Abstract
The O antigen of the Pseudomonas aeruginosa lipopolysaccharide is the optimal target for protective antibodies, but the unusual and complex nature of their sugar substituents has made it difficult to define the range of these structures needed in an effective vaccine. Most clinical isolates of P. aeruginosa can be classified into 10 O-antigen serogroups, but slight chemical differences among O polysaccharides within a serogroup give rise to subtype epitopes. These epitopes could impact the reactivity of O-antigen-specific antibodies, as well as the susceptibility of a target strain to protective, opsonic antibodies. To define parameters of serogroup and subtype-epitope immunogenicity, antigenicity, and surface expression on P. aeruginosa cells, we prepared high-molecular-weight O-polysaccharide vaccines from strains of P. aeruginosa serogroup O2, for which eight structurally variant O antigens expressing six defined subtype epitopes (O2a to O2f) have been identified. A complex pattern of immune responses to these antigens was observed following vaccination of mice. The high-molecular-weight O polysaccharides were generally more immunogenic at low doses (1 and 10 μg) than at a high dose (50 μg) and usually elicited antibodies that opsonized the homologous strain for phagocytic killing. Some of the individual polysaccharides elicited cross-opsonic antibodies to a variable number of strains that express all of the defined serogroup O2 subtype epitopes. Combination into one vaccine of two antigens that individually elicited cross-reactive opsonic antibodies to most members of the O2 serogroup inhibited, instead of enhanced, the production of antibodies broadly reactive with most serogroup O2 subtype strains. Thus, immune responses to P. aeruginosa O antigens may be restricted to a limited range of epitopes on structurally complex O antigens, and combining multiple related antigens into a single vaccine formulation may inhibit the production of those antibodies best able to protect against most P. aeruginosa strains within a given O-antigen serogroup.
It has been established through animal and human experimentation that the lipopolysaccharide (LPS) O antigen of Pseudomonas aeruginosa is a target for protective antibodies (3, 36, 38). The studies of Knirel and colleagues (17, 19) on the chemical composition and structure of the major O-side-chain polysaccharides have provided important insights into the immunochemical properties of these antigens, but our understanding of their antigenic and immunogenic properties is incomplete. This point is highlighted by the inability to date to develop effective, LPS-specific immunotherapies for human P. aeruginosa infection (7).
Results obtained with animals by using immunogens and antibodies specific to the O polysaccharides have indicated that slight chemical differences among bacterial strains with otherwise closely related O-side-chain structures can produce a complex pattern of reactions between antibodies and related antigens (13). With standard serologic methods using whole-cell agglutinations, strains of P. aeruginosa can be classified as members of one serogroup (serotype); members of each serogroup share a group-specific antigen. Further subdivision into subtypes, which correlate with structural variants determined by Knirel and colleagues (17), can be accomplished with appropriate antisera (22).
To develop safe and effective O-antigen-specific P. aeruginosa vaccines, we have utilized the high-molecular-mass (>100,000-Da) fraction of O polysaccharides. These antigens are safe and immunogenic in humans and animals (13, 27, 37) and elicit protective antibodies to the strains from which they are isolated. However, in recent studies of animals immunized with a heptavalent high-molecular-weight O-polysaccharide vaccine whose individual components were isolated from single strains representative of the major serogroups causing P. aeruginosa infection, opsonic antibody responses to the group-specific antigens were not commonly elicited (13). Thus, in spite of chemical and serologic relatedness among subtype strains within a P. aeruginosa serogroup, single antigens isolated from one subtype strain do not always elicit opsonic antibodies to all of the strains within the serogroup (13). Previous results showed that a particular O antigen from a given serogroup may elicit group-specific immunity, while an O antigen from another serogroup may elicit only immunity specific to the subtype epitopes expressed on that particular O antigen.
To explore this situation further and gain additional insight into the serologic diversity among P. aeruginosa LPS O antigens, we prepared high-molecular-weight O-polysaccharide immunogens from five strains of P. aeruginosa serogroup O2 that, together, express all six of the identified subtype antigens (Table 1). These polysaccharides were used to immunize mice, and the resultant sera were assessed by enzyme-linked immunosorbent assay (ELISA) and for opsonic killing activity. The results showed a complex interaction among the strains with regard to high-molecular-weight O-polysaccharide immunogenicity, antigenicity, serogroup and subtype epitope density, and susceptibility to opsonic killing. These findings indicate that the current serogroup classifications of P. aeruginosa are probably inadequate to define the full range of LPS antigens needed to elicit comprehensive immunity to a wide range of clinical isolates.
TABLE 1.
Strains used for immunogen production, their serologic classification by subtype epitope, and chemical structures of the associated O antigens
Boldface type indicates a feature of a structure that distinguishes it from a related structure of the same serogroup. Abbreviations: FucNAc, 2-acetamido-2,6-dideoxygalactose (N-acetylfucosamine); Man(NAc)2A, 2,3-diacetamido-2,3-dideoxymannuronic acid; Man(2NAc3N)A, 2-acetamido-3-acetamidino-2,3-dideoxymannuronic acid; Gul(NAc)2A, 2,3-diacetamido-2,3-dideoxyguluronic acid.
The lower structure is also part of the O antigen of strain 170007; there is about a 2:1 ratio of the upper and lower structures.
MATERIALS AND METHODS
Bacterial strains.
As representatives of serogroup O2, we utilized the P. aeruginosa strains whose O-polysaccharide structures had been elucidated by Knirel et al. (17, 18). The strains used to produce antigens for immunization are listed with their associated subtype antigenic determinants in Table 1, which also shows the structures of the associated O antigens (17, 19).
Antigen preparation.
LPS and high-molecular-weight O polysaccharides were prepared from strains 170003, 170006, 170007, Fisher IT-3, PAO1, Fisher IT-7, and IATS O16 as described elsewhere (13).
Immunization of mice.
