Abstract
Tumor necrosis factor (TNF) ligand family members are synthesized as transmembrane proteins, and cleavage of the membrane-anchored proteins from the cell surface is frequently observed. The TNF-related ligands APRIL and BLyS and their cognate receptors BCMA/TACI form a two ligand/two receptor system that has been shown to participate in B- and T-cell stimulation. In contrast to BLyS, which is known to be cleaved from the cell surface, we found that APRIL is processed intracellularly by furin convertase. Blockage of protein transport from the endoplasmic reticulum to the Golgi apparatus by Brefeldin A treatment abrogated APRIL processing, whereas monensin, an inhibitor of post-Golgi transport, did not interfere with cleavage of APRIL, but blocked secretion of processed APRIL. Thus, APRIL shows a unique maturation pathway among the TNF ligand family members, as it not detectable as a membrane-anchored protein at the cell surface, but is processed in the Golgi apparatus prior to its secretion.
INTRODUCTION
The tumor necrosis factor (TNF) family has an important role in inducing various biological responses such as differentiation, cell death, survival and proliferation. TNF family ligands are known to be synthesized as membrane-bound proteins, of which several have been shown to be proteolytically cleaved into a soluble form (Smith et al., 1994). For TNFα, a physiological role has been reported for both transmembrane and soluble forms (Grell et al., 1995). In the case of Fas ligand, it has been shown that the transmembrane, but not the soluble species, is a potent inducer of apoptosis, and it was concluded that cleavage of Fas ligand allows down-regulation of the activity of the membrane form (Schneider et al., 1998). The physiological consequences of the shedding of TNF cytokines thus seem to differ for each protein.
Two new members of the TNF family, BLyS (also called BAFF, TALL-1, THANK-1 and zTNF4) (Mackay et al., 1999; Moore et al., 1999; Gross et al., 2000; Khare et al., 2000) and a proliferation-inducing ligand (APRIL), have recently been described (Hahne et al., 1998). Both ligands promote B-cell proliferation by binding to the B-cell maturation antigen (BCMA) and transmembrane activator and CAML-interactor (TACI) receptors (Gross et al., 2000; Marsters et al., 2000; Wu et al., 2000), which are expressed on resting and activated B cells; in addition, TACI has been found on a subset of activated T cells. Nonetheless, the biological role of APRIL does not seem to be restricted to proliferation induction (Hahne et al., 1998), but to be more complex, as a proapoptotic effect of APRIL has been demonstrated as well (Kelly et al., 2000). Furthermore, elevated expression levels of APRIL in tumour tissues and an increased tumour growth rate of APRIL-transfected NIH 3T3 cells in nude mice suggest a regulatory role for APRIL in tumour growth (Hahne et al., 1998).
Many proteins, including hormones, viral proteins, growth factors and receptors, are synthesized as inactive precursor proteins that must be proteolytically processed to become biologically active. Furin, a ubiquitously expressed pro-protein convertase, has been shown to process many pro-proteins (Molloy et al., 1999). Here we report that APRIL is processed intracellularly by the action of furin convertase, and that the secreted form stimulates proliferation of Jurkat cells.
RESULTS AND DISCUSSION
APRIL is processed by a furin convertase
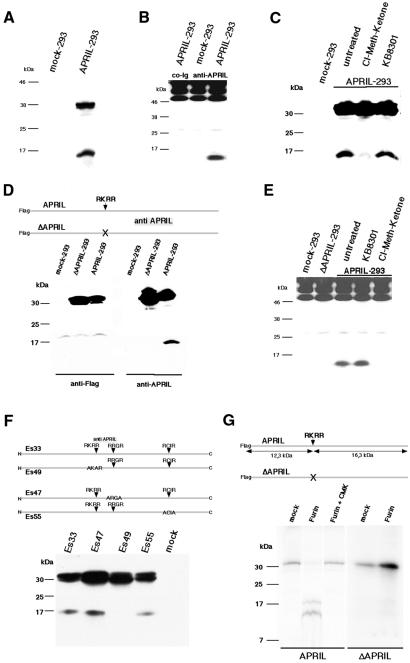
APRIL is a type II membrane protein with a predicted cytoplasmic domain, a hydrophobic transmembrane region and an extracellular domain of 201 amino acids. To study APRIL processing, human embryonic kidney (HEK) 293T cells were transfected with full-length human APRIL, and cell extracts were analysed for APRIL expression using an affinity-purified polyclonal antibody against amino acids 124–250 of the APRIL extracellular domain, which was generated as described (see Methods). The polyclonal antibody recognised the 30 kDa unprocessed form of APRIL in NP-40 extracts of APRIL-transfected HEK 293T cells, but showed no reactivity with NP-40 extracts of mock-transfected cells, confirming its specificity (Figure 1A). In addition to the 30 kDa species, the anti-APRIL antibody recognized a lower molecular weight form of ∼17 kDa. This species comigrates with a soluble form of APRIL, which was immunoprecipitated from culture supernatants of APRIL-transfected, but not mock-transfected, cells (Figure 1B). We therefore conclude that the 17 kDa species is the processed form of APRIL.
