Abstract
Evidence-based treatments for binge eating disorder (BED), such as cognitive behavioral therapy (CBT) lead to successful outcomes only about half the time. Individuals with BED often have measurable deficits in executive function (EF) that may challenge adherence to or impact of cognitive behavioral intervention components. The aim of this study was to evaluate the impact of adding EF training to CBT by combining CBT with a compensatory cognitive training approach (EF-CBT). Participants were 32 adults with BED, overweight/obesity, and comorbid anxiety or depression who were randomly assigned to four months of group treatment in either standard CBT or EF-CBT. Outcomes were assessed at baseline, post-treatment, and at 2-month follow-up. Results showed that EF-CBT was feasible and acceptable, comparable to CBT. Both groups significantly decreased loss of control (LOC) days, clinical impairment, and depression at post-treatment and 2-month follow-up; though there were no differences between groups. Neither group significantly reduced anxiety or weight. Exploratory analyses found that participants with lower EF treated with EF-CBT were less likely to have LOC at post-treatment than those with lower EF treated with CBT. Higher self-monitoring rates during treatment were associated with lower LOC at post-treatment and participants with lower EF were more likely to self-monitor in the EF-CBT arm relative to the CBT arm. These findings suggest that EF-CBT is feasible, acceptable and efficacious, although larger scale research is needed. EF-CBT may be particularly suited for individuals with BED who have lower EF.
Keywords: Loss of control, Cognitive function, Overweight, Obesity, Cognitive training
1. Introduction
Binge eating disorder (BED) is characterized by recurrent binge eating (consumption of an objectively large amount of food accompanied with a sense of loss of control [LOC]) and the absence of compensatory behaviors. BED is associated with several negative health sequelae including psychiatric comorbidity, medical complications, and decreased quality of life (Kessler et al., 2013; Mitchell, 2016; Rieger et al., 2005). Additionally, most individuals with BED (~70 %) have overweight or obesity (OW/OB) (Abraham et al., 2014; Dingemans & van Furth, 2012; Kessler et al., 2013). BED is associated with the metabolic complications associated with OW/OB, although research has shown that BED conveys unique risk above OW/OB (Hudson et al., 2010; Wassenaar et al., 2019). Evidence-based psychotherapies for BED such as cognitive behavioral therapy (CBT) have been established; however, only about 50 % abstain from binge eating following treatment (Linardon, 2018; Peat et al., 2017). Thus, it is important to identify mechanisms related to treatment failure to improve outcomes and reduce the significant burden associated with BED.
Support for training executive function (EF) in the treatment of eating disorders and OW/OB has emerged (Eichen et al., 2017; Eichen et al., 2021; Juarascio et al., 2015; Szabo-Reed & Donnelly, 2021; Yang et al., 2019). EF refers to higher-order cognitive functions, typically associated with pre-frontal cortex functioning, that are essential for goal-directed behavior and self-regulation (Hofmann et al., 2012; Miyake et al., 2000). There are three core domains of EF (cognitive flexibility, working memory, and inhibitory control) and several related EFs (planning, problem solving, decision making, prospective memory, organization). Research has consistently shown that OW/OB is associated with lower EF compared to those with healthy weight (Fitzpatrick et al., 2013; Smith et al., 2011; Yang et al., 2019). Several studies have shown that individuals with BED and comorbid OW/OB had lower EF, at least on some domains (problem solving, planning, inhibitory control, preference for immediate reward) than adults with OW/OB without BED (Blume et al., 2019; Cury et al., 2020; Grant & Chamberlain, 2020; Kollei et al., 2018; Manasse et al., 2015; Prunell-Castañé et al., 2021; Rouel et al., 2016; Svaldi et al., 2010). However, some studies failed to find differences in EF between these groups (Aloi et al., 2020; Davis et al., 2010; Galioto et al., 2012; Lavagnino et al., 2016). Some of the mixed results may be due to the confounding factor of OB. In several of the studies with no differences, the samples had higher BMIs (>35) (Aloi et al., 2020; Davis et al., 2010; Galioto et al., 2012). Additionally, given the numerous domains and various tasks administered, those can contribute to some heterogeneous results. Other psychiatric disorders that have high comorbidity rates with BED, like anxiety and mood disorders, have also been associated with lower EF and poorer BED treatment outcomes (Cotrena et al., 2016; Dingemans et al., 2020; Lydecker & Grilo, 2021; Shields et al., 2016). The link between lower EF and BED is apparent. For example, decreased inhibitory control can lead to binge eating in response to triggers by not having the ability to resist acting on urges. Similarly, preference for immediate reward may result in binge eating to obtain immediate relief rather than consideration of the long-term consequences. Difficulty with problem solving may lead to maladaptive eating patterns (i.e., irregular eating). Furthermore, EF is required to adhere to treatment recommendations in CBT for binge eating. For instance, self-monitoring requires substantial organization and memory, while establishing regular eating requires planning, problem solving and cognitive flexibility. Taken together, evidence suggests that targeting EF in the treatment of BED, particularly among those with other psychiatric comorbidities, is warranted.
Several research groups have utilized computerized cognitive training to address EF to reduce binge eating and eating disorder pathology (Boutelle et al., 2016; Brockmeyer et al., 2019; Chami et al., 2022; Das et al., 2022; Giel et al., 2017; Turton et al., 2018). These interventions often use a repetitive task (e.g., Go/NoGo, dot-probe) to increase inhibition toward food cues or direct attention away from food cues. Several studies showed promising preliminary effects at reducing binge eating (Boutelle et al., 2016; Brockmeyer et al., 2019; Chami et al., 2022; Giel et al., 2017), although sample sizes were small and maintenance effects were not durable. Despite these positive studies, others have failed to show any effect (Das et al., 2022; Turton et al., 2018). Computerized training programs have been criticized for their lack of generalizability to real-world behavior outside of a laboratory. Compensatory cognitive training (CCT), such as Cognitive Symptom Management and Rehabilitation Therapy (CogSMART), is a different type of training approach that focuses on teaching skills to compensate for weaknesses in cognitive functioning. These manualized interventions have been effective for individuals with a history of traumatic brain injury or other psychiatric illnesses such as psychosis and schizophrenia (Mendella et al., 2015; Storzbach et al., 2017; Twamley et al., 2012; Twamley et al., 2014; Twamley et al., 2015; Twamley et al., 2019). CCT interventions teach both internal strategies, including ways to categorize information, as well as external strategies, such as relying on tools such as calendars. Sessions incorporate both learning the cognitive strategies and practice during the session. Homework is assigned to emphasize turning skills into habits in the real world. CCT/CogSMART have been used as an adjunct with behavioral therapy for hoarding disorder (Ayers et al., 2014, 2017) and with behavioral weight loss for adults OW/OB (Eichen et al., 2021). These approaches have demonstrated feasibility, acceptability, and preliminary efficacy. Accordingly, combining CCT/CogSMART with CBT could enhance outcomes for the treatment of BED.
The current study aimed to evaluate incorporating CCT/CogSMART into standard CBT (EF-CBT) compared to standard CBT in a pilot randomized controlled trial for adults with BED and comorbid mood or anxiety symptoms. The primary aim of this study was to evaluate the feasibility and acceptability of EF-CBT. We hypothesized that EF-CBT would be at least as feasible and acceptable as CBT alone. Secondarily, we aimed to explore preliminary efficacy by evaluating change in LOC, clinical impairment, anxiety, depression, and body mass index (BMI). We hypothesized that EF-CBT would show greater preliminary efficacy than CBT. Exploratory moderator analyses aimed to evaluate the impact of EF on LOC outcomes. We hypothesized that those with lower EF would have better outcomes in the EF-CBT arm.
