Abstract
Objective:
Impaired adipogenic differentiation exacerbates metabolic disease in obesity. We reported that high fat diet (HFD)-fed mice housed at thermoneutrality exhibited impaired adipogenic differentiation, attributed to increased expression of histone deacetylase 9 (HDAC9). However, the impact of HFD on adipogenic differentiation is reportedly variable, possibly reflecting divergent environmental conditions, such as housing temperature.
Methods:
C57bl/6J (WT) mice were housed at either thermoneutral (28–30°C) or ambient (20–22°C) temperature and fed HFD or chow diet (CD) for 12 weeks. For acute exposure experiments, WT or TRPM8 knockout mice housed under thermoneutrality were acutely exposed to ambient temperature for 6–24 hr.
Results:
WT mice fed HFD and housed at thermoneutrality, compared to ambient temperature, gained more weight despite reduced food intake. They likewise exhibited increased inguinal adipose tissue HDAC9 expression and reduced adipogenic differentiation in vitro and in vivo compared to CD-fed mice. Conversely, HFD fed mice housed at ambient temperature exhibited minimal change in adipose HDAC9 expression or adipogenic differentiation. Acute exposure of WT mice to ambient temperature reduced adipose HDAC9 gene expression independent of sympathetic β-adrenergic signaling via a TRPM8-dependent mechanism.
Conclusions:
Adipose HDAC9 expression is temperature sensitive, regulating adipogenic differentiation in HFD-fed mice housed under thermoneutrality.
Keywords: Housing temperature, HDAC9 and obesity
Introduction
Higher environmental temperature has been positively associated with prevalence of obesity and type 2 diabetes (1, 2, 3, 4). Humans and rodents are homeotherms, which are capable of expending energy to defend their core temperature (5). Accordingly, mice that are housed at ambient temperature (20–22°C), compared with thermoneutrality (28–30°C), exhibit increased energy expenditure, resistance to diet induced obesity (DIO) and protection against development of metabolic disease (6, 7).
In the context of obesity, adipose tissue can expand via adipocyte hyperplasia and/or hypertrophy. Hyperplasia, accomplished by differentiation of preadipocytes into new adipocytes, is considered a relatively healthy method to expand lipid storage capacity (8, 9). Alternatively, when pre-existing adipocytes hypertrophy, they become mechanically stressed, which promotes adipose tissue inflammation, fibrosis, insulin resistance and the development of type 2 diabetes (10, 11). Eventually, the capacity to store the excess energy in adipose tissues is exhausted, leading to ectopic fat deposition in liver, skeletal muscles, and other tissues, which further accelerates metabolic disease (12).
One factor that may contribute to adipose hypertrophy and metabolic disease in obesity is impairment of adipogenic differentiation. Indeed, evidence of impaired adipogenic differentiation has been detected in adipose tissues obtained from obese humans (13, 14). Moreover, we reported that diet induced obesity (DIO) in mice is associated with impaired in vivo and in vitro adipogenic differentiation in a cell-autonomous manner (15). Histone deacetylase 9 (HDAC9) is an epigenetic regulator that impedes adipogenic differentiation and has been implicated in the pathogenesis of obesity and other chronic diseases (16). We detected upregulated expression of HDAC9 in the subcutaneous adipose tissues of high fat diet fed mice housed at thermoneutrality, which was causally linked to impaired adipogenic differentiation (15, 17). In contrast to our findings, however, other investigators did not observe impaired adipogenic differentiation in mice fed a similar HFD (18, 19, 20). Here, we investigated the impact of housing temperature on HDAC9 expression and adipogenic differentiation in the context of DIO.
Methods
Animals
Protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia at Augusta University and complied with National Institute of Health guidelines. Six-week-old C57BL/6J male mice (#000664, Jackson Laboratories) were individually housed at thermoneutral temperature (28–30°C) or ambient temperature (20–22°C). Mice were assigned to experimental conditions at random. Mice were either maintained on CD (Teklad LM-485, Envigo) or switched to HFD (D12492, 60% calories from fat, Research Diets) for up to 12 weeks. Heterozygous TRPM8 global knockout (KO) mice (21) (#008198, Jackson Laboratories) were crossed to generate male WT and KO littermate mice. Mice were group housed and maintained on CD at thermoneutral temperature for 11–20 weeks. All procedures were performed at housing temperature, with exception of body composition analysis.
Acute ambient temperature exposure
Six-week-old C57BL/6J mice housed at thermoneutral temperature were placed in individual cages without bedding, with 2 toys for enrichment for 2 days. Cages were then transferred to the environmental chamber (Powers Scientific, Inc.) at 20–22°C for 6 or 24 hr or maintained at thermoneutral temperature. Mice were euthanized per protocol, and tissues were snap frozen.
Treatment of mice with pharmacological modulators of sympathetic activation
Thermoneutral-housed mice were treated with intraperitoneal injection of vehicle or propranolol at 10 mg/kg (Sigma Aldrich) and kept at thermoneutral temperature or subjected to ambient temperature for 6 hr, as described above. For sympathomimetic experiments, mice were housed at thermoneutrality for 2 weeks prior to a single intraperitoneal injection of vehicle or CL316243 at 5 mg/kg (Cayman Chemical). Mice were maintained at thermoneutrality for 6 hr post-injection and euthanized per protocol for tissue collection.
Topical treatment of mice with menthol
Twelve-week-old C57BL/6J thermoneutral single-housed mice were topically treated with 10% w/v menthol (Sigma-Aldrich) in Tween80 (Sigma-Aldrich) on the ventral surface using a cotton swab as previously described (22). Animals assigned to the control group were administered Tween80 vehicle. Mice were euthanized 1 hr after treatment.
Body fat composition, food intake, locomotor activity and energy expenditure measurements in mice
Body weights were obtained weekly throughout the duration of the studies as previously described (15). Body fat composition was measured following 10–14 weeks of CD or HFD feeding in mice using nuclear magnetic resonance (NMR) spectroscopy (Minispec LF90II, Bruker) per manufacturer’s instructions. NMR spectroscopy measurements were conducted at ambient temperature within a 30 min period to minimize exposure in thermoneutral-housed mice. Following 10–12 weeks of CD or HFD feeding, locomotor activity and energy expenditure measurements were determined using a comprehensive laboratory animal monitoring system (CLAMS, Columbus Instruments) adjusted to 21°C or 28°C for 3 days (>48 hr of acclimation, followed by 24 hr of measurement) per manufacturer’s instructions as previously described (23). Food efficiency was calculated as body weight gain (g) x 100/food intake (g). Food intake was measured in home cages and averaged over a 4-day period.
Quantitative PCR
Total RNA was extracted from whole adipose tissues using QIAzol lysis reagent and purified with RNeasy lipid tissue mini kit (Qiagen) per manufacturer’s protocol. Real-time quantification of mRNA was performed using Power SYBR® Green RNA-to-CT™ 1-Step Kit (Applied Biosystems). Primer sequences are listed in Table 1. Ct values were normalized to acidic ribosomal phosphoprotein P0 (Arbp) mRNA and the fold difference was calculated by the ΔΔCt method.
