Abstract
Background:
Current ADAMTS13 activity assays are important for diagnosing thrombotic thrombocytopenic purpura (TTP) but are unreliable to assay ADAMTS13 activity in animal models. The Cattle-FRETS71 assay is capable of detecting ADAMTS13 activity in plasma from multiple animal species, making it a potentially useful reagent at all stages of clinical research. The performance of Cattle-FRETS71 in TTP diagnosis is not yet known.
Aims:
We evaluated the performance of the Cattle-FRETS71 substrate against the human FRETS-rVWF71 and the FRETS-VWF73 commercial substrates in human plasma and serum samples to validate its utility in diagnosing TTP in patients.
Methods:
Internal validation was performed using heparinized plasma samples (n=81). External validation was a blinded study using serum samples from the Oklahoma TTP Registry (n=118, collected 2004–2014) that had been initially assayed by FRETS-VWF73 within 1 year of collection. Additional validation was done with citrated plasma samples having variable ADAMTS13 activities (n=32) that were analyzed by FRETS-VWF73.
Results:
There was an excellent correlation (r=0.94) between Cattle-FRETS71 and FRETS-rVWF71 for assayed heparinized plasma samples (n=81). Assay results between Cattle-FRETS71 and FRETS-VWF73 of Oklahoma TTP Registry serum samples (n=118) as well as of citrated plasma samples (n=32) were comparably good (r=0.81 and r=0.85, respectively).
Conclusions:
The Cattle-FRETS71 assay is comparable with other assays in quantifying ADAMTS13 activity in human plasma collected from patients with documented or suspected TTP. The versatility of Cattle-FRETS71 combined with its specificity and sensitivity, makes it a useful tool for the standardization of ADAMTS13 activity across basic and clinical research paradigms.
Keywords: ADAMTS13, TTP, Animal Models, Enzyme Assays, von Willebrand Factor
Introduction
ADAMTS13 is a plasma enzyme responsible for cleaving von Willebrand factor (VWF), a large multimeric protein that tethers platelets to subendothelial collagen in the initial stages of hemostasis. A deficiency of ADAMTS13 activity results in uncontrolled platelet adhesion to ultra-large VWF causing thrombotic thrombocytopenic purpura (TTP) [1]. The most common etiology of TTP in adults is autoantibody-induced severe ADAMTS13 deficiency; hereditary deficiency of ADAMTS13 activity can also cause TTP [2, 3]. Rapid and accurate methods for diagnosing TTP are critical for patient management.
After the discovery of ADAMTS13, several first-generation methods were developed to assay its activity based on the cleavage of the Tyr1605-Met1606 bond within the VWF A2 domain [4]. These methods included electrophoretic assays, immunoradiometric assays, and assays based on assessing residual collagen-binding or ristocetin-cofactor function of cleaved multimeric VWF [5–9]. These assays were technically challenging, often confined to research laboratories, and often had long turn-around times. Kokame et al. addressed these assay shortcomings by developing FRETS-VWF73, a fluorogenically labeled substrate [10]. Its advantages include a simpler experimental setup and a shorter turnaround time of ~ 60 minutes. Despite its robustness, the FRETS-VWF73 assays use non-physiologic conditions and are susceptible to interference by bilirubin, hemoglobin, and other plasma proteins, which can falsely lower ADAMTS13 activity values [11, 12]. Moreover, patient samples in FRETS-VWF73 assays are routinely diluted >20 fold to minimize interference from plasma compounds but may cause dissociation of ADAMTS13-inhibitors thereby limiting sensitivity when run as end-point assays [13]. Dilution might also be necessary to counteract rapid substrate exhaustion in the case of higher ADAMTS13 activity, potentially reducing the wastage of an expensive substrate if assaying were repeated.
Muia et al. circumvented FRETS-VWF73 pitfalls by developing FRETS-rVWF71, a similar substrate but labeled with brighter fluorescent dyes that absorb and emit at near-infrared wavelengths (abs 638 nm, em 658 nm) where plasma is nearly transparent [14]. Assay results between FRETS-VWF73 and FRETS-rVWF71 were similar; however, inhibitory autoantibody titers by FRETS-rVWF71 were ~2.5-fold higher than with FRETS-VWF73. Additionally, the limit of detection (LoD) for FRETS-rVWF71 was ≤0.3% whereas that of FRETS-VWF73 was <5%. The improved properties of FRETS-rVWF71 led to the serendipitous discovery of Cattle-FRETS71, a “universal” ADAMTS13 substrate whose peptide backbone is derived from the VWF A2 domain of cattle VWF (Bos taurus) (Fig. 1, Fig. S1) [15].