Six- to 8-week-old female C3H/HeN mice (Harlan Sprague-Dawley Farms, Chicago, Ill.) housed under virus-free conditions were immunized intraperitoneally three times, 5 days apart, with a 1-, 10-, or 50-μg dose of high-molecular-weight O polysaccharide dissolved in 0.2 ml of 0.1 M phosphate–0.015 M sodium chloride, pH 7.2. Blood taken 12 days after the final injection of antigen was used for analysis of immune responses.
ELISA.
Binding of antibodies to the different LPS antigens was evaluated by standard ELISA methods as described previously (13). For simplicity of analysis, optical density (O.D.) values obtained with 1:10 dilutions of mouse antisera and goat-anti-mouse immunoglobulin G (IgG) as the detection reagent are presented.
Opsonic assay.
Opsonophagocytic killing of P. aeruginosa strains was determined as described previously (26). The negative control serum for each assay was normal rabbit serum, and the positive control serum was a 1:10 dilution of rabbit sera raised to boiled whole cells of Fisher strain IT-3. Results are given as the mean of quadruplicate determinations of the maximal percentage of killing obtained at a final mouse serum dilution of 1:8. Under routine conditions, killing of >40% is considered statistically significant at the 0.05 level and thus serves to classify a serum as positive for opsonic killing activity. For all of the values presented, the standard error of the mean (SEM) was <5%.
RESULTS
Properties of high-molecular-weight O polysaccharides.
Each high-molecular-weight O-polysaccharide antigen purified from the strains listed in Table 1 contained low levels of protein and nucleic acid contaminants (<1%), and comparable antigen concentrations reacted by immunodiffusion with antisera raised to boiled whole cells of the Fisher IT-3 strain. This rabbit serum contains high-titered antibodies to the shared O2a determinant, indicating preservation of this epitope during purification of the high-molecular-weight O-polysaccharide antigens (36). The O polysaccharides had no detectable 2-keto-3-deoxyoctonate. The LPS antigens generally contained between 2 and 5% 2-keto-3-deoxyoctonate. Chemical analysis indicated the presence of the sugars given in Table 1 in each antigen in the correct molar ratios.
Immune response to homologous antigens.
We have previously published our results on the immune responses of mice and rabbits following immunization with the Fisher IT-3 antigen (serogroups O2a and O2c) (13, 31). This antigen elicited good opsonic responses to the homologous strain. When this antigen was used in a heptavalent vaccine that also contained the high-molecular-weight O-polysaccharide antigen from a clinical strain of the Fisher IT-7 serogroup with an unknown array of subtype epitopes, there were no opsonic antibodies produced in mice or rabbits to any of the heterologous strains of serogroup O2 (13). The implication was that the Fisher IT-3 component of the vaccine principally elicited antibody to the IT-3-specific subtype O2c epitope. We therefore assessed the immunogenicity of high-molecular-weight O-polysaccharide antigens isolated from the remaining strains of P. aeruginosa serogroup O2 that express all of the other defined subtype determinants for the ability to elicit opsonic antibodies. Preliminary results comparing site of injection and number of immunizations indicated that optimal antibody responses were present in sera obtained 7 to 12 days after the last of three closely spaced intraperitoneal immunizations.
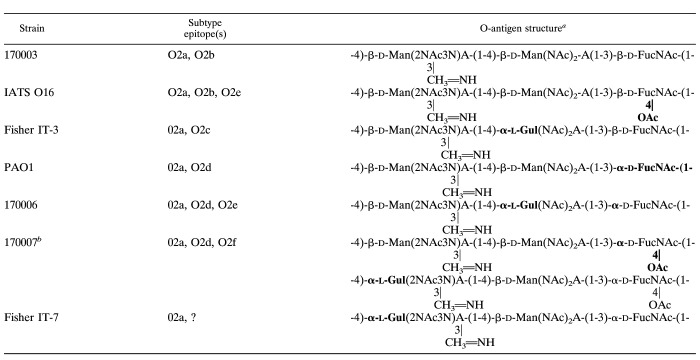
We first analyzed the opsonic killing response of C3H mice to the homologous strain in sera obtained 12 days after the last of three intraperitoneal injections of antigen (Fig. 1). Opsonic killing is considered to be an excellent in vitro correlate of protective immunity to P. aeruginosa (14, 42, 43). Except for the antigens isolated from strains PAO1 and 170006, each O polysaccharide elicited excellent levels of opsonic antibodies to the homologous strain. Although for most of the strains, the lower doses (1 and 10 μg) were superior, for some strains (e.g., 170007 and Fisher IT-7) a 50-μg dose was also highly effective. Immune responses to strain 170006 were modest, and reasonable killing was achieved only with the 10-μg dose. Overall, it appeared that the 10-μg dose elicited nearly maximal immune responses in mice with all but one of these chemically related antigens.
FIG. 1.
Opsonic antibody responses elicited by three different doses of high-molecular-weight O-polysaccharide antigens isolated from the individual strains designated on the y axis and tested against the homologous target strain. The bars indicate mean results of quadruplicate determinations. The SEM was <5% for all determinations.
Immune responses to heterologous strains.
Seeking a clear pattern of association between subtype determinants and immunogenicity, we next evaluated the immune sera for the ability to mediate opsonic killing of heterologous strains of serogroup O2 P. aeruginosa. No serum mediated opsonic killing of all of the strains. This result indicated that the serologically defined, shared O2a epitope either was not immunodominant in any high-molecular-weight O-polysaccharide antigen or was not expressed on the surface of the target strains at a sufficient density for effective antibody binding leading to opsonic killing.
As an example of an opsonic antibody response restricted to shared subtype determinants, we found that antisera raised against 1- and 10-μg doses of the high-molecular-weight O polysaccharide from strain 170003 had high levels of opsonic killing activity (>60% killing) against strain IATS O16, which shares the subtype O2b epitope with strain 170003. For strain 170003, antibodies to the O2b epitope are both immunodominant and targets of opsonic antibodies. Opsonic killing of other strains by antibodies in sera raised to strain 170003 was minimal.
