Abstract
Chlamydia trachomatis is an obligate intracellular eubacterial parasite capable of infecting a wide range of eucaryotic host cells. Purified chlamydiae contain several lipids typically found in eucaryotes, and it has been established that eucaryotic lipids are transported from the host cell to the parasite. In this report, we examine the phospholipid composition of C. trachomatis purified from host cells grown under a variety of conditions in which the cellular phospholipid composition was altered. A mutant CHO cell line, with a thermolabile CDP-choline synthetase, was used to show that decreased host cell phosphatidylcholine levels had no significant effect on C. trachomatis growth. However, less phosphatidylcholine was transported to the parasite and purified elementary bodies contained decreased levels of phosphatidylcholine. Brefeldin A, fumonisin B1, and exogenous sphingomyelinase were used to alter levels of host cell sphingomyelin. None of the agents had a significant effect on C. trachomatis replication. Treatment with fumonisin B1 and exogenous sphingomyelinase resulted in decreased levels of host cell sphingomyelin. This had no effect on glycerophospholipid trafficking to chlamydiae; however, sphingomyelin trafficking was reduced and elementary bodies purified from treated cells had reduced sphingomyelin content. Exposure to brefeldin A, which had no adverse effect on chlamydia growth, resulted in an increase in cellular levels of sphingomyelin and a concomitant increase in the amount of sphingomyelin in purified chlamydiae. Under the experimental conditions used, brefeldin A treatment had only a small effect on sphingomyelin trafficking to the host cell surface or to C. trachomatis. Thus, the final phospholipid composition of purified C. trachomatis mimics that of the host cell in which it is grown.
Chlamydiae are obligate intracellular gram-negative eubacterial parasites that infect a wide range of eucaryotic host cells and cause a variety of human diseases (27, 36). Chlamydiae have evolved a complex biphasic life cycle to facilitate their survival in two discontinuous habitats. The elementary body (EB) is the metabolically dormant, structurally rigid extracellular spore-like form that initiates infection by attaching to a suitable host cell. Following internalization, EBs differentiate into reticulate bodies (RBs), which are of the metabolically active, fragile intracellular form that grows within the confines of a vacuole, termed the chlamydial inclusion, which avoids fusion with cellular lysosomes. By 16 to 20 h into the infection, while some RBs are still replicating, others have begun the process of differentiation back into EBs. Approximately 48 to 72 h after infection, RB replication is essentially complete, EBs predominate, and the host cell lyses, releasing EBs to begin a new infection cycle.
Like other gram-negative bacteria, chlamydiae possess a cell envelope composed of an outer membrane (OM) and inner membrane (IM). The outer leaflet of the OM contains a unique deep rough lipopolysaccharide which is synthesized by chlamydiae (4, 5). The inner leaflet of the OM and both leaflets of the IM are made up of various phospholipids. In addition to containing lipids typically present in procaryotes (phosphatidylethanolamine [PE], phosphatidylglycerol [PG], and phosphatidylserine [PS]), purified chlamydiae contain lipids more frequently associated with eucaryotes (phosphatidylcholine [PC], phosphatidylinositol [PI], sphingomyelin [SM], and cholesterol) (28, 46). The presence of these lipids implies that eucaryotic lipids are transported to chlamydiae. Recent work by Hackstadt and colleagues (13–15, 33) with fluorescent 6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]hexanoyl (C6-NBD)–ceramide, which is converted to C6-NBD–SM by the host cell, clearly established that host cell SM is transported to chlamydiae. Hackstadt et al. (13–15) proposed that the chlamydial inclusion can best be described as an aberrant Golgi apparatus-derived vesicle situated distal to the trans-Golgi apparatus, such that it receives host-derived lipids from an exocytic pathway. The SM obtained from Golgi apparatus-derived vesicles fusing with the inclusion are ultimately sequestered by chlamydiae.
Unlike mammalian cell glycerophospholipids, which contain straight-chain fatty acids, chlamydial phospholipids possess branched-chain fatty acids (28, 46). Isoleucine is the precursor of the α-keto acid primer needed to initiate branched-chain fatty acid biosynthesis in prokaryotes (19). We have used radiolabelled isoleucine to study lipid metabolism in chlamydia-infected cells (46). Our results indicate that chlamydiae can synthesize PE, PS, and PG de novo and that host cell glycerophospholipids are transported to chlamydiae. Host glycerophospholipids are modified by Chlamydia trachomatis, such that a host-synthesized straight-chain fatty acid is replaced with a chlamydia-synthesized branched-chain fatty acid. Although it is established that chlamydiae obtain phospholipids from the host, it is not clear whether the host cell regulates which phospholipids are transported or whether chlamydiae can control which lipids they accept, thereby regulating their final lipid compositions.
In this study we used a temperature-sensitive mutant Chinese hamster ovary cell line (CHO 58) with a thermolabile CDP-choline synthetase, as well as wild-type mouse L929 cells treated with a variety of compounds that affect cellular phospholipid metabolism, to alter host cell phospholipid composition. The effects of altered host cell phospholipid composition on chlamydial growth, phospholipid trafficking, and metabolism were then determined. Our results indicate that the types of lipids trafficked are not strictly regulated by the host or chlamydiae and, as a result, that the final phospholipid compositions of chlamydiae mimic that of the host cell.
MATERIALS AND METHODS
Materials.
[2,8-3H]adenine (23 Ci mmol−1), [methyl-3H]choline (81 Ci mmol−1), l-[U-14C]isoleucine (342 mCi mmol−1), [1-14C]palmitic acid (57 mCi mmol−1), and [U-14C]serine (150 mCi mmol−1) were obtained from New England Nuclear, Dupont Canada Inc. Brefeldin A, Bacillus cereus sphingomyelinase, fumonisin B1, and phospholipid standards were obtained from Sigma.
C. trachomatis strains and propagation.
C. trachomatis L2/434/Bu was used throughout this study and was grown as previously described in monolayers (25) or in suspension culture (12). Unless otherwise indicated, 1 μg of cycloheximide ml−1 was present in the postinfection (p.i.) growth medium. The various cell lines were infected with C. trachomatis at a multiplicity of infection of 3 to 5 infection-forming units per cell. Mock-infected host cell cultures were treated in a manner identical to that used with infected cells except that chlamydiae were not added.
Cell lines and culture conditions.
Wild-type HeLa cells and mouse L929 cells are routinely maintained in our laboratory and were cultured in minimal essential medium containing 10% fetal bovine serum at 37°C (12, 25). The wild-type CHO K1 cells and the thermolabile CDP-choline synthetase CHO K1 cell line (strain 58) (10, 11) were provided by D. E. Vance, University of Alberta, Edmonton, Alberta, Canada. Both the wild-type CHO K1 and the mutant CHO strain 58 cells were cultured in minimal essential medium containing 10% fetal bovine serum and 300 μM proline at 33°C, the permissive temperature, or at 40°C, the nonpermissive temperature for the CHO 58 cells (10, 11).
Incorporation of radiolabelled adenine into host cell and C. trachomatis DNA.
