Abstract
Background and Aims:
While depletion of neuronal nitric oxide synthase (NOS1)-expressing neurons contributes to gastroparesis, stimulating nitrergic signaling is not an effective therapy. We investigated whether hypoxia-inducible factor 1α (HIF1A), which is activated by high O2 consumption in central neurons, is a Nos1 transcription factor in enteric neurons and whether stabilizing HIF1A reverses gastroparesis.
Methods:
Streptozotocin-diabetic mice, human and mouse tissues, NOS1+ mouse neuroblastoma cells, and isolated nitrergic neurons were studied. Gastric emptying of solids and volumes were determined by breath test and single-photon emission computed tomography, respectively. Gene expression was analyzed by RNA-sequencing, microarrays, immunoblotting, and immunofluorescence. Epigenetic assays included chromatin immunoprecipitation-sequencing (13 targets), chromosome conformation capture-sequencing, and reporter assays. Mechanistic studies utilized Cre-mediated recombination, RNA interference, and CRISPR-Cas9-mediated epigenome editing.
Results:
HIF1A signaling from physiological intracellular hypoxia was active in mouse and human NOS1+ myenteric neurons but reduced in diabetes. Deleting Hif1a in Nos1-expressing neurons reduced NOS1 protein by 50–92% and delayed gastric emptying of solids in female but not male mice. Stabilizing HIF1A with FG-4592/roxadustat, which is approved for human use, restored NOS1 and reversed gastroparesis in female diabetic mice. In nitrergic neurons, HIF1A upregulated Nos1 transcription by binding and activating proximal and distal cis-regulatory elements including newly discovered super-enhancers, facilitating RNA polymerase loading and pause-release, and by recruiting cohesin to loop anchors to alter chromosome topology.
Conclusion:
Pharmacological HIF1A stabilization is a novel, translatable approach to restoring nitrergic signaling and treating diabetic gastroparesis. The newly recognized effects of HIF1A on chromosome topology may provide insights into physioxia- and ischemia-related organ function.
Keywords: physioxia, Hypoxyprobe, RAD21, CTCF
Lay Summary
This study identifies a transcription factor that is required for the normal production of the main inhibitory neurotransmitter in the gastrointestinal tract and demonstrates that increasing its levels using a drug that is already approved for humans can reverse diabetic gastroparesis in female mice.
Graphical Abstract
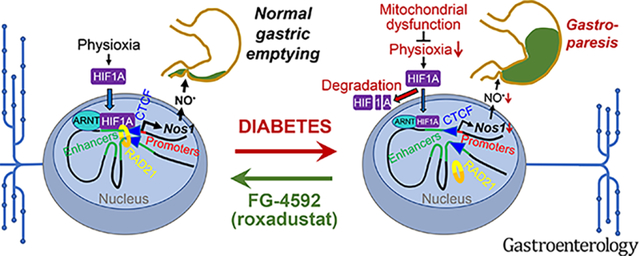
Introduction
Released by enteric neurons expressing neuronal nitric oxide synthase (NOS1), the inhibitory non-adrenergic, non-cholinergic neurotransmitter, nitric oxide (NO) regulates relaxation of the lower esophageal sphincter, gastric accommodation, and gastric emptying (GE).1,2
NOS1+ neurons are preferentially depleted in gastroparesis (GP),3–5 which is characterized by upper gastrointestinal symptoms and delayed GE without mechanical obstruction.6 GP is approximately four times more common in women than men.7 Delayed GE may be asymptomatic, accompanied by mild to moderate symptoms of dyspepsia, or with severe symptoms, nutritional compromise, anxiety, depression, and poor quality of life, which is referred to as gastroparesis.7 GP is most frequently associated with and impairs glycemic control in diabetes mellitus (DM).5,7 Loss of nitrergic neurons, due in part to repressed Nos1 transcription,8 has been causally linked to GP.3,9,10 However, pharmacological stimulation of nitrergic signaling also inhibits gastric contractility, facilitates storage rather than emptying, and can cause side effects.6,11,12 Neuron transplantation13,14 may have limited efficacy in DM due to the compromised tissue microenvironment. Therefore, we propose to restore nitrergic signaling by stimulating Nos1 transcription in cells where Nos1 is normally expressed. However, the mechanisms regulating Nos1 expression are poorly understood.
Due to the high O2 demands of oxidative phosphorylation (OXPHOS),15 which energetically sustains synaptic functions, central neurons experience physiological hypoxia (“physioxia”: partial O2 tensions (pO2) of 0.13–5.33 kPa or 0.13–5.26% O2 at sea level16). These levels are sufficient to activate hypoxic signaling through the stabilization of hypoxia-inducible transcription factors (HIFs).17–19 HIFs consist of a shared β subunit (ARNT; aryl hydrocarbon receptor nuclear translocator) and one of the three α subunits, HIF1A, HIF2A (Entrez Gene: EPAS1), and HIF3A.17–19 Although HIFs are constitutively expressed, at high pO2, α subunits are targeted for proteasomal degradation (t1/2~4–6 min20) by HIF prolyl hydroxylases (PHDs). HIF PHDs are inhibited at low pO2, allowing the α subunits’ stabilization (t1/2~200 min20) and translocation to the nucleus, where they complex with ARNT and bind their target promoters and enhancers.21 Pharmacological inhibition of HIF PHDs can upregulate HIF1A and its targets and increase neuronal survival.22 The HIF1A-stabilizing drug FG-4592 (roxadustat) has been approved in many countries for the treatment of anemia from chronic kidney disease, where DM and GP also occur.23 Since subphysiological hypoxia upregulates Nos1 mRNA in the nodose ganglion, cerebellum,24 and vascular smooth muscle cells,25 Nos3 (endothelial NOS) and Nos2 (inducible NOS) are targets for HIF1A,26 and mitochondrial dysfunction and neuropathy are associated with delayed GE in DM,27 we hypothesized that the neuronal transcriptional regulation of Nos1 involves HIF1A, and that stabilizing HIF1A can restore NOS1 levels and correct GP.
Methods
Standard methods and additional details are described in the Supplementary Methods.
Human Tissues
Under the aegis of two protocols approved by the Mayo Clinic Institutional Review Board (IRB 13–008138 and 20–012002), gastric corpus tissues representing most of the greater curvature of the stomach were obtained from 7 female and 3 male nondiabetic (28–69 years of age) and 11 female diabetic patients (30–59 years of age) who underwent sleeve gastrectomy for obesity. Consent was obtained and recorded in the patients’ electronic medical record.
Animal Studies
Animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines (see Supplementary Methods). The protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee (A48315–15 and A2508–08). Except for the GE and gastric volume (GV) studies, which were performed in female mice, both male and female animals were used. None of the mice were used in unrelated experiments. Littermates were used as controls. Before GE and GV tests, mice were fasted overnight in a metabolic cage with free access to water. Animals were handled during the light phase and euthanized by CO2 inhalation or by decapitation performed under deep isoflurane (Baxter Healthcare, Deerfield, IL) inhalation anesthesia.
The following mouse strains were used (all from The Jackson Laboratory, Bar Harbor, ME): C57BL/6J; B6.129-Hif1atm3Rsjo/J (Hif1afl) mice, which have loxP sites flanking exon 2 of Hif1a; Mapttm1(EGFP)Klt/J (Mapt-EGFP) mice, which carry EGFP (enhanced green fluorescent protein) knocked in the first exon of the microtubule-associated protein tau (Mapt) gene; B6.129-Nos1tm1(cre)Mgmj/J (Nos1cre) mice, which express Cre recombinase from the 3’ untranslated region of Nos1; and B6;129S-Nos1tm1.1(cre/ERT2)Zjh/J (Nos1creERT2/+) mice, which express CreERT2 fusion protein from the Nos1 promoter/enhancer elements. Mice expressing switchable, membrane- or nucleus-targeted tandem dimer Tomato-EGFP constructs (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (R26mTmG) and B6;129S6-Gt(ROSA)26Sortm1(CAG-tdTomato*,-EGFP*)Ees/J (R26nTnG), respectively) were used as reporters. In Nos1cre mice, recombination occurred not only in NOS1+ myenteric neurons but also in NOS1− cells (Supplementary Figure 2C), possibly due to germline recombination.28 Since recombination in Nos1creERT2/+ mice was limited to NOS1+ neurons, we used this strain for the conditional deletion of Hif1a.
To label hypoxic cells, mice were injected with 60 mg/kg pimonidazole (Hypoxyprobe™; Hypoxyprobe, Inc. Burlington, MA), which forms immunohistochemically detectable adducts with cellular proteins at pO2<1.33 kPa (<0.28% O2), intraperitoneally 60 min prior to euthanasia.29 Cre-mediated recombination was induced with tamoxifen (Sigma-Aldrich, St. Louis, MO; 0.075 mg/g body weight intraperitoneally once daily for 3–5 days). Induction, monitoring, and management of streptozotocin diabetes was performed using published methods.30
GE of solids was determined by our previously published 13C-octanoic acid breath test.30,31 In female and male Nos1creERT2/+;Hif1afl/fl mice, 2 weekly GE t1/2 values were averaged before and after tamoxifen or vehicle treatment. In female streptozotocin-diabetic mice, weekly measurements were taken (Supplementary Methods). Animals with two consecutive delayed GE readings (>97.5th percentile from 144 tests in 72 nondiabetic C57BL/6J females or from 120 tests in 60 nondiabetic C57BL/6J males) were considered to have GP.32 Female diabetic mice with our without GP were treated with FG-4592 (roxadustat; 10 mg/kg intraperitoneally once daily for 4 weeks)33 or vehicle (DMSO:PBS 1:49) for 4 weeks. The GE t1/2 values obtained during the last 3 weeks of the treatment were averaged for each mouse. GVs were measured noninvasively by single-photon emission computed tomography (SPECT)34 using intraperitoneally injected 99mTc-pertechnetate (100 μCi/50 μl) (Supplementary Figure 6A, Supplementary Videos 1–2) under isoflurane anesthesia.
Statistical Analysis
Hypothesis testing on low-throughput data was performed with GraphPad Prism 9.5.1 (Dotmatics, Boston, MA, USA) or SigmaPlot 14.5 (SPSS, Chicago, IL, USA) using nonparametric or parametric tests as appropriate (see Supplementary Methods). P values are exact values except in the case of Kruskal-Wallis ANOVA on ranks, where approximate P values were computed. For high-throughput data, P values adjusted for false discovery rate (FDR) are reported as Q values.
