Abstract
Differentiated service delivery (DSD) models, such as adherence clubs (ACs), are client-centred approaches where clinically stable people living with HIV (PLHIV) meet to receive various services, including psychosocial support, brief symptoms screening, and refills of antiretroviral medications, among others. We conducted a review to assess the impact of DSD models, including ACs, on sustaining retention in care (RC) and achieving viral suppression (VS) among PLHIV in sub-Saharan Africa. The review protocol was registered in PROSPERO (CRD42023418988). We searched the literature from PubMed, Scopus, Web of Science, Embase and Google Scholar from their inception through May 2023. Eligible randomised controlled trials of adherence clubs were reviewed to assess impact on retention and viral suppression. Random effect models were used to estimate the risk ratios (RR) and 95% confidence intervals (CI). The literature search yielded a total of 1596 records of which 16 randomised clinical trials were determined to be eligible. The trials were conducted in diverse populations among adults and children with a total of 13,886 participants. The RR between any DSD models and standard of care (SoC) was 1.09 (95% CI: 1.08–1.11, I2: 0%, p: <0.96) and 1.01 (95% CI: 1.00–1.02, I2: 0%, p: <0.85) for RC and VS, respectively. The RR between ACs and SoC was 1.01 (95% CI: 0.96–1.07, I2: 84%, p: <0.01) and 1.02 (95% CI: 0.98–1.07, I2: 77%, p: <0.01) for RC and VS, respectively. DSD models, including ACs, show comparable effectiveness to SoC in maintaining care and achieving viral suppression for stable PLHIV. To maximise adoption, an implementation science approach is crucial for designing effective strategies and overcoming challenges.
Keywords: adherence clubs, adherence to antiretroviral treatment, retention in HIV care, sub-Saharan Africa, viral suppression
1 |. INTRODUCTION
In 2021, approximately 38.4 million people globally were living with HIV, of whom 1.5 million became infected in 2021. A total of 28.7 million (74.7%) were accessing antiretroviral therapy (ART).1 Sub-Saharan region continues to be the most affected region by HIV with approximately, 25.6 million people living with HIV (PLHIV).2 To end the AIDS epidemic by 2030,3 the Joint United Nations programme on HIV/AIDS (UNAIDS) set the 95-95-95 target in 2014,4 that aims for HIV testing, treatment, and viral suppression rates all to exceed 95% by 2025. Data shows countries especially with limited resources and poor health systems are struggling to achieve the 95-95-95 targets.1,4 Factors limiting access and retention to care at the patient level include congestion in health care facilities with long waiting hours and spending long hours to reach the nearest clinic, transportation cost to collect antiretroviral medications and attending clinic appointments, time conflict between attending HIV clinic and work and community and self-stigmatisation5,6 remain to be the main challenges with negative impact on retention in HIV care and adherence to lifelong ART.7
The World Health Organization (WHO) recommends the implementation of Differentiated Service Delivery (DSD) models,8 especially for clinically stable individuals living with HIV.9 It has been shown that DSD models help in reducing unnecessary burden on HIV health care system.10 Adherence clubs (ACs) are one of the DSD model that has been implemented in different countries in sub-Saharan Africa.11,12 Generally, adherence clubs are comprised of 20–30 PLHIV, who meet every 2–3 months at health facilities or community locations, during off-hours (evening or weekends).11 During adherence club meeting, in programs participants may receive group psychosocial support, brief symptoms screening, re-fills of their ART medications and other interventions.12,13 These ACs are managed by lay staff or community health care worker or a health care provider. Furthermore, some ACs can allow members to choose between decentralised medication delivery, including picking at locations other than the clinic pharmacy or at the clinic pharmacy.10 Club members can be referred from the AC to a health care facility in case of acute illness or positive symptom screening or become pregnant or on the basis of a patient’s decision.
The impact of implementing DSD such as ACs include an expanded access to ART, sustained retention in HIV care, reduced late ART pick-up, attainment and sustaining of viral suppression and decongested primary health facilities.11–14 Ongoing roll-out of ACs in sub-Saharan African countries, could be seen as part of the effort to address the problem of poor retention in HIV care and sub-optimal adherence to ART.10,15 Therefore, we conducted a review to obtain a pooled effect of DSD intervention from randomised clinical trials with a special focus on ACs using studies conducted in the sub-Saharan Africa region.
2 |. MATERIALS AND METHODS
2.1 |. Study design and search strategy
This review was registered in The International Prospective Register of Systematic Reviews (PROSPERO: CRD42023418988). The review team prepared the protocol according to preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P).16 The review team searched for articles from PubMed, Embase, Scopus, Web of Science and Google Scholar, from database inception through 4th May 2023. A search was developed for PubMed with assistance from the research librarian and adapted to each database. The search query was developed with the guidance from PRESS peer review of electronic search strategies17 then adapted to other databases. The keywords used included ‘adherence clubs’, ‘retention in HIV care’, ‘viral suppression’, ‘adherence’, ‘heterosexual’, ‘decentralised care’, ‘differentiated service’ ‘virologic* response’, ‘people living with HIV’, ‘viral load’ and ‘medication adherence’. The review team used MeSH (medical sub-heading terms) for PubMed search, while Boolean operator ‘AND’ combined both or all of the keywords, while ‘OR’ searched articles containing at least one of the keywords.
2.2 |. Study selection
A review team (BJN and GMB) independently conducted title and abstract screening and performed a full-article screening. Covidence software (Veritas Health Innovation, Melbourne, Australia) was used to manage the duplicates and perform title and abstract screening. During title and abstract screening, we included studies reported on PLHIV, adherence clubs, decentralised medication delivery, differentiated service delivery, viral suppression, adherence to ART and retention in HIV care; while with the full-article screening we excluded studies that were not randomised clinical trials (RCTs), studies conducted in non-humans and studies not conducted in sub-Saharan African countries (Figure 1). Disagreements during the screening process were resolved by a third reviewer (RZS).