Fig. 1. APRIL is processed by a furin convertase. (A) Characterization of APRIL transfectants. HEK 293T cells were transfected with APRIL or empty vector (mock); cell extracts were analysed by western blot employing affinity-purified polyclonal anti-APRIL antibody. (B) Immunoprecipitates of culture supernatants derived from APRIL- and mock-transfected cells were analysed by western blot. Note that the same affinity-purified polyclonal anti-APRIL antibody was used for immunoprecipitations as well as for western blot; precipitated immunoglobulins (>46 and 25 kDa) were developed in western blot as well. An additional control immunoprecipitation of transfectant culture supernatants using non-specific rabbit immunoglobulin is thus shown. (C) An inhibitor for furin convertase, but not for metalloprotease, abrogates APRIL processing. Cells were treated for 5 h with 50 µM chloromethylketone derivative (furin convertase inhibitor) or 10 µM KB8301 (metalloprotease inhibitor) and subsequently analysed. (D) Deletion of amino acids 101–104 abrogates APRIL processing. HEK 293 cells stably transfected with Flag-tagged APRIL, Flag-tagged ΔAPRIL mutant lacking amino acids 101–104 or empty vector (mock), and cell extracts were analysed by western blot employing affinity-purified polyclonal anti-APRIL antibody. (E) Soluble APRIL cannot be immunoprecipitated from culture supernatants of chloromethylketone-treated APRIL transfectants and ΔAPRIL-transfected HEK 293 cells. Immunoprecipitates of culture supernatants derived from APRIL-, ΔAPRIL- and mock-transfected cells were analysed by western blot. (F) Analysis of APRIL processing during maturation. HEK 293T cells were transiently transfected with truncated or mutated APRIL constructs and cell extracts were analysed by western blot using polyclonal anti-APRIL antibody affinity purified on a peptide spanning amino acids 124–146. (G) APRIL digestion by furin in vitro. APRIL and deleted APRIL (ΔAPRIL) were in vitro translated, immunoprecipitated and either mock digested or digested with furin, alone or in the presence of the furin inhibitor chloromethylketone (CMK). Proteins were separated on 15% SDS–polyacrylamide gels.
Two members of the TNF cytokine family, TNFα and Fas ligand, are known to be processed by metalloproteinases. Two other members of the family, BAFF and TWEAK, are reported to be cleaved at a multibasic motif R-X-K/R-R (Chicheportiche et al., 1997; Schneider et al., 1999). An arginine-rich motif is also the cleavage site for several growth factors and, in the case of TGF, it has been reported that processing is mediated by furin convertase (Molloy et al., 1999). As APRIL contains an RKRR motif in the stalk region at amino acids 101–104, APRIL-transfected HEK 293 cells were cultured in the presence of a chloromethylketone derivative, a specific inhibitor of furin convertase (Hallenberger et al., 1992). Chloromethylketone treatment of APRIL-transfected cells almost completely abolished the cleavage of APRIL, whereas treatment with the metalloproteinase inhibitor KB8301 (Kayagaki et al., 1995) had no effect on APRIL processing (Figure 1C). In agreement with this, soluble APRIL could be immunoprecipitated from culture supernatants of KB8301-treated, but not of chloromethylketone-treated, cells (Figure 1E). These results suggest that APRIL is processed by a furin convertase and not by metalloproteases, and that this processing is required for secretion.