C. trachomatis growth was assessed by measuring the incorporation of radiolabelled adenine into parasite-specific DNA. Labelling conditions, cell harvesting, and quantitation of radiolabel incorporated into DNA were conducted as previously described (25). Radiolabel was added at 22 h p.i., when C. trachomatis DNA synthesis is at a maximum. All results were normalized to 106 cells.
Extraction and purification of lipids.
For radiolabelling of phospholipids, mock-infected or C. trachomatis-infected cells were incubated with a given radiolabelled precursor (3 μCi/5-cm-diameter dish) beginning at 20 h p.i., the time when chlamydial phospholipid metabolism is at a maximum (46). Five hours later, the cell layer was washed once with ice-cold phosphate-buffered saline and scraped into 2 ml of methanol-water (1:1 [vol/vol]). For cultures treated with brefeldin A (5 μg ml−1), fumonisin B1 (20 μM), and/or sphingomyelinase (0.05 U ml−1), the compounds were added at the start of the infection (2 h p.i.) and maintained throughout the entire growth period unless otherwise indicated. For cultures treated with brefeldin A, fresh inhibitor was added every 5 h. Procedures for extraction and purification of lipids were carried out as previously described (16, 46). Lipids were separated by a two-dimensional thin-layer-chromatography procedure, which gave good separation of all the major phospholipids (16, 17). The organic phase containing the extracted lipids was dried under a stream of N2 and resuspended in 100 μl of chloroform-methanol (2:1, [vol/vol]). Fifty-microliter aliquots, with appropriate standards, were spotted on 10- by 10-cm-diameter thin-layer Silica Gel 60 plates (Whatman) that had been previously treated with 0.4 M boric acid. The plates were developed in the first dimension with chloroform-methanol-water-ammonium hydroxide (70:30:3:2) and in the second dimension with chloroform-methanol-water (65:35:5). Individual lipids were visualized with iodine vapor. Areas corresponding to individual lipids were removed and placed in vials containing Universol scintillation fluor (ICN Biomedicals, Inc.) for quantitation. The results were standardized based on the number of milligrams of protein per dish. Protein was assayed by the method of Lowry et al. (23), with bovine serum albumin as the standard.
Total phospholipid compositions of mock-infected host cells, C. trachomatis-infected host cells at 40 h p.i., and highly purified chlamydial EBs were determined as previously described (16). Highly purified EBs were prepared from suspension cultures of infected host cells (CHO K1, CHO 58, and mouse L929) at 40 h p.i. For CHO 58 cells, a 1-liter suspension culture was grown at 33°C until cell density reached 106 ml−1. One-half of the culture was left at 33°C and maintained at a cell density of 106 ml−1. The other half was shifted to 40°C and left for 60 h. The suspension cultures were then infected with C. trachomatis (12), and after a further 40 h, the infected cells were harvested. Suspension cultures of other cell types, mock infected or C. trachomatis infected, were grown at 37°C for 40 h. For mock-infected and C. trachomatis-infected cultures treated with brefeldin A (5 μg ml−1), fumonisin B1 (20 μM), and/or sphingomyelinase (0.05 U ml−1) the inhibitors were added at the start of the infection and maintained in the culture for the entire 40-h chlamydial growth cycle unless otherwise indicated. EBs were purified by density gradient centrifugation (7). The phospholipids were quantitated by analysis of phospholipid phosphorous (31) after separation on two-dimensional thin-layer-chromatography plates as described above. The lipids on the developed plate were visualized by spraying with 0.2% orcinol in 2 N sulfuric acid followed by heating at 120°C.
Reaction of cell surface SM with sphingomyelinase.
Delivery of natural SM to the cell surface was assayed according to the procedure of Shiao and Vance (34) with the following modifications. All cultures were set up in quadruplicate. Two cultures each of mock-infected and C. trachomatis-infected cells were left as untreated controls. Treated cultures had sphingomyelinase (0.05 U ml−1) and/or brefeldin A (5 μg ml−1) added to duplicate mock-infected and duplicate C. trachomatis-infected cultures at the start of the chlamydia infection cycle. Twenty hours later cellular SM was labelled for 5 h with [3H]choline (15 μCi/5-cm-diameter dish), [14C]serine (3μCi/5-cm-diameter dish), or [14C]palmitate (3 μCi/5-cm-diameter dish), during which period transport of the newly synthesized radiolabelled lipids occurred. At the end of the labelling period, lipids were extracted, separated by two-dimensional thin-layer chromatography, and counted for radioactivity as described above. The percentage of radiolabelled SM that is hydrolyzable, 100 × {1 − ([3H]SMsphingomyelinase treated/[3H]SMuntreated control)}, is used as a measure of natural SM transport to the cell surface.
RESULTS
C. trachomatis growth under various experimental conditions.
A CHO cell line with a mutation in CDP-choline synthetase (10, 11) and a variety of agents, namely, brefeldin A, an inhibitor of exocytic vesicular transport from the Golgi apparatus (21, 22); fumonisin B1, an inhibitor of SM biosynthesis (26); and sphingomyelinase, an enzyme that degrades SM, were used throughout this study to alter host phospholipid metabolism and composition. As an initial experiment, we assessed the effects of these various agents on C. trachomatis growth and the ability of the mutant CHO cell line to support chlamydia replication at both the permissive (33°C) and nonpermissive (40°C) temperatures. As a control for the effect of temperature alone on C. trachomatis growth, wild-type CHO K1 cells cultured at 33 and 40°C were used as the host. C. trachomatis growth was monitored by measuring the incorporation of radiolabelled adenine into chlamydia-specific DNA (25).
The results presented in Table 1 indicate that the mutant CHO 58 cell line was just as good a host for supporting chlamydial growth as the wild-type CHO K1 cell line. In both cell lines, about 30% more radiolabel was incorporated into chlamydial DNA at 33°C than at 40°C, suggesting that chlamydial growth was somewhat compromised at the higher temperature. Similarly, titration experiments, quantitating infectious EB progeny, indicated that there was no significant difference between chlamydial replication in wild-type CHO K1 cells and that in mutant CHO 58 cells, regardless of the incubation temperature. There was about a 20% decrease in the yield of infectious progeny in both cell lines when incubations were carried out at 40°C compared to that at 33°C (data not shown).
TABLE 1.
Effects of various treatments on the incorporation of adenine into host and C. trachomatis DNAs
| Cell linea | Temp (°C) | Addition(s)b | Incorporation of [3H]adenine into DNA (103 dpm/106 cells)c
|
|
|---|---|---|---|---|
| Mock infected | C. trachomatis infected | |||
| CHO K1 | 33 | 2.1 ± 0.8 | 163 ± 11.9 | |
| CHO K1 | 40 | 1.8 ± 1.0 | 111 ± 10.4 | |
| CHO 58 | 33 | 1.9 ± 0.6 | 153 ± 9.4 | |
| CHO 58 | 40 | 0.9 ± 0.5 | 102 ± 9.8 | |
| Mouse L | 37 | 2.8 ± 1.1 | 147 ± 13.6 | |
| Mouse L | 37 | Fumonisin B1 | 3.1 ± 1.3 | 154 ± 14.9 |
| Mouse L | 37 | Brefeldin A | 2.4 ± 0.9 | 139 ± 9.4 |
| Mouse L | 37 | Sphingomyelinase | 2.9 ± 1.3 | 144 ± 11.5 |
| Mouse L | 37 | Brefeldin A + sphingomyelinase | 3.3 ± 1.6 | 137 ± 12.3 |
Triplicate cultures of wild-type CHO K1, CDP-choline synthetase mutant CHO 58, and wild-type mouse L 929 cells were seeded, cultured, and infected with C. trachomatis L2 or left as mock-infected controls as described in Materials and Methods and the report of McClarty and Tipples (25).