Results
HIF1A is required for normal NOS1 protein levels and normal gastric emptying in female mice
We assessed hypoxia-related gene expression by Gene Set Enrichment Analysis (GSEA) in Affymetrix Mouse Genome 430.2 microarray data generated in-house from colonic Mapt-EGFP+ neurons (Supplementary Figure 1A, Supplementary Data 1), total RNA-sequencing (RNA-seq) results from duodenal, ileal, and colonic Phox2b-CFP+ neurons,35 and single-cell RNA-seq data from small intestinal Baf53b-Tom+ neurons (all clusters).36 Using a matrix created by searching the Molecular Signatures Database (MSigDB) v.7.4 for neuronal or neurodegenerative disease-related gene sets containing Nos1 (Nos1&Neuron; Supplementary Data 2) we first verified the strong representation of nitrergic neurons in these datasets (Supplementary Figure 1B–C, Supplementary Data 3). GSEA using the MSigDB Hallmark v.7.4 matrix revealed a significant enrichment of the Hypoxia and OXPHOS gene sets in all preparations and the Glycolysis gene set in all but the Baf53b-Tom+ neurons (Figure 1A, Supplementary Figure 1D–E, Supplementary Data 3).
Figure 1. HIF1A from intracellular hypoxia is required for normal NOS1 levels in enteric neurons and for normal gastric emptying in female mice and is reduced in diabetes.
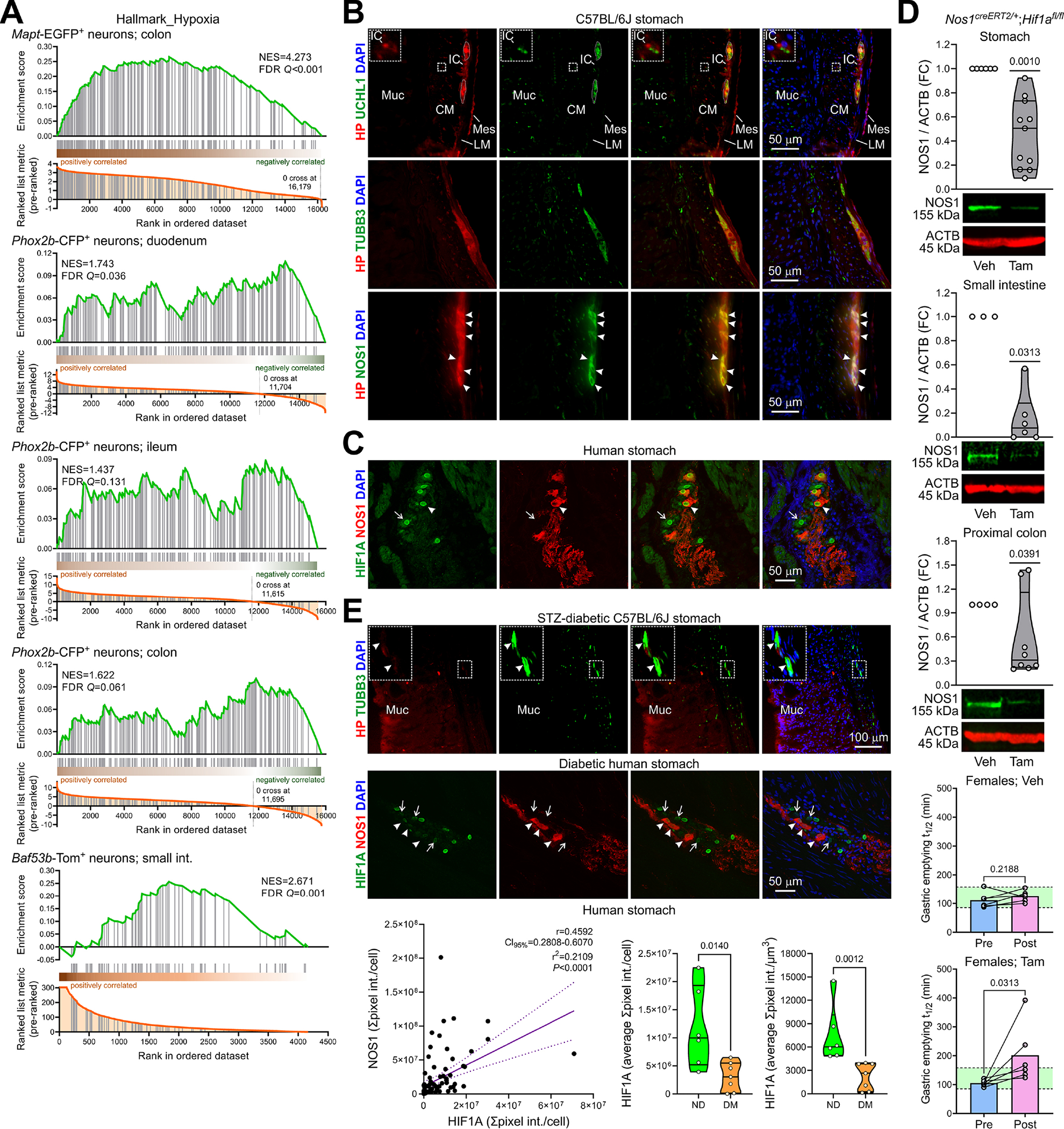
(A) Pre-ranked GSEA of hypoxia-related genes in enteric neurons. (B) Wide-field images of cryosections from nondiabetic mouse stomachs (n=2) showing Hypoxyprobe™ (HP)+ cells. UCHL1, TUBB3, NOS1: neuronal markers. DAPI, 4’,6-diamidino-2-phenylindole. IC, interstitial cells; Muc, mucosa; CM, circular muscle; LM, longitudinal muscle; Mes, mesothelial cells. Inset: enlargement of the outlined area showing a hypoxic IC adjacent to a nerve fiber. Dotted lines: enteric ganglia. Arrowheads: hypoxic NOS1+ neurons. (C) Confocal images of cryosections from 4 nondiabetic patients showing predominantly nuclear HIF1A (antibody: Thermo Fisher 700505) in NOS1+ (arrowhead) and NOS1− (arrow) neurons. (D) Top panels: Reduction of NOS1 by genomic deletion of Hif1a in tamoxifen (Tam) vs. vehicle (Veh)-treated Nos1creERT2/+;Hif1afl/fl mice (nVeh=6, 3, 4; nTam=11, 6, 8). P, Wilcoxon signed rank tests. Bottom panels: Increase in GE t1/2 by genomic deletion of Hif1a in female mice (n=6/group). Green area: strain- and sex-specific normal range. P, ratio-paired t tests. (E) Top: Wide-field images of cryosections from 2 STZ-diabetic mouse stomachs. Note reduced HP in TUBB3+ enteric neurons (outlined area enlarged in the inset) and preserved HP in the luminal epithelium. Middle: Confocal images of cryosections from 4 diabetic patients showing reduced HIF1A (Thermo Fisher 700505) in NOS1+ (arrowhead) and NOS1− (arrow) neurons. Bottom left: Direct linear relationship between HIF1A and NOS1 immunofluorescence in nitrergic neurons of 6 nondiabetic (n=37) and 7 diabetic patients (n=55). CI95%, 95% confidence interval. P is from linear regression and Pearson correlation. Right: HIF1A immunofluorescence/cell and immunofluorescence concentration were reduced in nitrergic neurons of the diabetic (DM) vs. nondiabetic (ND) patients. P, Mann-Whitney tests.
In the stomach of Hypoxyprobe™-injected mice, we detected intracellular hypoxia in luminal epithelial cells29 and in mesothelial cells, KIT− interstitial cells, and myenteric (including nitrergic) neurons (Figure 1B, Supplementary Figure 2A). In gastric corpus tissues from nondiabetic patients, using two different antibodies we found overall stronger and even nuclear HIF1A staining in myenteric ganglion cells including nitrergic neurons than in non-neuronal cells (Figure 1C, Supplementary Figure 2B. Deleting Hif1a in Nos1creERT2/+ mice, where tamoxifen treatment recombined 2 reporter constructs specifically and with ~70% efficiency (Supplementary Figure 2C), led to a 50% (stomach), 92% (small intestines), and 69% (proximal colon) reduction in median NOS1 protein levels 6 days post-tamoxifen and caused a delay in solid GE in females, with 50% showing gastroparetic GE t1/2 values (Figure 1D). These results are nearly identical to the 48% prevalence of diabetic GP detected in females of the same background (see below). In male mice, which we found resistant to diabetic GP in a recent unpublished study (n=14), GE remained unaffected (Supplementary Figure 2D).
These results show that OXPHOS-driven intracellular hypoxia is prevalent and HIF1A signaling is active in NOS1+ myenteric neurons. Our data also indicate that HIF1A is required for normal NOS1 levels and, in females, for normal GE.
Diabetes reduces HIF1A in NOS1+ gastric myenteric neurons
Twelve weeks after the onset of diabetes in streptozotocin (STZ)-diabetic mice, Hypoxyprobe™ injection identified a substantial loss of physioxia in gastric myenteric ganglia, but no effect on the environmentally determined hypoxia in the epithelium (Figure 1E, top). In diabetic patients, HIF1A immunofluorescence was reduced in myenteric (including nitrergic) neurons of the gastric corpus (Fig. 1E, middle). Quantitative image analysis (Supplementary Figure 3) revealed a direct linear relationship between HIF1A and NOS1 immunofluorescence and reduced HIF1A in nitrergic neurons of diabetic patients (Fig. 1E, bottom). These findings support our hypothesis that nitrergic enteric neuropathy in DM may arise from impaired hypoxic signaling.
HIF1A stabilization restores physioxic control of NOS1 and reverses diabetic gastroparesis
First, we validated the mouse neuroblastoma line N1E-11537 for our studies. GSEA of total RNA-seq data (Supplementary Data 4) indicated enrichment of Nos1- and hypoxia-related gene sets in cells cultured for ≥3 days at either 20% or 4% O2 (Supplementary Figure 4, Supplementary Data 5). Together with the (weak) nuclear HIF1A protein levels in cells maintained at 20% O2 (Fig. 2A, Supplementary Figure 5A), these findings indicate that cellular metabolism is an important driver of intracellular hypoxia in N1E-115 cells.15
Figure 2. HIF1A stabilization restores NOS1 and reverses diabetic gastroparesis.
