A young boy touches a hot oven plate. Heat sensors in his palm realise that the temperature exceeds a minimum safety threshold and activate sensory nerves in the hand. Within milliseconds, the information is passed on through the arm and the spinal cord to the brain. The brain interprets the incoming information as pain, registers that the hand is in danger of being burned and orders it to retract. Motor nerves in the spinal cord and the arm then transmit this order to the muscles that contract and pull the hand away from the oven plate. The hand is now safe, but the experience of pain caused by the heat remains. The child begins to cry and runs to his mother for consolation. He will remember this painful experience and, on the whole, steer clear of hot oven plates.
Pain is good for us. Over millions of years, the nervous system has developed the ability to experience pain as a protective system to warn us of imminent dangers and to keep us out of trouble. But although this data-processing system is sensitive, it is prone to errors: unlike motor nerves that cannot re-grow once cut, sensory nerves are trickier. And if something goes haywire in the delicate wiring of the sensory nervous system, it can create problems of its own. Injured nerves can grow back erroneously or start firing erratically and thus produce the sensation of pain with no physical external influence. Or the processing of painful sensations in the brain is short-circuited and results in a permanent sensation of pain. These feelings are what is known as chronic pain or, in some instances, neuropathic pain, reflecting that it is a disease state of the nervous system. Of course, there are other causes, such as inflammation or diseases of the muscle, but many instances of chronic pain are now believed to have a neurological component.
Indeed, any injury or serious disease creates painful emotions for the patient, and physicians routinely use the experience of pain to their advantage. The localisation of pain and how it is perceived reveals a lot about the nature of the disease—‘Where does it hurt?’ is one of the common questions doctors ask. Treatment of pain is usually achieved by treating the underlying disease, assuming that healing will take care of the pain as well. But when patients experience chronic pain with no apparent cause, doctors have been at a loss, especially as most common painkillers are not effective against many forms of chronic pain.
This is a serious disease that slowly destroys patients, as they are increasingly unable to control their lives. And it is surprisingly widespread. Estimates in the USA put the number of Americans suffering from chronic pain between 30 and 50 million, which not only causes major problems for these individuals, but also has economic and social implications. The costs of treatment and lost productivity total an estimated loss of US$100 billion for the US economy.
Recently, however, progress has been made in uncovering exactly what has gone awry with the nervous system in chronic pain patients. Here, investigating the mechanisms of phantom limb pain that is ‘caused’ by a part of the body that no longer exists has largely contributed to our understanding of the development and maintenance of pain perceptions. These studies have shown that the brain itself plays a major role in chronic pain when it reacts to past experiences and reorganises itself following amputation. This increasing knowledge about the underlying biochemical and physiological processes is paramount for devising effective treatments, both pharmacological as well as psychological, to help those who suffer from chronic pain.
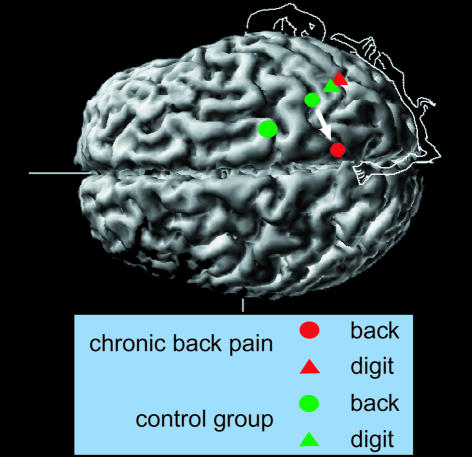
Any sensory information, including painful sensations, is eventually processed in the outside layer of the brain, the primary somatosensory (S1) cortex. This part of the brain is organised in ‘maps’ that represent a point-to-point connection between well-defined parts of the body and their respective anatomical location in the cortex. These maps and connections were thought to be established during learning and skill acquisition in early child development and then fixed for life. But in the late 1980s and early 1990s, psychologists accumulated evidence that this dogma of the hard-wired brain was no longer tenable. They found that the maps representing parts of the body are still pliable in adult mammals and that they change in response to experience and injury. For instance, after the amputation of a finger, neuronal input from the zones adjacent to the territory that represent the now-absent finger expands into the vacated space in the S1 cortex (Figure 1). Such changes can be quite major: for example, dorsal rhizotomies that enlarge the representation of the mouth into the region that formerly represented the hand and arm can span a distance of several centimetres (Kaas, 2000). This is supported by findings that a certain brain region will expand if it receives behaviourally relevant input, whereas no use will lead to a reduced representation (Recanzone, 2000).
Fig. 1. Representation in primary somatosensory cortex of the mouth and two digits in upper extremity amputees with and without phantom limb pain. Note how the mouth representation of the amputation side has shifted into the hand region in the amputees with pain.