For mock-infected and C. trachomatis-infected cultures treated with brefeldin A (5 μg ml−1), fumonisin B1 (20 μM), and/or sphingomyelinase (0.05 U ml−1), the inhibitors were added at the start of the infection (2 h p.i.).
Incorporation of radiolabelled adenine into host cell or C. trachomatis L2 DNA was determined after addition of radiolabel at 22 h p.i. and continuing incubation for 3 h. Cells were harvested and processed as described previously (25). Results are expressed as means ± standard deviations of results from three separate experiments.
At the concentrations used in this study, i.e., 5 μg of brefeldin A ml−1, 20 μM fumonisin B1, and 0.05 U of sphingomyelinase ml−1, none of the agents employed had a significant effect on C. trachomatis growth (Table 1). In all cases the agents were added at the beginning of the infection (2 h p.i.) and retained in the medium until growth was measured by the addition of radiolabelled adenine at 22 h p.i. C. trachomatis growth was also assessed by titration of infectious progeny, after incubation in the presence of the various agents for 44 h. There was no significant difference in the numbers of infectious EBs produced in the absence and presence of the agents (data not shown).
Phospholipid trafficking to C. trachomatis grown in wild-type CHO K1 and mutant CHO 58 cells.
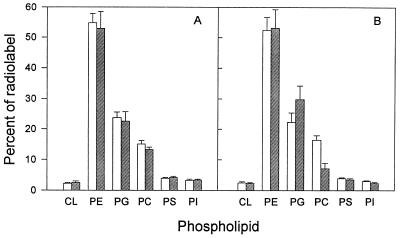
When CDP-choline synthetase mutant CHO 58 cells are shifted to the nonpermissive temperature of 40°C, de novo synthesis of PC rapidly ceases and the level of PC in the cells declines to approximately 50% of control values (10, 11). To determine the effect of altered host cell phospholipid composition on C. trachomatis phospholipid metabolism, we incubated confluent monolayers of CHO 58 cells at 33 or 40°C for 60 h and then infected them with C. trachomatis. At 20 h p.i. [14C]isoleucine was added, and 5 h later total lipids were extracted from the cultures. As a control for the effects of temperature alone, an identical series of cultures was set up with wild-type CHO K1 cells. We have previously shown that isoleucine is specifically incorporated into chlamydial branched-chain fatty acids and can serve as a tracer of phospholipid biosynthesis and trafficking in C. trachomatis-infected cells (46). In contrast, mock-infected cells incubated with radiolabel showed no incorporation of isoleucine into phospholipids, verifying that any observed incorporation into lipids reflects C. trachomatis-specific activity. Results presented in Fig. 1 show that the patterns of isoleucine incorporation into glycerophospholipids in C. trachomatis-infected CHO K1 cells are essentially identical at 33 and 40°C. This indicates that temperature alone has little effect on the pattern of de novo chlamydial phospholipid synthesis or trafficking of host-derived lipids to the organism. In contrast, infected CHO 58 cells show decreased and increased levels of isoleucine incorporation into PC and PG, respectively, at 40°C compared to levels at 33°C. This change is consistent with decreased trafficking of host-derived PC to chlamydiae and increased de novo synthesis of PG by the parasite at the nonpermissive temperature.
FIG. 1.
Incorporation of [U-14C]isoleucine into glycerophospholipids of C. trachomatis-infected wild-type CHO K1 (A) and thermolabile CDP-choline synthetase CHO strain 58 (B) cells at 33°C (open bars) and 40°C (hatched bars). The host cells were held at their respective temperatures for 60 h prior to infection. The temperature was maintained after infection. Infected cultures were radiolabelled at 20 h p.i. and harvested 5 h later. The results for each phospholipid are expressed as percentages of the total radioactivity incorporated into phospholipids. CL, cardiolipin. Results are expressed as means ± standard deviations of results from three separate experiments.
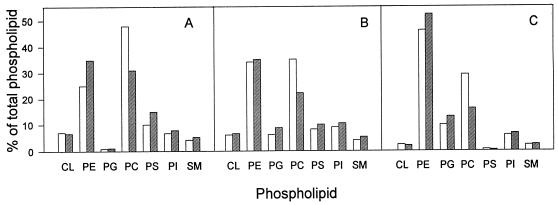
Decreased trafficking of host PC to chlamydiae should result in a concomitant decrease in the mass of PC in the organism. To determine if this was the case, we used CHO 58 cells grown in suspension culture as the host for support of chlamydial growth. CHO 58 cells were grown at 33°C to a density of 106 cells ml−1. The culture was split in two; one-half was placed at 40°C, and the other was kept at 33°C. The cells were held at their respective temperatures for 60 h, and the density of the 33°C culture was maintained at 106 cells ml−1 during this time. The cells were then mock infected or infected with C. trachomatis and harvested 40 h later. The phospholipid compositions of mock-infected cells, chlamydia-infected cells, and highly purified EBs from cultures at the permissive and nonpermissive temperatures are shown in Fig. 2. In agreement with previous reports (10), the PC content of the mock-infected CHO 58 cells decreased by 42% after incubation at 40°C. Similarly, the PC contents of C. trachomatis-infected CHO 58 cells and highly purified EBs also decreased, 44 and 47%, respectively, when the cells and EBs were incubated at 40°C compared to levels at 33°C. This result is consistent with the above-described finding of decreased isoleucine labelling of PC in C. trachomatis-infected CHO 58 cells at 40°C. There were also changes in PE, PS, and PG content; however, interpretation of these results is complicated by the fact that chlamydiae can synthesize these three phospholipids (but not PC) de novo.
FIG. 2.
Phospholipid composition of mock-infected CHO strain 58 cells (A), C. trachomatis-infected CHO strain 58 cells at 40 h p.i. (B), and highly purified EBs prepared from infected CHO strain 58 cells at 40 h p.i. (C) grown at 33°C (open bars) or 40°C (hatched bars). Phospholipids were quantitated by measuring the phosphorous associated with a given phospholipid and are expressed as percentages of the total phospholipid phosphorous. Results are averages of results from two separate experiments; duplicate results varied by less than 10%. CL, cardiolipin.
Effect of fumonisin B1 on phospholipid trafficking to C. trachomatis.