FIGURE 1.
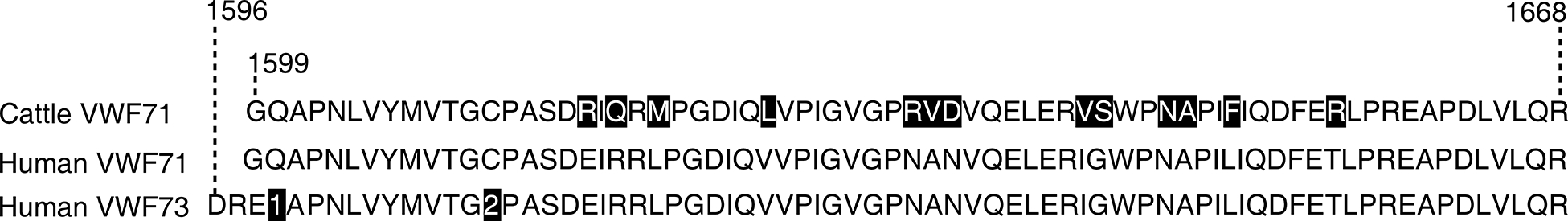
The amino acid sequence of FRET substrates. Recombinant cattle VWF71 peptide backbone differs from humans by 13-amino acid residues (shaded boxes). The human VWF73 peptide is like the human VWF71, except it has 3 additional von Willebrand factor (VWF) residues at the N-terminus. The synthetically prepared FRETS-VWF73 substrate has the VWF amino acid residues Q1599 (1) and N1610 (2) substituted with the highly fluorescent 2-(N-methylamino)benzoyl (Nma) group and a quencher 2,4-dinitrophenyl group, respectively. Cluster residues that make contact with M, D, T, and S exosites in the unfolded VWF A2 domain are indicated. Size = 178 × 25 mm (1200 × 1200 DPI).
Cattle-FRETS71 is cleaved by serum or plasma ADAMTS13 from humans and many animals used in research. For example, Cattle-FRETS71 substrate was cleaved with improved efficiency compared to human FRETS-rVWF71 by plasmas from humans (~2-fold), rats (~15-fold), FVB mouse (~8-fold), cattle (~5-fold), pig (~7.6-fold), and C57BL/6 mouse (~8-fold) [15]. Why Cattle-FRETS71 is preferentially cleaved by ADAMTS13 from different species remains unknown. However, the properties of Cattle-FRETS71 make it an assay of choice to measure and standardize ADAMTS13 activity across mammals. This study aimed to validate the utility of the Cattle-FRETS71 assay with clinically relevant plasma and serum human samples.
Methods
Protection of Human Subjects
All samples used in this study had been de-identified and contained no patient-identifiable information, and therefore the study was exempt from Institutional Review Board (IRB) protocol. However, the samples were previously obtained at the study sites following respective IRB protocols.
Plasmid construct
The cloning of the cattle VWF A2 sequence Gln1599-Arg1668 has been described before [15]. In brief, the DNA encoding Gln1599-Arg1668 was amplified from cattle genomic DNA template by PCR primers:
Forward, GGTATTGAGGGTCGCGAGAACCTTTATTTCCAGGGCCAGGCGCCCAACCTGGTC
Reverse, AGAGGAGAGTTAGAGCCTCACCGCTGCAGCACCAGATCTGG and ligated into the pET-32 Xa/LIC vector (Millipore Sigma) by a ligation independent method [16]. Mutations S1610C and K1615R were introduced by QuickChange II site-directed mutagenesis (Agilent Technologies) using primers:
Forward, GGTGACAGGATGCCCGGCCTCGGACAGGATCCAGCGGATG
Reverse, CATCCGCTGGATCCTGTCCGAGGCCGGGCATCCTGTCACC. The plasmid open reading frame (ORF) encodes thioredoxin, a his-tag, a TEV-cleavage site, a Gly residue, and cattle VWF Gln1599-Arg1668. Other sequences included and transcribed but not utilized in the current work are for S-tag, thrombin, and Factor Xa cleavage sites (Fig. S2).