2. Methods
2.1. Participants and procedures
Participants were 39 adults (see Fig. 1) recruited from the community through online recruitment resources such as ResearchMatch and clinicaltrials.gov (NCT04242550), advertising for a study called Binge Eating Anxiety and Mood (BEAM). Additionally, individuals who inquired about clinical BED treatment services were referred if they expressed interest in the research study. All procedures were conducted remotely due to COVID-19, recruitment occurred between June of 2020 and February of 2021 and participants were aware all assessments and treatment would take place remotely. Participants were adults aged 18–65 years with English fluency who met criteria for clinical or subclinical BED and a comorbid mood or anxiety disorder. Exclusion criteria included a known cognitive disability, psychiatric condition that would interfere with program participation (e.g., significant substance abuse, suicide attempt within previous 6 months, active suicidality, active purging, psychosis,); pregnant, lactating or planning to become pregnant during study or follow-up; change in psychotropic medications or other medications that could impact weight, binge eating, anxiety, or mood disorder symptoms within the past three months, participating in an organized program for binge eating or overeating.
Fig. 1.
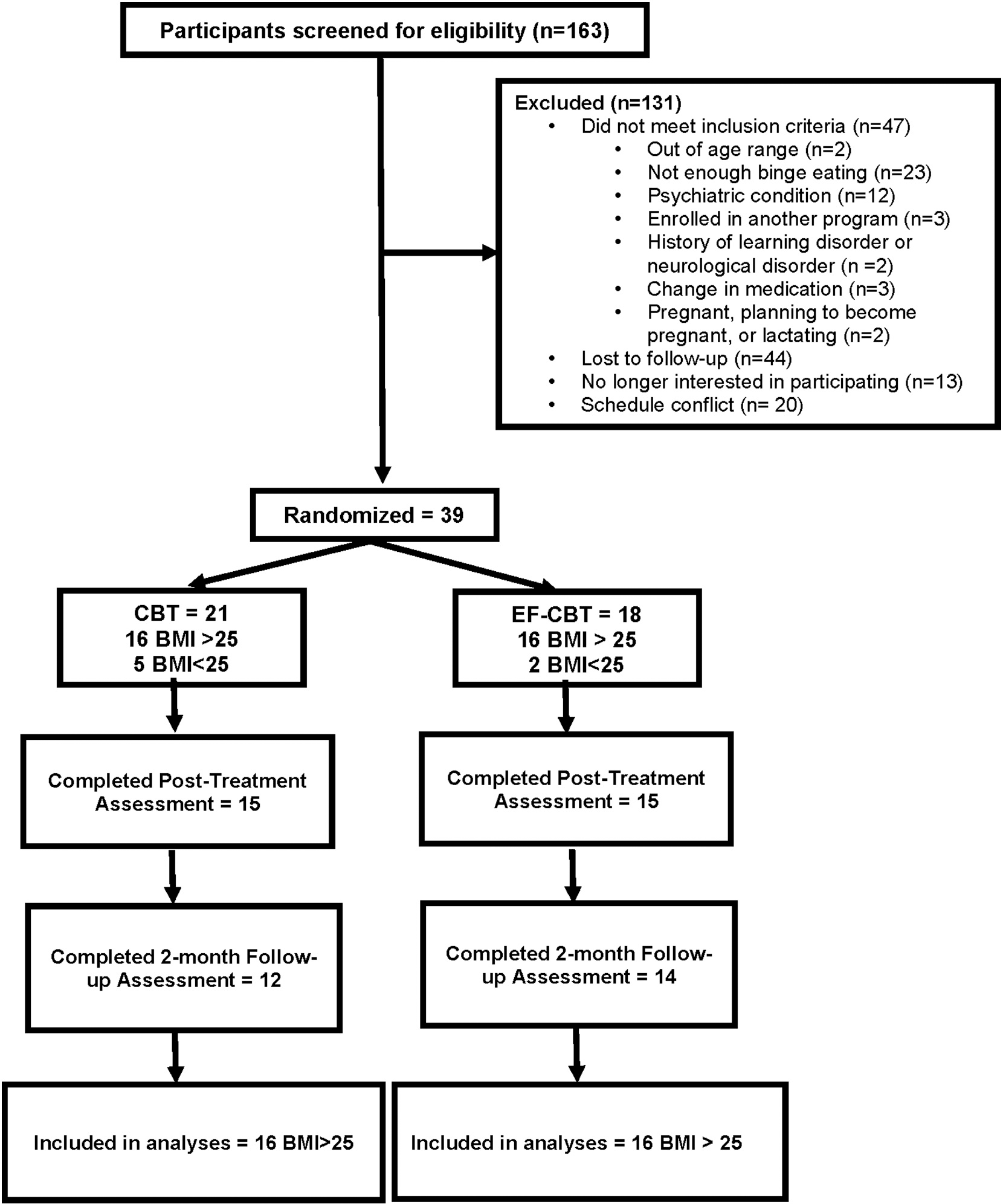
Flow of participants in the Binge Eating Anxiety and Mood (BEAM) Study.
Note: CBT = Cognitive Behavioral Therapy; EF-CBT = CBT + Executive Function Training;
All procedures were approved by the UC San Diego Institutional Review Board and all participants provided informed consent. Following consent, participants completed baseline assessments remotely via HIPAA-compliant videoconference meetings. Participants who remained eligible were randomized to one of the two groups, CBT or EF-CBT via block randomization by sex assigned at birth and BMI status (<25 kg/m2 or ≥ 25 kg/m2). Group treatment occurred via 14, 60-minute HIPAA-compliant videoconference sessions over the course of four months (weekly for first 12 sessions; then biweekly). Participants completed additional remote assessments immediately post-treatment and at 2-month follow-up. The current analyses focused on the 32 adults with OW/OB, as seven participants with BMI <25 were misallocated disproportionally to one arm (5 vs 2) due to errors within this randomization block. Additionally, previous research suggested that individuals with BED without OW/OB have distinct characteristics and thus a disproportionate allocation risked design balance (Goldschmidt et al., 2011). See Table 1 for demographic characteristics.
Table 1.
Demographic characteristics.
| CBT (n = 16) | EF-CBT (n = 16) | All (N = 32) | |
|---|---|---|---|
|
| |||
| Age M (SD) | 43.69 (13.71) | 46.60 (13.17) | 45.10 (13.31) |
| Sex | |||
| Female | 14 | 12 | 26 |
| % | 88 % | 75 % | 81 % |
| Male | 2 | 4 | 6 |
| % | 12 % | 25 % | 19 % |
| Race-ethnicity | |||
| White | 9 | 13 | 22 |
| % | 56 % | 81 % | 69 % |
| Hispanic or Latino | 3 | 1 | 4 |
| % | 19 % | 6 % | 12 % |
| Other | 4 | 2 | 6 |
| % | 25 % | 12 % | 19 % |
| Education | |||
| Less than college graduate | 6 | 1 | 7 |
| % | 38 % | 6 % | 22 % |
| College graduate | 3 | 8 | 11 |
| % | 19 % | 50 % | 34 % |
| Advanced degree | 7 | 7 | 14 |
| % | 44 % | 44 % | 44 % |
| BMI (kg/m2) M (SD) | 36.32 (9.26) | 37.76 (9.91) | 37.04 (9.46) |
| Total loss of control days | 58.38 | 63.19 (27.91) | 60.78 |
| (EDE) | (28.54) | (27.87) | |
2.2. Intervention
Both interventions were conducted by a licensed clinical psychologist (DME) and a registered dietician who were both experienced in the delivery of CBT. Each of the 14 sessions were 60 min long and groups met weekly for 12 weeks and biweekly for the last 2 sessions. Treatment materials for both groups included weekly PowerPoint sessions with the didactic content which was shared via email to participants and shown on screen to help ensure fidelity.