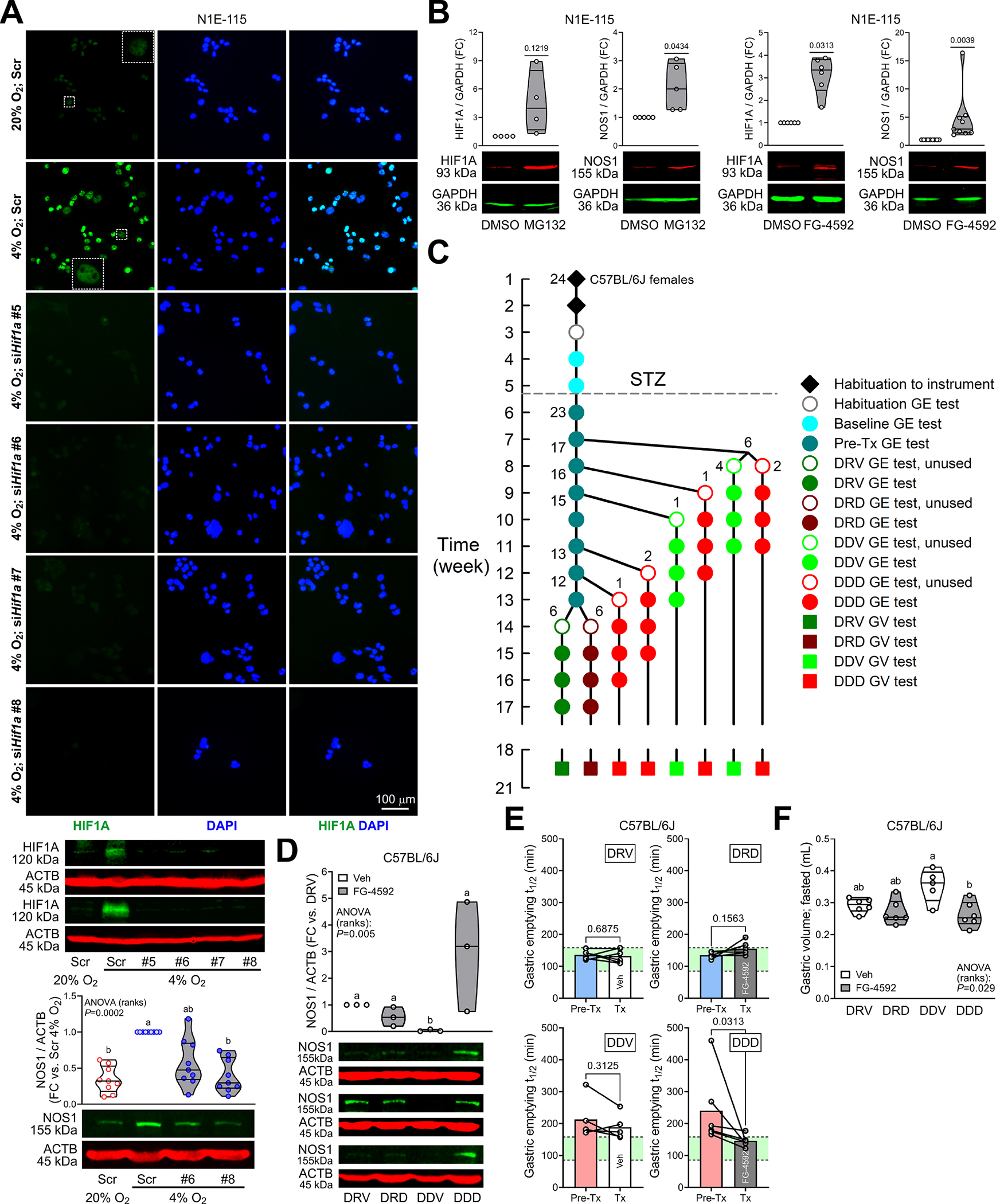
(A) Top and middle: Validation of Hif1a knockdown in N1E-115 cells by HIF1A immunofluorescence and immunoblotting (HIF1A antibody: Cell Signaling Technology #36169). Top: Wide-field images from 2 experiments. Scr, scrambled. Insets are enlargements of the outlined areas. Bottom: Effects of Hif1a knockdown on 4% O2-induced upregulation of NOS1 protein (n=9). P, Kruskal-Wallis ANOVA on ranks. Groups not sharing the same superscript are different by Dunn’s test. (B) Upregulation of HIF1A (Novus Biologicals NB100–134) and NOS1 protein by HIF1A stabilization with the proteasome inhibitor MG132 (10μM, 4h) or the PHD inhibitor FG-4592 (20μM, 2 days) (HIF1A: nMG132/Veh=4, nFG-4592/Veh=6; NOS1: nMG132/Veh=5; nFG-4592/Veh=9). P, one sample t or Wilcoxon signed rank tests. NOS1 was upregulated even relative to the HIF1A target GAPDH. (C) Design of longitudinal GE study. One mouse did not develop diabetes. DRV, diabetic, resistant to GP, Veh-treated (n=6); DRD, diabetic, resistant to GP, FG-4592-treated (n=6); DDV, diabetic, delayed GE, Veh-treated (n=5); DDD, diabetic, delayed GE, FG-4592-treated (n=6). (D) Upregulation of NOS1 in GP mouse gastric corpus+antrum tunica muscularis by FG-4592 (n=3). P, Kruskal-Wallis ANOVA on ranks. Groups not sharing the same superscript are different by Tukey’s test. (E) Significant reduction of GE t1/2 from pretreatment values in GP mice treated with FG-4592 (DDD) but not in Veh-treated animals (DDV). GE t1/2 did not change significantly in the non-GP mice (DRV, DRD). Green area: strain- and sex-specific normal range. P, Wilcoxon matched-pairs signed rank tests. (F) Normalization of fasting GVs by FG-4592. P, Kruskal-Wallis ANOVA on ranks. Groups not sharing the same superscript are different by Dunn’s test.
Environmental hypoxia approximating physiological O2 in postcapillary tissues (4% O2)16 increased the expression of hypoxia-related genes and Nos1 (Supplementary Figure 5B–C), the representation of Nos1-related gene sets (Supplementary Figure 4C–D), and HIF1A and NOS1 proteins by immunoblotting and immunofluorescence using two different antibodies for HIF1A (Figure 2A, Supplementary Figure 5A, C). We also verified the upregulation of Nos1 mRNA and NOS1 protein in the conditionally immortalized fetal enteric neuronal precursor cells IM-FEN38 (Supplementary Figure 5C). QIAGEN ingenuity Pathway Analysis of the differentially expressed genes in N1E-115 cells also supported a strong link between hypoxic and nitrergic signaling as NOS1-related pathways and networks were robustly represented among canonical pathways and upstream regulators, as well as causal, disease-, biological function-, and toxicology-related networks (Supplementary Data 6, Supplementary Figure 5D–E).
Reducing HIF1A protein by RNA interference (RNAi) using small interfering (si) RNAs suppressed hypoxia (4% O2)-induced upregulation of NOS1 protein (Figure 2A). Conversely, pharmacological stabilization of HIF1A with the proteasome inhibitor MG-132 and the clinically approved PHD inhibitor FG-4592 increased NOS1 (Figure 2B, Supplementary Figure 5F).
In our validated GP model,30,32 11/23 (48%) female STZ-diabetic C57BL/6J mice developed GP (Figure 2C). GP mice treated with vehicle for ≥4 weeks (DDV group: diabetic, delayed, vehicle-treated) remained gastroparetic and had lower HIF1A and NOS1 protein and higher GV results than vehicle-treated mice with normal pretreatment GE (DRV group: diabetic, GP-resistant, vehicle-treated) (Figure 2D–F, Supplementary Figure 6A–C). In GP mice, FG-4592 (DDD: diabetic, delayed, drug-treated) increased HIF1A and NOS1, significantly reduced GE t1/2 from pretreatment levels and restored it to normal in 5 of 6 mice, and normalized GVs. Pretreatment GE t1/2 values were not different between GP or GP-resistant mice randomly assigned to FG-4592 or Veh treatment (Supplementary Figure 6D). Blood glucose did not differ among groups or correlate with GE t1/2 (Supplementary Figure 6E).
These findings indicate that pharmacological stabilization of HIF1A can restore NOS1 protein levels and alleviate GP.
HIF1A binds promoters and enhancers of the Nos1 locus
To understand how HIF1A regulates Nos1 expression, we performed chromatin immunoprecipitation-sequencing (ChIP-seq) in N1E-115 cells cultured for 3 days at 20% or 4% O2 (Supplementary Figures 7–8, Supplementary Data 7). HIF1A and ARNT binding was evident at 20% O2, likely reflecting high O2 consumption.15 Culturing at 4% O2 caused a robust increase in HIF1A and ARNT peak numbers, signals, and coincidence (Supplementary Figure 8A–B). Increased HIF1A and ARNT binding was detected near genes including established hypoxia-regulated genes in the KEGG “HIF-1 signaling pathway–Mus musculus” (mmu04066) pathway and the MSigDB Hallmark v.7.4 Hypoxia gene set (Supplementary Figure 8A, C and Supplementary Data 8) and was associated with increased gene expression (Figure 3A) including, in the case of HIF1A, of Nos1. Analysis of transcription factor binding motifs within the genomic footprints of the HIF1A and ARNT peaks in the vicinity of the KEGG mmu04066 genes indicated a robust representation of canonical HIF1A, EPAS1, and ARNT motifs, confirming the peaks’ authenticity (Figure 3B, Supplementary Data 9). Consistent with the preference of HIF1A for promoters,21 HIF1A binding sites located in promoters and exons increased at 4% O2 (Supplementary Figure 8D). HIF1A and ARNT peaks were colocalized with the enhancer-associated histone mark monomethylated histone H3 lysine 4 (H3K4me1), the promoter-associated mark trimethylated H3K4 (H3K4me3), the active enhancer mark acetylated H3K27 (H3K27ac), and the active chromatin mark H3K9ac but not with the Polycomb-mediated repressive histone mark H3K27me3, indicating their predominant association with active chromatin (Figure 3C).
Figure 3. Hypoxia approximating physioxia increases HIF1A and ARNT binding to cis-regulatory elements of the Nos1 locus.