Recombinant peptide preparation
The protocol for the preparation of Cattle-FRETS71 fluorogenic substrate was similar to the protocol previously described for FRETS-rVWF71 [14]. The recombinant cattle VWF71 peptide was expressed in E. coli BL21(DE3) (Sigma Millipore) after transformation with a pET32-Xa-Cattle-VWF71 plasmid. The cells were grown at 37°C in LB medium supplemented with 50 μg/mL ampicillin. Induction of log-phase cultures (optical density = 0.6) with 1 mM isopropyl b-D-1-thiogalactopyranoside (IPTG) was carried out for 4 hours. The media was centrifuged, and the cell pellet was lysed with 20 mL of B-PER Protein Extraction Reagent (ThermoFisher Scientific), supplemented with 5 μL benzonase (Millipore Sigma), and 100 μL of Halt Protease Inhibitor Cocktail (ThermoFisher Scientific). After incubation at room temperature for 15 minutes, the cell debris was cleared by centrifugation (12,000 rpm, 4 °C, 10 min). The supernatant was adsorbed on 5 mL Ni-NTA agarose (Agarose Beads Technologies) and calibrated with His-buffer (20 mM sodium phosphate, pH 7.4, 300 mM sodium chloride, 10 mM imidazole). After 5 column washes with equilibration buffer, the protein was eluted with 300 mM imidazole in His-buffer. The eluate was concentrated to ~ 5 mL using 10 kDa ultrafiltration membrane and desalted into tobacco etch virus protease (TEV) buffer 50 mM Tris-HCL, pH 8.0, 1 mM dithiothreitol, 0.5 mM EDTA using a PD-10 column (Cytiva). A His-tagged TEV protease, ~100 μg per 10 mg of the fusion protein, was added to the cleavage reaction and incubated for 16–24 h at room temperature. The cleaved products were adsorbed on Ni-NTA agarose removing thioredoxin and TEV protease and the crude peptide was concentrated ~5 mL using a 3 kDa ultrafiltration membrane. Dithiothreitol (10 mM) was added to the crude peptide and purified further by reverse phase using a C18 column (300 Å, 5 μm, 150 × 10 mm, GraceVydac); solvent system buffer A (50 mM triethylammonium acetate (TEAA, pH 6.0, Millipore Sigma), buffer B (60% acetonitrile/40% 50 mM TEAA, pH 6.0), and a flow rate of 2 mL/min for 28 mins at a gradient of 40–80% buffer B. The purified cattle-VWF71 peptide was lyophilized and desalted in the labeling buffer, 100 mM sodium phosphate, 0.5 mM EDTA, pH 7.0, using a PD-10 desalting column packed with Sephadex G-25 resin (Cytiva).
Fluorescent dye labeling
DyLight 633 maleimide (absorbance 638 nm, emittance 658 nm, ε 170,000 M−1 cm−1, ThermoFisher Scientific), 1 mg per 100 μL in dimethyl sulfoxide, was added to ~5–10 mg of the peptide in 100 mM sodium phosphate, 0.5 mM EDTA, pH 7.0 and stirred for 2 hours in the dark. The DyLight 633 labeled peptide was purified as outlined for the crude peptide, lyophilized, and desalted in the quencher labeling buffer (100 mM sodium phosphate, 0.5 mM EDTA, pH 8.0). IR-Dye QC-1 N-hydroxysuccinimide ester (abs 737 nm, ε 96 000 M −1 cm −1, LI-COR), 0.5 mg in 100 μL dimethyl sulfoxide, was added to the singly labeled peptide and stirred for 2 hours in the dark. The crude Cattle-FRETS71 was further purified, lyophilized, and ion-exchanged using Amberlite IR120 sodium form (Millipore Sigma) in a 7 × 230 mm column. The concentration of Cattle-FRETS71 substrate was determined spectroscopically by measuring absorbance at 627 nm and 819 nm.
