Abstract
We have investigated the potential of PAC-based vectors as a route to the incorporation of a gene in a mammalian artificial chromosome (MAC). Previously we demonstrated that a PAC (PAC7c5) containing α-satellite DNA generated mitotically stable MACs in human cells. To determine whether a functional HPRT gene could be assembled in a MAC, PAC7c5 was co-transfected with a second PAC containing a 140 kb human HPRT gene into HPRT-deficient HT1080 cells. Lines were isolated containing a MAC hybridizing with both α-satellite and HPRT probes. The MACs segregated efficiently, associated with kinetochore proteins and stably expressed HPRT message after 60 days without selection. Complementation of the parental HPRT deficiency was confirmed phenotypically by growth on HAT selection. These results suggest that MACs could be further developed for delivering a range of genomic copies of genes into cells and that stable transgene expression can be achieved.
INTRODUCTION
Formation of mammalian artificial chromosomes (MACs) in cultured human cells following transfection of naked DNA including candidate sequences considered essential for chromosome function has been reported by several groups (Harrington et al., 1997; Ikeno et al., 1998; Henning et al., 1999). Common to the approaches taken was the inclusion of telomere repeats, which are capable of de novo telomere formation in transfection assays (Farr et al., 1992) and α-satellite DNA, which is present at all normal human centromere/kinetochores (Lee et al., 1997) as a centromere seeding component.
The sequence elements were introduced either as synthetic arrays in a transfection mixture including human genomic DNA (Harrington et al., 1997) or as cloned DNA fragments in pre-assembled YAC constructs (Ikeno et al., 1998; Henning et al., 1999). The MACs generated were mitotically stable, associated with functionally important kinetochore proteins and were in the 1–10 Mb size range. MACs formed after transfection with a 100 kb YAC were shown to consist of multimers of the input DNA (Ikeno et al., 1998). The MACs were either linear, reflecting normal human chromosome structure, or remained uncharacterized in this respect. While the MACs were shown to be replication competent, sequences conferring replication origin function remain to be identified.
Recently we have shown that telomeric sequences are not essential for de novo chromosome formation, as a circular PAC (PAC7c5) containing 70 kb of homogeneous 21-I α-satellite DNA (α21-I) generated low copy number, mitotically stable circular MACs in 50–90% of metaphase spreads in ∼50% of human HT1080 transformants tested (Ebersole et al., 2000). The simplicity, ease of preparation and efficient MAC formation in human cells of PAC-based vectors makes them amenable to further development as gene delivery vectors.
The use of MACs as gene delivery vectors offers considerable advantages. First, since MACs are in the megabase size range, large inserts spanning genes and their regulatory elements could be incorporated without the size constraints imposed by conventional vectors. Secondly, since artificial chromosomes are extrachromosomal and do not integrate into the host genome, potentially harmful mutational events associated with transgene insertion could be avoided. Thirdly, positional silencing of integrated transgenes, which has proved to be a common problem in the production of transgenic cell lines and animals, might be overcome by the use of a MAC vector. A consideration for MAC vector applications is centromere-associated gene silencing, which has been described in yeast, flies and mice (Festentein et al., 1996; Karpen and Allshire, 1997). However, this phenomenon has not been reported in human cells (Bayne et al., 1994).
The present report details the incorporation of a functional 140 kb HPRT gene into an artificial chromosome by means of a co-transfection procedure with a PAC containing α-satellite DNA from human chromosome 21 in human HPRT-deficient HT1080 cells. Sub-cloned lines containing an HPRT-expressing MAC in a majority of cells were identified. The MACs were mitotically stable and produced HPRT message at a consistent level during extended periods in culture under non-selective conditions. Phenotypic confirmation of HPRT expression was obtained by growth under positive selection conditions. These results provide new evidence that MACs could be used to complement a deficiency in a gene of medical relevance (Fujimori et al., 1989) in human cells.