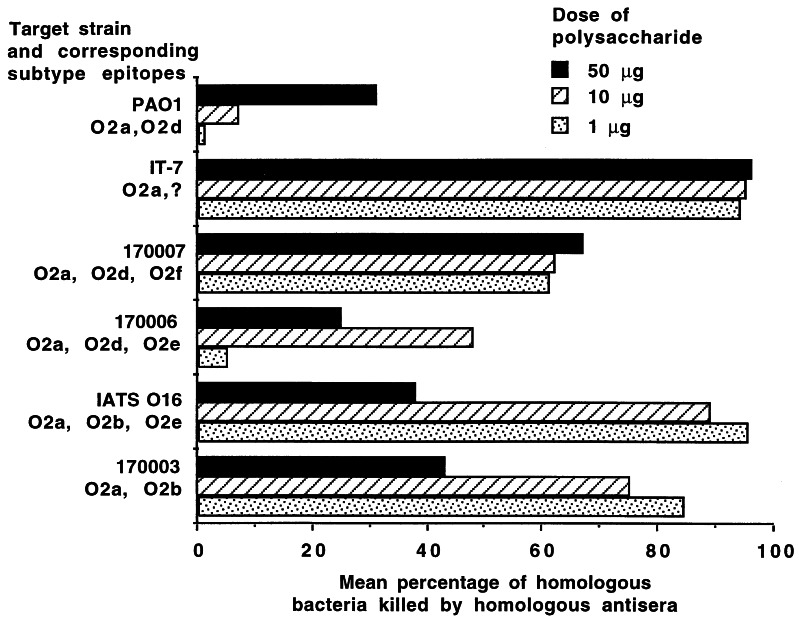
Strain IATS O16 elicited the highest level of cross-reactive opsonic killing of strain 170003 at the 10- and 50-μg doses, whereas homologous strain killing was highest at the 1- or 10-μg dose (Fig. 2). Thus, for strain IATS O16, the immunogenicity of the O2b epitope was maximal at 10 to 50 μg, but the O2b-specific antibodies were not the most effective ones giving opsonic killing of the homologous strain. In addition, immunization with IATS O16 elicited excellent opsonic killing of the Fisher IT-3 strain and good (40 to 85%) levels of killing of the Fisher IT-7 and PAO1 strains. Examination of the O-antigen structures of the five strains killed well (>40% killing) by antisera to IATS O16 revealed no clear chemical structure shared by these strains that was absent from less susceptible strains. Overall, the antigen from strain IATS O16 elicited a broader range of opsonic antibodies able to mediate the killing of five of the seven chemically distinct serogroup O2 strains.
FIG. 2.
Opsonic antibody responses elicited by three different doses (50 μg [▪], 10 μg [▨], and 1 μg [ ]) of the high-molecular-weight O-polysaccharide antigen isolated from P. aeruginosa IATS O16 tested against the target strains indicated on the y axis. The bars indicate mean results of quadruplicate determinations. The SEM was <5% for all determinations.
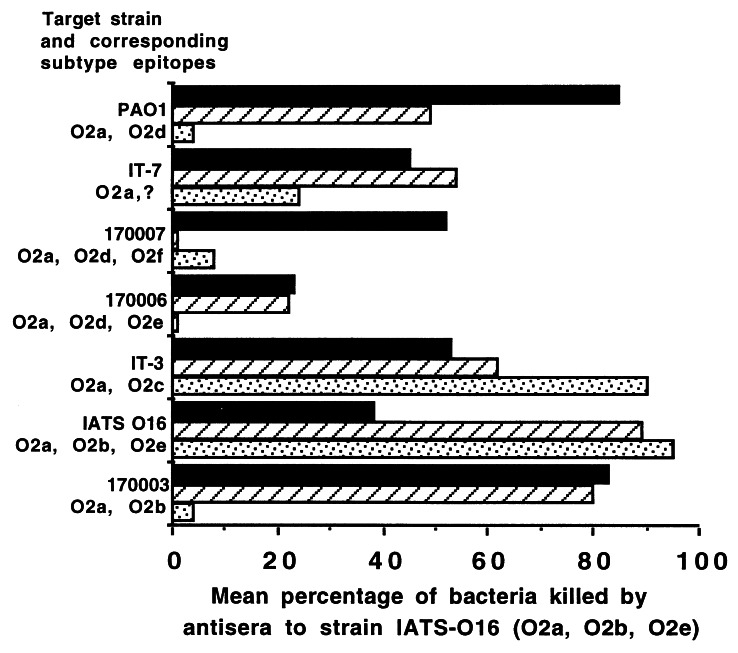
The serogroup O2d epitope is expressed by three strains (PAO1, 170006, and 170007), and results of studies with these strains emphasized the complex nature of the serology of P. aeruginosa LPS O antigens. Although antisera raised to strain PAO1 exhibited a high level of binding to the homologous antigen by ELISA (data not shown), the sera were poorly opsonic for the homologous strain, suggesting that the immunodominant epitopes for this strain were not good targets for opsonic antibody (Fig. 3A). The high-molecular-weight O polysaccharide from strain PAO1 was, however, able to elicit opsonic antibodies to the other two strains expressing the O2d epitope. It was interesting that the polysaccharide doses resulting in maximal killing of the two strains expressing the O2d epitope occurred in sera from animals given different doses. This result suggested that epitopes other than the O2d epitope were targets of opsonic antibodies in sera from mice immunized with the strain PAO1 high-molecular-weight O polysaccharide. Strain IATS O16 was also modestly killed by antisera to PAO1. This result probably represents one of the more confusing aspects of P. aeruginosa O-antigen immunology; i.e., immunodominant epitopes are not necessarily sufficiently available on the surface of the bacterial strain from which they were isolated to serve as a target for opsonic antibodies.
FIG. 3.
Opsonic antibody responses elicited by three different doses of high-molecular-weight O-polysaccharide antigens isolated from three P. aeruginosa strains carrying the O2d subtype epitope and tested against the target strains indicated on the y axis. The bars indicate mean results of quadruplicate determinations. The SEM was <5% for all determinations.