Cattle-FRETS71 ADAMTS13 activity assay
The ADAMTS13 activity assay protocol for Cattle-FRETS71 was slightly modified from the FRETS-rVWF71 protocol [14]. This was necessary because Cattle-FRETS71 is cleaved ~2-fold faster than FRETS-rVWF71 by human ADAMTS13. The assay buffer (50 mM HEPES, 150 mM NaCl, 10 mM CaCl2, 0.05% Tween-20, 1 mg/ml BSA, 1 μM ZnCl2 pH 7.4), the microplate reader, microplates, and data analysis methodology remained unchanged. Standard assays by FRETS-rVWF71 can accommodate plasma volumes up to 100 μL but we adjusted plasma volumes up to 50 μL for Cattle-FRETS71 due to the increased cleavage rate. The standard calibration curve for FRETS-rVWF71 consisted of 7 data points of pooled plasma or serum (0, 5 μL, 10 μL, 25 μL, 50 μL, 75 μL, and 100 μL), which were both not standardized against the International Standard ADAMTS13 plasma [17]. We found 6 data points (0, 5 μL, 15 μL, 25 μL, 35 μL, and 50 μL) were sufficient for Cattle-FRETS71 assays. All assays were supplemented with 2 μL of 10 mM phenylmethylsulfonyl fluoride, and 3 μL of EDTA-free Halt Protease Inhibitor Cocktail (ThermoFisher Scientific) irrespective of the substrate type. For standard normal serum or plasma samples, the Cattle-FRETS71 assays were completed in ~15 minutes compared to 30–60 minutes for FRETS-rVWF71. While we have routinely used plasma volumes 25 μL and 100 μL as two standard data points to assay test plasma ADAMTS13 activity using FRETS-rVWF71, we chose to use 5 μL and 25 μL equivalently for Cattle-FRETS71 assays. Of note, the volume of test plasma becomes a factor only in the instances of limited sample availability and severe deficiency of ADAMTS13 activity. However, fluorogenic dyes used in our substrates are capable of strong signals at very low ADAMTS13 activity, resulting in a limit of detection (LoD) ≤0.3% for plasmic ADAMTS13 activity in humans.
Plasma and serum samples
Since our main objective was to assess the performance of Cattle-FRETS71 against the validated FRETS-rVWF71 and the commonly used FRETS-VWF73 substrate, we sought plasma samples from healthy subjects and TTP patients that had been previously assayed with these two methods. ADAMTS13 activity assays are routinely performed with citrated plasma in which the citrate can chelate metal ions and impair its optimal natural catalytic activity, even when recalcification appears to be sufficient [4]. In this regard, serum and heparinized plasma would be samples of choice to assay ADAMTS13 activity. However, plasma and/or serum can be associated with the activation of leukocyte proteases that can also cleave ADAMTS13 substrates and lead to inaccurate measures of ADAMTS13 activity [18]. Also, a similar situation can occur when some proteases in plasma and serum cleave off ADAMTS13 distal domains (CUB domains) resulting in the hyperactive variant MDTCS [19]. Since we previously demonstrated that ADAMTS13 activity can be assayed in serum or plasma samples anticoagulated with citrate or heparin without any difficulties [14], we obtained all three kinds of assay samples. Most of the patient samples in this study were from immune-mediated TTP (iTTP) patients, both in the acute and remission phases (Supplemental Table S4–S6). The rest were from congenital TTP, other thrombotic microangiopathy (TMA) forms, and non-TTP diagnoses. Additionally, we were unable to obtain clinical diagnoses and/or status of some heparinized plasma samples for which ADAMTS13 activity was available. Internal validation was performed using 81 heparinized plasma samples from healthy donors (n=49, BioIVT, USA) and from TTP patients (n=32) collected between 2012–2018 that were previously analyzed using FRETS-rVWF71 in a multisite study [20]. External validation was performed on serum and citrated anticoagulated samples whose ADAMTS13 activity had been assayed by FRETS-VWF73 and/or quantitative immunoblotting assay. The 118 serum samples used in the current study had been obtained and biobanked by the Oklahoma TTP Registry, Oklahoma City, OK, from 2004–2014. The citrated plasma (n=32) had been collected and assayed at the Bern University Hospital, University of Bern, Bern, Switzerland between 2021–2022. ADAMTS13 activity in serum samples was extrapolated from the standard curve of pooled normal serum (PNS, BioIVT, USA). ADAMTS13 activity in citrated plasma was standardized with the same pooled normal citrated plasma (PNP) that was used for FRETS-VWF73 assays. To avoid bias, the Cattle-FRETS71 assays were carried out in a blinded manner for both internal and external samples, i.e., ADAMTS13 activity levels of test samples determined previously at the time of collection using other ADAMTS13 assays were disclosed after ADAMTS13 activity levels had been determined by Cattle-FRETS71 assay. ADAMTS13 activity was then compared between assays, with an activity <10% described as low, which commonly supports the diagnosis of TTP, versus an activity ≥10%, which commonly indicates an absence of TTP.