Results
Incorporation of a 140 kb human HPRT gene into an artificial chromosome
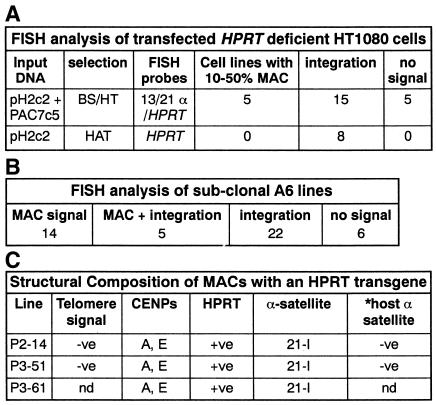
PAC7c5 contains a 70 kb array of a uniform higher order repeat (11mer) derived from the human chromosome 21-I α-satellite locus (α21-I) (Ebersole et al., 2000). Following transfection of PAC7c5 into HT1080 cells, mitotically stable MACs were detected in a high proportion of spreads in half of the transformants analyzed using fluorescence in situ hybridization (FISH) (Ebersole et al., 2000). To determine whether a human gene could be assembled into a MAC, a PAC (pH2c2) containing a 140 kb genomic DNA insert spanning the human HPRT gene was co-transfected with PAC7c5 into HPRT-deficient HT1080 cells. Cells resistant to the blasticidin-S (BS) resistance marker gene present only on PAC7c5 were selected to avoid possible influences on chromosome formation associated with selection for HPRT expression. Although integration of the transfected DNA was a frequent event, a MAC was detected in 10–50% of metaphases in five out of 25 BS-resistant clones analyzed by FISH using both an α-satellite probe, α13/21 (which detects chromosome 13 and 21 α-satellite DNA) and HPRT probes (Table IA).
Table I. Artificial chromosome formation rates and MAC properties.
(A) Fate of PAC DNA transfected into HPRT-deficient HT1080 cells. In the remaining 50–90% of metaphases in cell lines where a MAC formed, the transfected DNA either integrated into a host chromosome or was undetectable. (B) FISH analysis of sub-cloned A6 lines. MAC: lines with a MAC as the only detectable fate of the transfected DNA. MACs were present at a copy number of usually one and occasionally two per spread. MAC+ integration: lines with a MAC and integration(s) within the same metaphase spread. In the remaining lines the transfected DNA either integrated or was undetectable. (C) Summary of the structural properties of MACs in three sub-clones (P2-14, P3-51 and P3-61) containing a MAC (and no detectable integrations) using FISH (Telomere, HPRT and α-satellite probes) and immunofluorescence (CENPs: centromere protein localization using anti CENP-A and anti CENP–E antibodies) analysis.
*The absence of detectable host α-satellite DNA on the MACs in the P2-14 and P3-51 cell lines (other than those present on the input PAC) was demonstrated using FISH analysis (detailed in Figure 1B).
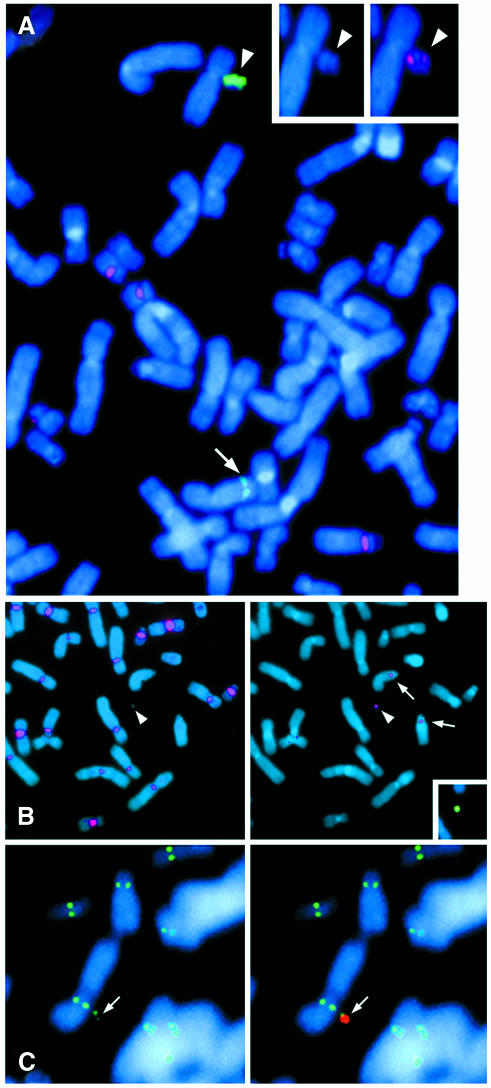
The MACs hybridized at similar levels with the α13/21 probe but variation in signal intensity was detected with the HPRT probe among clonal lines. In one transformant, A6, a MAC was detected in 40% of spreads that hybridized strongly with both HPRT and α13/21 probes. The A6 line was single-cell cloned to produce sub-clonal lines containing MACs in the majority (75–100%) of cells at a copy number of 1 or 2 per spread (Table IB). Whilst a stronger HPRT signal was detected on the MACs compared with that of the host HPRT signal (Figure 1A), copy number comparisons of HPRT DNA content in three sub-clonal lines (P2-14, P3-51 and P3-61) containing a MAC in 75–100% of spreads with that of the parental HT1080 line using Southern analysis indicated the presence of only an additional 1 or 2 unrearranged copies of the HPRT transgene on the artificial chromosomes (data not shown). At least part of the HPRT signal intensity in Figure 1A will reflect vector sequences present in the HPRT probe and could reflect a less condensed chromatin conformation permitting better probe access.