To confirm these results, we constructed a mutated form of APRIL (ΔAPRIL) lacking the RKRR motif (Figure 1D). APRIL and ΔAPRIL were Flag-tagged at the N-terminal end. Western blot analysis of ΔAPRIL-transfected HEK 293 cells showed that the processed form of APRIL could neither be detected in cell extracts nor immunoprecipitated from culture supernatant (Figure 1E), suggesting that the unprocessed form of APRIL is indeed cleaved at the RKRR motif. In agreement with this, anti-Flag antibody revealed only the unprocessed form of APRIL, but not the processed species, suggesting rapid turnover of the N-terminal fragment.
APRIL contains two additional putative cleavage sites for furin at amino acids 143–146 (RRGR) and 195–198 (RCIR). These two motifs do not correspond to the consensus furin site -R-X-R/K-R-, although it was stated that the -R-X-X-R- motif can serve as a minimal furin cleavage site (Molloy et al., 1999). Neither replacement of the -R-R-G-R- motif by -A-R-G-A- (construct Es47) nor substitution of the -R-C-I-R- motif by -A-C-I-A- (construct Es55) abrogated APRIL processing. In contrast, replacement of the -R-K-R-R- motif by -A-K-R-A- (construct Es49) resulted in the predicted blocking of APRIL processing (Figure 1F).
To test whether cleavage of APRIL is mediated directly by furin, in vitro-translated APRIL was exposed to purified furin. Furin treatment converted unprocessed APRIL into the cleavage products, which was abrogated in the presence of the furin inhibitor chloromethylketone (Figure 1G). In addition, furin did not cleave in vitro-translation products of APRIL in which the cleavage site was deleted, providing evidence that APRIL is cleaved directly by furin at amino acids 101–104 (Figure 1G).
Processing of APRIL is localized in the Golgi apparatus
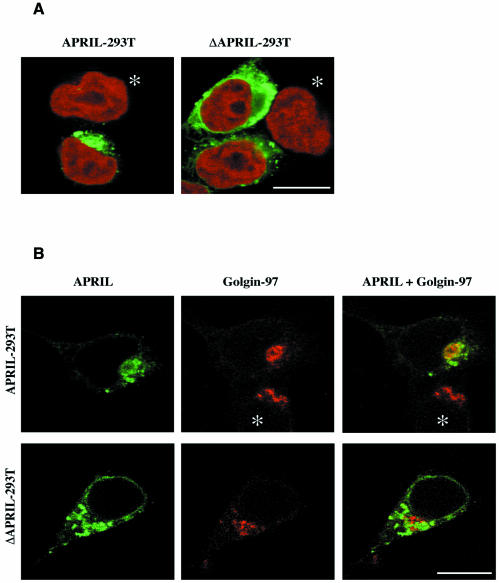
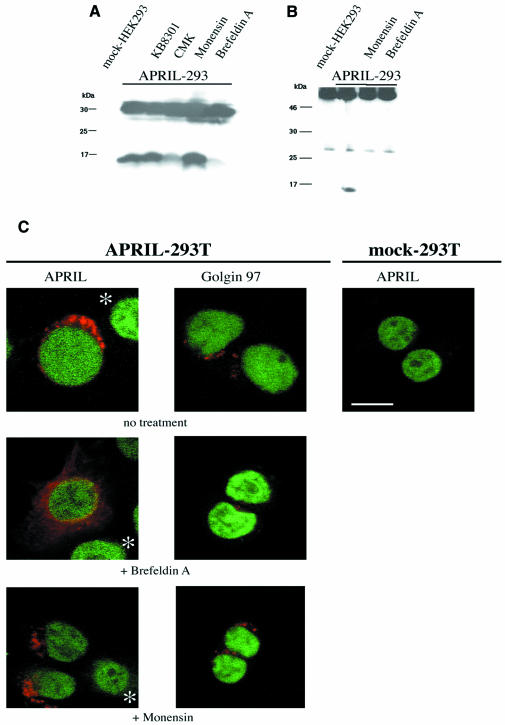
Furin has been described as a secretory pathway endoprotease that catalyses maturation of growth factors, including insulin-like growth factor-2 (IGF-2) and transforming growth factor (TGF) (Dubois et al., 1995; Duguay et al., 1997). The fact that the processed form of APRIL could be detected in NP-40 lysates suggests that APRIL processing occurs intracellularly rather than on the cell surface. Furin is predominantly localized within the trans-Golgi network (TGN)/endosomal system, but has also been detected on the cell surface and extracellularly (Molloy et al., 1999). To determine the site of APRIL processing, we took advantage of the APRIL constructs containing a Flag-tag on the N-terminal end and employed an anti-Flag antibody in immunofluorescence analysis. Figure 2A shows the intracellular localization of APRIL, whereas no APRIL was detected on the cell surface. This concurs with the finding that no surface staining was observed in FACS analysis using the anti-APRIL antibody (not shown). Double immunofluorescence labelling was employed to identify the subcellular localization of APRIL, using a monoclonal antibody against golgin-97 as specific marker for the Golgi apparatus (Figure 2B) (Griffith et al., 1997). Colocalization of APRIL immunostaining with golgin-97 became apparent when both images were superposed and fluorescence merged, demonstrating that APRIL is localized in the Golgi apparatus. In ΔAPRIL transfectants lacking the RKRR motif, the subcellular distribution of mutated APRIL was