Abstract
Inborn errors of purine metabolism are rare syndromes with an array of complex phenotypes in humans. One such disorder, adenylosuccinate lyase deficiency (ASLD), is caused by a decrease in the activity of the bi-functional purine biosynthetic enzyme, adenylosuccinate lyase (ADSL). Mutations in human ADSL cause epilepsy, muscle ataxia, and autistic-like symptoms. Although the genetic basis of ASLD is known, the molecular mechanisms driving phenotypic outcome are not. Here, we characterize neuromuscular and reproductive phenotypes associated with a deficiency of adsl-1 in Caenorhabditis elegans. We demonstrate that adsl-1 function contributes to regulation of spontaneous locomotion, that adsl-1 functions acutely for proper mobility, and that aspects of adsl-1-related dysfunction are reversible. Using pharmacological supplementation, we correlate phenotypes with distinct metabolic perturbations. The neuromuscular defect correlates with accumulation of a purine biosynthetic intermediate whereas reproductive deficiencies can be ameliorated by purine supplementation, indicating differing molecular mechanisms behind the phenotypes. Because purine metabolism is highly conserved in metazoans, we suggest that similar separable metabolic perturbations result in the varied symptoms in the human disorder and that a dual-approach therapeutic strategy may be beneficial.
Keywords: adsl-1, C. elegans, purine metabolism, locomotion, reproduction
Introduction
Inborn errors of purine metabolism are understudied syndromes that arise from mutation of purine biosynthetic or catabolic enzymes. Although rare, these disorders are thought to be underdiagnosed because the varied clinical symptoms mimic other disorders (Jurecka 2009; Mastrogiorgio et al. 2021). Purine disorders can have devastating health effects and often result in early death. Not only are there few therapeutic options available to patients, but the biological mechanisms linking defects in purine biosynthesis to phenotypic outcomes have also been difficult to decipher. Our aim is to use a fast, inexpensive and yet applicable model to explore the relationships between perturbations in purine biosynthesis and physiological and behavioral outcomes.
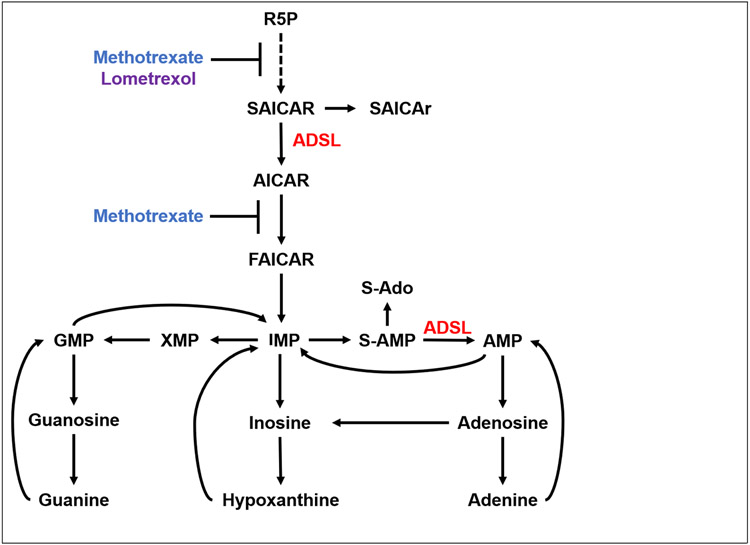
Purine nucleotides are monomers that polymerize with pyrimidine nucleotides to form nucleic acids. They also serve critical roles in cell signaling, energy storage and transfer, and metabolic regulation (Fang et al. 2013; Huang et al. 2021). Purines are synthesized via two biosynthetic pathways: de novo and salvage. De novo purine synthesis (DNPS) forms purine monomers from the components of intracellular amino acids and sugars. This pathway is comprised of eleven steps to convert ribose-5-phosphate (R5P) to inosine monophosphate (IMP), the precursor for other purine monomers (Fig. 1). The salvage biosynthetic pathway uses nucleic acid constituents from the diet or purine catabolism to recreate new purine nucleotides.
Figure 1. Purine biosynthesis pathways.
ADSL functions twice in de novo purine synthesis (DNPS). Methotrexate is an antimetabolite that indirectly inhibits DNPS. Lometrexol directly inhibits DNPS as an antagonist of GART activity. Abbreviations: R5P, ribose-5-phospate; SAICAR/r, succinylaminoimidazole carboxamide ribotide/ riboside; ADSL, adenylosuccinate lyase; AICAR, aminoimidazole carboxamide ribotide; IMP, inosine monophosphate; S-AMP, adenylosuccinate; S-Ado, succinyladenosine AMP, adenosine monophosphate; XMP, xanthine monophosphate; GMP, guanosine monophosphate.
Adenylosuccinate lyase (ADSL) is an enzyme with dual functions in DNPS. It catalyzes the cleavage of succinyl groups to yield fumarate twice; it converts succinylaminoimidazole carboxamide ribotide (SAICAR) to aminoimidazole carboxamide ribotide (AICAR) in the primary backbone of the de novo pathway. ADSL also catalyzes production of AMP from succinyladenosine monophosphate (S-AMP) in the branch of the de novo pathway specific to AMP, which overlaps with the purine nucleotide cycle and functions to maintain adenosine monophosphate (AMP) levels and favor the production of adenosine triphosphate (ATP).
Adenylosuccinate lyase deficiency (ASLD) is a human syndrome associated with a spectrum of symptoms including seizures, ataxia and motor impairment, cognitive impairment, and neurobehavioral phenotypes, including autistic-like behaviors (Jaeken 1983; Jaeken et al. 1988; Mastrogiorgio et al. 2021; Stone et al. 1992). Symptoms range in severity from mild to severe and are negatively correlated with the degree of residual ADSL activity (Zikanova et al. 2010). In the most extreme cases, ASLD is neonatally fatal due to prenatal growth restriction, encephalopathy, and intractable seizures (van den Bergh et al. 1998; Zikanova et al. 2010).
There are varied hypotheses about the etiology of ASLD symptoms. Severity of symptoms has been positively correlated with the level of accumulation of two succinylnucleosides, SAICAr and S-Ado, in the urine and cerebrospinal fluid (Jurecka et al. 2008; Krijt et al. 1999a). These nucleosides are the dephosphorylated forms of the ADSL substrates SAICAR and S-AMP, respectively, and their accumulation in body fluids is the biochemical marker of the disorder. A lower ratio of S-Ado/SAICAr is associated with more severe symptoms, and it was hypothesized that S-Ado is protective while SAICAr is toxic (Jurecka et al. 2008; Krijt et al. 1999b). Others have suggested that this ratio may not be predictive of phenotype severity, instead, it may correlate to the patient’s development and age during sample collection (Zikanova et al. 2010). Dephosphorylation of SAICAR to SAICAr has also been proposed to be a detoxification mechanism to reduce the toxic accumulation of SAICAR in affected cells (Hürlimann et al. 2011). There are, thus, outstanding questions about the role of ADSL substrates in disease etiology.
A second hypothesis is that suboptimal DNPS specifically contributes to ASLD symptoms. Deficiency of ADSL is expected to result in decreased levels of purine products, particularly adenine nucleotides, due to the dual function of this enzyme in the biosynthesis of AMP. However, no deficit in purines has been detected in patients; measurements of purine levels in kidney, liver, and muscle cells of ASLD patients are normal (Van den Berghe and Jaeken 1986a). Residual enzyme activity in patients likely contributes to the conservation of purine levels. Measurements of ADSL enzyme activity indicate that 3% residual activity is sufficient to convert S-AMP to AMP; although metabolic flux is greatly hindered (Van den Berghe and Jaeken 1986b). Moreover, a reduction in ADSL activity can be circumvented via supply of purines through the salvage pathway and dietary intake (Van Den Berghe, Vincent, and Jaeken 1997).