The high-molecular-weight O polysaccharide from strain 170006 was poorly immunogenic and failed to elicit opsonic antibodies that killed any strain at a level of >50% (Fig. 3B). This antigen elicited high levels of IgM antibodies to itself at all three doses (O.D. at 450 nm of a 1:10 serum dilution, >1.0), but IgG antibodies were not elevated over those in preimmune sera. This result provides an example of an immunogen with poor overall immunogenicity in mice which therefore might not be considered to be a strong candidate for inclusion in a multivalent vaccine.
The high-molecular-weight O polysaccharide from strain 170007 showed good opsonic killing against itself at all three antigen doses and even somewhat higher killing of the IATS O16 strain (Fig. 3C). The low killing of the two other strains expressing the O2d epitope (PAO1 and 170006) indicates that this epitope is either not very immunogenic on the antigen obtained from strain 170007 or not expressed on strain PAO1 or 170006 as a target for opsonic antibodies. There was good killing of the Fisher IT-3 strain following immunization with 50 μg of the O polysaccharide from strain 170007. Strain IATS O16 was killed at a level of >40% by at least one serum from each group of mice immunized with a representative strain carrying the O2d epitope. This result suggests some cross-reactivity or additional shared antigenicity between IATS O16 and the O2d-expressing strains.
The final antigen we evaluated as a monovalent preparation was isolated from the Fisher IT-7 strain, which, in addition to the O2a epitope, expresses a strain-specific epitope not defined by the Lanyi serotyping system (18, 22; designated by a question mark in Table 1). This antigen elicited high levels of opsonic killing (>90% of bacterial cells killed [Table 1]) against itself at all three doses examined, and a 50-μg dose of this antigen elicited a high level of opsonic killing of strain PAO1 (90% of cells killed; data not shown). When P. aeruginosa serogrouping is performed with antisera to the prototypic Fisher IT strains, PAO1 is classified as an IT-7 strain. Thus, these two strains appear to share an immunogenic and antigenic epitope. Opsonic killing of the homologous IT-7 strain by sera from mice given any of the three doses of antigen suggests maximal immunogenicity of additional epitopes expressed only on the IT-7 strain and not shared by the other serogroup O2 strains. These IT-7-specific epitopes likely form the basis for classifying this strain separately from IT-3 in the Fisher immunotyping system (9).
In all of the opsonic assays using serogroup O2 strains, each strain was killed at a level of >90% when a 1:10 dilution of rabbit serum raised to boiled cells of strain IT-3 was used as a positive control. Normal rabbit serum always exhibited no killing activity. Thus, in hyperimmune rabbit sera raised to boiled cells, opsonic antibodies with high levels of activity against all of the strains could be elicited. This finding suggests that the form, route, and dose of an antigen, along with the species used to produce antibodies, can give rise to different populations of antibodies with various abilities to mediate opsonic killing of related P. aeruginosa strains.
ELISA responses to antigens.
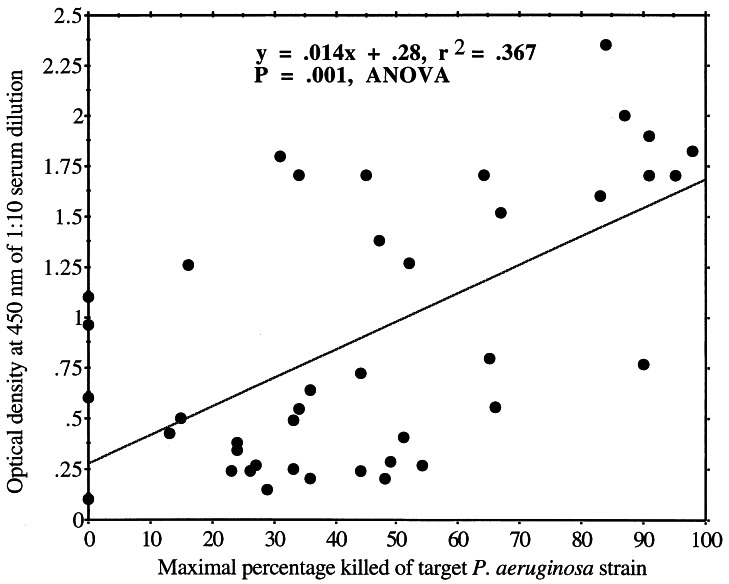
There was a weak (r = 0.367) but significant (P < 0.001, analysis of variance) correlation between ELISA values obtained with a 1:10 serum dilution and opsonic killing (Fig. 4). All of the strains but one that was killed at a level of >80% had an LPS antigen that gave a high ELISA reading (O.D. of >1.5) when tested against the opsonically active antiserum, although a fair number of strains had high O.D. values but only modest levels of killing (30 to 70%). These results indicate that antibody binding to the antigen was necessary but not sufficient for a high level of opsonic killing. Sera with high levels of binding but poor-to-modest killing most likely contained antibodies directed at epitopes that were not good targets for opsonic antibodies. Antibody isotype may also have been a factor, as previously reported by Schreiber et al. (39), and analysis of five selected sera representing a cross-section of highly to poorly opsonic sera confirmed a restriction of the mouse IgG response to high-molecular-weight O polysaccharides to the IgG3 subclass.
FIG. 4.
Antibody responses in a 1:10 dilution of serum from mice immunized with one of three different doses of high-molecular-weight O polysaccharide correlated with the percentage of target P. aeruginosa bacteria killed in an opsonophagocytosis assay. Each O.D. value represents IgG reactivity (measured by ELISA) to the LPS antigen expressed by different serogroup O2 target strains.
However, we did not determine the subclass-specific immune responses in all of the sera and thus cannot totally exclude the possibility of an effect of subclass on some of the opsonic activities measured. We did measure IgM responses in all of the sera and found no correlation between IgM responses and opsonic activity. As one might expect from classic immunologic teaching, most of the strains that bound antibodies in immune sera poorly also were not killed in the opsonic assay by these antisera. However, we did note that three strains for which good levels of opsonic killing (>60%) were obtained had relatively low ELISA readings (O.D. of <0.75), indicating the existence of modest levels of potent antibodies in some sera. The ELISA results are consistent with the opsonic results in that there is no obvious relationship among the chemical structure, antigenic epitopes, and immunogenicity of high-molecular-weight O-polysaccharide antigens expressed by strains representing the P. aeruginosa O2 serogroup.