Statistical methods
Results from the Cattle-FRETS71 and the FRETS-rVWF71 or the FRETS-VWF73 assays were compared using a Pearson correlation coefficient (r). The Kappa agreement statistic was calculated to compare the classification of the results by normal (≥10%) versus low (<10%) ADAMTS13 activity. Patients with ADAMTS13 activity <10% were classified as having TTP.
Results
Cattle-FRETS71 peptide substrate
The Cattle-FRETS71 peptide backbone differs from the human FRETS-rVWF71 by 13 amino acid residues (Fig. 1, Fig. S1). Unlike some species-specific ADAMTS13, the cleavage of Cattle-FRETS71 by human ADAMTS13 was ~2-fold faster than the human FRETS-rVWF71 [15]. This may indicate that the difference in peptide sequence does not confer a major advantage for human ADAMTS13. The yield of the Cattle-FRETS71 substrate was comparable to the reported yield of FRETS-rVWF71 [14]. For example, 15 mg of fusion protein would yield ~2.5 mg of the labeled peptide. Solubility of the Cattle-FRETS71 after the ion exchange procedure is comparable to that reported for FRETS-rVWF71, (>350 μM). We did not determine the cleavage kinetic parameters of Cattle-FRETS71 as we did not expect them to significantly differ from those of the human FRETS-rVWF71 (Km = 1.8 μM, Kcat = 6.8 min−1 at 30 °C for human plasma ADAMTS13) [14]. For example, Cattle-FRETS71 and FRETS-rVWF71 share similar spectral properties (conjugated dyes) and therefore the analytical interferences and the limit of detection should be the same. In our future studies, we plan to assess the intra‐assay coefficient of variation (CV) and the inter‐assay CV for Cattle-FRETS71; nevertheless, these parameters were remarkable for FRETS-rVWF71, 1.8% and 1.7%, respectively [14]. The labeled Cattle-FRETS71 substrate can undergo multiple freezing and thawing cycles without deterioration as determined by internal quenching (data not shown).
Improved performance of the Cattle-FRETS71 assay
A pooled normal plasma (PNP) assay comparing Cattle-FRETS71 and FRETS-rVWF71 revealed that Cattle-FRETS71 had an average reaction rate ~ 2.5 times greater than FRETS-rVWF71 (Fig. 2) when using identical parameters as outlined previously [14]. The increased cleavage of Cattle-FRETS71 enabled the adjustment of assay time as well as the volumes of assay plasma. Therefore, assays by Cattle-FRETS71 are faster than with FRETS-rVWF71.
FIGURE 2.