The pathological mechanisms causing the specific symptoms also remain unknown (Ciardo, Salerno, and Curatolo 2001; Jurecka et al. 2015; Spiegel, Colman, and Patterson 2006). However, recent studies are beginning to shed light on possible mechanisms. Dutto et al have tied ADSL function to DNA damage repair and to ciliogenesis in cultured cells, and specifically link ADSL function in production of purines to the impact on damage repair and the role of ADSL in preventing SAICAR accumulation to the effect on ciliogenesis (Dutto et al. 2022). Other genetic model systems expand the experimental approaches available to study ASLD to developmental, physiological, and behavioral analyses not available in models such as mammalian cell culture and yeast (Chen et al. 2016; Gooding et al. 2015a). Such models are shedding light on perturbations in both the musculature and the nervous system upon loss of adenylosuccinate lyase activity. Using chicken and zebrafish models, Dutto et al. document effects of loss of ADSL on neurodevelopment (Dutto et al. 2022). Marsac et al have studied purine homeostasis in C. elegans and observed that loss of adsl-1 results in slow mobility and disorganization of myosin fibers in muscle (Marsac et al. 2019). However, they found no evidence of neuronal dysfunction after examining sensory responses and the response of the neuromuscular junction to pharmacological manipulation.
We are interested in probing the etiology of ASLD outcomes using Caenorhabditis elegans, an established organism for studying metabolism and associated metabolic disorders (Kuwabara and O’Neil 2001). The purine metabolic pathways are highly conserved across all eukaryotes, including C. elegans (Kappock, Ealick, and Stubbe 2000). This level of conservation supports the functionality of C. elegans as a model for probing inborn errors of purine metabolism. In addition to metabolic conservation, C. elegans provides a well-characterized nervous system that is essential for studying aspects of ASLD. By using a model with a simple and fully identified neural network (White et al. 1986), nervous system function can be studied under conditions of ADSL depletion.
We report here on our development of C. elegans as a model for ASLD using a mutant allele and RNAi knockdown of the gene that encodes adenylosuccinate lyase, adsl-1 (Marsac et al. 2019). Analysis of mobility and reproductive phenotypes reveals that adsl-1 activity plays an acute role in fertility and functional and regulatory roles in locomotion. We also shed light on the specific metabolic disruptions that give rise to phenotypes. We examine metabolite levels in control and adsl-1 animals and use metabolite supplementation to associate purine homeostasis with fertility and pharmacological approaches to link substrate accumulation to dysregulation of neuromuscular motility patterns.
Results
adsl-1 function is required for fertility and promotes embryonic viability
We used adsl-1(tm3328) and adsl-1(RNAi) in the RNAi hypersensitive, eri-1(mg366), and control N2 genetic backgrounds to characterize phenotypes caused by disruption of ADSL-1 function. The tm3328 allele is a large deletion that removes the majority of the coding sequence of adsl-1, from the start codon through codon 365, out of 478 total, resulting in a putative null allele. We observed that adsl-1(tm3328) animals are small and sterile as previously reported (Marsac et al. 2019). While Marsac et al. observed mild levels of sterility upon RNAi of adsl-1 (Marsac et al. 2019), we found that treatment with RNAi targeting adsl-1 from the first larval stage, which reduces adsl-1 mRNA levels an average of 75% (Fig. 2A), causes fully penetrant sterility in the eri-1(mg366) and N2 backgrounds (n>100). Animals exposed to RNAi beginning at the fourth larval stage produce F1 progeny that are completely sterile (n>100).
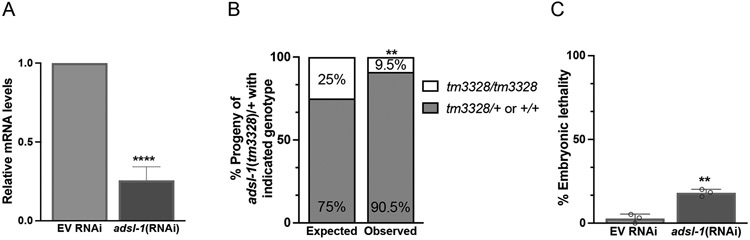
Figure 2. adsl-1 function promotes embryonic viability.
(A) RNAi results in robust knockdown of adsl-1 expression. qRT-PCR was used to measure relative mRNA levels of adsl-1 in the empty vector RNAi control and adsl-1(RNAi) animals. Values are averages from five biological replicates performed with two technical replicates each. Error bars are standard deviation S.D. **** represents p<0.0001 by Student’s two-tailed t test. (B) Homozygous adsl-1(tm3328) mutants are less prevalent than expected in the population of progeny that segregate from adsl-1(tm3328)/+ hermaphrodites. Observed data is derived from nine replicate matings and a total of 1,345 progeny. ** represents p<0.01 using chi squared test. (C) Embryos of eri-1 animals exposed to adsl-1(RNAi) at the fourth stage display a high degree (18%) of embryonic lethality compared to the EV control (3%). Eat dot represents the average lethality derived from an RNAi replicate. Each RNAi experiment included between 144 and 213 eggs laid by 3 to 5 hermaphrodites per condition. Error bars are standard deviation. ** represents p<0.01 using Student’s two-tailed t test.
The sterility of adsl-1(tm3328) requires the strain to be maintained using a balancer chromosome. To assess viability of animals with loss of adsl-1(tm3328) function, we first removed the balancer chromosome from the strain via mating of adsl-1(tm3328)/ hT2 hermaphrodites to control N2 males, and subsequently examined the progeny of the resulting adsl-1(tm3328)/+ heterozygotes. Only 9.5% of the progeny, as opposed to the expected 25%, were homozygous for adsl-1(tm3328), as assessed by their phenotypes, including sluggishness and sterility (Fig. 2B). This altered genotypic ratio indicates that more than 60% of the adsl-1(tm3328) population is missing. We also observed a mild degree of embryonic lethality (18%) in the progeny produced by animals treated with adsl-1 RNAi from the fourth larval stage (Fig. 2C).
adsl-1 function is required to execute normal swimming and crawling locomotion
adsl-1(tm3328) are noticeably sluggish compared to control animals, crawl more slowly when prodded and display a disorganized network of muscle myosin (Marsac et al. 2019). We further characterized mobility of animals with loss and reduction of ADSL-1 function by quantifying spontaneous crawling speed and swimming locomotion (thrashing) of adsl-1(tm3328) and adsl-1(RNAi) animals. Animals switch abruptly between crawling and swimming locomotion when moved between solid media and a liquid environment. These forms of locomotion are distinguished by distinct underlying patterns of neuromuscular activity (Pierce-Shimomura et al. 2008; Vidal-Gadea et al. 2011), but likely represent the extremes in modulation of a single gait pattern (Boyle, Berri, and Cohen 2012; Vidal-Gadea et al. 2011).
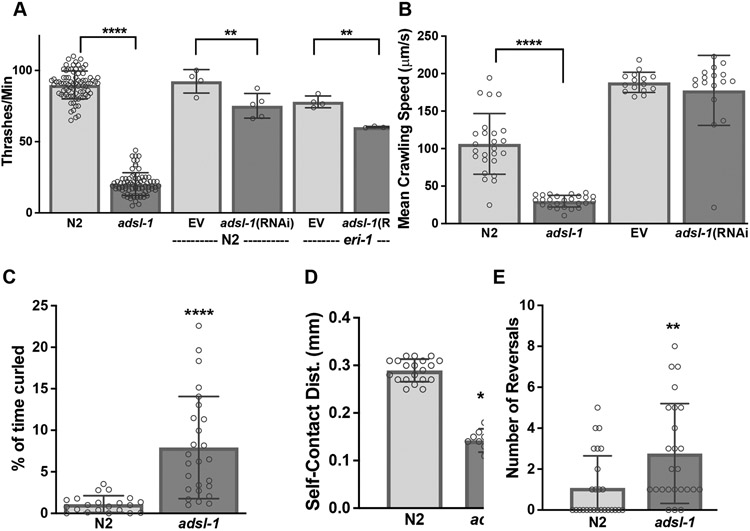
The thrashing rate of adsl-1(tm3328) and adsl-1(RNAi) animals is significantly slower than controls, although the RNAi treatment produces a more subtle phenotype (Fig. 3A), adsl-1(tm3328) animals exhibit a 77% reduction in thrashing rate relative to N2, and adsl-1(RNAi) causes 18% to 23% reduction in thrashing rate relative to N2 or eri-1 controls, respectively (Fig. 3A). Crawling speed for adsl-1(tm3328) animals is also reduced, but adsl-1(RNAi) animals are not, on average, detectably slow during crawling locomotion (Fig. 3B).
Figure 3. Disruption of ADSL function causes locomotion defects.