Fig. 1. Structural analysis of HPRT-expressing MACs. (A–C) FISH analysis. (A) Sub-clone P3-51 was hybridized with a 13/21 α-satellite (α13/21) probe (red) and HPRT probe (green). The MAC hybridized more strongly with the HPRT probe (arrowhead) than the endogenous gene (arrow). Left insert: DAPI stained MAC. Right insert: MAC hybridized with α13/21. (B) A pan-centromere probe was annealed with cold p11-4 to compete α13/21 out sequences (Ebersole et al., 2000) and hybridized to P2-14 chromosomes (red, left panel). The α13/21-depleted probe did not hybridize with centromeres from 13 (arrow) or 21 (not shown) as expected or on the MAC (arrowhead). The right panel shows the same spread subsequently hybridized with an α13/21 probe (red), which confirms the identity of two copies of chromosome 13 and the MAC. An HPRT probe (green, insert) further confirmed MAC identity. (C) CENP-A signals (green) were detected using anti-CENP-A antibodies (left panel, arrow indicates MAC in P2-14). The right hand panel shows the merged image with an HPRT probe (red). Similar results were obtained with anti-CENP-E antibodies (data not shown). (A–C) Chromosomes were counterstained with DAPI.
Mitotic stability and structural composition of MACs in sub-clonal lines
The properties of the artificial chromosomes in three A6 sub-clones containing a MAC and no integrated copies of the transfected DNA were further investigated (summarized in Table IC). There was no evidence for telomere seeding using FISH with a telomere probe suggesting a circular MAC structure (data not shown), nor for acquisition of host α-satellite DNA (Figure 1B), indicating that only α21-I sequences introduced on PAC7c5 were incorporated into MACs during chromosome formation.
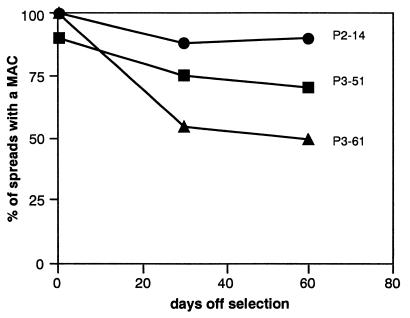
Mitotic stability of the MACs was measured by FISH analysis after passage in non-selective (HT supplemented) medium which permits growth of either HPRT expressing or non-expressing cells. After 60 days growth off selection, the MACs in lines P2-14 and P3-51 were retained in a high proportion (70–90%) of cells, indicating that a functional kinetochore had been established on the MACs (Figure 2). This was further supported by the presence of the functionally indispensable kinetochore proteins CENP-A (Valdivia et al., 1998) and CENP-E (Yen et al., 1991) in each sub-clone (Figure 1C and data not shown). The MAC in sub-clone P3-61 exhibited a lower mitotic stability level and was retained in only 50% of cells after 30 days passage off selection. No further decline in this frequency was observed during a further 30 days in culture (Figure 2).
