Abstract
Aging is characterized by progressive loss of organ and tissue function, and the immune system is no exception to that inevitable principle. Of all the age-related changes in the body, reduction of the size of, and naïve T (Tn) cell output from, the thymus occurs earliest, being prominent already before or by the time of puberty. Therefore, to preserve immunity against new infections, over much of their lives, vertebrates dominantly rely on peripheral maintenance of the Tn cell pool in the secondary lymphoid organs (SLO). However, SLO structure and function subsequently also deteriorate with aging. Several recent studies have made a convincing case that this deterioration is of major importance to the erosion of protective immunity in the last third of life. Specifically, the SLO were found to accumulate multiple degenerative changes with aging. Importantly, the results from adoptive transfer and parabiosis studies teach us that the old microenvironment is the limiting factor for protective immunity in old mice. In this review, we discuss the extent, mechanisms, and potential role of stromal cell aging in the age-related alteration of T cell homeostatic maintenance and immune function decline. We use that discussion to frame the potential strategies to correct the SLO stromal aging defects - in the context of other immune rejuvenation approaches, - to improve functional immune responses and protective immunity in older adults.
Keywords: Immune aging, Lymphoid stromal cells, Lymph node involution, Secondary lymphoid organs, T cell homeostasis
1. Introduction.
Immune defenses decline in the last third of life, resulting in a pronounced susceptibility of older adults to infectious diseases [1]. This susceptibility is most evident in the case of infections mediated by new, previously unencountered, microbial pathogens, where there is generally a log-linear accumulation of mortality and morbidity by age. For example, compared to the population between 18–39 years of age, morbidity and mortality from epidemics or pandemics caused by viruses such as West Nile virus (WNV), chikungunya (CHIKV), severe acute respiratory syndrome (SARS), and SARS-CoV-2 increases up to tenfold in those >50 years of age [2–5], increasing steeply thereafter. Indeed, in the case of SARS-CoV-2, people older than 80 died at 270–300-fold higher rates compared to those between 18 and 39 years of age [6]. While the changes affecting other organ systems may have contributed to such age-related predisposition, the primary reason for this vulnerability is recognized to be the aging of the immune system, often also called immunosenescence [7]. Successful immune defense against new intracellular infections (viruses, intracellular bacteria, and intracellular parasites) requires that naïve T (Tn) lymphocytes, specific for the invading microbe, are present in sufficient numbers and are activated in a timely manner to robustly proliferate and differentiate into anti-pathogen effector cells that will directly attack infected cells and/or inhibit microbe’s multiplication to curtail and eradicate the infection. Other Tn cells similarly must divide and differentiate to help B cells make strong antibody (Ab) responses.
Within the immune system, many components must act in concert to ensure immune defense, and most, if not all, have been shown to be affected by aging [7–9]. One of the main challenges for the field has been to identify key and proximal changes occurring with immune aging and to discern which of them may provide promising targets and avenues for intervention to delay immune aging and ameliorate immune function in older adults. In that regard, aging of the immune system first affects the T cell lineage, beginning exceptionally early with clear signs of thymic involution well before puberty [10]. This results in the reduction of new Tn cell production to 10% by puberty [11] and to ~1% by the age of 50 [12] relative to thymic output in childhood. As the production declines rapidly, maintenance of existing Tn cells produced in youth becomes a primary mechanism that preserves the numbers of these cells [13, 14]. Such “peripheral” maintenance (denoting the fact that it does not happen in the thymus or bone marrow, which are considered primary, or “central” lymphoid organs) occurs in the secondary lymphoid organs (SLO), most notably in the lymph nodes (LN), and depends on LN stromal cells [15–17]. However, in the last third of life, peripheral maintenance of Tn cells begins to shows signs of age-related deterioration, leading to an absolute loss of two-thirds or more of Tn cells in blood and SLO [18–22]. Molecular and cellular drivers of this deterioration remain incompletely understood.
Important questions concerning the loss of protective immunity in older adults include understanding not only the drivers of each facet of the aging process but more importantly whether and how deeply each component of the immune system is affected by aging. It has been long thought that T cells lose proliferative and effector ability with aging [23, 24]. Many of the experiments giving support to that view did not separate naïve from memory cells. As a consequence, their readouts simply reflected unequal representations of highly proliferative Tn cells (higher numbers in young and much lower in old) relative to terminally differentiated, and poorly- or non-proliferating, effector memory cells (including the human, effector memory cells reexpressing CD45RA, a.k.a. Temra, cells). More recent rigorous experiments from our group showed that on a cell-by-cell basis, highly purified CD8 Tn cells from old mice exhibit no discernible proliferation and effector differentiation defects when transferred into, and activated in, adult recipients [25]. Conversely, adult Tn cells responded poorly to infection when transferred into old recipients [25]. Major defects were observed when pathogen detection, uptake, and generation of the initial inflammatory response were assessed in the old environment [8, 25–27], and most of the depressed CD8 Tn cell response could be corrected in old mice by providing exogenous IL-12 and IL-18 [25]. This paralleled the prior findings from Swain, Haynes, and colleagues showing improvement of CD4 Tn priming in old mice in the presence of proinflammatory adjuvants or recombinant IL-2 and IL-4 [28, 29].
The corollary of the above results is that while Tn numbers are depressed, their function remains fairly strong, implying that, beyond the defects of Tn generation and maintenance, the defects in function largely lie outside of Tn cells themselves. We want to stress that we do not imply that there are no age-related defects in Tn cells themselves at all, but rather point to the fact that in well-controlled experimental models, such defects appear mild and are functionally dwarfed by the defects in the Tn cell environment. To that effect, this review deals with Tn cell-extrinsic changes with aging, that affect the environment in which Tn cells operate, and that provide critical signals to coordinate Tn cell maintenance and their initial immune responses. In that regard, parallels exist between stromal age-related changes found in primary (thymus, bone marrow) and secondary (LN, spleen) lymphoid organs, although thymic and LN stroma are made of different types of cells (epithelium in the thymus, mesenchymal cells in the LN). We will also examine the age-related changes in soluble factors that guide Tn cell maintenance, migration, and activation and will finally discuss potential modes of intervention to improve Tn cell maintenance and function with aging.
2. Impact of thymic involution on protective immunity against infections.
2.1. Age-related thymic involution.
As mentioned, thymic involution, characterized by a gradual decline in thymic cellularity and size, is the earliest characteristic of age-related immunosenescence, conserved in a wide variety of vertebrate animals, including humans, non-human primates, and mice. Thymic involution starts somewhere between 4–6 weeks in mice, and as early as 1 year in humans and continues at a much faster rate (approximately 3% loss in size per year) until midlife before it slows down to 1% loss per year [30]. Structurally and morphologically there is a loss of thymic stromal cells, particularly of cortical and medullary thymic epithelial cells (cTEC and mTEC, respectively), and concomitant accumulation and expansion of fibroblasts, senescent cells, adipocytes, and perivascular space [31]. Both mTEC and cTEC experience downregulation of genes associated with epithelial specification (including FoxN1, the master specification factor for thymic epithelia) and cellular growth and proliferation. Concomitant upregulation of genes encoding pro-inflammatory mediators also occurs at the time corresponding to the onset of thymic involution [32]. Thymus-seeding precursors derived from hematopoietic stem cells seed equally in thymi of young adult and old mice [33], and the niches available to accept early T-lineage progenitors (ETPs) seem to be equally available in the adult and old environment [34]. However, microenvironmental issues accumulate with aging. The loss of thymic stromal structure integrity, as evident from diffused cortico-medullary regions, and dysfunctional cTEC and mTEC likely underlie aberrant thymocyte development, maturation, and selection during thymus involution. At the output level, thymic involution manifests in a decline in recent thymic emigrants (RTEs), which are post-thymic, relatively, but not completely, mature CD4+ or CD8+ single-positive T cells exported out of the thymus [35, 36]. RTE seed secondary lymphoid organs and further undergo maturation to become fully functional Tn cells [36]. As a result, a decline in thymic export of RTE leads to a decline in the Tn cell pool [7, 14]. Along these lines, a recent mathematical model that integrates immunology and epidemiology data suggested that the diminution of the Tn cell pool is a major risk factor for an increased incidence of infectious diseases and cancer in the elderly [37]. Fortunately, the thymus itself has considerable regenerative potential, and it is this capability that provides thymus the privilege to rejuvenate itself after various insults. However, the endogenous thymic regenerative mechanisms also begin to diminish in adulthood and further deteriorate as we age. Reinitiating thymic production of Tn cells has been considered a potentially promising strategy to restore the Tn cell pool [31].
2.2. Thymic rejuvenation strategies and protective immunity.
Over the past decades, several damage-and-regeneration pathways have been investigated for the potential to improve thymic function in different experimental and clinical settings, and some have shown thymic rejuvenation efficacy [31]. The rising levels of sex steroid hormones, such as testosterone and estrogen, correlate well with the timeline of thymic involution at puberty, and their physical (castration) or pharmacological ablation, also called sex steroid blockade (SSB) has long been known to cause transient thymic regrowth in rodents, non-human primates, and humans ([38], and references therein). The SSB has been shown to successfully induce thymic recovery, either after radiation or chemotherapeutic damage, hematopoietic stem cell transplantation (HSCT) or to curtail age-related thymic involution [39–41]. It is important to note, however, that sex steroids themselves are not the cause of thymic involution, as elegantly shown using androgen receptor knockout animals [42], in which thymic involution nonetheless readily occurs. The exact mechanisms by which SSB transiently regenerates thymic Tn cell production remain unclear. In old mice, SSB restores thymic weight, cellularity, maturation into CD4 or CD8 single-positive (SP) T cells, and export of RTEs to the levels seen in young adult mice. Sutherland et. al. showed that castration in 18 months-old male mice leads to the generation of cytotoxic T cells (CTL) with superior in vitro cytolytic function against Herpes Simplex Virus (HSV)-1-infected cells [38]. Another study from the same group has demonstrated that surgical SSB in mid-age (9 months) and old (18–24 months) mice improved numbers of Tn cells and influenza A virus (IAV)-antigen-specific CTL in the spleen, and lead to enhanced IAV clearance. These mice showed no improvement in the ratio of naïve to memory T cells or in the numbers of antigen-specific CTL, making the mechanism of this improvement less clear [43].
Exogenous administration of the human growth hormone (HGH) and insulin-like growth factor-1 (IGF-1) have shown beneficial effects on thymic rejuvenation and peripheral T cell diversity in old mice receiving allogeneic bone marrow transplants [44, 45]. A randomized controlled trial has shown that GH administration improved thymic mass and restored peripheral naive and total CD4+ T cell pool in HIV-1 infected individuals [46].
Keratinocyte growth factor (KGF), a member of the fibroblast growth factor family, has been shown to have a key role in postnatal thymic regeneration in mice and non-human primates, especially following radiation- or chemotherapy-induced immunosuppression prior to bone marrow transplantation [47, 48]. KGF’s ability to improve thymic function and T cell output is mainly attributed to its role in promoting TECs survival and proliferation through induction of bone morphogenetic protein (BMP) 2, BMP4, Wnt5b, and Wnt10b [49]. Although studies in mice, non-human primates, and humans have demonstrated the beneficial role of KGF in promoting thymopoiesis and peripheral T cell reconstitution in recipients of allogeneic HSCT [48, 50], KGF did not reduce the incidence of cytomegalovirus (CMV) reactivation or fungal infection [51], and treatment in non-human primate resulted in poor antibody responses against tetanus toxoid vaccination [52]. Therefore, whether KGF-mediated improvement in thymic T cell output is capable of providing protective immunity against infection remains to be conclusively demonstrated.
Various genetic mouse models have been developed to improve the size and cellularity of the thymus. Some examples of strategies to generate these models include: mitochondria-targeted catalase in mCAT mice [53], MYC expression in the TEC, either constitutive (FoxN1MycTg mice) or inducible (K5-CreERT2 x flox-stop-flox-MYC mice) [32]; and cyclin D1 expression in TEC and keratinocytes (K5.D1Tg mice) [54]. These transgenic models typically resulted in enlargement of the thymus and improvement in thymic output, although the extent to which this improved thymic function effectively boosts functional immunity against infections and vaccinations is still being investigated. However, potential issues associated with these models include spontaneous hyperplasia, outright carcinoma development, and impaired central tolerance [55–57], which tend to complicate both the interpretation and the applicability of results.
Exciting discoveries have been made recently on the mechanisms of thymic endogenous regeneration in response to irradiation that operate via the damage-associated molecular pattern (DAMP) axis, and include both the Zn++ and ATP-mediated pathways [58, 59]. Whether such mechanisms remain active in an older thymus and whether they may be able to improve protective immunity in older organisms remains to be established.
Recently, our group directly examined whether some strategies that induce thymic regeneration confer protective immunity to the host against intracellular pathogens. In this study, we used two pharmacologic interventional strategies: (i) SSB in old mice using degarelix, an antagonist of the luteinizing hormone-releasing hormone receptor, and (ii) administration of KGF, to rejuvenate thymus in old mice and non-human primates. Both degarelix- and KGF-treated old mice exhibited robust thymic regeneration, as judged by the increase in thymic size, mass, total cellularity, double-positive thymocytes, and improved export of RTE in the blood [21]. Despite the successful thymic rejuvenation, degarelix-treated old mice failed to show improvement in survival upon challenge with West Nile virus (WNV) infection, and these mice did not show improvement in the WNV-specific CTL or antibody response [21]. Similarly, KGF treatment of old mice and rhesus macaques failed to improve the numbers of CD8+ or CD4+ Tn cells in the blood; or in mice to improve immune responses against Listeria monocytogenes. Using Rag2-pGFP reporter mice, in which RTE are marked with GFP to track thymic export [60], we demonstrated that degarelix-mediated thymic rejuvenation in old mice did improve the numbers of GFP+ RTE, however, it did not improve the ability of SLO to accept and retain incoming newly generated T cells or naive T cells [21]. This phenomenon was more evident in LN than in the spleen [21]. This study further highlighted the presence of potential fibrotic changes in old LN as a result of age-related accumulation of excessive collagen [21]. The excessive collagen implies a higher structural stiffness and loss of structural integrity [61], collectively capable of hampering the critical intercellular communication in the old pLN. This raises the possibility that, regardless of the influx of new Tn cells from the rejuvenated thymus, other age-related changes may affect LN architecture and organization in a manner to disallow support of T cell homeostasis, intranodal cell positioning and trafficking, and cross-talk between lymphoid and stromal cells, which is required for homeostatic maintenance and orchestration of immune response. These observations highlight the contribution of SLO aging to immune deficiency with aging and single it out as a limiting factor for the successful restoration of functional and protective immunity following thymic rejuvenation.
3. Age-related changes in the host microenvironment are the limiting factor for functional immunity.
As discussed above, SLO, especially LN, experience multiple age-related changes that interfere with peripheral T cell homeostatic maintenance and reduce the efficacy of immune responses to new and reemerging infections and vaccination. These include the decreased homing and retention of lymphocytes, a decline in the ability of SLO stromal cells to sense infection-associated inflammatory signals (directly or indirectly via activated dendritic cells (DCs) and macrophages), reduced stromal cell stretching and expansion, and impairment in stromal microenvironment affecting intranodal trafficking cues that facilitate rapid DCs-T cells and T-B cell interactions, T follicular helper (Tfh) cell differentiation, B cell activation, and germinal center (GC) formation [19–21, 62–64].