FIGURE 1.
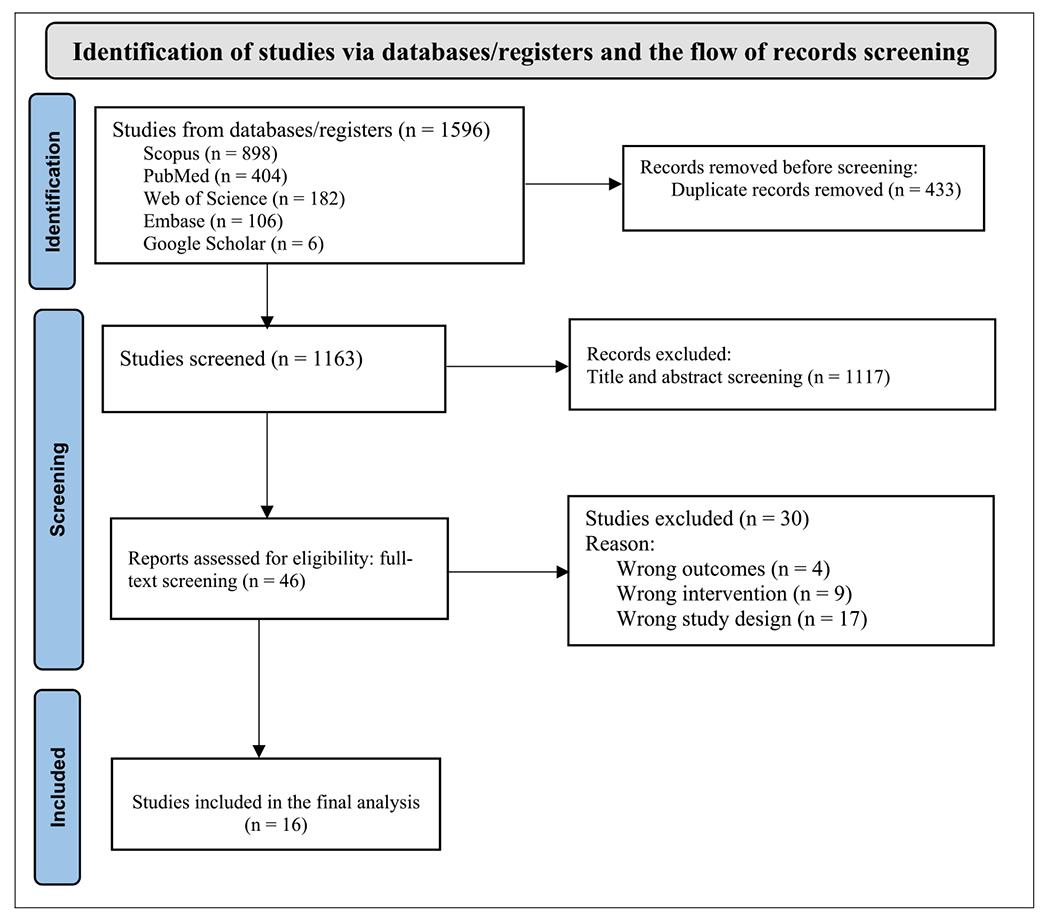
PRISMA flow chart, describing the process involved in study screening.
2.3 |. Quality of the reviewed studies
Two authors (George M. Bwire and Belinda J. Njiro) evaluated the quality of studies using version 2 of the Cochrane risk-of-bias tool for randomised trials (RoB2). The judgement was low risk of bias, high risk of bias or unclear risk (some concerns).18,19 Disagreement was resolved using arbitration by a third reviewer. A total of 16 RCTs were assessed, of which seven studies had low risk,11,14,20–24 seven studies had some concerns,12,13,25–28 and two articles had high risk of bias.29,30 (Figure 2).
FIGURE 2.
