Abstract
Rationale
Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) has been shown to be safe and efficacious in people with cystic fibrosis (pwCF) aged 2 years and older with at least one F508del-CFTR allele or more. After U.S. approval in 2019, reports emerged of depression-related adverse events in pwCF treated with ELX/TEZ/IVA.
Objectives
To review available evidence on depression-related events in pwCF treated with ELX/TEZ/IVA in the context of background epidemiology in pwCF.
Methods
Safety data from 14 ELX/TEZ/IVA clinical trials and 10 trials of CF transmembrane conductance regulator (CFTR) modulators in which placebo was administered, along with data from CF registries in the United States and Germany and cumulative postmarketing adverse event data from 61,499 pwCF who initiated ELX/TEZ/IVA after initial approval in the United States (October 2019) through October 2022, were reviewed and used to calculate exposure-adjusted rates of depression-related adverse events and prevalence of depression. In addition, a scientific literature review was conducted to identify ELX/TEZ/IVA publications reporting depression-related events or changes in depressive symptoms after treatment initiation.
Measurements and Main Results
In clinical trials, the exposure-adjusted rate of any depression-related adverse event was 3.32/100 person years (PY) in the pooled ELX/TEZ/IVA group (n = 1,711) and 3.24/100 PY in the pooled placebo group (n = 1,369). The exposure-adjusted rates of suicidal ideation and suicide attempt were also similar between the pooled ELX/TEZ/IVA group and pooled placebo group (ideation: 0.23/100 PY vs. 0.28/100 PY; attempt: 0.08/100 PY vs. 0.14/100 PY). In the postmarketing setting, the exposure-adjusted reporting rates of depression-related events were low in context of the background prevalence in pwCF (all depression-related events: 1.29/PY; suicidal ideation: 0.12/100 PY; and suicide attempt: 0.05/100 PY). Assessments of individual case reports were confounded by preexisting mental health conditions, intercurrent psychosocial stressors (including coronavirus disease [COVID-19] lockdowns), and the heterogeneous and fluctuating nature of depression. Data from CF registries in the United States and Germany showed that patterns of depression prevalence in pwCF exposed to ELX/TEZ/IVA did not change after treatment initiation. Published studies utilizing the nine-item Patient Health Questionnaire did not show evidence of worsening depression symptoms in pwCF treated with ELX/TEZ/IVA.
Conclusions
Our review of data from clinical trials, postmarketing reports, an ongoing registry-based ELX/TEZ/IVA postauthorization safety study, and peer-reviewed literature suggests that depression symptoms and depression-related events reported in pwCF treated with ELX/TEZ/IVA are generally consistent with background epidemiology of these events in the CF population and do not suggest a causal relationship with ELX/TEZ/IVA treatment.
Keywords: CFTR modulator, elexacaftor/tezacaftor/ivacaftor, mental health, depression
At a Glance Commentary
Scientific Knowledge on the Subject
After U.S. approval of the use of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) in adolescents and adults with CF, reports emerged of depression-related adverse events (AEs) in individuals treated with commercially available ELX/TEZ/IVA.
What This Study Adds to the Field
We summarized and reviewed safety data on depression-related events from completed ELX/TEZ/IVA clinical trials, an ongoing ELX/TEZ/IVA postauthorization safety study using registry data, postmarketing safety reports, and published literature. In clinical trials, exposure-adjusted rates of depression-related AEs in people with CF (pwCF) taking ELX/TEZ/IVA were similar to rates seen in those given placebo. Postmarketing reports of depression-related events were low in the context of the high background prevalence of these events in pwCF, with assessments of individual cases confounded by preexisting mental health conditions, intercurrent psychosocial stressors (including coronavirus disease [COVID-19]), and the heterogeneous and fluctuating nature of depression. Registry data showed that patterns of depression prevalence did not change after initiation of ELX/TEZ/IVA treatment. Finally, published studies with validated instruments did not indicate worsening of depression symptoms with ELX/TEZ/IVA treatment. These data suggest that depression-related events observed in pwCF taking ELX/TEZ/IVA are generally consistent with background epidemiology of these events in the CF population.
Cystic fibrosis (CF) is an autosomal recessive disorder that results from mutations in the CF transmembrane conductance regulator (CFTR) gene (1). Over the past decade, the advancement of small-molecule CFTR modulator therapies has changed the CF treatment landscape (2). CFTR potentiators, such as ivacaftor (IVA), act by augmenting the gating of mutant CFTR proteins, whereas CFTR correctors, such as tezacaftor (TEZ) and elexacaftor (ELX), act by remedying the defects in CFTR protein processing and trafficking associated with CFTR misfolding mutations, including F508del, the most common CFTR mutation present on at least one allele in up to 90% of people with CF (pwCF) in many parts of the world (3–7).
Clinical trials have shown that the triple-combination CFTR modulator regimen ELX/TEZ/IVA is safe and efficacious in pwCF ages 2 years and older who have at least one F508del allele (8–13), leading to robust and clinically meaningful improvements in lung function, CFTR function, and respiratory symptoms. Longer-term data from a 192-week open-label extension study of ELX/TEZ/IVA showed that lung function improvements were sustained with no mean decline in pulmonary function (14), whereas real-world data showed profound decreases in pulmonary exacerbations, lung transplantation, and mortality (15). Taken together, these findings have established ELX/TEZ/IVA as a standard of care in countries where it has been approved for use.