Such shifts in the S1 cortex are quite common in amputees who have lost an upper extremity—up to 80% experience pain from the region of the amputated limb. Thus, it is reasonably safe to assume that cortical reorganisation and phantom sensation in those parts of the cortex that formerly represented the amputated limb are linked to each other. Indeed, Ramachandran et al. (1992) suggested that the phantom limb sensations that occured when certain areas of the face of human amputees were stimulated were linked to reorganisations similar to those that had earlier been observed in the S1 cortex of primates.
Based on their research, psychologists did similar experiments in amputees and showed that cortical reorganisation also occurs in humans and that it is highly correlated with phantom limb pain (Flor et al., 1995). Amputees suffering from phantom limb pain showed a shift of adjacent areas into the zone that formerly represented the amputated limb in the S1 cortex, whereas pain-free amputees displayed a cortex structure that was not significantly different from that of healthy controls. Further studies using functional magnetic resonance imaging and transcranial magnetic stimulation revealed similar changes in the cortical maps of the motor system in those amputees who suffered from phantom limb pain. But these cortical alterations are clearly not the only cause. Similar changes are also present in lower levels of the neuraxis, such as the spinal cord, the brain stem and the thalamus, but it is not known yet whether cortical reorganisation is a cause or a consequence of these alterations. Indeed, the S1 cortex is only one important part of the cortical network involved in pain processing, which includes other parts of the brain as well—the secondary somatosensory cortex, the posterior parietal cortex, the prefrontal cortex, the anterior cingulate and the insula (Treede et al., 1999).
The relationship between phantom limb pain and cortical changes was further substantiated by Birbaumer et al. (1997), who reported that eliminating sensory input from the amputation stump reversed cortical reorganisation and phantom limb pain in 50% of the amputees they studied. This prompted the authors to suggest that this phenomenon may be maintained in some amputees due to input from peripheral nerves that had been damaged as a result of the amputation and that continued to send random signals to the brain.
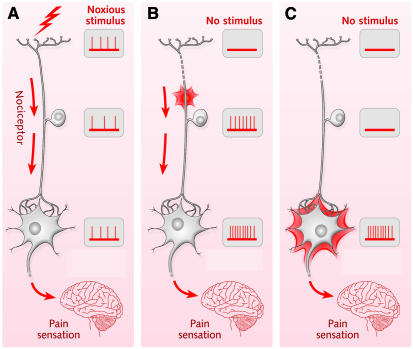
Indeed, changes in the peripheral sensory nervous system may also explain why many patients suffer from chronic pain after surgery. Here, cortical reorganisation may be a consequence of damaged nerves that continue to send signals and trigger further changes in the sensory-processing system. Loss of inhibitory C-fibre nerves, for instance, leads to an expansion of receptive fields in the S1 cortex and might trigger further reorganisation, as those sensory nerves that were normally inhibited by the C-fibres increase their activity. Moreover, when C-fibres are lost, terminals of Aβ-fibres may sprout in the dorsal horn and connect to pain-transmitting neurones. Activation of sympathetic nerves may also act on both peripheral nociceptors and the dorsal root ganglion and contribute to chronic pain (Figures 1 and 2; Woolf and Mannion, 1999). Indeed, microneurographic recordings from nerves supplying the former hand region have shown that considerable spontaneous activity of a random nature takes place in these nerves. This might be an additional cause of cortical reorganisation, as random input seems to increase shifts in the cortical map.
Fig. 2. Nociceptors (specialised sensory receptors that respond to pain) can cause chronic pain if they are damaged. (A) In the normal state, if a nociceptor is activated by a noxious stimulus, the nerve cell transmits the information via the sensory system to create a painful sensation in the brain. (B) If the nociceptor is damaged, it can start firing randomly and activate other nerves that eventually cause phantom pain. (C) If the nociceptor was an inhibitory nerve, its inactivation through damage could activate other nerves in the sensory network that eventually cause phantom pain.
Several other mechanisms that may cause changes in the S1 cortex on the physiological and cellular level have been discussed. Short-term plastic alterations may come from the activation of normally silent connections, whereas long-term changes may be related to long-term potentiation, Hebbian learning and nerve sprouting. Florence et al. (1998) have shown recently that nerve axons sprout in the cortex of amputated monkeys with cortical reorganisation. Animal studies where nerve destruction led to reduced long-term depression also revealed reorganisational changes in the anterior cingulate. Thus, it is likely that, in addition to the S1 cortex, other cortical areas undergo malleable changes as well and contribute to chronic pain.