Table 1:
Primer sequences used for quantitative PCR
| Gene Name | Forward | Reverse |
|---|---|---|
| Arbp | AGCTGAAGCAAAGGAAGAGTCGGA | ACTTGGTTGCTTTGGCGGGATTAG |
| C/ebpɑ | AGAAGTCGGTGGACAAGAACAGCA | GCGTTGTTTGGCTTTATCTCGGCT |
| Fabp4 | ATGAAATCACCGCAGACGACAGGA | TGTGGTCGACTTTCCATCCCACTT |
| Hdac9 | AGGATGATGATGCCTGTGGTGGAT | GAGTTGTGCTTGATGCTGCCTTGT |
| Pparɣ | ACATAAAGTCCTTCCCGCTGACCA | AAATTCGGATGGCCACCTCTTTGC |
| Ucp1 | AAGCTGTGCGATGTCCATGT | AAGCCACAAACCCTTTGAAAA |
Isolation of preadipocytes and in vitro adipogenic differentiation
Preadipocytes were isolated from inguinal fat tissues of mice as previously described (15). Briefly, adipose tissues were minced, digested with collagenase type I (Worthington Biochemical Corporation), filtered, and centrifuged to separate floating mature adipocyte fraction (MAF) from the pelleted stromal vascular fraction (SVF). SVF pellets were resuspended, plated, and grown in preadipocyte growth medium (Cell Applications). The cells were differentiated in the presence of adipocyte differentiation medium (Cell Applications) as previously described (15).
Oil-Red O (ORO) staining
Neutral lipids, stained with ORO (Sigma-Aldrich), were measured as previously described (15). Briefly, cells were washed with PBS and fixed with 4% paraformaldehyde for 1 hr. Cells were washed with ddH2O and incubated with ORO working solution (3 parts 0.4% ORO stock solution: 2 parts ddH2O) for 10 min. After incubation, cells were washed with ddH2O, imaged under light microscopy, and optical density (520 nm) of ORO eluted with isopropanol was measured by.
Statistical Analysis
Unless otherwise stated, all statistical analysis was performed using GraphPad Prism version 9.4.1 for Windows (GraphPad Software, www.graphpad.com) and results expressed as mean ± SD. Differences between two groups were analyzed by unpaired two-tailed Student’s t-test. Welch’s correction was used for two sample comparison between groups with unequal variances. Multiple group datasets were evaluated for normality, and differences were analyzed by one-way ANOVA followed by Tukey post-hoc analysis. Multiple group datasets with repeated time point measurements were analyzed by two-way ANOVA. p values less than 0.05 were considered significant. For data of energy expenditure, a one-way analysis of covariance (ANCOVA) was performed using the Statistical Package for the Social Sciences (SPSS, version 29) software to assess the effect of the independent variable (housing temperature) on the dependent variable (24 hr energy expenditure) while controlling for a covariate (body weight).
Results
Thermoneutral housing exacerbates DIO in C57BL/6J mice
A protective effect of ambient temperature housing against weight gain in the DIO model is well documented in the literature (7, 24). However, the effects of housing temperature on adipogenic differentiation in the setting of DIO are unknown. We began by assessing body weight and food intake of mice fed either a HFD or CD and housed at thermoneutral (28°C) versus ambient (21°C) temperature. Consistent with previous reports (25, 26, 27), mice housed at thermoneutrality and fed a HFD for 10 weeks gained significantly more weight when compared to those housed at ambient temperature (Figure 1A), despite consuming similar amounts of food (Figure 1B) and exhibiting no difference in locomotor activity (Supplemental Figure S1A). Likewise, CD-fed mice housed at thermoneutrality weighed more than those housed at ambient temperature and fed the same diet (Figure 1A). Additionally, food efficiency was higher in HFD-fed mice housed at thermoneutrality compared with ambient temperature (Figure 1C). The increased body weights observed under thermoneutral conditions were attributed entirely to increases in fat mass in CD- or HFD-fed mice (Figure 1D). Ambient temperature housing did not alter respiratory exchange ratio in CD or HFD fed mice (Supplemental Figure S1B). As expected, energy expenditure was higher in both resting and non-resting periods in mice housed at ambient temperature, particularly in those fed HFD (Figure 1E). Even after controlling for body weight, housing temperature had a significant effect on 24 hr energy expenditure as assessed by ANCOVA analysis [F(3, 12)=34.934, p<0.001] (Supplemental Figure S2). Taken together, these findings point to a significant contribution of thermogenesis to energy expenditure in mice housed at ambient temperature, as previously reported (26, 27, 28).
Figure 1:
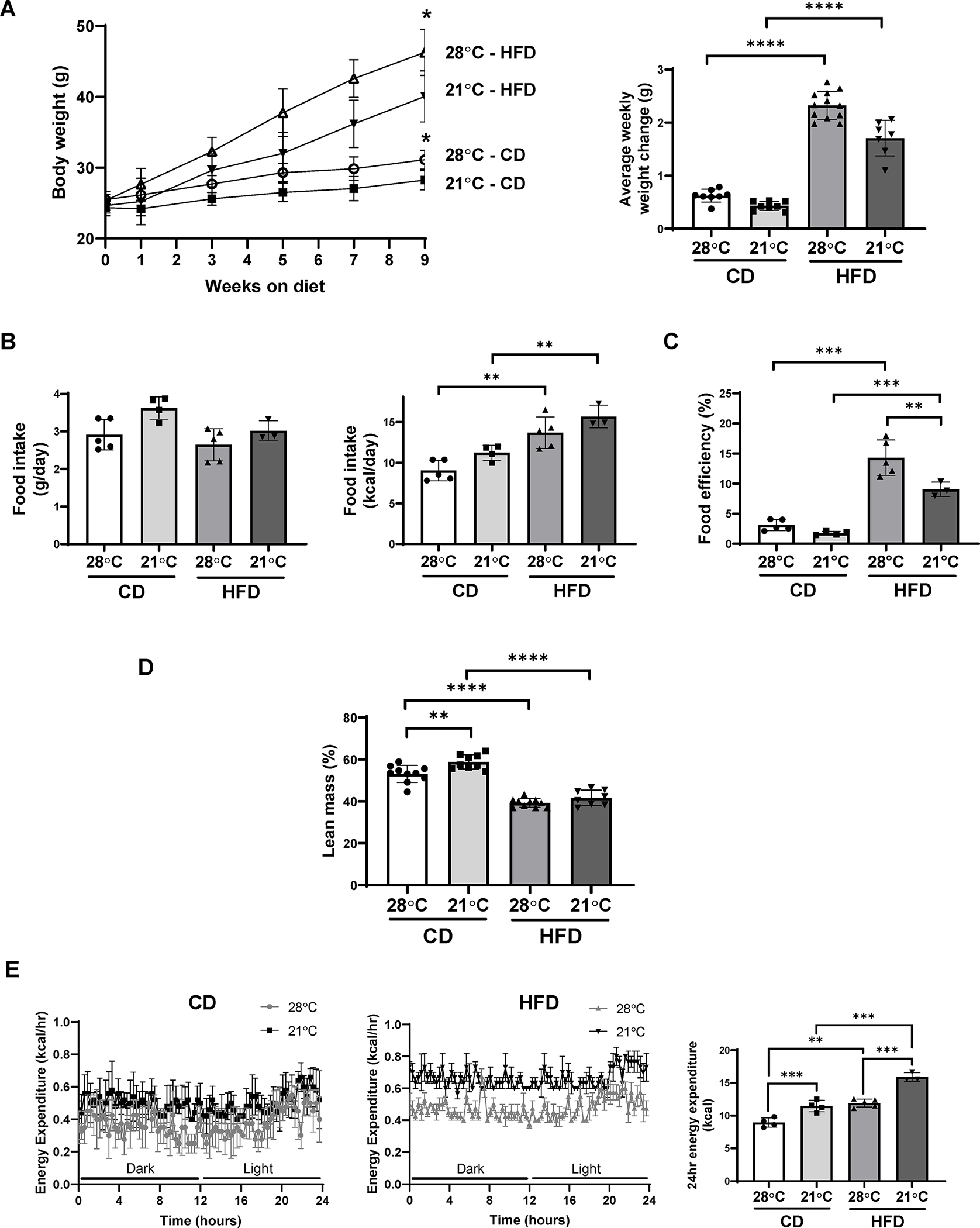
Thermoneutral housing exacerbates DIO in C57BL/6J mice. (A) Growth curves (left panel) and average weekly weight change (right panel) of mice fed either CD or HFD under ambient or thermoneutral housing (n=7–12). (B) Food intake presented as g/day/mouse (left panel) and kcal/day/mouse (right panel) in mice fed a CD or HFD under ambient or thermoneutral housing (n=3–5). (C) Food efficiency was calculated as body weight gain (g) x 100 / food intake (g) (n=3–5). (D) Fat mass (left panel) and lean mass (right panel) in mice fed a CD or HFD under ambient or thermoneutral housing as measured by whole body composition by NMR (n=8–10). (E) Energy expenditure over a 24 hr measurement period in mice fed a CD or HFD under ambient or thermoneutral housing. Data are represented as mean ± SD. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Differential effects of housing temperature on expression of adipogenic differentiation markers and HDAC9
We reported that HFD feeding for 12 weeks led to impaired adipogenic differentiation in subcutaneous adipose tissues in vivo and in vitro, in a cell-autonomous manner, in conjunction with increased expression of HDAC9, a negative regulator of adipogenic differentiation (15). In contrast, several studies of chronic HFD feeding in mice did not report impaired adipogenic differentiation (18, 19, 20). To test whether differences in housing temperature could explain these discrepant results, we compared parameters of adipogenic differentiation and HDAC9 expression in adipose tissues from CD and HFD-fed mice housed at thermoneutral versus ambient temperature (Figure 2). In inguinal adipose tissue of HFD versus CD-fed mice housed at thermoneutral temperature (compare columns 1 vs. 3), expression of adipogenic differentiation markers CCAAT/enhancer-binding protein alpha (C/ebpα) and fatty acid-binding protein 4 (Fabp4) was reduced, while peroxisome proliferator activated receptor gamma (Pparγ) trended toward reduced expression (Figure 2A). Notably, HDAC9 expression was markedly upregulated in inguinal adipose tissue of HFD compared to CD-fed mice housed at thermoneutral temperature (Figure 2A&2B). These findings are consistent with our previously published findings that implicated HDAC9 as a mediator of impaired adipogenic differentiation in HFD-fed mice housed at thermoneutral temperature (15, 17).