After the United States granted approval of ELX/TEZ/IVA in 2019, several published case reports and studies described depression-related events in pwCF who took ELX/TEZ/IVA (16–21). When evaluating these reports, it is important to account for the well-acknowledged high background prevalence of depression symptoms and depression-related events (suicidal ideation, attempts) in the CF population, with the prevalence of depression symptoms estimated to be two to three times higher than that in the general population (22, 23). Elevated rates of suicidal ideation have also been reported in pwCF, with a prevalence of up to 11% in adults and even higher (up to 22%) in adolescents (24–26). Recognizing the importance of monitoring mental health in pwCF, in 2016, the U.S. Cystic Fibrosis Foundation, in collaboration with the European Cystic Fibrosis Society, developed clinical guidelines for the screening and treatment of depression and anxiety (27). Finally, market availability of ELX/TEZ/IVA coincided with the onset of the coronavirus disease (COVID-19) pandemic, when multiple reports described the detrimental impact of the pandemic and associated lockdowns on mental health in the general population, suggesting a more than threefold higher prevalence of depression and an approximately twofold higher prevalence of suicidal ideation at the peak of the pandemic compared with that during the prepandemic period (28–30).
To better understand the incidence of depression symptoms and depression-related events in pwCF treated with ELX/TEZ/IVA in the context of background epidemiology, we systematically reviewed and evaluated depression-related data in individuals taking ELX/TEZ/IVA from clinical trials, postmarketing reports, an ongoing registry-based study, and available scientific literature.
Methods
Data sources for this review included clinical trials of ELX/TEZ/IVA in pwCF ages 6 years and older, postmarketing reports, interim results from an ongoing registry-based ELX/TEZ/IVA postauthorization safety study, and peer-reviewed literature.
All authors contributed to the design of this review, had access to and reviewed clinical trial and postmarketing data, critically edited the manuscript, and approved it for submission.
Clinical Trials
The standardized Medical Dictionary for Regulatory Activities query “Depression and suicide/self-injury” was used to identify and analyze depression-related adverse events in CFTR modulator clinical trials. Use of this validated query, which contains multiple preferred terms related to depression and suicide/self-injury, provided a means to identify safety signals in the CFTR modulator clinical trial datasets. Exposure-adjusted rates of depression-related adverse events (including suicidal ideation and suicide attempts) were calculated per 100 person years (PY) of exposure in the ELX/TEZ/IVA and placebo arms of the pivotal Phase 3 Study 445–102. In addition, rates of depression-related events were calculated for the pooled population of patients ages 6 years and older who received ELX/TEZ/IVA across 14 clinical trials (see Table E1 in the online supplement) and were compared with rates for the pooled population of patients ages 6 years and older who were randomized to placebo across 10 randomized, placebo-controlled trials of CFTR modulators (see Table E2 in the online supplement). In all ELX/TEZ/IVA clinical trials where safety was a primary endpoint, sample sizes were deemed sufficient such that the probability of observing at least one participant with an adverse event that had an incidence of 5% or 10% was >94% or >99%, respectively.
Postmarketing Reports
Adverse events experienced by pwCF while taking commercially available ELX/TEZ/IVA are reported to the sponsor safety database. Using the standardized Medical Dictionary for Regulatory Activities query “Depression and suicide/self-injury,” we conducted a search of the Vertex Pharmaceuticals global safety database to identify relevant postmarketing cases that were received through October 20, 2022. Reporting rates were calculated as the number of reported events per 100 PY of postmarketing exposure.
Registry-based Postauthorization Safety Study
Depression prevalence data were reviewed from the second interim analysis of the ongoing registry-based, 5-year postauthorization safety study (VX20–445–120). This study is being conducted as a commitment to the European Medicines Agency to understand the long-term effects of ELX/TEZ/IVA under real-world use conditions (European Union electronic Register of Post-Authorisation Studies, or EU PAS, register number 43022). Data are being collected from the U.S. Cystic Fibrosis Foundation Patient Registry and German Cystic Fibrosis Registry for pwCF who initiate ELX/TEZ/IVA treatment during patient accrual periods (United States: 2019–2020; Germany: 2020–2021). Posttreatment patterns in depression prevalence (calculated as the annual proportion of pwCF with depression noted as a complication in the registry) were compared within a 5-year pretreatment period. The second interim analysis included data through December 31, 2021.
Review of Published Literature
A search of published literature using the Medline and Embase databases was conducted on October 4, 2023, to identify case reports, case series, or cohort studies describing the occurrence of depression events after the initiation of ELX/TEZ/IVA treatment (additional details on the literature search and PRISMA diagram can be found in the online supplement). Studies that systematically evaluated depression symptoms using validated patient-reported instruments, such as the 9-item Patient Health Questionnaire (PHQ-9), were summarized.