Earlier in the 1990s, Katz and Melzack (1990) observed that patients frequently experience pain they had commonly felt before the amputation and suggested that somatosensory memories play an important role in establishing cortex reorganisation and chronic phantom pain. According to their hypothesis, the long-term experience of pain leads to a memory trace in the brain. Such a process would only be partially subject to conscious experience: it is much more likely that pain memories change behaviour and experience with the patient not necessarily being aware of them. Thus, intense and/or long-standing states of pain might lead to an enhanced representation of the pain in the S1 cortex and other brain areas, which will expand the area devoted to the processing of painful input. After the amputation, the zones that originally processed neuronal input from the now-missing hand or arm would still code for pain. These memories can be stimulated by neuronal activity in neighbouring areas and are more likely to lead to phantom pain than to non-painful sensations. Furthermore, as adjacent zones in the cortex expand into the areas that harbour these memories of pain, they further increase the probability of activating these memories. Thus, signals coming from the face, for instance, would now be processed in the S1 area that formerly represented the amputated limb, which would explain why facial stimulation can trigger phantom pain.
Chronic pain patients often show hyperalgesia (exaggerated pain perception) and allodynia (perception of pain with innocuous stimulation). For example, pain thresholds were found to be significantly lower in patients with chronic back pain and episodic headaches, increasingly so the more the chronic pain had manifested. Although peripheral as well as spinal and thalamic mechanisms have been implicated, cortical changes might also play a major role in these alterations in sensitivity. We compared the representation of a finger (unaffected site) and the back (affected site) in chronic back pain patients, a subchronic group and healthy controls and found a shift to occur in the representation of the back in the S1 cortex (Flor et al., 1997). In the chronic patients, but not the subchronic patients, this area in the cortex had shifted towards the leg area. In addition, the magnetic field relating to this stimulation was enhanced in the chronic patients, which implies increased activity in the S1 cortex. The amount of expansion of the back region was positively correlated with chronicity, suggesting that this pain-related cortical reorganisation develops over time. The more chronic the pain, the more reactive the patients’ S1 cortex had become, which may be due to a stimulation-induced reorganisation. These data suggest that chronic pain indeed leads to an expansion of the cortical representation zone related to nociceptive input similar to the expansions observed with other types of behaviourally relevant stimulation. Nociceptive input is of high relevance for the organism, as it enhances the representation of this form of painful stimulation in order to generate an adequate response. It should be emphasised again that this is a type of implicit memory that leads to behavioural and perceptual changes, such as hyperalgesia and allodynia, of which the patient is not aware. It is impossible, therefore, for the patient to counteract these pain memories.
Memories of pain, established over a period of time, might also explain why many patients suffer from chronic pain after a long-term injury or disease. As the experience of pain is implanted in the memory, it continues to torture the patient even after the disease or injury has been treated. The somatosensory pain memories manifest themselves in alterations in the S1 cortex and may contribute to hypersensitivity even in the absence of peripheral stimulation. Psychological processes, such as conditioning or attention, can further establish additional and more widespread memories or enhance existing ones. In addition to local changes in the cortex, chronic states of pain are also associated with increased cortical excitation that may significantly contribute to reorganisation.
Consequently, the prevention of pain memories will be an important task in pain management for chronic pain patients. There are at least four possible targets where therapists can intervene. First, chronic pain must be prevented as early as possible by pharmacological and psychological interventions in order to keep pain memories from being established. Secondly, cortical reorganisation as a consequence of amputation could further be suppressed by using pharmacological agents that are known to impair cortical reorganisation. Thirdly, chronic pain itself could be reversed by training procedures with the aim of influencing reorganisation of the cortex. And, finally, substances that play an important role in cortex reorganisation could be counteracted by using antagonists (Flor and Birbaumer, 2000).
There are pharmacological agents that can be used prior to amputation to prevent changes in the S1 cortex that represent pain memories and thus could suppress phantom limb pain. Among these substances, GABA agonists, NMDA receptor antagonists and anticholinergic substances seem to be the most promising. A recent double-blind placebo-controlled study that used the NMDA receptor antagonist memantine in the pre-operative phase reported a decrease of phantom limb pain from 72 to 20% within 1 year of amputation.