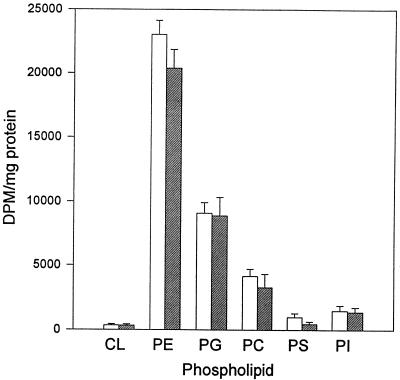
Fumonisin B1 is structurally similar to the SM precursor sphinganine and is a competitive inhibitor of sphinganine N-acyltransferase (26). Treatment of cultured cells with fumonisin B1 results in a rapid inhibition of SM biosynthesis, which ultimately leads to a decrease in SM mass (2, 32, 45). It has also been reported that fumonisin B1 treatment of cells causes an increase in serine incorporation into PE (2, 35, 43). We determined the effects of fumonisin B1 on phospholipid biosynthesis and trafficking in mock-infected and C. trachomatis-infected mouse cells. We found that the antibiotic caused no significant change in the extent of isoleucine incorporation into any of the glycerophospholipids extracted from treated or untreated C. trachomatis-infected cells (Fig. 3).
FIG. 3.
Incorporation of [14C]isoleucine into glycerophospholipids of C. trachomatis-infected mouse L cells in the absence (open bars) and presence (hatched bars) of 20 μM fumonisin B1. Fumonisin B1 was added at the start of the infection (2 h p.i.), and radiolabelled isoleucine was added at 20 h p.i. The cells were harvested 5 h later. Results are expressed as means ± standard deviations of results from three separate experiments. CL, cardiolipin.
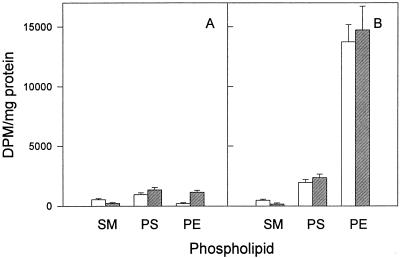
Radioactive serine, an SM precursor, was used to directly assess the effects of fumonisin B1 on SM synthesis. As reported previously (2, 32, 45), we found that fumonisin B1 treatment of mock-infected mouse L cells caused decreased and increased incorporation of serine into SM and PE, respectively (Fig. 4A). Furthermore, there was no significant effect on serine incorporation into PS. Serine was incorporated into SM to about the same extent in C. trachomatis-infected cells as in mock-infected controls. This finding is consistent with the fact that chlamydiae are incapable of de novo SM synthesis (37, 46). Fumonisin B1 treatment of C. trachomatis-infected mouse cells caused a similar decrease in serine labelling of SM and had little or no effect on PS labelling. We have previously shown that chlamydiae synthesize PE from PS (46), a reaction catalyzed by PS decarboxylase; therefore, there is a large increase in serine labelling of PE in C. trachomatis-infected cells compared to the level in mock-infected controls (Fig. 4B). In contrast to results with mock-infected controls, fumonisin B1 had little effect on serine labelling of PE in chlamydia-infected mouse cells.
FIG. 4.
Incorporation of [14C]serine into phospholipids of mock-infected mouse L cells (A) and C. trachomatis-infected mouse L cells (B) in the absence (open bars) and presence (hatched bars) of 20 μM fumonisin B1. Fumonisin B1 was added at the start of the infection (2 h p.i.), and radiolabelled serine was added at 20 h p.i. The cells were harvested 5 h later. Results are expressed as means ± standard deviations of results from three separate experiments.
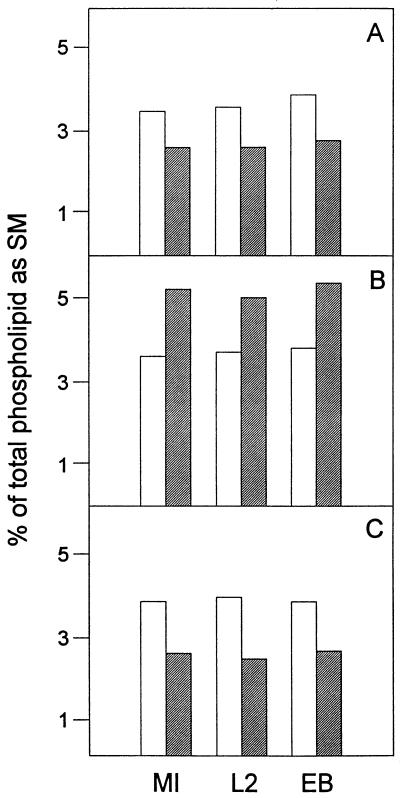
To determine if the fumonisin B1-induced decrease in SM biosynthesis results in a decrease in SM mass, we examined the phospholipid compositions of treated and untreated wild-type mouse cells, C. trachomatis-infected mouse cells at 40 h p.i., and highly purified EBs. Fumonisin B1 treatment had no effect on cardiolipin, PG, PS, PI, PC, and PE composition in mock-infected cells, C. trachomatis-infected cells, and purified EBs (data not shown). In contrast, fumonisin B1 treatment reduced levels of SM in all cultures by 25 to 30% (Fig. 5A). This result suggests that decreased content of host cell SM results in decreased trafficking of SM to chlamydiae.
FIG. 5.
SM composition of mock-infected mouse L cells (MI), C. trachomatis-infected mouse L cells at 40 h p.i. (L2), and highly purified EBs prepared from infected mouse L cells at 40 h p.i. (EB) grown in the absence (open bars) or presence (hatched bars) of 20 μM fumonisin B1 (A), 5 μg of brefeldin A ml−1 (B), or 0.05 U of sphingomyelinase ml−1 (C). Phospholipids were quantitated by measuring the phosphorous associated with a given phospholipid. Results for SM are expressed as percentages of the total phospholipid phosphorous. Results are averages of results from two separate experiments; duplicate results varied by less than 10%.
Effect of brefeldin A on SM trafficking to C. trachomatis.
While chlamydia growth is not significantly affected by the presence of brefeldin A (Table 1) (3, 14, 46), Hackstadt and colleagues (13–15, 33) have shown that a fluorescent SM analog, C6-NBD–SM synthesized by the host cell from exogenously added C6-NBD–ceramide, is transported to chlamydiae via a brefeldin A-sensitive pathway. Using radiolabelled isoleucine as a tracer for branched-chain fatty acids, we have previously shown that trafficking of host glycerophospholipids to chlamydiae occurs by a mechanism that is not affected by brefeldin A (46). In the above-mentioned studies, it was not determined if there was a difference in the SM and/or glycerophospholipid compositions of EBs purified from host cells cultured in the presence and absence of brefeldin A. The following experiments were carried out to address this issue.
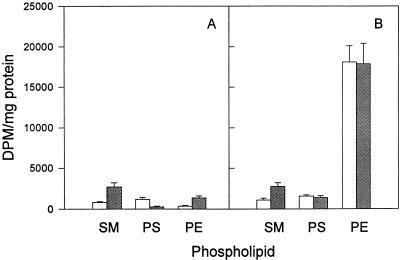
We first determined the effects of brefeldin A on the incorporation of radiolabelled serine into SM, PS, and PE in mock-infected and C. trachomatis-infected mouse cells. We chose serine as a precursor because, in addition to being used by the host cell for phospholipid synthesis, it can also be used by chlamydiae for the synthesis of PS and PE (46). In agreement with the results of numerous reports (1, 6, 17, 18, 34), we found that brefeldin A stimulated (three- to fourfold) host cell SM biosynthesis (Fig. 6A). A similar increase in SM biosynthesis was detected when radiolabelled choline was used as the precursor (data not shown). In addition, levels of serine incorporation into PS and PE were decreased and increased, respectively, in mock-infected cells treated with brefeldin A (Fig. 6A). Brefeldin A treatment of C. trachomatis-infected mouse cells caused a similar increase in serine incorporation into SM (Fig. 6B). In contrast, the antibiotic had no significant effect on PS and PE synthesis. This result is not surprising, since serine can be directly used by chlamydiae for PS and PE, but not SM, biosynthesis. The effects of brefeldin A on [14C]isoleucine incorporation into glycerophospholipids in C. trachomatis-infected mouse cells was also assessed, and as reported previously (46), we found that there were no significant changes (data not shown).
