contribute to vascular inflammation. AngII, angiotensin II; KIR2.1, inwardly rectifying potassium channel 2.1; Nox, NADPH oxidase; TRPV4, transient receptor potential vanilloid 4; Nox2, NADPH oxidase 2.
Endothelial cells
Endothelial cells participate in CBF regulation by modulating vasomotor tone through the release of vasoactive signals, including both constrictor and relaxing factors44. Hypertension has profound effects on endothelial-mediated vasodilation8, particularly associated with reduced nitric oxide (NO) bioavailability7. Experimental studies have found that endothelial NO synthase (eNOS) function is impaired in various models of hypertension, ranging from reduced expression, mislocalization, modulation of activating and inhibitory phosphorylation sites, and eNOS uncoupling; thus resulting in reduced NO production45. Furthermore, superoxide may scavenge NO, thus vascular oxidative stress further limits NO bioavailability8,44. Finally, although studies have suggested a link between endothelial dysfunction and cognitive impairment46, the question remains whether this association is mediated by a direct effect of CBF or via correlation with CSVD severity47.
Endothelial cells are also important in sensing and transmitting neurovascular signals during neurovascular coupling39. Neural activity is postulated to activate endothelial KIR2.1 channels, resulting in endothelial cell hyperpolarization, and retrograde propagation of vasodilation48. Increased aldosterone, induced by long-term treatment with an angiotensin receptor blocker (ARB), may damage KIR2.1 signaling in hypertension, resulting in impaired capillary-to-arteriolar signaling49 contributing to neurovascular uncoupling. However, ARB use is not detrimental to cognitive function, therefore this mechanism may not be widespread in hypertensive cognitive decline. On the other hand, the recent description of a prevalent continuum of renin-independent aldosterone production that parallels hypertension severity in essential hypertensive patients raises the possibility that this mechanism may be more relevant than previously recognized. Given the regional heterogeneity observed in brain endothelial cells both at the level of vascular segments50 and brain regions, future investigation should evaluate the effects of hypertension, and associated molecules (Ang II, aldosterone), on different vascular segments as well as across brain regions.
Endothelial cells also form the first layer of the BBB, a unique property of the brain vasculature regulating the bidirectional transport of molecules between the brain and the blood45. BBB disruption in hypertension has been suggested to be mediated by AngII51 and reactive oxygen species52, rather than the increase in systolic pressure itself51,53. AngII in the circulation activates cerebral endothelial AT1R leading to tight junction remodeling, increased vesicular transport, and BBB disruption53. However, studies have also shown that venules are a key site of BBB disruption during inflammation and are particularly sensitive to increases in pressure in both acute and chronic hypertension54,55. In humans, BBB breakdown is an early biomarker of cognitive impairment56, specifically BBB breakdown in the hippocampus worsened mild cognitive impairment57. Although BBB disruption is present in some patients with hypertension58, and may be associated with changes in daily blood pressure profile59, it has not been widely reported as a key feature of hypertension. Whether this is a result of the difficulty to evaluate the effect of hypertension in the presence of other comorbidities, or a true indication of the low prevalence of BBB disruption in the human patient population remains to be determined.
Mural cells
Smooth muscle cells are present in cerebral arteries and arterioles, and are important for the maintenance of cerebral autoregulation - the ability of the cerebral circulation to maintain CBF relatively constant over a range of arterial pressures44. Importantly, autoregulation is affected by the rate at which blood pressure fluctuates60, such that relatively slow changes in arterial blood pressure are associated with stable CBF, while rapid changes result in greater variability in CBF. Static autoregulation refers to the classic steady-state relationship between arterial blood pressure and CBF, while dynamic autoregulation refers to a CBF response occurring over seconds to minutes due to fast changes in blood pressure60. Static autoregulation is impaired in various animal models of hypertension60 potentially via increased myogenic tone44 as well as structural adaptations to the elevated blood pressure. These findings suggested that hypertension increases the risk for hypoxia if blood pressure were to drop below the lower limit of autoregulation and thus could be a contributing mechanism to neuronal injury. Although cerebral autoregulation was also thought to be impaired in human hypertension, increasing evidence indicates that static autoregulation is actually maintained in hypertensive patients60. On the other hand, whether hypertension affects dynamic cerebral autoregulation is still under debate60, as some studies suggest it is maintained, while others report impairments. It may be important to investigate the functional heterogeneity of dynamic autoregulation across brain regions to better understand the potential effects on cognitive function.