more disperse than that of wild-type APRIL. This included a distinct perinuclear localization, suggesting accumulation of the mutated, unprocessed protein, possibly in the endoplasmatic reticulum (ER) due to misfolding of the mutated form of APRIL. Nevertheless, the fact that in vitro-translated ΔAPRIL cannot be cleaved by purified furin indicates that the mutant protein is unlikely to be cleaved in the cell because it lacks the appropriate cleavage site, rather than being retained in the ER. To verify that APRIL processing takes place within the Golgi apparatus, we treated APRIL transfectants with the Golgi-disturbing agents monensin and Brefeldin A (BFA). Western blot analysis from cell lysates of APRIL transfectants showed that BFA abrogates cleavage of APRIL, whereas monensin had no effect on APRIL processing (Figure 3A). No soluble APRIL species could be immunoprecipitated from culture supernatants of BFA- or monensin-treated cells (Figure 3B), demonstrating that both Golgi-disturbing agents block secretion of APRIL. Monensin reversibly slows the intracellular transport rate of newly-synthesized proteins, interfering especially with transfer across Golgi compartments and compromising secretion from the trans-Golgi (Tartakoff, 1983). BFA, which inhibits protein transport between the ER and the Golgi apparatus, is reported to cause a disappearance of the Golgi structure (Lippincott-Schwartz et al., 1989). Concurring with this, immunostaining for golgin-97 was scattered following BFA treatment (Figure 3C) and immunostaining for APRIL was dispersed in APRIL transfectants after BFA treatment, whereas monensin caused no APRIL redistribution (Figure 3C).
Fig. 2. APRIL associates with the Golgi apparatus. (A) APRIL is intracellularly localized. APRIL staining is shown in green, DNA staining with Topro-3 in red. (B) APRIL colocalizes with anti-human golgin-97 (CF4) monoclonal antibody, a marker for Golgi apparatus. APRIL staining is shown in green, staining for golgin-97 (CF4) in red. Yellow visualizes the overlap of the two fluorescent antibodies. Images are shown of transiently transfected 293T cells. Similar images were obtained on stably transfected cells. Asterisks indicate non-transfected cells serving as negative controls. Bar, 50 µm.
Fig. 3. Processing of APRIL takes place in the Golgi apparatus. (A) BFA abrogates cleavage of APRIL. HEK 293T cells were transiently transfected with APRIL, and treated for 5 h with BFA (3.5 µM), monensin (3.5 µM), chloromethylketone derivative (CMK; furin convertase inhibitor, 50 µM) or KB8301 (metalloprotease inhibitor, 10 µM) and subsequently analysed by western blot employing affinity-purified polyclonal anti-APRIL antibody. Proteins were separated in 15% SDS–PAGE. (B) Soluble APRIL cannot be immunoprecipitated from culture supernatants of BFA- or monensin-treated APRIL transfectants. Immunoprecipitates of culture supernatants from APRIL-transfected cells treated with BFA or monensin for 5 h were analysed by western blot. Proteins were separated on a 15% SDS–polyacrylamide gel. (C) Treatment with BFA, but not monensin, causes delocalization of APRIL. Cells were incubated with BFA (3.5 µM) or monensin (3.5 µM) for 5 h prior to fixing. Staining for golgin-97 or APRIL is shown in red, DNA staining with SybrGreen in green. Asterisks indicate non-transfected cells serving as negative controls. Bar, 10 µm.