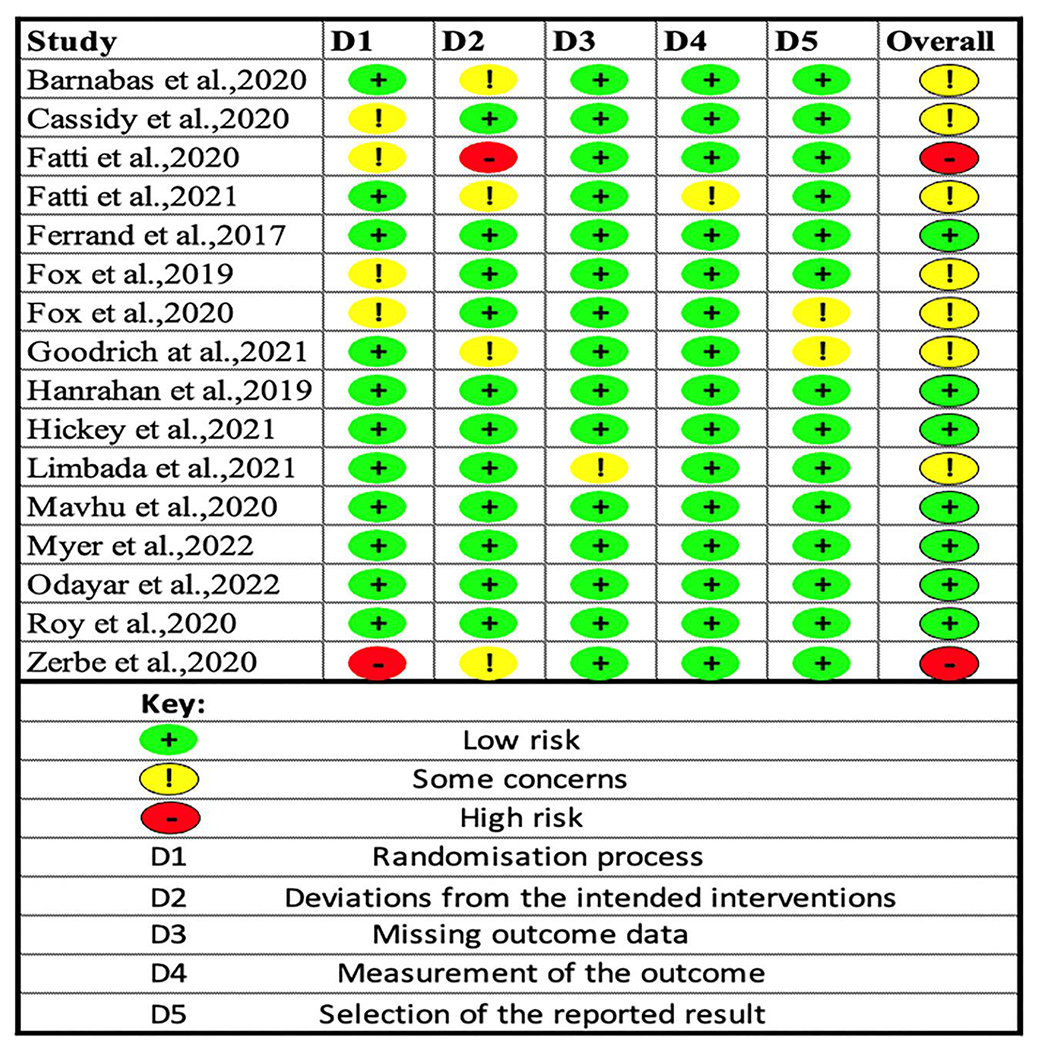
Risk of bias assessed using version 2 of the Cochrane risk-of-bias tool for randomised trial.
2.4 |. Data extraction and analysis
Data that were extracted from study documents by two independent authors (George M. Bwire and Belinda J. Njiro), and included information on year of publication, country, description of the intervention and control, description of the type of DSD model, retention in care and sustained viral load suppression and other outcomes of the interventions (Table 1). Unavailable, unclear information and additional details were requested from the study investigators. Data were recorded in Excel spread sheet 2015 (Microsoft Corporation, Redmond, WA).
TABLE 1.
Characteristics of the included randomized clinical trials and the risk of bias.
| Author | Country | Population | Description of differentiated service delivery (DSD) model | |
|---|---|---|---|---|
| Control | Intervention | |||
| Cassidy et al. (2020)13 | South Africa | Adults (>18 years) | The standard of care (SoC)- adherence clubs (ACs); two-monthly refills | ACs; (six monthly ART refills) |
| Fatti et al. (2021)26 | Zimbabwe and Lesotho | Adults (≥18 years) | SoC; three-monthly refill | Three-monthly community antiretroviral refill and, six-monthly community ART group |
| Fatti et al. (2020)30 | Zimbabwe | Adults (≥18 years) | SoC; three-monthly refill | Three-monthly community antiretroviral refill and, six-monthly community ART group |
| Barnabas et al. (2020)25 | South Africa and Uganda | Adults (≥18 years) | SoC with quarterly monitoring and ART refills | Community-based ART initiation and hybrid (community and clinic) quarterly monitoring and ART refills through mobile vans |
| Ferrand et al. (2017)24 | Zimbambwe | Children and adolescents (6–15 years) | SoC | Community-based HIV service |
| Fox et al. (2021)31 | South Africa | Adults (≥18 years) | SoC | ACs |
| Fox et al. (2019)12 | South Africa | Adults (≥18 years) | SoC | ACs |
| Goodrich et al. (2021)27 | Kenya | Adults (≥18 years) | SoC | Community-based HIV service |
| Hanrahan et al. (2019)14 | South Africa | Adults (≥18 years) | Clinic-based ACs | Community-based ACs |
| Hickey et al. (2020)20 | Kenya | Adults (≥18 years) | SoC | Community microclinic social network |
| Limbada et al. (2022)28 | Zambia | Adults (≥18 years) | SoC | Home-based ART delivery and ACs |
| Mavhu et al. (2020)21 | Zimbambwe | Adolescents and adults (13–19 years) | SoC | Community model with peer-support |
| Myer et al. (2022)22 | South Africa | Adults postpartum women (≥18 years) | SoC | ACs |
| Odayar et al. (2022)23 | South Africa | Adults | SoC | ACs |
| Roy et al. (2020)11 | Zambia | Adolescents and adults (≥14 years) | SoC | ACs |
| Zerbe et al. (2020)29 | South Africa | Adults postpartum women | SoC | ACs |
A pooled effect estimate (risk ratio) calculated by comparing the DSD model or adherence clubs and the standard of care at the 95% confidence interval (95% CI), I2 >75% represented high heterogeneity. Estimation of the effect was done using a random effect model.32,33 Retention in HIV care was defined as attending the scheduled visits after 12 months. The viral load suppression was defined as any viral load count below 1000 copies/mL taken any time after 6-months from the baseline. However, we noted the differences in viral load thresholds from various study settings.
Clinically stable patient9 was defined as a PLHIV receiving ART for at least 6 months with no adverse medication reactions requiring regular monitoring, no current illnesses or pregnancy, the patient with two consecutive undetectable viral load measures or CD4 counts above 200 cells/mm3. We performed meta-analysis for studies which compared any DSD or ACs models as an intervention and standard of care (SoC) as a control, all statistics were performed using R software version 4.2.3.
