The diagnosis of pulmonary arterial hypertension (PAH) incorporates a pulmonary artery wedge pressure (PAWP) ⩽ 15 mm Hg to exclude pulmonary hypertension (PH) due to left heart diseases (PH-LHD) (1). In this issue of the Journal (pp. 316–324), Harder and colleagues report data suggesting that the PAWP cutoff of 15 mm Hg may need to be reduced (2).
Harder and colleagues performed a longitudinal cluster analysis in 301 patients with PAH that revealed two distinct patterns of PAWP progression: in one group the PAWP remained <15 mm Hg (76% of the patients), and in another group the PAWP increased to >15 mm Hg (24% of the patients). Baseline PAWP was around 9 and 12 mm Hg for the former and latter groups, respectively. A baseline PAWP > 12 mm Hg predicted a higher PAWP during follow-up, which was associated with a greater number of heart failure with preserved ejection fraction risk factors and reduced transplant-free survival. Thus, patients with PAH with a baseline PAWP ⩾ 12 mm Hg show characteristics of PH-LHD during follow-up (2).
A recent report by Gerges and colleagues in 593 patients with chronic thromboembolic PH found that a PAWP > 15 mm Hg or >11 mm Hg was present in 11% and 37% of the patients, respectively (3). A PAWP above either cutoff was associated with a greater number of cardiovascular risk factors and worse long-term survival, further supporting that a PAWP cutoff value ⩽15 mm Hg fails to exclude a substantial proportion of patients with PH-LHD.
The PAWP cutoff to define postcapillary PH has evolved over time. It was initially set at 10–11 mm Hg, up to 12 mm Hg at the first World Symposium on Pulmonary Hypertension in 1973 (4, 5). In fact, a PAWP < 12 mm Hg was one of the inclusion criteria required to enroll patients with idiopathic PAH (at that time called “primary PH”) in the National Institute of Health registry in the 1980s (6). However, the PAWP cutoff increased to 15 mm Hg in PH guidelines in the 2000s. Why? The main argument was not to preclude patients with PAH with concomitant LHD from receiving the newly developed PAH-specific therapies.
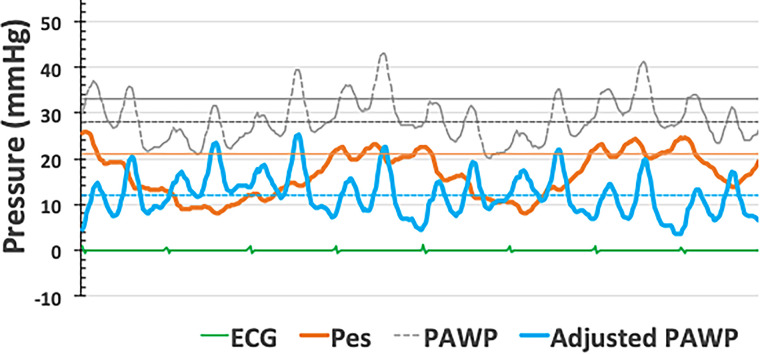
The heart and pulmonary circulation are exposed to subatmospheric pressure generated by the opposing elastic recoil of the lungs and chest wall. Intrathoracic pressure further decreases during inspiration; nevertheless, pressure changes are generally small (around 1–2 mm Hg in resting supine healthy subjects) because of the high compliance of the respiratory system. Hyperventilation is associated with more negative inspiratory and more positive expiratory pressures and may result in dynamic hyperinflation, with a disproportionate increase in the magnitude and duration of end-expiratory pulmonary pressures. Dynamic hyperinflation may occur in healthy subjects during moderate to strenuous exercise, but it may also be observed in patients with airway obstruction or obesity during low-intensity exercise or even at rest (7–9). In severe obesity, altered abdominal and chest wall mechanics may result in positive intrathoracic pressure throughout the respiratory cycle (9). In these circumstances, PAWP and left ventricular end-diastolic pressure (LVEDP) measured at end-expiration increasingly reflect the contribution of a higher intrathoracic pressure (10, 11). Figure 1 shows PAWP and esophageal pressure (a reflection of the intrathoracic pressure) in an obese patient with airway obstruction.
Figure 1.
Pulmonary artery wedge pressure (PAWP) and esophageal pressure (Pes) measurements in a patient with obesity and obstructed airways. Pressure tracings show large respiratory swings, with end-expiratory pressures being the highest. Pes is positive during the entire respiratory cycle in relation to the mechanical effects of morbid obesity in the supine position. Adjusting for Pes reduces PAWP from 33 mm Hg at end-expiration (gray solid line) or 27 mm Hg mean (gray dashed line) to 12 mm Hg (blue solid line).