Results
Depression-related Events in Clinical Trials
In the Phase 3 pivotal study 445–102, which formed the basis of the safety profile for ELX/TEZ/IVA, the exposure-adjusted rate of depression-related adverse events was 2.0 per 100 PY for those taking ELX/TEZ/IVA and 5.0 per 100 PY in those given placebo (Table 1). An integrated analysis of pooled clinical trial data showed the exposure-adjusted rate of depression-related adverse events was 3.32 per 100 PY in the pooled ELX/TEZ/IVA group (n = 1,711 across 14 trials) and was 3.24 per 100 PY in the pooled placebo group (n = 1,369 across 10 trials) (Table 1). Overall, three cases of suicide attempts (0.08 events per 100 PY) and nine cases of suicidal ideation (0.23 events per 100 PY) were reported in the pooled ELX/TEZ/IVA group compared with one case of suicide attempt (0.14 events per 100 PY) and two cases of suicidal ideation (0.28 events per 100 PY) in the pooled placebo group. No completed suicides were reported in the clinical trials. Of the 12 cases of suicide attempt or suicidal ideation reported in the ELX/TEZ/IVA group, 11 resolved with continued ELX/TEZ/IVA treatment, and in the remaining case, the participant was not taking ELX/TEZ/IVA at the time of the event onset.
Table 1.
Incidence of Depression and Depression-related Adverse Events in ELX/TEZ/IVA Pivotal Phase 3 Trial (Study 445-102) and Pooled Clinical Trial Data
| Event | Pivotal Study 445-102 |
Pooled Data |
||
|---|---|---|---|---|
| Placebo (n = 201), 100 PY Events/100 PY |
ELX/TEZ/IVA (n = 202), 100 PY Events/100 PY |
Placebo* (n = 1,369), 709 PY Events/100 PY |
ELX/TEZ/IVA† (n = 1,711), 3,857 PY Events/100 PY |
|
| Any depression AE | 5.0 | 2.0 | 3.24 | 3.32 |
| Suicide attempt | 0 | 0 | 0.14 | 0.08 |
| Suicidal ideation | 1.00 | 0 | 0.28 | 0.23 |
| Completed suicide | 0 | 0 | 0 | 0 |
Definition of abbreviations: AE = adverse event; ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor; PY = person years.
*The placebo column includes data from 10 studies in the VX-445, VX-659, VX-809, VX-661, and VX-770 programs in participants 6 yr and older (see online supplement for the list of included trials).
The ELX/TEZ/IVA column contains data from 14 completed studies in participants 6 yr and older (see online supplement for the list of included trials).
Postmarketing Reports of Depression-related Events
A search of the Vertex Pharmaceuticals global safety database identified 899 cases reporting 1,056 depression-related adverse events out of 61,499 pwCF treated with ELX/TEZ/IVA (representing over 82,053 PY) (Table 2). Overall, this represents an exposure-adjusted rate of 1.29 depression-related adverse events per 100 PY (Table 2). The exposure-adjusted rates of suicidal ideations, suicide attempts, and completed suicides were 0.12 events per 100 PY, 0.05 events per 100 PY, and 0.02 events per 100 PY, respectively. Individual cases generally included limited information in a narrative format without objective psychiatric assessment and were confounded by preexisting mental health conditions, intercurrent psychosocial stressors, and the heterogeneous and fluctuating nature of depression.
Table 2.
Incidence of Depression and Depression-related Adverse Events in ELX/TEZ/IVA Postmarketing Reports
| Preferred Term | Number of Events | Reporting Rate/100 PY |
|---|---|---|
| Any depression AE | 1,056 | 1.3 |
| Suicide attempt | 98 | 0.12 |
| Suicidal ideation | 38 | 0.05 |
| Completed suicide | 14 | 0.02 |
Definition of abbreviations: AE = adverse event; CF = cystic fibrosis; ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor; PY = person years.
The cumulative exposure (e.g., number of people with CF taking ELX/TEZ/IVA who could report an event) in the postmarketing setting was 61,449 patients (82,053 PY) through October 20, 2022.
ELX/TEZ/IVA Registry-based Postauthorization Safety Study
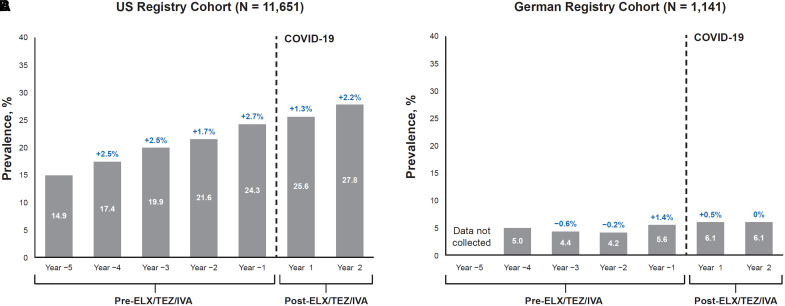
In the ELX/TEZ/IVA postauthorization safety study, data on depression prevalence in pwCF taking ELX/TEZ/IVA were recorded by CF center physicians during each of the pretreatment years (5 yr) and each of the years after ELX/TEZ/IVA initiation (2 yr) in the U.S. CF registry cohort (N = 11,651) and German CF registry cohort (N = 1,141). The annual change in depression prevalence after ELX/TEZ/IVA initiation was consistent with the annual change observed during the 5-year period before starting ELX/TEZ/IVA in both registries, although differences in prevalence estimates were observed between the regions that could be due to differences in the background rates of depression and depression diagnoses in the United States and Germany (Figure 1). The U.S. and German CF registries do not collect data on suicidal ideation and suicide attempts but may record suicide as a cause of death. No suicide deaths were reported in the smaller German CF registry cohort, and the rate of suicide deaths in the larger U.S. CF registry cohort was 0.01 events per 100 PY.