3 |. RESULTS
3.1 |. Characteristics of the included randomised clinical trials
In total 1596 records were screened for inclusion, of which 16 RCTs met eligibility criteria.10,11,13,14,20–31 The trials included more than 13,800 PLHIV (Table 1). Most of the trials were conducted in South Africa,12–14,22,23,25,29,31 with most (11 studies) conducted among adult men and women (≥18 years).12–14,20,23,25–28,30,31 Two studies were conducted in postpartum women,22,29 and three were conducted in mixed adolescent and adult populations (Ferrand et al. 201724 Mavhu et al. 202021 and Roy et al. 202011) used both adolescents and adult population, Ferrand et al. 201724 investigated the impact of differentiated service delivery (DSD) model in children and adult population.
3.2 |. Differentiated service delivery (DSD) models
Ten RCTs12,20,24–31 compared the retention in care and viral suppression between any DSD model and the standard of care. For viral suppression the risk ratio (RR) was 1.02 (95% CI: 0.98–1.07, I2: 77%, p-value for heterogeneity <0.01) while for retention in HIV care the RR was 1.01 (95% CI: 0.96–1.07, I2: 84%, p-value for heterogeneity<0.01). The RR in terms of retention in care and viral suppression between the standard of care group (SoP) and intervention group (ACs) were almost similar (Figure 3).
FIGURE 3.
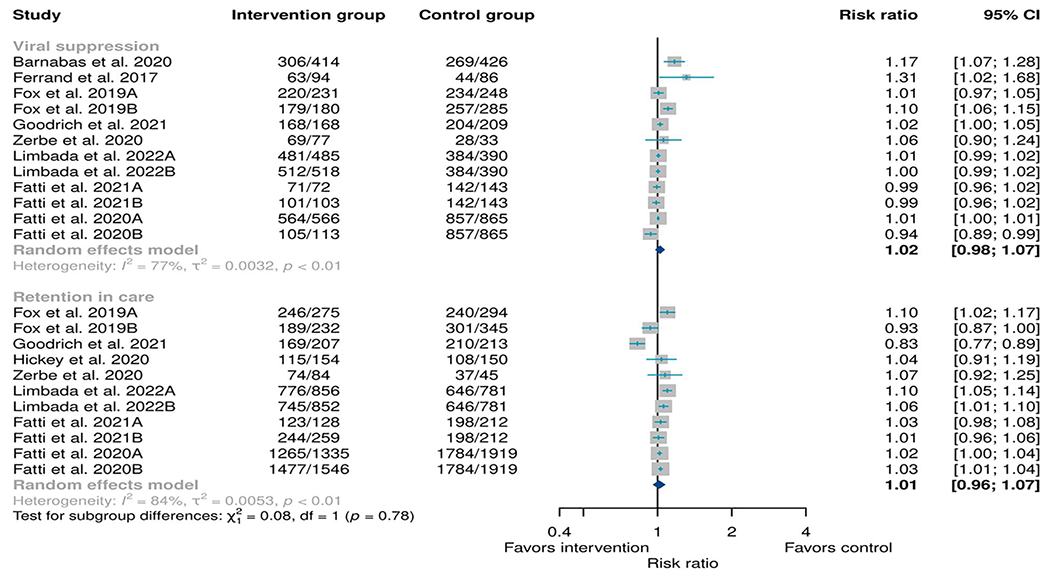
Meta-analysis of any type of differentiated service delivery model as an intervention when compared to any type of standard of care as a control. Retention was determined 12 months from the baseline while viral suppression was defined as any viral load below 1000 copies/mL taken any time above 6 months from the baseline.
3.3 |. Adherence club models
We describe the impact of ACs in the retention in HIV care, timely refill and viral suppression. Generally, the ACs comprised of 15–30 members per group,11–14,22,23,29,31 club members could choose the meeting place either be at the clinic or community locations. Club members met for 1 hour every 2–6 months11–14,22,23,29,31 to receive psychosocial counselling, symptoms screening and pre-packed ART medications re-fill. Members who were not able to attend the meeting in person were allowed to send someone else (treatment buddy) to collect their ART,11,14,23
Roy et al. 202011 did not use the attendance as the criteria for being referred to the standard care from the club while two consecutive buddy pickups were one of the reasons used by Hanrahan et al. 201914 to exclude participants from participating in the ACs. Roy et al. 202011 described that members met during off-hours at the facility (evenings or weekends) to receive medication refills, symptom screening, and group psychosocial support. In most cases, the groups were led by lay staff and nurses at the facility with support from community health workers, nurse/doctor could see patients, mostly once a year to conduct a clinical assessment and viral load testing.11–14,22,23,29,31
Seven studies compared the standard of care (SoC) and adherence clubs (ACs) as an intervention,11,12,22,23,29,31 Cassidy et al. 202013 compared ACs one arm refilling their medications after every 2-month while the other arm participants refilled their ART medications after every 6-month, Hanrahan et al. 201914 compared clinic based ACs versus community-based ACs. Limbada et al. 202228 randomised participants in SoC group, home-based delivery group and ACs. In most cases, participants were referred back to the facility-based care when became clinically unstable, diagnosed with tuberculosis (TB), viral rebound or chronic illness requiring clinical management (Table 2). Except Hanrahan et al. 2019,14 the study which did not include SoC as a control, the remaining studies reported comparable outcomes (retention in care, timely refill and viral suppression) between the SoC and ACs as an intervention.
TABLE 2.