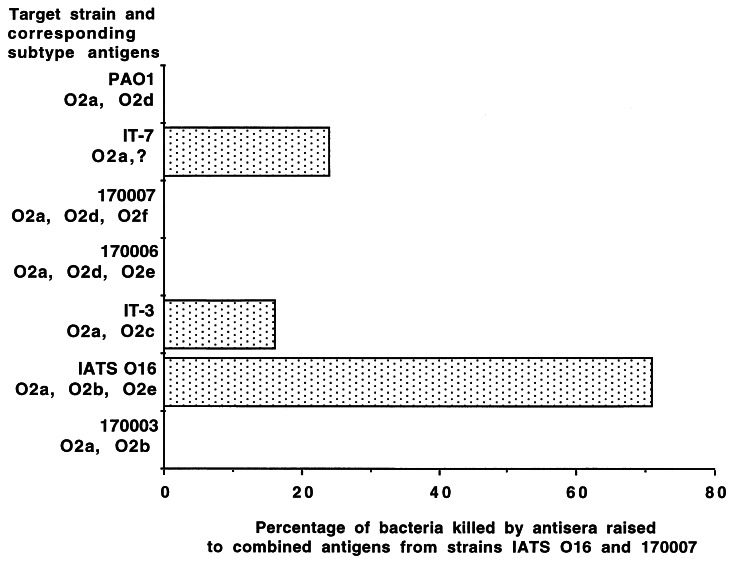
Immune response to divalent IATS O16 and 170007 vaccine.
Examination of our opsonic activity and ELISA results indicated that the level of killing of all of the representative strains of the P. aeruginosa O2 serogroup was reasonable in antisera raised to 10 μg of strain IATS O16 or 170007. In addition, these two strains expressed all but one (O2c) of the subtype epitopes defined among the serogroup O2 strains. We therefore combined these two immunogens and vaccinated mice three times with the optimal 10-μg dose of each to see if we could obtain antisera capable of mediating opsonic killing of all or most of the serogroup O2 strains. When combined into a divalent vaccine, the IATS O16 and 170007 antigens elicited good levels of opsonic antibodies only to strain IATS O16 and not to the other serogroup O2 strains (Fig. 5). Thus, combining what appeared to be the best formulation of serogroup O2 immunogens into a single vaccine resulted in antagonistic as opposed to additive or synergistic immunogenicity, consistent with the previous result obtained with a heptavalent vaccine containing two serogroup O2 antigens. ELISA analysis closely followed the opsonic pattern, wherein high levels of IgG antibodies were only elicited to the IATS O16 component of the vaccine (data not shown).
FIG. 5.
Opsonic antibody responses elicited by combining 10-μg doses of high-molecular-weight O-polysaccharide antigens isolated from P. aeruginosa IATS O16 (O2a, O2b, and O2e) and 170007 (O2a, O2d, and O2f) against the target strains indicated on the y axis. The bars indicate mean results of quadruplicate determinations. The SEM was <5% for all determinations.
DISCUSSION
The quest for an O-antigen-specific P. aeruginosa vaccine is nearing the 30-year mark (1, 2, 9) with little evidence of success in human clinical trials. It now appears clear that serogroup definitions of O-antigenic activity do not correlate with the range of antigens needed for an effective vaccine based on immunogenic, nontoxic, high-molecular-weight versions of the O polysaccharides (13, 33). The Fisher immunotyping schema was based on definitions of protective antigens obtained by immunization of mice with over 200 strains of P. aeruginosa and subsequent challenge with live organisms, but these studies used an extract later shown to be composed mostly of LPS (15). Detoxification of LPS by acid hydrolysis to eliminate the lipid A portion and isolation of the immunogenic high-molecular-weight polysaccharide O antigens, originally described in 1978 (33, 35), have consistently resulted in monovalent preparations of O antigens that are immunogenic in mice (30, 31, 34, 35) and humans (26, 28). These antigens elicit protective immunity in a variety of animal models (23, 29, 30, 31, 37) when the challenge strain is the one from which the immunogen was obtained. However, combining multiple high-molecular-weight O polysaccharides into one heptavalent preparation has resulted in only limited levels of opsonic antibodies to nonvaccine strains. The goal of our investigation was to elucidate the basis for this restricted immunogenicity.
While, on the surface, our results seem somewhat confusing, their importance lies in the demonstration of the fallibility of using serogroup classifications of P. aeruginosa as a basis for vaccine formulations. While most high-molecular-weight serogroup O2 polysaccharides elicited antibodies to themselves when used as monovalent preparations, their ability to elicit opsonic antibodies to other serogroup O2 strains was not readily correlated with either subtype epitope expression or chemical relatedness. Most critically, combining two related serogroup O2 antigens into one vaccine and immunizing mice with the dose of each individual component determined to be optimal when used as a monovalent preparation inhibited the production of broadly reactive opsonic antibodies to other serogroup O2 strains. Although we did not evaluate other dose combinations of these related immunogens, our results indicate that for the formulation of a vaccine for human use it will be necessary to evaluate both various combinations of antigens and various combinations of doses of chemically related O antigens to define the optimal formulation for generating antibodies able to protect against a sufficient array of clinically important strains.