Figure 1.
Prevalence of depression reported in the elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) postauthorization safety study (PASS) longitudinal cohort before and after initiation of ELX/TEZ/IVA use. (A and B) Results using data from people with cystic fibrosis (pwCF) in the U.S. Cystic Fibrosis Foundation Patient Registry (A) and the German Cystic Fibrosis Registry (Mukoviszidose eV) (B). Time periods include 5 years before ELX/TEZ/IVA initiation and 2 years (Year 1 and Year 2) after starting ELX/TEZ/IVA treatment. Change in prevalence of depression from the previous year is indicated in blue. COVID-19 = coronavirus disease.
Literature Search for ELX/TEZ/IVA and Depression-related Events
Overall, a literature search identified publications that fell into one of the following categories: individual case reports of depression-related events, including suicidal ideation and attempts (16, 18, 31); cohort studies reporting depression-related events in some cohort participants (19–21), and studies using the validated depression instrument PHQ-9 or the eight-item PHQ (PHQ-8) to evaluate changes in depression symptoms before and after ELX/TEZ/IVA initiation (Table 3), as well as studies using any other validated instruments (e.g., the Center for Epidemiologic Studies–Depression Scale) (32).
Table 3.
Literature Search Results Showing Changes in PHQ-9 Scores After the Initiation of ELX/TEZ/IVA Treatment in People with CF
| Author, Year (Reference No.) | Country | Age | N | Follow-up Assessment Duration After ELX/TEZ/IVA Treatment | Change in PHQ-9 Scores After ELX/TEZ/IVA Initiation |
|---|---|---|---|---|---|
| Borawska-Kowalczyk, 2023 (36) | Poland | Not specified (abstract) | 52 | 3 mo | No change |
| Ergenekon, 2023 (37) | Turkey | 11–22 yr (median, 18 yr) | 86 | 6 mo | Decrease |
| Graziano, 2023 (38) | Italy | 12–59 yr (mean, 25.4 yr) | 92 | 1, 3, and 6 mo | Decrease |
| Piehler, 2023 (33) | Germany | Median, 27.9 yr (IQR, 22.5–34.1) | 70 | 2–4 mo | Decrease |
| Blackwelder, 2022 (39) | United States | Adult | 172 | 12 mo | Decrease |
| Zhang, 2022 (40) | United States | Mean, 35.3 ± 11.3 yr | 100 | 12 mo | No change |
| Vance, 2021 (41) | United States | 12–20 yr | 62 | 3, 6, 9, and 12 mo | No change |
| Pudukodu, 2021 (42) | United States | ⩾18 yr | 39 | Not specified | No change |
| George, 2020 (43) | United States | Pediatric | 14 | 1, 3, and 6 mo | Decrease |
| Sakon, 2022 (35) | United States | Mean, 29 ± 12 yr | 56 | 3–6 mo | Decrease (median: −1.11) |
| Dell, 2022 (44) | United States | Adolescents and adults | 184 | 1, 3, 6, 9, and 12 mo | Decrease |
| Allgood, 2021 (45) | United States | Median, 35.6 yr | 24 | 98 d | No change |
Definition of abbreviations: CF = cystic fibrosis; ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor; IQR = interquartile range; PHQ-9 = nine-item Patient Health Questionnaire.
A literature search identified published studies (n = 3) and congress presentations (n = 9) that used the PHQ-9 assessment tool to evaluate changes in depression symptoms after starting ELX/TEZ/IVA treatment.
For the individual case reports, assessments of any causal role of ELX/TEZ/IVA were confounded by preexisting mental health conditions; intercurrent psychosocial stressors, including the coronavirus disease (COVID-19) pandemic; the heterogenous and fluctuating nature of depression; and the lack of objective evidence. For instance, Arslan and colleagues reported two cases of suicide attempt in adolescents treated with ELX/TEZ/IVA, both of whom attended online schooling in 2020–2021 (the pandemic period) and reported a decline in grades. In addition, one of the cases had reported marijuana abuse, a family history of depression, declining mood since the beginning of the pandemic, and parent-reported concerns over a new peer group associated with worsening mood (18). Tindell and colleagues reported a case of worsening mood and passive suicidal ideation in a young adult treated with ELX/TEZ/IVA who had a history of generalized anxiety disorder, social anxiety disorder, major depressive disorder, attention deficit/hyperactivity disorder, and sleep paralysis and who also reported that their worsening mood and suicidal thoughts were related to interpersonal conflicts in a relationship (16). Hufton and colleagues additionally reported on a case of an adolescent who struggled with mental health issues since the start of the COVID-19 pandemic that escalated during lockdowns and who subsequently started ELX/TEZ/IVA treatment in late 2020 (31).