Pericytes are mural cells present in the microvasculature (pre-capillary arterioles, capillaries, and post-capillary venules) involved in regulating blood flow61 as well as maintenance of the BBB62. Pericytes vary in their location, morphology, protein expression, and related functions, many of which are still being identified. Morphologically, they are surrounded by basement membrane and extend processes both along and around capillaries, with more circumferential processes at the arteriole end of the capillary bed, more longitudinal processes in the middle of the capillary bed, and a stellate morphology at the venule end of the capillaries63. These morphological differences have led to “zone-specific” nomenclature: on precapillary arterioles (e.g., ensheathing pericytes, transitional pericytes), on capillaries (e.g., thin-strand or helical pericytes), and on post-capillary venules (e.g., mesh pericytes). Notably, there is variation in pericyte gene expression too, wherein downstream mural cells from capillary to venule zones are characterized by high expression of membrane transporters, such as abcc9 (ATP binding cassette subfamily C member 9) and pericytes that give out more circumferential processes expressing more alpha-smooth muscle actin (α-SMA)64. Pericyte loss has been associated with impairment of neurovascular coupling, BBB disruption, and cognitive impairment. Pericytes may also be playing an important mechanistic role in the breakdown of the BBB31, white matter lesions22, and development of cognitive impairment. Early studies have reported both increased and decreased brain pericyte numbers in hypertensive rodent models65,66. However, limitations in the methods of pericyte identification used prevented definitive conclusions from being drawn. More recent studies have found a loss of pericyte coverage of capillaries in hypertension, which is further exacerbated by aging31, and can be prevented by interferon-gamma blockade67. AngII can directly stimulate pericyte migration and contraction68 and may also upregulate Nox4 expression69. While Nox4 has been considered protective in endothelial cells70, pericyte Nox4 is associated with promoting blood-brain barrier damage through activation of NF-Kb and matrix metalloproteases71 and mediating vasoconstriction of capillaries72. Thus, Ang II-induced Nox4 upregulation in pericytes may contribute to the neurovascular unit dysfunction observed in hypertension. RNA-sequencing of cerebral microvascular pericytes from normotensive and hypertensive rats identified several differentially expressed genes and signaling pathways related to cell adhesion, extracellular matrix interactions, and inflammation73, consistent with their possible role in BBB disruption. However, whether disruption of pericyte function in hypertension contributes to cognitive impairment has not been determined.
Astrocytes
Astrocytes are important members of the NVU contributing to both BBB integrity and neurovascular coupling. Swelling of astrocytic endfeet can be observed around capillaries in hypertensive rats and may be an indication of BBB disruption66. With regards to neurovascular coupling, astrocytes may respond to neuronal activity by releasing vasoactive mediators74, and two recent studies reported that AngII augments astrocytic calcium signaling75,76. These findings raise the possibility that astrocytes contribute to the increased vascular tone and disruption of neurovascular coupling in hypertension. However, the importance of altered astrocytic calcium signaling or astrocytic endfeet swelling on cognitive function in hypertension remains to be determined.