2.2.1. CBT
All groups followed the same structure and started with check in/review of homework, followed by introduction of the new topic covered with time for skill practice, and ended with a summary of content reviewed and homework assigned. The CBT content was derived from established CBT programs (Fairburn et al., 1993; Mitchell et al., 2007). To help reinforce the CBT content, all participants were provided the book Overcoming Binge Eating (Fairburn, 2013) and were assigned to read the corresponding material in the book as homework after the content was reviewed in group. All participants were encouraged to self-monitor their eating episodes daily via the Recovery Record app (Tregarthen et al., 2015) or on paper and weigh themselves weekly. Participants were encouraged to establish regular eating of meals and snacks and were taught to identify cues and triggers of binge eating as well as consequences of binge eating. Focus was placed on the link between thoughts, emotions, and behaviors, with a focus on modifying thoughts. Additional content was provided on managing emotions, self-esteem, body checking behaviors, body avoidance, food avoidance, addressing strict dieting, and creating a relapse prevention plan.
2.2.2. EF-CBT
Participants in the EF-CBT group received all elements of the CBT group described above. Additionally, cognitive training exercises adapted from CCT/CogSMART (Twamley et al., 2010) were integrated each week and were tied to the CBT topic. Domains addressed included: cognitive flexibility, inhibition, problem solving, planning, organization, and prospective memory. For example, participants were taught about time management and prioritization. Then, participants were encouraged to use a calendar to help ensure there were no barriers to group attendance. Participants were taught the benefits of weekly planning and how to incorporate reminders such as alarms, can’t miss reminders, and linking tasks to help them remember to utilize skills (e. g., setting alarms to cue self-monitoring of meals). Participants worked on establishing routines that incorporated CBT recommendations such as regular eating to facilitate habit development. Strategies to help improve problem-solving such as utilizing self-talk and learning how to effectively brainstorm were also included.
2.3. Measures
2.3.1. Feasibility and acceptability
Feasibility was assessed by attendance at group sessions. Acceptability was assessed by responses to questions created for this study on the post-treatment survey. Questions were asked on a 5-point Likert scale ranging from 0-Strongly Disagree to 4- Strongly Agree and included “The program was helpful to me overall”, “The program helped me reduce my binge eating”, and “I would recommend the program to someone with binge eating”.
2.3.2. Loss of control/binge eating
The Eating Disorder Examination (Cooper & Fairburn, 1987; Fairburn et al., 2008) version 17.0 was administered to all participants at all timepoints. At baseline, it was used as a screening measure to ensure participants met inclusion criteria. All participants needed to endorse at least 12 binge eating episodes (objectively large amount of food plus sense of LOC) at baseline over the past three months. The total number of days with LOC over the past 3 months (regardless of size) was used as the main outcome to assess change in LOC symptoms over the course of treatment and follow-up. LOC days were collapsed into different intervals of levels of days of LOC over the past 3 months for analysis as an ordered categorical index of increasing frequency of LOC days. The levels included a ‘no LOC’ level and then the remaining categories consisted of increasing intervals of LOC over the past 3 months (e.g., 1–7 days, 8–14 days, 15–21 days, 22–35 days and > 35 days) to balance the number of observations across categories. Conceptually, results should be interpreted as a change in the level of number of days of LOC over the past 3 months.
2.3.3. Mood and anxiety
The MINI International Neuropsychiatric Interview (Sheehan et al., 1998) (MINI) 7.0 was a psychodiagnostic interview conducted at baseline to evaluate inclusion/exclusion criteria. Additionally, the Patient Health Questionnaire-9 (Kroenke et al., 2001) (PHQ-9) was administered to assess depressive symptomatology and the General Anxiety Disorder-7 (Spitzer et al., 2006) (GAD-7) was administered to assess anxiety symptomatology across all timepoints. Participants met inclusion criteria for mood or anxiety disorder if they met criteria on the MINI for a mood or anxiety disorder and/or if their PHQ-9 or GAD-7 scores were in at least the range for mild depression or anxiety symptoms. Despite evaluating for this criterion, no participants were solely excluded for this reason. For both the PHQ-9 and GAD-7 higher scores equate to greater psychopathology.
2.3.4. Executive function
The Wisconsin Card Sorting Test – 64 Card Version (Kongs et al., 2000) (WCST) was administered remotely over Zoom following telehealth administration guidelines (PAR Staff, 2020). The total errors t-score (corrected for age and education) was used with lower scores representing lower EF. Reliability (split-half) of WSCT scores has been estimated to be ≥0.90 for in-person and computer administrations (Steinke et al., 2021). The Behavior Rating Inventory of Executive Function-Adult Version (Roth et al., 2005) (BRIEF-A) was administered to capture participants’ views of their own EF in their everyday environment. The Global Executive Composite (GEC) or overall summary score age-adjusted t-score was used with higher scores representing lower EF. The GEC and BRIEF-A scales have demonstrated internal consistency reliability of 0.93–0.96 (Roth et al., 2005). The Problem-Solving Inventory (Heppner, 1988) (PSI) – was administered to assess participants’ views of their problem solving abilities. The inventory produces three scores: problem solving confidence (coefficient alpha = 0.89), approach-avoidance style (coefficient alpha = 0.88), and personal control (coefficient alpha = 0.74) with higher score reflective of lower EF. Lastly, the Detail and Flexibility Questionnaire (Roberts et al., 2011)(DFlex) was administered to assess cognitive rigidity (coefficient alpha = 0.82) and attention to detail (coefficient alpha = 0.81). Higher scores represent lower EF.
2.3.5. Anthropometrics
Participants self-reported their height at baseline and weight at all assessments. Height and weight were converted to BMI (kg/m2). If participants did not have a home scale, one was provided.
2.3.6. Demographics
Participants self-reported their demographics, including age, sex assigned at birth, race, ethnicity, and years of education.
2.4. Analyses
We used ordinal mixed effects models with a cumulative link (ordinal - Regression models for ordinal data. Version R package version 2019.12–10, 2019) to assess ordered categories of increasing frequency of LOC days from baseline to post-treatment and 2-month follow-up assessments. Covariates included sex at birth, baseline BMI, age and race-ethnicity in all models evaluating differences between EF-CBT and CBT treatments. Covariate adjusted effects reflecting probability of LOC episodes were generated from models using the ‘effects’ package (Fox, 2003; Fox & Hong, 2009). Moderators of treatment effects on outcomes were evaluated with an interaction term reflecting potential differences in level of change in outcomes for each treatment across time (moderator*treatment*time). Linear mixed effects models were used for the other continuous outcomes using the lme4 package (Bates et al., 2015) with the same covariates. Multi-way interactions always included lower order terms.
3. Results
3.1. Feasibility and acceptability
Both groups were well attended. On average, participants in the EF-CBT group attended 11.6 sessions (SD = 3.4; median = 13), while participants in the CBT group attended 10.1 sessions (SD = 4.7; median = 12). Both groups were also well accepted. In total, 92.9 % (13 of 14) of participants in EF-CBT and 83.3 % (10 of 12) in CBT responded that they agreed or strongly agreed that the treatment was helpful overall. 73.3 % (11 of 15) of participants in EF-CBT and 58.3 % (7 of 12) of participants in CBT responded that they agreed or strongly agreed that the treatment was helpful to reduce their binge eating. 93.3 % (14 of 15) of participants in EF-CBT and 100 % (13 of 13) in CBT agreed or strongly agreed that they would recommend the program to someone else.
3.2. Loss of control
Both groups significantly decreased levels of LOC days over the past 3 months from baseline to post-treatment (B = −4.28; SE = 0.92; p < 0.001) and baseline to 2-month follow-up (B = −5.34; SE = 1.04; p < 0.001). Model estimates of covariate-adjusted post-treatment probability of 7 or more LOC days over the past 3 months was 0.52 (95%CI: 0.32–0.72) and 0.82 (95%CI: 0.66–0.97) for CBT and EF-CBT, respectively. At 2-months, the covariate-adjusted probability of 7 or more LOC days was 0.27 (95%CI: 0.10–0.45) and 0.56 (95%CI: 0.35–0.77) for CBT and EF-CBT, respectively. There were no differences in LOC between the groups at post-treatment (B = 0.87, SE = 1.04; p = 0.40) or 2-month follow-up (B = 0.65; SE = 1.07; p = 0.55). See Fig. 2 & Table 2.