Figure 2:
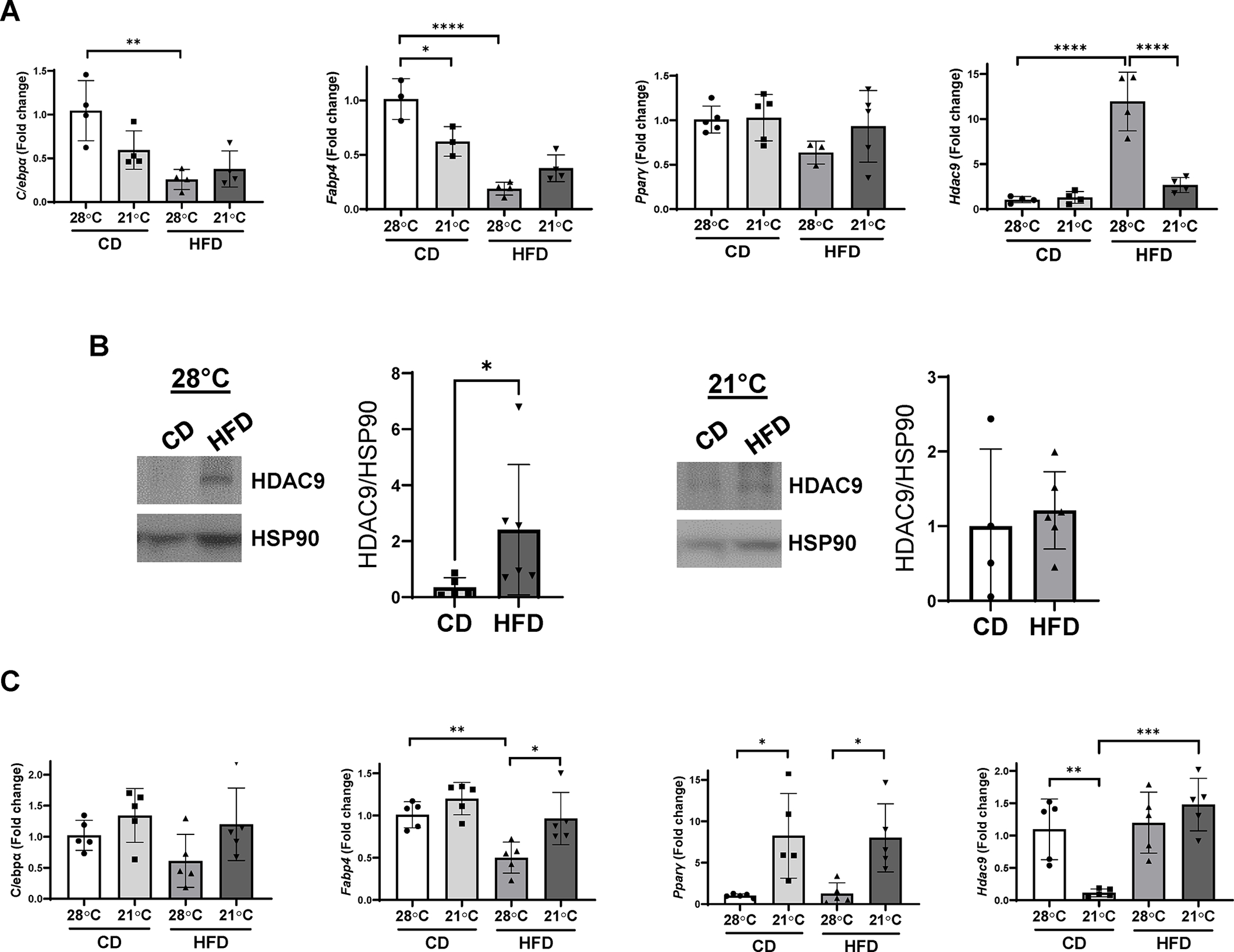
Differential effects of housing temperature on gene expression in inguinal and epididymal adipose tissue in CD and HFD fed mice. mRNA expression of Pparγ, C/ebpα, Fabp4 and Hdac9, shown as fold change indexed to CD fed mice housed at thermoneutral temperature in inguinal (A) and epididymal (C) adipose tissues (n=3–5). (B) Expression of HDAC9 protein in inguinal adipose tissue from mice housed at thermoneutral and ambient temperature. Data are represented as mean ± SD. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In contrast, in mice housed at ambient temperature, there was no significant change in C/ebpα or Pparγ, and ~50% reduction in Fabp4, in HFD versus CD-fed mice (compare columns 2 vs. 4, Figure 2A). Moreover, ambient temperature-housed HFD-fed mice exhibited minimal upregulation of inguinal adipose HDAC9 expression (Figure 2A&2B). Even when fed a HFD for 40 weeks, we were unable to demonstrate impairment of adipogenic differentiation in mice housed at ambient temperature (data not shown), suggesting that differences in body weight do not explain the discrepant findings in mice housed at ambient versus thermoneutral temperature. These findings suggest that housing temperature is a key determinant of HDAC9 expression in subcutaneous adipose tissue, which impacts adipogenic differentiation state in the setting of DIO.
We also examined expression of adipogenic genes and Hdac9 in epididymal adipose tissues. As was observed in inguinal adipose tissue, Fabp4 expression was lower in HFD-fed mice housed at thermoneutral compared to ambient temperature (Figure 2C). However, Pparγ expression was lower in thermoneutral- compared to ambient-housed mice, independent of the diet, while C/ebpα expression was variable, with no significant differences amongst the four groups. Notably, Hdac9 expression in epididymal adipose tissue was lower in CD-fed mice housed at ambient temperature as compared with the other three groups. These findings suggest that the relationship between temperature, HDAC9 expression and adipogenic differentiation is inconsistent in visceral adipose tissues.