Microglia
While microglia are commonly known in neurodegeneration for their role in neuroinflammatory processes, and particularly contributing to Alzheimer’s disease pathology77, they have recently been implicated in cerebrovascular function78. A subpopulation of microglia, known as juxtavascular or capillary-associated microglia78, are located in close proximity of brain microvessels, specifically in direct contact with the capillary basement membrane without disrupting astrocytic endfeet or pericyte coverage of microvessels. Microglia depletion with the CSF1 receptor inhibitor PLX3397 triggered capillary diameter dilation by approximately 15%, an increase in blood flow of about 20% assessed by laser-speckle, and impaired vasodilation to hypercapnia78. However, it’s important to note that PLX3397 targets all microglia, not only the capillary-associated subpopulation, while CSF1 receptor inhibition also depletes about 60% of brain macrophages79. Thus, it is not possible at this time to rule out the contribution of parenchymal microglia or brain macrophages to mediating the observed effects. Nonetheless, given their implication in neuroinflammation and neurovascular regulation, determining their contribution to neurovascular dysfunction and cognitive impairment in hypertension should be a focus of investigation in coming years.
Perivascular macrophages
Found in the perivascular space surrounding cerebral arterioles and venules, perivascular macrophages (PVM) are innate resident immune cells that have emerged as new players of the NVU and are increased in aging and hypertension80. PVM are one of three types of brain resident macrophages, and together with leptomeningeal and choroid plexus macrophages, they are known by various nomenclatures including central nervous system-associated macrophages, brain- or border-associated macrophages, or parenchymal border macrophages. However, given their localization to perivascular spaces, PVM are uniquely positioned to influence the cerebral vasculature. In rodent models of hypertension, PVM mediate the neurovascular dysfunction and disruption of the BBB via AT1R activation and Nox2-derived radicals53,81. Given the sensitivity of the venular BBB54, the presence of PVM-derived radicals may be particularly detrimental in this vascular segment. Depletion of PVM protects hypertensive mice from developing cognitive impairment53,79,81, suggesting that PVM not only have a detrimental role in regulating cerebrovascular function in hypertension, but may also be key mediators in hypertensive cognitive decline. A recent report identified a new role of these cells in regulating CSF flow dynamics82, raising the possibility that dysfunctional PVM53,79,81 may be associated with impaired CSF flow in hypertension83,84. This warrants future investigation on whether CSF flow and clearance mechanisms are affected in hypertension and may contribute to cognitive decline.
COGNITIVE DEFICITS IN RODENT MODELS OF HYPERTENSION
Given that no animal model can fully recapitulate the complex etiology of human hypertension85, utilizing various models is necessary for thorough investigation of the mechanisms underlying hypertension-induced cognitive impairment. Supplementary Table S1 presents potential mechanisms of cognitive deficits in selected rodent models of hypertension as a resource for future investigation. It is important to note that large animal models, such as Rhesus monkeys86, have also demonstrated hypertension-associated memory decline.
Genetic models of hypertension have a progressive rise in blood pressure with age, and thus are beneficial to investigate the effects of chronic spontaneous hypertension. Various genetic models develop cognitive deficits, including the spontaneously hypertensive rat (SHR), Schlager/blood pressure high (BPH/2) mouse, and human renin and angiotensinogen transgenic mice (R+/A+). Both the SHR and BPH/2 models develop such deficits at 12 weeks of age53,81,87, and the R+/A+ transgenic mice at 16 weeks of age88. In the BPH/2 mouse, impairments in spatial learning and memory81,89, working memory53,81, and disinhibition53,89 have been observed. However, it is important to note that we cannot rule out that the phenotypic effects observed are due to genetic differences unrelated to the elevation of blood pressure.
One of the most widely used models of hypertension, long-term subcutaneous infusion of AngII, varies widely in both dose and length of treatment85. In rats, 2 months of AngII hypertension did not affect spatial learning and memory, but did impair cognitive flexibility assessed by a complex task90. In mice, high doses of AngII administration for longer than 4 weeks is needed to induce cognitive deficits91. However, these high doses may also induce anxiety92, and thus could make data interpretation more difficult. Despite extensive cerebrovascular structural and functional alterations induced in the AngII model of hypertension, and given that less than 15% of patients have elevated circulating renin-angiotensin system (RAS) activity, AngII-induced hypertension may not be the best suited model for assessing the effects of hypertension on cognitive function.