Fig. 2.
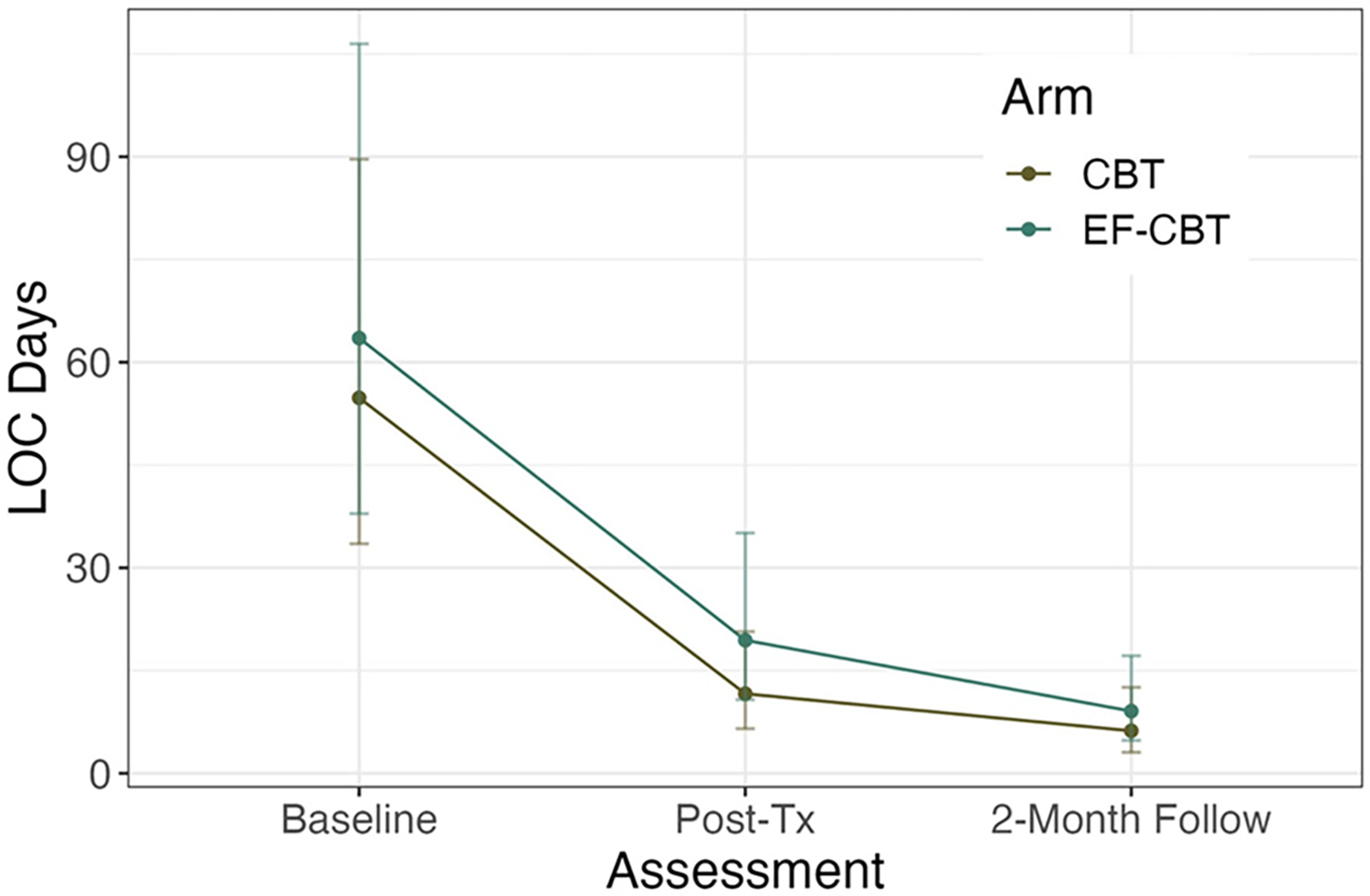
Both CBT and EF-CBT significantly decreased loss of control (LOC) at post-treatment and 2-month follow-up.
Note: CBT = Cognitive Behavioral Therapy; EF-CBT = CBT + Executive Function Training; Graph depicts average LOC days at each assessment.
Table 2.
Means and Standard Deviations of Examined Outcomes at each Timepoint.
| Baseline |
Post-treatment |
2-Month follow-up |
|
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
|
| |||
| LOC days | |||
| CBT | 58.38 (28.54) | 14.21 (15.34) | 8.09 (11.41) |
| EF-CBT | 63.19 (27.91) | 20.14 (20.74) | 12 (22.34) |
| CIA | |||
| CBT | 23.62 (8.27) | 14.93 (11.45) | 13.05 (9.32) |
| EF-CBT | 23.03 (11.44) | 12.40 (9.58) | 10.07 (7.08) |
| PHQ-9 | |||
| CBT | 13.25 (6.57) | 9.31 (6.29) | 6.42 (3.82) |
| EF-CBT | 10.31 (7.00) | 7.33 (4.73) | 4.50 (4.01) |
| GAD-7 | |||
| CBT | 8.69 (6.41) | 7.00 (6.18) | 6.00 (4.69) |
| EF-CBT | 8.11 (6.54) | 6.40 (5.04) | 5.07 (4.98) |
| BMI | |||
| CBT | 36.32 (9.26) | 35.44 (8.12) | 34.63 (6.97) |
| EF-CBT | 37.76 (9.91) | 37.95 (9.66 | 38.09 (9.25) |
Note: CBT = Cognitive Behavioral Therapy; EF-CBT = CBT + Executive Function; LOC = loss of control; CIA = Clinical Impairment Assessment; PHQ-9 = Patient Health Questionnaire-9; GAD-7 = Generalized Anxiety Disorder-7; BMI = body mass index.
3.2.1. Moderators of LOC
Given that EF-CBT was developed to improve outcomes for individuals with lower EF, we explored EF variables as moderators of LOC outcome. We found that participants who had lower EF at baseline, as measured by the GEC on the BRIEF-A, were less likely to have LOC at post-treatment in the EF-CBT compared to CBT group (B = −0.37; SE = 0.12; p = 0.002; see Fig. 3a). Among those with lower EF at baseline (i.e., GEC >75th percentile) who received EF-CBT compared to CBT had an estimated 0.26 lower probability of 7 or more LOC days at post-treatment. Conversely, those with higher EF at baseline (i.e., GEC <25th percentile) who received EF-CBT compared to CBT had an estimated 0.87 higher probability of 7 or more LOC days at post-treatment. Similarly, participants who had lower EF at baseline as measured by WCST total were less likely to have LOC at post-treatment in the EF-CBT compared to CBT group (B = 0.30; SE = 0.12; p = 0.02; see Fig. 3b).
Fig. 3.