(A) adsl-1(tm3328) mutants and adsl-1(RNAi) have significantly decreased thrashing rate. In this panel and those below, dots represent the value for a single animal in experiments using the null allele and the average of all animals in an experiment when using RNAi. Sample size for each RNAi experiment was between 9 and 21 animals. RNAi was performed in both N2 and eri-1(mg366) animals. We used the hypersensitive eri-1(mg366) for all RNAi experiments unless otherwise noted. (B) Average crawling speed for adsl-1(tm3328) mutants is significantly slower than controls. However adsl-1(RNAi) does not impact crawling speed. (C) adsl-1(tm3328) mutants spend significantly more time in a contracted, curled position while swimming. (D) Swimming adsl-1(tm3328) mutants have an average shorter distance between the head and tail compared to control animals. (E) The number of spontaneous reversals observed in 1 minute of crawling is greater in adsl-1(tm3328) mutants. Error bars indicate S.D. **, ***, and **** represent p<0.01, p<0.001, and p<0.0001, respectively, calculated using ANOVA with Šidák’s multiple comparison test for (A) and (B) and Student’s two-tailed t test for (C-E).
We also characterized aspects of locomotion expected to be independent of slowness or weakness: protracted contractions and number of reversals. While swimming, C. elegans will sometimes stop and hold a contracted curled position that resembles the letter ‘O’ or the number ‘6’. adsl-1(tm3328) spend 7-fold more time in the curled position (Fig. 3C), likely contributing to a decrease in the average self-contact distance between the head and the tail (Fig. 3D). During crawling locomotion, animals periodically stop, reverse, and then continue forward, adsl-1(tm3328) animals execute an average of over 4-fold more reversals per minute than control animals during their spontaneous crawling program (Fig. 3E). Thus, adsl-1 animals are not merely weak due to lack of muscle cell mass or integrity, they aberrantly execute aspects of the locomotory pattern during crawling and swimming, suggesting defects beyond previously observed muscle structural disorganization (Marsac et al. 2019).
ADSL-1 is required acutely to promote locomotion
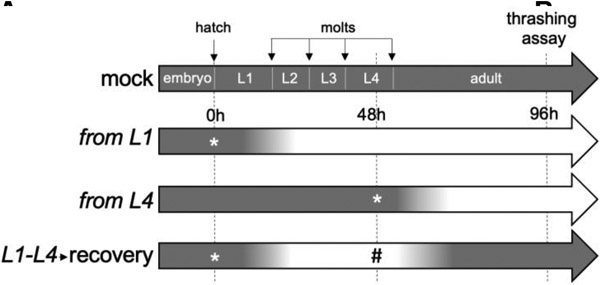
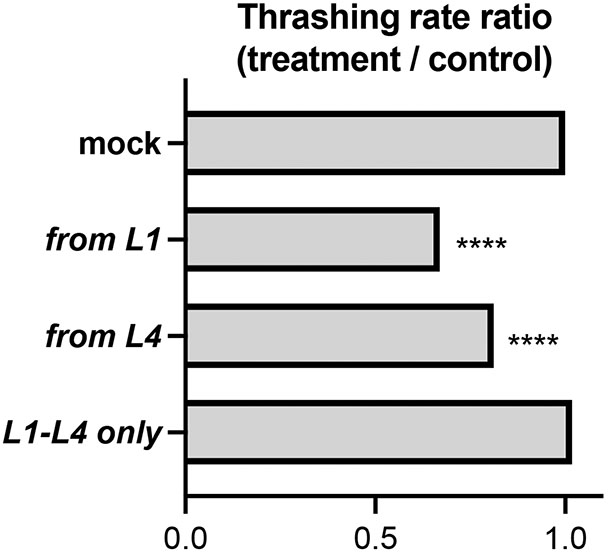
ADSL-1 could have effects in development that affect neuromuscular structure or morphology resulting in indirect effects on motility. Alternatively, or in combination, ADSL-1 could act acutely to affect physiology and function of the neuromuscular system. Developmental effects of ADSL have been documented in both C. elegans and zebrafish (Dutto et al. 2022; Marsac et al. 2019). However, the adsl-1(RNAi) phenotypes suggest that the locomotion defects that are readily induced by RNAi (such as thrashing) are not an indirect result of aberrant development; application of RNAi beginning in the first larval stage, after embryonic differentiation and development of the musculature, consistently produces a thrashing defect, although less extreme than that of the null allele (Fig. 4A). We further investigated the acute need for ADSL-1 with RNAi experiments designed to remove and then restore function on a temporal timeline. We synchronized N2 animals as arrested L1s and initiated larval development by feeding on mock RNAi plates at time 0 and assayed thrashing rate at 96 hours and then compared their thrashing rates to animals exposed to RNAi under three distinct temporal protocols (Fig. 4A): 1. exposure from L1 (adsl-1(RNAi) positive control) 2. exposure from L4 (late RNAi treatment) and 3. transient exposure from L1-L4 followed by a 24h recovery period. In this experiment, adsl-1(RNAi) positive control animals produced thrashing rates that were about 30% lower than that of the mock RNAi control (Fig. 4B). Similarly, late RNAi exposure for 48 hours beginning two days after embryogenesis induces a thrashing rate decrease of almost 20%. Animals exposed to RNAi for 48 hours followed by a recovery period thrash at indistinguishable rates to controls (Fig. 4B). Thus, we conclude that ADSL-1 has an acute function in thrashing beyond any requirement during embryogenesis, and that the effects of loss of ADSL-1 on thrashing are likely reversible.
Figure 4.
Temporal requirements for adsl-1 function in thrashing.
(A) Experimental design. Four distinct treatment protocols are labeled according to timing of RNAi. Gradients on the timelines represent expected levels of ADSL-1 activity from fully active (dark) to maximally inhibited by RNAi (light). * indicates a timepoint when animals were moved to an adsl-1 RNAi plate and # indicates a timepoint when animals were moved off of adsl-1 RNAi plates. Thrashing was assessed at 96 hours in each experiment. (B) Ratio of experimental to control thrashing rate when treated as indicated in (A). Data represents averages from 3 biological replicates with 17-20 animals in each replicate. **** represents p<0.0001, calculated using Student’s two-tailed t test.
Reduction of adsl-1 function alters substrate levels but not steady state purine levels
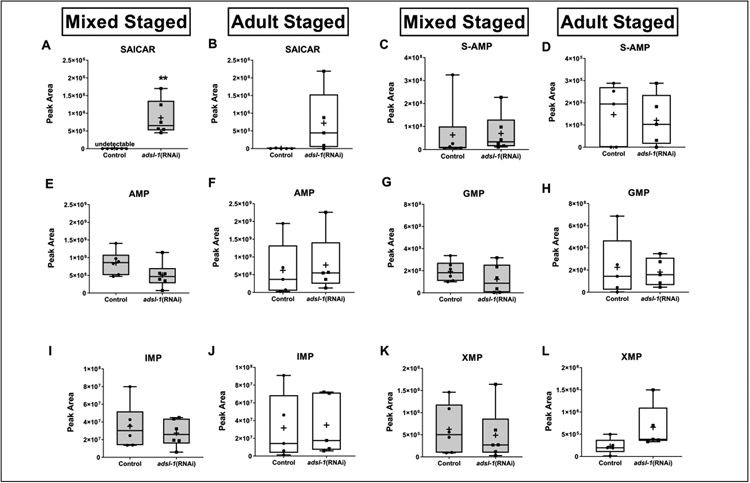
To begin to investigate how changes in ADSL substrate or purine levels may relate to phenotypes, we quantified metabolite levels in adsl-1(RNAi) animals using LC-MS. We first determined the relative levels of both ADSL substrates, SAICAR and S-AMP, in six biological replicate samples of mixed-stage adsl-1(RNAi) and control animals. There were no detectable peaks for SAICAR in any of the control RNAi replicates (Fig. 5A), indicating that the amount of SAICAR is typically below the threshold for metabolite detection via our methods. In all six replicates of adsl-1(RNAi), SAICAR was easily detected, indicating that there is an increase in SAICAR levels when adsl-1 is knocked down (Fig. 5A). Surprisingly, global steady-state levels of S-AMP are not significantly altered, although there is an upward trend in mixed stage adsl-1(RNAi) animals compared to the control (Fig. 5C). Thus, knockdown of adsl-1 leads to the accumulation of at least one ADSL-1 substrate.