Salt-sensitivity affects up to 50% of patients with hypertension and has been identified as an independent risk factor for cardiovascular events93. Rodent studies commonly use 4 or 8% NaCl, representing an 8-fold to 16-fold increase from a normal mouse diet94. Although extremely high, these diets are comparable to the high end of the spectrum of human salt consumption95. These high doses have been used historically because many rodent strains are resistant to salt treatment85. A historical perspective and extensive list of salt-sensitive rodent models has been previously published93, and thus will not be reiterated here. Both Dahl salt-sensitive96 and DOCA-salt rats97 have been reported to develop cognitive deficits in various domains, although the results are not always consistent. Given the independent link between salt and cognitive impairment in animals94 and humans98, further investigation on models of salt-sensitive hypertension is warranted.
Lastly, cognitive impairment has also been reported in surgical models of hypertension including transverse aortic coarctation (TAC)99 and renovascular hypertension100. TAC may be a particularly useful model because it presents with alterations to cerebrovascular reactivity, endothelial dysfunction, as well as neurodegenerative pathology, brain amyloid accumulation and BBB dysfunction99.
CONCLUSIONS
While the relationship between hypertension and cognitive decline is undeniable, the mechanisms underlying this link are less well understood. Importantly, there are several big questions which remain in the field. First, how does impaired CBF regulation lead to cognitive impairment? As mentioned earlier, much of the studies investigating cerebrovascular mechanisms of cognitive impairment work under the assumption that cognitive impairment occurs when the metabolic demands of neurons are not met due to neurovascular uncoupling40,41. However, the exact mechanisms linking impaired CBF to neuronal dysfunction and cognitive impairment are not clear. There are several hypotheses to explain this association: (1) the lack of oxygen availability resulting from reduced CBF could directly lead to neuronal death, (2) neuroinflammation in response to the leakage of plasma proteins in the brain parenchyma may be responsible for neuronal dysfunction, and (3) a neurotoxic milieu due to impaired clearance of byproducts of neuronal activity may impair neuronal function. Given that there is evidence for each of these in the literature, it is unlikely that only one of these hypotheses will explain the association. Instead, each of these mechanisms may be present to different degrees in individuals, and thus the combination of all three is important to cognitive health.
The second main question which remains is does restoring CBF in hypertension improve cognitive function? The answer may not lie exclusively in antihypertensive therapy. This is because even if the pharmacological lowering of blood pressure may improve CBF101, it does not always rescue cognition102. Thus, even though hypertension treatment is essential and undoubtedly beneficial to cardiovascular health, it is important to recognize that there may be other processes operating in parallel that may contribute to cognitive decline in hypertension. This is complicated by the slow temporal progression of pathology, as the onset of hypertension and cognitive decline are separated by several decades7. Furthermore, aging exacerbates the detrimental effects of hypertension on the brain, and the interaction of aging and hypertension is a point of active investigation6. Therefore, protecting cognitive health will require early identification of patients at risk and prevention of CBF impairment.
Lastly, women are at much greater risk for dementia than men103. This has historically been attributed to women living longer, though cardiovascular risk factors like hypertension may play a role in these differences. Women are more prone to hypertension after the age of 60104, increasing the burden of elevated blood pressure and aging. This could be due to reduced estrogen signaling post-menopause as estrogen is important in nitric oxide signaling and a reduction in this could impact vasodilation105. Estrogen is also important in the availability of elastin and collagen in the arterial walls, highlighting its importance in keeping compliant vessels walls105. Additional, estrogen is neuroprotective and plays a role in inflammatory regulation106. Despite its essential roles, the effect of menopause on cardiovascular health and how that may contribute to dementia risk is understudied.
Supplementary Material
SOURCES OF FUNDING
MMS was supported by grant number K22-NS123507 from the National Institutes of Health (NIH). FEC was supported by training grant T32-AG058524 (NIH). CEB, FEC, and ALJ were supported by grant number R01-AG034962 and K24-AG046373 from NIH. Figures were created on Biorender.com.