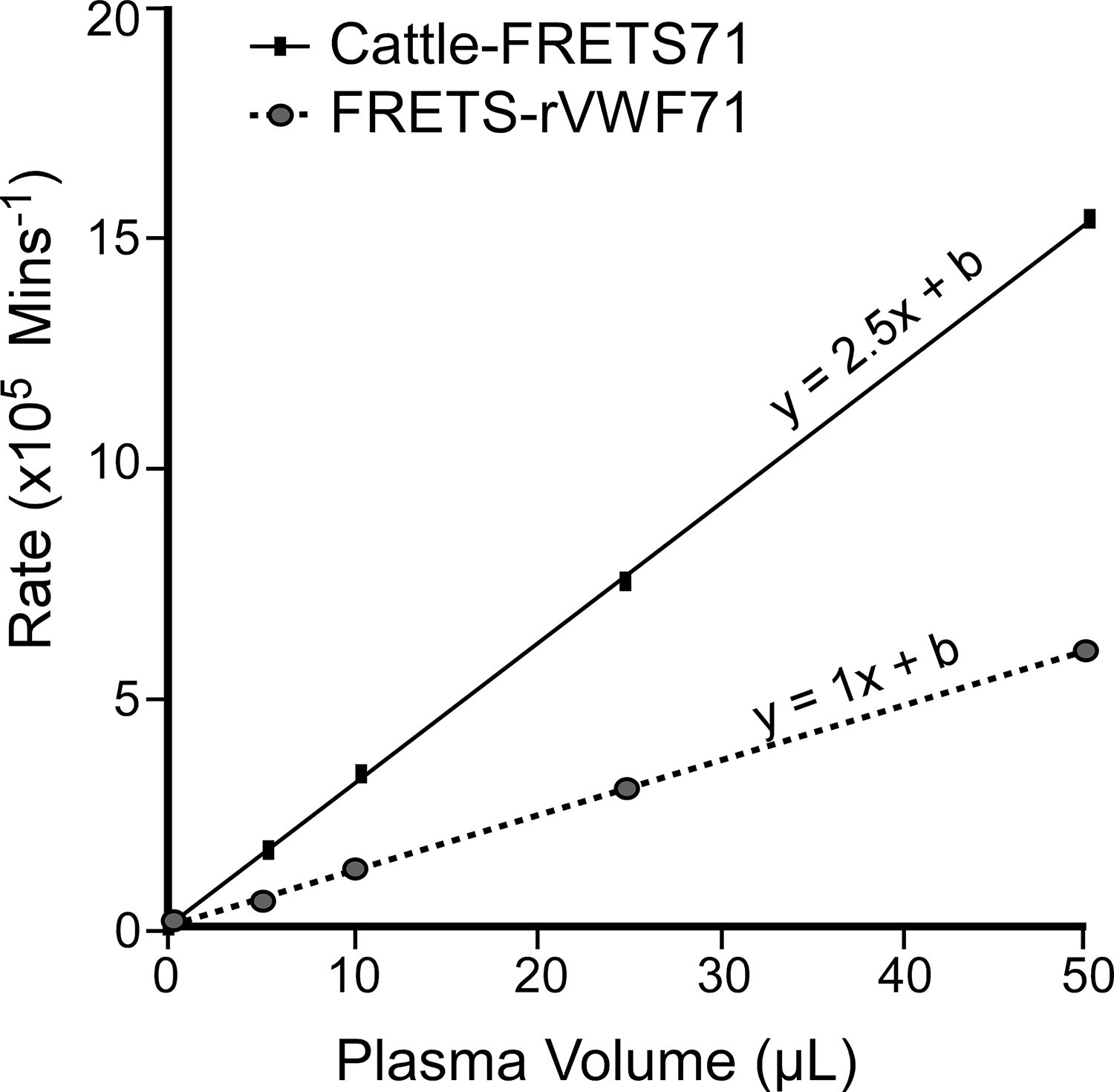
Cleavage of species-specific VWF71 substrate by human plasma ADAMTS-13. Cattle-FRETS71 vs human FRETS-rVWF71, the enzymatic reaction velocity of Cattle-FRETS71 is ~2.5 times (the slope) greater than the human FRETS-rVWF71. Size = 95 × 93 mm (600 × 600 DPI).
In-house heparinized plasma validation samples
We sought to validate Cattle-FRETS71 using 81 heparinized plasma samples from 32 TTP patients and 49 healthy donors. Results from these samples using Cattle-FRETS71 were correlated with previously obtained results using FRETS-rVWF71, which yielded an r = 0.94, p<0.0001 (Fig. 3A). Cattle-FRETS71 accurately classified all heparinized plasma samples having low ADAMTS13 activity (<10%), which identifies the presence of TTP, or TTP cutoff threshold ≥10%, respectively (Kappa = 1.0). Further analysis of plasma samples with low (<10%) ADAMTS13 activity yielded an r of 0.35, p=0.204, indicating that values between Cattle-FRETS71 and FRETS-rVWF71 had a less linear correlation in these samples (Fig. 3B, Table S1). This is possibly due to Cattle-FRETS71 having a higher signal-to-noise ratio at detecting residual ADAMTS13 activity, causing ADAMTS13 measurements to be greater in Cattle-FRETS71 assays than in FRETS-rVWF71 assays for samples with very low ADAMTS13 values.
FIGURE 3.