Fig. 2. Mitotic stability. Cells from P2-14, P3-51 and P3-61 were passaged under non-selective conditions. MAC mitotic stability was measured at 30 day intervals using FISH with α13/21 and HPRT probes on metaphase spreads. y-axis: percentage of spreads containing a MAC. x-axis: time points at which MAC frequency was determined. The MAC in P3-61 was either retained in 50% of spreads after 60 days off selection or recombined with a host chromosome (40% of spreads) or was undetectable (10% of spreads).
Stable HPRT expression from artificial chromosomes
To determine whether HPRT gene activity was maintained after passaging in culture, RNA was extracted from P2-14 and P3-51 after 0 and 60 days growth under non-selective conditions (HT medium). Northern analysis revealed that HPRT message was produced at similar levels in both P2-14 (Figure 3) and P3-51 (data not shown) at each time point tested, indicating stable transgene expression from the artificial chromosomes. Complementation of the parental HPRT deficiency was confirmed phenotypically in P2-14 and P3-51 by growth under positive HPRT selection (HAT medium) of cells replated from 0 and 60 day time points on HT medium. Under HAT selection, an increase in HPRT mRNA levels was detected by northern analysis in each sub-clonal line (Figure 3 and data not shown).
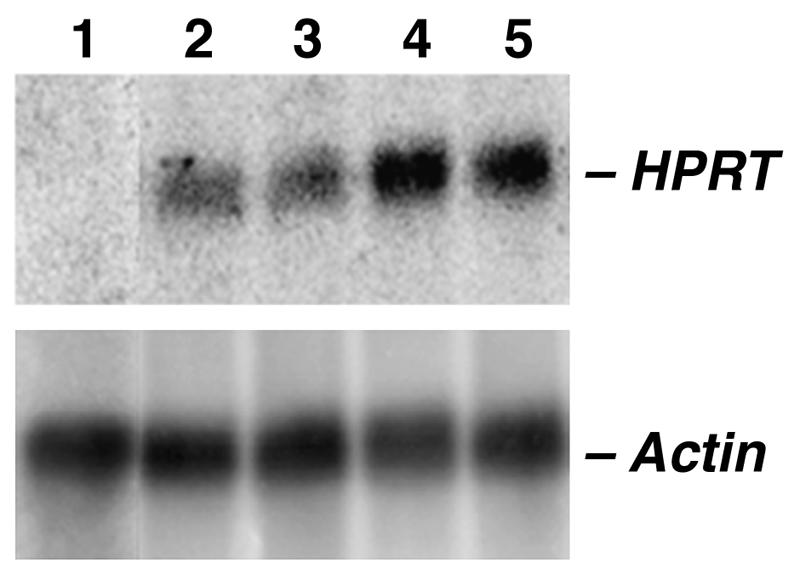
Fig. 3. Stable HPRT expression from MACs. (Top) Northern analysis of RNA extracted from P2-14 at time point 0 (lane 2) and after 60 days passage off selection (HT medium) (lane 3) using an HPRT probe. P2-14 cells from each of the 0 and 60 day time points were subsequently shown to grow when replated on positive HPRT selection (HAT medium) and under these conditions a similar increase in HPRT mRNA levels as detected (lane 4: RNA from P2-14 cells plated into HAT medium from time point 0; lane 5: RNA from P2-14 cells plated into HAT medium from the 60 day time point). RNA from HPRT-deficient cells is loaded in lane 1. (Bottom) RNA loading control using an actin probe..