Obvious candidates for the underlying mechanism include limited immunogenicity in the absence of conjugation to a carrier, competition among related antigens for a limited membrane immunoglobulin (mIg) repertoire on B cells, evolutionary pressures on the organism to select for strains making O antigens with immunodominant but nonprotective epitopes, and antigenic variation by which opsonic antibodies are rendered ineffective. Addition of a carrier protein might change the immunodominant epitopes on high-molecular-weight O polysaccharides. However, the results of Cryz and colleagues, who have prepared a multivalent P. aeruginosa O-antigen conjugate (6), suggest that conjugation does not enhance the immunogenicity of serogroup-specific epitopes. These investigators found that an octavalent O-antigen conjugate vaccine was not effective in eliciting antibodies that protected humans at risk for P. aeruginosa infection following passive transfer of IgG (7). While failure in a clinical trial can be due to a multitude of factors, one possible basis for the clinical failure of this vaccine was insufficient coverage against the protective epitopes of common clinical isolates of P. aeruginosa. Published results from preclinical evaluations of this octavalent conjugate vaccine documented opsonic antibody responses and protective efficacy only against strains used to manufacture the vaccine (4–6).
Evolutionary pressure to select for immunodominant but nonprotective epitopes on O antigens could explain some of the results we obtained, but this is a difficult hypothesis to test. Along these lines, we have proposed that the mucoid exopolysaccharide or alginate antigen that coats the isolates of P. aeruginosa obtained from cystic fibrosis patients principally elicits nonopsonic, nonprotective antibodies in patients (32). The evidence that this scenario might also apply to some P. aeruginosa O antigens consists of results obtained with the high-molecular-weight O-polysaccharide antigen from strain PAO1, which did not elicit a good opsonic antibody to itself but did elicit a good one to strain 170006. Overall, we consider it unlikely that production of immunodominant but nonprotective O-antigen epitopes is a common mechanism of immune evasion by P. aeruginosa.
Antigenic variation is the basis for the classification of P. aeruginosa into subtypes within serogroups, but whether a given strain can change its major protective epitopes during infection is unknown and is also difficult to ascertain. The fact that strain 170007 produces two variant O-antigen structures suggests that antibody-mediated selection could promote the survival of a variant of this strain expressing an O antigen that evades antibodies to a related structure. Mucoid isolates from cystic fibrosis patients are well known to lose the ability to produce LPS O antigens and a complete outer core oligosaccharide (10, 12) during chronic infection; the same has been reported to occur in a rat model of chronic P. aeruginosa infection (41). In vitro conversion of the O-antigen structure of P. aeruginosa PAO1 by bacteriophage D3 has been reported (20), but this is also unlikely to be a common mechanism for in vivo changes in O antigens.
An explanation most consistent with our results regarding the complex serology of P. aeruginosa O antigens and their interaction with the mammalian immune system relates to the binding of polysaccharide antigens to mIg on B cells with the consequent activation and differentiation of these cells into antibody-secreting plasma cells. Usually, polysaccharides activate B cells by cross-linking mIg via the presentation of the same epitope multiple times on the same molecule (25). However, if similar epitopes are present on related but distinct molecules, then cross-linking of the mIg would be less efficient. Thus, combining chemically related antigens into one vaccine could result in suboptimal immune responses to epitopes shared by O antigens from different strains. This would occur if B cells bound identical epitopes on different polysaccharide molecules, resulting in less cross-linking of mIg and decreased B-cell activation. Consistent with this explanation is the observation that, for most of the serogroup O2 high-molecular-weight O polysaccharides, immune responses were optimal at a dose of 1 or 10 μg and not at a dose of 50 μg. Presumably, there was more binding of antigen to mIg but less cross-linking at the higher dose due to the presence of a greater number of molecules with relevant epitopes. Similarly, simultaneous immunization with multiple serogroup O2 antigens inhibits antibody production to shared epitopes, leaving only highly restricted, strain-specific epitopes to activate their cognate B cells to a sufficient degree to drive them into becoming plasma cells. We obtained this result when we combined the antigens from strains IATS O16 and 170007, which individually gave rise to antibodies opsonic against most of the O2 strains. As noted above, lower doses or a different ratio of these two antigens might be more immunogenic, but this could only be determined in a large-scale trial of multiple combinations of these vaccine components.
We tested the vaccines on only one strain of mouse. The results might be different with other mouse strains or other animal species. We have previously shown the disparity of mouse and rabbit responses to high-molecular-weight O polysaccharides (13). However, there is no way to know which animal will yield results most predictive of human immune responses to these antigens; thus, immunizing additional strains of mice or different species of animals would not reveal which system would be most useful for formulating a human vaccine. Indeed, the results obtained with C3H mice are probably the most important and relevant to human vaccine design. Both the complexity of the response and the antagonistic immunogenicity obtained when two serogroup O2 vaccines were combined suggest that comprehensive coverage of P. aeruginosa strains by an O-antigen-specific vaccine may require multiple immunizations, with the serologically related components separated into different vaccine formulations in order to avoid competition and inhibition of the antibody response. Alternative approaches include vaccinating different humans with different noninterfering polysaccharide antigens and combining immune IgG from these individuals into a pool of hyperimmune IgG for passive therapy or use of lymphocytes from immunized individuals for development of human monoclonal antibodies directed at important protective epitopes.