Effects of housing temperature on in vitro adipogenic differentiation of preadipocytes isolated from HFD-fed mice
In thermoneutral-housed mice, chronic HFD feeding led to impaired in vitro differentiation of subcutaneous preadipocytes into mature adipocytes in a cell-autonomous manner (15). Moreover, this impaired differentiation phenotype persisted despite multiple passages in cell culture (15). Notably, HDAC9 expression was upregulated in adipose tissues of these mice, and deletion of Hdac9 prevented impairment of adipogenic differentiation in HFD-fed mice housed at thermoneutral temperature (15). Since, in the current study, our HFD-fed mice housed at ambient temperature exhibited preserved expression of adipogenic differentiation markers, and comparatively little upregulation of HDAC9 in inguinal adipose tissues, we examined the in vitro differentiation capacity of preadipocytes isolated from these mice. Notably, preadipocytes isolated from inguinal adipose tissues of HFD-fed mice housed at ambient temperature readily differentiated into mature adipocytes capable of efficiently forming lipid droplets, similar to their CD-fed counterparts (Figure 3A). Furthermore, similar levels of Pparγ and Fabp4 were observed in differentiating preadipocytes isolated from HFD and CD-fed ambient-housed mice (Figure 3B). Importantly, HDAC9 expression was virtually identical in the HFD and CD groups at day 0 and day 7 of differentiation (Figure 3C). In contrast, as we reported previously, mice maintained on HFD under thermoneutrality exhibited impaired in vitro adipogenic differentiation (15), as evidenced by markedly reduced lipid droplet accumulation compared to CD-fed mice (Figure 3A). These data point to the importance of housing temperature on regulation of HDAC9 expression and differentiation of preadipocytes from HFD-fed mice.
Figure 3:
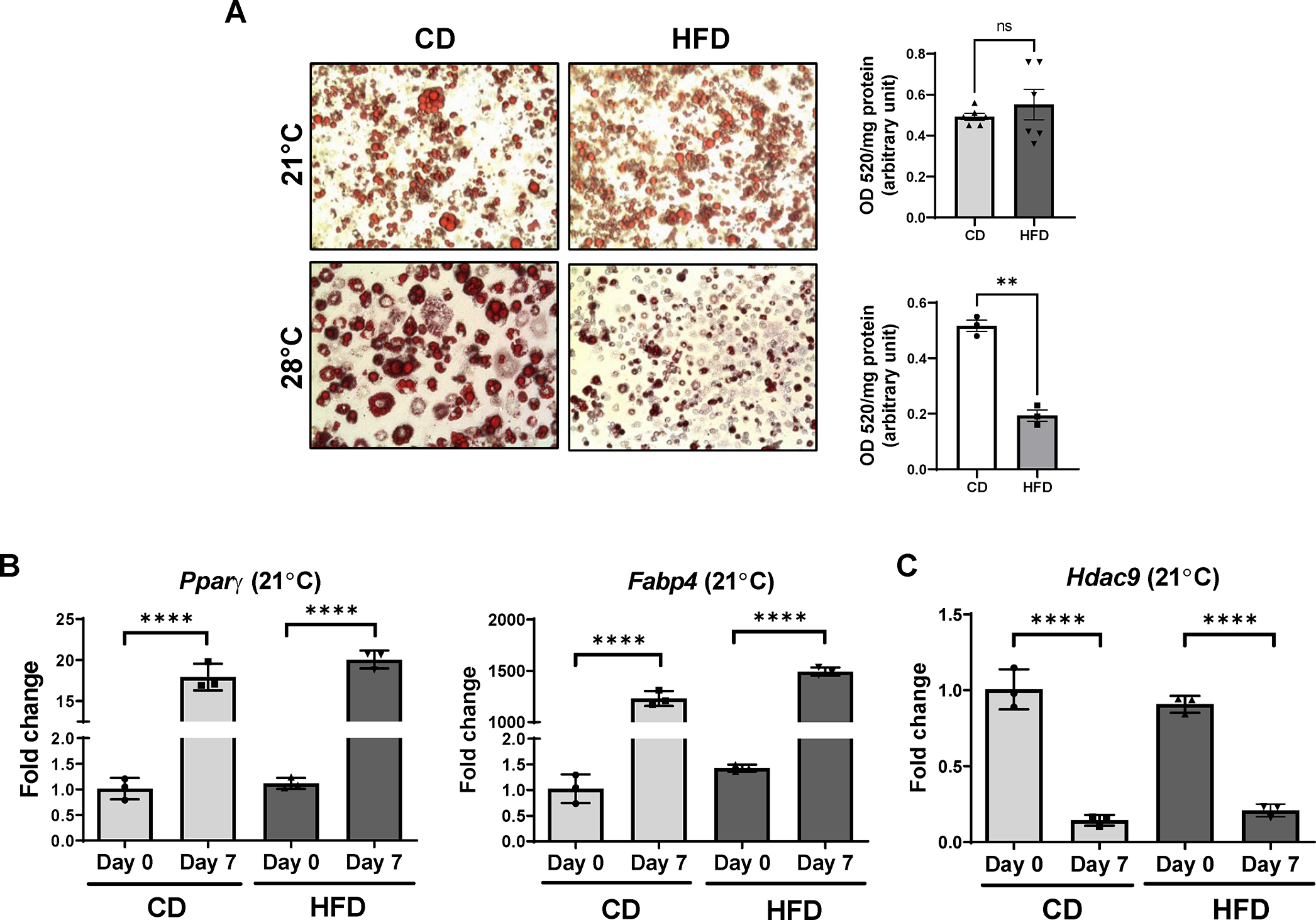
Impact of housing temperature on in vitro adipogenic differentiation of preadipocytes isolated from inguinal adipose tissues of mice. (A) Representative images of ORO staining (left panel) and quantification (OD520/mg protein, right panel, n=4–6). Primary preadipocytes isolated from inguinal fat of CD or HFD fed mice housed at ambient or thermoneutral temperature were differentiated into mature adipocytes for 7 days. (B) Effects of ambient housing on mRNA expression of adipogenic differentiation-specific genes (Pparγ, Fabp4) at day 0 and day 7 of in vitro adipogenic differentiation (n=3). (C) Expression of Hdac9 at day 0 and day 7 of in vitro adipogenic differentiation (n=3). Data are represented as mean ± SD. **p<0.01, ****p<0.0001.
Acute ambient temperature exposure selectively downregulates HDAC9 expression in adipose tissues
The above data suggest that ambient temperature housing prevented Hdac9 upregulation in inguinal adipose tissues, which in turn alleviated impairment of adipogenic differentiation in HFD-fed mice. Subsequently, we tested the impact of acute ambient temperature exposure on Hdac9 expression in adipose tissues. CD-fed mice were moved from their thermoneutral housing to 21°C for 6 or 24 hr, after which they were euthanized, and tissues collected for measurement of Hdac9 expression. We detected a reduction in Hdac9 expression in inguinal adipose tissue within 6 hr of ambient temperature exposure (Figure 4A), with no significant changes in expression noted in epididymal adipose tissue (Figure 4B) or skeletal muscle (Figure 4C); Hdac9 expression in brown adipose tissue (BAT) was more variable but also significantly reduced at the 6 hr time point (Figure 4D). As expected, expression of uncoupling protein 1 (Ucp1) was upregulated in inguinal adipose tissue (Figure 4E) and BAT (Figure 4F) at the 24 hr time point. To determine whether the impact of acute exposure to ambient temperature was specific for Hdac9 in inguinal adipose tissues, we examined expression of all 11 members of the HDAC family. None of the other HDAC family members was downregulated in adipose tissues following 6 hr ambient temperature exposure (Figure 4G).