Description of the eligibility criteria, intervention and the outcomes following the implementation of adherence club.
| Author | Eligibility criteria for club membership | The place of the club meeting | Description of the intervention | Outcome of the intervention |
|---|---|---|---|---|
| Cassidy et al. (2020)13 | Adherence clubs (ACs) members were >18 years; not pregnant, free from opportunistic infection, on ART for more than 6 months with one viral load (VL) below 400 copies/mL, and already participating in AC. | Clinic location | The standard of care (SoC)-ACs received (two-monthly refills) and the intervention (six-monthly refills). Intervention ACs met twice a year and were given ART for 6-month use. One of two visits included a clinical consultation led by nurse. Intervention patients were assigned a VL appointment date and allowed to come anytime 2–6 weeks before their clinical consultation AC visit. Those who did not come for their scheduled VL test had their VL taken at their clinical consultation AC visit and those with high VLs (400 copies/mL and more) were contacted. | There were no differences in term of viral suppression and retention in care between AC members receiving six-monthly ART refills and those who received two-monthly ART refill. |
| Fox et al. (2019)12 | Participants over 18 years old, neither pregnant nor eligible for prevention of mother-to-child transmission services were included, being on the same ART regimen for at least 12 months, having had their VL in the past 3 months, having had two consecutive undetectable viral loads (<400 copies/mL). | Clinic or community location | AC had approximately, 30 members who met every 2–3 months to receive group counselling, have a brief symptom screen, and receive prepacked ART medications. Clubs were led by lay staff and nurses at the facility with support from community health workers. Pre-packed ART was done either at the clinic or community. | There were comparable outcomes between the intervention group and standard of care at the facilities. |
| Fox et al. (2021)31 | Non-pregnant adults ≥18 years old on ART between 12 and 48 months, and being on the same ART treatment regimen for ≥12 months, with two consecutive VL (<400 copies/mL) taken within the past 6 months. | Clinic or community location | ACs composed of adherent and stable patients on ART who met in groups of up to 30 every 2–3 months to receive group counselling, have a clinical assessment, and receive pre-packed ART medications. Patients return to the clinic every 6 months for a clinical exam. ACs were managed by a nurse with a facilitation from a lay staff and support from community health workers. | There was a timely medication refills among members of the AC. |
| Hanrahan et al. (2019)14 | Inclusion criteria adults ≥18 years, on the same ART regimen for adults ≥12 months, HIV suppressed for at least 12 months (2 most recent viral load results were <400 copies/mL). Exclusion criteria were being on stavudine-containing regimen, pregnant, chronic illness, active tuberculosis (TB), cancer, or mental illness, attending ART care with an HIV-positive child. | Clinic or community location | AC consisted of 25 to 30 members, who met every other month and led by a lay HIV counsellor. Once annually, the club visit was replaced by a visit to a medical doctor at the clinic. Participants met briefly as a group to discuss for about 1 hour on adherence- or ART-related topic. At the end of the club visit, each participant received their 2-month prepacked supply of ART medication, a repeat blood draw was taken if viral load was 50–400 copies/mL. Participants ask a ‘buddy’ to pick up their ART medication at a club visit. Participants who were unable to attend a club visit were allowed to pick up their medication at the clinic within 5 days. Clinic-based clubs were held at a space separate from the clinical exam rooms while community-based clubs were held at community venues. Club members were weighed and screened for current TB symptoms and those experiencing any TB symptom were referred to the clinic for further management. Reasons for referral from club-based care to standard care included; two consecutive buddy pickups, two consecutives ART medications pickups, becoming pregnant, infected with TB, ART regimen changes and viral rebound (1 viral load measurement of >400 copies/mL or 2 measurements of 50–400 copies/mL). | There was a loss (poor retention in care and viral rebound) from adherence club intervention, the loss was even high in community-based adherence clubs compared to those based at the clinic. |
| Limbada et al. (2022)28 | Adults living with HIV (≥18 years) who were receiving first-line ART for at least 6 months, virally suppressed (<1000 copies/mL) in the last 12 months, had no other health conditions requiring the clinicians attention were eligible for inclusion. | Community locations | Home-based delivery group, community HIV care providers visited the participants in their homes once every 3 months to provide adherence support, symptom screening and dispensed prepacked medications. In the AC group, consisting of 15–30 participants who met at an agreed communal venue for adherence support, symptom screening, and prepacked medications delivered by a community HIV care provider. In both intervention groups, participants returned to the clinic at 6 and 12 months for a clinical review, ART refill, and laboratory monitoring. | Differentiated service delivery models on ART refill were as effective as facility-based care in terms of viral suppression. |
| Myer et al. (2021)22 | Women living with HIV on their first postnatal clinic visit, initiated ART during pregnancy with age 18 years or older, had a live infant at the time of screening, viral load VL less than 400 copies/mL within the last 3 months and clinically stable. | Clinic or community location | AC comprises 25–30 individuals who met for 1–2 h on weekdays every 2 months, except for the end-of-year period when they meet once in 4 months. During the meeting community health care workers weigh each patient and perform tuberculosis (TB) symptom screening. A nurse sees patients once a year to conduct a clinical assessment and VL testing. | Early differentiated service delivery was associated with reduced viraemia through 24 months postpartum. |