Figure 5. adsl-1 knockdown causes substrate accumulation, but does not affect purine levels.
Grey box plots represent LC-MS peak area data from mixed-stage, whole animal lysates, and white box plots represent data from synchronized day one adult, whole animal lysates. (A,B) SAICAR is undetectable in empty vector control (EV) animals from mixed stage populations and undetectable in four of five EV samples from synchronized adults. There is a statistically significant increase in SAICAR levels upon RNAi of adsl-1 in mixed-stage animals (A), and an upward trend in synchronized adult animals (B). (C,D) We detected no statistically significant changes in S-AMP levels in adsl-1(RNAi) animals compared to the empty vector control. (D) One of the data points for the empty vector control was a statistical outlier and not included in statistical analysis. Outliers are defined as any value greater than Q3+(1.5xIQR). (E,F) AMP (G,H) GMP, and (I,J) IMP levels do not change upon adsl-1(RNAi). (K,L) XMP levels do not change upon adsl-1(RNAi) compared to the empty vector control in mixed stage animals (K) but trend upward in synchronized adults (L, results are not statistically different p=?). For all plots, each data point represents one biological replicate. Boxes show the upper and lower quartile values, + indicates the mean value, and lines indicate the median. Error bars indicate the maximum and minimum of the population distribution. **, 0.01<p<0.05, calculated using Welch’s t test.
We also measured steady state levels of purine monophosphate metabolites in mixed-stage populations of adsl-1(RNAi) and control animals. Interestingly, none of these metabolites showed statistically significant changes upon adsl-1 knockdown. AMP is the only metabolite that shows a downward trend (Fig. 5E) with an average 29% decrease in the adsl-1 samples, relative to controls. Levels of IMP do not exhibit any difference in adsl-1(RNAi) compared to the control (Fig. 5I). Levels of XMP are more variable than that of AMP or IMP, but do not display any relative difference when comparing adsl-1(RNAi) to controls (Fig 6K). The relative levels of GMP have the largest variance of the examined metabolites for adsl-1 RNAi, but did not exhibit a statistically significant difference from the control (Fig. 5G). Overall, this data indicates that there is not a global decrease in purine metabolite levels that might be expected upon knockdown of adsl-1.
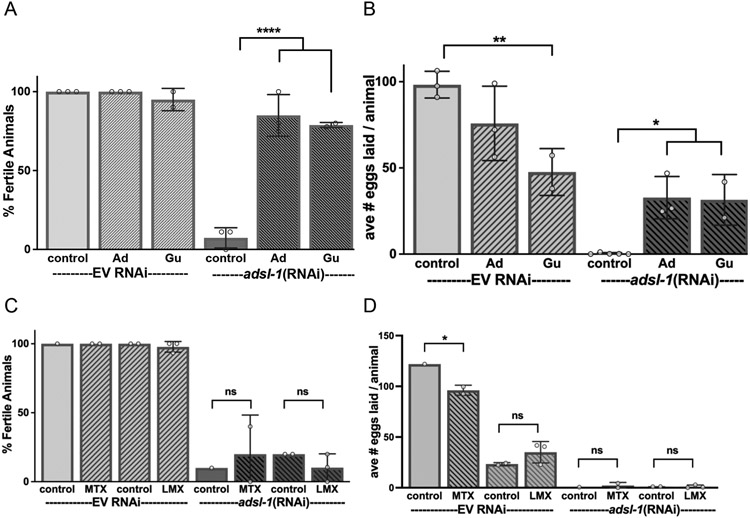
Figure 6. Purine supplementation restores fertility and fecundity to adsl-1.
Supplementation with 10 mM adenosine (Ad), or guanosine (Gu) restores fertility (A) and fecundity (B) of adsl-1(RNAi) animals. Supplementation with drugs to interfere with DNPS, including 22 μM methotrexate or 29 nM lometrexol, had no significant effect on (C) fertility or (D) fecundity of adsl-1(RNAi) animals. The controls for methotrexate and lometrexol are 8 μL and 155 μL of DMSO, respectively. Evidence for the uptake and inhibitory effect of methotrexate and lomotrexol is in Fig. 7. Each dot represents the value from an RNAi experiment with between 9 and 20 animals. Error bars indicate S.D. *, **, and **** represent p<0.05, p<0.01, and p<0.0001, respectively. Significance was calculated using ANOVA with Šidák’s multiple comparison test.
Because we found no significant decrease in purine levels upon reduction of adsl-1 function in a mixed stage population of animals, we also investigated the hypothesis that purine levels are decreased in adsl-1(RNAi) during only specific developmental stages. We compared ADSL substrate and global purine levels in synchronized day-one adult adsl-1(RNAi) animals, which are sterile, and control animals, which are in the peak of the fertile period where we expect a high demand for purines. The results were similar to those from mixed-stage animals (Fig. 5B) with the exception of no detection of an increase in S-AMP (Fig. 5D). Notably, steady state levels of purine monophosphate metabolites again did not show any significant decrease upon adsl-1 knockdown in the synchronized population relative to the fertile control animals. The levels are variable in the adsl-1(RNAi) population but do not show any noticeable trends (Fig. 5F, H, J) aside from XMP, which shows an upward trend relative to controls (Fig. 5L). Overall, this data indicates that there is no significant decrease in steady state purine metabolite levels upon knockdown of adsl-1 even though the animals are completely sterile and significantly slow in the thrashing assay.
Purine homeostasis contributes to the reproductive phenotype
We next examined the effect of treatments designed to prevent adsl-1 substrate buildup or to enhance purine availability on adsl-1 phenotypic outcomes. To determine if purine homeostasis was correlated to any of the phenotypic outcomes, we supplemented cultures with the purine nucleosides adenosine and guanosine, which can be used to produce purine nucleotides via the salvage pathways and assessed phenotypes. To determine if substrate accumulation may contribute to phenotypic outcome, we treated the animals with drugs to inhibit DNPS at steps prior to ADSL-1 function and assessed phenotypes (Fig. 1). To block substrate accumulation, we used methotrexate, an inhibitor of dihydrofolate reductase, and lometrexol, an inhibitor of phosphoribosylglycinamide transformylase (Allegra et al. 1987; Beardsley et al. 1989; Fairbanks et al. 1999).
In control animals, supplementation with adenosine or guanosine had no marked effect on fertility but guanosine did reduce brood size (Fig. 6A,B). In contrast, in adsl-1(RNAi) animals, supplementation had striking effects on fertility, robustly restoring fertility from completely sterile to over 80% fertile (Fig. 6A). Fecundity is also robustly restored, brood sizes of the supplemented adsl-1(RNAi) animals approached the levels observed upon supplementation of control animals with these purine metabolites (Fig. 6B). The positive effect of purine supplementation on fertility does not extend to the loss-of-function adsl-1(tm3328) animals; no adsl-1(tm3328) animals lay eggs when supplemented with purines (n>100).
Supplementation of cultures with methotrexate had no effect on the fecundity or fertility of adsl-1 RNAi animals (Fig. 6C,D). Thus, RNAi-induced sterility is linked to a deficit in purine homeostasis, and we detected no strong role for substrate accumulation in etiology of the sterility.
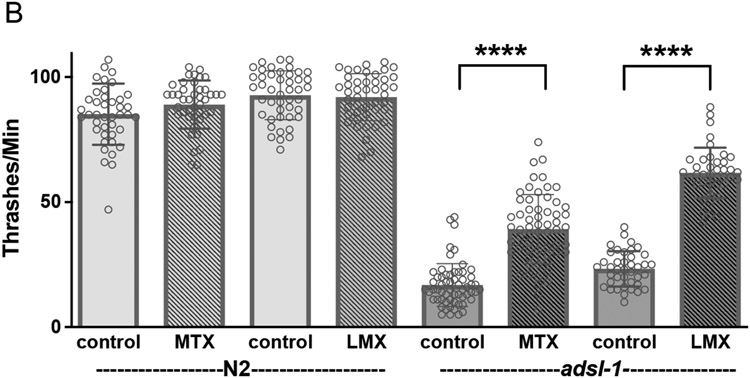
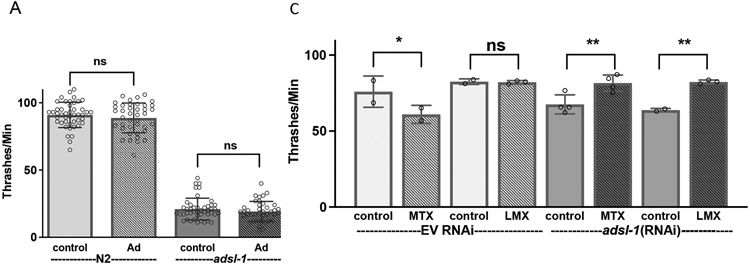
We also analyzed mobility using thrashing assays after supplementation with adenosine. Supplementation with adenosine levels sufficient to restore fertility and fecundity had no effect on thrashing rate for adsl-1(tm3328) or the N2 control (Fig. 7A).