FIG. 6.
Incorporation of [14C]serine into phospholipids of mock-infected mouse L cells (A) and C. trachomatis-infected mouse L cells (B) in the absence (open bars) and presence (hatched bars) of 5 μg of brefeldin A ml−1. Brefeldin A was added at the start of the infection (2 h p.i.), and radiolabelled serine was added at 20 h p.i. The cells were harvested 5 h later. Results are expressed as means ± standard deviations of results from three separate experiments.
To see if the brefeldin A-induced increase in SM synthesis resulted in an increase in SM mass, we compared the phospholipid compositions of untreated and treated mock-infected mouse cells, C. trachomatis-infected mouse cells at 40 h p.i., and purified EBs. While there was no change in glycerophospholipid composition (data not shown), there was a consistent increase (≈40%) in SM in brefeldin A-treated samples (Fig. 5B). The magnitudes of the increases were similar for mock-infected cells, C. trachomatis-infected cells at 40 h p.i., and purified EBs. An identical set of experiments was carried out with HeLa cells, instead of mouse cells, and the findings were in complete agreement (data not shown).
Effect of extracellular sphingomyelinase treatment on SM content.
The majority of cellular SM is located in the exoplasmic face of a plasma membrane (44). Numerous studies have shown that treatment of cells in culture with exogenous sphingomyelinase results in a substantial decrease in SM content, and such treatment has been used to study the transport of SM to the cell surface (18, 29, 34, 39). As a result of their studies with labelled C6-NBD–ceramide in chlamydia-infected cells, Hackstadt and colleagues (13, 14) concluded that a substantial portion of the SM endogenously synthesized from the added fluorescent ceramide analog is transported to the chlamydial inclusion rather than to the host cell plasma membrane. We determined whether exogenous sphingomyelinase had any effect on phospholipid metabolism and/or trafficking in C. trachomatis-infected mouse L cells.
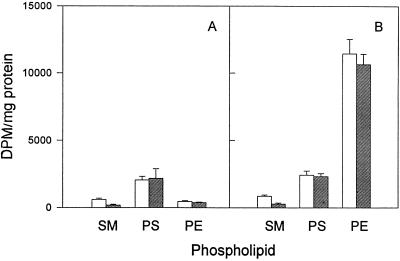
Mouse L cells were infected with C. trachomatis; one culture was left as a control, and sphingomyelinase was added to the other at the start of the infection. The infected cells were labelled with [14C]isoleucine and harvested 5 h later. No significant change was found in the extents of isoleucine incorporation into any of the phospholipids, suggesting that sphingomyelinase treatment caused no major alteration in chlamydial growth or glycerophospholipid metabolism (data not shown). A similar experiment was conducted with [14C]serine as the phospholipid precursor. Since serine can be used by both the host and C. trachomatis for phospholipid synthesis, an appropriate mock-infected control was added. As expected with the mock-infected controls, exposure to sphingomyelinase resulted in a decrease in the amount of radiolabel associated with SM but had little or no effect on PS and PE (Fig. 7A). We reasoned that if a significant portion of the de novo-synthesized SM in a C. trachomatis-infected cell was transported to the inclusion membrane and chlamydia cell wall (13, 14), where it would be protected from exogenous sphingomyelinase, then there would be an increase in the amount of [14C]serine associated with SM. The results shown in Fig. 7B indicate that there was no significant difference between the amount of radiolabelled SM protected from sphingomyelinase degradation in the C. trachomatis-infected cells and that in mock-infected controls. [3H]choline and [14C]palmitate were also used to label SM, and the results obtained were similar to those found with radiolabelled serine (data not shown).
FIG. 7.
Incorporation of [14C]serine into phospholipids of mock-infected mouse L cells (A) and C. trachomatis-infected mouse L cells (B) in the absence (open bars) and presence (hatched bars) of 0.05 U of sphingomyelinase ml−1. Sphingomyelinase was added at the start of the infection (2 h p.i.), and radiolabelled serine was added at 20 h p.i. The cells were harvested 5 h later. Results are expressed as means ± standard deviations of results from three separate experiments.
The effect of exogenous sphingomyelinase treatment on SM mass was assessed by determining the phospholipid compositions of mock-infected cells, C. trachomatis-infected mouse cells at 40 h p.i., and purified EBs. The results of the compositional analysis show that, while there was little or no change in amounts of glycerophospholipids (data not shown), there was consistently less SM (30 to 40%) in all three sphingomyelinase-treated samples (Fig. 5C).
Effect of brefeldin A on SM trafficking in C. trachomatis-infected cells.
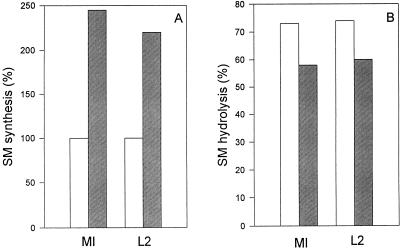
The percentage of total cellular SM hydrolyzed by exogenously added sphingomyelinase has been used to monitor SM and fluorescent C6-NBD–SM transport to the plasma membrane (18, 34, 39). Conflicting results have been reported for the effects of brefeldin A on SM transport, ranging from no effect (34) to essentially complete inhibition (18). In a final set of experiments, we determined the effect of brefeldin A on SM trafficking to the plasma membrane in mock-infected and C. trachomatis-infected mouse L cells. Three precursors ([14C]serine, [14C]palmitate, and [3H]choline) were used to label SM. Similar results were obtained with all three; results for [3H]choline are shown in Fig. 8. Brefeldin A treatment alone resulted in an increase in [3H]choline incorporation into SM in both mock-infected and C. trachomatis-infected mouse L cells (Fig. 8A).
FIG. 8.
(A) Incorporation of [3H]choline into SM of mock-infected mouse L cells (MI) and C. trachomatis L2-infected mouse L cells (L2) grown in the absence (open bars) and presence (hatched bars) of 5 μg of brefeldin A ml−1. [3H]SM synthesis was 18,347 dpm mg−1 in mock-infected control cells and 16,926 dpm mg−1 in L2-infected cells. (B) Transport of natural [3H]choline-labeled SM to the surfaces of mock-infected mouse L cells and C. trachomatis L2-infected mouse L cells grown in the absence (open bars) and presence (hatched bars) of 5 μg of brefeldin A ml−1. Hydrolyzable cell surface SM was quantitated with an exogenous sphingomyelinase treatment as described in Materials and Methods. Results are averages of results from two separate experiments; duplicate results varied by less than 10%.