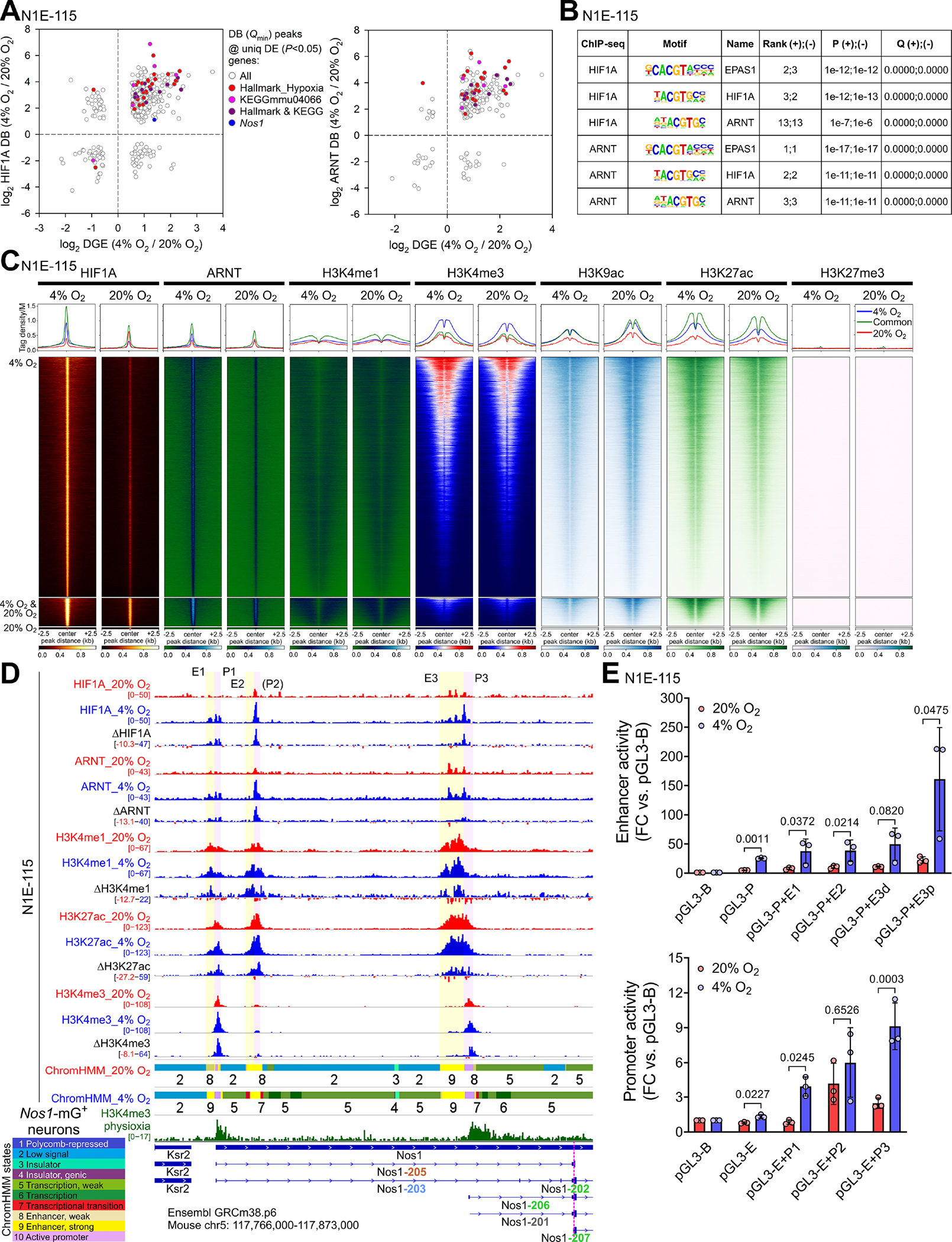
(A) Correlation between differential binding (DB; FDR Q<0.1) of HIF1A and ARNT within peaks with the lowest FDR Q value among the peaks assigned to the same gene and differential gene expression (DE; P<0.05) in N1E-115 cells cultured for 3 days at 4% or 20% O2. (B) Motifs within the HIF1A and ARNT peaks assigned to KEGG mmu04066 genes. (C) Heatmaps and average tag density plots of ChIP-seq data aligned to the centers of HIF1A peaks±2.5 kb are shown for HIF1A peaks unique to cells cultured at 4% O2 (blue lines, top heatmap panels) or 20% O2 (red lines, bottom heatmap panels) or common to cells cultured at 4% or 20% O2 (green lines, middle heatmap panels). (D) Binding profiles of HIF1A, ARNT, and key histone marks in the Ensembl GRCm38.p6 Nos1 locus of N1E-115 cells cultured at 20% (red tracks) or 4% O2 (blue) for 3 days. Aggregate and subtracted (4% O2−20% O2) occupancy data from two replicates are displayed. Colored ribbons: epigenetic states discovered by ChromHMM (10-state model). Green track: H3K4me3 in FACS-isolated Nos1-mG+ enteric neurons. (E) Activities of the indicated candidate Nos1 cis-regulatory elements cloned into the pGL3-Promoter (pGL3-P; top) or the pGL3-Enhancer (pGL3-E; bottom) vectors vs. the pGL3-Basic (pGL3-B) plasmid. The E3 putative enhancer was separated into a Nos1-distal (E3d) and Nos1-proximal (E3p) fragment. P, ratio-paired t tests.
In the Ensembl Nos1 locus (ENSMUSG00000029361), HIF1A and ARNT peaks were detected in three clusters with 3’ ends located 0, 50, and 59 kb upstream of the transcription start site (TSS) of the nearest Nos1 transcript encoding for a functional NOS1 isoform (Nos1–206, Figure 3D, Supplementary Figure 9A). Analysis of the binding profiles of key histone marks (Figure 3D), RNA polymerase II (POLR2), and POLR2 phosphorylated on serine 5 (POLR2S5p) or serine 2 (POLR2S2p) of its C-terminal repeat domain (Figure 4A, Supplementary Figure 9B), which are associated with transcriptional initiation, elongation, and termination,39,40 together with epigenetic state modeling by ChromHMM (Supplementary Figure 8E) and enhancer/promoter reporter assays (Figure 3E) revealed the occurrence of these peaks within three cognate enhancer-promoter pairs (E1-P1, E2-P2, E3-P3). Except for P2, which, despite being occupied by initiating POLR2S5p (Figure 4A), had little H3K4me3 binding, all these cis-regulatory elements showed increased occupancy by their active histone marks in response to 4% O2 (Figure 3D). We also observed prominent H3K4me3 occupancy of genomic sites corresponding to P1 and P3 in nitrergic neurons isolated from the small intestines of tamoxifen-treated Nos1creERT2/+;R26mTmG mice (Figure 3D, Supplementary Figure 8F). These results suggest a physiological role for the HIF-binding regulatory elements detected within the Nos1 locus in the hypoxic control of Nos1 expression.
Figure 4. HIF1A controls Nos1 transcription through multiple cis-regulatory elements of the Nos1 locus.
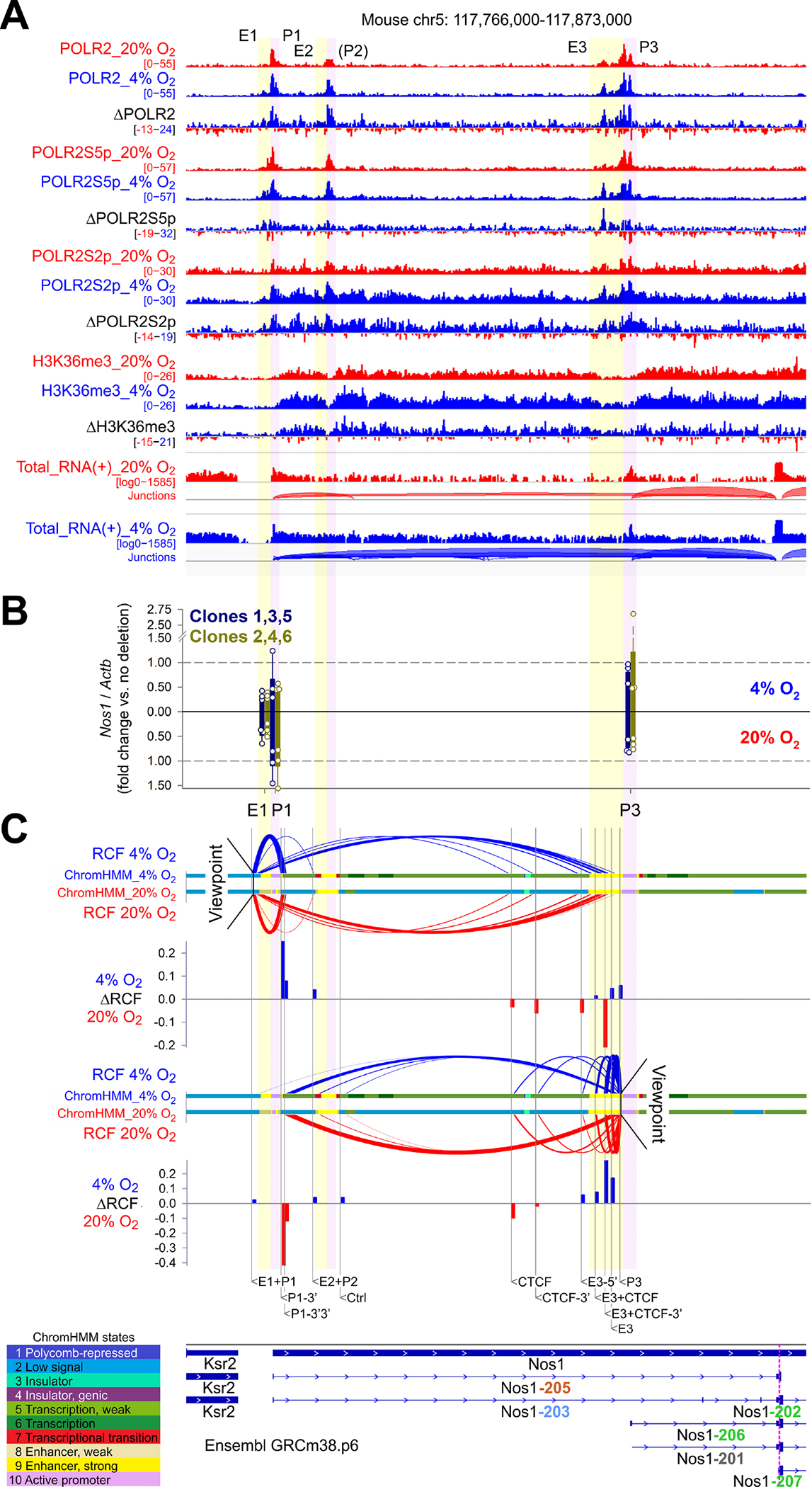
(A) ChIP-seq and total RNA-seq (+ strand) profiles in the Ensembl GRCm38.p6 Nos1 locus of N1E-115 cells cultured at 20% O2 (red) or 4% O2 (blue) for 3 days. Aggregate data from 2 (ChIP-seq) or 3 (RNA-seq) replicates and subtracted (4% O2−20% O2) data are displayed. (B) Nos1 expression in N1E-115 cells bearing CRISPR-Cas9 deletions in E1, P1, and P3 (two clones/position, n=3 cultures/clone). Mean±SD data from cells cultured at 4% (upright) and 20% O2 (inverted) are shown. (C) Enhancer-promoter loops detected by 3C using NcoI. qPCR data from 3 experiments were expressed relative to genomic DNA representing the same locus expressed in bacterial artificial chromosome (BAC). BAC-normalized data were scaled to a range of 0–1 across the loci tested to compare data obtained at 4% O2 (upright) and 20% O2 (inverted). RCF, relative crosslinking frequency (scaled 2ΔCT vs. BAC). The colored ribbons identify epigenetic states discovered by ChromHMM using a 10-state model. Bar plots show ΔRCF values (4% O2−20% O2).