Similarly, assessment of the causal role of ELX/TEZ/IVA was challenging in the cohort studies reporting depression-related events in some participants (including those leading to treatment discontinuation or dose adjustments) because of the limited case-level information, confounding factors, and lack of control population or period. For instance, Spoletini and colleagues described the single-center experience of 262 pwCF treated with ELX/TEZ/IVA, 19 of whom reported deterioration of mental health, including low mood; 12 of 19 (63%) had a medical history of anxiety and depression and received regular psychology input before ELX/TEZ/IVA treatment (20). In another single-center study of 148 pwCF treated with ELX/TEZ/IVA, 31 were referred to psychiatric evaluation (all with a medical history of psychiatric diagnoses, including depression). After therapy initiation, 15 of 31 participants had either clear improvement, no change, or worsening of neuropsychiatric symptoms consistent with their preexisting diagnosis, and 16 participants were deemed to have new or worsening symptoms that were unexpected in the context of their psychiatric history and probably related to ELX/TEZ/IVA (21). However, insufficient individual case-level details limited the ability to perform comprehensive assessment of these cases.
Last, we identified 12 studies (three published articles and nine congress presentations) that assessed changes in the relationship between depression symptoms and initiation of ELX/TEZ/IVA in pwCF, using the well-established PHQ-9 (one study used the PHQ-8), which is designed specifically to assess the severity of depression symptoms (Table 3). These studies, which involved 951 pwCF and reflected real-world experiences with up to 12 months of follow-up, did not indicate any systematic increases (i.e., worsening) in PHQ-9 scores on average, demonstrating either no change or a decrease (i.e., improvement) in assessed PHQ-9 (or PHQ-8) scores after ELX/TEZ/IVA initiation (Table 3). Notably, one of those studies, a prospective observational cohort of 70 adults with CF treated with ELX/TEZ/IVA in Germany, reported a trend toward a decrease in patients describing suicidal ideation: Before therapy initiation, four patients (5.6%) reported suicidal ideation on the PHQ-9, and after therapy initiation, only one patient (1.4%) reported suicidal ideation (33). One additional study of 84 adults with CF reported no significant change in score on the Center for Epidemiologic Studies–Depression Scale after 6 months post ELX/TEZ/IVA initiation (32). Finally, a recent 6-year retrospective longitudinal study of 150 pwCF ages 12 to 22 years demonstrated that, at the population level, the use of more effective modulator therapies such as ELX/TEZ/IVA was associated with lower PHQ-9 scores (34).
Discussion
Evaluation of depression-related events in pwCF treated with ELX/TEZ/IVA is challenging and needs to account for the high background rates of depression symptoms in the CF population, the heterogeneous and fluctuating nature of these events, and the impact of psychosocial stressors (including the COVID-19 pandemic) and other confounding factors. Additionally, the transformative clinical benefit of ELX/TEZ/IVA may change the life perspectives of pwCF, which could impact their psychological well-being.
In this review, the totality of data on depression-related events from the completed ELX/TEZ/IVA clinical trials, postmarketing safety reports, a registry-based postauthorization safety study, and published literature suggested that depression-related events observed in pwCF taking ELX/TEZ/IVA were generally consistent with the background rate of these events in the CF population.
The high background prevalence of mental health disorders, including depression-related events in pwCF, is well acknowledged. In the International Depression Epidemiological Study conducted by Quittner and colleagues before the market availability of ELX/TEZ/IVA and including data from 6,088 pwCF ages 12 years and older across nine countries, 5–19% of adolescents and 13–29% of adults had elevated symptoms of depression, two to three times higher than in community samples (22). Similarly, in a meta-analysis of 94 articles that were published between 1989 and 2020, depression prevalence in adolescents with CF ages 12–18 years was estimated at 18.7% (95% confidence interval [CI], 12.8–25.3%); in adults with CF, it was estimated at 27.2% (95% CI, 23.6–31.0%) (23). Elevated rates of suicidal ideation have been reported in pwCF, with a prevalence of up to 11% in adults and even higher (up to 22%) in adolescents (24–26). Although evidence is more limited for completed suicides in the CF population, registry data suggest that, although relatively rare, these events have been observed annually; for instance, in 2018 (the year before ELX/TEZ/IVA was available), five of the 30,922 (0.02%) pwCF in the U.S. Cystic Fibrosis Foundation patient registry died by committing suicide.
In addition, multiple reports noted increases in depression prevalence in the general population during the COVID-19 pandemic, compared with that during the prepandemic period (28–30). In a representative sample of U.S. adults, 24.3% reported symptoms of depression in June 2020, compared with 6.5% in the second quarter of 2019, suggesting a prevalence of depression more than threefold higher during the pandemic. In the same study, serious thoughts of suicide were reported by 10.7% of survey participants in June 2020 versus 4.3% in 2018 (a prevalence about twofold higher). Although robust data on the impact of the pandemic on the CF population are scarce, it is expected that pwCF were similarly impacted. For instance, in a survey of 82 pwCF that was performed in 2022, 40% of responders felt that COVID-19 contributed to worsening mental health, including depression (35).