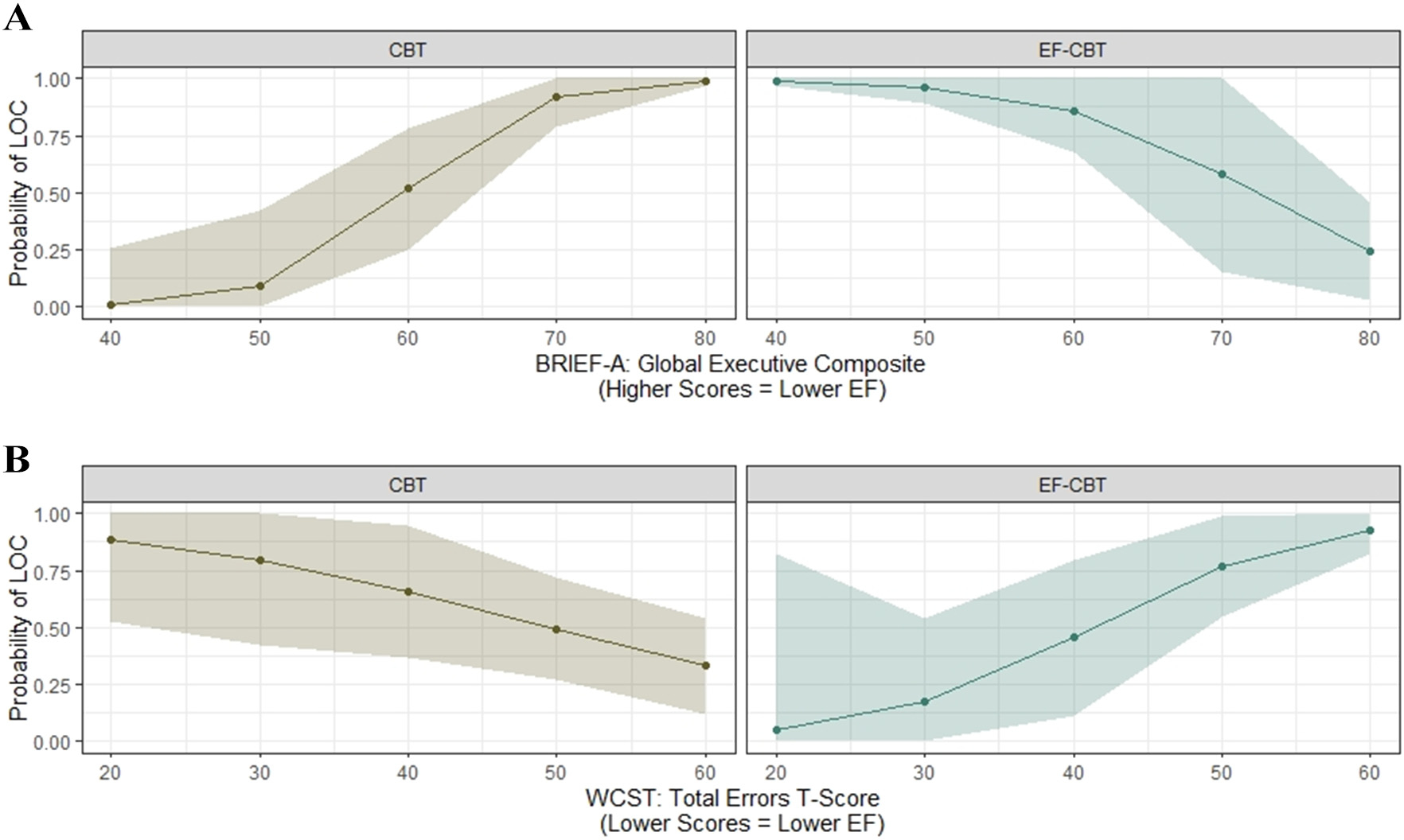
Participants with lower executive function at baseline were less likely to have loss of control at post-treatment. a BRIEF-A – Global Executive Composite. b WCST – total errors.
Note: CBT = Cognitive Behavioral Therapy; EF-CBT = CBT + Executive Function; BRIEF-A = Behavior Rating Inventory of Executive Function – Adult Version; WCST = Wisconsin Card Sorting Test; LOC = loss of control; EF = executive function.
3.2.2. Tracking as a potential mechanism
We evaluated self-monitoring as a potential mechanism across the sample. We found that increased self-monitoring over the course of treatment was significantly related to a lower probability of LOC at post-treatment (B = −1.32; SE = 0.62; p = 0.039). Adults with poorer EF as measured by higher rigidity scores on the DFlex questionnaire (B = 0.18; SE = 0.08; p = 0.03) were more likely to track in the EF-CBT compared to CBT condition (see Fig. 4A). Adults with poorer EF (DFlex scores at the 75th percentile) had a 0.79 (95%CI: 0.51–0.93) probability of reporting tracking 1 or more meals in EF-CBT compared to 0.28 (95%CI: 0.08–0.62) in CBT. Adults with higher EF (DFlex scores at the 25th percentile) had a similar probability of reporting tracking 1 or more meals in EF-CBT (prob = 0.51 (95%CI: 0.25–0.77)) compared to those in CBT (prob = 0.61 (95%CI: 0.34–0.82)). Similarly, adults with poorer EF as measured by higher scores on the PSI personal control subscale (B = 0.66; SE = 0.17; p < 0.001) were also more likely to track in EF-CBT compared to CBT (see Fig. 4B).
Fig. 4.
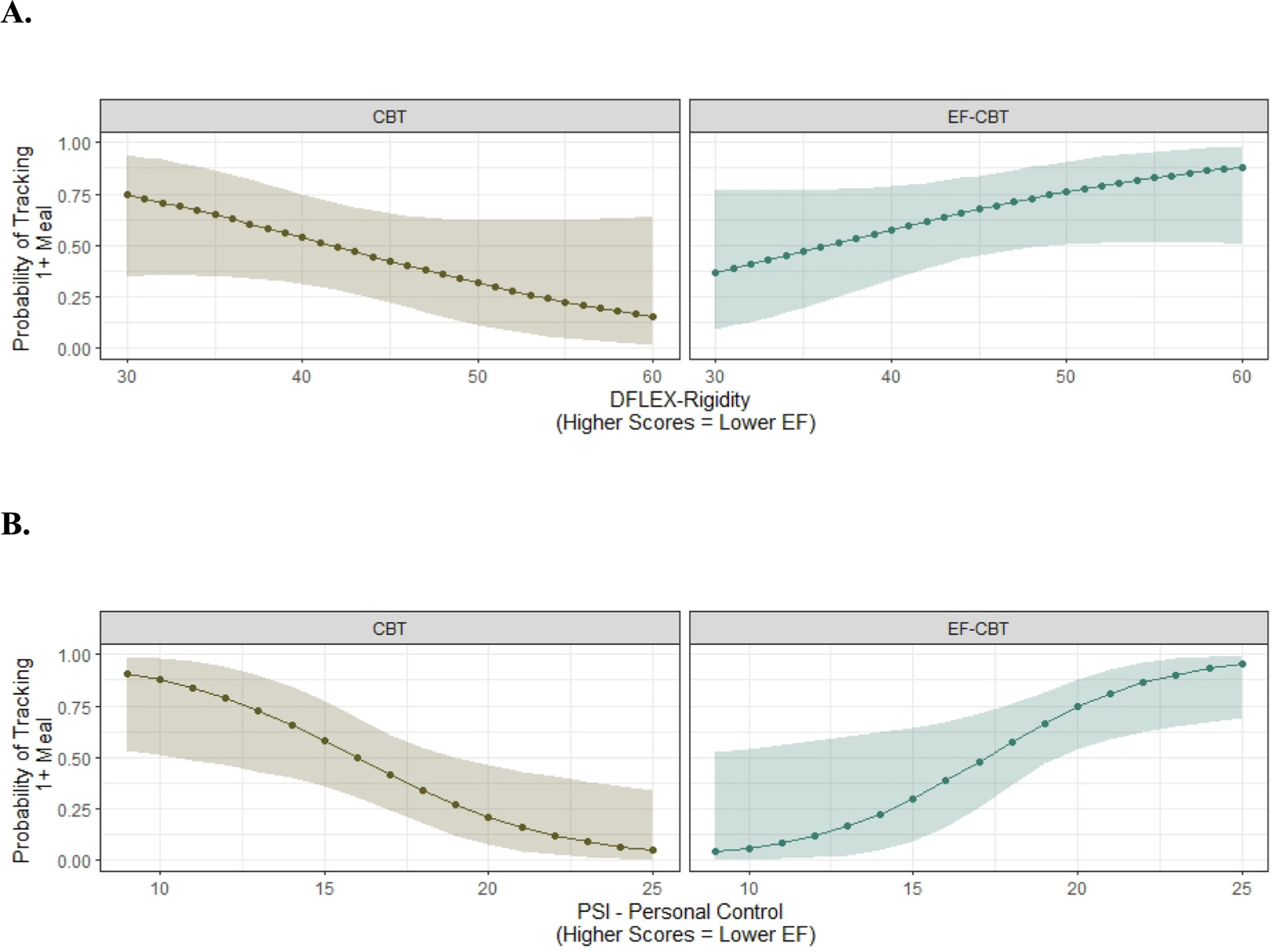
Participants with lower executive function were more likely to self-monitor in the EF-CBT compared to CBT group. a DFlex-Rigidity b PSI-Personal Control.