The effects of brefeldin A on the transport of natural SM to plasma membranes, as estimated by susceptibility to hydrolysis with exogenous sphingomyelinase, in mock-infected and C. trachomatis-infected mouse L cells are shown in Fig. 8B. Under our experimental conditions, for both mock-infected control cells and chlamydia-infected cells, brefeldin A caused approximately a 20% decrease in the amount of cellular SM susceptible to sphingomyelinase hydrolysis. This result indicates that the transport of natural SM to the plasma membrane is inhibited to some extent by brefeldin A in both mock-infected and C. trachomatis-infected cells. There was no significant difference in the percentages of SM hydrolyzed when mock-infected cultures were compared to C. trachomatis-infected cultures. This result implies that there is no appreciable sequestration of newly synthesized SM in chlamydia-infected cells.
DISCUSSION
In this study of a CHO cell line with a temperature-sensitive CDP-choline synthetase, fumonisin B1, brefeldin A, and sphingomyelinase were used to induce alterations in host cell phospholipid composition. The effects of these alterations on C. trachomatis growth, phospholipid metabolism, trafficking, and composition were then determined. In all cases, regardless of how the alterations in host phospholipid composition were brought about, we found that C. trachomatis growth and EB progeny infectivity were not adversely affected. In addition, in every instance the final phospholipid composition of purified chlamydiae mimicked that of the host cell in which the parasite was grown. Together, these results indicate that, at least for the limits within which we could vary host phospholipid composition, the final lipid composition of chlamydiae did not appear to be strictly controlled. However, since none of our treatments resulted in a complete depletion of any host cell phospholipid, it is not possible to determine, from this study, whether chlamydiae require host lipids for survival.
Previous studies have established that the phospholipid composition of purified chlamydiae is a mixture of lipids typically found in procaryotes and eucaryotes (28, 46). It is known that C. trachomatis can synthesize two major bacterial glycerophospholipids, PE and PS, de novo (37, 46). In a series of studies, Hackstadt and colleagues (13–15) have shown that fluorescently labelled short-chain SM analogs are transported from the eucaryotic host to the chlamydial inclusion membrane and are ultimately incorporated into the parasite. Finally, Wylie et al. (46) used [14C]isoleucine to specifically label chlamydial branched-chain fatty acids and showed that eucaryotic glycerophospholipids were transported to and then modified by C. trachomatis. Together, these studies clearly indicate that, at least for phospholipids, there is an intimate association between chlamydiae and the host cell.
In contrast to PS and PE, C. trachomatis cannot synthesize PC or SM de novo, and as a result, it is easiest to monitor the effects of alterations in the compositions of these two host-specific phospholipids on trafficking to chlamydiae. The mutant CHO 58 cell line, with a thermolabile CDP-choline synthetase, provided an excellent model system for studying the effects of decreased PC content on PC trafficking to chlamydiae. Our results indicate that C. trachomatis grows just as well in this cell line as it does in the wild-type CHO K1 parent at both 33 and 40°C. Trafficking of PC from the host to chlamydiae was not appreciably affected in wild-type cells but was greatly reduced in CHO 58 cells at 40°C. The decrease in trafficking of PC was also reflected by the fact that C. trachomatis EBs, purified from CHO 58 cells cultured at the nonpermissive temperature, had a substantially decreased PC content.
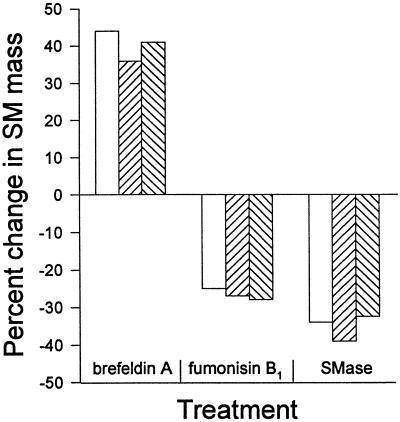
Three separate approaches were used to alter host cell SM content. A summary of the effects of the various treatments on the SM contents of mock-infected cells, C. trachomatis-infected cells, and highly purified chlamydial EBs is shown in Fig. 9. Fumonisin B1 treatment had little effect on C. trachomatis replication or isoleucine labelling of glycerophospholipids, which suggests that phospholipid trafficking from the host to chlamydiae occurs normally. Similar to results with mock-infected controls, fumonisin B1 did cause a significant decrease in SM biosynthesis and mass in C. trachomatis-infected cells. This, in turn, resulted in decreased trafficking of SM and a decline in SM content in highly purified EBs.
FIG. 9.
Summary of the effects of brefeldin A, fumonisin B1, and sphingomyelinase (SMase) treatment on SM content of mock-infected mouse L cells (open bars), C. trachomatis-infected mouse L cells at 40 h p.i. (upward hatched bars), and highly purified EBs prepared from infected mouse L cells at 40 h p.i. (downward hatched bars).
We found that treatment of mock-infected or C. trachomatis-infected cells with exogenous sphingomyelinase, which hydrolyzes only cell surface SM (18, 29, 34, 39), had little effect on chlamydial growth, glycerophospholipid biosynthesis, or trafficking. Similar to the findings with uninfected controls, sphingomyelinase treatment of C. trachomatis-infected cells resulted in a decrease in radiolabelled serine incorporated into newly synthesized SM and a decrease in SM mass. Previously, Hackstadt and colleagues (13, 14, 33) had concluded that C. trachomatis inhabits a unique vesicle which interrupts an exocytic pathway to intercept host SM in transit from the Golgi apparatus to the plasma membrane. Quantitative estimates made by microphotometry or thin-layer chromatography of extracted fluorescent lipids suggested that up to 40 to 50% of the C6-NBD–SM endogenously synthesized from C6-NBD–ceramide is retained by chlamydiae. Given these findings, we anticipated that a significant portion of the newly synthesized SM in a C. trachomatis-infected cell would be transported to the parasite and protected from exogenous sphingomyelinase. This should have resulted in but did not result in an increase in the amount of radiolabelled SM isolated from sphingomyelinase-treated chlamydia-infected cells compared to that isolated from mock-infected controls.
Differences in the experimental systems used by Hackstadt et al. (13, 14, 33) and ourselves may, at least in part, account for this variance. In our study, radiolabelled precursors were used to follow natural SM trafficking, whereas in the studies of Hackstadt et al. (13, 14), fluorescent C6-NBD–ceramide and –SM analogs were used. It is known that the fluorescent short-chain fatty acid renders these analogs more water soluble than their natural counterparts (30). As a result, these molecules can undergo spontaneous transfer between donor and acceptor membranes much more readily.
Brefeldin A was also used as an agent to alter host cell SM content. While it is clearly established that brefeldin A prevents vesicular transport of proteins to the cell surface by promoting retrograde transport of Golgi apparatus components back to the endoplasmic reticulum (20–22), studies of the effects of the antibiotic on SM transport have been less conclusive. Results varying from essentially complete inhibition (18), partial inhibition (39, 44), and virtually no inhibition (34) have been reported. In addition, brefeldin A affects the trafficking of natural SM and short-chain fatty acid analogs, C6-NBD–SM, differently. Both van Meer and van’t Hof (40) and van Helvoort et al. (39) found that C6-NBD–SM transport was not inhibited by brefeldin A whereas natural SM transport was blocked (39). Clearly, the effects of brefeldin A on SM trafficking to the cell surface vary depending on the experimental system used.