In this context, the integrated review of data from clinical trials showed that rates of depression-related adverse events, including suicidal ideation and suicide attempts, were relatively low and were similar between the participants who received ELX/TEZ/IVA and those who received placebo. Similarly, reported rates of depression-related events in pwCF treated with ELX/TEZ/IVA, including suicidal ideation and attempts, in the postmarketing setting were low in context of the background prevalence of these events in pwCF before ELX/TEZ/IVA approval, and the rate of completed suicide postmarketing (0.02/100 PY) was consistent with the U.S. CF registry rate before the commercial availability of ELX/TEZ/IVA (0.02% in 2018). Furthermore, postmarketing case reports generally included limited information, and assessments were confounded by preexisting mental health conditions, intercurrent psychosocial stressors, the heterogenous and fluctuating nature of depression, and lack of formal psychiatric assessments.
After approval of ELX/TEZ/IVA, a long-term, postauthorization safety study was initiated to collect and analyze data from pwCF in the U.S. and German CF registries. An interim analysis of the first 2 years of this study showed that the annual change in depression prevalence after ELX/TEZ/IVA initiation was consistent with the annual change observed during the 5-year period before ELX/TEZ/IVA initiation in both registries. It is notable that the pattern in depression prevalence after ELX/TEZ/IVA initiation remained unchanged despite the COVID-19 pandemic coinciding with the posttreatment period. No suicide deaths were reported in the smaller German cohort in the study, and the rate of suicide death in the larger U.S. cohort was 0.01 events per 100 PY, consistent with the U.S. registry rate before the commercial availability of ELX/TEZ/IVA (0.02%) in 2018. These results suggest that patterns of depression-related events in pwCF taking ELX/TEZ/IVA are consistent with the background epidemiology in the CF population.
As part of this review, we identified 12 published articles and congress presentations that utilized the well-validated PHQ-9 (or PHQ-8) to evaluate depression symptoms both before and after ELX/TEZ/IVA initiation in more than 951 pwCF (33, 35–45). These studies found that, on average, PHQ-9 (or PHQ-8) scores either decreased (indicating an improvement in depression symptoms) or showed no change after ELX/TEZ/IVA initiation.
There are limitations inherent to the data sources evaluated in the present review. Data on depression-related events in clinical trials came from adverse event reports and not from formal assessments using standardized tests such as the PHQ-9. In contrast to clinical trials in which there is complete reporting of adverse events, it is likely that there is underreporting of events in the postmarketing setting, with individual case reports generally including limited information in a narrative format without objective psychiatric assessment. Given the high background rates of depression symptoms in pwCF and the fluctuating nature of depression, as well as other confounding factors, the evaluation of causality for individual postmarketing adverse events is challenging. Data from CF patient registries included depression reported as one of CF’s complications on the basis of clinician assessment rather than patient-reported assessment using standardized measures. In addition, the number of ELX/TEZ/IVA interruptions or discontinuations caused by depression-related events in the registry-based study is not known and could impact trends in event rates. Although some of the ELX/TEZ/IVA clinical trials used for this analysis included children ages 6 years and older, there are very limited data on depression symptoms in young children with CF, as there are few established measures to assess depression symptoms in younger children, making the interpretation of the background rates in this age group challenging. Finally, this study specifically assessed the incidence of depression, depression symptoms, and depression-related events in pwCF taking ELX/TEZ/IVA in the context of background epidemiology, but the incidence of other behavioral and mental health issues that also have higher prevalence in pwCF than the general population, such as anxiety, could be a focus for further study.
ELX/TEZ/IVA provides transformative benefits in pwCF, with improvements in lung function, pulmonary exacerbations, and respiratory symptoms, along with significant reduction in lung transplantations and improvement in survival. Depression is a common and serious comorbidity in pwCF that requires careful evaluation and management. Although depression has been reported in pwCF treated with ELX/TEZ/IVA, data from clinical trials, postmarketing reports, an ongoing registry-based study, and published peer-reviewed literature suggest that people taking ELX/TEZ/IVA have rates of depression-related adverse events that are consistent with background epidemiology of depression in the CF population and do not suggest a causal relationship between ELX/TEZ/IVA treatment and depression-related adverse events.
Acknowledgments
Acknowledgment
The authors thank Nathan Blow, Ph.D., of Vertex Pharmaceuticals Incorporated, who may own stock or options in the company, for providing medical writing and editorial support under the guidance of the authors; Nucleus Global for quality control assurance and graphics support; and the Cystic Fibrosis Foundation and the German CF Registry (Mukoviszidose eV) for the use of registry data.
Footnotes
Supported by Vertex Pharmaceuticals Incorporated.
Author Contributions: The study sponsor (Vertex Pharmaceuticals Incorporated) designed the study analyses in collaboration with the academic authors. All authors had full access to the study data. B.R. and S.T. developed the initial draft of the manuscript, with writing assistance from the sponsor. All authors participated in subsequent revisions. All authors approved the final version that was submitted for publication.