Several additional aspects of our findings are relevant to the development of a vaccine for LPS-smooth P. aeruginosa. While none of the serogroup O2 vaccines we tested elicited serogroup-specific opsonic activity directed at the shared O2a epitope, this result does not rule out this epitope as a target for opsonic antibody. It is possible that the conditions or doses we evaluated affected the immunogenicity of the O2a epitope. Thus, strategies evaluating either different monovalent antigens from serogroup O2 strains in humans or monoclonal antibodies to this epitope may identify broadly reactive and protective antibodies with potential as immunotherapeutic agents. Alternative antigenic targets to LPS O antigens have been intensely investigated, with outer membrane proteins (OMPs) (8, 11, 16, 40) and flagella (21) receiving the most attention. However, levels of antibodies to O antigens are clearly correlated with resistance to lethal outcomes during human infection (38). In a comparison of the efficacy of antibody to OMP F and LPS, OMP F-specific antibodies protected only 30 to 95% of burned mice against P. aeruginosa challenge doses of 2 × 105 to 2 × 106 CFU (2 to 8 50% lethal doses), whereas an anti-O-antigen LPS vaccine gave 90% protection against a challenge dose of 3 × 1011 CFU of the O-antigen-homologous strain (24). Thus, while OMP-directed vaccines offer broader coverage of strains, it is not clear that they elicit antibodies of sufficient potency to protect humans against infection. Given the obvious superiority of O-antigen-specific antibodies in providing protection against P. aeruginosa, it seems reasonable to continue to pursue the development of vaccines that elicit serogroup- or subtype-specific immunity. However, our results suggest that to be most effective, O-antigen-specific P. aeruginosa immunization may require (i) the administration to a given individual of multiple vaccine formulations at different injection sites with no related serogroup antigens in the same injection and/or (ii) the administration of related antigens at different times to the same individual or (iii) the administration of different antigen formulations to different individuals for manufacture of a hyperimmune IgG product for passive immunotherapy. Trials of polysaccharide vaccines involving multiple structurally and serologically related antigen components needed for comprehensive immunity to the common serologic variants of the pathogen that cause disease have been rare. Insights gained from murine immunization studies using P. aeruginosa high-molecular-weight O polysaccharides should provide guidance for the formulation of future vaccines incorporating multiple variants of antigens with slight but important structural differences that relate to antigenicity and immunogenicity of the polysaccharide.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 22535 and, in part, by the NIH Biomedical Resource Center Program (grant RR05351 to the Complex Carbohydrate Research Center, The University of Georgia).
REFERENCES
- 1.Alexander J W, Fisher M W, MacMillan B G. Immunological control of Pseudomonasinfection in burn patients: a clinical evaluation. Arch Surg. 1971;102:31–35. doi: 10.1001/archsurg.1971.01350010033008. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J W, Fisher M W, MacMillan B G, Altemeier W A. Prevention of invasive Pseudomonasinfection in burns with a new vaccine. Arch Surg. 1969;99:249–256. doi: 10.1001/archsurg.1969.01340140121018. [DOI] [PubMed] [Google Scholar]
- 3.Cryz S J, Jr, Furer E, Germanier R. Protection against fatal Pseudomonas aeruginosaburn wound sepsis by immunization with lipopolysaccharide and high-molecular-weight polysaccharide. Infect Immun. 1984;43:795–799. doi: 10.1128/iai.43.3.795-799.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryz S J, Jr, Furer E, Que J U. Synthesis and characterization of a Pseudomonas aeruginosaalginate-toxin A conjugate vaccine. Infect Immun. 1991;59:45–50. doi: 10.1128/iai.59.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryz S J, Jr, Furer E, Sadoff J C, Fredeking T, Que J U, Cross A S. Production and characterization of a human hyperimmune intravenous immunoglobulin against Pseudomonas aeruginosa and Klebsiellaspecies. J Infect Dis. 1991;163:1055–1061. doi: 10.1093/infdis/163.5.1055. [DOI] [PubMed] [Google Scholar]
- 6.Cryz S J, Jr, Sadoff J C, Fürer E. Octavalent Pseudomonas aeruginosaO-polysaccharide–toxin A conjugate vaccine. Microb Pathog. 1989;6:75–80. doi: 10.1016/0882-4010(89)90010-7. [DOI] [PubMed] [Google Scholar]
- 7.Donta S T, Peduzzi P, Cross A S, Sadoff J, Haakenson C, Cryz S J, Kauffman C, Bradley S, Gafford G, Elliston D, Beam T R, John J F, Ribner B, Cantey R, Welsh C H, Ellison R T, Young E J, Hamill R J, Leaf H, Schein R M H, Mulligan M, Johnson C, Abrutyn E, Griffiss J M, Hamadeh R, Eliasson A H, Mcclain J B, Melcher G P, Kelly J W, Byrne W R, Wallace M, Amundson D, Gumpert B, Slagle D. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosainfections. J Infect Dis. 1996;174:537–543. doi: 10.1093/infdis/174.3.537. [DOI] [PubMed] [Google Scholar]
- 8.Finke M, Duchene M, Eckhardt A, Domdey H, von Specht B U. Protection against experimental Pseudomonas aeruginosa infection by recombinant P. aeruginosa lipoprotein I expressed in Escherichia coli. Infect Immun. 1990;58:2241–2244. doi: 10.1128/iai.58.7.2241-2244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher M W, Devlin H B, Gnabasik F. New immunotype schema for Pseudomonas aeruginosabased on protective antigens. J Bacteriol. 1969;98:835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fomsgaard A, Conrad R S, Galanos C, Shand G H, Hoiby N. Comparative immunochemistry of lipopolysaccharides from typable and polyagglutinable Pseudomonas aeruginosastrains isolated from patients with cystic fibrosis. J Clin Microbiol. 1988;26:821–826. doi: 10.1128/jcm.26.5.821-826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilleland H E, Gilleland L B, Fowler M R. Vaccine efficacies of elastase, exotoxin-A, and outer-membrane protein F in preventing chronic pulmonary infection by Pseudomonas aeruginosain a rat model. J Med Microbiol. 1993;38:79–86. doi: 10.1099/00222615-38-2-79. [DOI] [PubMed] [Google Scholar]
- 12.Hancock R E W, Mutharia L M, Chan L, Darveau R P, Speert D P, Pier G B. Pseudomonas aeruginosaisolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatano K, Boisot S, DesJardins D, Wright D C, Brisker J, Pier G B. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect Immun. 1994;62:3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidbrink P J, Toews G B, Gross G N, Pierce A K. Mechanisms of complement-mediated clearance of bacteria from the murine lung. Am Rev Respir Dis. 1982;125:517–520. doi: 10.1164/arrd.1982.125.5.517. [DOI] [PubMed] [Google Scholar]