Note: CBT = Cognitive Behavioral Therapy; EF-CBT = CBT + Executive Function; D-Flex = Detail and Flexibility Questionnaire; PSI = Problem Solving Inventor.
3.3. Clinical impairment assessment
Both groups significantly decreased clinical impairment from baseline to post-treatment (B = −7.32; SE = 2.55; p = 0.006) and to the 2-month follow-up (B = −8.58; SE = 2.54; p = 0.001) but there were no differences in the amount of change in clinical impairment between the groups at post-treatment (B = −2.77, SE = 3.52; p = 0.44) or 2-month follow-up (B = −4.23; SE = 3.56; p = 0.24); see Table 2.
3.4. Depression/anxiety
Both groups reported significantly decreased depression from baseline to post-treatment (B = −3.16; SE = 1.21; p = 0.011) and to the 2-month follow-up (B = −5.41; SE = 1.23; p = 0.001) but there were no differences in the amount of change in depression between the groups at post-treatment (B = 0.19, SE = 1.76; p = 0.92) or 2-month follow-up (B = −0.55; SE = 1.80; p = 0.76). Anxiety did not significantly change at any assessment (ps > 0.05). See Table 2.
3.5. BMI
There were no significant changes in weight across the entire sample at post-treatment or two-month follow-up (ps > 0.05). Furthermore, there was no main effect of group on change in weight. See Table 2.
4. Discussion
This pilot randomized controlled trial was the first to evaluate the impact of adding compensatory executive function training, by incorporating CCT/CogSMART strategies into CBT. As hypothesized, results showed that EF-CBT was feasible and acceptable, comparable or better than CBT. Both EF-CBT and CBT similarly decreased levels of LOC and depression at post-treatment and two-month follow-up. Neither treatment was related to any significant decreases in anxiety or BMI.
Exploratory moderator analyses were conducted to better understand if levels of EF prior to treatment were associated with differences in response to EF-CBT and CBT interventions. These analyses showed that individuals with lower EF within this sample were less likely to have LOC at post-treatment if they were in the EF-CBT rather than CBT group. These findings suggest that adding EF training to CBT may promote skills that aid individuals with lower EF in managing binge behavior. Thus, EF-CBT may potentially help a subset of those 50 % who do not respond in the long term. Interestingly, the converse also appeared to be true such that those with higher EF were more likely to have LOC following treatment in EF-CBT. Accordingly, these exploratory moderator analyses may suggest that EF-CBT only be offered to those with lower EF. Again, as this is a small pilot study, these findings should all be interpreted with caution without replication in larger samples. However, it is possible that providing EF strategies to those who may not need them as much may cause participants to lose interest and perhaps not pay attention to some of the other core curriculum to reduce binge eating.
Not surprisingly, this study found that greater compliance with self-monitoring over the course of treatment resulted in greater reductions of LOC eating. This finding is consistent with previous research that showed that greater compliance with self-monitoring was related to greater reductions in binge eating (Srivastava et al., 2021). Self-monitoring can be challenging for many participants and requires considerable amounts of EF. Importantly, we found that participants with lower EF were more likely to self-monitor in EF-CBT compared to CBT. Thus, self-monitoring represents a potential mechanism of action which can be improved by using targeted treatments, such as EF-CBT.
As with previous studies (Agras et al., 1995; Wilfley et al., 2002), despite decreases in LOC, there were no changes in weight in either arm of the study. Following the principles of CBT, both groups were informed that the goal of treatment was to focus on reducing binge eating first rather than weight change. The only recommendation regarding weight was for participants to weigh themselves weekly. Additionally, this treatment was only four months long with a two-month follow-up and this study was underpowered to detect differences between two active treatment arms. However, almost all participants stated that they wanted to lose weight. Given the strong desire by most participants to lose weight, new treatments that both reduce binge eating and help with weight management may be warranted for longer term remission of BED.
Results of this study should also be considered in the context of the timing of the study. This study was conducted early during the COVID-19 pandemic when many individuals were under mandated quarantines. Thus, some individuals may have experienced fewer triggers for binge eating or LOC (eating out; drive thru) while others may have experienced more (due to loneliness/isolation, increased anxiety, and financial stress). Relatedly, it is possible that the lack of change in anxiety and weight may have been due to the timing of the trial as many individuals experienced fluctuations of anxiety during the pandemic and policies related to quarantines and social distancing resulted in reduced physical activity behaviors for many individuals and increased isolation.
Strengths of this study include the development and initial evaluation of a treatment aimed at targeting a potential mechanism, executive function, which may be related to failure in CBT. The treatment was delivered virtually, which allows for ease of dissemination and increased reach. Despite this novel treatment development, there are limitations that need to be noted. This is a very small pilot study and should be viewed as a proof-of-concept study for EF-CBT and the findings should be interpreted with caution. Furthermore, all treatment for this study was conducted during the COVID-19 pandemic, which may have impacted outcomes. This treatment was tested among participants with BED and OW/OB and may not apply to those without OW/OB. Additionally, all participants had a comorbid mood or anxiety disorder making this a challenging sample for a proof-of-concept pilot; however, the majority of individuals with BED have a comorbid psychiatric disorder (Udo & Grilo, 2019) so it may be more representative of BED patients attending treatment. Lastly, we did not include a measure of IQ and thus we cannot say for sure whether the EF results may be confounded with IQ. Future research should continue to evaluate EF-CBT in large samples to explore whether it continues to confer additional benefits for individuals with lower EF and whether this is through the mechanism of self-monitoring. Additionally, future research could explore whether EF-CBT has any benefits for individuals with BED without OW/OB.
Overall, this study was the first to evaluate adding CCT to CBT for BED. Results suggest that EF-CBT was acceptable and feasible. Furthermore, it was efficacious in reducing LOC, anxiety, and depression. Specifically, EF-CBT may benefit a specific phenotype of individuals with BED, those with lower EF. EF-CBT may help a subset of individuals achieve improved outcomes via increasing adherence to self-monitoring. Future larger clinical trials are needed to better understand this mechanism and evaluate for whom EF-CBT may be more beneficial. If proven to be efficacious, EF-CBT could provide a treatment for those who may not respond to CBT, specifically those with lower EF. In conclusion, results suggest that EF-CBT is a promising treatment worthy of more exploration.
Acknowledgements
We would like to acknowledge all of the research participants, as well as the staff at the Center for Healthy Eating and Activity Research (CHEAR) at UC San Diego, who made this study possible - especially the study coordinators, Ellen Pasquale and Allison Tietz and Dr. Saori Obayashi for her help with treatment.
Role of funding sources
Funding for the research study was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation # 27943 awarded to Dr. Dawn Eichen and the project was partially supported by UL1TR001442. Dr. Eichen’s effort was also funded by National Institute of Health award K23DK114480. Dr. Twamley gratefully acknowledges the support of a VA Rehabilitation Research and Development Research Career Scientist Award. The funding sources had no involvement in the study design, collection, analysis, interpretation of data, writing of manuscript and decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent official views of the funding sources.
Footnotes
Declaration of competing interest
The authors have no conflict of interest to report.
CRediT authorship contribution statement
DME, EWT, KNB were responsible for the design of the study; DRS & DME conducted analyses; DME drafted the original manuscript; DME, EWT, KNB, and DRS all edited the manuscript. All authors have approved the final manuscript.
Data availability
Data will be made available on request.