Footnotes
The authors declare no conflicts.
REFERENCES
- 1.WHO. Global status report on the public health response to dementia. World Health Organization; 2021. [Google Scholar]
- 2.Bakris G, Sorrentino M. Redefining hypertension - assessing the new blood-pressure guidelines. N Engl J Med. 2018;378:497–499 [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: A scientific statement from the american heart association. Hypertension. 2016;68:e67–e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santisteban MM, Iadecola C, Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension. 2023;80:22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C, Smith EE, Anrather J, et al. The neurovasculome: Key roles in brain health and cognitive impairment: A scientific statement from the american heart association/american stroke association. Stroke. 2023;54:e251–e271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: Epidemiology, pathobiology and treatment. Circ Res. 2019;124:1025–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey JD. Mechanisms of vascular remodeling in hypertension. Am J Hypertens. 2021;34:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for cuzn superoxide dismutase. Stroke. 2006;37:1850–1855 [DOI] [PubMed] [Google Scholar]
- 11.Chan SL, Baumbach GL. Deficiency of nox2 prevents angiotensin ii-induced inward remodeling in cerebral arterioles. Front Physiol. 2013;4:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Earley S, Li YS, Chien S. Vascular mechanotransduction. Physiol Rev. 2023;103:1247–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigsby CS, Ergul A, Portik Dobos V, et al. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011;24:708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Yang H, Zheng LY, et al. Downregulation of tmem16a calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707 [DOI] [PubMed] [Google Scholar]
- 15.Safar ME, Asmar R, Benetos A, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805 [DOI] [PubMed] [Google Scholar]
- 16.Muhire G, Iulita MF, Vallerand D, et al. Arterial stiffness due to carotid calcification disrupts cerebral blood flow regulation and leads to cognitive deficits. J Am Heart Assoc. 2019;8:e011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidelis HG, Mageski JGA, Goes SCE, et al. Blockade of angiotensin at(1) receptors prevents arterial remodelling and stiffening in iron-overloaded rats. Br J Pharmacol. 2020;177:1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore EE, Liu D, Li J, et al. Association of aortic stiffness with biomarkers of neuroinflammation, synaptic dysfunction, and neurodegeneration. Neurology. 2021;97:e329–e340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadekova N, Iulita MF, Vallerand D, et al. Arterial stiffness induced by carotid calcification leads to cerebral gliosis mediated by oxidative stress. J Hypertens. 2018;36:286–298 [DOI] [PubMed] [Google Scholar]
- 20.Eglit GML, Weigand AJ, Nation DA, et al. Hypertension and alzheimer’s disease: Indirect effects through circle of willis atherosclerosis. Brain Commun. 2020;2:fcaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696 [DOI] [PubMed] [Google Scholar]
- 22.Ding R, Hase Y, Ameen-Ali KE, et al. Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and alzheimer’s disease. Brain Pathol. 2020;30:1087–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam KW, Kwon HM, Jeong HY, et al. Cerebral small vessel disease and stage 1 hypertension defined by the 2017 american college of cardiology/american heart association guidelines. Hypertension. 2019;73:1210–1216 [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat Rev Neurol. 2020;16:137–153 [DOI] [PubMed] [Google Scholar]
- 25.Ungvari Z, Tarantini S, Kirkpatrick AC, et al. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–h1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrea RE, O’Donnell A, Beiser AS, et al. Mid to late life hypertension trends and cerebral small vessel disease in the framingham heart study. Hypertension. 2020;76:707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toth P, Tarantini S, Springo Z, et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: Role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyúl-Tóth Á, Tarantini S, Kiss T, et al. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of alzheimer’s disease. Geroscience. 2020;42:1685–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: The rotterdam scan study. Neurology. 2012;78:326–333 [DOI] [PubMed] [Google Scholar]