HIF1A coordinates the activities of multiple cis-regulatory elements of the Nos1 locus
StringTie2 assembly of total RNA-seq reads indicated that of the transcripts encoding for functional NOS1 isoforms (green fonts in the figures), Nos1–206, whose TSS maps within P3, was most abundantly expressed and responsive to environmental hypoxia (Supplementary Figure 9A). While P1 included the TSSs of Nos1–205, which encodes for a truncated protein, and Nos1–203, a processed transcript, neither was expressed. We deleted P1 and P3 and also E1 using CRISPR-Cas9 in two N1E-115 clones each (Figure 4B, Supplementary Figure 9C, Supplementary Table 5) and analyzed Nos1 expression using primers specific for the transcripts encoding for functional NOS1 and Nos1–201, a transcript subject to nonsense-mediated decay, which was not detectable (Supplementary Figure 9A). Deletion of E1 reduced Nos1 expression at both 20% O2 and 4% O2 as did the deletion of P1 at 4% O2, whereas deleting P3 had modest effects, possibly due to the incomplete elimination of the 3’ end of the H3K4me3 peak (Supplementary Figure 9C). Chromosome conformation capture (3C) assays utilizing primers targeting genomic sites immediately upstream of E1 and P3 as “viewpoints” and target loci near the enhancers and promoters, flanking a binding site of the loop anchor/insulator CCCTC-binding factor (CTCF), and a control site (Figure 4C) revealed an interaction between E1-P1 and P3 at both 20% and 4% O2 and short-range interactions between E1 and P1 and E3 and P3. There was a subtle shift from long-range to short-range interactions at 4% O2, indicating that HIF1A binding may reinforce the interactions between cognate enhancers and promoters and suggesting a function for E1-P1 in stimulating Nos1–206 expression beyond enhancing P3 activity through looping (Supplementary Figure 9D). Examining the RNA-seq data and ChIP-seq profiles for the transcriptional elongation-associated histone mark H3K36me3, POLR2, POLR2S5p, and POLR2S2p (Figure 4A) we noted the Nos1 locus bore the characteristics of paused-and-expressed genes40 with POLR2 and POLR2S5p accumulation at all 3 promoters, a sign of transcription initiation and pausing, and occupancy by POLR2S2p and H3K36me3 throughout the locus indicating elongation. All these signals increased in response to 4% O2, consistent with HIF1A’s role in facilitating transcriptional pause-release.41 POLR2S2p also accumulated at sites with POLR2 and POLR2S5p peaks and having low H3K36me3 occupancy, suggesting a coincidence between transcription termination and initiation sites.39,40 This is consistent with “preloading” of elongating POLR2 onto successive initiation sites, which could potentially result in more robust expression of Nos1 encoding for functional NOS1 protein, particularly at low pO2 (Supplementary Figure 9E).
HIF1A-binding remote super-enhancers interact with cis-regulatory elements of the Nos1 locus
To discover new remote cis-regulatory elements controlling Nos1 expression, we performed two independent chromosome conformation capture-sequencing (HiC) studies utilizing the Arima, Inc. 2-enzyme chemistry and a published HiC protocol42 (Supplementary Figure 10A). Both localized Nos1 within a 5’ subdomain of an ~800 kb topologically associating domain (TAD) (Supplementary Figure 10B). Within this TAD we identified, using Rank Ordering of Super-Enhancers (ROSE), 2 (at 20% O2) and 3 (at 4% O2) HIF1A-bound super-enhancers (SEs) located 5’ of the Nos1 locus and one SE, which was detectable at both pO2 levels, 3’ of Nos1 (Figure 5A). Using HiCCUPS we detected >30,600 unique loops (Supplementary Figure 10C, Supplementary Data 10). Chromosome-wise assessment of the enrichment of the HiC signal in loops by aggregate peak analysis (APA)43 indicated concordant peak-to-lower-left (P2LL) enrichment values in the Arima and NcoI HiC data (Supplementary Figure 10D). The numbers of HiCCUPS loops and genome-wide APA enrichment values were similar at 20% and 4% O2 (Supplementary Figures 10C, E and 11A). Within the Nos1-containing subdomain, the Arima HiC data revealed 14 loops shared between the HiC matrices obtained at 20% O2 and 4% O2 and 6 and 7 loops that were unique to the 20% O2 and 4% O2 datasets, respectively (Figure 5A–C, Supplementary Data 11). Notably, the shared loops included the loop connecting E3 and the more distal cis-regulatory elements of the Nos1 locus we previously identified by 3C (Figure 4C). Other notable changes included the replacement of a loop connecting E3 with the 3’ SE at 20% O2 with a loop between E3 and one of the 5’ SEs and newly formed connections between E1 and E2 and upstream enhancers/SEs. Considering peaks of the loop anchor protein CTCF, the cohesin component RAD21, and the orientation of CTCF motifs at the 12 clusters of loop anchor sites,44 we classified these loops as architectural (loops with significant, coincident RAD21 and CTCF peaks at both anchors, with CTCF peaks occupying motifs with convergent or left/right tandem configurations) or transient/functional (loops lacking one or more component or having a divergent CTCF motif orientation)45 (Figure 5A–C). This analysis identified 6, 3, and 3 of the shared, 20% O2-only, and 4% O2-only loops, respectively, as architectural and revealed that culturing at 4% O2 changed the loop connecting the Nos1-proximal E3 with a 5’ SE from functional to architectural due to the recruitment of RAD21 to the CTCF peak at anchor site #3’ (Figure 5B–C, Supplementary Figure 10F). These findings indicate that the cis-regulatory elements of the Nos1 locus are coupled to remote SEs within its TAD in a pO2-dependent manner and that the effects of environmental hypoxia involve substantial reconfiguration and strengthening of these interactions.
Figure 5. HIF1A binds remote super-enhancers that interact with cis-regulatory elements of the Nos1 locus in a pO2-dependent manner.
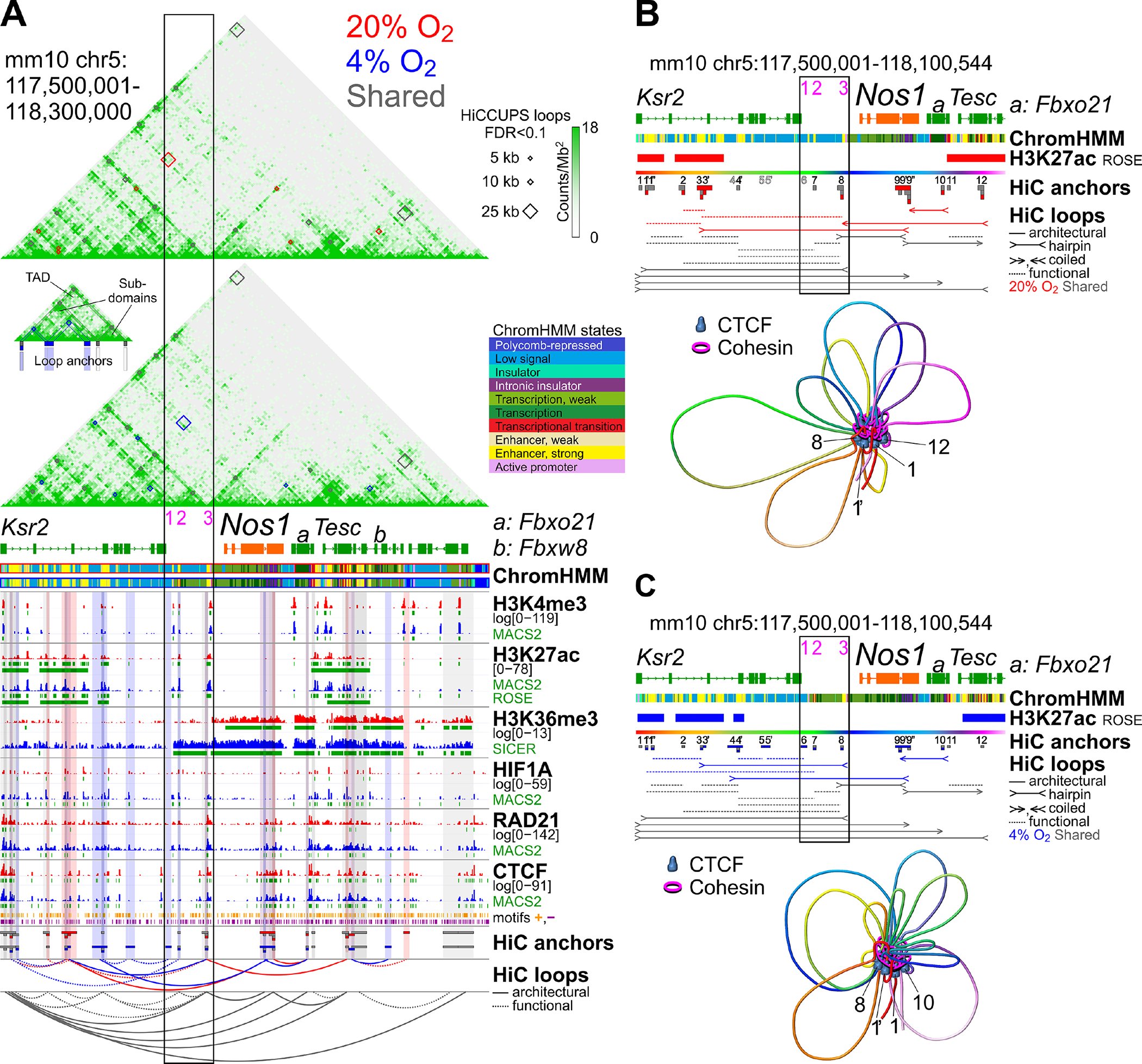
(A) Upper panel: HiC heatmaps of the TAD containing Nos1 in N1E-115 cells cultured at 20% O2 or 4% O2 for 3 days. Chromosome loops detected at 5-, 10-, and 25-b resolution are displayed as red (loops unique to 20% O2), blue (loops unique to 4% O2), and gray diamonds (loops detected at both 20% and 4% O2). Lower panel: ChromHMM and ChIP-seq tracks. Anchors and unscaled arc plots are color-coded as above. CTCF motif orientations are from ref.44 Solid and dashed lines: architectural and functional/transient loops, respectively. Black rectangle: cis-regulatory elements of the Ensembl Nos1 locus. (B) Upper panel: loops in N1E-115 cells cultured at 20% O2. Arrows: CTCF motif orientations permissive to architectural loop formation (convergent, left tandem, right tandem). Dashed lines without arrows: functional/transient loops. Lower panel: 3D chromatin structure of the locus inferred from the loop data. Numbered CTCF molecules correspond to the numbering of loop anchors in the upper panel. (C) Loops, and proposed chromosome conformation in N1E-115 cells cultured at 4% O2. See legend to panel B.