Adoptive T cells transfers, in which adult and old lymphocytes or T cells can be systematically introduced into adult or old recipient hosts, and parabiosis, wherein adult and old hosts can be surgically joined in a way that they can share the blood and circulating immune cells but not stationary stromal cells, have been informative to discern the impact of old immune cell-intrinsic and microenvironment-driven changes underlying immune function decline during aging [65, 66]. Also, approaches that allow genetic labeling of T cells at the time of their development, such as indelible labeling of newly generated T cells in TCRδCreER.ZsGreen mice [67, 68], further facilitated our understanding of the exact time when a specific defect starts building in T or stromal cells during aging. Regardless of the models used, recent studies unequivocally pinpointed that old microenvironment-driven changes are the main culprit underlying the age-related defect in adaptive immune function. Surh’s group originally demonstrated that the aged murine SLO microenvironment failed to support the homeostatic proliferation of adoptively transferred young adult T cells, whereas the proliferation potential of 18 mo old donor T cells was robust in 2–3 mo young adult recipients, suggesting that age-related intrinsic changes in the old T cells do not affect their inherent homeostatic proliferation capacity [18]. Rather, it was the aged microenvironment that had a decisive impact [19]. Another study reported that an aged SLO microenvironment underlies multiple defects in homing, priming, and effector differentiation that led to poor CD4+ T cell response and hindered the ability to support germinal center (GC) B cells in response to immunization [69]. These authors showed that fewer adoptively transferred young adult OT-II CD4+ T cells trafficked to the T cell zone in the old spleen, coinciding with reduced protein levels of homeostatic chemokines, CCL19 and CCL21 [69]. Moreover, OT-II cells that did make it to the old spleen exhibited a significant delay in their priming and proliferation due to impaired DCs function, and subsequently fewer T cells differentiated into Tfh, which closely corresponded to reduced numbers of GC B cells [69]. Similar defects in priming, proliferation, and effector differentiation of transferred young adult antigen-specific T cells were reported in response to infection with mouse-adapted influenza PR8 and lymphocytic choriomeningitis virus (LCMV) Armstrong strain in old recipient mice [70, 71]. Our group has reported that in the young host, adoptively transferred adult and old OT-I CD8+ T cells responded similarly to Ovalbumin-expressing L. monocytogenes (Lm-OVA) infection, whereas adult OT-I CD8+ T cells transferred into old recipient mice responded poorly as measured by T-bet induction, differentiation into short-lived effector cells, proliferation and effector molecule production [25]. This defect was mapped to the underproduction of proinflammatory cytokines in old spleen early after Listeria infection, and could be corrected by provision of exogenous immunostimulatory IL-12 and IL-18 [72]. Similar priming defects were also reported in CD4+ T cells, where 2–3 mo young adult TCR transgenic TCR7- or OT-II- CD4+ T cells that recognizes hen egg lysozyme (HEL) or OVA, respectively, were adoptively transferred in 3 mo young adult and 2224 mo old mice [73]. Fewer young adult TCR transgenic CD4+ T cells proliferated in old recipients and poorly differentiated into Tfh cells upon immunization [73]. Of note, during the vaccination, such DCs priming defects in old mice [73] and the human [74, 75] were reversed by administration of a TLR7 agonist, imiquimod.
Recently experiments using the parabiosis pairing of adult and old mice have revealed that the age of the host microenvironment underlies the limited immune functionality observed in old mice, and further pinpointed that poor LN stromal cell responses contribute to the mechanisms that limit the GC B cell and antibody response to vaccination [64]. Supporting the notion that age-related adverse changes in the host microenvironment are the key limiting factors for T cell proliferation and function with aging, a recent pioneer study, using repeated T cell transfer from one young host to the next for about 10 years, demonstrated that T cells intrinsically have the capability for unlimited proliferation and possess longevity beyond the life of host organism [76]. Similarly, adoptively transferred old B cells performed well in adults but not the old recipient mice, as judged by equivalent levels of B cell activation, somatic hypermutation, affinity maturation, and isotype class switching, indicative of normal GC B cell responses, in adult and old B cells in the adult host [77–79]. Collectively, this data strongly suggests that age-related changes in the microenvironment have the potential to affect SLO homeostasis and immune function.
4. Stromal cells of the secondary lymphoid organ microenvironment.
During embryonic development, highly dynamic and spatiotemporally regulated cross-talks between hematopoietic lymphoid tissue inducer (LTi) and mesenchymal lymphoid tissue organizer (LTo) cells dictate the development and maturation of the LN. These interactions facilitate the survival, maturation, proliferation, and differentiation of LTo cells into functional stromal compartments that deliver membrane-bound and soluble- survival and trophic signals to the lymphocytes and dendritic cells (DCs) [80, 81]. The stromal cells constitute about 1–2% of total LN cells. They are diverse and heterogeneous non-hematopoietic cells that form several distinct cellular niches specialized in supporting the function of different types of innate and adaptive immune cells. In the mature LN, major stromal cell types are distinguished by surface expression of podoplanin (PDPN) and CD31; the fibroblastic reticular cells (FRC; PDPN+CD31−) are mesenchymal in origin, lymphatic- (LEC; PDPN+CD31+) and blood- (BEC; PDPN−CD31+) endothelial cells represent the endothelial lineage, and double negative (DN; PDPN−CD31−) cells represent precursors of the fibroblastic stroma.
The FRC are a major stromal cell type in the LN, accounting for approximately 30–50% of the total CD45− stromal fraction. They are known to produce various extracellular matrix (ECM) molecules that are structurally arranged in a conduit, through which antigens from the lymph flow are sampled. FRC are interconnected to form a specialized reticular network, on which various tropic (chemokine C-C motif ligand (CCL)19, CCL21, CXCL12, CXCL13) and survival (IL-7, IL-15 and B-cell activating factor of the tumor necrosis factor superfamily, BAFF) factors are embedded [15] to facilitate the lymphocyte homing, retention, survival, and function. FRC rapidly respond to innate immune triggers, growth factor(s), and cytokines, and also possess the antigen presentation capabilities that make them ideal candidates to balance tolerance and immunity in the LN [82]. The FRC undergo complex remodeling in response to immune activation that involves sequential events of activation, stretching, and proliferation resulting in LN expansion to accommodate incoming and proliferating lymphocytes. Genetic deletion of FRC in CCL19-Cre-iDTR [83] or fibroblast activation protein-α (FAPα)-iDTR [84] mice or selective depletion of lymphotoxin beta receptor (LTβR) in FRC [85] led to a substantial reduction in lymphocyte numbers, alterations in the reticular network, and loss of T and B cell localization. Mice with such deletions exhibited reduced expression of PDPN, IL-7, CCL19, and CCL21, and failed to confer protection against viral infections [85]. Recent single-cell RNA-sequencing (scRNA-Seq) approaches have revealed at least nine distinct FRC subpopulations that possess unique phenotypes and functions underlying distinct cellular niches in the human and mice LN [86–88]. The phenotypic characteristics, spatial localization, and function of these FRC subpopulations are described in Table 1.
Table 1.
Heterogeneity of Fibroblastic reticular cells and their alterations during aging.
| Type of reticular cells | FRC subsets | Localization | Unique transcriptomic signature | Surface phenotype | Function | Age-related changes | References |
|---|---|---|---|---|---|---|---|
| T cell zone reticular cells (TRC) | Ccl19high reticular cells | Primarily in T cell zone | Ccl19hi, Ccl21+, Il7+, Bst1+, Slc7a11+ | CD31− PDPN+PDGFRα+Bst1+CD21/35−MAdCAM1− CD34− (does not differentiate between Ccl19hi and Ccl19lo cells) | Support T cell and DCs survival and maintenance via IL-7 and homeostatic chemokines. Produce collagens and ECM components and form a conduit network. | (1) Old mice LN exhibit reduced CCL19 and CCL21 protein levels. (2). Reduction in FRC homeostatic and survival signal, LTb in old LN may affect the integrity of the PDPN+ reticular network in the T cell zone (various FRC subsets, like Ccl19hi, Ccl19lo, and Cxcl9+ cells, in T cell zone may get affected). (3) FRC in the paracortical T cell zone exhibit denser ECM (increased accumulation of collagen and alpha-smooth muscle actin, α-SMA), suggestive of fibrosis. | [68, 86, 117, 118] |
| T/B border and Interfollicular reticular cells (IFRC) | Ccl19low reticular cells | Mainly at interfollicular (IF) region, B cell follicles, and T cell zones | Ccl19lo, Ccl21+, Il7+, Cxcl13+, Baff+ | CD31− PDPN+PDGFRα+Bst1+CD21/35−MAdCAM1− CD34− | Support T cell (IL-7 and CCL21) and B cell (CXCL13, BAFF) survival and maintenance, and may be their interaction at T/B borders and in the B cell follicles. A proportion of these cells produce cholesterol-25-hydroxylase (Ch25h), an enzyme that catalyzes the production of 7 a, 25-hydroxy cholesterol, a ligand known to bind with GPR183. | (1) Loss of CCL21 and CXCL13 protein levels, correspondingly, altered T cell and B cell localization and diffused T/B border suggest the possible alteration of these stromal cells at the T/B border and IF region. (2) FRC in cortical region (that may include Ccl19lo cells at T/B borders and IF region) exhibit increased collagen and α-SMA accumulation. | [86, 88, 117] |
| Grem1+ reticular cells | T cell zone, T/B border | Ccl19hi, Ccl21+, Il7+, Bst1+, Grem1+ | Currently, there is no available surface marker(s) that can distinguish Grem1+ cells from the rest of the TRC. Grem1-CreERT2::Rosa26-LSL-EYFP reporter mice allow the isolation and characterization of Grem1-EYFP+ stroma. | Support the survival and homeostatic proliferation of LN-resident conventional DCs (cDCs). | ? | [88] | |
| Cxcl9+ reticular cells | T cell zone, IF region, Medullary area | Cxcl9+, Cxcl10+, Ccl19+, Ccl21+, Il7+ | ? | (1) Cxcl9+ FRC express class II MHC genes and machinery for peptide generation (chaperons) and loading (CD74) onto MHC molecules, and reduced expression of co-stimulatory molecules, indicating their possible role in tolerance. (2) Gene signature of Cxcl9+ FRC (Gpb3–9, STAT1, Irgm2, Ifit3, Irf1 and 8) indicate that they likely are capable of responding to IFN-signaling (type I and IFN-γ) and might represent an activated subpopulation of TRC. | (1) Loss of CCL21 and CXCL13 protein levels, correspondingly, altered T cell and B cell localization and diffused T/B border suggest the possible alteration of stromal cells at the IF region. However, no definitive evidence exists that suggest the impairment in the structure and/or function of Cxcl9+ stromal cells. | [86, 117, 118] | |
| Medullary reticular cells (MedRC) | Inmt+ reticular cells | Medullary cord | Inmt+, Nr4a1lo, Bst1lo, Ccl19lo, Cxcl1lo, Cxcl2lo, Cxcl9lo, Cxcl10lo, Cxcl12+, Cxcl13+, Desminhi, Baff+, lepr+, CD34− | ? | (1) Localization and gene expression data suggest that Inmt+ stroma may represent a population of MedRC. (2) Inmt+ stromal cells can be distinguished from Nr4a1+ MedRC by Cxcl1, 2 and 10 expression. (3) Produce BAFF, IL-6, and APRIL and help maintain macrophage and plasma cells at medullary regions. | (1) In old human LN, PDPN+Bst1− CD34− supposedly MedRC (that may contain Inmt+ and Nr4a1+ cells in addition to other unknown MedRC) have been reported to be particularly sensitive for transdifferentiation into adipocytes. (2) The loss of lymphatic network and dilation of HEV have been found within a medullary area with increased adipocyte accumulation, and correspondingly, altered localization of plasma cells and reduced Naive T cells around HEV. | [86, 87, 102, 119] |
| Nr4a1+ reticular cells | Mostly in the medullary cord. Since Nr4a1 is also expressed by other stromal cells (TRC, CD34+, MRC, and PvRC), Nr4a1+ staining can be located in IF region, T/B cell borders, T cell zones, and SCS. | Nr4a1hi, Inmt+, Bst1lo, Ccl21lo, Cxcl1hi, Cxcl2hi, Cxcl9lo, Cxcl10hi, Baff+, lepr+, CD34− | CD31− PDPN+PDGFRa+Bst1− CD21/35−MAdCAM1− CD34− (Nr4a1-GFP reporter mice may allow isolation and characterization of Nr4a1-GFP+ cells. Gating out cells that express CD21/35, MAdCAM1, and Bst1 could help distinguish Nr4a1+ MedRC from FDC, MRC, and TRC that express Nr4a1). | (1) Like Inmt+ cells, Nr4a1+ stroma may represent a population of MedRC. (2) Express early response genes (Junb, Egr1–2, Fos, Nr4a1, Nfkbia, Nfkbiz, and Zfp36) indicative of their possible activated state. (3) Produce BAFF, IL-6, and APRIL and help maintain macrophage and plasma cells at medullary regions. | |||
| CD34+ reticular cells | Capsular area and surrounding large blood vessels in the medullary region, In the vicinity of efferent lymphatics | CD34+, CD248hi, Pdgfrβ+, Inmt+ Desminlo, CD31−, Bst1−, Acta2− | CD31− PDPN+PDGFRα+CD2 1/35−MAdCAM1− CD34+ | (1) CD34+ fibroblastic cells serve as a progenitor for stromal cell development. (2) CD34+ stromal cells exhibit a gene signature enriched in angiogenic factors (Igf1, Igfbp3, 4, and 6), indicating their possible for in new lymph and blood vessel formation. (3) They may facilitate LN expansion during immune response via CD248. | In the medullary region of old human LN, CD31− PDPN+BSt1− CD34+ stromal cells have been found to transdifferentiate into adipocytes, leading to increased lipomatosis, and gradual loss of lymphatic network and dilation of HEV within an area with increased lipomatosis. | [85–88, 102] | |
| B cell zone reticular cells (BRC) | Cxcl12+ reticular cells | Exclusively in the dark zone of the germinal center (GC) | CD21/35lo, Baff+, Cxcl13−, Cxcl12+ | CXCL12 reporter strains could be used to isolate CD31− PDPN+Bst1+CD21/35− MAdCAM1−CD34− cells expressing reporter driven by Cxcl12 promoter. | (1) CXCL12+CD 21/35lo stromal cells form the structure of the GC dark zone. (2) Attract CXCR4+ B cells to the dark zone of GC and may provide microenvironment support for the B cell somatic hypermutation process. | ? | [83, 86, 130, 200] |
| Follicular dendritic cells (FDC) | B cell follicle and light zone of GC | CD21+, CD35+, Baff+, Cxcl13+, Cxcl12− | CD31− PDPN+CD21/35+MAd CAM1+/− PDGFRα+Bst1+ | (1) CXCL13+CD 21/35hi FDC facilitate homing of CXCR5+ B cells and Tfh to form B cell follicle. (2) Support B cell survival and maintenance via CXCL13 and BAFF. (3) Present antigens to B cells via immunocomp lexes bound to Fc-receptors (CD16 and CD32). (4) Maintain the microenvironment of light zone of GC where class-switching and affinity maturation occurs. | (1) FDC numbers decline with age, correspondin gly fewer and smaller GC form in old LN. (2) FDC's Fc-receptor expression reduced and their ability to capture antigens and present them to B cells also diminished. (3) The proliferative response of FDC is reduced in the draining LN of vaccinated old mice and found to be linked to mislocalization of Tfh cells to the dark zone of GC. | [64, 86, 116, 130] | |
| Marginal reticular cells (MRC) | Lining the subcapsularsinus (SCS) | MAdCAM1+, Bst1+, RANKL+, Cxcl13+, Enpp2+, CD21/CD3 5- | CD31− PDPN+PDGFRα+CD2 1/35−MAdCAM1+ | (1) Perform barrier defense by capturing antigens from SCS space and communicate this information to B cells. (2) Support and maintain a niche for sinusoidal macrophages via RANKL. (3) Maintain type 3 innate lymphoid cells via IL-7 signaling. (4) MRC also serve as precursors for FDC and other FRC subtypes during immune activation. | (1) The numbers of MRC reduced with age. (2) Aged MRC activate and proliferate less in response to vaccination. (3) Aged MRC need a boost of TLR4-mediated signal(s) to be able to mount similar response to their adult counterparts. (4) Poor MRC response to vaccine correspond to fewer GC B cells and reduced antigen-specific antibody production. | [12, 64, 86] | |
| Perivascular reticular cells (PvRC) | Ensheath HEV and other blood vessels | Itgα7+, Pdgfrβ+, Acta2+, Esam1+, Mcam+, Fabp4+, Myh11hi, Myl9hi, Ccl19− Ccl21− | CD31− PDPN+PDGFRβ+CD2 1/35−MAdCAM1−CD34− Integrin α7+ | (1) Secrete ECM molecules and wrap arround the HEV and other vessels to maintain endothelial structure and functon. (2) Produce integrins and cell adhesion molecules to support lymphocyte migration across HEV and other endothelium. | ? | [86, 88] |
The lymphatic endothelial cells (LEC) form specialized endothelium of the lymphatic vessels that facilitate the transport of lymph fluid and lymphocytes from the tissue to LN via afferent lymphatics. LEC secrete various growth factors, chemokines, and cytokines, and express class I and II MHC, and co-stimulatory molecules. LEC can capture, process, and present the antigens to T cells and are also reported to acquire peptide-MHC complexes from DCs, and are known to maintain tolerance and immunity [89, 90]. During infection and inflammation, LEC undergo rapid proliferation, modulate local chemotactic gradient, and expand the lymphatic network required to increase the lymph flow and promote lymphocyte and DCs entry and retention in the LN [91, 92]. Both in humans and mice, multiple distinct subsets of LEC have been identified [93–95] that reside in specific niches and perform unique functions (Table 2). Blood endothelial cells (BEC) form the lining of blood vessels that facilitate the transport of soluble mediators and leukocyte trafficking to the LN. High endothelial venule (HEV) is a specialized BEC that produces CCL19 and CCL21, forming a gradient required to chemoattract CCR7+ DCs and lymphocytes into the LN. HEV expression of cell adhesion molecules, such as PECAM-1, ICAM-1, VCAM-1, MAdCAM-1, GlyCAM-1, P-selectin, peripheral node vascular addressin (PNAd), and integrins facilitate the trafficking of lymphocytes in the LN. The BEC and HEV rapidly respond to the immunological challenge by contributing to the expansion and contraction of the LN. Recently, scRNA-seq has revealed at least eight distinct molecular clusters that encompass capillary, venous, and arterial EC within a BEC compartment (Table 3) [96, 97].