Figure 4:
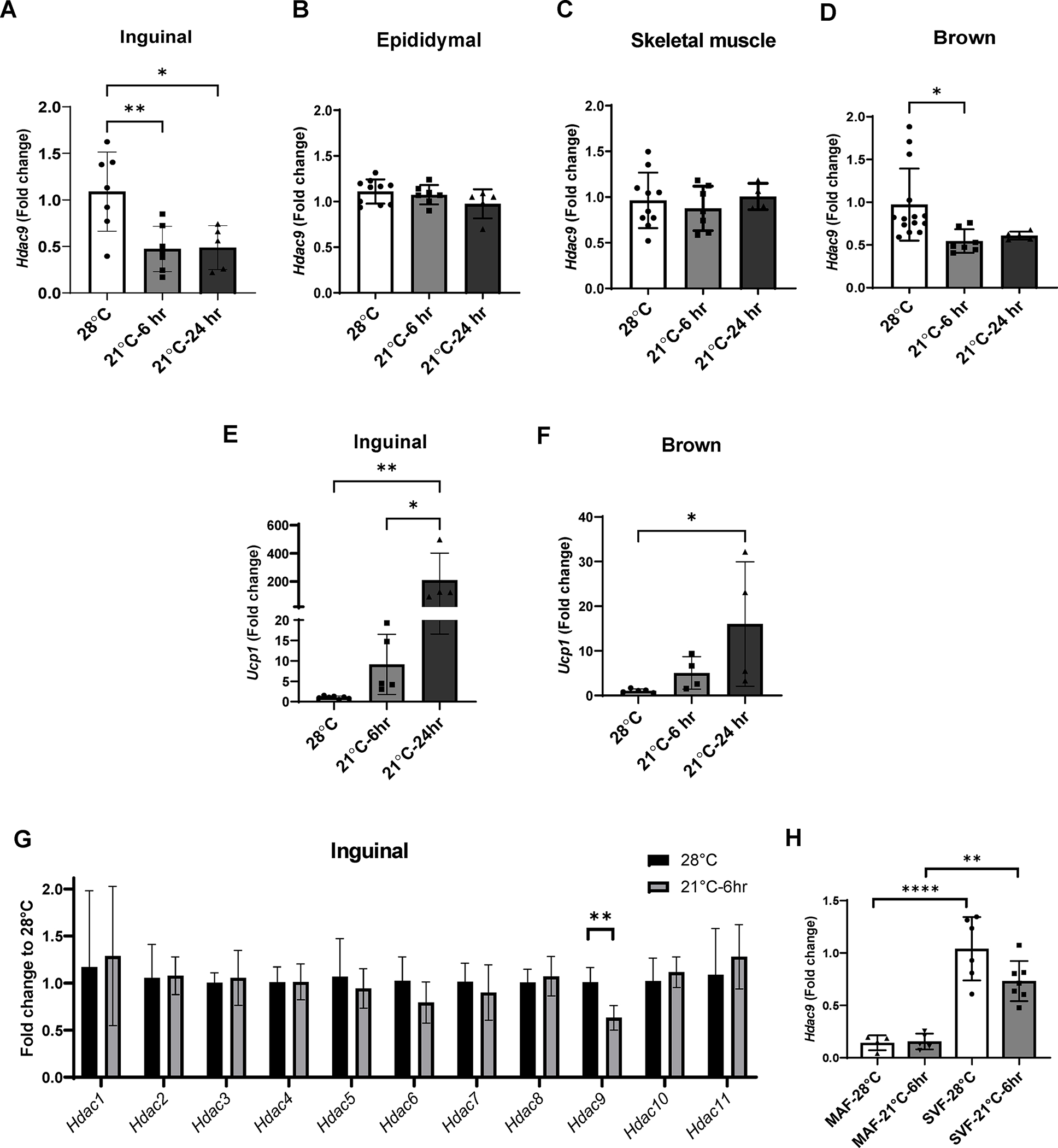
Acute ambient temperature exposure selectively downregulates Hdac9 expression in adipose tissues. (A-D) Effects of acute ambient exposure on Hdac9 mRNA expression in inguinal fat (A), epididymal fat (B), skeletal muscle (C) and brown fat (D) of CD fed mice housed at thermoneutral temperature (n=4–8). (E, F) Effects of acute ambient exposure on Ucp1 mRNA expression in inguinal (E) and brown (F) fat (n=4–7). (G) Effects of 6 hr ambient exposure on mRNA expression of Hdac family members (n=6–7). (H) Effects of 6 hr ambient exposure on expression of Hdac9 mRNA in the MAF and SVF of inguinal adipose tissue from CD fed mice (represented as fold change to SVF, n=5–7). Data are represented as mean ± SD. *p<0.05, **p<0.01, ****p<0.001.
We previously reported that HDAC9 is selectively downregulated during in vitro adipogenic differentiation of preadipocytes (17). Therefore, we collected inguinal adipose tissue from mice exposed to 21°C for 6 hr, or kept at thermoneutral temperature, and separated into the MAF and SVF, which is enriched in preadipocytes. While acute exposure to 21°C for 6 hr did not change Hdac9 mRNA expression in the MAF (Figure 4H), expression in the SVF trended lower (Figure 4H), although the data were variable and not statistically significant. These findings suggest that moderate changes in environmental temperature can selectively modulate HDAC9 expression in adipose tissue, which may promote adipogenic differentiation and healthy metabolic responses to obesity.
Effects of pharmacological modulators of sympathetic activation on adipose tissue HDAC9 expression
Cold exposure in mammals is associated with activation of the sympathetic nervous system, which has biological effects on adipose tissues through β3-adrenergic receptors to promote thermogenesis. Thus, we conducted experiments to investigate the potential role of adrenergic signaling in adipose HDAC9 following ambient temperature exposure. First, we treated thermoneutral-housed mice intraperitoneally with a selective β3-adrenergic receptor agonist, CL316243. As with exposure to ambient temperature, CL316243 downregulated Hdac9 in inguinal adipose tissues (Figure 5A); however, it also downregulated Hdac9 in epididymal fat (Figure 5B), and there was a strong trend towards downregulation in brown fat (Figure 5C). Moreover, unlike ambient temperature exposure, CL316243 indiscriminately downregulated multiple HDAC family members in inguinal fat (Figure 5D). Next, we tested the effect of treatment with propranolol, a non-selective β-adrenergic receptor antagonist, on ambient temperature-induced downregulation of Hdac9 in inguinal adipose tissues. Propranolol pretreatment was unable to prevent the downregulation of Hdac9 in mice exposed to ambient temperature (Figure 5E), although it completely blocked upregulation of Ucp1 (Figure 5F). Together, these results suggest that transient exposure to ambient temperature did not promote downregulation of Hdac9 expression in adipose tissues through β-adrenergic signaling.