| Odayar et al. (2022)23 | HIV-positive adults ≥18 years on 4-month post-ART, not on treatment for tuberculosis (TB). Participants who were pregnant, had co-morbidities requiring regular clinical follow-up were excluded. Those with VL <400 copies/mL were eligible for inclusion in this trial. | Clinic location | AC comprised of 25–30 patients who meet approximately 1 hour for every 2 months, except during the end of the year when the appointment interval was 4 months. Clubs were run by community health care workers (CHWs) who provided a group health education and adherence talk. CHWs conducted a weight check and symptom screen and dispense pre-packed ART for each club member. A nurse attended an annual visit per club to withdraw blood for routine VL and clinical assessment. Patients could ask someone (‘a buddy’) to collect their ART medication on their behalf. | Stable adults referred to differentiated service delivery models at 4 months post-ART initiation had comparable virologic outcomes at 12 months on ART as those attended in primary health facility clinics. |
| Roy et al. (2020)11 | HIV positive individuals, age ≥14 years, on ART >6 months, not acutely ill, and CD4 count not <200 cells/mm3. | Clinic location | AC comprised of 30 HIV-positive individuals on ART who meet every 2 months in the first 6 months and every 3 months thereafter, during off-hours at the facility (evenings or weekends) to receive medication refills, symptom screening, and group psychosocial support. AC was led by pharmacy technologists, who were responsible for prepackaging ART medication prior to the meeting and dispensing the ART medication to the members. Groups were supported by 2 community lay health workers who were responsible for conducting symptom screening and leading a group counselling session. Blood samples were taken for analysis at the meeting, and follow-up with a medical officer would occur only once a year. Patients were allowed to send ‘a buddy’ for medication pickup on their behalf. Patients were referred back to facility-based care because of acute illness, positive symptoms screening or became pregnant. | ACs were associated with timely ART medications pick-ups among HIV-infected adults. |
| Zerbe et al. (2020)29 | Newly postpartum, breastfeeding women who were on ART. | Community location | Clubs met every 2–4 months. Patients were weighed, screened for symptom such as TB and participated in health education sessions. Community-based ACs were facilitated by lay counsellors and housed within community venues. Pre-packed ART was dispensed at all visits to patients or ‘treatment buddies’ sent by the patient. Clinical review and viral load testing were conducted annually by a nurse. Patients were referred back to routine primary health facility if they missed a scheduled club visit, had a detectable viral load (>50 copies/mL) or chronic illness requiring clinical management. | The study found comparable outcomes related to retention and viral suppression at 12 months postpartum between women choosing adherence clubs and those choosing primary health care facilities. |
Note: Clinically stable patients were defined as a people living with HIV receiving ART for at least 1 year with no adverse medication reactions requiring regular monitoring, no current illnesses or pregnancy, the patient with two consecutive undetectable viral load measures or CD4 counts above 200 cells/mm3.
3.4 |. Outcomes between adherence clubs versus standard of care
Three RCTs compared the retention in care and viral suppression between ACs and the SoC.12,28,29 The RR for retention in care was 1.09 (95% CI: 1.08–1.11, I2: 0%, p-value for heterogeneity: <0.96) while for viral suppression the RR was 1.01 (95% CI: 1.00–1.02, I2: 0%, p-value for heterogeneity: <0.85). The RR in terms of retention of care viral suppression between the standard of care (SoP) group and intervention group (ACs) were comparable (Figure 4).
FIGURE 4.
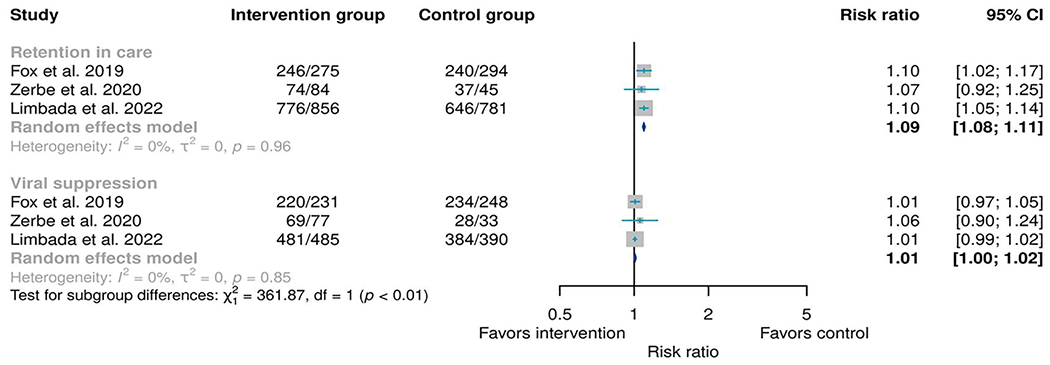
Meta-analysis of any type of adherence club model as an intervention when compared to any type of standard of care as a control. Retention was determined 12 months from the baseline while viral suppression was defined as any viral load below 1000 copies/mL taken any time above 6 months from the baseline.
4 |. DISCUSSION
We systematically reviewed and quantitatively analysed the current evidence related to clinical outcomes of Differentiated Service Delivery (DSD) models, especially Adherence clubs (ACs). We identified 16 studies describing clinical outcomes for different DSD models implemented in six sub-Saharan African countries. Seven studies used ACs models to determine clinical outcomes, of which five studies compared the ACs as an intervention versus standard of care (SoC) as a control.
We found comparable clinical outcomes in terms of sustained retention in care and viral suppression between patients on DSD models and those undergoing facility-based care (SoP). In contrary to the review by Long et al. 2020,34 this review used the most recent high-quality evidence from the randomised controlled clinical trials where most of the studies compared the SoC and the intervention (DSD models). Although Long et al. 2020,34 concluded that the then existing evidence on clinical outcomes of DSD models for HIV treatment in sub-Saharan Africa had limited data in terms of both quantity and quality, their findings on retention in care and viral suppression concurred and were roughly equivalent to those in conventional models of care (SoC). It appears that providing six-monthly ART refills in ACs did not compromise viral suppression or retention in care when compared to the standard practice of two-monthly ART refills.13 This result suggests that the implementation of six-monthly refills could be a viable option for managing HIV patients in ACs, potentially reducing the frequency of clinic visits and the burden on both patients and the healthcare system.