- 15.Horton D, Riley D A, Samreth S, Schweitzer M G. Lipopolysaccharide antigens of Pseudomonas aeruginosa. In: Anderson L, Unger F M, editors. Bacterial lipopolysaccharides: structure, synthesis and biological activities. New York, N.Y: American Chemical Society; 1983. pp. 21–47. [Google Scholar]
- 16.Hughes E E, Gilleland H E., Jr Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against P. aeruginosainfection in a murine acute pneumonia model. Vaccine. 1995;13:1750–1753. doi: 10.1016/0264-410x(95)00166-x. [DOI] [PubMed] [Google Scholar]
- 17.Knirel Y A. Polysaccharide antigens of Pseudomonas aeruginosa. Crit Rev Microbiol. 1990;17:273–304. doi: 10.3109/10408419009105729. [DOI] [PubMed] [Google Scholar]
- 18.Knirel Y A, Paramonov N A, Vinogradov E V, Shashkov A S, Dmitriev B A, Kochetkov N K, Kholodkova E V, Stanislavsky E S. Somatic antigens of Pseudomonas aeruginosa. The structure of O-specific polysaccharide chains of lipopolysaccharides of P. aeruginosaO3 (Lanyi), O25 (Wokatsch) and Fisher immunotypes 3 and 7. Eur J Biochem. 1987;167:549–561. doi: 10.1111/j.1432-1033.1987.tb13372.x. [DOI] [PubMed] [Google Scholar]
- 19.Knirel Y A, Vinogradov E V, Kocharova N A, Paramonov N A, Kochetkov N K, Dmitriev B A, Stanislavsky E S, Lányi B. The structure of O-specific polysaccharides and serological classification of Pseudomonas aeruginosa. Acta Microbiol Hung. 1988;35:3–24. [PubMed] [Google Scholar]
- 20.Kuzio J, Kropinski A M. O-antigen conversion in Pseudomonas aeruginosaPAO1 by bacteriophage D3. J Bacteriol. 1983;155:203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landsperger W J, Kellywintenberg K D, Montie T C, Knight L S, Hansen M B, Huntenburg C C, Schneidkraut M J. Inhibition of bacterial motility with human antiflagellar monoclonal antibodies attenuates Pseudomonas aeruginosa-induced pneumonia in the immunocompetent rat. Infect Immun. 1994;62:4825–4830. doi: 10.1128/iai.62.11.4825-4830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanyi B, Bergan T. Serological characterization of Pseudomonas aeruginosa. Methods Microbiol. 1978;10:94–168. [Google Scholar]
- 23.Markham R B, Pier G B. Immunologic basis for mouse protection provided by high-molecular-weight polysaccharide from immunotype 1 Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:S957–S962. doi: 10.1093/clinids/5.supplement_5.s957. [DOI] [PubMed] [Google Scholar]
- 24.Matthews-Greer J M, Gilleland H E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosaas a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987;155:1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- 25.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 26.Pier G B. Safety and immunogenicity of a high molecular weight polysaccharide vaccine to immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982;69:303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pier G B. Immunochemistry of Pseudomonas aeruginosalipopolysaccharides and high-molecular weight polysaccharides. Rev Infect Dis. 1983;5:S950–S956. doi: 10.1093/clinids/5.supplement_5.s950. [DOI] [PubMed] [Google Scholar]
- 28.Pier G B, Bennett S E. Structural analysis and immunogenicity of Pseudomonas aeruginosaimmunotype 2 high molecular weight polysaccharide. J Clin Invest. 1986;77:491–495. doi: 10.1172/JCI112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pier G B, Cohen M, Jennings H. Further purification and characterization of high-molecular weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1983;42:936–941. doi: 10.1128/iai.42.3.936-941.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pier G B, Pollack M. Isolation, structure, and immunogenicity of Pseudomonas aeruginosaimmunotype 4 high-molecular-weight polysaccharide. Infect Immun. 1989;57:426–431. doi: 10.1128/iai.57.2.426-431.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pier G B, Pollack M, Cohen M. Immunochemical characterization of high-molecular-weight polysaccharide from Fisher immunotype 3 Pseudomonas aeruginosa. Infect Immun. 1984;45:309–313. doi: 10.1128/iai.45.2.309-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pier G B, Saunders J M, Ames P, Edwards M S, Auerbach H, Goldfarb J, Speert D P, Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosamucoid exopolysaccharide in older, non-colonized cystic fibrosis patients. N Engl J Med. 1987;317:793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 33.Pier G B, Sidberry H F, Sadoff J C. Protective immunity induced in mice by immunization with a high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978;22:919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pier G B, Sidberry H F, Sadoff J C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosaimmunotype 2. Infect Immun. 1981;34:461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pier G B, Sidberry H F, Zolyomi S, Sadoff J C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978;22:908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pier G B, Thomas D M. Lipopolysaccharide and high molecular weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982;145:217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- 37.Pollack M, Pier G B, Prescott R K. Immunization with Pseudomonas aeruginosa high-molecular-weight polysaccharides prevents death from Pseudomonasburn infections in mice. Infect Immun. 1984;43:759–760. doi: 10.1128/iai.43.2.759-760.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack M, Young L S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosasepticemia in man. J Clin Invest. 1979;63:276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber J R, Cooper L J N, Diehn S, Dahlhauser P A, Tosi M F, Glass D D, Patawaran M, Greenspan N S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosalipopolysaccharide O-specific side chain function differently. J Infect Dis. 1993;167:221–226. doi: 10.1093/infdis/167.1.221. [DOI] [PubMed] [Google Scholar]
- 40.Von Specht B U, Knapp B, Muth G, Broker M, Hungerer K D, Diehl K D, Massarrat K, Seemann A, Domdey H. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosaouter membrane protein F and outer membrane protein I fusion proteins. Infect Immun. 1995;63:1855–1862. doi: 10.1128/iai.63.5.1855-1862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods D E, Sokol P A, Bryan L E, Storey B G, Mattingly S J, Vogel H J, Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosaassociated with genetic rearrangement. J Infect Dis. 1991;163:143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- 42.Young L S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972;126:277–284. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]
- 43.Young L S. Role of antibody in Pseudomonas aeruginosainfections. J Infect Dis. 1974;130:S111–S116. doi: 10.1093/infdis/130.supplement.s111. [DOI] [PubMed] [Google Scholar]