- 30.van Dinther M, Voorter PH, Jansen JF, et al. Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J Cereb Blood Flow Metab. 2022;42:718–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toth P, Tucsek Z, Sosnowska D, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin ii-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzoni D, De Ciuceis C, Porteri E, et al. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens. 2009;27:838–845 [DOI] [PubMed] [Google Scholar]
- 33.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown WR, Thore CR. Review: Cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes AD, Martinez-Perez E, Jabbar AS, et al. Quantification of topological changes in retinal vascular architecture in essential and malignant hypertension. J Hypertens. 2006;24:889–894 [DOI] [PubMed] [Google Scholar]
- 36.Jordão MT, Ceroni A, Michelini LC. Perfusion of brain preautonomic areas in hypertension: Compensatory absence of capillary rarefaction and protective effects of exercise training. Front Physiol. 2021;12:773415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freitas F, Estato V, Reis P, et al. Acute simvastatin treatment restores cerebral functional capillary density and attenuates angiotensin ii-induced microcirculatory changes in a model of primary hypertension. Microcirculation. 2017;24 [DOI] [PubMed] [Google Scholar]
- 38.van Dinther M, Bennett J, Thornton GD, et al. Evaluation of microvascular rarefaction in vascular cognitive impairment and heart failure (crucial): Study protocol for an observational study. Cerebrovasc Dis Extra. 2023;13:18–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Montgolfier O, Pinçon A, Pouliot P, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension. 2019;73:217–228 [DOI] [PubMed] [Google Scholar]
- 41.Tarantini S, Hertelendy P, Tucsek Z, et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain. 2014;137:2960–2972 [DOI] [PubMed] [Google Scholar]
- 43.Kang EJ, Prager O, Lublinsky S, et al. Stroke-prone salt-sensitive spontaneously hypertensive rats show higher susceptibility to spreading depolarization (sd) and altered hemodynamic responses to sd. J Cereb Blood Flow Metab. 2023;43:210–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cipolla MJ. The cerebral circulation. San Rafael (CA): Morgan & Claypool Life Sciences; 2016. [PubMed] [Google Scholar]
- 45.Santisteban MM, Iadecola C. Hypertension, dietary salt and cognitive impairment. J Cereb Blood Flow Metab. 2018;38:2112–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleem M, Herrmann N, Dinoff A, et al. Association between endothelial function and cognitive performance in patients with coronary artery disease during cardiac rehabilitation. Psychosom Med. 2019;81:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nezu T, Hosomi N, Aoki S, et al. Endothelial dysfunction is associated with the severity of cerebral small vessel disease. Hypertens Res. 2015;38:291–297 [DOI] [PubMed] [Google Scholar]
- 48.Longden TA, Dabertrand F, Koide M, et al. Capillary k(+)-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koide M, Harraz OF, Dabertrand F, et al. Differential restoration of functional hyperemia by antihypertensive drug classes in hypertension-related cerebral small vessel disease. J Clin Invest. 2021;131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanlandewijck M, He L, Mae MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480 [DOI] [PubMed] [Google Scholar]
- 51.Biancardi VC, Son SJ, Ahmadi S, et al. Circulating angiotensin ii gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakagawa T, Hasegawa Y, Uekawa K, et al. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2:e000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santisteban MM, Ahn SJ, Lane D, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. 2020;76:795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension. 1987;9:Iii101–105 [DOI] [PubMed] [Google Scholar]