BFA treatment provokes disassembly of the Golgi apparatus and redistribution of various Golgi enzymes to the ER (Wood et al., 1991), whilst a portion of the trans-Golgi region remains separate (Chege and Pfeffer, 1990; Berger et al., 1995). The unprocessed form of APRIL relocalizes after BFA treatment and is thus not exposed to furin, which resides in the trans-Golgi compartment (Molloy et al., 1999). Monensin, a known inhibitor of post-Golgi transport, did not interfere with APRIL cleavage, but blocked secretion of processed APRIL. In summary, we demonstrated that APRIL processing takes place in the Golgi apparatus rather than at the cell surface. This describes for APRIL a new, distinct maturation pathway within the TNF ligand family.
The secreted form of APRIL is biologically active
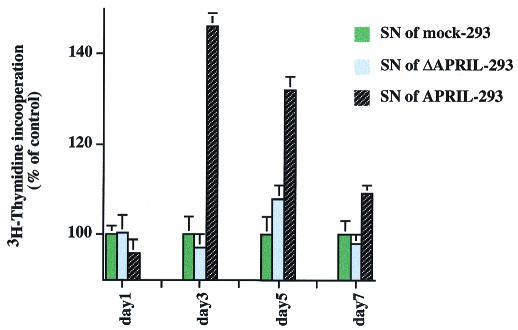
Many proteins, including hormones and growth factors, are synthesized as inactive precursors that must be proteolytically processed to release the bioactive polypeptide. To test whether the secreted form of APRIL displays biological activity, Jurkat cells, which are susceptible to APRIL, were cultured in the presence of supernatants from APRIL transfectants. Culture supernatants of mock and ΔAPRIL transfectants served as controls. Supernatants derived from APRIL transfectants stimulated proliferation of Jurkat cells as measured by thymidine incorporation, whereas conditioned media of mock or ΔAPRIL transfectants had no effect on Jurkat cells (Figure 4). This confirms that the secreted form of APRIL is biologically active and that this activity is regulated by the intracellular processing described above.
Fig. 4. Secreted APRIL induces additional proliferation of Jurkat cells. Jurkat cells were incubated for indicated time points in the presence of culture supernatant derived from mock-, ΔAPRIL- and APRIL-transfected HEK 293 cells (1:2 dilution with fresh RPMI medium). Proliferation was measured by [3H]thymidine incorporation. Data are the mean ± SEM of triplicate determinations. Proliferation of control treated cells is taken as 100%. Using ANOVA analysis of variance, the following values were obtained: p >0.05 for day 1; p <0.01 for days 3, 5 and 7.
BLyS and APRIL have several features in common: they are both expressed in monocytes and macrophages, and both participate in B- and T-cell stimulation by binding to the receptors TACI and BCMA (Marsters et al., 2000; Yu et al., 2000; Khare and Hsu, 2001). Moreover, BLyS has been shown to be cleaved at a multibasic motif -R-N-K-R-, presumably by furin (Schneider et al., 1999; Nardelli et al., 2001). In contrast to APRIL, however, it has been clearly demonstrated that soluble BLyS is released from the cell surface by processing of membrane-bound BLyS (Schneider et al., 1999; Nardelli et al., 2001), and both cell surface-bound as well as secreted BLyS were shown to have biological activity (Schneider et al., 1999). So far, no functional differences between APRIL and BLyS are known (Khare and Hsu, 2001), and their different post-translational regulation suggests a distinct, but complementary, role for these two proteins.
METHODS
Materials. The anti-Flag M2 monoclonal antibodies, uncoupled or biotinylated, were purchased from Sigma Chemical, the anti-human golgin-97 antibody CDF4 from Molecular Probes and secondary antibodies from Jackson ImmunoResearch. BFA and monensin were obtained from Sigma, furin convertase inhibitor chloromethylketone from Alexis Corp. and metalloproteinase inhibitor KB8301 from PharMingen. Recombinant human furin convertase was purchased from Alexis Corp.
Plasmids. EST clone AA29304 was used to clone the coding region of APRIL into the expression pCRIII vector (Invitrogen). N-terminal Flag and site-specific mutations were introduced by performing PCR using modified primers (primer sequences available on request).
Cells and transfections. HEK 293 and HEK 293T cells were maintained in DMEM containing 10% heat-inactivated fetal calf serum (FCS). Human T leukaemia Jurkat cells were grown in RPMI supplemented with 10% FCS. Media contained antibiotics (penicillin and streptomycin at 5 µg/ml each). Constructs containing the APRIL open reading frame were cloned into the pCRIII expression vector (Invitrogen) and transfected into HEK 293T cells using the Lipofectamine method (Life Technologies). For the generation of stably transfected cell lines, HEK 293 cells were used and selected after transfection for neomycin resistance (800 µg/ml; Sigma).