Figure 5:
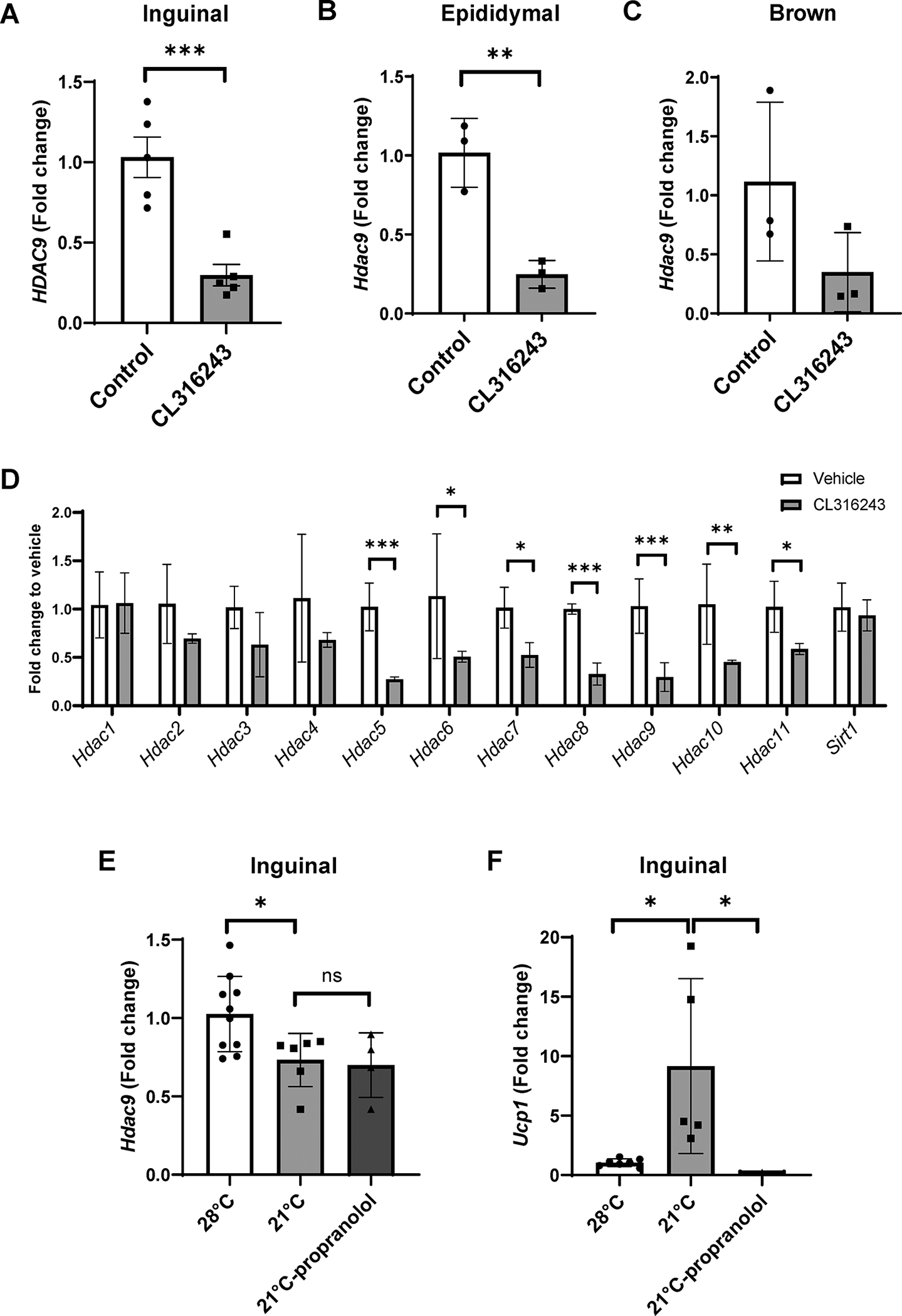
Effects of pharmacological modulators of sympathetic activation on adipose tissue Hdac9 expression. (A-C) Effects of intraperitoneal injection of CL316243 on adipose Hdac9 mRNA expression in inguinal (A), epididymal (B) and brown (C) adipose tissue of mice housed at thermoneutrality (n=3–5). (D) Effects of CL316243 on expression of Hdac family members (n=3). (E, F) Effects of intraperitoneal injection of propranolol on Hdac9 mRNA expression (E) and Ucp1 induction (F) in inguinal fat following 6 hr ambient temperature exposure (n=3–10). Data are represented as mean ± SD. *p<0.05, **p<0.01, ***p<0.001.
Activation of cold-sensitive TRPM8 channels downregulates Hdac9 expression in adipose tissue
Cold exposure (8–26°C) and compounds such as menthol activate transient receptor potential cation channel subfamily M member 8 (TRPM8) channels (21, 29). Activation of TRPM8 channels by topical menthol administration has previously been shown to increase thermogenesis and reduce weight gain in HFD-fed mice (30, 31, 32). To test the role of TRPM8 channels on HDAC expression in adipose tissue, we treated thermoneutral-housed CD-fed mice with topical menthol. Similar to the effect of 6 hr ambient temperature exposure (Figure 4C), Hdac9 was selectively downregulated in inguinal adipose tissue following menthol treatment (Figure 6A), suggesting that activation of TRPM8 channels may lead to the downregulation of Hdac9 in adipose tissue. Therefore, we subjected WT and TRPM8 KO mice to acute ambient temperature exposure. Ucp1 was significantly upregulated in inguinal (Figure 6B), brown (Figure 6C) and epididymal fat (Figure 6D) of WT mice following acute ambient temperature exposure. Ucp1 was also upregulated in brown fat of TRPM8 KO mice, and there were strong trends towards upregulation in inguinal and epididymal adipose tissues of these mice. However, TRPM8 gene deletion completely prevented the downregulation of Hdac9 in inguinal (Figure 6E) and brown (Figure 6F) adipose tissues following acute ambient temperature exposure. These data suggest that TRPM8 channels are obligatory for downregulation of adipose Hdac9 expression in response to short-term ambient temperature exposure.
Figure 6:
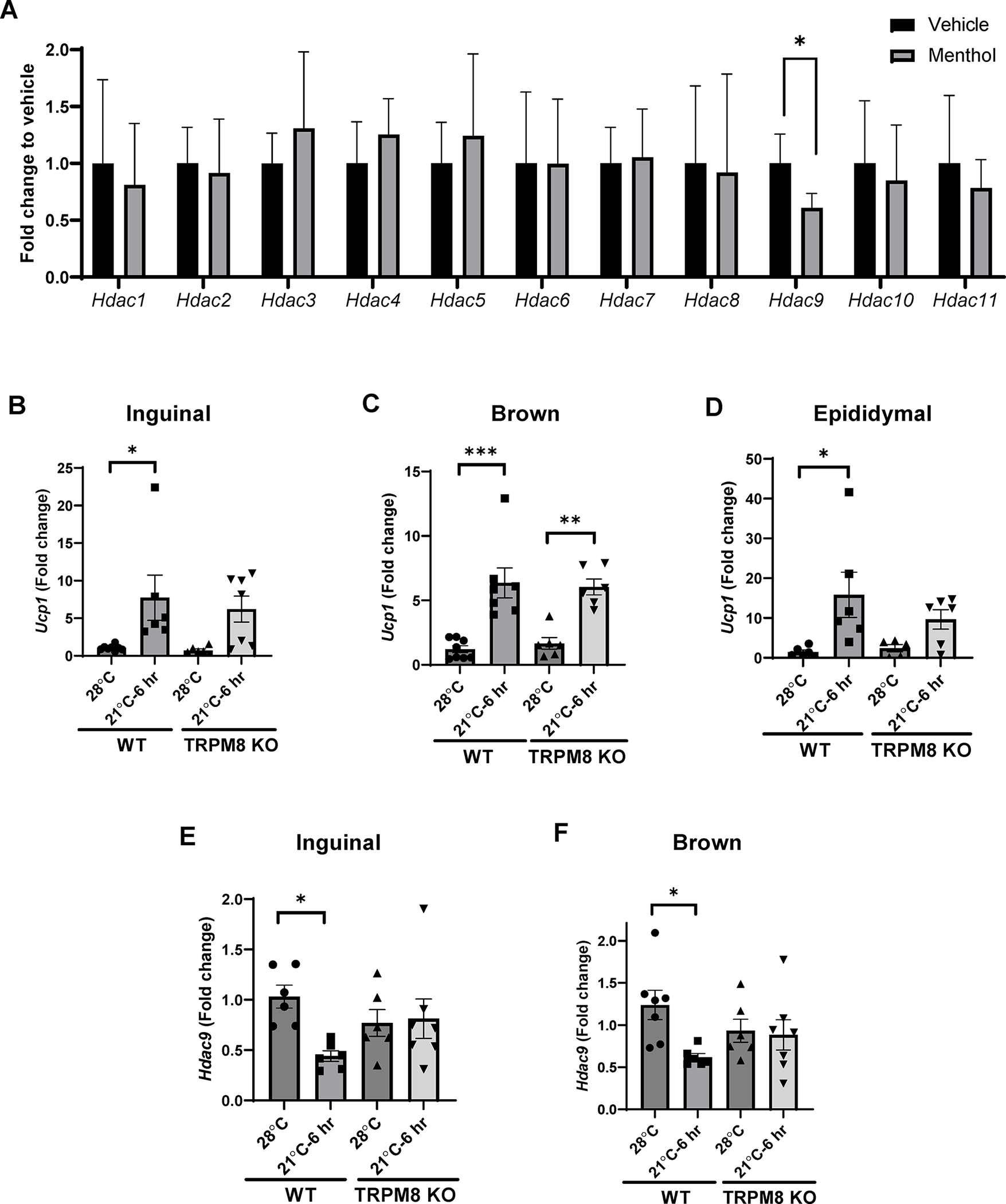
Acute ambient temperature exposure downregulates Hdac9 expression in inguinal and brown adipose tissue via activation of TRPM8 channels. (A) Effects of 1 hr topical menthol exposure on mRNA expression of Hdac family members compared to vehicle (n=4–5). (B-D) Effects of acute ambient exposure on Ucp1 in inguinal (B), brown (C) and epididymal (D) adipose tissue of WT and TRPM8 KO mice. (E, F) Hdac9 mRNA expression in inguinal (E) and brown (F) adipose tissues of WT and TRPM8 KO mice (n=4–7). Data are represented as mean ± SD. *p<0.05, ***p<0.001.