Hackstadt et al. (13, 14) used conventional fluorescence and confocal microscopy to show that brefeldin A blocked transport of C6-NBD–SM, endogenously synthesized from C6-NBD–ceramide, to chlamydiae. In addition, C6-NBD–SM incorporated into the plasma membrane was not transported to the inclusion to a significant extent (14). Our results with radiolabelled natural SM indicate that brefeldin A treatment of both mock-infected cultures and C. trachomatis-infected cells results in an increase in SM biosynthesis compared to that in the appropriate untreated controls. Furthermore, transport of newly synthesized SM to the cell surface, as estimated by susceptibility to exogenous sphingomyelinase, was only partially inhibited by brefeldin A in both mock-infected and C. trachomatis-infected cells. Finally, brefeldin A treatment resulted in an increase in the SM content of mock-infected mouse cells, C. trachomatis-infected mouse cells at 40 h p.i., and purified EBs. In total, these results suggest that, under our experimental conditions, brefeldin A has little effect on SM trafficking to chlamydiae. This finding agrees with our previous observation that brefeldin A had essentially no effect on glycerophospholipid trafficking to chlamydiae (46). A possible explanation for the differences in the findings of Hackstadt et al. (13, 14) and ourselves, in addition to the different types of SM tracer used (natural versus short-chain fatty acid analog), is the length of time C. trachomatis-infected cultures were exposed to the tracer in the presence of brefeldin A. Hackstadt et al. (13, 14) treated C. trachomatis-infected cultures at 18 h p.i. with brefeldin A for 90 min before a short labelling period (≈1 h with back exchange) with C6-NBD–ceramide. Fluorescent C6-NBD–SM, endogenously synthesized from C6-NBD–ceramide, rapidly accumulated in chlamydiae in untreated cultures but was prevented from trafficking in the presence of brefeldin A. However, upon longer incubation with C6-NBD–ceramide, brefeldin A-treated cultures eventually displayed fluorescent lipid in chlamydia cell walls (14). In our experiments, we routinely incubated cultures for 5 h with radiolabelled SM precursors.
The complexity of the system limits the level of our interpretation. Chlamydial infection alone causes changes in a variety of host cell processes (15, 24, 27, 36), and the addition of exogenous agents (brefeldin A, fumonisin B1, and sphingomyelinase) which directly affect host cell lipid metabolism compounds the problem. All our findings support the central conclusion that the final phospholipid composition of purified C. trachomatis EBs mimics that of the host cell. Neither the host nor the parasite appears to regulate the composition of the lipid mixture transported to or accepted by chlamydiae. Free-living bacteria, such as Escherichia coli and Bacillus subtilis, are known to tolerate wide variations in their final phospholipid compositions (8, 9); therefore, the fact that chlamydiae remain viable with an altered phospholipid makeup is not unusual.
The trafficking of glycerophospholipids, whether they are fluorescent short-chain fatty acid analogs (14) or radiolabelled natural lipids (46), is unaffected by brefeldin A. SM trafficking gives various results, which may be related to the fact that while the majority of SM is synthesized in the cis-medial Golgi apparatus (39), some is made in the plasma membrane (42) and in recycling endosomes (29). Interestingly, it has recently been shown that the chlamydial vacuole interacts with the endocytic pathway of the host and shares several characteristics with recycling endosomes (41). The results of Hackstadt and colleagues (13–15) indicate that at least part of the SM transported to chlamydiae comes from fusion of trans Golgi exocytic vesicles with the inclusion membrane. Previously, we have suggested that C. trachomatis obtains glycerophospholipids from the host through transient direct membrane contact with host cell subcellular organelles (46). In this model the chlamydial vacuole is free to accept lipids from any of the host subcellular structures (endoplasmic reticulum, Golgi apparatus, plasma membrane, mitochondria, nucleus, or early endosomes) with which it may transiently interact as it expands, ultimately occupying a major portion of the cellular cytoplasm. This explanation accounts for most of our observations to date regarding phospholipid trafficking in chlamydia-infected cells. In addition, it may also explain why, despite the fact that many host cell organelles are closely associated with the chlamydial inclusion, no proteins from any organelle have been found in the inclusion membrane (14, 33, 38, 41).
ACKNOWLEDGMENTS
We thank J. L. Wylie, L. L. Wang, and S. G. Cao for technical assistance.
This work was supported by grants from the Medical Research Council of Canada to G.M. (GR-13301) and G.M.H. (MT-14261). G.M.H. is a Heart and Stroke Foundation of Canada scholar.
REFERENCES
- 1.Andrieu N, Salvayre R, Levade T. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem. 1996;236:738–745. doi: 10.1111/j.1432-1033.1996.00738.x. [DOI] [PubMed] [Google Scholar]
- 2.Badiani K, Byers D M, Cook H W, Ridgway N D. Effect of fumonisin B1 on phosphatidylethanolamine biosynthesis in Chinese hamster ovary cells. Biochim Biophys Acta. 1996;1304:190–196. doi: 10.1016/s0005-2760(96)00119-1. [DOI] [PubMed] [Google Scholar]
- 3.Beatty P R, Stephens R S. CD+ T-lymphocyte mediated lysis of Chlamydia-infected L cells using an endogenous antigen pathway. J Immunol. 1994;153:4588–4595. [PubMed] [Google Scholar]
- 4.Belunis C J, Mdluli K E, Raetz C R H, Nano F E. A novel 3-deoxy-d-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus specific epitope. J Biol Chem. 1992;267:18702–18707. [PubMed] [Google Scholar]
- 5.Brade H, Brade L, Nano F E. Chemical and serological investigations on the genus-specific lipopolysaccharide epitope of Chlamydia. Proc Natl Acad Sci USA. 1987;84:2508–2512. doi: 10.1073/pnas.84.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruning A, Karrenbauer A, Schnabel E, Wieland F T. Brefeldin A-induced increase of sphingomyelin synthesis. J Biol Chem. 1992;267:5052–5055. [PubMed] [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 612–636. [Google Scholar]
- 9.de Mendoza D, Grau R, Cronan J E., Jr . Biosynthesis and function of membrane lipids. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C: American Society for Microbiology; 1993. pp. 411–424. [Google Scholar]
- 10.Esko J D, Raetz C R H. Autoradiographic detection of animal cell membrane mutants altered in phosphatidylcholine synthesis. Proc Natl Acad Sci USA. 1980;77:5192–5196. doi: 10.1073/pnas.77.9.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esko J D, Wermuth M M, Raetz C R H. CDP-choline synthetase in an animal cell mutant defective in lecithin formation. J Biol Chem. 1981;256:7388–7393. [PubMed] [Google Scholar]