Proliferation assays. Cells were plated at 5 × 104 cells/well and proliferation quantified by 3H-thymidine uptake (1 µCi/200 µl) during the final 24 h of culture.
Polyclonal antibody production. Polyclonal anti-APRIL antibodies were generated in rabbits after immunization with a recombinant soluble form of APRIL spanning extracellular amino acids 105–250. Purified IgG fractions were affinity purified on recombinant APRIL coupled to CNBr-activated Sepharose (Amersham Pharmacia Biotech). Subsequent affinity purification was performed on a peptide encompassing amino acids 124–146.
Western blot analysis and immunoprecipitation. Cell lysates were prepared in NP-40 buffer (1% NP-40, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 5 mM natrium fluoride, 1 mM PMSF in 50 mM Tris pH 7.4). Following quantification of protein content to ensure equal loading of the various cell samples, lysates were electrophoretically separated by SDS–PAGE and subsequently transferred to nitrocellulose. Equal loading and transfer was verified in each experiment by Ponceau S staining of the membrane (Sigma). Immunoblots were probed using either 2 µg/ml of affinity-purified anti-APRIL antibody or 5 µg/ml anti-Flag M2 mAb (Sigma) and developed using the ECL system (Amersham Pharmacia Biotech). Immunoprecipitations were performed by overnight incubation of cell culture supernatants with 2.5 µg of affinity-purified anti-APRIL antibody, followed by a 4 h incubation with 5 µl of Protein A CL–4B Sepharose beads (Amersham Pharmacia Biotech). After three washes with phosphate-buffered saline (PBS)/100 mM NaCl, beads were boiled in sample buffer and analysed by western blotting.
Immunofluorescence and image acquisition. Cells were cultured overnight in poly-l-lysine (Sigma) coated chamber slides, washed with PBS, fixed in 4% paraformaldehyde (15 min at room temperature) and permeabilized for 15 min with 0.1% Triton-X 100 in PBS (Sigma). After washing three times with PBS/Tween 20 (0.1%), slides were pre-incubated in 5% bovine serum albumen (BSA), 0.1% Tween 20 (Sigma), 0.1% Triton X-100, 10% goat serum in PBS, then incubated for 1 h with 20 µg/ml of primary golgin-97 antibody (Molecular Probes) or 0.5 µg/ml anti-Flag mAb M2. Primary antibodies were diluted in dilution buffer (0.5% BSA, 0.1% Tween 20, 0.1% Triton X-100, 3% goat serum in PBS). Cells were washed three times in washing buffer (dilution buffer without goat serum) and incubated for 1 h with Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch). In some experiments, samples were incubated with Topro-3 or SybrGreen (Molecular Probes) for DNA staining. Images were obtained using an Ar–Kr laser and a TCS-NT Leica confocal imaging system (Leica Microsystems). When indicated, cells were incubated for 5 h with 3.5 µM BFA or monensin before fixing.
In vitro translation and cleavage assays. In vitro translation experiments were performed as described (Muellner and Garcia-Sanz, 1997); the protein products were separated in 15% SDS–PAGE and detected by subsequent fluorography. In some cases, in vitro translation products were immunoprecipitated using the anti-Flag M2 mAb and resuspended in 100 mM HEPES at pH 7.6, 1 mM CaCl2, 0.5% Triton X-100 and 1 mM 2-mercaptoethanol. Enzyme assays were performed by adding 1 U furin convertase alone or with the furin convertase inhibitor chloromethylketone at 30°C for 1 h.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs S. Mañes, B. Pradet-Balade, J.A. Garcia-Sanz, J.P. Medema and M. Campanero for critical reading of the manuscript, Cathy, Keith and Lucio for their invaluable assistance and the excellent services of the CNB. M.L.F. is supported by the Consejería de Educación y Cultura de la Comunidad de Madrid financed by the Fondo Social Europeo. The Department of Immunology and Oncology was founded and is supported by the Spanish Council for Scientific Research and by the Pharmacia Corporation.