HIF1A alters chromosome topology genomewide
Besides a RAD21 peak, the loop anchor site #3’ also gained a HIF1A peak at 4% O2 (Supplementary Figure 10F). Within all 12 clusters of Nos1-relevant loop anchors, apart from the intra-Nos1 anchor #9, HIF1A binding coinciding with RAD21 and CTCF peaks increased at low pO2 (Figure 6A). The pO2-dependent concordance among CTCF, RAD21, and HIF1A peaks was also evident genome-wide (Figure 6B) including within loop anchors (Figure 6C–D) and accompanied by an increase in the number of overlapping HIF1A, RAD21, and CTCF peaks at 4% O2 (Figure 6E). ChromHMM analysis using all ChIP-seq data (Supplementary Figure 11B–C) identified an active distal enhancer and an active promoter state (states 11 and 12, respectively) that had the strongest HIF1A and ARNT enrichment among all states and could be classified as loop anchor-associated. Analysis of chromatin state changes by Sankey plot (Figure 6F) revealed an expansion of these states at the expense of enhancers and promoters not associated with chromosome loops (states 9 and 13). These results suggest that the conversion of a functional loop to architectural in the Nos1 TAD reflected genome-wide actions of HIF1A stabilization and recruitment.
Figure 6. HIF1A alters chromosome topology genomewide.
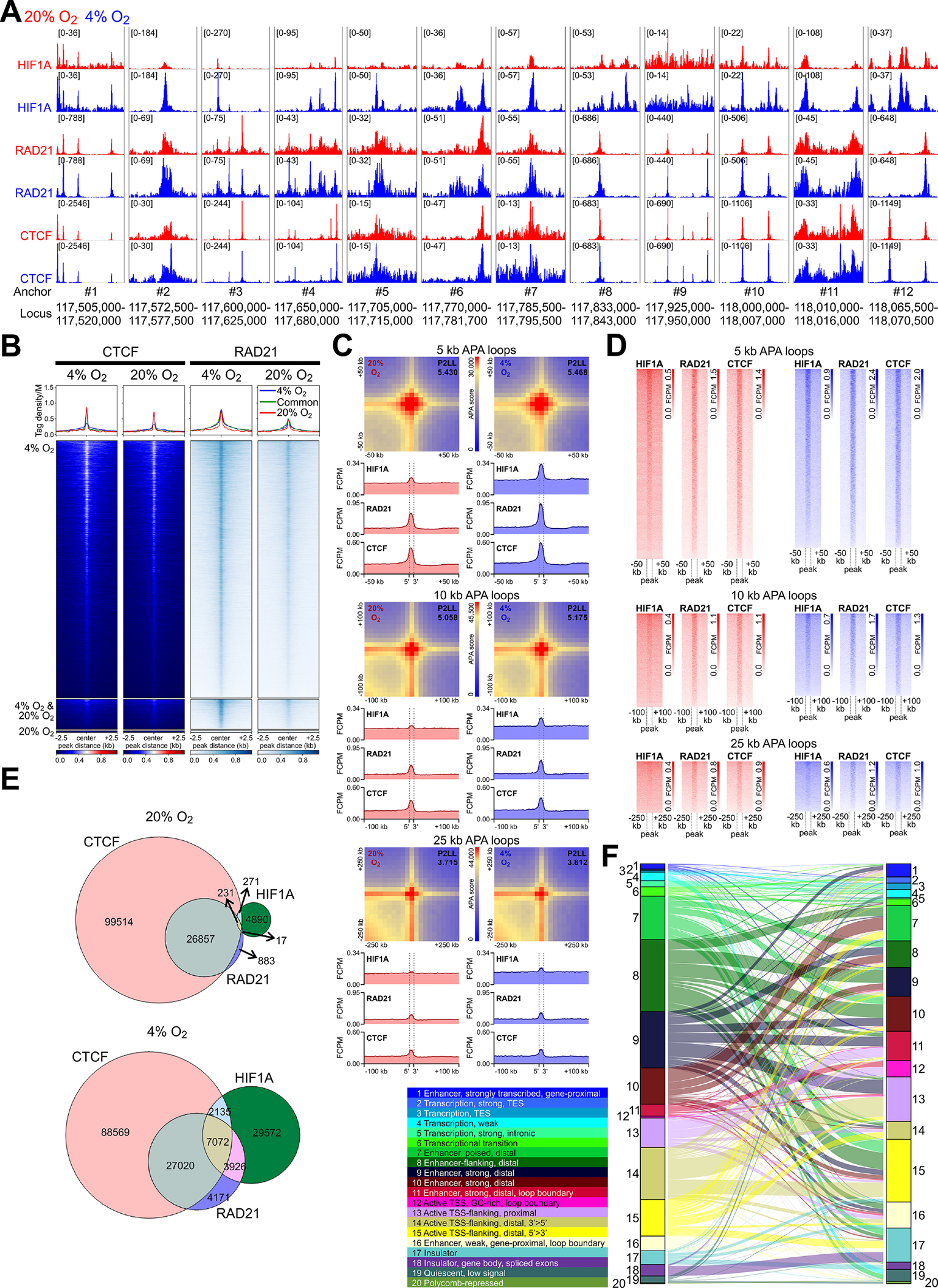
(A) HIF1A, RAD21, and CTCF binding (aggregate data from 2 replicates/condition) in N1E-115 cells within 12 clusters of HiC loop anchors identified in Figure 5. (B) Heatmaps and average tag density plots of CTCF and RAD21 ChIP-seq data aligned to the centers of HIF1A peaks±2.5 kb (see in Figure 3C) are shown for HIF1A peaks unique to cells cultured at 4% O2 (blue lines, top heatmap panels) or 20% O2 (red lines, bottom heatmap panels) or common to cells maintained at 4% O2 or 20% O2 (green lines, middle heatmap panels). (C) Heatmaps of standard APA results obtained at 5-, 10-, and 25-kb resolution. P2LL, peak-to-lower-left-quadrant enrichment values. Below the APA plots, HIF1A, RAD21, and CTCF average tag density plots centered around all detected APA loop anchors are shown. Dashed vertical lines demarcate the central APA bins. (D) Heatmaps corresponding to the average tag density plots in C. (E) Venn diagrams showing overlaps of HIF1A, CTCF, and RAD21 peaks at 20% O2 and 4% O2. (F) Sankey plot showing chromatin state changes from 20% O2 (left) to 4% O2 (right) in N1E-115 cells. The colored ribbons show the relative frequencies of epigenetic states discovered by ChromHMM using a 20-state model.
HIF1A recruits RAD21 to CTCF-bound sites to upregulate NOS1
Considering that low pO2 affected CTCF binding less than HIF1A and RAD21 occupancy and neither Rad21 nor Ctcf expression was modified by pO2 (Supplementary Data 4), we investigated whether HIF1A could regulate chromosome conformation by binding to CTCF and recruiting RAD21. Consistent with the ability of HIF1A to bind noncanonical motifs,46 we detected enrichment of CTCF motifs within the HIF1A-bound cis-regulatory elements of the Nos1-containing sub-TAD (Figure 7A, Supplementary Data 12). RAD21 and HIF1A co-immunoprecipitation with CTCF and RAD21 verified HIF1A binding to both CTCF and RAD21 along with RAD21-CTCF binding (Figure 7B). At 4% O2, RNAi-mediated depletion of HIF1A binding within key enhancers/SEs of the Nos1-containing sub-TAD (Supplementary Figure 11D) and the promoter of the HIF1A target Slc2a1 (dotted pink line in Supplementary Figure 8A) also caused the reduction of RAD21 binding to the same sites (Figure 7C). RNAi-mediated depletion of RAD21 inhibited the hypoxia-induced gain in NOS1 protein and reduced NOS1 at 20% O2 (Figure 7D). Together, these results indicate that besides upregulating enhancer/promoter activities, HIF1A facilitates enhancer-promoter interactions by binding to CTCF sites and promoting the formation of architectural chromosome loops by the recruitment of RAD21 (Figure 7E).
Figure 7. HIF1A recruits RAD21 to CTCF-bound sites to upregulate NOS1.