A possible explanation for the lower level of HPRT message observed under non-selective conditions would be the existence of a sub-population of cells harboring an HPRT transgene(s) that has been epigenetically silenced. This was investigated in five A6 sub-clonal lines (including P2-14 and P3-51) with a high MAC content that had been maintained on HT medium but had been shown independently to be capable of growth on HAT selection. Following transfer of 105 cells from each sub-clonal line on to HPRT counter selection (6-TG supplemented medium) and BS selection (to maintain selective pressure for the MACs) most cells died, as would be expected if they were HPRT expressing. Each cell line produced a small set (60–200) of BS/6-TG resistant colonies that were pooled and analyzed by FISH. However, results of this analysis only revealed MAC rearrangement and/or MAC recombination with a host chromosome (data not shown). Confirmation that cells with epigenetically silenced HPRT genes were not present within the BS/6-TG resistant cell population was demonstrated by failure of viable colonies following replating in HAT selection. While the existence of MACs harboring both an active and epigenetically silenced HPRT transgene cannot be ruled out, their existence seems unlikely since MACs where both HPRT copies were silenced have not been identified thus far.
Discussion
This study has demonstrated that artificial chromosomes can be used to deliver a functional human HPRT gene into HPRT-deficient human cells. The HPRT transgene maintained stable expression under non-selective conditions from mitotically stable mini-chromosomes and complemented the parental HPRT deficiency. A simple co-transfection procedure using a circular PAC containing α-satellite DNA from human chromosome 21 as a centromere seeding component in the presence of a second circular PAC containing a human HPRT gene on a 140 kb genomic DNA fragment was used to incorporate the HPRT transgene into a MAC. Although MAC formation was not the sole fate of the transfected DNA and integrations were frequently detected, sub-clones could be isolated containing MACs in most cells that hybridized with both HPRT and α13/21 probes.
Two of the three sub-clonal lines were shown to contain highly mitotically stable MACs using FISH analysis of cells grown without selection. This suggests that the MACs have a functional centromere, a conclusion supported by the association of functionally important kinetochore proteins CENP-A and CENP-E on the MACs. The absence of telomere sequences on the artificial chromosomes points to a circular MAC structure as predicted since telomere sequences were not included on the input DNA. The HPRT-expressing MACs were estimated cytologically to be similar in size (1–10 Mb) to the MACs generated when PAC7c5 was introduced as the sole input DNA. Previous analysis indicated that PAC7c5-based MACs formed as a result of input DNA multimerization. Consistent with these findings we saw no evidence for acquisition of host telomere or α-satellite DNA on MACs harboring HPRT transgenes, indicating a mechanism of chromosome formation involving amplification/multimerization of the input PACs. Despite a stronger HPRT hybridization signal with an HPRT probe on the MAC relative to the endogenous gene, quantitation by Southern analysis indicated only an additional 1 or 2 unrearranged copies of the HPRT transgene on the MACs (data not shown) implying more extensive amplification of α21-I DNA and/or PAC sequences during chromosome formation.
Following transfection into the HPRT-deficient HT1080 cell line, colonies were selected for uptake of the α-satellite- containing PAC using BS selection to rule out possible influences on centromere/chromosome formation that may have occurred under positive selection conditions for HPRT expression. Sub-clonal lines with a high MAC content were subsequently passaged under non-selective conditions for either BS resistance or HPRT expression and HPRT message levels were assessed using northern hybridization. Since similar levels of HPRT expression were detected at the start of the time course and after 60 days off selection we concluded that the HPRT transgene was being stably expressed from the MACs. Cells from the 0 and 60 day time points were subsequently shown to grow on HAT selection medium, which phenotypically confirmed the production of HPRT protein, and under these conditions an increase in HPRT mRNA levels was detected. Since the existence of a sub-population of cells with epigenetically silenced transgenes was ruled out as an explanation for the lower HPRT message levels under non-selective conditions, the increased HPRT mRNA steady-state levels upon HAT selection could reflect the operation of a transcriptional or post-transcriptional regulatory feedback mechanism.