Vertex Pharmaceuticals Incorporated is committed to advancing medical science and improving the health of people with cystic fibrosis. This includes the responsible sharing of clinical trial data with qualified researchers. Proposals for the use of these data will be reviewed by a scientific board. Approvals are at the discretion of Vertex and will be dependent on the nature of the request, the merit of the research proposed, and the intended use of the data. Please contact CTDS@vrtx.com if you would like to submit a proposal or need more information.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202308-1525OC on October 27, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science . 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2. Lopes-Pacheco M. CFTR modulators: the changing face of cystic fibrosis in the era of precision medicine. Front Pharmacol . 2020;10:1662. doi: 10.3389/fphar.2019.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med . 2013;1:158–163. doi: 10.1016/S2213-2600(12)70057-7. [DOI] [PubMed] [Google Scholar]
- 4. Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med . 2020;201:1193–1208. doi: 10.1164/rccm.201910-1943SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA . 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature . 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 7. Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. for the VX16-445-001 Study Group VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med . 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barry PJ, Mall MA, Álvarez A, Colombo C, de Winter-de Groot KM, Fajac I, et al. for the VX18-445-104 Study Group Triple therapy for cystic fibrosis Phe508del-gating and -residual function genotypes. N Engl J Med . 2021;385:815–825. doi: 10.1056/NEJMoa2100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. on behalf of the VX17-445-103 Trial Group Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet . 2019;394:1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. for the VX17-445-102 Study Group Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med . 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zemanick ET, Taylor-Cousar JL, Davies J, Gibson RL, Mall MA, McKone EF, et al. VX18-445-106 Study Group A phase 3 open-label study of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del allele. Am J Respir Crit Care Med . 2021;203:1522–1532. doi: 10.1164/rccm.202102-0509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mall MA, Brugha R, Gartner S, Legg J, Moeller A, Mondejar-Lopez P, et al. VX19-445-116 Study Group Efficacy and safety of elexacaftor/tezacaftor/ivacaftor in children 6 through 11 years of age with cystic fibrosis heterozygous for F508del and a minimal function mutation. A phase 3b, randomized, placebo-controlled study. Am J Respir Crit Care Med . 2022;206:1361–1369. doi: 10.1164/rccm.202202-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutharsan S, McKone EF, Downey DG, Duckers J, MacGregor G, Tullis E, et al. on behalf of the VX18-445-109 Study Group Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: a 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir Med . 2022;10:267–277. doi: 10.1016/S2213-2600(21)00454-9. [DOI] [PubMed] [Google Scholar]
- 14.Polineni D, Daines CL, Tullis E, Costa S, Linnemann RW, Mall MA, et al.
- 15. Bower JK, Volkova N, Ahluwalia N, Sahota G, Xuan F, Chin A, et al. Real-world safety and effectiveness of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis: interim results of a long-term registry-based study. J Cyst Fibros . 2023;22:730–737. doi: 10.1016/j.jcf.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 16. Tindell W, Su A, Oros SM, Rayapati AO, Rakesh G. Trikafta and psychopathology in cystic fibrosis: a case report. Psychosomatics . 2020;61:735–738. doi: 10.1016/j.psym.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 17. Heo S, Young DC, Safirstein J, Bourque B, Antell MH, Diloreto S, et al. Mental status changes during elexacaftor/tezacaftor/ivacaftor therapy. J Cyst Fibros . 2022;21:339–343. doi: 10.1016/j.jcf.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 18. Arslan M, Chalmers S, Rentfrow K, Olson JM, Dean V, Wylam ME, et al. Suicide attempts in adolescents with cystic fibrosis on elexacaftor/tezacaftor/ivacaftor therapy. J Cyst Fibros . 2023;22:427–430. doi: 10.1016/j.jcf.2023.01.015. [DOI] [PubMed] [Google Scholar]
- 19. Bathgate CJ, Muther E, Georgiopoulos AM, Smith B, Tillman L, Graziano S, et al. Positive and negative impacts of elexacaftor/tezacaftor/ivacaftor: healthcare providers’ observations across US centers. Pediatr Pulmonol . 2023;58:2469–2477. doi: 10.1002/ppul.26527. [DOI] [PubMed] [Google Scholar]
- 20. Spoletini G, Gillgrass L, Pollard K, Shaw N, Williams E, Etherington C, et al. Dose adjustments of elexacaftor/tezacaftor/ivacaftor in response to mental health side effects in adults with cystic fibrosis. J Cyst Fibros . 2022;21:1061–1065. doi: 10.1016/j.jcf.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 21. Baroud E, Chaudhary N, Georgiopoulos AM. Management of neuropsychiatric symptoms in adults treated with elexacaftor/tezacaftor/ivacaftor. Pediatr Pulmonol . 2023;58:1920–1930. doi: 10.1002/ppul.26412. [DOI] [PubMed] [Google Scholar]
- 22. Quittner AL, Goldbeck L, Abbott J, Duff A, Lambrecht P, Solé A, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax . 2014;69:1090–1097. doi: 10.1136/thoraxjnl-2014-205983. [DOI] [PubMed] [Google Scholar]