- 55.Mayhan WG, Faraci FM, Heistad DD. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am J Physiol. 1986;251:H1171–1175 [DOI] [PubMed] [Google Scholar]
- 56.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dankbaar JW, Hom J, Schneider T, et al. Age- and anatomy-related values of blood-brain barrier permeability measured by perfusion-ct in non-stroke patients. J Neuroradiol. 2009;36:219–227 [DOI] [PubMed] [Google Scholar]
- 59.Dobrynina LA, Shamtieva KV, Kremneva EI, et al. Daily blood pressure profile and blood-brain barrier permeability in patients with cerebral small vessel disease. Sci Rep. 2022;12:7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561 [DOI] [PubMed] [Google Scholar]
- 63.Hartmann DA, Underly RG, Grant RI, et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman IM, Jacobson S. In situ analysis of microvascular pericytes in hypertensive rat brains. Tissue Cell. 1988;20:1–12 [DOI] [PubMed] [Google Scholar]
- 66.Tagami M, Nara Y, Kubota A, et al. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke. 1990;21:1064–1071 [DOI] [PubMed] [Google Scholar]
- 67.Apaydin DC, Zakarauskas-Seth BI, Carnevale L, et al. Interferon-γ drives macrophage reprogramming, cerebrovascular remodeling, and cognitive dysfunction in a zebrafish and a mouse model of ion imbalance and pressure overload. Cardiovasc Res. 2023;119:1234–1249 [DOI] [PubMed] [Google Scholar]
- 68.Matsugi T, Chen Q, Anderson DR. Contractile responses of cultured bovine retinal pericytes to angiotensin ii. Arch Ophthalmol. 1997;115:1281–1285 [DOI] [PubMed] [Google Scholar]
- 69.Kuroda J, Ago T, Nishimura A, et al. Nox4 is a major source of superoxide production in human brain pericytes. J Vasc Res. 2014;51:429–438 [DOI] [PubMed] [Google Scholar]
- 70.Schröder K, Zhang M, Benkhoff S, et al. Nox4 is a protective reactive oxygen species generating vascular nadph oxidase. Circ Res. 2012;110:1217–1225 [DOI] [PubMed] [Google Scholar]
- 71.Nishimura A, Ago T, Kuroda J, et al. Detrimental role of pericyte nox4 in the acute phase of brain ischemia. J Cereb Blood Flow Metab. 2016;36:1143–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nortley R, Korte N, Izquierdo P, et al. Amyloid β oligomers constrict human capillaries in alzheimer’s disease via signaling to pericytes. Science. 2019;365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan X, Wu Q, Liu X, et al. Transcriptomic profile analysis of brain microvascular pericytes in spontaneously hypertensive rats by rna-seq. Am J Transl Res. 2018;10:2372–2386 [PMC free article] [PubMed] [Google Scholar]
- 74.Tran CHT, Peringod G, Gordon GR. Astrocytes integrate behavioral state and vascular signals during functional hyperemia. Neuron. 2018;100:1133–1148.e1133 [DOI] [PubMed] [Google Scholar]
- 75.Diaz JR, Kim KJ, Brands MW, Filosa JA. Augmented astrocyte microdomain ca(2+) dynamics and parenchymal arteriole tone in angiotensin ii-infused hypertensive mice. Glia. 2019;67:551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boily M, Li L, Vallerand D, Girouard H. Angiotensin ii disrupts neurovascular coupling by potentiating calcium increases in astrocytic endfeet. J Am Heart Assoc. 2021;10:e020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jorfi M, Maaser-Hecker A, Tanzi RE. The neuroimmune axis of alzheimer’s disease. Genome Med. 2023;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bisht K, Okojie KA, Sharma K, et al. Capillary-associated microglia regulate vascular structure and function through panx1-p2ry12 coupling in mice. Nat Commun. 2021;12:5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerkhofs D, van Hagen BT, Milanova IV, et al. Pharmacological depletion of microglia and perivascular macrophages prevents vascular cognitive impairment in ang ii-induced hypertension. Theranostics. 2020;10:9512–9527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Jacobowitz DM, Barone F, et al. Quantitation of perivascular monocytes and macrophages around cerebral blood vessels of hypertensive and aged rats. J Cereb Blood Flow Metab. 1994;14:348–352 [DOI] [PubMed] [Google Scholar]