These results show that MACs can be used to introduce a human housekeeping gene into cultured human cells, which subsequently can be stably expressed and can lead to phenotypic correction of a human HPRT deficiency. Further challenges in the development of MAC vectors will be to demonstrate retention of appropriate tissue and temporal regulation of MAC-borne transgenes with more complex expression patterns. Also important will be to determine the permissive host range of the MAC formation process, as, to date, experiments have focused on defining the minimal requirements for generating chromosomes in the HT1080 cell line. In addition, however, we have now shown that MACs can form in HeLa cells using PAC7c5, indicating that the capacity to form MACs may be a more general property of cultured cells (B.R. Grimes and H.J. Cooke, unpublished data). The demonstration in this study that de novo circular chromosomes expressing a human gene can form without a requirement for telomerase is significant because it suggests that MACs may be generated in primary cell lines lacking telomerase. This could be important for gene therapy applications. If improved vectors permit the introduction of MAC-borne transgenes to a range of cell types and species, it seems possible that they may become a useful tool in the areas of gene therapy and biotechnology as well as increasing our understanding of the basic structure and function of chromosomes.
METHODS
Co-transfection of Pac7c5 and pH2c2 into HPRT-deficient HT1080 cells. PAC7c5 contains 70 kb of 21-I α-satellite DNA and has a BS resistance marker gene. pH2c2 (clone co-ordinate 71G4) was isolated from a human PCYPAC2N PAC library and contains a 140 kb insert spanning the human HPRT locus. PAC DNA was transfected into HPRT-deficient HT1080 cells maintained on non-selective HT medium [DMEM (Gibco-BRL)/10% calf serum (Hyclone); HT supplement: 10 µM hypoxanthine, 20 µM thymidine]. Co-transfection experiments were carried out using 2 µg each of PAC 7c5 and pH2c2 in the presence of Lipofectamine (Gibco-BRL). BS-resistant clones were picked after 10 days in 4 µg/ml BS (Blasticidin-S Hydrochloride, ICN Biochemicals)/HT medium.
FISH analysis and immunofluorescence. Metaphase chromosomes were analyzed by FISH using standard protocols with biotin or digoxigenin-labeled probes. The 13/21 α-satellite probe was p11-4 (Ikeno et al., 1994) and the HPRT probe was pH2c2. Telomere signals were detected using a direct FITC-labeled PNA telomere probe (Lansdorp et al., 1996). Centromere proteins were localized as described previously (Ebersole et al., 2000).
HPRT expression analysis. Cells were grown on either HT, HAT (HT as above supplemented with 9 µM aminopterin) or 6-TG (20 µM 6-thioguanine) supplemented DMEM media with or without BS selection as indicated. Total RNA was extracted using RNAZol B reagent (AMS Biotechnology). Ten micrograms of total RNA were loaded per lane, northern blotted and hybridized using standard protocols. The 0.5 kb HPRT probe was amplified from human cDNA with HPRT primers V241 (5′-CTTCCTCCTCCTGAGGAGTC-3′) and V242 (5′-CCTGACCAAGGAAAGCAAAG-3′), and the 0.5 kb human actin probe used as a loading control was amplified from human cDNA using the primers X710 (5′-GGCTACAGCTTCACCACCAC-3′) and X711 (5′-ACTCCTGCTTGCTGATCCAC-3′). Absence of HPRT message in the parental HPRT-deficient HT1080 cell line was confirmed by the failure to amplify a product from cDNA prepared from this line with V241 and V242 primers (data not shown).
Acknowledgments
ACKNOWLEDGEMENTS
We thank C. Farr (Cambridge University) for providing HPRT-deficient HT1080 cells, P. Lansdorp for the telemore probe (University of British Columbia, and Boston Probes Inc., Bedford, MA), T. Yen (Fox-Chase Cancer Institute) for anti-CENP-E antibody, B. Sullivan for advice with immunofluorescence protocols and R. Slee for comments on the manuscript. This work was supported by the UK MRC.