Table 2.
Transcriptional heterogeneity of lymphatic endothelial cells.
| Type of LEC | LEC subpopulations | Localization | Unique transcriptomic signature | Function | References |
|---|---|---|---|---|---|
| Subcapsular sinus LEC | Ceiling LEC (cLEC) | Lining the ceiling of SCS space | Lyve1+, Cav1+, Ackr3+, Ackr4+, Cd36+ | (1) cLEC express chemokine scavenging receptors (ACKR3 and 4) that might shape chemokine gradient by scavenging homeostatic chemokines, CCL19, CCL21, and CCL25, close to the capsular region. (2) cLEC also expresses lipid transporters (CD36) that may help uptake the low-density lipoproteins (LDLs) from incoming lymph from afferent lymphatics. | [93–95, 201] |
| Floor LEC (fLEC) | Lining the floor of SCS space | Lyve1+, Cd44+, cd74+, Cd274hi, Madcam1+, Ccl20+, Cxcl1+, Cxcl5+ | (1) fLEC express Class II MHC molecules, invariant chain CD74, and high levels of PD-L1, suggesting their possible role in maintaining tolerance. (2) fLEC are capable of chemoattraction of CCR6+ Th17, γδT cells and ILC3s via CCL20. (3) fLEC may interact with incoming immune cells from afferent lymphatics via multiple cell adhesion molecules (MAdCAM1, Lyve1, GlyCAM1) and integrins. | [93–95] | |
| Medullary sinus LEC | Ptx3+ LEC | Cortical and medullary sinus lymphatics | Lyve1+, Ptx3+, Cd274lo, Marco−, Itih5+, Mrc1+, Reln+ | Ptx3+ LEC identified in mice but not human LN. Ptx3 is known to bind to soluble pattern recognition and altered self-molecules that help promote innate recognition and phagocytic activity. (3) Ptx3 LEC also interact with CD44hi activated/memory T cells via MRC-1. (4) Ptx3+ LEC may vigorously respond to the angiogenic signals due to their higher expression of VEGFR3. | [93–95] |
| Marco+ LEC | Medullary sinus lymphatics | Lyve1+, Marco+, Cd209+, Clec1b+, Clec4g+, Clec4m+ | Marco+ LEC expresses CLEC2 (Clec1b), a ligand for PDPN, and like CLEC2+ DCs, these cells may have a role in FRC stretching and expansion. (2) Marco LEC abundantly express a gene signature indicative of their possible role in maintaining innate immune response in the medullary region. (3) These cells are also shown to sequester virus particles to limit dissemination. | [93–95, 202] | |
| Valve LEC (vLEC) | Throughout the lymphatic network | Lyve1+, Foxc2+, Cav1+, Esam+,Cldn11+ | Prevent the backflow of lymph and facilitate the unidirectional flow of lymph though lymphatics. | [93–95, 203] |
Table 3.
Transcriptional heterogeneity of lymph node blood endothelial cells.
| Type of BEC | BEC subpopulations | Unique transcriptomic signature | Function | References |
|---|---|---|---|---|
| Capillary endothelial cells (CapEC) | Capillary endothelial cell type I (Cap-EC1) | Igfbp3+, Col4a1+, Col4a2+, Id1+, Id3+ | (1) Express molecules that facilitate the maintenance of vascular tone and blood pressure. (2) Higher expression of Id1 and Id3 help control cell differentiation and senescence via inhibiting the DNA binding of basis helix-loop-helix proteins. | [97, 204] |
| Capillary endothelial cell type II (Cap-EC2) | Erg1+, Cxcl1+, Sgk1+, Nr4a1+, Jun+, Junb+, Jund+, Fos+, Nfkbia+ | CapEC2 transcriptome suggests that they are capable of responding to cytokines and other inflammatory mediators, possibly in response to disturbance in shear stress. | [97, 204] | |
| Interferon-stimulated gene-enriched CapEC (CapIfn EC) | Ifit1+, Ifit2+, Irf7+, Cxcl9+, Cxcl10+ | CapIfn represents CapEC subset particularly responsive to IFN-signaling. | [97] | |
| Activated CapEC (CRP) | Angpt2+, Apln+, Esm1+, Pgf+, Nid2+, Kit+, Sox7+, Ets2+ | (1) CRP represents a rare pluripotent EC subset, which acts as a precursor for other differentiated EC. (2) CRP EC possesses a gene signature indicative of activated capillaries that are capable of forming new blood vessels. | [97] | |
| Transitional phenotype EC (TrEC) | Chst2+, St3Gal6+, Fut7+ | (1) Express genes signature indicative of transitory phenotype shared between the HEV and CapEC. (2) TrEC connects capEC to venous EC. (3) May help tether the lymphocytes to the vessel wall under shear stress and facilitate the recruitment of lymphocytes. | [97, 204] | |
| Arterial endothelial cells | Arterial EC (ArtEC) | Cd31+, Gja4 (Connexin 40)+, Gja5(Connexin37)+, Bmx+, Sox17+, Gkn3+ | Maintain blood flow and pressure in arteries. | [97, 204] |
| Venous endothelial cells | High endothelial venule (HEV) | Cd31+, PNAd+, Ccl21+, Chst4+, Glycam1+ | (1) HEV produces CCL19 and CCL21 gradient and adhesion molecules required to chemoattract CCR7+ DCs and lymphocytes into the LN. (2) HEV vigorously responds to immune activation signals, thereby contributing to the structural change required for the LN expansion and accumulation of inflammatory cells. | [96, 97, 205] |
| Non-high endothelial cell venule (non-HEV) | Nr2f2+, Ackr1+, Sele+, Selp+, Vcam1+ | Facilitate the rolling and crawling of circulating lymphocytes and myeloid cells via L- and P-selectin, ICAM1, and VCAM1 molecules. | [96, 97] |
4.1. Age-related changes in steady-state lymph node structure and organization.
Unlike thymic involution, SLO atrophy as a function of age has been much less explored. Nonetheless, accumulating evidence suggests that LN, and to some extent spleen, with increasing age experience various degenerative changes that affect their overall structure and integrity (Figure 1) [98–101]. The key histopathological changes reported in the aged LN include the increased accumulation of adipose tissue and perivascular connective tissue, thickening of the capsule, and fibrotic changes, characterized by excessive fibrous tissue and collagen deposition, and such changes were evident in functional niches in the cortical and medullary regions of the LN [99, 100]. Age-related increased adipose deposition (lipomatosis) and replacement of perivascular smooth muscle cells with the connective tissue (hyaline degeneration) can affect several critical functions in the LN and might lead to the constriction of lymphatics, impairing lymph flow and inducing lymphedema. Adipose tissue production of adipokines can influence the interaction of immune cells and stromal cells [102–104]. Fibrotic changes can further lead to loss of LN flexibility [21, 101], potentially negatively influencing the normal migratory behavior of lymphocytes and DCs and also their cross-talk with stromal cells [105]. The LN from older humans and mice exhibit a sign of atrophy and appear smaller with a significant reduction in total cellularity. Of note, such LN atrophy is not uniform across all the LN. Specifically, in mice [68] and humans [106] the skin-draining LN appeared to be affected quite early compared to LN draining the deeper tissue. Using time-stamp labeling of RTE, we recently demonstrated that skin-draining LN in mice undergo early (6 to 9 mo) atrophy, characterized by reduced ability to maintain Tn cells, disturbance in FRC structural network, and numerical loss of FRC and LEC, which all precipitated reduced immunity to cutaneous vaccination [68]. In line with the findings in aged LN [68, 99, 101], a recent report has demonstrated the waning of PDPN+ area, reduction in FRC numbers in the T-cell zone, and reduced levels of CCL19 and CCL21 proteins per mg of total proteins in the spleen of naturally aged mice [107]. With the increasing age, the reduced or aberrant CCL19, CCL21, and CXCL13 expression by the stromal cells is thought to contribute to the disorganization of T and B cell zones in the LN [99, 108]. Similar disorganization in the T cell zone and B cell follicles and altered T-B cell interaction have also been reported in the human LN [109]. Such disorganization led to the accumulation of increasing numbers of B cells in a paracortical T cell zone, where intruding B cells may alter the structural integrity of CCL21-producing FRC [110].
Figure 1. Age-related changes in the stromal cells and lymph node structure and organization.
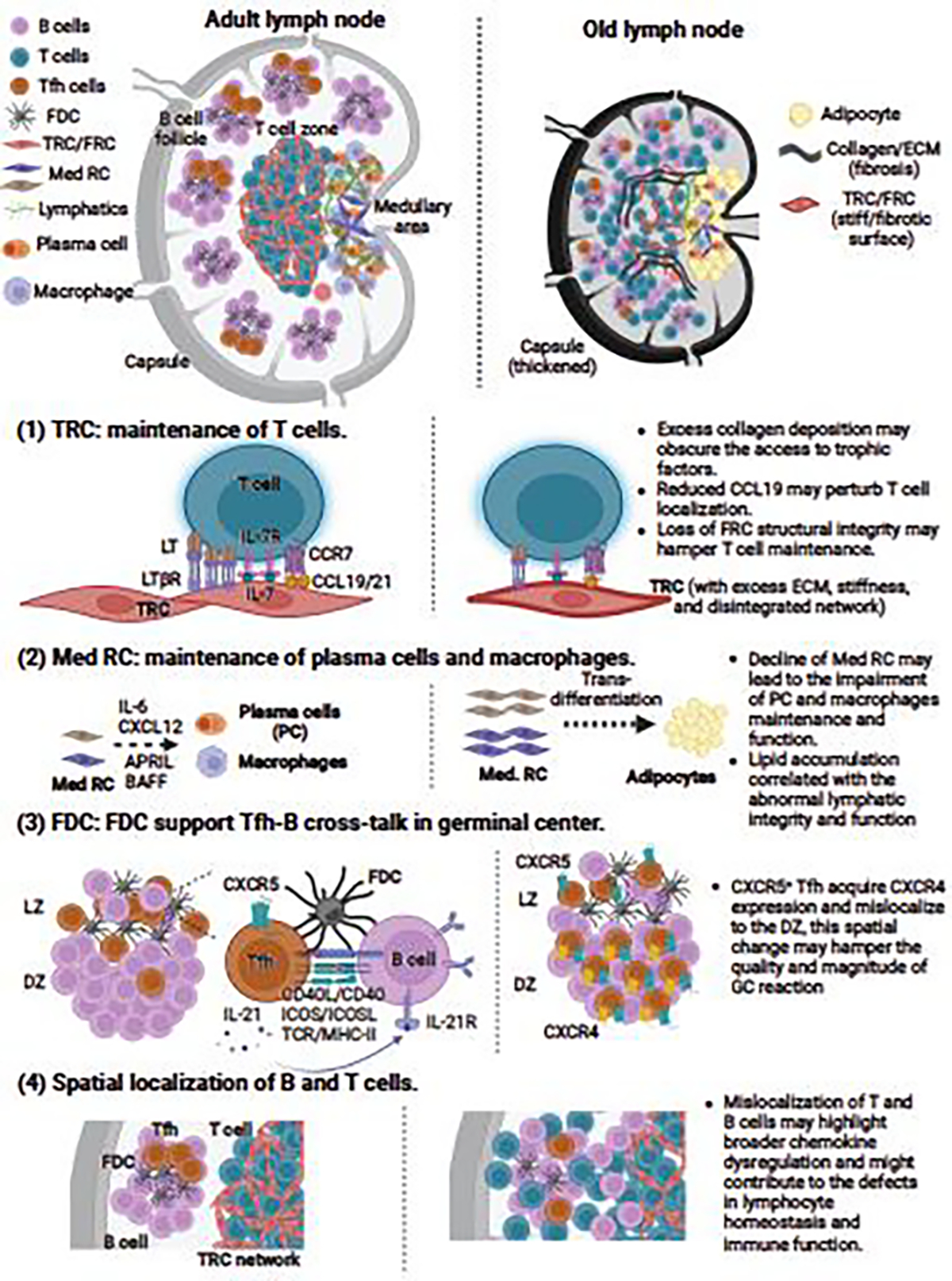
Lymph node stromal cell compartments form the structure, organization, and compartmentalization of the lymph node, and significantly contribute to the homeostatic maintenance of peripheral naïve T cells. The lymph nodes in aged mice and humans exhibit several degenerative changes, characterized by loss of size and total cellularity, capsule thickening, impaired cellular compartmentalization, and accumulation of collagen and other extracellular matrix components (fibrosis) and adipocytes (lipomatosis). Specific defects that contribute to impairment in homeostatic maintenance of lymphocytes involve- (1) Impairment in the ability of T cell zone fibroblastic reticular cells (TRC) to maintain naïve T cells. This issue may be triggered by the loss of FRC/TRC structural integrity, dysregulation of homeostatic chemokines (CCL19 and CCL21), and fibrosis and other structural stiffness issues that may hamper the bioavailability of trophic and homeostatic molecules [19, 21, 61, 68]. (2) Recent evidence from aging human lymph node studies indicates that medullary reticular cells located in the medullary region are highly prone to undergo transdifferentiation into adipocytes [102], which may in part explain increased lipomatosis often found in the old lymph nodes [103]. Such structural change has broader consequences, such as loss of lymphatic integrity and function and aberrant plasma cell and macrophage maintenance in the medulla [102]. (3) Chemokine dysregulation in the aged mouse lymph nodes can mislocalize follicular helper T cells to the dark zone of the germinal center [131], which affects the FDC proliferation and quality of germinal center response during vaccination [64, 131]. (4) Similarly, alteration in homeostatic chemokine gradient is thought to underlie the phenotype of diffused T and B cell compartments often seen in the old human and mice lymph nodes [68, 99, 100, 118]. (Created with BioRender.com)
FRC and LEC are the major producers of the T cell survival factor, IL-7. The IL-7 stimulation in T cells integrates multiple signaling pathways, such as Janus kinase (JAK1 and JAK3); phosphorylation of STAT5, STAT1, and STAT3; and the phosphoinositide 3 kinase (PI3K)-AKT, to induce the anti-apoptotic and pro-survival proteins, such as Bcl-2, Bcl-xL and Mcl-1, and glucose-dependent metabolic regulators [111]. Reduced T cell survival and homeostatic proliferation in aged SLO might likely be a result of suboptimal IL-7 signaling, as CD4+ and CD8+ T cells exhibited increased IL-7Rα expression and concomitantly reduced pSTAT5 [19, 112]. However, IL-7 mRNA and protein levels remained unchanged in the aged SLO [21, 107] and similar serum IL-7 levels were reported in the adult and older humans [19]. Boosting the IL-7 bioavailability using recombinant IL-7 complexed with neutralizing monoclonal antibody M25 (IL-7c) significantly improved the homeostatic proliferation of transferred donor young adult CD4+ and CD8+ T cells in the aged SLO [19], suggesting that altered stromal architectural or macroscopic alterations, such as fibrosis, might limit the bioavailability of the survival signals. However, whether or not IL-7c influence the structure and function of the SLO stromal compartment is unclear and needs systematic investigation. Moreover, it will be important to test whether strategies that control the fibrosis or inhibition of pro-fibrogenic signaling may improve the accessibility of IL-7 and other stroma-derived survival factors to T and B cells.