Discussion
There are four major findings in this study. First, we demonstrate that chronic HFD impairs adipogenic differentiation in inguinal fat of mice housed at thermoneutral, but not ambient, temperature. The impaired adipogenic differentiation observed in thermoneutral housed HFD-fed mice appears to be cell-autonomous and dependent on upregulated expression of HDAC9, as indicated by our prior publication. Second, brief exposure to ambient temperature selectively downregulates HDAC9 expression in inguinal and brown adipose tissues, without affecting gene expression of other HDAC family members. Third, the effects of ambient temperature exposure on Hdac9 expression in inguinal adipose tissues are independent of β-adrenergic receptor activation. Finally, we demonstrate that TRPM8 channels are required for downregulation of Hdac9 in response to acute ambient temperature exposure. Collectively, these findings have important implications regarding how environmental temperature modulates obesity-related metabolic disease.
Mounting evidence points to the importance of controlling housing temperatures to mimic human physiology in studies of metabolic disease (33, 34, 35), as humans typically live in climate-controlled environments, akin to thermoneutral housing in mice. The thermoneutral zone is defined as the temperature range at which mice maintain normal body temperature without expending energy above the basal metabolic rate, usually considered 28–32°C (33). However, most vivarium set housing temperature at 20–22°C, below the thermoneutral range, which causes mice to utilize energy to maintain homeostasis. Despite similar or greater caloric consumption, ambient temperature-housed mice gain significantly less weight and have lower total body adiposity when compared to thermoneutral-housed mice. Conversely, thermoneutral housing has been associated with impaired insulin sensitivity and glucose tolerance, altered immune responses and adipokine release, and a higher burden of atherosclerotic disease (26). However, the mechanisms by which thermoneutral housing mediates these pathophysiologic changes are largely unknown.
The findings of this study provide important insight into the potentially detrimental effects of thermoneutral housing on metabolic health in the context of HFD. We report that thermoneutral housing dramatically upregulates HDAC9 in inguinal adipose tissues of HFD-fed mice, in association with impaired adipogenic differentiation and reduced energy expenditure. The impact of housing temperature and diet on HDAC9 expression and adipogenic differentiation in epididymal adipose tissues was less consistent. Nevertheless, adipogenic differentiation of preadipocytes derived from human subcutaneous adipose tissue has been negatively correlated with body mass index (BMI) (36). Accordingly, we recently showed that BMI positively correlates with subcutaneous adipose tissue HDAC9 expression in humans, who typically live in climate-controlled environments (37), suggesting that our findings are potentially translationally relevant.
To begin to address the mechanisms whereby environmental temperature regulates HDAC9 expression, we performed short-term experiments wherein CD-fed mice were transferred from a thermoneutral to an ambient temperature environment. Interestingly, we detected selective downregulation of Hdac9 in inguinal adipose tissues within 6 hr of ambient temperature exposure. Rodents exposed to acute cold stress respond with activation of the sympathetic nervous system, which in turn leads to stimulation of adrenergic receptors on adipocytes. Thus, we investigated whether acute ambient temperature exposure downregulates Hdac9 through β-adrenergic activation. Mice injected with selective β3-adrenergic agonist, CL316243, at thermoneutrality exhibited downregulation of adipose Hdac9, as was observed with transient exposure to ambient temperature. However, unlike ambient temperature exposure, CL316243 downregulated numerous HDAC family members in inguinal fat. Furthermore, non-selective β-adrenergic receptor antagonist, propranolol, blocked induction of Ucp1 but was unable to prevent downregulation of adipose Hdac9 expression in response to ambient temperature exposure, suggesting that short-term ambient temperature exposure does not downregulate adipose HDAC9 expression via β-adrenergic receptor signaling. In contrast, the effects of ambient temperature exposure on Hdac9 expression in inguinal adipose tissues were mimicked by the TRPM8 agonist menthol and blocked by global TRPM8 gene deletion. These findings suggest that TRPM8 is required for downregulation of HDAC9 in adipose tissues following short-term ambient temperature exposure. How long-term exposure to ranges of environmental temperatures affects adipose HDAC9 expression is unknown and will require further investigation. Interestingly, impaired adipogenic differentiation in preadipocytes from long-term thermoneutral-housed HFD-fed mice was maintained after days in culture, in conjunction with upregulated HDAC9 expression (15). This raises the possibility of epigenetic alterations in HDAC9 expression that impede adipogenic differentiation capacity of preadipocytes during long-term thermoneutral housing and high fat feeding. Roh et al., previously demonstrated that adipocytes can maintain epigenomic memory of cold exposure (38), supporting a potential long-term effect of housing temperature on adipocyte function via epigenetic alterations. Further studies are required to identify the molecular mechanisms and signaling pathways that underlie dysregulated HDAC9 expression and adipogenic differentiation under such conditions.
In our previous study, we observed that global HDAC9 KO mice fed a CD and maintained in a thermoneutral environment were leaner than their wild-type counterparts (15). Moreover, HDAC9 KO mice exhibited higher basal metabolic rate and body temperature, and they were more proficient at adaptive thermogenesis. These findings were attributed in part to increased “beiging” of subcutaneous adipose tissues. It is tempting to speculate that adipose HDAC9 may function as a metabolic switch: in cooler temperatures, diminished adipose HDAC9 levels favor energy expenditure to promote thermogenesis, whereas in warmer temperatures, when environmental temperature is optimal for comfort and food is abundant, increased HDAC9 levels promote energy storage. The latter is more akin to current human living conditions, suggesting that adipose HDAC9 upregulation might contribute to impaired adipogenic differentiation and metabolic disease in human obesity.