- 23. Lord L, McKernon D, Grzeskowiak L, Kirsa S, Ilomaki J. Depression and anxiety prevalence in people with cystic fibrosis and their caregivers: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol . 2023;58:287–298. doi: 10.1007/s00127-022-02307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Latchford G, Duff AJ. Screening for depression in a single CF centre. J Cyst Fibros . 2013;12:794–796. doi: 10.1016/j.jcf.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 25. Quon BS, Bentham WD, Unutzer J, Chan YF, Goss CH, Aitken ML. Prevalence of symptoms of depression and anxiety in adults with cystic fibrosis based on the PHQ-9 and GAD-7 screening questionnaires. Psychosomatics . 2015;56:345–353. doi: 10.1016/j.psym.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 26. Garcia G, Snell C, Sawicki G, Simons LE. Mental health screening of medically-admitted patients with cystic fibrosis. Psychosomatics . 2018;59:158–168. doi: 10.1016/j.psym.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 27. Quittner AL, Abbott J, Georgiopoulos AM, Goldbeck L, Smith B, Hempstead SE, et al. International Committee on Mental Health; EPOS Trial Study Group International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax . 2016;71:26–34. doi: 10.1136/thoraxjnl-2015-207488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open . 2020;3:e2019686. doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ettman CK, Cohen GH, Abdalla SM, Sampson L, Trinquart L, Castrucci BC, et al. Persistent depressive symptoms during COVID-19: a national, population-representative, longitudinal study of U.S. adults. Lancet Reg Health Am . 2022;5:100091. doi: 10.1016/j.lana.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Czeisler ME, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep . 2020;69:1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hufton M, Hussaini Y, Desai M, Saleem N, Srikantaiah R, Fairbank J, et al. Cystic fibrosis, lockdown and CFTR modulators – a perfect storm. J Cyst Fibros . 2022;21(Suppl 1):S40. [Google Scholar]
- 32. Rieubet L, Rossello N, Haesebaert J, Perceval M, Josserand RN, Durieu I, et al. Evolution of psychic symptoms before and after 6 months of treatment with elexacaftor/tezacaftor/ivacaftor (ETI) in French adults patients with cystic fibrosis (pwCF) J Cyst Fibros . 2023;22:S55. [Google Scholar]
- 33. Piehler L, Thalemann R, Lehmann C, Thee S, Röhmel J, Syunyaeva Z, et al. Effects of elexacaftor/tezacaftor/ivacaftor therapy on mental health of patients with cystic fibrosis. Front Pharmacol . 2023;14:1179208. doi: 10.3389/fphar.2023.1179208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hjelm M, Hente E, Miller J, Moore S, Peugh J, Swetland DV, et al. Longitudinal mental health trends in cystic fibrosis. J Cyst Fibros . 2023;22:1093–1099. doi: 10.1016/j.jcf.2023.06.009. [DOI] [PubMed] [Google Scholar]
- 35. Sakon C, Vogt H, Brown CD, Tillman EM. A survey assessing the impact of COVID-19 and elexacaftor/tezacaftor/ifavacaftor on both physical and mental health in adults with cystic fibrosis. Pediatr Pulmonol . 2023;58:662–664. doi: 10.1002/ppul.26260. [DOI] [PubMed] [Google Scholar]
- 36. Borawska-Kowalczyk U, Walicka-Serzysko K, Postek M, Milczewska J, Sands D. Mental health after initiating triple CFTR modulators in a Polish paediatric cystic fibrosis centre—a preliminary report. J Cyst Fibros . 2023;22:S170–S171. [Google Scholar]
- 37. Ergenekon AP, Erdem Eralp E, Sakalli AK, Yanaz M, Baskan AK, Gulieva A, et al. Modulatory therapy experience in patients with cystic fibrosis in Turkey: a multi-centre study. J Cyst Fibros . 2023;22:S117–S118. [Google Scholar]
- 38.Graziano S, Boldrini F, Milo F, Pellicano GR, Majo F, Cristiani L, et al. Is the new modulator affecting global health outcomes over time? J Cyst Fibros 202322(Suppl 2S88 [Google Scholar]
- 39. Blackwelder J, Indihar V, Finke J, Oder A, Riddle J, Meyers M, et al. Depression and anxiety scores after highly effective cmodulator therapy in pandemic times in an adult cystic fibrosis clinic. J Cyst Fibros . 2022;21(Suppl):S188. [Google Scholar]
- 40. Zhang L, Albon D, Jones M, Bruschwein H. Impact of elexacaftor/tezacaftor/ivacaftor on depression and anxiety in cystic fibrosis. Ther Adv Respir Dis . 2022;16:17534666221144211. doi: 10.1177/17534666221144211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vance T, Finch M, Bauer D, Sachs M, Landvik S, McNamara J. Mental health implications of genetic modulator therapy in CF: depression and anxiety screening for pediatric patients prescribed elexacaftor/tezacaftor/ivactor during the COVID-19 pandemic. J Cyst Fibros . 2021;20(Suppl 2):S142–S143. [Google Scholar]
- 42. Pudukodu H, Howe K, Donaldson S, Goralski J, Powell M, Wendel K, et al. Worsening anxiety after initiation of elexacaftor/tezacaftor/ivacaftor in an adult cohort of patients with cystic fibrosis. J Cyst Fibros . 2021;20(Suppl 2):S135–S136. [Google Scholar]
- 43.George A, Sliemers S, Johnson M, Pasley K, Nemastil CJ, Hunt AL, et al. 2020.
- 44. Dell M, May A, Pasly KE, Johnson M, Sliemers S, Nemastil CJ, et al. Depression and anxiety in patients with cystic fibrosis after six months on elexacaftor-tezacaftor-ivacaftor. J Acad Consult Liaison Psychiatry . 2022;63:S119–S120. [Google Scholar]
- 45. Allgood S, Psoter K, Levy R, Bubaris D, Lechtzin N. Impact of highly effective modulator therapy on patient-reported outcomes in CF. J Cyst Fibros . 2021;20(Suppl):S140. [Google Scholar]