Recent studies have suggested that different stromal cell subsets experience age-related changes differently, and such differences may underlie specific changes in LN structure and organization. For example, CD34+ stromal cells and Bst1− medullary reticular cells were recently shown to be more sensitive to adipogenic signals, with a high propensity to transdifferentiation into adipocytes and age-related lipomatosis [102]. Within a medullary area of lipomatosis, increased loss of lymphatic vessels and complex remodeling of HEV have been observed. The HEV vessels, in particular, appeared dilated with thinning of endothelium walls and loss of PNAd expression, leading to a reduction in homing of T cells and abnormal distribution of CD38+ plasma cells around HEV in the lipomatosis-rich medullary region [102]. The follicular dendritic cells (FDC) also experience multiple age-related changes that collectively contribute to weakened Tfh and GC B cell activity and poor high-affinity antibody response [113]. In old mice and humans, the proportion and numbers of FDC were consistently found reduced and fewer and less dense GC were reported in the aged LN [73, 99, 114]. Moreover, aged FDC exhibit reduced surface Fc-receptors (CD21, CD35, FcγRII, and FDC-M2), become rigid, and lose flexibility which may affect their antigen archiving and/or antigen presentation to B cells and ability to effectively communicate simultaneously with Tfh and B cells in the follicle [114, 115]. Old FDC poorly support in vitro activation of the adult B cells [114], and transferred old B cells underwent normal affinity maturation and isotype switching in adult but not old recipient mice [77–79]. Some of these functional problems could be reversed by complement-mediated augmentation of immune complex formation that facilitated antigen archiving by FDC [115, 116].
Some studies reported that steady-state old murine LN exhibit less dense FRC and FDC reticular network and even noted fewer FRC, FDC, and LEC compared to adult LN [19, 68, 117]. However, other studies have provided mixed results on the age-related changes in FRC numbers and organization, with some reporting no change [117, 118], and others even finding an increased proportion [99]. Careful consideration is required when interpreting these studies. First, not all old peripheral LN exhibit reduction in FRC numbers; specifically, axillary and inguinal LN experienced higher FRC loss while brachial LN showed no change [68], and pooling all peripheral LN together obviously would result in mixed or inaccurate results; second, we found a loss of FRC network integrity much earlier than the measurable numerical decline [68] and sometimes even without any change in cell numbers [118]; third, subtle population-level differences may not be picked up or withstand rigorous statistical methods when FRC are defined merely via the CD45−CD31−PDPN+ phenotype; and finally, use of different mechanical/enzymatic digestion methods might result in different results across the studies. When comparing stromal cell steady-state or infection/immunization responses of adult and old LN, we encourage the inclusion of as many as possible reliable markers, including CD21/CD35, MAdCAM-1, CD157 (Bst-1), PDGFR-α (CD140a), PDGFR-β (CD140b), Sca-1, CD29, CD34, CD44, CD73, CD106, LepR, Integrin α7, PNAd, ICAM-1, VCAM-1, Glycam-1, Lyve-1, CD209, CD272, Claudin-3, -5, and -11, and ER-TR7, in addition to CD31 and PDPN to be able to define various FRC, LEC, and BEC subpopulations [80, 88, 119, 120]. This granular approach would help in dissecting the stromal cell subpopulation-specific age-related difference at homeostasis and infection or vaccination settings.
4.2. Impact of age-related changes in lymph node structure and organization on functional immunity to infection and vaccination.
Regardless of the changes at a population level at steady-state, old FRC often display delayed activation, reduced proliferative responses, and loss of flexibility that impair the ability of LN to expand in response to infection to accommodate expansion of antigen-responsive cells to generate high-quality T cell and high-affinity B cell responses [64, 118, 121]. The infection and related inflammation are known to remodel the LN stromal network, facilitating the expansion and contraction of the LN during the activation and resolution, phases of the immune response, respectively [122]. During such conditions, FRC relieve their contractile force and undergo extensive stretching via the interaction of FRC’s PDPN and CLEC2 on resident and migratory DCs, leading to uncoupling of PDPN from ezrin, radixin, and moesin family proteins (ERM proteins), the downstream Ras homolog family member A (RhoA) and clustering in the membrane lipid rafts [123]. This response enables FRC proliferation, makes room for lymphocyte proliferation, and facilitates lymphadenopathy as a key response to infection [122, 124]. Although the exact trigger(s) for FRC proliferation during such immune activation is not known, the lymphotoxin beta (LTβ) and receptor activator of nuclear factor kappa-B ligand (RANKL) are known regulators of FRC proliferation during development and therefore could be also involved during the response to infection [122, 125]. Whether and to what extent such mechanism(s) still operate in old mice and human remains to be determined. Some studies suggest that increased accumulation of DCs and CD4+ Tn cells could be a possible trigger [124, 126]. However, whether and to what extend age-related lymphopenia (especially reduced Tn cells) affect FRC phenotype, mechanobiology, and function is not known. Along these lines, it has been reported that aged peripheral LN lose their ability to undergo remodeling and expansion in response to viral infections [20, 127], and correspondingly, exhibit defects in CD4+ T cell trafficking, and Tfh and GC B cells function, with a consequent reduction in WNV neutralizing antibody serum levels [20]. Similar defects in the LN expansion (as evident with reduced total cellularity) and reduced humoral response were observed in old mice immunized with OVA/alum [20] and Chikungunya virus (CHIK) infection [127]. Interestingly, age-related increased susceptibility to CHIK infection was found to be TGF-β-dependent, and anti-TGF-β treatment of old mice successfully restored the neutralizing antibody response to adult levels, alleviating the clinical symptoms (arthritic or metatarsal inflammation). TGF-β is a key driver of fibrosis and its blockade might in part lead to a reduction of fibrotic changes in old LN, resulting in improved bioavailability of trophic factors and intranodal trafficking that benefit FRC and lymphocyte function. FRC are a major producer of collagen and other ECM molecules, and the fact that they are particularly responsive to cytokine stimulation [87], makes it likely that elevated TGF-β and other fibrotic stimuli in the aging host might adversely affect the regulation of ECM protein production by FRC [105], thus contributing to fibrotic changes. Moreover, TGF-β and IL-7 are antagonistic to each other, and elevated levels of TGF-β [127] and biased Treg response [128] in old mice might in part drive the impairment in FRC- lymphocyte-DCs cross-talks by this antagonism. Such changes may deprive lymphocytes and DCs of stromal-derived chemotactic and survival signals, while FRC may also experience the scarcity of hematopoietic cell-derived homeostatic signals, including LT-β and RANKL. Collectively, these events have a great potential to cause numerical and functional loss of stromal cells and lymphocytes, specifically CD4+ and CD8+ Tn cells, in the aged LN. Consistent with this assumption, a study has reported the correlation of CD4+ T cell loss with reduced numbers of FRC and FDC in HIV-1 infected individuals regardless of the administration of anti-retroviral therapy [129]. Our group has previously documented that aged mice and non-human primates exhibit excessive collagen deposition in the LN [21], which might in part help explain the observed reduction in the acceptance or retention of incoming new T cells after a successful thymic rejuvenation, and subsequent, poor immune defense against lethal WNV infection. Recently, the Linterman group has reported that poor response of marginal reticular cells (MRC; as defined by CD45−CD31−PDPN+MAdCAM-1+CD21/35−) underlie the reduced GC B cell response and impaired antibody response to NP-KLH (4-hydroxy, 3-nitrophenyl acetyl linked to keyhole limpet hemocyanin) in Alum in LN of aged mice [64]. The adult and old mice used in this study showed comparable FRC numbers, and instead, the proliferation and expansion of MRC and FDC (CD45−CD31−PDPN+MAdCAM-1−CD21/35+) was severely reduced in the draining LN of old mice. The authors further demonstrated that TLR4-adjuvanted vaccination boosted the old MRC response and transiently increased the GC B cell and antibody responses [64]. Of note, in addition to serving as gatekeepers at SCS, MRC also act as a precursor for FDC and some FRC subsets, and therefore, any age-related defect in MRC phenotype or function may have a broader downstream effect on T and B cell responses [130]. Recently, the same group has elegantly demonstrated the critical role chemokine dysregulation plays in the altered spatial localization of Tfh cells in aged mice LN. In an adult healthy LN, Tfh cells express CXCR5 and are primarily confined to the light zone of the GC, where they support the cross-talk between FDC and GC B cells. However, in an aged mouse LN, these Tfh cells acquired CXCR4 expression and mislocalized to the CXCL12-expressing reticular cells in the dark zone of the GC [131]. Such mislocalization resulted in a poor expansion of the FDC network and severely compromised the quality of the Ab response and correction of chemokine receptors on Tfh cells restored the defects [131]. These results suggest that alterations in LN architecture and stromal function strongly impact the T cell and humoral response, and targeting such age-related changes would pave new ways to restore functional immunity in older adults.
4.3. Age-related changes in endothelial stromal cells.
The lymphatics system that connects various organs to the regional LN, including those that drain the skin and other mucosal surfaces, also undergoes age-related changes, [132, 133]. Aged lymphatic vessels exhibit reduced contractility, less dense lymphatic vasculature, and impaired drainage to the local LN [134, 135]. In old rats, the lymphatic vessels appeared leaky and highly permeable and exhibited an impairment in the transport of bacterial and fungal pathogens [136]. Similarly, impaired in the trafficking of DC to the draining LN through lymphatic vessels was reported in old mice [137]. Such lymphatic permeability issues might contribute to lymphedema and tissue swelling observed in older adults [138], which in the CNS may lead to amyloid deposition and cognitive defects [139]. Further, aging might affect lymphangiogenesis, a process of forming new lymphatic vessels, due to age-related reduction in vascular endothelial growth factors (VEGF-C and VEGF-D) [140] or increase in TGF-β, an inhibitor of lymphangiogenesis [141]. Multiple distinct LEC subpopulations were identified (Table 2) and it would be critical to understand if specific subsets exhibit differential sensitivity to aging. At steady-state population levels with aging, both murine skin lymphatics [137] and murine skin-draining LN [68] harbored fewer LEC and proliferated poorly in response to acute infections [118, 121]. Aged LEC were reported to be highly sensitive to apoptosis and senescence [142]. A state of age-associated chronic, low-grade inflammation, often referred to as “inflammaging” [143] appeared to negatively affect the LEC. Specifically, reduced CCL21+ LEC area and increased infiltration of inflammatory cells were found around the skin lymphatics [137]. In some tissues and cell types, aging is characterized by the accumulation of senescent cells [144, 145] a state of permanent cell cycle arrest, sometimes also accompanied by the production of senescence-associated secretory proteins, named the senescence-associated secretory phenotype (SASP) [146]. Both inflammaging and SASP are known to drive senescence-like phenotype in the endothelium, including LEC and BEC [144, 147]. Senescent cells and SASP act in a paracrine manner and drive the age-related defects in surrounding cells. As lymphatics carry tissue fluid to the LN, they can likely drain SASP products into the LN and during this process also get affected themselves, which may in part drive LEC dysfunction [148, 149].