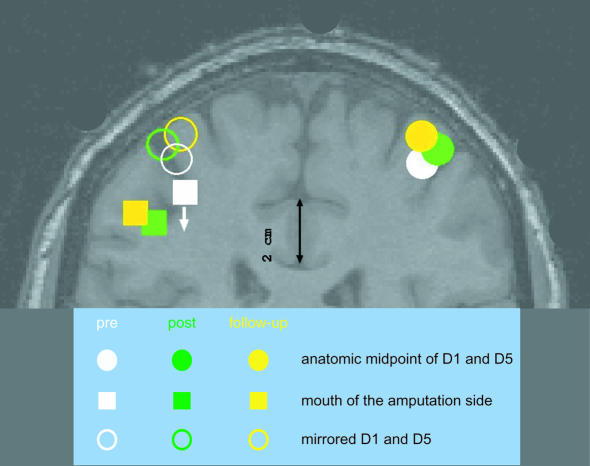
Apart from pharmaceutical interventions, therapies may also involve psychological treatment to counteract established pain memories. In particular, extensive training with a prosthesis could be useful in reducing phantom limb pain. Such stimulation-related procedures have already shown some efficiency in treating amputees with chronic pain. Intense sensory input to the cortical zone that represented the amputated limb through the use of a myoelectric hand or arm prosthesis was found to reduce both cortical reorganisation and phantom limb pain. Where the use of a prosthesis is not possible, sensory discrimination training might also be beneficial. In one study, electrodes were closely spaced over the amputation stump to excite the nerves that formerly supplied the amputated arm. Patients then had to discriminate the frequency and the location of the stimulation in an extended training schedule that encompassed 90 min per day over a 2-week period. This programme led to substantial improvements for the trained patients. The progress in pain management was accompanied by changes in cortical reorganisation, indicating that the representation of the mouth that expanded after the amputation shifted back to the normal state (Figure 3; Flor et al., 2001). These findings are in line with other evidence suggesting that behavioural training can have immense effects on cortical representations. In the future, direct modification of cortical activity by a biofeedback application to target slow cortical potentials, somatosensory evoked potentials, EEG rhythms or blood flow changes related to the experience of heightened pain might thus become effective tools in pain treatment.
Fig. 3. Sensory discrimination training with amputees can lead to a reversal of cortex reorganisation. Here, the representation of the mouth in the primary somatosensory cortex reversed to its original position after 2 weeks of follow-up training (see arrow).
Even so, these therapies will probably not be sufficient to help those patients whose lives are slowly being destroyed by chronic pain. The brain and the sensory nervous system are highly intricate networks and we are only just beginning to understand the physiological and biochemical processes that govern their function. In order to treat states of chronic and phantom pain, we must learn more about the nervous system and the changes it undergoes when responding to sensations from the outside world. Given the seriousness of chronic pain and the large number of people whose lives are dominated by it, this is a worthwhile undertaking.

References
- Birbaumer N., Lutzenberger, W., Montoya, P., Larbig, W., Unertl, K., Töpfner, S., Grodd, W., Taub, E. and Flor, H. (1997) Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J. Neurosci., 17, 5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. and Birbaumer, N. (2000) Phantom limb pain: cortical plasticity and novel therapeutic approaches. Curr. Opin. Anesth., 13, 561–564. [DOI] [PubMed] [Google Scholar]
- Flor H., Elbert, T., Knecht, S., Wienbruch, C., Pantev, C., Birbaumer, N., Larbig, W. and Taub, E. (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature, 375, 482–484. [DOI] [PubMed] [Google Scholar]
- Flor H., Braun, C., Elbert, T. and Birbaumer, N. (1997) Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett., 224, 5–8. [DOI] [PubMed] [Google Scholar]
- Flor H., Denke, C., Schäfer, M. and Grüsser, S. (2001) Sensory discrimination training alters both cortical reorganization and phantom limb pain. Lancet, 357, 1763–1764. [DOI] [PubMed] [Google Scholar]
- Florence S.L., Taub, H.B. and Kaas, J.H. (1998) Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science, 282, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. (2000) The reorganization of sensory and motor maps after injury in adult mammals. In Gazzaniga, M.S. (ed.), The New Cognitive Neurosciences. The MIT Press, Cambridge, MA, pp. 223–236.
- Katz J. and Melzack, R. (1990) Pain ‘memories’ in phantom limbs: review and clinical observations. Pain, 43, 319–336. [DOI] [PubMed] [Google Scholar]
- Ramachandran V.S., Steward, M. and Rogers-Ramachandran, D. (1992) Perceptual correlates of massive cortical reorganization. Neuroreport, 3, 583–586. [DOI] [PubMed] [Google Scholar]
- Recanzone G.H. (2000) The cerebral cortical plasticity: perception and skill acquisition. In Gazzaniga, M.S. (ed.), The New Cognitive Neurosciences. The MIT Press, Cambridge, MA, pp. 237–247.
- Treede R.D., Kenshalo, D.R., Gracely, R.H. and Jones, A.K. (1999) The cortical representation of pain. Pain, 79, 105–111. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. and Mannion, R.J. (1999) Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet, 353, 1959–1964. [DOI] [PubMed] [Google Scholar]