Our review included a pragmatic randomised controlled trial14 which evaluated the impact of community-based versus clinic-based ACs and found adverse outcomes in terms of retention in care, the loss was even more for patients who participated in the community-based model. Hanrahan et al. 201914 urged that studies need to have better understanding of patient-level factors associated with successful retention in care. However, these findings were limited to lack of SoC as a comparison group. In support of Hanrahan et al. observation, a scooping review40 found various barriers to implementing DSD models; patient-level factors included fear of stigma and discrimination, low knowledge on the DSD model, ARV drug stockouts, and supply chain inconsistencies. Additionally, there was low level of knowledge on DSD model among health care provider, lack of resources and lack of model adoption from providers.
On the other hand, several factors such as perceived reduction in cost of visit for patients, reduction in staff workload and overburdening of health facilities, and improved or maintained adherence and retention were reported as facilitators.35 This warrants the design of effective strategies through the Implementation Science approach.36 A study conducted in Zambia found that ACs helped in reducing the late ART drug pick up and decongestion at the primary health facilities. This model was effective and yielded positive outcomes in terms of acceptability, appropriateness and feasibility.11 In this regard, more evidence is needed to explore other outcomes such as adoption, penetration, cost and feasibility of implementing these models in resources-limited settings.36,37 As recommended by the WHO our findings were limited to clinically stable patients.9
5 |. CONCLUSIONS
Clinical outcomes from DSD models, especially ACs, were found to be almost similar to the conventional models or standard of care. AC models have been proven to sustain retention in HIV care and adherence to antiretroviral therapy, particularly for clinically stable patients. If implemented, adherence clubs could help reduce the unnecessary burden on the HIV healthcare system, including congestion at health facilities. Therefore, we recommend undertaking Implementation research to explore effective strategies that could facilitate the uptake and scaling-up of this evidence-based intervention. The implementation science approach could help evaluate the outcomes (e.g., adoption, penetration, cost, and sustainability) for implementing this evidence-based intervention in resource-limited settings.
ACKNOWLEDGEMENTS
Authors thank Covidence, a not-for-profit organisation dedicated to quality evidence synthesis and its contribution to evidence-based decision making for assigning a free review to the account managed by (Belinda J. Njiro). George M. Bwire, Christopher R. Sudfeld and Muhammad Bakari were partially supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009775. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
Fogarty International Center of the National Institutes of Health, Grant/Award Number: D43TW009775
Abbreviations
- AC
adherence club
- ART
antiretroviral therapy
- ARV
antiretroviral
- DSD
differentiated service delivery
- RC
retention in care
- SoC
standard of care
- VS
viral suppression
Footnotes
CONFLICT OF INTEREST STATEMENT
Authors declare that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
Data used to draw this conclusion are available from the corresponding author on reasonable request
REFERENCES
- 1.UNAIDS. Global HIV & AIDS Statistics — Fact Sheet; 2022. Published May 3, 2022. Accessed 3 May 2023. https://www.unaids.org/en/resources/fact-sheet
- 2.WHO African Region. World AIDS Day; 2022. Published May 2, 2022. Accessed 3 May 2023. https://www.afro.who.int/regional-director/speeches-messages/world-aids-day-2022
- 3.De Lay PR, Benzaken A, Karim QA, et al. Ending AIDS as a public health threat by 2030: time to reset targets for 2025. PLoS Med. 2021; 18(6):e003649. 10.1371/journal.pmed.1003649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath K, Levi J, Hill A. The Joint United Nations Programme on HIV/AIDS 95-95-95 targets: worldwide clinical and cost benefits of generic manufacture. AIDS. 2021;35(Suppl 2):S197–S203. 10.1097/QAD.0000000000002983 [DOI] [PubMed] [Google Scholar]
- 5.Kiekens A, Mosha IH, Zlatić L, et al. Factors associated with HIV drug resistance in Dar es Salaam, Tanzania: analysis of a complex adaptive system. Pathogens. 2021;10(12):1535. 10.3390/pathogens10121535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiekens A, Dierckx de Casterlé B, Pellizzer G, et al. Exploring the mechanisms behind HIV drug resistance in sub-Saharan Africa: conceptual mapping of a complex adaptive system based on multidisciplinary expert insights. BMC Publ Health. 2022;22(1):1–15. 10.1186/s12889-022-12738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fl K, Decroo T, van den Borne B, van de Pas R. ART adherence clubs in the Western Cape of South Africa: what does the sustainability framework tell us? A scoping literature review. J Int AIDS Soc. 2019;22(3):e25235. 10.1002/jia2.25235/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Updated Recommendations on Service Delivery for the Treatment and Care of People Living with HIV; 2021. Accessed 11 May 2023. https://openwho.org/courses/hiv-treat [PubMed]
- 9.Waldrop G, Doherty M, Vitoria M, Ford N. Stable patients and patients with advanced disease: consensus definitions to support sustained scale up of antiretroviral therapy. Trop Med Int Health. 2016;21(9):1124–1130. 10.1111/tmi.12746 [DOI] [PubMed] [Google Scholar]