Figure 7. Upstream inhibition of DNPS improves mobility of adsl-1.
(A) Supplementation with 10 mM adenosine has no effect adsl-1 mutant thrashing rate. (B-C) Supplementation with 22 μM methotrexate or 29 nM lometrexol partially restores thrashing rate in adsl-1 mutants (B) and fully restores thrashing rate in adsl-1(RNAi) (C). Controls for methotrexate and lometrexol are 8 μL and 155 μL of DMSO, respectively. Dots represent the value for a single animal in experiments using the null allele (A and B) and the average of all animals in an experiment when using RNAi (C). Sample size for each RNAi experiment was between 16 and 20 animals. Error bars indicate S.D. *, **, and **** represent p<0.05, p<0.01, and p<0.0001, respectively. ns is not significant. Significance was calculated using ANOVA with Šidák’s multiple comparison test.
In contrast, both adsl-1(tm3328) and adsl-1(RNAi) displayed improved locomotion upon either methotrexate or lometrexol supplementation (Fig. 7). The methotrexate supplemented mutants displayed a 212% increase in thrashing rate compared to the control mutants, but are only restored to ~45% of the N2 control; the lometrexol supplemented mutants displayed a 265% increase in thrashing rate compared to the control mutants, but are only restored to 67% of the N2 control (Fig. 7B). The attenuation of the milder phenotype of adsl-1(RNAi) is more robust than that of the mutants; the RNAi-treated animals thrash at a rate indistinguishable from the empty vector control after both methotrexate and lometrexol supplementation (Fig. 7C). We conclude that reproductive and neuromuscular deficits observed in adsl-1 are of distinct etiology.
Discussion
Given the myriad roles of purines in cellular metabolism, it is unsurprising that perturbations to purine biosynthesis would have wide-ranging effects on cellular and organismal function. However, the etiologies of the distinct and unique neurobehavioral phenotypes associated with reduction or loss of function of various enzymes involved in purine biosynthesis are still mysterious. We are using C. elegans as a tractable model for specifically investigating the etiology of neurobehavioral outcomes associated with adenylosuccinate lyase deficiency. Both reduction of function via RNAi of adsl-1 in C. elegans and loss-of-function adsl-1(tm3328) homozygotes present amenable genetic systems to model this disorder. Corroborating the work of Marsac et al. (Marsac et al. 2019), we find that C. elegans with reduction or loss of adsl-1 function have impaired mobility, sterility, and the expected metabolic signature of substrate accumulation. We add to the analysis of adsl-1 function in C. elegans, revealing an acute role of adsl-1 in locomotion and a role for adsl-1, beyond the recognized disorganized musculature, in regulation of mobility patterns. Finally, we demonstrate that drugs that inhibit DNPS upstream of adsl-1 function ameliorate mobility phenotypes but not fertility whereas supplementation with purines ameliorates fertility issues but not mobility.
Locomotion
C. elegans have two distinct modes of locomotion (Pierce-Shimomura et al 2008), and loss of adsl-1 function causes slow phenotypes in both swimming (measured by thrashing rate) and crawling (measured by speed). Marsac et al. have previously shown that loss of adsl-1 affects speed and muscle integrity (Marsac et al. 2019). Our analysis of additional locomotory parameters reveals that mobility deficits are more complicated than a simple problem with muscle weakness. First, the mutant animals are capable of strong muscle contraction, as illustrated by their tendency to hold a periodic protracted curling contraction while swimming. Second, crawling adsl-1(tm3328) animals also have a mobility defect that is unlikely to be attributable to muscle weakness; the normal periodic reversals are increased in frequency in adsl-1(tm3328). We conclude that while muscle cells structure is disorganized in adsl-1(tm3328) (Marsac et al. 2019), the mutant animals display phenotypes associated with a defect in neural control of coordination. The finding that loss of adsl-1 function has affects in the nervous system is supported by other results from our lab, including altered gustatory plasticity (Moro et al. 2023). Thus, our data suggest a flaw in the neural-driven muscle activation strategy behind the sinusoidal motion of C. elegans. While locomotion was affected in both adsl-1(tm3328) and adsl-1(RNAi) animals, the phenotype was more severe in adsl-1(tm3328) mutants. The milder locomotory phenotype of adsl-1(RNAi) likely reflects more residual enzyme activity.
Because onset of reduction of gene function can be controlled by timing the RNAi application, we were also able to test the developmental versus acute requirements for adsl-1 function in locomotion. All 95 C. elegans body wall muscle cells are generated during embryogenesis (Sulston and Horvitz 1977). However, the number of motor neurons increases post-embryonically by more than three-fold, and significant remodeling of the motor neuron circuitry occurs during the first three larval stages (Sulston and Horvitz 1977; White, Albertson, and Anness 1978). We first clearly show that post-embryonic loss (beginning at L1) of adsl-1 function results in a thrashing defect and that 48 hours is sufficient time to observe the effects of RNAi knockdown. We also show that 48 hour application of RNAi beginning at the middle of the L4 stage, after the period of neurogenesis and remodeling (Mulcahy et al. 2022), results in a thrashing deficit. Moreover, the mobility phenotype of a treatment known to result in reduced thrashing, 48 hours of RNAi exposure beginning at hatching, is not observed when the animals are given 48 hours to recover from this RNAi exposure. Our results support the conclusion that adsl-1 has acute function(s) in mobility and that the effects of reduction of adsl-1 function are reversible.
Marsac et al. have speculated that developmental delay associated with loss of adsl-1 might contribute to the muscle defects (Marsac et al. 2019). We can’t rule out developmental functions for adsl-1 in locomotion during early larval stages because it is possible that these early larval events occur in our experiments in the presence of sufficient levels of adsl-1 function even when RNAi is applied at hatching.
Using drug treatments to block DNPS at steps upstream of adsl-1 function has ameliorating effects on swimming ability suggesting that adsl-1 substrate accumulation contributes to the disruption of the swimming pattern. In contrast supplementation of cultures with purines did not reverse the thrashing deficit. Using genetic approaches to block purine biosynthesis upstream of adsl-1 function, Marsac et al deduced that the effects of adsl-1 on crawling speed were independent of SAICAR accumulation. Divergent effects on the distinct locomotory patterns is possible, but reconciling these results will involve examining both swimming and crawling locomotory patters in genetic and drug treatment experiments. In separate results in our lab, we have also found that both LMX and MTX ameliorate gustatory plasticity phenotypes associated with loss of adsl-1 function (Moro et al. 2023), supporting a role for SAICAR toxicity in neural function.
Fertility
We observe that the development of the gonad is severely hindered in adsl-1(tm3328) animals and our observations are consistent with previous findings that adsl-1 is required for germline stem cell maintenance (Marsac et al. 2019). Marsac et al. documented a weakly penetrant fertility effect of adsl-1(RNAi) (Marsac et al. 2019). However, in our hands adsl-1(RNAi) beginning at hatching induces an almost completely penetrant effect on fertility. We found that supplementation with individual purine products results in restoration of fertility in adsl-1(RNAi) animals, but not adsl-1(tm3328). Each supplement can be converted to IMP, the central metabolite of purine synthesis, through the salvage pathways. In this way, these supplementations might overcome the blockage of IMP biosynthesis that results from the first function of ADSL, conversion of SAICAR to AICAR. However, adenosine is the only supplement that overcomes the second blockage of ADSL function, conversion of S-AMP to AMP. For this reason, it is interesting that both the tested purine supplements are able to independently reverse the sterility of adsl-1(RNAi). This result suggests that compensation for the second enzymatic function of ADSL is not as crucial for restoring fertility to adsl-1(RNAi) or that residual levels of ADSL-1 more easily suffice for this biochemical step. These results highlight the distinct etiology of the fertility and mobility phenotypes. We conclude that a perturbation to purine homeostasis contributes to the fertility phenotypes.