REFERENCES
- Bayne R.A., Broccoli, D., Taggart, M.H., Thomson, E.J., Farr, C.J. and Cooke, H.J. (1994) Sandwiching of a gene within 12 kb of a functional telomere and α-satellite does not result in silencing. Hum. Mol. Genet., 3, 539–546. [DOI] [PubMed] [Google Scholar]
- Ebersole T.A., Ross, A., Clark, E., McGill, N., Schindelhauer, D., Cooke, H. and Grimes, B. (2000) Mammalian artificial chromosome formation from circular alphoid input DNA does not require telomere repeats. Hum. Mol. Genet., 9, 1623–1631. [DOI] [PubMed] [Google Scholar]
- Farr C., Fantes, J., Goodfellow, P. and Cooke, H. (1992) Functional reintroduction of human telomeres into mammalian cells. Proc. Natl Acad. Sci. USA, 88, 7006–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festentein R., Tolaini, M., Corbella, P., Mamalaki, C., Parrington, J., Fox, M., Miliou, A., Jones, M. and Kioussis, D. (1996) Locus control region function and heterochromatin-induced position effect variegation. Science, 271, 1123–1125. [DOI] [PubMed] [Google Scholar]
- Fujimori S., Davidson, B.L., Kelley, W.N. and Palella, T.D. (1989) Identification of a single nucleotide change in the hypoxanthine-guanine phosphoribosyltransferase gene (HPRT) responsible for Lesch-Nyhan syndrome. J. Clin. Invest., 83, 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington J.J., Van Bokkelen, G., Mays, R.W., Gustashaw, K. and Willard, H.F. (1997) Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nature Genet., 15, 345–355. [DOI] [PubMed] [Google Scholar]
- Henning K.A., Novotny, E.A., Compton, S.T., Guan, X.Y., Liu, P.P. and Ashlock, M.A. (1999) Human artificial chromosomes generated by modification of a yeast artificial chromosome containing both human α-satellite and single-copy DNA sequences. Proc. Natl Acad. Sci. USA, 96, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno M., Masumoto, H. and Okazaki, T. (1994) Distribution of CENP-B boxes reflected in CREST centromere antigenic sites on long-range α-satellite DNA arrays of chromosome 21. Hum. Mol. Genet., 3, 1245–1257. [DOI] [PubMed] [Google Scholar]
- Ikeno M., Grimes, B., Okazaki, T., Nakano, M., Saitoh, K., Hoshino, H., McGill, N.I., Cooke, H. and Masumoto, H. (1998) Construction of YAC-based mammalian artificial chromosomes. Nature Biotechnol., 16, 431–439. [DOI] [PubMed] [Google Scholar]
- Karpen G.H. and Allshire, R.C. (1997) The case for epigenetic effects on centromere identity and function. Trends Genet., 13, 489–496. [DOI] [PubMed] [Google Scholar]
- Lansdorp P.M., Verwoerd, N.P., van de Rijke, F.M., Dragowska, V., Little, M.T., Dirks, R.W., Raap, A.K. and Tanke, H.J. (1996) Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet., 5, 685–691. [DOI] [PubMed] [Google Scholar]
- Lee C., Wevrick, R., Fisher, R.B., Ferguson-Smith, M.A. and Lin, C.C. (1997) Human centromeric DNAs. Hum. Genet., 100, 291–304. [DOI] [PubMed] [Google Scholar]
- Mejia J.E. and Larin, Z. (2000) The assembly of large BACs by in vivo recombination. Genomics, 70, 165–170. [DOI] [PubMed] [Google Scholar]
- Valdivia M.M., Figueroa, J., Iglesias, C. and Ortiz, M. (1998) A novel centromere monospecific serum to a human autoepitope on the histone H3-like protein CENP-A. FEBS Lett., 422, 5–9. [DOI] [PubMed] [Google Scholar]
- Yen T.J., Compton, D.A., Wise, D., Zinkowski, R.P., Brinkley, B.R., Earnshaw, W.C. and Cleveland, D.W. (1991) CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J., 10, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]