Like other stromal cells, the BEC population also experiences multiple age-related changes that might contribute to observed defects in homing and recirculation of the naïve and memory T cells and the quality and magnitude of immune activity in old LN. The aging of BEC has been considered a wide spread phenomenon, and BEC are sensitive to a variety of age-related systemic and local changes, including SASP and inflammaging. The BEC responses to these changes are thought to drive some tissue-specific aging phenotypes, including in the spleen [150, 151]. Aged splenic BEC are sensitive to hypoxia and oxidative stress [150]. Within the aged spleen and other tissue, a loss of PDGFRβ+ pericytes that adhere to the endothelial vessels and correspondingly, a loss of BEC network density was evident. Importantly, fate-mapping experiments have shown that pericytes can transdifferentiate into fibroblasts and promote fibrotic changes in the aged spleen and lung [150, 152, 153]. In older human LN, the proportion and numbers of BEC and HEV are reduced [104]. Although the numeric decline in BEC LN was not evident in old mice [68, 118, 121], HEV appeared to be structurally altered with a loss of cuboidal morphology and the presence of altered vessel walls [99]. Unlike LEC, proliferation and expansion kinetics of aged BEC in draining LN were not affected in response to viral infection [118, 121]. During WNV infection, adoptively transferred old CD4+ Tn cells experienced delayed transmigration through HEV in adult recipients [20]. However, a direct comparison of the ability of adult and old HEV to support lymphocyte trafficking to the LN has yet to be performed. A recent study showed that ex vivo expanded adult and old mice-derived LEC and BEC were capable of recognizing the WNV, and responded similarly to their adult counterparts by inducing the equivalent early transcriptional response, indicative of type I IFN signaling [121]. Like LN BEC and LEC, aged bone-marrow (BM)-derived endothelial cells (EC) exhibited similar leakiness and sensitivity to oxidative stress, and overall vascular niche issues [154], and transplantation of young BM-EC in old mice has demonstrated a beneficial role in the rejuvenation of hematopoietic stem cells and immune reconstitution [155]. Similar rejuvenation potential for thymic EC in the adult response to irradiation has been demonstrated [156]. It will be important to establish whether young LEC or BEC population possess similar rejuvenation capacity that can help alleviate some of the age-related changes in the LN.
It becomes clear from the discussion above that LN stromal cells experience multiple age-related changes that precipitate in LN structural and functional impairment. However, which of these changes, and to what degree, are targetable is not known. While studies directly addressing this question are yet to be performed, a recent report has demonstrated that TLR-4 adjuvant boosted the response of MAdCAM1+ marginal reticular cells (MRC) in the aged LN during vaccination, resulting in improvement in GC activity [64]. Another study recently showed that localization and functional response of FDC in the GC region is altered by aging in both old murine and human LN, and that an age-related upregulation of CXCR4 on Tfh underlies these defects [131]. Specifically, transfer of young Tfh cells that express CXCR5 but not CXCR4 restored these defects, and improved FDC response and quality of GC during vaccination [131]. While the authors showed an improvement in antibody “quality” by assessing rates of affinity maturation, some of the effects were discrete and the authors did not test antibody “quality” in microbial challenge models to test improvements in immune defense. Nonetheless, these findings indicate that some of the age-related changes in the LN stromal cells can be targeted for correction, and it will be interesting to see if such strategies help restore the stromal cell network and structural issues.
Some of the age-related changes in the aged LN and/or stromal cells, such as senescence, fibrosis, and metabolic alterations appear to be (at least in part) reversible by senolytics, anti-fibrosis treatment, and dietary interventions, respectively [144, 146, 157, 158]. Senolytics, compounds that selectively eliminate senescent cells and reduce SASP in some (particularly mesenchymal) tissues, are one of the promising strategies to improve physiological function and physical performance in older adults [144, 157]. However, studies assessing their role in an immunosenescence setting have yielded mixed results, with some demonstrating benefits [159–161] while others reporting no improvement [162] in restoring functional immunity. Therefore, a more direct assessment of improvement in aged LN stromal cell structural and functional aspects through senolytic treatments is needed.
Similarly, anti-fibrosis and dietary interventions have improved age-related fibrosis [158, 163] and metabolic dysregulation [164–166], respectively, in other tissues. However, whether and to what extent such approaches could delay or ameliorate fibrotic or metabolic changes in the old LN stromal cells is not known and warrants direct examination. As is the case for other tissues during aging, incisive examination is needed of the potential to delay or reverse accumulation of macromolecular damage, cellular dysfunction, and structural changes that accumulate over time, and may be fueled by several feed-forward mechanisms [167]. Such investigations should address the alterations in epigenetic states [168], mitochondrial and lysosomal dysfunction [169, 170], impaired proteostasis [171], increased genomic instability [172], and loss of cellular function [167]. Moreover, age-related structural changes in the LN involve alterations in the ECM and tissue organization, and reestablishing such intricate tissue architecture needs specific attention, possibly exploiting approaches from tissue engineering technologies and innovative biological interventions.
4.4. Possible contribution of aged innate lymphoid cells in SLO defects?
Innate lymphoid cells (ILC type 1–3) have emerged as a key cellular player in regulating tissue homeostasis, repair, and immunity. They are also found in SLO, predominantly in the T cell and B cell zones and interfollicular regions of the LN, and experimental evidence suggests that they might play a critical role in regulating adaptive immunity [173]. A critical role of ILC3 in regulating lymphocyte entry to the LN has been suggested. Depletion of IL-7 in the adult mice resulted in a reduction of ILC3 numbers in the peripheral LN, concomitant with reduced lymphocyte homing and without affecting stromal cell abundance [174]. This effect was found to be independent of positive regulators (chemokines, cell adhesion molecules, and glycans) of HEV function, instead, the mechanism appeared to lie at the level of HEV barrier integrity [174]. A critical role of LTi cells, a specific type of ILC3, is well established in LN development, and ILC2 and ILC3 are often found located close to FRCs and MRCs in adult life [175, 176]. It has been shown that cytotoxic CD8+ T cells dismantle the TRC network during viral infection, which could be restored by ILC response mediated by LTα1β2 [176]. Splenic ILC3s are capable of integrating stromal and hematopoietic signals to help B cell function and antibody response [175]. ILC3 has been shown to establish a bi-directional cross-talk with MRCs. ILC3 production of LTα1β2 and TNF induce the expression of IL-7, CCL20, and MAdCAM-1 in MRCs, which in turn support the survival of ILCs via cell-cell contact-dependent and soluble (IL-7) signals [175]. ILC3 has also been reported to express BAFF, CD40L, and DLL1, and co-culture of ILC3 and CpG-stimulated marginal zone B cells induced the IgM, IgG, and IgA production of B cells, the level of which was further enhanced by additive signals from ILCs and MRCs [175]. Unraveling the exact role (and identification of associated molecular cues) of ILCs in the maintenance of LN structure and organization warrants urgent attention. Like other immune cells, ILCs also experience age-related changes, and understanding whether such ILC-mediated LN stromal conditioning, bi-directional cross-talks and maintenance function(s) deteriorate with age, and to what extent they fuel the age-related decline of functional immunity would pave a way to understand and modulate SLO aging.
5. Systemic Changes in Circulating Factors with Immune Aging.
Heterochronic parabiosis has been used in many studies to establish whether circulating cells and soluble factors may modulate the aging process in animals, chiefly mice [177]. For example, when an old mouse was surgically connected to a younger mouse, the old parabiont showed improvement in brain function, tissue regeneration, vascular function, and other aging phenotypes [178–182]. In 2023, Zhang et al. showed that old mice, who underwent heterochronic parabiosis for 3 months and the detachment, exhibited improved physiological parameters and lived longer than control isochronic mice [183]. The growth differentiation factor 11 (GDF11) has been identified as one candidate responsible for remodeling the hypertrophied heart in the old parabiont [179–181], although other studies contradict that finding [184]. In addition to GDF11, oxytocin [185], insulin-like growth factor-1 [186, 187], and tissue inhibitor of metalloproteinase (TIMP)-2 [188] were also implied as anti-aging circulatory factors responsible for improved function and increased regeneration of the brain, vascular system or muscle. Several studies reported that young plasma transfer can improve certain age-related conditions, but immunological changes in those models were not assessed [189–191]. Overall, to this date, to the best of our knowledge, no study with parabiosis or young plasma transfer ever demonstrated an improvement in immune parameters of the old host.
On the other hand, a young parabiont surgically connected to an older mouse developed aging phenotypes, such as decreased neurogenesis and reduced cognitive functions and C-C motif chemokine ligand 11 [192], β2-microglobulin [193, 194] and vascular cell adhesion molecule 1 [195] were identified as the progeroid factors. So far, heterochronic parabiosis has not been extensively applied to study circulatory factors involved in immunosenescence. In 2015, Shytokov et al. showed that the capacity of splenic adherent cells from younger parabionts to co-stimulate T cells was impaired to the levels seen in old mice [196]. Using heterochronic parabiosis, Kim et al. showed that thymic size was not regenerated in the old parabiont [65]. We confirmed this finding and extended it to show that old lymph node cellularity similarly was not regenerated by heterochronic parabiosis [66]. Moreover, we found that the cellularity of both stromal cells and T cells in LN declined in adult parabionts and that this effect was transient, being reversible upon parabiont separation [66]. In the same study, decay analysis of T cells after the separation from parabiosis showed the age-related alteration in the old LN made them unable to maintain T cells from young animals [66].
The key question here is the identity of the circulating factors in the old mice that drive adverse changes in the adult immune system over a relatively short time of parabiosis (4–5 weeks). In parabiosis, both cells and soluble factors will be shared between two parabionts. To figure out if the soluble progeroid factors alone can cause the changes in the immune system of younger mice, we performed plasma exchange experiments. We injected 3-month-old adult mice with PBS or with plasma from old mice and 18-month-old mice with PBS or plasma from the adult mice every 3 days for one month. Our preliminary data showed the injection of old plasma caused a pro-aging effect on the CD8+ Tn cell number in adult mice, while injection of adult plasma did not alter the aging phenotype in the spleens of old mice. However, further research will need to be done to identify the responsible factor(s) and the mechanism, particularly because SLO stromal aging plays such a prominent role in immune senescence. So far, no rejuvenating soluble factor which can reverse immune aging has been identified. Instead, immune-related factors, including inflammatory factors, are elevated in plasma from older mice or humans. This list includes but is not limited to, many chemokines, such as CCL2, CCL11, CCL12, and CCL19, cytokines, such as IL-3, IL-5, IL-16, IL-18, and other factors, such as haptoglobin, TIMP-1, and C-reactive protein [192, 195]. The elevation of those pro-inflammatory markers could be the result of immune aging, and it also could be its partial cause. The immune dysfunction seen in a young parabiont who shares the circulation with an old mouse happens rather rapidly (over 4–5 weeks) and is reversible, suggesting the action of a short-lived (likely soluble) component. Systematic dysregulation in chemokines and other pro-inflammatory factors can impair cell trafficking, cytokine production, or receptor expression. And prolonged elevated inflammatory factors may damage immune and/or stromal cells in SLO and may disturb the interaction between immune cells and stromal cells by causing irreversible structural changes. It is possible that senescent cell production of SASP-related molecules contributes to production of circulating pro-aging factors (reviewed in [157] and [197]). Palacio et al. showed that in irradiation-induced senescence, SASP was activated in the spleen to impair immune cell function in the splenic environment. The effect was reversible with the removal of senescence cells [198]. Therefore, the targeting of senescent cells in SLO needs to be assessed for its ability to improve immune function with aging. However, in the lymph node stroma, other mechanisms could be at work. For example, CCL19 and 21 are the key chemokines produced by FRC that provide a chemotactic gradient to Tn and Tcm cells cells to migrate to LN. In the (relative) absence of Tn cells, FCR may elevate production of CCL19 and 21 to “call in” more Tn cells to the LN. Prolonged overproduction of such chemokines, however, will eventually have a negative effect due to their accumulation in circulation (see parabiotic studies above, [192, 195], and the loss of a chemotactic gradient.
Overall, when considering the totality of stromal cell as well as soluble factor dysregulation with aging in SLO biology, it is likely that the Tn cell defect is the primary and initiating factor in the cascade. That leads to the initial compensatory changes in soluble factor secretion and stromal cell biology (retraction of the FRC network), that eventually further feed forward into impaired migration of Tn cells into the LN as well as their inferior maintenance by an already disorganized stromal networks.”
6. Concluding remarks and outstanding questions.
While the knowledge on the importance of non-lymphoid cell aging in thymic involution has been present for a while, the past decade brought us key insights on the importance of stromal cells in immune aging of other lymphoid compartments, including the bone marrow [199] and, as discussed above, SLO. Despite all the evidence pointing out the critical role of microenvironment SLO defects, the direct mechanistic evidence that age-related changes in the SLO stromal cells underlie defective immunity with aging is still limited. Specifically, the field now needs the development of stromal cell-type-specific, conditional, or even better, inducible gene overexpression models to directly address the role of key stromal-lymphocyte cross-talks during aging. If indeed such interactions are as important as suggested by the literature reviewed here, one should be able to demonstrate the reversal of age-related LN, and also protective immunity, defects by manipulation of key maintenance factors for LN stroma and of the stromal: lymphocyte cross-talk. Such experiments are already in progress and we are confident that we will soon be privy to new and exciting insights that should in turn pave the way to human clinical trials to improve protective immunity in older adults.
Acknowledgments
Supported in part by the USPHS National Institutes of Health awards AG-020719, AG-052359 and AG-057701 from the National Institute on Aging and by the Bowman Endowed Professorship in Medical Sciences to J.Ž.N
Abbreviations.