For the interpretation of PAWP, a correction for esophageal pressure as shown in Figure 1 would be ideal, but this is not practical. A generally acceptable compromise, except in morbid obesity, is to measure PAWP throughout the respiratory cycle (10, 11). End-expiratory and respiratory cycle–averaged PAWP most often differ by an average of 3 to 4 mm Hg, but with pronounced individual differences in patients with cardiorespiratory conditions (7–9). It is difficult to speculate by how much PAWP would have increased at end-expiration in the studies by Harder and colleagues (2) or Gerges and colleagues (3)
Would LVEDP determination overcome the PAWP limitations? The answer is no. The Pascal principle applies; therefore, PAWP and LVEDP have to be equal, based on the stop-flow phenomenon downstream to a wedged pulmonary artery catheter (10). As a matter of fact, Gerges and colleagues demonstrated no significant bias in Bland-Altman analysis when PAWP and LVEDP determinations were compared (3). However, this similarity is only present when PAWP is measured at end-diastole, at the “pre–c wave” nadir, as was actually done by Gerges and colleagues (12). In cases in which “a or c waves” are not clearly identifiable, the measure may be timed with the QRS (10, 13). Electronic PAWP averaging, including “v waves,” slightly underestimates LVEDP but overestimates LVEDP in the presence of significant mitral valve disease and/or heart failure (10). Harder and colleagues measured the PAWP as an electronic mean (2), excluding patients with valvular heart disease or heart failure; hence, the difference between averaged and end-diastolic PAWP was probably negligible.
So, where do we go from here? Mounting evidence supports the revision of the current end-expiration PAWP cutoff of ⩽15 mm Hg to exclude PH-LHD in the diagnostic workup of patients with PAH. Recent PH guidelines use the upper limit of normal for mean pulmonary artery pressure and pulmonary vascular resistance but not PAWP (1). The upper limit of normal of PAWP is 12 mm Hg. The PAWP measurement should be averaged over several respiratory cycles and strictly taken at end-diastole. When the PAWP determination is not consistent with the clinical scenario, which may be related to technical factors and/or inherent physiological variability (14), it is advised to repeat the PAWP measurement during exercise or after a fluid challenge (1). Qaiser and colleagues showed that PAWP measurements around the upper limit of normal often disclose occult PH-LHD after a fluid challenge (15). In general, one should be wary of clinical decisions based in a single number and carefully consider the applicability in a specific patient of guideline cutoffs derived from population studies.
Harder and colleagues (2) are to be commended for revitalizing the debate about PAWP measurement methodology and the upper limit of normal. Their study convincingly shows that current guidelines do not sufficiently exclude patients with heart failure who benefit less from PAH-specific therapies.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202311-2037ED on December 5, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. ESC/ERS Scientific Document Group 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J . 2023;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 2. Harder EM, Divo MJ, Washko GR, Leopold JA, Rahaghi FN, Waxman AB. Implications of mean pulmonary arterial wedge pressure trajectories in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2024;9:316–324. doi: 10.1164/rccm.202306-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerges C, Pistritto AM, Gerges M, Friewald R, Hartig V, Hofbauer TM, et al. Left ventricular filling pressures in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol . 2023;81:653–664. doi: 10.1016/j.jacc.2022.11.049. [DOI] [PubMed] [Google Scholar]
- 4. Hellems HK, Haynes FW, Dexter L. Pulmonary capillary pressure in man. J Appl Physiol . 1949;2:24–29. doi: 10.1152/jappl.1949.2.1.24. [DOI] [PubMed] [Google Scholar]
- 5.Hatano S, Strasser T, editors. Primary pulmonary hypertension: report on a WHO meeting, Geneva, 15–17 October 1973. Geneva: World Health Organization; 1975. [Google Scholar]
- 6. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med . 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 7. LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J . 2014;44:425–434. doi: 10.1183/09031936.00209313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boerrigter BG, Waxman AB, Westerhof N, Vonk-Noordegraaf A, Systrom DM. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J . 2014;43:1316–1325. doi: 10.1183/09031936.00016913. [DOI] [PubMed] [Google Scholar]
- 9. Khirfan G, Melillo CA, Al Abdi S, Lane JE, Dweik RA, Chatburn RL, et al. Impact of esophageal pressure measurement on pulmonary hypertension diagnosis in patients with obesity. Chest . 2022;162:684–692. doi: 10.1016/j.chest.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naeije R, Chin K. Differentiating precapillary from postcapillary pulmonary hypertension. Circulation . 2019;140:712–714. doi: 10.1161/CIRCULATIONAHA.119.040295. [DOI] [PubMed] [Google Scholar]
- 11. Olschewski H, Zeder K, Douschan P, Sassmann T, Foris V, Olschewski A, et al. Let’s talk about respiratory swings! Am J Respir Crit Care Med . 2023;208:1338–1340. doi: 10.1164/rccm.202309-1637LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerges C, Lang IM. Reply about left ventricular filling pressures in CTEPH. J Am Coll Cardiol . 2023;81:e183. doi: 10.1016/j.jacc.2023.03.417. [DOI] [PubMed] [Google Scholar]
- 13. Houston BA, Tedford RJ. What we talk about when we talk about the wedge pressure. Circ Heart Fail . 2017;10:e004450. doi: 10.1161/CIRCHEARTFAILURE.117.004450. [DOI] [PubMed] [Google Scholar]
- 14. Naeije R, D’Alto M, Forfia PR. Clinical and research measurement techniques of the pulmonary circulation: the present and the future. Prog Cardiovasc Dis . 2015;57:463–472. doi: 10.1016/j.pcad.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 15. Qaiser KN, Almoushref A, Mehta AK, Alkhayyat M, Lane JE, Tonelli AR. Fluid loading during the hemodynamic evaluation of pulmonary hypertension: a cross-sectional study. Cardiovasc Diagn Ther . 2023;13:833–842. doi: 10.21037/cdt-23-59. [DOI] [PMC free article] [PubMed] [Google Scholar]