REFERENCES
- Berger E.G., Burger, P., Hille, A. and Bachi, T. (1995) Comparative localization of mannose-6-phosphate receptor with 2,6-sialyltransferase in HepG2 cells: an analysis by confocal double immunofluorescence microscopy. Eur. J. Cell Biol., 67, 106–111. [PubMed] [Google Scholar]
- Chege N.W. and Pfeffer, S.R. (1990) Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J. Cell Biol., 111, 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicheportiche Y., Bourdon, P.R., Xu, H., Hsu, Y.M., Scott, H., Hession, C., Garcia, I. and Browning, J.L. (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem., 272, 32401–32410. [DOI] [PubMed] [Google Scholar]
- Dubois C.M., Laprise, M.H., Blanchette, F., Gentry, L.E. and Leduc, R. (1995) Processing of transforming growth factor β 1 precursor by human furin convertase. J. Biol. Chem., 270, 10618–10624. [DOI] [PubMed] [Google Scholar]
- Duguay S.J., Milewski, W.M., Young, B.D., Nakayama, K. and Steiner, D.F. (1997) Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J. Biol. Chem., 272, 6663–6670. [DOI] [PubMed] [Google Scholar]
- Grell M. et al. (1995) The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell, 83, 793–802. [DOI] [PubMed] [Google Scholar]
- Griffith K.J., Chan, E.K., Lung, C.C., Hamel, J.C., Guo, X., Miyachi, K. and Fritzler, M.J. (1997) Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren’s syndrome. Arthritis Rheum., 40, 1693–1702. [DOI] [PubMed] [Google Scholar]
- Gross J.A. et al. (2000) TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature, 404, 995–999. [DOI] [PubMed] [Google Scholar]
- Hahne M. et al. (1998) APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J. Exp. Med., 188, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenberger S., Bosch, V., Angliker, H., Shaw, E., Klenk, H.D. and Garten, W. (1992) Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature, 360, 358–361. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Kawasaki, A., Ebata, T., Ohmoto, H., Ikeda, S., Inoue, S., Yoshino, K., Okumura, K. and Yagita, H. (1995) Metalloproteinase-mediated release of human Fas ligand. J. Exp. Med., 182, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Manos, E., Jensen, G., Nadauld, L. and Jones, D.A. (2000) APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res., 60, 1021–1027. [PubMed] [Google Scholar]
- Khare S.D. and Hsu, H. (2001) The role of TALL-1 and APRIL in immune regulation. Trends Immunol., 22, 61–63. [DOI] [PubMed] [Google Scholar]
- Khare S.D. et al. (2000) Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc. Natl Acad. Sci. USA, 97, 3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan, L.C., Bonifacino, J.S., and Klausner, R.D. (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell, 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F., Woodcock, S.A., Lawton, P., Ambrose, C., Baetscher, M., Schneider, P., Tschopp, J. and Browning, J.L. (1999) Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med., 190, 1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsters S.A., Yan, M., Pitti, R.M., Haas, P.E., Dixit, V.M. and Ashkenazi, A. (2000) Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Biol., 10, 785–788. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Anderson, E.D., Jean, F. and Thomas, G. (1999) Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell. Biol., 9, 28–35. [DOI] [PubMed] [Google Scholar]
- Moore P.A. et al. (1999) BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science, 285, 260–263. [DOI] [PubMed] [Google Scholar]
- Muellner E.W. and Garcia-Sanz, J.A. (1997) Additional uses of RNA probes. Immunology Methods Manual. Academic Press, London, 7.8, 464–476. [Google Scholar]
- Nardelli B. et al. (2001) Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood, 97, 198–204. [DOI] [PubMed] [Google Scholar]
- Schneider P., Holler, N., Bodmer, J.L., Hahne, M., Frei, K., Fontana, A. and Tschopp, J. (1998) Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med., 187, 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P. et al. (1999) BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med., 189, 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.A., Farrah, T. and Goodwin, R.G. (1994) The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell, 76, 959–962. [DOI] [PubMed] [Google Scholar]
- Tartakoff A.M. (1983) Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell, 32, 1026–1028. [DOI] [PubMed] [Google Scholar]
- Wood S.A., Park, J.E. and Brown, W.J. (1991) Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell, 67, 591–600. [DOI] [PubMed] [Google Scholar]
- Wu Y. et al. (2000) Tumor necrosis factor receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J. Biol. Chem., 275, 35478–35485. [DOI] [PubMed] [Google Scholar]
- Yu G. et al. (2000) APRIL and TALL-1 and receptors BCMA and TACI: system for regulation humoral activity. Nature Immunol., 1, 252–256. [DOI] [PubMed] [Google Scholar]