- BAFF
B-cell activating factor of the tumor necrosis factor superfamily
- BEC
blood endothelial cells
- BMP
bone morphogenetic protein
- CHIK
Chikungunya virus
- CMV
cytomegalovirus
- cTEC
cortical thymic epithelial cells
- CTL
Cytotoxic T lymphocytes
- DC
dendritic cells
- ECM
extra cellular matrix
- ETPs
early T-lineage progenitors
- FDC
follicular dendritic cells
- FRC
fibroblastic reticular cells
- GC
germinal center
- GDF11
growth differentiation factor 11
- GH
growth hormone
- HEV
high endothelial venule
- HSV-1
herpes simplex virus 1
- IFRC
inter follicular reticular cells
- KGF
keratinocyte growth factor
- LCMV
lymphocytic choriomeningitis virus
- LEC
lymphatic endothelial cells
- LT
lymphotoxin
- LTβR
lymphotoxin beta receptor
- LTi
lymphoid tissue inducer
- LTo
lymphoid tissue organizer
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- MedRC
medullary reticular cells
- MRC
marginal reticular cells
- mTEC
medullary thymic epithelial cells
- NAC
N-acetyl cysteine
- OVA
ovalbumin
- PDGFR
platelet-derived growth factor receptor
- PDPN
podoplanin
- pLN
peripheral lymph nodes
- PNAd
peripheral node addessin
- PROX1
prospero homeobox protein 1
- RANKL
receptor activator of nuclear factor kappa-B ligand
- RTE
recent thymic emigrants
- SASP
senescence-associated secretory phenotype
- SLO
secondary lymphoid organs
- TBRC
T-B border reticular cells
- Tfh cells
follicular helper T cells
- TGF-β
Transforming growth factor beta
- Tn cells
naïve T cells
- TRC
T-cell zone reticular cells
- WNV
West Nile virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
7. References.
- [1].Keilich SR, Bartley JM, Haynes L, Diminished immune responses with aging predispose older adults to common and uncommon influenza complications, Cell Immunol 345 (2019) 103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL, Epidemiology and transmission dynamics of West Nile virus disease, Emerg Infect Dis 11(8) (2005) 1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, Germonneau P, Quatresous I, Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Reunion, Epidemiol Infect 137(4) (2009) 534–41. [DOI] [PubMed] [Google Scholar]
- [4].Martin-Sanchez FJ, Del Toro E, Cardassay E, Valls Carbo A, Cuesta F, Vigara M, Gil P, Lopez Picado AL, Martinez Valero C, Miranda JD, Lopez-Ayala P, Chaparro D, Cozar Lopez G, Del Mar Suarez-Cadenas M, Jerez Fernandez P, Angos B, Diaz Del Arco C, Rodriguez Adrada E, Montalvo Moraleda MT, Espejo Paeres C, Fernandez Alonso C, Elvira C, Chacon A, Garcia Brinon MA, Fernandez Rueda JL, Ortega L, Fernandez Perez C, Gonzalez Armengol JJ, Gonzalez Del Castillo J, Clinical presentation and outcome across age categories among patients with COVID-19 admitted to a Spanish Emergency Department, Eur Geriatr Med 11(5) (2020) 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mueller AL, McNamara MS, Sinclair DA , Why does COVID-19 disproportionately affect older people?, Aging (Albany NY) 12(10) (2020) 9959–9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bonanad C, Garcia-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-Gonzalez V, Facila L, Ariza A, Nunez J, Cordero A, The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects, J Am Med Dir Assoc 21(7) (2020) 915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nikolich-Zugich J, The twilight of immunity: emerging concepts in aging of the immune system, Nat Immunol 19(1) (2018) 10–19. [DOI] [PubMed] [Google Scholar]
- [8].Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T, Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans, Semin Immunol 24(5) (2012) 331–41. [DOI] [PubMed] [Google Scholar]
- [9].Kogut I, Scholz JL, Cancro MP, Cambier JC, B cell maintenance and function in aging, Semin Immunol 24(5) (2012) 342–9. [DOI] [PubMed] [Google Scholar]
- [10].Steinmann GG, Klaus B, Muller-Hermelink HK, The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study, Scand J Immunol 22(5) (1985) 563–75. [DOI] [PubMed] [Google Scholar]
- [11].Chinn IK, Blackburn CC, Manley NR, Sempowski GD, Changes in primary lymphoid organs with aging, Semin Immunol 24(5) (2012) 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ, The influence of age on T cell generation and TCR diversity, J Immunol 174(11) (2005) 7446–52. [DOI] [PubMed] [Google Scholar]
- [13].den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K, Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans, Immunity 36(2) (2012) 288–97. [DOI] [PubMed] [Google Scholar]
- [14].Goronzy JJ, Weyand CM, Mechanisms underlying T cell ageing, Nat Rev Immunol 19(9) (2019) 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA, Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells, Nat Immunol 8(11) (2007) 1255–65. [DOI] [PubMed] [Google Scholar]
- [16].Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ, Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes, Nat Immunol 12(11) (2011) 1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, Cupedo T, Coles M, Ludewig B, IL-7-producing stromal cells are critical for lymph node remodeling, Blood 120(24) (2012) 4675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Zugich D, Kaye J, Nikolich-Zugich J, Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans, J Immunol 192(5) (2014) 2143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, Spasova DS, Sarkisyan G, LeRoy E, Tan JT, Wahlus H, Bondi-Boyd B, Luther SA, Surh CD, The aged lymphoid tissue environment fails to support naive T cell homeostasis, Sci Rep 6 (2016) 30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Richner JM, Gmyrek GB, Govero J, Tu Y, van der Windt GJ, Metcalf TU, Haddad EK, Textor J, Miller MJ, Diamond MS, Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection, PLoS Pathog 11(7) (2015) e1005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thompson HL, Smithey MJ, Uhrlaub JL, Jeftic I, Jergovic M, White SE, Currier N, Lang AM, Okoye A, Park B, Picker LJ, Surh CD, Nikolich-Zugich J, Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates, Aging Cell 18(1) (2019) e12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uhrlaub JL, Jergovic M, Bradshaw CM, Sonar S, Coplen CP, Dudakov J, Murray KO, Lanteri MC, Busch MP, van den Brink MRM, Nikolich-Zugich J, Quantitative restoration of immune defense in old animals determined by naive antigen-specific CD8 T-cell numbers, Aging Cell 21(4) (2022) e13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, Loh L, Olshanksy M, Naeem H, Tsyganov K, Wiede F, Webster R, Blyth C, Sng XYX, Tiganis T, Powell D, Doherty PC, Turner SJ, Kedzierska K, La Gruta NL, Age-Related Decline in Primary CD8(+) T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8(+) T Cells, Cell Rep 23(12) (2018) 3512–3524. [DOI] [PubMed] [Google Scholar]
- [24].Lee KA, Shin KS, Kim GY, Song YC, Bae EA, Kim IK, Koh CH, Kang CY, Characterization of age-associated exhausted CD8(+) T cells defined by increased expression of Tim-3 and PD-1, Aging Cell 15(2) (2016) 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jergovic M, Thompson HL, Renkema KR, Smithey MJ, Nikolich-Zugich J, Defective Transcriptional Programming of Effector CD8 T Cells in Aged Mice Is Cell-Extrinsic and Can Be Corrected by Administration of IL-12 and IL-18, Front Immunol 10 (2019) 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Desai A, Grolleau-Julius A, Yung R, Leukocyte function in the aging immune system, J Leukoc Biol 87(6) (2010) 1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li G, Smithey MJ, Rudd BD, Nikolich-Zugich J, Age-associated alterations in CD8alpha+ dendritic cells impair CD8 T-cell expansion in response to an intracellular bacterium, Aging Cell 11(6) (2012) 968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L, Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells, J Immunol 182(10) (2009) 6129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Baldwin SL, Hsu FC, Van Hoeven N, Gage E, Granger B, Guderian JA, Larsen SE, Lorenzo EC, Haynes L, Reed SG, Coler RN, Improved Immune Responses in Young and Aged Mice with Adjuvanted Vaccines against H1N1 Influenza Infection, Front Immunol 9 (2018) 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liang Z, Dong X, Zhang Z, Zhang Q, Zhao Y, Age-related thymic involution: Mechanisms and functional impact, Aging Cell 21(8) (2022) e13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR, Thymus: the next (re)generation, Immunol Rev 271(1) (2016) 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cowan JE, Malin J, Zhao Y, Seedhom MO, Harly C, Ohigashi I, Kelly M, Takahama Y, Yewdell JW, Cam M, Bhandoola A, Myc controls a distinct transcriptional program in fetal thymic epithelial cells that determines thymus growth, Nat Commun 10(1) (2019) 5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM, The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells, Int Immunol 19(10) (2007) 1201–11. [DOI] [PubMed] [Google Scholar]
- [34].Srinivasan J, Vasudev A, Shasha C, Selden HJ, Perez E Jr., LaFleur B, Sinari SA, Krueger A, Richie ER, Ehrlich LIR, The initial age-associated decline in early T-cell progenitors reflects fewer pre-thymic progenitors and altered signals in the bone marrow and thymus microenvironments, Aging Cell (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ, Continued maturation of thymic emigrants in the periphery, Nat Immunol 5(4) (2004) 418–25. [DOI] [PubMed] [Google Scholar]
- [36].Hale JS, Boursalian TE, Turk GL, Fink PJ, Thymic output in aged mice, Proc Natl Acad Sci U S A 103(22) (2006) 8447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Palmer S, Albergante L, Blackburn CC, Newman TJ, Thymic involution and rising disease incidence with age, Proc Natl Acad Sci U S A 115(8) (2018) 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL, Activation of thymic regeneration in mice and humans following androgen blockade, J Immunol 175(4) (2005) 2741–53. [DOI] [PubMed] [Google Scholar]
- [39].Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL, Effects of castration on thymocyte development in two different models of thymic involution, J Immunol 175(5) (2005) 2982–93. [DOI] [PubMed] [Google Scholar]
- [40].Olsen NJ, Watson MB, Henderson GS, Kovacs WJ, Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice, Endocrinology 129(5) (1991) 2471–6. [DOI] [PubMed] [Google Scholar]
- [41].Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, Hubbard VM, Kochman A, Willis LM, Greenberg AS, Tjoe KH, Sutherland JS, Chidgey A, van den Brink MR, Boyd RL, Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation, J Immunol 178(11) (2007) 7473–84. [DOI] [PubMed] [Google Scholar]
- [42].Lai KP, Lai JJ, Chang P, Altuwaijri S, Hsu JW, Chuang KH, Shyr CR, Yeh S, Chang C, Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy, Mol Endocrinol 27(1) (2013) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Heng TS, Reiseger JJ, Fletcher AL, Leggatt GR, White OJ, Vlahos K, Frazer IH, Turner SJ, Boyd RL, Impact of sex steroid ablation on viral, tumour and vaccine responses in aged mice, PLoS One 7(8) (2012) e42677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen BJ, Cui X, Sempowski GD, Chao NJ, Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice, Exp Hematol 31(10) (2003) 953–8. [DOI] [PubMed] [Google Scholar]
- [45].Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O’Reilly RJ, van den Brink MR, Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation, Transplantation 75(12) (2003) 1977–83. [DOI] [PubMed] [Google Scholar]
- [46].Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, Li K, Killian ML, Bacchetti P, McCune JM, Growth hormone enhances thymic function in HIV-1-infected adults, J Clin Invest 118(3) (2008) 1085–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR, Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration, Blood 107(6) (2006) 2453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Seggewiss R, Lore K, Guenaga FJ, Pittaluga S, Mattapallil J, Chow CK, Koup RA, Camphausen K, Nason MC, Meier-Schellersheim M, Donahue RE, Blazar BR, Dunbar CE, Douek DC, Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques, Blood 110(1) (2007) 441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, Gudkov AV, Takahama Y, Krenger W, Blazar BR, Hollander GA, Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells, Blood 109(9) (2007) 3803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Seggewiss R, Einsele H, Hematopoietic growth factors including keratinocyte growth factor in allogeneic and autologous stem cell transplantation, Semin Hematol 44(3) (2007) 203–11. [DOI] [PubMed] [Google Scholar]
- [51].Levine JE, Blazar BR, DeFor T, Ferrara JLM, Weisdorf DJ, Long-term follow-up of a phase I/II randomized, placebo-controlled trial of palifermin to prevent graft-versus-host disease (GVHD) after related donor allogeneic hematopoietic cell transplantation (HCT), Biol Blood Marrow Transplant 14(9) (2008) 1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wils EJ, Aerts-Kaya FS, Rombouts EJ, van Mourik I, Rijken-Schelen A, Visser TP, Braakman E, Wagemaker G, Cornelissen JJ, Keratinocyte growth factor and stem cell factor to improve thymopoiesis after autologous CD34+ cell transplantation in rhesus macaques, Biol Blood Marrow Transplant 18(1) (2012) 55–65. [DOI] [PubMed] [Google Scholar]
- [53].Griffith AV, Venables T, Shi J, Farr A, van Remmen H, Szweda L, Fallahi M, Rabinovitch P, Petrie HT, Metabolic Damage and Premature Thymus Aging Caused by Stromal Catalase Deficiency, Cell Rep 12(7) (2015) 1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, Jorcano JL, Conti CJ, Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia, Proc Natl Acad Sci U S A 93(15) (1996) 7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Semwal MK, Hester AK, Xiao Y, Udeaja C, Cepeda S, Verschelde JS 2nd, Jones N, Wedemeyer SA, Emtage S, Wimberly K, Griffith AV, Redox status regulates autophagy in thymic stromal cells and promotes T cell tolerance, Proc Natl Acad Sci U S A 119(40) (2022) e2204296119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV, Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice, Nature 369(6482) (1994) 669–71. [DOI] [PubMed] [Google Scholar]
- [57].Deane NG, Parker MA, Aramandla R, Diehl L, Lee WJ, Washington MK, Nanney LB, Shyr Y, Beauchamp RD, Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice, Cancer Res 61(14) (2001) 5389–95. [PubMed] [Google Scholar]
- [58].Kinsella S, Evandy CA, Cooper K, Iovino L, deRoos PC, Hopwo KS, Granadier DW, Smith CW, Rafii S, Dudakov JA, Attenuation of apoptotic cell detection triggers thymic regeneration after damage, Cell Rep 37(1) (2021) 109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Iovino L, Cooper K, deRoos P, Kinsella S, Evandy C, Ugrai T, Mazziotta F, Ensbey KS, Granadier D, Hopwo K, Smith C, Gagnon A, Galimberti S, Petrini M, Hill GR, Dudakov JA, Activation of the zinc-sensing receptor GPR39 promotes T-cell reconstitution after hematopoietic cell transplant in mice, Blood 139(25) (2022) 3655–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC, Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization, Nature 400(6745) (1999) 682–7. [DOI] [PubMed] [Google Scholar]
- [61].Kwok T MS, Silva-Junior IA, Brown EM, Haug JC, Barrios MR, Morris KA and Lancaster JN, Age-Associated Changes to Lymph Node Fibroblastic Reticular Cells, Frontiers in Aging (3) (2022) 838943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Moysi E, Paris RM, Le Grand R, Koup RA, Petrovas C, Human lymph node immune dynamics as driver of vaccine efficacy: an understudied aspect of immune responses, Expert Rev Vaccines 21(5) (2022) 633–644. [DOI] [PubMed] [Google Scholar]