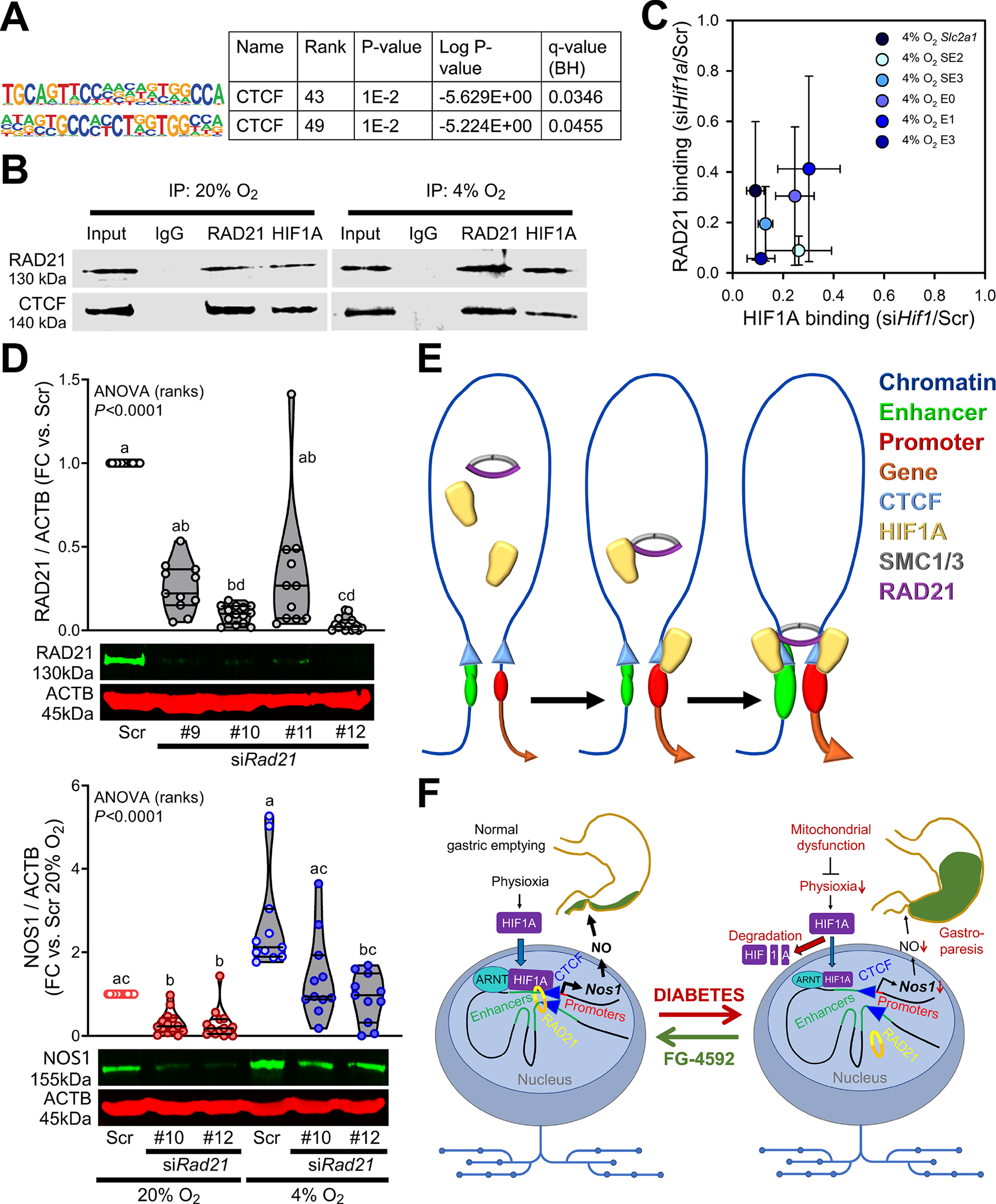
(A) Enrichment of known CTCF motifs in HIF1A-bound cis-regulatory elements of the Nos1 TAD in N1E-115 cells. (B) Co-immunoprecipitation of RAD21 and CTCF with RAD21 and HIF1A. Representative immunoblots from 3 (RAD21) or 2 (CTCF) experiments. (C) Concordant reduction in HIF1A and RAD21 binding in the promoter of the canonical HIF1A target Slc2a1 gene and key HIF1A-bound SEs and enhancers of the sub-TAD containing Nos1 in HIF1A-silenced N1E-115 cells cultured at 4% O2. The loci targeted for analysis are indicated by pink bars in Supplementary Figure 10D and a pink vertical line in Supplementary Figure 7A. (D) Top: Silencing efficiencies of 4 siRNAs targeting Rad21. nScr=14, n#9=11, n#10=14, n#11=11, n#12=14. P, Kruskal-Wallis one-way ANOVA. Groups not sharing the same superscript are different by Dunn’s test. Bottom: Effects of Rad21 knockdown using the two most efficacious siRNAs on NOS1 protein in N1E-115 cells. nScr;20%=14, n#10;20%=14, n#12;20%=14, nScr;4%=11, n#10;4%=11, n#12;4%=11. P, Kruskal-Wallis one-way ANOVA. Groups not sharing the same superscript are different by Dunn’s test. (E) Schematic illustration showing the formation of architectural enhancer-promoter loops triggered by HIF1A recruitment of RAD21 to CTCF-bound sites. (F) Overview of the proposed main roles of HIF1A in gastroparesis.
Discussion
While NOS1 is central to GP,3,4,9,10 supplementing NO via pharmacotherapy is ineffective,6,11,12 and transplantation of nitrergic neurons13,14 may have limited efficacy in DM due to the compromised tissue microenvironment. Here, we demonstrate that HIF1A is required to maintain normal NOS1 levels and, in female mice, also GE. Conceivably, this may explain the poorly understood finding that over 65% of patients with diabetic GP are women.7 We also observed that diabetes reduces hypoxia/HIF1A in NOS1+ gastric myenteric neurons in mice and humans. FG-4592/roxadustat, which has been used in chronic kidney disease patients,23 stabilized HIF1A, reversed the downregulation of NOS1 protein, and restored GE in female diabetic mice (Figure 7F). Besides stimulating gene expression by binding to promoter-proximal elements,21 we also found HIF1A to upregulate transcription from the 5’ end of the Nos1 locus to increase the number of POLR2 complexes available at the TSS of the main Nos1 transcript encoding for functional NOS1 protein. Finally, we demonstrate that HIF1A facilitates long-range enhancer/SE-promoter interactions by altering chromosome topology through the recruitment of cohesin to CTCF-bound sites within the Nos1 TAD and genomewide. The recognition of HIF1A’s role in 3D chromosome conformation may lead to better understanding of organ functions at physioxia and in ischemic conditions47 and the pathobiology of cancers.17–19
In enteric neurons, hypoxic signaling is activated under physiological conditions, likely reflecting high oxygen consumption.15,16 This physioxic signaling, also evident in N1E-115 cells, was reduced in diabetic mice and humans. These findings are consistent with the association of DM-associated mitochondrial dysfunction with neuropathy and delayed GE.27 High oxidative stress from downregulation of macrophage heme oxygenase-132 may also contribute to the impaired expression and function of HIF1A in DM.19 Stabilizing HIF1A normalized GE and GVs in diabetic mice, reflecting a robust stimulation of NOS1. Indeed, NOS1 was more sensitive to HIF1A in diabetic mice with vs. without GP. This may have reflected reduced production of NO in GP, which at high concentrations can inhibit HIF1A function.19 We did not study HIF1A/EPAS1 or HIF3A, which are not expressed by N1E115 cells, but they may have contributed to the effects of FG-4592 in mice. Stabilizing HIFs may also enhance the function of transplanted nitrergic neurons.14 Arguably, hypobaric hypoxia or carbon monoxide-releasing molecules (CORMs) may have effects similar to FG-4592.48 However, hypobaric hypoxia therapy has practical limitations, and CORMs may aggravate mitochondrial dysfunction by disabling cytochromes.48
Akin to mice with targeted disruption of Nos1,9 the stomach was larger in diabetic mice, arguably due to pyloric dysfunction and functional gastric outlet obstruction secondary to reduced Nos1 expression.6 In these mice, FG-4592 reduced gastric volume, which contrasts with the pan-gastric relaxation of drugs that upregulate NO levels in humans.6,11,12 Although interstitial cells of Cajal, whose depletion contributes to GP,6,49 are not naturally hypoxic (Supplementary Figure 2A), their numbers could be increased/normalized by the restoration of NO signaling with FG-4592.50
We also discovered 4 pO2-regulated SEs within the Nos1 TAD. These SEs are more strongly coupled to proximal Nos1 enhancers under physioxia. These interactions included de-novo transient loops, reorganization of architectural loops, and the conversion of a transient to an architectural loop, with the latter involving recruitment of HIF1A and RAD21 to an existing CTCF peak. Evident throughout the genome, these findings are consistent with the model that physioxia leads to HIF1A-directed formation of cohesin-mediated, CTCF-delimited architectural loops. In DM, there is less intracellular hypoxia and these interactions dissolve (Figure 7E). Future studies will address whether the HIF1A-RAD21 interactions involve other members of the cohesin complex and whether HIF1A interacts with transcription factors important for the specification of the NOS1 neuronal lineage such as Etv136 to limit HIF1A-mediated Nos1 upregulation to nitrergic neurons.
Supplementary Material
What You Need to Know.
BACKGROUND AND CONTEXT:
Neuronal nitric oxide synthase contributes to the pathogenesis of gastroparesis. So far, measures to stimulate nitrergic signaling have been ineffective, necessitating alternative approaches.
NEW FINDINGS:
Hypoxia-inducible factor 1α (HIF1A) stimulated neuronal nitric oxide synthase gene transcription by binding and activating proximal and distal regulatory elements and by modifying 3-dimensional chromatin topology. Pharmacological stabilization of this protein with FG-4592/roxadustat, a drug approved for human use, reversed gastroparesis in diabetic mice.
LIMITATIONS:
Roxadustat upregulates nitric oxide synthase levels but does not ensure optimal functioning of this protein. Future studies will examine whether roxadustat can alleviate gastroparesis in patients.
CLINICAL RESEARCH RELEVANCE:
Reduced HIF1A resulting from mitochondrial dysfunction may contribute to gastric nitrergic neuropathy and diabetic gastroparesis in females. Pharmacological stabilization of HIF1A with a repurposed drug may be useful for treating diabetic gastroparesis in the future.
BASIC RESEARCH RELEVANCE:
Physiological intracellular hypoxia engenders HIF1A, which is required for normal transcription of the neuronal nitric oxide synthase gene. The newly recognized effects of HIF1A on chromosome topology may provide insights into physioxia- and ischemia-related organ function and the pathobiology of cancers.
Acknowledgements:
The authors thank Dr. Shanthi Srinivasan, Emory University, Atlanta, Georgia, USA, for her generous gift of the conditionally immortalized mouse IM-FEN cells line; Teresa D. Decklever (Small Animal Imaging Core, Mayo Clinic, Rochester, MN, USA) for the gastric volume measurements; Natalie R. Wertish (Enteric NeuroScience Program and Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, USA) for her assistance with the gastric emptying tests; the Center for Individualized Medicine Medical Genome Facility (Mayo Clinic, Rochester, MN, USA) for the next-generation sequencing; and Pritha Chanana, Krutika S. Gaonkar, Gavin R. Oliver (Center for Individualized Medicine Bioinformatics Core and Division of Computational Biology, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, USA) for their contribution to the bioinformatic analyses.
Grant Support:
This study was supported in part by National Institutes of Health grants R01 DK126827, R01 DK131455, P01 DK068055, P30 DK084567, and the Mayo Clinic Center for Individualized Medicine. The funding agencies had no role in the study analysis or writing of the manuscript. Its contents are solely the responsibility of the authors.