References
- Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, & Fox CS (2014). Metabolic characterization of adults with binge eating in the general population: The Framingham heart study. Obesity, 22(11), 2441–2449. 10.1002/oby.20867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agras WS, Telch CF, Arnow B, et al. (1995). Does interpersonal therapy help patients with binge eating disorder who fail to respond to cognitive-behavioral therapy? Journal of Consulting and Clinical Psychology, 63, 356–360. 10.1037/0022-006X.63.3.356 [DOI] [PubMed] [Google Scholar]
- Aloi M, Rania M, de Filippis R, & Segura-Garcia C (2020). Weight and age do not account for a worse executive functioning among BED-obese patients. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 25(2), 373–377. 10.1007/s40519-018-0608-9, 2020/04/01. [DOI] [PubMed] [Google Scholar]
- Ayers C, Dozier M, Twamley E, et al. (2017). Cognitive rehabilitation and exposure/sorting therapy (CREST) for hoarding disorder in older adults: A randomized clinical trial. The Journal of Clinical Psychiatry, 79(2), 16m11072. 10.4088/JCP.16m11072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers CR, Saxena S, Espejo E, Twamley EW, Granholm E, & Wetherell JL (2014). Novel treatment for geriatric hoarding disorder: An open trial of cognitive rehabilitation paired with behavior therapy. The American Journal of Geriatric Psychiatry : official journal of the American Association for Geriatric Psychiatry., 22(3), 248–252. 10.1016/j.jagp.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01, 10/07. [DOI] [Google Scholar]
- Blume M, Schmidt R, & Hilbert A (2019). Executive functioning in obesity, food addiction, and binge-eating disorder. Nutrients, 11(1), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Monreal T, Strong DR, & Amir N (2016). An open trial evaluating an attention bias modification program for overweight adults who binge eat. Journal of Behavior Therapy and Experimental Psychiatry, 52, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmeyer T, Friederich H-C, Küppers C, et al. (2019). Approach bias modification training in bulimia nervosa and binge-eating disorder: A pilot randomized controlled trial. The International Journal of Eating Disorders, 52(5), 520–529. 10.1002/eat.23024 [DOI] [PubMed] [Google Scholar]
- Chami R, Cardi V, Lawrence N, et al. (2022). Targeting binge eating in bulimia nervosa and binge eating disorder using inhibitory control training and implementation intentions: A feasibility trial. Psychological Medicine, 52(5), 874–883. 10.1017/S0033291720002494 [DOI] [PubMed] [Google Scholar]
- Cooper Z, & Fairburn C (Jan 1987). The eating disorder examination - A semistructured interview for the assessment of the specific psychopathology of eating disorders. The International Journal of Eating Disorders, 6(1), 1–8. [DOI] [Google Scholar]
- Cotrena C, Branco LD, Shansis FM, & Fonseca RP (2016). Executive function impairments in depression and bipolar disorder: Association with functional impairment and quality of life. Journal of Affective Disorders, 190, 744–753. 10.1016/j.jad.2015.11.007, 2016/01/15/. [DOI] [PubMed] [Google Scholar]
- Cury MEG, Berberian A, Scarpato BS, Kerr-Gaffney J, Santos FH, & Claudino AM (2020). Scrutinizing domains of executive function in binge eating disorder: A systematic review and meta-analysis. systematic review. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.00288, 2020-April-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Cawley EA, Simeonov L, et al. (Jun 3 2022). The effects of response inhibition training following binge memory retrieval in young adults binge eaters: a randomised-controlled experimental study. Scientific Reports, 12(1), 9281. 10.1038/s41598-022-12173-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, & Reid C (2010). Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite, 54 (1), 208–213. [DOI] [PubMed] [Google Scholar]
- Dingemans AE, & van Furth EF (2012). Binge eating disorder psychopathology in normal weight and obese individuals. The International Journal of Eating Disorders, 45 (1), 135–138. 10.1002/eat.20905 [DOI] [PubMed] [Google Scholar]
- Dingemans AE, van Son GE, Vanhaelen CB, & van Furth EF (2020). Depressive symptoms rather than executive functioning predict group cognitive behavioural therapy outcome in binge eating disorder. European Eating Disorders Review, 28(6), 620–632. 10.1002/erv.2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichen DM, Matheson BE, Appleton-Knapp SL, & Boutelle KN (2017). Neurocognitive treatments for eating disorders and obesity. Current Psychiatry Reports, 19(9), 62. 10.1007/s11920-017-0813-7, 2017/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichen DM, Pasquale EK, Twamley EW, & Boutelle KN (2021). Targeting executive function for weight loss in adults with overweight or obesity. Physiology & Behavior, 240, 113540. 10.1016/j.physbeh.2021.113540, 2021/10/15/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG (2013). Overcoming binge eating: The proven program to learn why you binge and how you can stop, 2nd ed. In Overcoming binge eating: The proven program to learn why you binge and how you can stop, 2nd ed. Guilford Press. [Google Scholar]
- Fairburn CG, Cooper Z, & OC. M. (2008). Eating disorder examination (16.0D). In Fairburn CG (Ed.), Cognitive behavior therapy and eating disorders. Guilford Press. [Google Scholar]
- Fairburn CG, Marcus MD, & Wilson GT (1993). Cognitive-behavioral therapy for binge eating and bulimia nervosa: A comprehensive treatment manual. In Fairburn CG, & Wilson GT (Eds.), Binge eating: Nature, assessment, and treatment (pp. 361–404). Guilford Press. [Google Scholar]
- Fitzpatrick S, Gilbert S, & Serpell L (2013). Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychology Review, 23(2), 138–156. [DOI] [PubMed] [Google Scholar]
- Fox J (2003). Effect displays in R for generalised linear models. Journal of Statistical Software, 8(15), 1–27. 10.18637/jss.v008.i15, 07/22. [DOI] [Google Scholar]
- Fox J, & Hong J (2009). Effect displays in R for multinomial and proportional-odds logit models: Extensions to the effects package. Journal of Statistical Software, 32(1), 1–24. 10.18637/jss.v032.i01, 10/14. [DOI] [Google Scholar]
- Galioto R, Spitznagel MB, Strain G, et al. (2012). Cognitive function in morbidly obese individuals with and without binge eating disorder. Comprehensive Psychiatry, 53(5), 490–495. 10.1016/j.comppsych.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giel KE, Speer E, Schag K, Leehr EJ, & Zipfel S (Jun 2017). Effects of a food-specific inhibition training in individuals with binge eating disorder-findings from a randomized controlled proof-of-concept study. Eating and Weight Disorders, 22(2), 345–351. 10.1007/s40519-017-0371-3 [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Le Grange D, Powers P, et al. (Jul 2011). Eating disorder symptomatology in normal-weight vs. obese individuals with binge eating disorder. Obesity, 19(7), 1515–1518. 10.1038/oby.2011.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, & Chamberlain SR (2020). Neurocognitive findings in young adults with binge eating disorder. International Journal of Psychiatry in Clinical Practice, 24(1), 71–76. 10.1080/13651501.2019.1687724, 2020/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner PP (1988). The problem-solving inventory: Manual. Consulting Psychologists Press. [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. 10.1016/j.tics.2012.01.006, 2012/03/01/. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lalonde JK, Coit CE, et al. (Jun 2010). Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. The American Journal of Clinical Nutrition, 91(6), 1568–1573. 10.3945/ajcn.2010.29203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarascio AS, Manasse SM, Espel HM, Kerrigan SG, & Forman EM (2015). Could training executive function improve treatment outcomes for eating disorders? Appetite, 90, 187–193. 10.1016/j.appet.2015.03.013, 2015/07/01/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, et al. (2013). The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biological Psychiatry, 73(9), 904–914. 10.1016/j.biopsych.2012.11.020, 2013/05/01/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollei I, Rustemeier M, Schroeder S, Jongen S, Herpertz S, & Loeber S (2018). Cognitive control functions in individuals with obesity with and without binge-eating disorder. The International Journal of Eating Disorders, 51(3), 233–240. 10.1002/eat.22824 [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, & Heaton RK (2000). Wisconsin card sorting test-, 64 card version: WCST-64. FL: PAR Lutz. [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino L, Arnone D, Cao B, Soares JC, & Selvaraj S (2016). Inhibitory control in obesity and binge eating disorder: A systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neuroscience and Biobehavioral Reviews, 68, 714–726. 10.1016/j.neubiorev.2016.06.041, 2016/09/01/. [DOI] [PubMed] [Google Scholar]
- Linardon J (2018). Rates of abstinence following psychological or behavioral treatments for binge-eating disorder: Meta-analysis. The International Journal of Eating Disorders, 51(8), 785–797. 10.1002/eat.22897 [DOI] [PubMed] [Google Scholar]
- Lydecker JA, & Grilo CM (2021). Psychiatric comorbidity as predictor and moderator of binge-eating disorder treatment outcomes: An analysis of aggregated randomized controlled trials. Psychological Medicine, 1–9. 10.1017/S0033291721001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, & Fitzpatrick KK (2015). Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. The International Journal of Eating Disorders, 48(6), 677–683. 10.1002/eat.22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendella PD, Burton CZ, Tasca GA, Roy P, St Louis L, & Twamley EW (Mar 2015). Compensatory cognitive training for people with first-episode schizophrenia: Results from a pilot randomized controlled trial. Schizophrenia Research, 162(1–3), 108–111. 10.1016/j.schres.2015.01.016 [DOI] [PubMed] [Google Scholar]
- Mitchell JE (2016). Medical comorbidity and medical complications associated with binge-eating disorder. International Journal of Eating Disorders, 49(3), 319–323. 10.1002/eat.22452 [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Devlin MJ, de Zwaan M, Crow SJ, & Peterson CB (2007). Binge-eating disorder: Clinical foundations and treatment. Guilford Press. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (Aug 2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- ordinal - Regression models for ordinal data. Version R package version 2019.12–10. https://CRAN.R-project.org/package=ordinal, (2019).