- 10.Long L, Kuchukhidze S, Pascoe S, et al. Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: a rapid systematic review. J Int AIDS Soc. 2020;23(11). 10.1002/jia2.25640/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy M, Bolton-Moore C, Sikazwe I, et al. Participation in adherence clubs and on-time medication pickup among HIV-infected adults in Zambia: a matched-pair cluster randomized trial. PLoS Med. 2020;17(7):e1003116. 10.1371/journal.pmed.1003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MP, Pascoe S, Huber AN, et al. Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. PLoS Med. 2019;16(7):e1002874. 10.1371/journal.pmed.1002874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy T, Grimsrud A, Keene C, et al. Twenty-four-month outcomes from a cluster-randomized controlled trial of extending antiretroviral therapy refills in ART adherence clubs. J Int AIDS Soc. 2020;23(12). 10.1002/jia2.25649/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanrahan CF, Schwartz SR, Mudavanhu M, et al. The impact of community-versus clinic-based adherence clubs on loss from care and viral suppression for antiretroviral therapy patients: findings from a pragmatic randomized controlled trial in South Africa. PLoS Med. 2019;16(5):e1002808. 10.1371/journal.pmed.1002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberer JE, Sabin L, Amico KR, et al. Improving antiretroviral therapy adherence in resource-limited settings at scale: a discussion of interventions and recommendations. J Int AIDS Soc. 2017;20(1):21371. 10.7448/IAS.20.l.21371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev Española Nutr Humana Dietética. 2016;20(2):148–160. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019:205–228. 10.1002/9781119536604.ch8 [DOI] [Google Scholar]
- 19.Cochrane. Assessing Risk of Bias in a Randomized Trial; 2023. Published May 5, 2023. Accessed 4 May 2023. https://training.cochrane.org/handbook/current/chapter-08
- 20.Hickey MD, Ouma GB, Mattah B, et al. The Kanyakla study: randomized controlled trial of a microclinic social network intervention for promoting engagement and retention in HIV care in rural western Kenya. PLoS One. 2021;16(9 September):e0255945. 10.1371/journal.pone.0255945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavhu W, Willis N, Mufuka J, et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. Lancet Global Health. 2020;8(2):e264–e275. 10.1016/S2214-109X(19)30526-1 [DOI] [PubMed] [Google Scholar]
- 22.Myer L, Odayar J, Malaba TR, et al. Improved virologic outcomes in postpartum women living with HIV referred to differentiated models of care. AIDS. 2022;36(15):2203–2211. 10.1097/QAD.0000000000003385 [DOI] [PubMed] [Google Scholar]
- 23.Odayar J, Malaba TR, Allerton J, et al. Virologic outcomes after early referral of stable HIV-positive adults initiating ART to community-based adherence clubs in Cape Town, South Africa: a randomised controlled trial. PLoS One. 2022;17(11 November):e0277018. 10.1371/journal.pone.0277018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrand RA, Simms V, Dauya E, et al. The effect of community-based support for caregivers on the risk of virological failure in children and adolescents with HIV in Harare, Zimbabwe (ZENITH): an open-label, randomised controlled trial. Lancet Child Adolesc Health. 2017;1(3):175–183. 10.1016/S2352-4642(17)30051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnabas RV, Szpiro AA, van Rooyen H, et al. Community-based antiretroviral therapy versus standard clinic-based services for HIV in South Africa and Uganda (DO ART): a randomised trial. Lancet Global Health. 2020;8(10):e1305–e1315. 10.1016/S2214-109X(20)30313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatti G, Ngorima-Mabhena N, Tiam A, et al. Community-based differentiated service delivery models incorporating multi-month dispensing of antiretroviral treatment for newly stable people living with HIV receiving single annual clinical visits: a pooled analysis of two cluster-randomized trials in southern Africa. J Int AIDS Soc. 2021;24(S6). 10.1002/jia2.25819/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich S, Siika A, Mwangi A, et al. Assessment, and Outcomes of a Community-Based Model of Antiretroviral Care in Western Kenya through a Cluster-Randomized Control Trial; 2021. www.jaids.com [DOI] [PMC free article] [PubMed]
- 28.Limbada M, Macleod D, Situmbeko V, et al. Rates of viral suppression in a cohort of people with stable HIV from two community models of ART delivery versus facility-based HIV care in Lusaka, Zambia: a cluster-randomised, non-inferiority trial nested in the HPTN 071 (PopART) trial. Lancet HIV. 2022;9(1):e13–e23. 10.1016/S2352-3018(21)00242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerbe A, Brittain K, Phillips TK, et al. Community-based adherence clubs for postpartum women on antiretroviral therapy (ART) in Cape Town, South Africa: a pilot study. BMC Health Serv Res. 2020;20(1):621. 10.1186/s12913-020-05470-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatti G, Ngorima-Mabhena N, Mothibi E, et al. Outcomes of Three-Versus Six-Monthly Dispensing of Antiretroviral Treatment (ART) for Stable HIV Patients in Community ART Refill Groups: A Cluster-Randomized Trial in Zimbabwe; 2020. www.jaids.com [DOI] [PMC free article] [PubMed]
- 31.Fox MP, Pascoe S, Huber AN, et al. Short-term outcomes from a cluster randomized evaluation of adherence clubs as part of differentiated HIV care in South Africa. JAIDS J Acquir Immune Defic Syndromes. 2021. Publish Ahead of Print. 10.1097/QAI.0000000000002728 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JPT. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158–1160. 10.1093/ije/dyn204 [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses Testing for Heterogeneity. BMJ. 2003:327:557. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long L, Kuchukhidze S, Pascoe S, et al. Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: a rapid systematic review. J Int AIDS Soc. 2020;23(e25640). 10.1002/jia2.25640/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belay YA, Yitayal M, Atnafu A, Taye FA. Barriers and facilitators to the implementation and scale up of differentiated service delivery models for HIV treatment in Africa: a scoping review. BMC Health Serv Res. 2022;22(1):1431. 10.1186/s12913-022-08825-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churruca K, Ludlow K, Taylor N, Long JC, Best S, Braithwaite J. The time has come: embedded implementation research for health care improvement. J Eval Clin Pract. 2019;25(3):373–380. 10.1111/jep.l3100 [DOI] [PubMed] [Google Scholar]
- 37.Theobald S, Brandes N, Gyapong M, et al. Implementation research: new imperatives and opportunities in global health. Lancet. 2018;392(10160):2214–2228. 10.1016/S0140-6736(18)32205-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to draw this conclusion are available from the corresponding author on reasonable request