Abbreviations:
- 3C
chromosome conformation capture
- APA
aggregate peak analysis
- ARNT
aryl hydrocarbon receptor nuclear translocator
- BAC
bacterial artificial chromosome
- BAF53B
alias of ACTL6B, actin-like 6B
- CFP
cyan fluorescent protein
- ChIP-seq
chromatin immunoprecipitation-sequencing
- CORM
carbon monoxide-releasing molecule
- CM
circular muscle
- CTCF
CCCTC-binding factor
- CTD
C-terminal repeat domain
- DAPI
4’,6-diamidino-2-phenylindole
- DB
differential binding
- DDD
diabetic, delayed gastric emptying, FG-4592-treated
- DDV
diabetic, delayed gastric emptying, vehicle-treated
- DM
diabetes mellitus
- DRD
diabetic, resistant to gastroparesis, FG-4592-treated
- DRV
diabetic, resistant to gastroparesis, vehicle-treated
- E
enhancer
- EGFP
enhanced green fluorescent protein
- EPAS1
endothelial PAS domain protein 1 (alias: HIF2A)
- FACS
fluorescence-activated cell sorting
- FDR
false discovery rate
- GE
gastric emptying
- GP
gastroparesis
- GSEA
Gene Set Enrichment Analysis
- GV
gastric volume
- H3K27ac
acetylated histone H3 lysine 27
- H3K27me3
trimethylated histone H3 lysine 27
- H3K36me3
trimethylated histone H3 lysine 36
- H3K4me1
monomethylated histone H3 lysine 4
- H3K4me3
trimethylated histone H3 lysine 4
- H3K9ac
acetylated histone H3 lysine 9
- HiC
chromosome conformation capture-sequencing
- HIF
hypoxia-inducible factor
- HIF1A
hypoxia inducible factor 1, α subunit
- HP
Hypoxyprobe™
- HuC/D
alias of ELAV like neuron-specific RNA binding protein 3 and ELAV like RNA binding protein 4
- IC
interstitial cell
- IM-FEN
immortalized fetal enteric neuron
- LM
longitudinal muscle
- MAPT
microtubule-associated protein tau
- Mes
mesothelial cell
- MSigDB
Molecular Signatures Database
- Muc
mucosa
- ND
nondiabetic
- NO
nitric oxide
- NOS1
nitric oxide synthase 1, neuronal
- NOS2
nitric oxide synthase 2, inducible
- NOS3
nitric oxide synthase 3, endothelial cell
- OXPHOS
oxidative phosphorylation
- P
promoter
- P2LL
peak to lower left quadrant
- PHD
prolyl hydroxylase domain
- PHOX2B
paired-like homeobox 2b
- pO2
partial oxygen tension
- POLR2
RNA polymerase II
- POLR2S2p
RNA polymerase II phosphorylated on C-terminal repeat domain serine 2
- POLR2S5p
RNA polymerase II phosphorylated on C-terminal repeat domain serine 5
- R26
ROSA26 locus
- RAD21
RAD21 cohesin complex component
- RCF
relative crosslinking frequency
- RNAi
RNA interference
- RNA-seq
RNA-sequencing
- Scr
scrambled
- SE
super-enhancer
- siRNA
small interfering RNA
- SLC2A1
solute carrier family 2 (facilitated glucose transporter), member 1 (alias: GLUT1)
- SPECT
single-photon emission computed tomography
- STZ
streptozotocin
- TAD
topologically associating domain
- Tam
tamoxifen
- Tom
Tomato fluorescent protein
- TSS
transcription start site
- TUBB3
tubulin, beta 3 class III
- UCHL1
ubiquitin carboxy-terminal hydrolase L1
- Veh
vehicle
Footnotes
Disclosures: The authors disclose no conflicts.
Transcript Profiling: The microarray and RNA-sequencing (RNA-seq) data generated in this study have been deposited in a public database (National Center for Biotechnology Information, U.S. National Library of Medicine Gene Expression Omnibus (GEO), https://www.ncbi.nlm.nih.gov/gds/) as part of SuperSeries GSE139483, SubSeries GSE199990 (microarray) and GSE139472 (RNA-seq).
Writing Assistance: Not applicable.
Data Transparency Statement: The microarray, RNA-seq, chromatin immunoprecipitation-sequencing (ChIP-seq), and chromosome conformation capture-sequencing (HiC) data generated in this study have been deposited in a public database (National Center for Biotechnology Information, U.S. National Library of Medicine Gene Expression Omnibus (GEO), https://www.ncbi.nlm.nih.gov/gds/) as part of SuperSeries GSE139483, SubSeries GSE199990 (microarray), GSE139472 (RNA-seq), GSE139477, GSE139478, GSE139479, and GSE139481 (ChIP-seq), and GSE139470 (HiC). Processed microarray and sequencing results and outputs of gene expression and epigenomic data analysis software packages have been provided in Supplementary Data files.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 2016;13:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera LR, Poole DP, Thacker M, et al. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil 2011;23:980–988. [DOI] [PubMed] [Google Scholar]
- 3.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest 2000;106:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest 2006;116:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011;140:1575–1585 e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Sanders KM. Gastroparesis. Gastroenterology 2022;162:68–87 e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Kudva YC, Prichard DO. Diabetic Gastroparesis. Endocr Rev 2019;40:1318–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes 2003;52:2353–2362. [DOI] [PubMed] [Google Scholar]
- 9.Huang PL, Dawson TM, Bredt DS, et al. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 1993;75:1273–1286. [DOI] [PubMed] [Google Scholar]
- 10.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology 2000;119:766–773. [DOI] [PubMed] [Google Scholar]
- 11.Bortolotti M, Mari C, Lopilato C, et al. Sildenafil inhibits gastroduodenal motility. Aliment Pharmacol Ther 2001;15:157–161. [DOI] [PubMed] [Google Scholar]
- 12.Dishy V, Cohen Pour M, Feldman L, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther 2004;76:281–286. [DOI] [PubMed] [Google Scholar]
- 13.Micci MA, Pasricha PJ. Neural stem cells for the treatment of disorders of the enteric nervous system: strategies and challenges. Dev Dyn 2007;236:33–43. [DOI] [PubMed] [Google Scholar]
- 14.Stavely R, Hotta R, Picard N, et al. Schwann cells in the subcutaneous adipose tissue have neurogenic potential and can be used for regenerative therapies. Sci Transl Med 2022;14:eabl8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim J, Cowburn AS, Palazon A, et al. The Factor Inhibiting HIF Asparaginyl Hydroxylase Regulates Oxidative Metabolism and Accelerates Metabolic Adaptation to Hypoxia. Cell Metab 2018;27:898–913 e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 2001;128:263–276. [DOI] [PubMed] [Google Scholar]
- 17.Pugh CW, Ratcliffe PJ. New horizons in hypoxia signaling pathways. Exp Cell Res 2017;356:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivan M, Kaelin WG Jr. The EGLN-HIF O2-Sensing System: Multiple Inputs and Feedbacks. Mol Cell 2017;66:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol 1999;15:551–578. [DOI] [PubMed] [Google Scholar]
- 20.Moroz E, Carlin S, Dyomina K, et al. Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One 2009;4:e5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smythies JA, Sun M, Masson N, et al. Inherent DNA-binding specificities of the HIF-1alpha and HIF-2alpha transcription factors in chromatin. EMBO Rep 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Cui XX, Chen YJ, et al. Therapeutic Potential of a Prolyl Hydroxylase Inhibitor FG-4592 for Parkinson's Diseases in Vitro and in Vivo: Regulation of Redox Biology and Mitochondrial Function. Front Aging Neurosci 2018;10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N, Hao C, Liu BC, et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med 2019;381:1011–1022. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakar NR, Pieramici SF, Premkumar DR, et al. Activation of nitric oxide synthase gene expression by hypoxia in central and peripheral neurons. Brain Res Mol Brain Res 1996;43:341–346. [DOI] [PubMed] [Google Scholar]
- 25.Ward ME, Toporsian M, Scott JA, et al. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest 2005;115:3128–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benita Y, Kikuchi H, Smith AD, et al. An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 2009;37:4587–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puthanmadhom Narayanan S, O'Brien D, Sharma M, et al. Duodenal mucosal mitochondrial gene expression is associated with delayed gastric emptying in diabetic gastroenteropathy. JCI Insight 2021;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo L, Ambrozkiewicz MC, Benseler F, et al. Optimizing Nervous System-Specific Gene Targeting with Cre Driver Lines: Prevalence of Germline Recombination and Influencing Factors. Neuron 2020;106:37–65 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and Mucosal Inflammation. Annu Rev Pathol 2016;11:77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cipriani G, Gibbons SJ, Miller KE, et al. Change in Populations of Macrophages Promotes Development of Delayed Gastric Emptying in Mice. Gastroenterology 2018;154:2122–2136 e2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller KE, Bajzer Z, Hein SS, et al. High temporal resolution gastric emptying breath tests in mice. Neurogastroenterol Motil 2018:e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme Oxygenase-1 Protects Interstitial Cells of Cajal From Oxidative Stress and Reverses Diabetic Gastroparesis. Gastroenterology 2008;135:2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Yu X, Zhang Y, et al. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 2018;132:825–838. [DOI] [PubMed] [Google Scholar]
- 34.Wang XJ, Burton DD, Breen-Lyles M, et al. Gastric accommodation influences proximal gastric and total gastric emptying in concurrent measurements conducted in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2021;320:G759–G767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May-Zhang AA, Tycksen E, Southard-Smith AN, et al. Combinatorial Transcriptional Profiling of Mouse and Human Enteric Neurons Identifies Shared and Disparate Subtypes In Situ. Gastroenterology 2021;160:755–770 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morarach K, Mikhailova A, Knoflach V, et al. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat Neurosci 2021;24:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A 1972;69:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anitha M, Joseph I, Ding X, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology 2008;134:1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev 2006;20:2922–2936. [DOI] [PubMed] [Google Scholar]
- 40.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet 2012;13:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Lu H, Chen C, et al. HIF-1 Interacts with TRIM28 and DNA-PK to release paused RNA polymerase II and activate target gene transcription in response to hypoxia. Nat Commun 2022;13:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imakaev M, Fudenberg G, McCord RP, et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat Methods 2012;9:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao SS, Huntley MH, Durand NC, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 2014;159:1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y, Xu Q, Canzio D, et al. CRISPR Inversion of CTCF Sites Alters Genome Topology and Enhancer/Promoter Function. Cell 2015;162:900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Z, Luo OJ, Li X, et al. CTCF-Mediated Human 3D Genome Architecture Reveals Chromatin Topology for Transcription. Cell 2015;163:1611–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allan KC, Hu LR, Scavuzzo MA, et al. Non-canonical Targets of HIF1a Impair Oligodendrocyte Progenitor Cell Function. Cell Stem Cell 2021;28:257–272 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroka DM, Burkhardt T, Desbaillets I, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J 2001;15:2445–2453. [DOI] [PubMed] [Google Scholar]
- 48.Almeida AS, Figueiredo-Pereira C, Vieira HL. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front Physiol 2015;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ordog T, Takayama I, Cheung WK, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000;49:1731–1739. [DOI] [PubMed] [Google Scholar]
- 50.Choi KM, Gibbons SJ, Roeder JL, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil 2007;19:585–595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