- [63].Frasca D, Blomberg BB, Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination, Immun Ageing 17(1) (2020) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Denton AE, Dooley J, Cinti I, Silva-Cayetano A, Fra-Bido S, Innocentin S, Hill DL, Carr EJ, McKenzie ANJ, Liston A, Linterman MA, Targeting TLR4 during vaccination boosts MAdCAM-1(+) lymphoid stromal cell activation and promotes the aged germinal center response, Sci Immunol 7(71) (2022) eabk0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kim MJ, Miller CM, Shadrach JL, Wagers AJ, Serwold T, Young, proliferative thymic epithelial cells engraft and function in aging thymuses, J Immunol 194(10) (2015) 4784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Davies JS, Thompson HL, Pulko V, Padilla Torres J, Nikolich-Zugich J, Role of Cell-Intrinsic and Environmental Age-Related Changes in Altered Maintenance of Murine T Cells in Lymphoid Organs, J Gerontol A Biol Sci Med Sci 73(8) (2018) 1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang B, Jia Q, Bock C, Chen G, Yu H, Ni Q, Wan Y, Li Q, Zhuang Y, Glimpse of natural selection of long-lived T-cell clones in healthy life, Proc Natl Acad Sci U S A 113(35) (2016) 9858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sonar SA, Uhrlaub JL, Coplen CP, Sempowski GD, Dudakov JA, van den Brink MRM, LaFleur BJ, Jergovic M, Nikolich-Zugich J, Early age-related atrophy of cutaneous lymph nodes precipitates an early functional decline in skin immunity in mice with aging, Proc Natl Acad Sci U S A 119(17) (2022) e2121028119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, Haynes L, The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice, Aging Cell 11(5) (2012) 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jiang J, Fisher E, Bennett AJ, Murasko DM, Enhancement of virus-specific expansion of transgenic CD8 T cells in aged mice by dendritic cells, Mech Ageing Dev 131(9) (2010) 580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jiang J, Bennett AJ, Fisher E, Williams-Bey Y, Shen H, Murasko DM, Limited expansion of virus-specific CD8 T cells in the aged environment, Mech Ageing Dev 130(11–12) (2009) 713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jergovic M, Thompson HL, Renkema KR, Smithey MJ, Nikolich-Zugich J, Corrigendum: Defective Transcriptional Programming of Effector CD8 T Cells in Aged Mice Is Cell-Extrinsic and Can Be Corrected by Administration of IL-12 and IL-18, Front Immunol 11 (2020) 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stebegg M, Bignon A, Hill DL, Silva-Cayetano A, Krueger C, Vanderleyden I, Innocentin S, Boon L, Wang J, Zand MS, Dooley J, Clark J, Liston A, Carr E, Linterman MA, Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hung IF, Zhang AJ, To KK, Chan JF, Li C, Zhu HS, Li P, Li C, Chan TC, Cheng VC, Chan KH, Yuen KY, Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial, Clin Infect Dis 59(9) (2014) 1246–55. [DOI] [PubMed] [Google Scholar]
- [75].Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL, Zhang R, Chan TC, Chan BC, Wai HH, Chan LW, Fong HP, Hui RK, Kong KL, Leung AC, Ngan AH, Tsang LW, Yeung AP, Yiu GC, Yung W, Lau JY, Chen H, Chan KH, Yuen KY, Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial, Lancet Infect Dis 16(2) (2016) 209–18. [DOI] [PubMed] [Google Scholar]
- [76].Soerens AG, Kunzli M, Quarnstrom CF, Scott MC, Swanson L, Locquiao JJ, Ghoneim HE, Zehn D, Youngblood B, Vezys V, Masopust D, Functional T cells are capable of supernumerary cell division and longevity, Nature 614(7949) (2023) 762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yang X, Stedra J, Cerny J, Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice, J Exp Med 183(3) (1996) 959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dailey RW, Eun SY, Russell CE, Vogel LA, B cells of aged mice show decreased expansion in response to antigen, but are normal in effector function, Cell Immunol 214(2) (2001) 99–109. [DOI] [PubMed] [Google Scholar]
- [79].Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B, Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice, J Immunol 170(3) (2003) 1267–73. [DOI] [PubMed] [Google Scholar]
- [80].Grasso C, Pierie C, Mebius RE, van Baarsen LGM, Lymph node stromal cells: subsets and functions in health and disease, Trends Immunol 42(10) (2021) 920–936. [DOI] [PubMed] [Google Scholar]
- [81].Fletcher AL, Acton SE, Knoblich K, Lymph node fibroblastic reticular cells in health and disease, Nat Rev Immunol 15(6) (2015) 350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li L, Shirkey MW, Zhang T, Piao W, Li X, Zhao J, Mei Z, Guo Y, Saxena V, Kensiski A, Gavzy SJ, Song Y, Ma B, Wu J, Xiong Y, Wu L, Fan X, Roussey H, Li M, Krupnick AS, Abdi R, Bromberg JS, Lymph node fibroblastic reticular cells preserve a tolerogenic niche in allograft transplantation through laminin alpha4, J Clin Invest 132(13) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ, B cell homeostasis and follicle confines are governed by fibroblastic reticular cells, Nat Immunol 15(10) (2014) 973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Denton AE, Roberts EW, Linterman MA, Fearon DT, Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8+ T cells, Proc Natl Acad Sci U S A 111(33) (2014) 12139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C, Cupovic J, Danuser R, Sparwasser T, Luther SA, Thiel V, Rulicke T, Stein JV, Hehlgans T, Ludewig B, Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity, Immunity 38(5) (2013) 1013–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rodda LB, Lu E, Bennett ML, Sokol CL, Wang X, Luther SA, Barres BA, Luster AD, Ye CJ, Cyster JG, Single-Cell RNA Sequencing of Lymph Node Stromal Cells Reveals Niche-Associated Heterogeneity, Immunity 48(5) (2018) 1014–1028 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Perez-Shibayama C, Islander U, Lutge M, Cheng HW, Onder L, Ring SS, De Martin A, Novkovic M, Colston J, Gil-Cruz C, Ludewig B, Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8(+) T cells, Sci Immunol 5(51) (2020). [DOI] [PubMed] [Google Scholar]
- [88].Kapoor VN, Muller S, Keerthivasan S, Brown M, Chalouni C, Storm EE, Castiglioni A, Lane R, Nitschke M, Dominguez CX, Astarita JL, Krishnamurty AT, Carbone CB, Senbabaoglu Y, Wang AW, Wu X, Cremasco V, Roose-Girma M, Tam L, Doerr J, Chen MZ, Lee WP, Modrusan Z, Yang YA, Bourgon R, Sandoval W, Shaw AS, de Sauvage FJ, Mellman I, Moussion C, Turley SJ, Gremlin 1(+) fibroblastic niche maintains dendritic cell homeostasis in lymphoid tissues, Nat Immunol 22(5) (2021) 571–585. [DOI] [PubMed] [Google Scholar]
- [89].Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH, Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation, J Exp Med 207(4) (2010) 681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McGovern KE, Sonar SA, Watanabe M, Coplen CP, Bradshaw CM, Nikolich JZ , The aging of the immune system and its implications for transplantation, Geroscience (2023) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M, CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs, Nature 440(7083) (2006) 540–4. [DOI] [PubMed] [Google Scholar]
- [92].Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ, B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization, Immunity 24(2) (2006) 203–15. [DOI] [PubMed] [Google Scholar]
- [93].Fujimoto N, He Y, D’Addio M, Tacconi C, Detmar M, Dieterich LC, Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes, PLoS Biol 18(4) (2020) e3000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Takeda A, Hollmen M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lonnberg T, Bostrom P, Koskivuo I, Irjala H, Miyasaka M, Salmi M, Butcher EC, Jalkanen S, Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils, Immunity 51(3) (2019) 561–572 e5. [DOI] [PubMed] [Google Scholar]
- [95].Xiang M, Grosso RA, Takeda A, Pan J, Bekkhus T, Brulois K, Dermadi D, Nordling S, Vanlandewijck M, Jalkanen S, Ulvmar MH, Butcher EC, A Single-Cell Transcriptional Roadmap of the Mouse and Human Lymph Node Lymphatic Vasculature, Front Cardiovasc Med 7 (2020) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Veerman K, Tardiveau C, Martins F, Coudert J, Girard JP, Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes, Cell Rep 26(11) (2019) 3116–3131 e5. [DOI] [PubMed] [Google Scholar]
- [97].Brulois K, Rajaraman A, Szade A, Nordling S, Bogoslowski A, Dermadi D, Rahman M, Kiefel H, O’Hara E, Koning JJ, Kawashima H, Zhou B, Vestweber D, Red-Horse K, Mebius RE, Adams RH, Kubes P, Pan J, Butcher EC, A molecular map of murine lymph node blood vascular endothelium at single cell resolution, Nat Commun 11(1) (2020) 3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ahmadi O, McCall JL, Stringer MD, Does senescence affect lymph node number and morphology? A systematic review, ANZ J Surg 83(9) (2013) 612–8. [DOI] [PubMed] [Google Scholar]
- [99].Turner VM, Mabbott NA, Structural and functional changes to lymph nodes in ageing mice, Immunology 151(2) (2017) 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Turner VM, Mabbott NA, Influence of ageing on the microarchitecture of the spleen and lymph nodes, Biogerontology 18(5) (2017) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Cakala-Jakimowicz M, Kolodziej-Wojnar P, Puzianowska-Kuznicka M, Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview, Cells 10(11) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bekkhus T, Olofsson A, Sun Y, Magnusson PU, Ulvmar MH, Stromal transdifferentiation drives lipomatosis and induces extensive vascular remodeling in the aging human lymph node, J Pathol 259(3) (2023) 236–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nakagawa M, Hayashi S, Matsuo S, Yamazaki M, Kato A, Lipomatosis of axillary lymph nodes in a cynomolgus monkey (Macaca fascicularis), J Toxicol Pathol 35(1) (2022) 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hadamitzky C, Spohr H, Debertin AS, Guddat S, Tsokos M, Pabst R, Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance?, J Anat 216(5) (2010) 556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Li X, Zhao J, Kasinath V, Uehara M, Jiang L, Banouni N, McGrath MM, Ichimura T, Fiorina P, Lemos DR, Shin SR, Ware CF, Bromberg JS, Abdi R, Lymph node fibroblastic reticular cells deposit fibrosis-associated collagen following organ transplantation, J Clin Invest 130(8) (2020) 4182–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH, Human lymph node morphology as a function of age and site, J Clin Pathol 33(5) (1980) 454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Masters AR, Jellison ER, Puddington L, Khanna KM, Haynes L, Attrition of T Cell Zone Fibroblastic Reticular Cell Number and Function in Aged Spleens, Immunohorizons 2(5) (2018) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Aw D, Hilliard L, Nishikawa Y, Cadman ET, Lawrence RA, Palmer DB, Disorganization of the splenic microanatomy in ageing mice, Immunology 148(1) (2016) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B, Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes, Immunology 114(1) (2005) 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Daniel L, Bhattacharyya ND, Counoupas C, Cai Y, Chen X, Triccas JA, Britton WJ, Feng CG, Stromal structure remodeling by B lymphocytes limits T cell activation in lymph nodes of Mycobacterium tuberculosis-infected mice, J Clin Invest 132(21) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mazzucchelli R, Durum SK, Interleukin-7 receptor expression: intelligent design, Nat Rev Immunol 7(2) (2007) 144–54. [DOI] [PubMed] [Google Scholar]
- [112].Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, Ikuta K, Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice, J Immunol 189(4) (2012) 1577–84. [DOI] [PubMed] [Google Scholar]
- [113].Lee JL, Linterman MA, Mechanisms underpinning poor antibody responses to vaccines in ageing, Immunol Lett 241 (2022) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Aydar Y, Balogh P, Tew JG, Szakal AK, Follicular dendritic cells in aging, a “bottle-neck” in the humoral immune response, Ageing Res Rev 3(1) (2004) 15–29. [DOI] [PubMed] [Google Scholar]
- [115].Aydar Y, Balogh P, Tew JG, Szakal AK, Age-related depression of FDC accessory functions and CD21 ligand-mediated repair of co-stimulation, Eur J Immunol 32(10) (2002) 2817–26. [DOI] [PubMed] [Google Scholar]
- [116].Aydar Y, Balogh P, Tew JG, Szakal AK, Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation, J Immunol 171(11) (2003) 5975–87. [DOI] [PubMed] [Google Scholar]
- [117].Kwok T, Medovich SC, Silva-Junior IA, Brown EM, Haug JC, Barrios MR, Morris KA, Lancaster JN, Age-Associated Changes to Lymph Node Fibroblastic Reticular Cells, Front Aging 3 (2022) 838943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Masters AR, Hall A, Bartley JM, Keilich SR, Lorenzo EC, Jellison ER, Puddington L, Haynes L, Assessment of Lymph Node Stromal Cells as an Underlying Factor in Age-Related Immune Impairment, J Gerontol A Biol Sci Med Sci 74(11) (2019) 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Huang HY, Rivas-Caicedo A, Renevey F, Cannelle H, Peranzoni E, Scarpellino L, Hardie DL, Pommier A, Schaeuble K, Favre S, Vogt TK, Arenzana-Seisdedos F, Schneider P, Buckley CD, Donnadieu E, Luther SA, Identification of a new subset of lymph node stromal cells involved in regulating plasma cell homeostasis, Proc Natl Acad Sci U S A 115(29) (2018) E6826–E6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Cheng HW, Onder L, Novkovic M, Soneson C, Lutge M, Pikor N, Scandella E, Robinson MD, Miyazaki JI, Tersteegen A, Sorg U, Pfeffer K, Rulicke T, Hehlgans T, Ludewig B, Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp, Nat Commun 10(1) (2019) 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Bennett AK, Richner M, Mun MD, Richner JM, Type I IFN stimulates lymph node stromal cells from adult and old mice during a West Nile virus infection, Aging Cell (2023) e13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yang CY, Vogt TK, Favre S, Scarpellino L, Huang HY, Tacchini-Cottier F, Luther SA, Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes, Proc Natl Acad Sci U S A 111(1) (2014) E109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, Song K, Kondo Y, Woodruff MC, Gogineni A, Onder L, Ludewig B, Weimer RM, Carroll MC, Mooney DJ, Xia L, Turley SJ, The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture, Nat Immunol 16(1) (2015) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Acton SE, Farrugia AJ, Astarita JL, Mourao-Sa D, Jenkins RP, Nye E, Hooper S, van Blijswijk J, Rogers NC, Snelgrove KJ, Rosewell I, Moita LF, Stamp G, Turley SJ, Sahai E, Reis e Sousa C, Dendritic cells control fibroblastic reticular network tension and lymph node expansion, Nature 514(7523) (2014) 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hess E, Duheron V, Decossas M, Lezot F, Berdal A, Chea S, Golub R, Bosisio MR, Bridal SL, Choi Y, Yagita H, Mueller CG, RANKL induces organized lymph node growth by stromal cell proliferation, J Immunol 188(3) (2012) 1245–54. [DOI] [PubMed] [Google Scholar]
- [126].Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, Lu TT, Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells, J Immunol 187(11) (2011) 5558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Uhrlaub JL, Pulko V, DeFilippis VR, Broeckel R, Streblow DN, Coleman GD, Park BS, Lindo JF, Vickers I, Anzinger JJ, Nikolich-Zugich J, Dysregulated TGF-beta Production Underlies the Age-Related Vulnerability to Chikungunya Virus, PLoS Pathog 12(10) (2016) e1005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Palatella M, Guillaume SM, Linterman MA, Huehn J, The dark side of Tregs during aging, Front Immunol 13 (2022) 940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zeng M, Paiardini M, Engram JC, Beilman GJ, Chipman JG, Schacker TW, Silvestri G, Haase AT, Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution, Blood 120(9) (2012) 1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, Klauschen F, Bajenoff M, Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells, J Exp Med 211(6) (2014) 1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Silva-Cayetano A, Fra-Bido S, Robert PA, Innocentin S, Burton AR, Watson EM, Lee JL, Webb LMC, Foster WS, McKenzie RCJ, Bignon A, Vanderleyden I, Alterauge D, Lemos JP, Carr EJ, Hill DL, Cinti I, Balabanian K, Baumjohann D, Espeli M, Meyer-Hermann M, Denton AE, Linterman MA, Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging, Nat Immunol (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Rittie L, Fisher GJ, Natural and sun-induced aging of human skin, Cold Spring Harb Perspect Med 5(1) (2015) a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kaye AM, Shinkarenko L, Waisman A, Victor T, Degani H, Estradiol induction of accelerated energy metabolism in prepuberal rat uteri in vitro: mRNA hybridization and [13C]NMR studies, J Steroid Biochem 34(1–6) (1989) 289–92. [DOI] [PubMed] [Google Scholar]