- Staff PAR. (2020). Remote administration for the Wisconsin Card Sorting Test (WCST). https://www.parinc.com/Portals/0/Webuploads/samplerpts/WCST_Remote%20Administration_Digital%20Paper_Jun24.pdf.
- Peat CM, Berkman ND, Lohr KN, et al. (Sep 2017). Comparative effectiveness of treatments for binge-eating disorder: Systematic review and network meta-analysis. European Eating Disorders Review, 25(5), 317–328. 10.1002/erv.2517 [DOI] [PubMed] [Google Scholar]
- Prunell-Castañé A, Jurado MÁ, & García-García I (2021). Clinical binge eating, but not uncontrolled eating, is associated with differences in executive functions: Evidence from meta-analytic findings. Addictive Behaviors Reports, 13, 100337. 10.1016/j.abrep.2020.100337, 2021/06/01/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger E, Wilfley DE, Stein RI, Marino V, & Crow SJ (Apr 2005). A comparison of quality of life in obese individuals with and without binge eating disorder. The International Journal of Eating Disorders, 37(3), 234–240. 10.1002/eat.20101 [DOI] [PubMed] [Google Scholar]
- Roberts ME, Barthel FM, Lopez C, Tchanturia K, & Treasure JL (Aug 2011). Development and validation of the Detail and Flexibility Questionnaire (DFlex) in eating disorders. Eating Behaviors, 12(3), 168–174. 10.1016/j.eatbeh.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, & Gioia GA (2005). Behavioral rating inventory of executive function - Adult version. Psychological Assessment Resources, Inc. [Google Scholar]
- Rouel M, Raman J, Hay P, & Smith E (2016). Validation of the behaviour rating inventory of executive function – Adult version (BRIEF-A) in the obese with and without binge eating disorder. Eating Behaviors, 23, 58–65. 10.1016/j.eatbeh.2016.07.010, 2016/12/01/. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Shields GS, Moons WG, Tewell CA, & Yonelinas AP (2016). The effect of negative affect on cognition: Anxiety, not anger, impairs executive function. Emotion, 16, 792–797. 10.1037/emo0000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L, & Trollor JN (2011). A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obesity Reviews, 12(9), 740–755. 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166 (10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Parker MN, Presseller EK, Wons OB, Clark KE, & Juarascio AS (2021). A closer look at homework compliance in behavior therapy for bulimia nervosa: Does homework compliance in between-session period prospectively predict session-by-session change in bulimia symptoms? Eating Disorders, 1–20. 10.1080/10640266.2021.2014666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke A, Kopp B, & Lange F (Apr 21 2021). The Wisconsin Card Sorting Test: Split-half reliability estimates for a self-administered computerized variant. Brain Sciences, 11(5). 10.3390/brainsci11050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storzbach D, Twamley EW, Roost MS, et al. (2017). Compensatory cognitive training for operation enduring freedom/operation Iraqi freedom/operation new dawn veterans with mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 32(1), 16–24. 10.1097/htr.0000000000000228 [DOI] [PubMed] [Google Scholar]
- Svaldi J, Brand M, & Tuschen-Caffier B (Feb 2010). Decision-making impairments in women with binge eating disorder. Appetite, 54(1), 84–92. 10.1016/j.appet.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Szabo-Reed AN, & Donnelly JE (Spring 2021). Cognitive training: associations and implications for weight management and translational research. Translational Journal of the American College of Sports Medicine, 6(2). 10.1249/tjx.0000000000000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregarthen JP, Lock J, & Darcy AM (2015). Development of a smartphone application for eating disorder self-monitoring. The International Journal of Eating Disorders, 48(7), 972–982. 10.1002/eat.22386 [DOI] [PubMed] [Google Scholar]
- Turton R, Nazar BP, Burgess EE, et al. (Jan 2018). To go or not to go: A proof of concept study testing food-specific inhibition training for women with eating and weight disorders. European Eating Disorders Review, 26(1), 11–21. 10.1002/erv.2566 [DOI] [PubMed] [Google Scholar]
- Twamley EW, Jak A, Delis DC, Bondi MW, & Lohr JB (2014). Cognitive Symptom Management and Rehabilitation Therapy (CogSMART) for veterans with traumatic brain injury: Pilot randomized controlled trial. Journal of Rehabilitation Research and Development, 51, 59–70. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Noonan SK, Schiehser D, Salva GN, & Jak A (2010). Cognitive symptom management and rehabilitation therapy (CogSMART) for traumatic brain injury. www.cogsmart.com. [DOI] [PubMed]
- Twamley EW, Thomas KR, Burton CZ, et al. (Jan 2019). Compensatory cognitive training for people with severe mental illnesses in supported employment: A randomized controlled trial. Schizophrenia Research, 203, 41–48. 10.1016/j.schres.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Thomas KR, Gregory AM, et al. (2015). CogSMART compensatory cognitive training for traumatic brain injury: Effects over 1 year. The Journal of Head Trauma Rehabilitation, 30(6), 391–401. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Vella L, Burton CZ, Heaton RK, & Jeste DV (2012). Compensatory cognitive training for psychosis: Effects in a randomized controlled trial. The Journal of Clinical Psychiatry, 73(9), 1212–1219. 10.4088/JCP.12m07686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, & Grilo CM (Sep 1 2019). Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biological Psychiatry, 84(5), 345–354. 10.1016/j.biopsych.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar E, Friedman J, & Mehler PS (2019). Medical complications of binge eating disorder. Psychiatric Clinics of North America, 42(2), 275–286. 10.1016/j.psc.2019.01.010, 2019/06/01/. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Welch RR, Stein RI, et al. (Aug 2002). A randomized comparison of group cognitive-behavioral therapy and group interpersonal psychotherapy for the treatment of overweight individuals with binge-eating disorder. Archives of General Psychiatry, 59(8), 713–721. 10.1001/archpsyc.59.8.713 [DOI] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Wu Q, Liu Y, Chen H, & Guo C (Nov 2019). Cognitive training on eating behaviour and weight loss: A meta-analysis and systematic review. Obesity Reviews, 20(11), 1628–1641. 10.1111/obr.12916 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