We found that supplementation with individual purine products results in restoration of fertility in adsl-1(RNAi) animals but not adsl-1(tm3328) animals. Marsac et al. also report no effect of purine supplementation on fertility of adsl-1(tm3328) (Marsac et al. 2019). These populations differ in the timing of loss of adsl-1 function. Any maternally supplied product would be maintained in both the adsl-1(RNAi) and the adsl-1(tm3328) populations. The adsl-1(RNAi) animals would also maintain any zygotic contribution to adsl-1 function in embryogenesis, whereas the adsl-1(tm3328) would not. Finally both populations end up with compromised or absent adsl-1 function by late larval stages. These differences likely underlie the differential effects of purine supplementation on rescue of fertility in animals with post-embryonic reduction of function compared to those with pre-embryonic loss of function. Marsac et al conclude that there is no evidence that purine supplementation allows recovery of the germ line stem cell deficit phenotype. Our results are consistent with this conclusion but have revealed that purine supplementation does overcome loss of subsequent functions of adsl-1 in fertility.
Interestingly, knockdown of adsl-1 did not significantly affect any of the purine monophosphate products of de novo synthesis. We speculate that the salvage biosynthesis pathways likely contribute to homeostatic mechanisms that maintain global purine levels in the absence of efficient DNPS. It is likely that these animals are recycling enough purines from their diets to accommodate for the blockage of DNPS but this model remains to be tested. Our initial metabolite measurements are derived from mixed-stage, whole-animal lysates. Thus, it remains possible that certain cells, tissues or developmental stages do not successfully maintain purine levels. Increased demand for purines or low activity of the salvage enzymes could alter purine levels for specific cells or developmental stages; more affected cell types could be masked by the whole-animal scale of metabolite measurements. In order to begin to address the possibility that specific developmental stages do not successfully maintain purine levels, we also measured metabolites from synchronized day one adult, whole-animal lysates. These measurements revealed similar trends in global purine levels to our initial measurements. Although adult animals seem to successfully maintain purine levels, it is still possible that other developmental stages do not. Even with this possibility, the global maintenance of purine monophosphate levels still suggests compensation for the blockage of DNPS in adsl-1(RNAi) animals. This maintenance of global purine levels is consistent with findings regarding adenine and guanine concentrations in patients and disease models with decreased ADSL function (Van den Berghe and Jaeken 1986b; Gooding et al. 2015).
Materials and methods
C. elegans culture and strains
Strains were maintained on OP50 Escherichia coli as food under standard conditions at 20° C (Brenner 1974). We used the following strains; N2, eri-1(mg366), adsl-1(tm3328), unc-63(x13), and snt-1(md290). The N2, GR1373 eri-1(mg366), ZZ13 unc-63(x13), and NM204 snt-1(md290)strains were obtained from the Caenorhabditis Genetics Center (CGC). adsl-1(tm3328) was obtained from the National BioResource Project in Tokyo, Japan and outcrossed three times against N2. The outcrossed, balanced strain was named HV854. This allele is homozygous sterile and was balanced with hT2, a balancer for the first and third chromosomes of C. elegans; this balancer causes pharyngeal expression of GFP (McKim, Peters, and Rose 1993). Non-GFP homozygous adsl-1(tm3328) animals were used in phenotypic analysis.
RNAi
The adsl-1 RNAi clone was from the C. elegans RNAi Library (Source BioScience, Nottingham, UK). RNAi feeding assays were carried out as described (Ahringer 2006). Unless otherwise noted, we transferred L4 eri-1 animals to RNAi plates and scored their progeny for phenotypes. E. coli strain HT115 carrying the empty RNAi feeding vector L4440 was used as a control.
Metabolite Supplementation
Stock solutions of 117 mM adenosine (Sigma) in H2O with 10% 1 M NaOH, 150 mM guanosine (Sigma) in H2O with 25% 1 M NaOH. We added these solutions to OP50 seeded NGM plates to a final concentration of 10 mM for adenosine and guanosine. Following supplementation, we incubated the plates at room temperature for 1–2 days before use.
Filter sterilized stock solutions of 22mM methotrexate (Sigma) in DMSO and 1.5 μM lometrexol (Sigma) in DMSO. We added these solutions to OP50 seeded NGM plates for a final concentration of 22 μM methotrexate, 29 nM lometrexol. Following supplementation, we incubated the plates at room temperature for 1–2 days before use.
Metabolomics
LC-MS metabolomics analysis was done with the Metabolomics Core Facility at Penn State. ~50 μL of animals were collected in ddH2O, flash frozen in liquid nitrogen and stored at −80°C. 15 μL samples were extracted in 1 mL of 3:3:2 acetonitrile: isopropanol: H2O with 1 μM chlorpropamide as internal standard. Samples were homogenized using a Precellys™ 24 homogenizer. Extracts from samples were dried under vacuum, resuspended in HPLC Optima Water (Thermo Scientific) and divided into two fractions, one for LC-MS and one for BCA protein analysis. Samples were analyzed by LC-MS using a modified version of an ion pairing reversed phase negative ion electrospray ionization method (Lu et al. 2010). Samples were separated on a Supelco (Bellefonte, PA) Titan C18 column (100 x 2.1 mm 1.9 μm particle size) using a water-methanol gradient with tributylamine added to the aqueous mobile phase. The LC-MS platform consisted of Dionex Ultimate 3000 quaternary HPLC pump, 3000 column compartment, 3000 autosampler, and an Exactive plus orbitrap mass spectrometer controlled by Xcalibur 2.2 software (all from ThermoFisher Scientific, San Jose, CA). The HPLC column was maintained at 30°C and a flow rate of 200 uL/min. Solvent A was 3% aqueous methanol with 10 mM tributylamine and 15 mM acetic acid; solvent B was methanol. The gradient was 0 min., 0% B; 5 min., 20% B; 7.5 min., 20% B; 13 min., 55% B; 15.5 min., 95% B, 18.5 min., 95% B; 19 min., 0% B; 25 min 0% B. The orbitrap was operated in negative ion mode at maximum resolution (140,000) and scanned from m/z 85 to m/z 1000. Metabolite levels were corrected to protein concentrations determined by BCA assay (Thermo Fisher).
Quantitative RT-PCR
RNA was isolated from mixed stage worms using TRIZOL reagent (Invitrogen). 1 μg of RNA was converted to cDNA using the qScript cDNA Synthesis Kit (Quanta Biosciences). cDNA was diluted 1:10 and used for quantitative PCR using SYBR Green and Applied Biosciences RT-PCR machine. Three primer sets, cdc-42, tba-1, and pmp-3, were used as expression controls.
cdc-42 F: ctgctggacaggaagattacg; R: ctgggacattctcgaatgaag
tba-1 F: gtacactccactgatctctgctgaca; R: ctctgtacaagaggcaaacagccatg
pmp-3 F: gttcccgtgttcatcactcat; R: acaccgtcgagaagctgtaga
adsl-1 F: acagacaatggccgatcc; R: tgttggtttcaattccttggc
aprt-1 F: ggattcagttgctggcttaga; R: ttcccttcttgcggattgg
hprt-1 F: tcgtgctgtcattcctgatg; R: gacgacgaacacgatctctaac
Results represent the average of three to five independent biological samples amplified in duplicate.
Phenotypic Analysis
Thrashing Assay.
Mid-L4 hermaphrodites of each genotype were aged for 1 day at 20° C prior to the assay. Individual animals were placed in a drop of M9 solution on the surface of an unspotted, clean NGM plate. After 1 minute of acclimation at room temperature, thrashes, the number of body bends, were counted for 1 minute using a Nikon SMZ645 Stereoscope.
Linear Crawling Velocity.
Mid-L4 hermaphrodites were aged for 1 day at 20° C prior to the assay. Individual animals were tracked as they crawled on OP50 seeded NGM plates. 30 second videos were collected on a Nikon SMZ 1500 Stereoscope using NIS-Elements software from Nikon and analyzed using ImageJ (version 1.51). The mean linear crawling velocity was calculated for each animal by tracing the displacement of the animal’s midpoint. The displacement of the midpoint was tracked as a vector as the animal moved in a singular direction. Once the animal changed direction, a new vector was made to track movement in that direction; this process was repeated for the length of each video. Crawling velocity was determined by dividing each vector length by the corresponding time. The velocity values from all vectors in a video were averaged and adjusted for the time-fraction of each vector within the video.