There are several limitations in this study. First, we only used male mice, and the data cannot be extrapolated to females. In a prior study, male mice exhibited greater weight gain than female mice when housed at thermoneutrality and fed a high fat, high sucrose diet (39), suggesting sexual dimorphism in metabolic adaptation to housing temperature and diet composition. Second, the chow diet used in this study was sourced from a different manufacturer and thus was not precisely formulated as a control for the HFD. Third, we did not investigate the mechanism(s) of increased energy expenditure in mice housed at ambient temperature (i.e., UCP-1 dependent versus independent) (40), or its dependence on HDAC9 expression. Fourth, the absolute dose of menthol topically applied to the mice in the acute TRPM8 activation experiment cannot be quantified. Finally, whether TRPM8 expressed in sensory neurons versus adipocytes or preadipocytes regulates HDAC9 expression, and how this impacts energy expenditure, remains to be determined.
In summary, we report that HFD-fed mice housed chronically at thermoneutral temperature, in contrast to those housed at ambient temperature, exhibit impairment of adipogenic differentiation in inguinal adipose tissue. These findings are likely due, at least in part, to effects of housing temperature on adipose HDAC9 expression. Mechanistically, inguinal adipose Hdac9 expression is selectively downregulated following acute ambient temperature exposure, independent of sympathetic activation, but contingent on TRPM8 channels. Our findings may have important implications for the impact of environmental temperature on obesity and metabolic disease.
Supplementary Material
What is already known?
Impaired adipogenic differentiation has been reported in obese humans but inconsistently in mouse models of obesity.
Mice housed at thermoneutrality develop more severe obesity and metabolic disease as compared to mice housed at ambient temperature.
What are the new findings?
Impairment of adipogenic differentiation, in association with upregulated expression of adipose histone deacetylase 9 (HDAC9), occurs in inguinal adipose tissues of high fat fed mice housed at thermoneutrality (28–30°C) but not ambient (20–22°C) temperature.
Acute ambient temperature exposure or topical menthol treatment selectively downregulates HDAC9 in inguinal adipose tissues of wild type (WT) mice, which is abolished by deletion of TRPM8 channels.
How might these results change the direction of research of the focus of clinical practice?
This study emphasizes the impact of modest changes in environmental temperature on adipose gene expression, adipogenic differentiation and diet-induced obesity.
Exploiting the temperature sensitivity of HDAC9 in adipose tissues may provide a therapeutic avenue for improving metabolic health in obesity.
Funding:
This study was funded by grants AG076235 (NIH), 971459 and 863622 (AHA).
Footnotes
The authors declared no conflict of interest.
References
- 1.Voss JD, Masuoka P, Webber BJ, Scher AI, Atkinson RL. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int J Obes (Lond) 2013;37: 1407–1412. [DOI] [PubMed] [Google Scholar]
- 2.Speakman JR, Heidari-Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Sci Rep 2016;6: 30409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HK, Han K, Cho JH, Yoon KH, Cha BY, Lee SH. Ambient Temperature and Prevalence of Obesity: A Nationwide Population-Based Study in Korea. PLoS One 2015;10: e0141724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson F, Mavrogianni A, Ucci M, Vidal-Puig A, Wardle J. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes Rev 2011;12: 543–551. [DOI] [PubMed] [Google Scholar]
- 5.Moellering DR, Smith DL Jr. Ambient Temperature and Obesity. Curr Obes Rep 2012;1: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol 2015;6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitman ML. Of mice and men - environmental temperature, body temperature, and treatment of obesity. FEBS Lett 2018;592: 2098–2107. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab 2015;26: 193–200. [DOI] [PubMed] [Google Scholar]
- 9.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 2019;129: 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS One 2014;9: e105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muir LA, Neeley CK, Meyer KA, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring) 2016;24: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol Rev 2018;98: 1911–1941. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Jansson PA, Nagaev I, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun 2004;317: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee TK, Basford JE, Knoll E, et al. HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes 2014;63: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Cho EH, Lee JY. Histone Deacetylase 9: Its Role in the Pathogenesis of Diabetes and Other Chronic Diseases. Diabetes Metab J 2020;44: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee TK, Idelman G, Blanco V, et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. JBC 2011;286: 27836–27847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan JT, McLennan SV, Williams PF, et al. Connective tissue growth factor/CCN-2 is upregulated in epididymal and subcutaneous fat depots in a dietary-induced obesity model. Am J Physiol Endocrinol Metab 2013;304: E1291–1302. [DOI] [PubMed] [Google Scholar]
- 19.Shen L, Li Q, Wang J, et al. miR-144–3p Promotes Adipogenesis Through Releasing C/EBPalpha From Klf3 and CtBP2. Front Genet 2018;9: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Chen X, Qi T, et al. HSPA12A is required for adipocyte differentiation and diet-induced obesity through a positive feedback regulation with PPARgamma. Cell Death Differ 2019;26: 2253–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448: 204–208. [DOI] [PubMed] [Google Scholar]
- 22.Khare P, Mangal P, Baboota RK, et al. Involvement of Glucagon in Preventive Effect of Menthol Against High Fat Diet Induced Obesity in Mice. Front Pharmacol 2018;9: 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster GI, Henstridge DC. Body Composition and Metabolic Caging Analysis in High Fat Fed Mice. J Vis Exp 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastias-Perez M, Zagmutt S, Soler-Vazquez MC, Serra D, Mera P, Herrero L. Impact of Adaptive Thermogenesis in Mice on the Treatment of Obesity. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth LA, Trammell RA, Ilsley-Woods M. Interactions Between Housing Density and Ambient Temperature in the Cage Environment: Effects on Mouse Physiology and Behavior. J Am Assoc Lab Anim Sci 2015;54: 708–717. [PMC free article] [PubMed] [Google Scholar]
- 26.Giles DA, Ramkhelawon B, Donelan EM, et al. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab 2016;5: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui X, Nguyen NL, Zarebidaki E, et al. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol Rep 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 2011;214: 242–253. [DOI] [PubMed] [Google Scholar]
- 29.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell 2002;108: 705–715. [DOI] [PubMed] [Google Scholar]
- 30.McKie GL, Medak KD, Shamshoum H, Wright DC. Topical application of the pharmacological cold mimetic menthol stimulates brown adipose tissue thermogenesis through a TRPM8, UCP1, and norepinephrine dependent mechanism in mice housed at thermoneutrality. FASEB J 2022;36: e22205. [DOI] [PubMed] [Google Scholar]
- 31.Khare P, Chauhan A, Kumar V, et al. Bioavailable Menthol (Transient Receptor Potential Melastatin-8 Agonist) Induces Energy Expending Phenotype in Differentiating Adipocytes. Cells 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma S, Yu H, Zhao Z, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol 2012;4: 88–96. [DOI] [PubMed] [Google Scholar]
- 33.Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 2017;13: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankenson FC, Marx JO, Gordon CJ, David JM. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp Med 2018;68: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Yavar Z, Sun Q. Cardiovascular response to thermoregulatory challenges. Am J Physiol Heart Circ Physiol 2015;309: H1793–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009;58: 1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goo B, Ahmadieh S, Zarzour A, et al. Sex-Dependent Role of Adipose Tissue HDAC9 in Diet-Induced Obesity and Metabolic Dysfunction. Cells 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roh HC, Tsai LTY, Shao M, et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab 2018;27: 1121–1137 e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris EM, Noland RD, Allen JA, et al. Difference in Housing Temperature-Induced Energy Expenditure Elicits Sex-Specific Diet-Induced Metabolic Adaptations in Mice. Obesity (Silver Spring) 2020;28: 1922–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikeda K, Yamada T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front Endocrinol (Lausanne) 2020;11: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