- 12.Fan H, Brunham R C, McClarty G. Acquisition and synthesis of folates by obligate intracellular bacteria of the genus Chlamydia. J Clin Invest. 1992;90:1803–1811. doi: 10.1172/JCI116055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackstadt T, Scidmore M A, Rockey D D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackstadt T, Rockey D D, Heinzen R A, Scidmore M A. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 15.Hackstadt T, Fischer E R, Scidmore M A, Rockey D D, Heinzen R A. Origins and functions of the chlamydial inclusion. Trends Microbiol. 1997;5:288–293. doi: 10.1016/S0966-842X(97)01061-5. [DOI] [PubMed] [Google Scholar]
- 16.Hatch G M. Cardiolipin biosynthesis in the isolated heart. Biochem J. 1994;297:201–208. doi: 10.1042/bj2970201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatch G M, Vance D E. Stimulation of sphingomyelin biosynthesis by brefeldin A and sphingomyelin breakdown by okadaic acid treatment of rat hepatocytes. J Biol Chem. 1992;267:12443–12451. [PubMed] [Google Scholar]
- 18.Kallen K-J, Quinn P, Allan D. Effects of brefeldin A on sphingomyelin transport and lipid synthesis in BHK21 cells. Biochem J. 1993;289:307–312. doi: 10.1042/bj2890307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klausner R D, Donaldson J G, Lippinscott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippincott-Schwartz J, Yuan L G, Bonifacino J S, Klausner R D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippincott-Schwartz J, Donaldson J G, Schweizer A, Berger E G, Hauri H-P, Yaun L G, Klausner R D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.McClarty G. Chlamydiae and the biochemistry of intracellular parasitism. Trends Microbiol. 1994;2:157–164. doi: 10.1016/0966-842x(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 25.McClarty G, Tipples G. In situ studies on incorporation of nucleic acid precursors into Chlamydia trachomatis DNA. J Bacteriol. 1991;173:4922–4931. doi: 10.1128/jb.173.16.4922-4931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill A H, Wang E, Gilchrist D G, Riley R T. Fumonisins and other inhibitors of de novo sphingolipid biosynthesis. Adv Lipid Res. 1993;26:215–234. [PubMed] [Google Scholar]
- 27.Moulder J W. Interactions of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newhall W J. Macromolecular and antigenic composition of chlamydiae. In: Barron A L, editor. Microbiology of Chlamydia. Boca Raton, Fla: CRC Press Inc.; 1988. pp. 47–70. [Google Scholar]
- 29.Obradors M J M, Sillence D, Howitt S, Allan D. The subcellular sites of sphingomyelin biosynthesis in BHK cells. Biochim Biophys Acta. 1997;1359:1–12. doi: 10.1016/s0167-4889(97)00088-8. [DOI] [PubMed] [Google Scholar]
- 30.Rosenwald A G, Pagano R E. Intracellular transport of ceramide and its metabolites at the Golgi complex: insights from short-chain analogs. Adv Lipid Res. 1993;26:101–118. [PubMed] [Google Scholar]
- 31.Rouser G A, Siakotos A N, Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorous analysis of spots. Lipids. 1966;1:85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder J J, Crane H M, Xia J, Liotta D C, Merrill A H., Jr Disruption of sphingolipid metabolism and stimulation of DNA synthesis by fumonisin B1. A molecular mechanism for carcinogenesis associated with Fusarium moniliforme. J Biol Chem. 1994;269:3475–3481. [PubMed] [Google Scholar]
- 33.Scidmore M A, Fischer E R, Hackstadt T. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol. 1996;134:363–374. doi: 10.1083/jcb.134.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiao Y-J, Vance J E. Sphingomyelin transport to the cell surface occurs independently of protein secretion in rat hepatocytes. J Biol Chem. 1993;268:26085–26092. [PubMed] [Google Scholar]
- 35.Smith E R, Merrill A H., Jr Differential roles of de novo sphingolipid biosynthesis and turnover in the “burst” of free sphingosine and sphinganine, and their 1-phosphates and N-acyl-derivatives, that occur upon changing the medium of cells in culture. J Biol Chem. 1995;270:18749–18758. doi: 10.1074/jbc.270.32.18749. [DOI] [PubMed] [Google Scholar]
- 36.Stephens R S. Challenge of Chlamydia research. Infect Agents Dis. 1993;1:279–293. [PubMed] [Google Scholar]
- 37.Stephens R S, Kalman S, Fenner C, Davis R. Chlamydia genome project. 1997. http://chlamydia-www.berkeley.edu:4231 http://chlamydia-www.berkeley.edu:4231. . [Google Scholar]
- 38.Taraska T, Ward D M, Ajioka R S, Wyrick P B, Davis-Kaplan S R, Davis C H, Kaplan J. The late chlamydial inclusion membrane is not derived from the endocytic pathway and is relatively deficient in host proteins. Infect Immun. 1996;64:3713–3727. doi: 10.1128/iai.64.9.3713-3727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Helvoort A, Giudici M L, Thielemans M, van Meer G. Transport of sphingomyelin to the cell surface is inhibited by brefeldin A and in mitosis, where C6-NBD-sphingomyelin is translocated across the plasma membrane by a multidrug transporter activity. J Cell Sci. 1997;110:75–83. doi: 10.1242/jcs.110.1.75. [DOI] [PubMed] [Google Scholar]
- 40.van Meer G, van’t Hof W. Epithelial sphingolipid sorting is insensitive to the reorganization of the Golgi by nocodazole, but is abolished by monensin in MDCK cells and by brefeldin A in Caco-2 cells. J Cell Sci. 1993;104:833–842. doi: 10.1242/jcs.104.3.833. [DOI] [PubMed] [Google Scholar]
- 41.van Ooij C, Apodaca G, Engel J. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect Immun. 1997;65:758–766. doi: 10.1128/iai.65.2.758-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vos J P, Giudici M L, van der Bijl P, Magni P, Marchesini S, van Golde L M, Lopes-Cardozo M. Sphingomyelin is synthesized at the plasma membrane of oligodendrocytes and by purified myelin membranes: a study with fluorescent- and radiolabelled ceramide analogues. FEBS Lett. 1995;368:393–396. doi: 10.1016/0014-5793(95)00695-6. [DOI] [PubMed] [Google Scholar]
- 43.Wang E, Norred W P, Bacon C W, Riley R T, Merrill A H., Jr Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 44.Warnock D E, Roberts C, Lutz M S, Blackburn W A, Young W W, Jr, Baenziger J U. Determination of plasma membrane lipid mass and composition in cultured Chinese hamster ovary cells using high gradient magnetic affinity chromatography. J Biol Chem. 1993;268:10145–10153. [PubMed] [Google Scholar]
- 45.Wu W-I, McDonough V M, Nickels J T, Jr, Ko J, Fischl A S, Vales T R, Merrill A H, Jr, Carman G M. Regulation of lipid biosynthesis in Saccharomyces cerevisiae by fumonisin B1. J Biol Chem. 1995;270:13171–13178. doi: 10.1074/jbc.270.22.13171. [DOI] [PubMed] [Google Scholar]
- 46.Wylie J L, Hatch G M, McClarty G. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J Bacteriol. 1997;179:7233–7242. doi: 10.1128/jb.179.23.7233-7242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]