WormLab Software.
Speed, curling, self-contact distance and reversals were collected using WormLab software. Mid-L4 hermaphrodites were aged for 1 day at 20° C prior to the assays. Videos were recorded for 1-minute with raw data including measurements on a frame-by-frame basis. We used a Nikon lens attached to a Basler acA2440 Camera at video mode 2456x2052 Mono8 setting to record the animals movement for 1-minute. 30 frames per second were collected in a light background. First, we set the threshold, which is used to identify the worms from background, for each video. The software then uses the given length and width measurements to identify and track an individual worm for each frame (Restif & Ibanez-Ventoso et al 2014). Crawling Speed refers to the distance per second covered by the worm along its central axis. Individual animals were tracked as they crawled on unspotted, clean NGM plates. The speed of an animal for each frame was calculated based on WormLab calculations. To exclude outliers (Angstman et al 2016) we then averaged the absolute values recorded between 0 and 500 micrometers per second. Self-Contact Distance is the mean distance between the head and tail of the animal. A reversal is scored when an animals moves in a backward direction for at least 10 frames. Curling is a measure of the percentage (%) that an animal spends bent around so far that it overlaps with itself. Swimming C. elegans can sometimes stop and curl up into a shape that nearly resembles the letter “O” or the number “6”. We detect a curl by computing the distance from either extremity to the opposite side of the body; “O”, “6”, and shapes in between are counted.
Egg-laying, brood size and RNAi lethality.
For the egg-laying assay, ten eri-1 animals were placed on a plate containing the desired RNAi and supplementation. For each condition, ten second generation mid-L4 animals were placed onto individual plates. To quantify the number of eggs laid, L4 animals were singled onto plates and moved to a new plate daily for five days. The brood size was calculated by adding the total number of eggs in each plate. Embryonic lethality was calculated by adding the total number of eggs in each plate and comparing it to number of live animals two days later.
Lethality.
Heterozygous adsl-1(tm3328) worms were obtained by mating the balanced adsl-1(tm3328)/hT2 strain with the male N2 control strain. F1 offspring yields heterozygous adsl-1(tm3328)/+ and ht2/+ mutants. F1 adsl-1(tm3328)/+ heterozygotes were selected by obtaining non-GFP worms and cloned to produce progeny. F2 progeny were phenotypically scored for aberrant mobility and sterility to determine genotypic ratio.
Statistical Analysis
Two-tailed Student’s t tests were used to determine p values when comparisons were limited between two conditions. One-way or two-way ANOVA was carried out with appropriate post-tests to determine p values between three or more experimental conditions. In LC-MS analysis, we used Welch’s two sample t test to calculate p values. We substituted all undetectable measurements with zeros to statistically compare conditions for LC-MS. In all figures: ns, not significant; *, 0.01 <p< 0.05; **, 0.001<p< 0.01; ***, p<0.001; ****, p<0.0001.
Highlights.
adsl-1 function is required for neural regulation of spontaneous locomotion
adsl-1 functions acutely in locomotion
adsl-1-related dysfunction in swimming locomotion is reversible
locomotion defects correlate with accumulation of a purine biosynthetic intermediate
adsl-1 reproductive deficiencies can be ameliorated by purine supplementation
Acknowledgements
We thank A. Patterson and P. Smith in the Penn State Metabolomics Core Facility for technical assistance and advice, tm alleles were provided by the Mitani laboratory through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Japan. Other strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health Office of Research Infrastructure Programs (Grant P40 OD010440). We acknowledge a J. Lloyd Huck Biotechnology Mini-grant from the Huck Institutes of the Life Science for support for this work. This work was supported by the National Institutes of Health [#R03NS096451].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
References
- Ahringer J. 2006. “Reverse Genetics.” in WormBook, edited by The C. elegans Research Community. [Google Scholar]
- Allegra CJ, Hoang K, Yeh GC, Drake JC, and Baram J. 1987. “Evidence for Direct Inhibition of de Novo Purine Synthesis in Human MCF-7 Breast Cells as a Principal Mode of Metabolic Inhibition by Methotrexate.” Journal of Biological Chemistry 262(28):13520–26. [PubMed] [Google Scholar]
- Beardsley GP, Moroson BA, Taylor EC, and Moran RG. 1989. “A New Folate Antimetabolite, 5,10-Dideaza-5,6,7,8-Tetrahydrofolate Is a Potent Inhibitor of de Novo Purine Synthesis.” Journal of Biological Chemistry 264(l):328–33. doi: 10.1016/S0021-9258(17)31261-9. [DOI] [PubMed] [Google Scholar]
- van den Bergh FA, Bosschaart AN, Hageman G, Duran M, and Tien Poll-The B. 1998. “Adenylosuccinase Deficiency with Neonatal Onset Severe Epileptic Seizures and Sudden Death.” Neuropediatrics 29(1):51–53. doi: 10.1055/s-2007-973536. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, and Jaeken J. 1986a. “Adenylosuccinase Deficiency.” Advances in Experimental Medicine and Biology 195 Pt A:27–33. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, and Jaeken J. 1986b. “Adenylosuccinase Deficiency.” Advances in Experimental Medicine and Biology 195 Pt A:27–33. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G, Vincent MF, and Jaeken J. 1997. “Inborn Errors of the Purine Nucleotide Cycle: Adenylosuccinase Deficiency.” Journal of Inherited Metabolic Disease 20(2):193–202. doi: 10.1023/A:1005304722259. [DOI] [PubMed] [Google Scholar]
- Boyle Jordan H., Bern Stefano, and Cohen Netta. 2012. “Gait Modulation in C. Elegans: An Integrated Neuromechanical Model.” Frontiers in Computational Neuroscience 0(FEBRUARY 2012):10. doi: 10.3389/FNCOM.2012.00010/ABSTRACT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. 1974. “The Genetics of Caenorhabditis Elegans.” Genetics 77(l):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardo F, Salerno C, and Curatolo P. 2001. “Neurologic Aspects of Adenylosuccinate Lyase Deficiency.” Journal of Child Neurology 16(5):301–8. [DOI] [PubMed] [Google Scholar]
- Dutto Ilaria, Gerhards Julian, Herrera Antonio, Souckova Olga, Škopová Václava, Smak Jordann A., Junza Alexandra, Yanes Oscar, Boeckx Cedric, Burkhalter Martin D., Zikánová Marie, Pons Sebastian, Philipp Melanie, Lüders Jens, and Stracker Travis H.. 2022. “Pathway-Specific Effects of ADSL Deficiency on Neurodevelopment.” ELife 11. doi: 10.7554/ELIFE.70518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks LD, Rückemann K, Qiu Y, Hawrylowicz CM, Richards DF, Swaminathan R, Kirschbaum B, and Simmonds H. a. 1999. “Methotrexate Inhibits the First Committed Step of Purine Biosynthesis in Mitogen-Stimulated Human T-Lymphocytes: A Metabolic Basis for Efficacy in Rheumatoid Arthritis?” The Biochemical Journal 342(1):143–52. doi: 10.1042/0264-6021:3420143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Ye, French Jarrod, Zhao Hong, and Benkovic Stephen. 2013. “G-Protein-Coupled Receptor Regulation of de Novo Purine Biosynthesis: A Novel Druggable Mechanism.” Biotechnology & Genetic Engineering Reviews 29:31–48. doi: 10.1080/02648725.2013.801237. [DOI] [PubMed] [Google Scholar]