- 81.Faraco G, Sugiyama Y, Lane D, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drieu A, Du S, Storck SE, et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature. 2022;611:585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koundal S, Elkin R, Nadeem S, et al. Optimal mass transport with lagrangian workflow reveals advective and diffusion driven solute transport in the glymphatic system. Sci Rep. 2020;10:1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mortensen KN, Sanggaard S, Mestre H, et al. Impaired glymphatic transport in spontaneously hypertensive rats. J Neurosci. 2019;39:6365–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lerman LO, Kurtz TW, Touyz RM, et al. Animal models of hypertension: A scientific statement from the american heart association. Hypertension. 2019:e87–e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moss MB, Jonak E. Cerebrovascular disease and dementia: A primate model of hypertension and cognition. Alzheimers Dement. 2007;3:S6–15 [DOI] [PubMed] [Google Scholar]
- 87.Gattu M, Pauly JR, Boss KL, et al. Cognitive impairment in spontaneously hypertensive rats: Role of central nicotinic receptors. I. Brain Res. 1997;771:89–103 [DOI] [PubMed] [Google Scholar]
- 88.Inaba S, Iwai M, Furuno M, et al. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362 [DOI] [PubMed] [Google Scholar]
- 89.Hartman RE, Kamper JE, Goyal R, et al. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav. 2012;105:1092–1097 [DOI] [PubMed] [Google Scholar]
- 90.Levit A, Cheng S, Hough O, et al. Hypertension and pathogenic happ independently induce white matter astrocytosis and cognitive impairment in the rat. Front Aging Neurosci. 2020;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Foulquier S, Namsolleck P, Van Hagen BT, et al. Hypertension-induced cognitive impairment: Insights from prolonged angiotensin ii infusion in mice. Hypertens Res. 2018;41:817–827 [DOI] [PubMed] [Google Scholar]
- 92.Duchemin S, Belanger E, Wu R, et al. Chronic perfusion of angiotensin ii causes cognitive dysfunctions and anxiety in mice. Physiol Behav. 2013;109:63–68 [DOI] [PubMed] [Google Scholar]
- 93.Elijovich F, Weinberger MH, Anderson CA, et al. Salt sensitivity of blood pressure: A scientific statement from the american heart association. Hypertension. 2016;68:e7–e46 [DOI] [PubMed] [Google Scholar]
- 94.Faraco G, Brea D, Garcia-Bonilla L, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated th17 response. Nat Neurosci. 2018;21:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruiz-Opazo N, Tonkiss J. Genome-wide scan for quantitative trait loci influencing spatial navigation and social recognition memory in dahl rats. Physiol Genomics. 2006;26:145–151 [DOI] [PubMed] [Google Scholar]
- 97.Jabaris SS, Sumathy H, Girish R, et al. Phosphodiesterase-4 inhibitors ameliorates cognitive deficits in deoxycorticosterone acetate induced hypertensive rats via camp/creb signaling system. Brain Res. 2015;1622:279–291 [DOI] [PubMed] [Google Scholar]
- 98.Blumenthal JA, Smith PJ, Mabe S, et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology. 2019;92:e212–e223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carnevale D, Mascio G, D’Andrea I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan Y, Lan L, Zheng L, et al. Spontaneous white matter lesion in brain of stroke-prone renovascular hypertensive rats: A study from mri, pathology and behavior. Metab Brain Dis. 2015;30:1479–1486 [DOI] [PubMed] [Google Scholar]
- 101.van Rijssel AE, Stins BC, Beishon LC, et al. Effect of antihypertensive treatment on cerebral blood flow in older adults: A systematic review and meta-analysis. Hypertension. 2022;79:1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and alzheimer’s disease: A meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazure CM, Swendsen J. Sex differences in alzheimer’s disease and other dementias. Lancet Neurol. 2016;15:451–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghazi L, Annabathula RV, Bello NA, et al. Hypertension across a woman’s life cycle. Curr Hypertens Rep. 2022;24:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–249 [DOI] [PubMed] [Google Scholar]
- 106.Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: A matter of timing. Arterioscler Thromb Vasc Biol. 2012;32:2035–2042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