- [134].Thangaswamy S, Bridenbaugh EA, Gashev AA, Evidence of increased oxidative stress in aged mesenteric lymphatic vessels, Lymphat Res Biol 10(2) (2012) 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Nagai T, Bridenbaugh EA, Gashev AA, Aging-associated alterations in contractility of rat mesenteric lymphatic vessels, Microcirculation 18(6) (2011) 463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zolla V, Nizamutdinova IT, Scharf B, Clement CC, Maejima D, Akl T, Nagai T, Luciani P, Leroux JC, Halin C, Stukes S, Tiwari S, Casadevall A, Jacobs WR Jr., Entenberg D, Zawieja DC, Condeelis J, Fooksman DR, Gashev AA, Santambrogio L, Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance, Aging Cell 14(4) (2015) 582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kataru RP, Park HJ, Shin J, Baik JE, Sarker A, Brown S, Mehrara BJ, Structural and Functional Changes in Aged Skin Lymphatic Vessels, Front Aging 3 (2022) 864860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Dybicka A, Fabiszewska-Gorna D, Kozakiewicz A, [Eye injuries connected with industrial accidents of shipyeard workers according to the material of the Ophthalmic Clinic in Gdansk in the period of 20 years], Klin Oczna 38(1) (1968) 87–94. [PubMed] [Google Scholar]
- [139].Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, Viar KE, Powell RD, Baker W, Dabhi N, Bai R, Cao R, Hu S, Rich SS, Munson JM, Lopes MB, Overall CC, Acton ST, Kipnis J, Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease, Nature 560(7717) (2018) 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, Miyazono K, Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis, Blood 111(9) (2008) 4571–9. [DOI] [PubMed] [Google Scholar]
- [141].Hos D, Bachmann B, Bock F, Onderka J, Cursiefen C, Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis, Exp Eye Res 87(5) (2008) 427–32. [DOI] [PubMed] [Google Scholar]
- [142].Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K, Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3, EMBO J 20(17) (2001) 4762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Franceschi C, Campisi J, Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases, J Gerontol A Biol Sci Med Sci 69 Suppl 1 (2014) S4–9. [DOI] [PubMed] [Google Scholar]
- [144].Chaib S, Tchkonia T, Kirkland JL, Cellular senescence and senolytics: the path to the clinic, Nat Med 28(8) (2022) 1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Miwa S, Kashyap S, Chini E, von Zglinicki T, Mitochondrial dysfunction in cell senescence and aging, J Clin Invest 132(13) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wiley CD, Campisi J, The metabolic roots of senescence: mechanisms and opportunities for intervention, Nat Metab 3(10) (2021) 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Chala N, Moimas S, Giampietro C, Zhang X, Zambelli T, Exarchos V, Nazari-Shafti TZ, Poulikakos D, Ferrari A, Mechanical Fingerprint of Senescence in Endothelial Cells, Nano Lett 21(12) (2021) 4911–4920. [DOI] [PubMed] [Google Scholar]
- [148].Yin K, Patten D, Gough S, de Barros Goncalves S, Chan A, Olan I, Cassidy L, Poblocka M, Zhu H, Lun A, Schuijs M, Young A, Martinez-Jimenez C, Halim TYF, Shetty S, Narita M, Hoare M, Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance, Genes Dev 36(9–10) (2022) 533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Shrestha S, Cho W, Stump B, Imani J, Lamattina AM, Louis PH, Pazzanese J, Rosas IO, Visner G, Perrella MA, El-Chemaly S, FK506 induces lung lymphatic endothelial cell senescence and downregulates LYVE-1 expression, with associated decreased hyaluronan uptake, Mol Med 26(1) (2020) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Chen J, Sivan U, Tan SL, Lippo L, De Angelis J, Labella R, Singh A, Chatzis A, Cheuk S, Medhghalchi M, Gil J, Hollander G, Marsden BD, Williams R, Ramasamy SK, Kusumbe AP, High-resolution 3D imaging uncovers organ-specific vascular control of tissue aging, Sci Adv 7(6) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chen J, Lippo L, Labella R, Tan SL, Marsden BD, Dustin ML, Ramasamy SK, Kusumbe AP, Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system, EMBO J 40(1) (2021) e105242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Caporarello N, Meridew JA, Aravamudhan A, Jones DL, Austin SA, Pham TX, Haak AJ, Moo Choi K, Tan Q, Haresi A, Huang SK, Katusic ZS, Tschumperlin DJ, Ligresti G, Vascular dysfunction in aged mice contributes to persistent lung fibrosis, Aging Cell 19(8) (2020) e13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD, Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis, Cell Stem Cell 16(1) (2015) 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kusumbe AP, Ramasamy SK, Itkin T, Mae MA, Langen UH, Betsholtz C, Lapidot T, Adams RH, Age-dependent modulation of vascular niches for haematopoietic stem cells, Nature 532(7599) (2016) 380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, Butler JM, Endothelial transplantation rejuvenates aged hematopoietic stem cell function, J Clin Invest 127(11) (2017) 4163–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, Ottmuller KJ, Mokhtari Z, Brede C, deRoos P, Kinsella S, Palikuqi B, Ginsberg M, Young LF, Kreines F, Lieberman SR, Lazrak A, Guo P, Malard F, Smith OM, Shono Y, Jenq RR, Hanash AM, Nolan DJ, Butler JM, Beilhack A, Manley NR, Rafii S, Dudakov JA, van den Brink MRM, Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration, Sci Immunol 3(19) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F, Cellular senescence in ageing: from mechanisms to therapeutic opportunities, Nat Rev Mol Cell Biol 22(2) (2021) 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Han X, Ding C, Sang X, Peng M, Yang Q, Ning Y, Lv Q, Shan Q, Hao M, Wang K, Wu X, Zhang H, Cao G, Targeting Sirtuin1 to treat aging-related tissue fibrosis: From prevention to therapy, Pharmacol Ther 229 (2022) 107983. [DOI] [PubMed] [Google Scholar]
- [159].Delval L, Hantute-Ghesquier A, Sencio V, Flaman JM, Robil C, Angulo FS, Lipskaia L, Cobanoglu O, Lacoste AS, Machelart A, Danneels A, Corbin M, Deruyter L, Heumel S, Idziorek T, Seron K, Sauve F, Bongiovanni A, Prevot V, Wolowczuk I, Belouzard S, Saliou JM, Gosset P, Bernard D, Rouille Y, Adnot S, Duterque-Coquillaud M, Trottein F, Removal of senescent cells reduces the viral load and attenuates pulmonary and systemic inflammation in SARS-CoV-2-infected, aged hamsters, Nat Aging 3(7) (2023) 829–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pastor-Fernandez A, Bertos AR, Sierra-Ramirez A, Del Moral-Salmoral J, Merino J, de Avila AI, Olague C, Villares R, Gonzalez-Aseguinolaza G, Rodriguez MA, Fresno M, Girones N, Bustos M, Smerdou C, Fernandez-Marcos PJ, von Kobbe C, Treatment with the senolytics dasatinib/quercetin reduces SARS-CoV-2-related mortality in mice, Aging Cell 22(3) (2023) e13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Camell CD, Yousefzadeh MJ, Zhu Y, Prata L, Huggins MA, Pierson M, Zhang L, O’Kelly RD, Pirtskhalava T, Xun P, Ejima K, Xue A, Tripathi U, Espindola-Netto JM, Giorgadze N, Atkinson EJ, Inman CL, Johnson KO, Cholensky SH, Carlson TW, LeBrasseur NK, Khosla S, O’Sullivan MG, Allison DB, Jameson SC, Meves A, Li M, Prakash YS, Chiarella SE, Hamilton SE, Tchkonia T, Niedernhofer LJ, Kirkland JL, Robbins PD, Senolytics reduce coronavirus-related mortality in old mice, Science 373(6552) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Torrance BL, Cadar AN, Martin DE, Panier HA, Lorenzo EC, Bartley JM, Xu M, Haynes L, Senolytic treatment with dasatinib and quercetin does not improve overall influenza responses in aged mice, Front Aging 4 (2023) 1212750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ, Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance, Sci Transl Med 6(231) (2014) 231ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Okawa T, Nagai M, Hase K, Dietary Intervention Impacts Immune Cell Functions and Dynamics by Inducing Metabolic Rewiring, Front Immunol 11 (2020) 623989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Parkhitko AA, Jouandin P, Mohr SE, Perrimon N, Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species, Aging Cell 18(6) (2019) e13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ, The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice, Cell Metab 19(3) (2014) 418–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Baechle JJ, Chen N, Makhijani P, Winer S, Furman D, Winer DA, Chronic inflammation and the hallmarks of aging, Mol Metab 74 (2023) 101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Nardini C, Moreau JF, Gensous N, Ravaioli F, Garagnani P, Bacalini MG, The epigenetics of inflammaging: The contribution of age-related heterochromatin loss and locus-specific remodelling and the modulation by environmental stimuli, Semin Immunol 40 (2018) 49–60. [DOI] [PubMed] [Google Scholar]
- [169].Escrig-Larena JI, Delgado-Pulido S, Mittelbrunn M, Mitochondria during T cell aging, Semin Immunol 69 (2023) 101808. [DOI] [PubMed] [Google Scholar]
- [170].Guerrero-Navarro L, Jansen-Durr P, Cavinato M, Age-Related Lysosomal Dysfunctions, Cells 11(12) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Frankowska N, Lisowska K, Witkowski JM, Proteolysis dysfunction in the process of aging and age-related diseases, Front Aging 3 (2022) 927630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Saxena S, Zou L, Hallmarks of DNA replication stress, Mol Cell 82(12) (2022) 2298–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, Filbey KJ, Maizels RM, Hepworth MR, Sonnenberg GF, Milling S, Withers DR, CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes, Nat Commun 6 (2015) 5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Yang J, Cornelissen F, Papazian N, Reijmers RM, Llorian M, Cupedo T, Coles M, Seddon B, IL-7-dependent maintenance of ILC3s is required for normal entry of lymphocytes into lymph nodes, J Exp Med 215(4) (2018) 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, Barra CM, Comerma L, Chudnovskiy A, Gentile M, Llige D, Cols M, Serrano S, Arostegui JI, Juan M, Yague J, Merad M, Fagarasan S, Cerutti A, Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells, Nat Immunol 15(4) (2014) 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B, Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone, Nat Immunol 9(6) (2008) 667–75. [DOI] [PubMed] [Google Scholar]
- [177].Conboy IM, Rando TA, Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches, Cell Cycle 11(12) (2012) 2260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, Rejuvenation of aged progenitor cells by exposure to a young systemic environment, Nature 433(7027) (2005) 760–4. [DOI] [PubMed] [Google Scholar]
- [179].Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ, Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle, Science 344(6184) (2014) 649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors, Science 344(6184) (2014) 630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT, Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy, Cell 153(4) (2013) 828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, Ungvari Z, Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood, Geroscience 42(2) (2020) 727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Zhang B, Lee DE, Trapp A, Tyshkovskiy A, Lu AT, Bareja A, Kerepesi C, McKay LK, Shindyapina AV, Dmitriev SE, Baht GS, Horvath S, Gladyshev VN, White JP, Multi-omic rejuvenation and life span extension on exposure to youthful circulation, Nat Aging (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR, GDF11 does not rescue aging-related pathological hypertrophy, Circ Res 117(11) (2015) 926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP, Conboy IM, Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration, Nat Commun 5 (2014) 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N, Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle, Nat Genet 27(2) (2001) 195–200. [DOI] [PubMed] [Google Scholar]
- [187].Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z, Insulin-like growth factor-1 in CNS and cerebrovascular aging, Front Aging Neurosci 5 (2013) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, Shen JC, Zou B, Xie XS, Tingle M, Hinkson IV, Angst MS, Wyss-Coray T, Human umbilical cord plasma proteins revitalize hippocampal function in aged mice, Nature 544(7651) (2017) 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, Zhang H, Luo J, Stan T, Mosher KI, Masliah E, Wyss-Coray T, Preclinical Assessment of Young Blood Plasma for Alzheimer Disease, JAMA Neurol 73(11) (2016) 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Sahu A, Clemens ZJ, Shinde SN, Sivakumar S, Pius A, Bhatia A, Picciolini S, Carlomagno C, Gualerzi A, Bedoni M, Van Houten B, Lovalekar M, Fitz NF, Lefterov I, Barchowsky A, Koldamova R, Ambrosio F, Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles, Nat Aging 1(12) (2021) 1148–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice, Nat Med 20(6) (2014) 659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T, The ageing systemic milieu negatively regulates neurogenesis and cognitive function, Nature 477(7362) (2011) 90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA, beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis, Nat Med 21(8) (2015) 932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Rebo J, Mehdipour M, Gathwala R, Causey K, Liu Y, Conboy MJ, Conboy IM, A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood, Nat Commun 7 (2016) 13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, Zandstra J, Berber E, Lehallier B, Mathur V, Nair RV, Bonanno LN, Yang AC, Peterson T, Hadeiba H, Merkel T, Korbelin J, Schwaninger M, Buckwalter MS, Quake SR, Butcher EC, Wyss-Coray T, Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1, Nat Med 25(6) (2019) 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].M.S.S. Shytikov Dmytro W., Yankova Tetiana M., Peregudov Alex G., Artyuhov Igor V., Pishel. Iryna M., Splenic Niche Cells from Young Heterochronic Parabionts Have Decreased Capability to Amplify T-cell Proliferation in Vitro, American Journal of BioScience 3(2) (2015) 46–54. [Google Scholar]
- [197].Kumari R, Jat P, Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype, Front Cell Dev Biol 9 (2021) 645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Palacio L, Goyer ML, Maggiorani D, Espinosa A, Villeneuve N, Bourbonnais S, Moquin-Beaudry G, Le O, Demaria M, Davalos AR, Decaluwe H, Beausejour C, Restored immune cell functions upon clearance of senescence in the irradiated splenic environment, Aging Cell 18(4) (2019) e12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Montecino-Rodriguez E, Dorshkind K, The layered development of mouse B and T Cells, Immunol Rev 315(1) (2023) 79–88. [DOI] [PubMed] [Google Scholar]
- [200].Cosgrove J, Novkovic M, Albrecht S, Pikor NB, Zhou Z, Onder L, Morbe U, Cupovic J, Miller H, Alden K, Thuery A, O’Toole P, Pinter R, Jarrett S, Taylor E, Venetz D, Heller M, Uguccioni M, Legler DF, Lacey CJ, Coatesworth A, Polak WG, Cupedo T, Manoury B, Thelen M, Stein JV, Wolf M, Leake MC, Timmis J, Ludewig B, Coles MC, B cell zone reticular cell microenvironments shape CXCL13 gradient formation, Nat Commun 11(1) (2020) 3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K, Chan L, Lucas B, Novitzky-Basso I, Nakamura K, Rulicke T, Nibbs RJ, Worbs T, Forster R, Rot A, The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes, Nat Immunol 15(7) (2014) 623–30. [DOI] [PubMed] [Google Scholar]
- [202].Carpentier KS, Sheridan RM, Lucas CJ, Davenport BJ, Li FS, Lucas ED, McCarthy MK, Reynoso GV, May NA, Tamburini BAJ, Hesselberth JR, Hickman HD, Morrison TE, MARCO(+) lymphatic endothelial cells sequester arthritogenic alphaviruses to limit viremia and viral dissemination, EMBO J 40(22) (2021) e108966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Ortsater H, Hernandez-Vasquez MN, Ulvmar MH, Gow A, Makinen T, An inducible Cldn11-CreER(T2) mouse line for selective targeting of lymphatic valves, Genesis 59(7–8) (2021) e23439. [DOI] [PubMed] [Google Scholar]
- [204].Abe Y, Sakata-Yanagimoto M, Fujisawa M, Miyoshi H, Suehara Y, Hattori K, Kusakabe M, Sakamoto T, Nishikii H, Nguyen TB, Owada Y, Enomoto T, Sawa A, Bando H, Yoshida C, Tabata R, Terao T, Nakayama M, Ohshima K, Usuki K, Oda T, Matsue K, Chiba S, A single-cell atlas of non-haematopoietic cells in human lymph nodes and lymphoma reveals a landscape of stromal remodelling, Nat Cell Biol 24(4) (2022) 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Lee M, Kiefel H, LaJevic MD, Macauley MS, Kawashima H, O’Hara E, Pan J, Paulson JC, Butcher EC, Transcriptional programs of lymphoid tissue capillary and high endothelium reveal control mechanisms for lymphocyte homing, Nat Immunol 15(10) (2014) 982–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