- Gooding Jessica R., Jensen Mette V., Dai Xiaoqing, Wenner Brett R., Lu Danhong, Arumugam Ramamani, Ferdaoussi Mourad, MacDonald Patrick E., and Newgard Christopher B.. 2015. “Adenylosuccinate Is an Insulin Secretagogue Derived from Glucose-Induced Purine Metabolism.” Cell Reports 13(1):157–67. doi: 10.1016/j.celrep.2015.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Zhao, Xie Na, Illes Peter, Di Virgilio Francesco, Ulrich Henning, Semyanov Alexey, Verkhratsky Alexei, Sperlagh Beata, Yu Shu Guang, Huang Canhua, and Tang Yong. 2021. “From Purines to Purinergic Signalling: Molecular Functions and Human Diseases.” Signal Transduction and Targeted Therapy 6(1). doi: 10.1038/S41392-021-00553-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hürlimann Hans C., Laloo Benoît, Simon-Kayser Barbara, Saint-Marc Christelle, Coulpier Fanny, Lemoine Sophie, Daignan-Fornier Bertrand, and Pinson Benoît. 2011. “Physiological and Toxic Effects of Purine Intermediate 5-Amino-4- Imidazolecarboxamide Ribonucleotide (AICAR) in Yeast.” Journal of Biological Chemistry 286:30994–2. doi: 10.1074/jbc.M111.262659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J, Wadman SK, Duran M, van Sprang FJ, Beemer FA, Holl RA, Theunissen PM, de Cock P, van den Bergh F, and van den Berghe G. 1988. “Adenylosuccinase Deficiency: An Inborn Error of Purine Nucleotide Synthesis.” European Journal of Pediatrics 148:126–31. [DOI] [PubMed] [Google Scholar]
- Jaeken Jaak. 1983. “AN INFANTILE AUTISTIC SYNDROME Succinyladenosine Case-Reports.” Pediatric Clinics Of North America 1058–61. [Google Scholar]
- Jurecka A. 2009. “Inborn Errors of Purine and Pyrimidine Metabolism.” Journal of Inherited Metabolic Disease 32:247–63. doi: 10.1007/s10545-009-1094-z. [DOI] [PubMed] [Google Scholar]
- Jurecka Agnieszka, Zikanova Marie, Kmoch Stanislav, and Tylki-Szymańska Anna. 2015. “Adenylosuccinate Lyase Deficiency.” Journal of Inherited Metabolic Disease 38(2):231–42. doi: 10.1007/s10545-014-9755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurecka Agnieszka, Zikanova Marie, Tylki-Szymanska Anna, Krijt Jakub, Bogdanska Anna, Gradowska Wanda, Mullerova Karolina, Sykut-Cegielska Jolanta, Kmoch Stanislav, and Pronicka Ewa. 2008. “Clinical, Biochemical and Molecular Findings in Seven Polish Patients with Adenylosuccinate Lyase Deficiency.” Molecidar Genetics and Metabolism 94(4):435–42. doi: 10.1016/j.ymgme.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Kappock TJ, Ealick SE, and Stubbe J. 2000. “Modular Evolution of the Purine Biosynthetic Pathway.” Current Opinion in Chemical Biology 4(5):567–72. [DOI] [PubMed] [Google Scholar]
- Krijt J, Kmoch S, Hartmannová H, Havlícek V, and Sebesta I. 1999a. “Identification and Determination of Succinyladenosine in Human Cerebrospinal Fluid.” Journal of Chromatography. B, Biomedical Sciences and Applications 726(l–2):53–58. [DOI] [PubMed] [Google Scholar]
- Krijt J, Kmoch S, Hartmannová H, Havlícek V, and Sebesta I. 1999b. “Identification and Determination of Succinyladenosine in Human Cerebrospinal Fluid.” Journal of Chromatography. B, Biomedical Sciences and Applications 726(1–2):53–58. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, and O’Neil N. 2001. “The Use of Functional Genomics in C. Elegans for Studying Human Development and Disease.” J Inherit Metab Dis 24:127–38. [DOI] [PubMed] [Google Scholar]
- Lu Wenyun, Clasquin Michelle F., Melamud Eugene, Amador-Noguez Daniel, Caudy Amy A., and Rabinowitz Joshua D.. 2010. “Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer.” Analytical Chemistry 82:3212–21. doi: 10.1021/ac902837x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsac Roxane, Pinson Benoît, Saint-Marc Christelle, Olmedo María, Artal-Sanz Marta, Daignan-Fornier Bertrand, and Gomes José Eduardo. 2019. “Purine Homeostasis Is Necessary for Developmental Timing, Germline Maintenance and Muscle Integrity in Caenorhabditis Elegans.” Genetics 211(4):1297–1313. doi: 10.1534/GENETICS.118.301062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiorgio Gerarda, Macchiaiolo Marina, Buonuomo Paola Sabrina, Bellacchio Emanuele, Bordi Matteo, Vecchio Davide, Brown Kari Payne, Watson Natalie Karen, Contardi Benedetta, Cecconi Francesco, Tartaglia Marco, and Bartuli Andrea. 2021. “Clinical and Molecular Characterization of Patients with Adenylosuccinate Lyase Deficiency.” Orphanet Journal of Rare Diseases 16(1). doi: 10.1186/S13023-021-01731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim KS, Peters K, and Rose AM. 1993. “Two Types of Sites Required for Meiotic Chromosome Pairing in Caenorhabditis Elegans.” Genetics 134(3):749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro Corinna A., Franklin Latisha P., Wittig Kathryn E., Peifer Mia M., Dong Shirley, and Hanna-Rose Wendy. 2023. “Adenylosuccinate Lyase Deficiency Affects Neurobehavior via Perturbations to Tyramine Signaling in Caenorhabditis Elegans.” Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy Ben, Witvliet Daniel K., Mitchell James, Schalek Richard, Berger Daniel R., Wu Yuelong, Holmyard Doug, Lu Yangning, Ahamed Tosif, Samuel Aravinthan D. T., Chisholm Andrew D., Lichtman Jeff W., and Zhen Mei. 2022. “Post-Embryonic Remodeling of the C. Elegans Motor Circuit.” Current Biology: CB 32(21):4645–4659.e3. doi: 10.1016/J.CUB.2022.09.065. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura Jonathan T., Chen Beth L., Mun James J., Ho Raymond, Sarkis Raman, and McIntire Steven L.. 2008. “Genetic Analysis of Crawling and Swimming Locomotory Patterns in C. Elegans.” Proceedings of the National Academy of Sciences of the United States of America 105(52):20982–87. doi: 10.1073/PNAS.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébora Karine, Laloo Benoît, and Daignan-Fornier Bertrand. 2005. “Revisiting Purine-Histidine Cross-Pathway Regulation in Saccharomyces CerevisiaeA Central Role for a Small Molecule.” Genetics 170(1):61–70. doi: 10.1534/GENETICS.104.039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel Erin K., Colman Roberta F., and Patterson David. 2006. “Adenylosuccinate Lyase Deficiency.” Molecular Genetics and Metabolism 89(1–2):19–31. doi: 10.1016/j.ymgme.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Stone RL, Aimi J, Barshop B. a, Jaeken J, den Berghe G, Zalkin H, and Dixon JE. 1992. “A Mutation in Adenylosuccinate Lyase Associated with Mental Retardation and Autistic Features.” Nature Genetics 1(1):59–63. doi: 10.1038/ng0492-59. [DOI] [PubMed] [Google Scholar]
- Sulston J, and Horvitz HR. 1977. “Postembryonic Cell Lineages of the Nematode Caenorhabditis Elegans.” Developmental Biology 56:110–56. [DOI] [PubMed] [Google Scholar]
- Vidal-Gadea Andrés, Topper Stephen, Young Layla, Crisp Ashley, Kressin Leah, Elbel Erin, Maples Thomas, Brauner Martin, Erbguth Karen, Axelrod Abram, Gottschalk Alexander, Siegel Dionicio, and Pierce-Shimomura Jonathan T.. 2011. “Caenorhabditis Elegans Selects Distinct Crawling and Swimming Gaits via Dopamine and Serotonin.” Proceedings of the National Academy of Sciences of the United States of America 108(42):17504–9. doi: 10.1073/pnas.1108673108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Albertson DG, and Anness MAR. 1978. “Connectivity Changes in a Class of Motoneurone during the Development of a Nematode.” Nature 271:764–66. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, and Brenner S. 1986. “The Structure of the Nervous System of the Nematode Caenorhabditis Elegans.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sci Series B, Biological Sciences 314(1165):1–340. [DOI] [PubMed] [Google Scholar]
- Zikanova Marie, Skopova Vaclava, Hnizda Ales, Krijt Jakub, and Kmoch Stanislav. 2010. “Biochemical and Structural Analysis of 14 Mutant ADSL Enzyme Complexes and Correlation to Phenotypic Heterogeneity of Adenylosuccinate Lyase Deficiency.” Human Mutation 31(4):445–55. doi: 10.1002/humu.21212. [DOI] [PubMed] [Google Scholar]