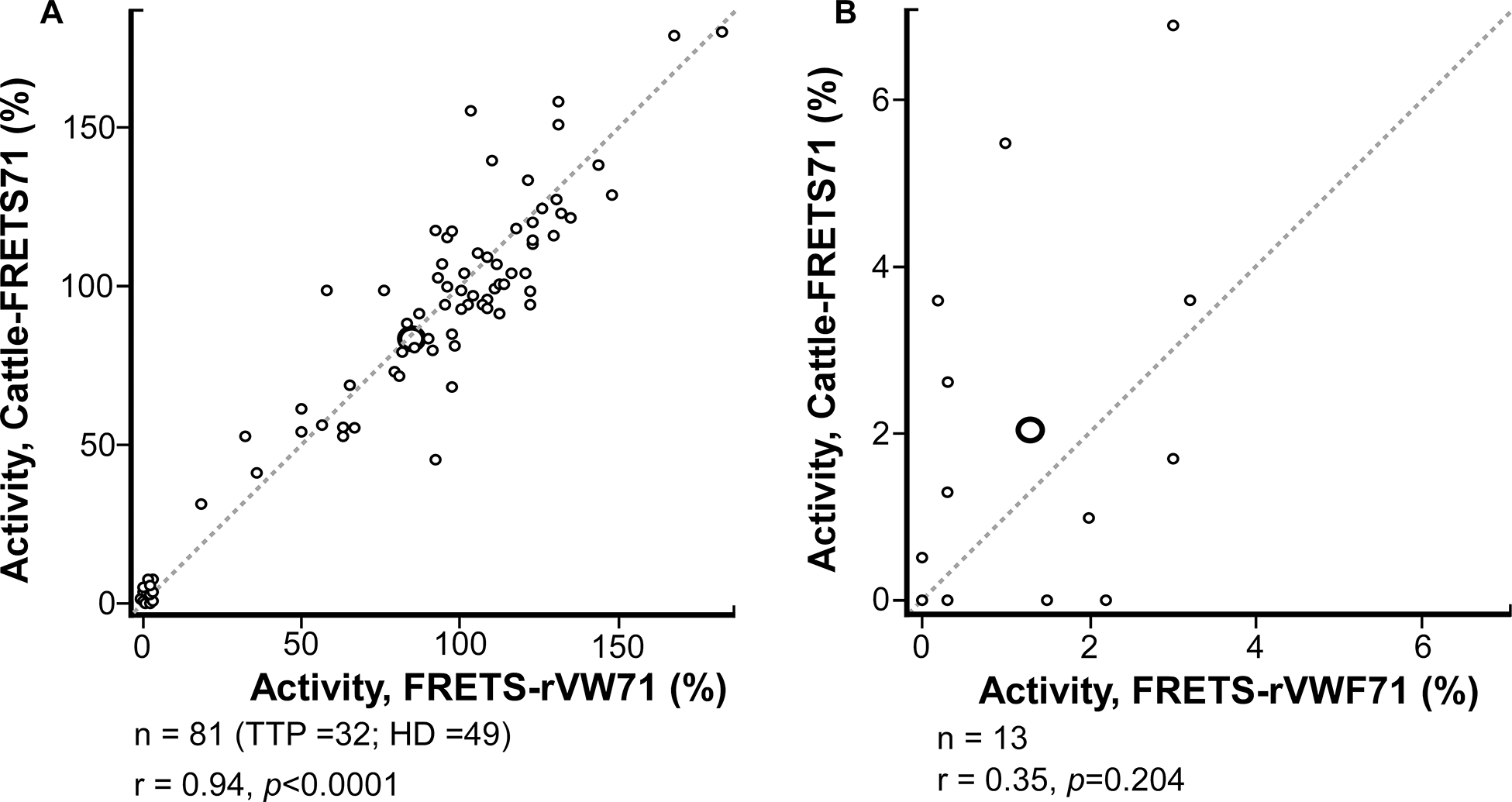
Assays of internal heparinized plasma samples. (A) Correlation between Cattle-FRETS71 and FRETS-rVWF71 for heparinized plasma samples (n = 81, 32 unique patients with thrombotic thrombocytopenic purpura [TTP] and 49 healthy donors [HD] plasma samples; r =.94: P <.0001). (B) Comparison between Cattle-FRETS71 and FRETS-rVWF71 for heparinized plasma samples with ADAMTS-13 activity below 10% (n = 13; r =.35; P =.204). Size = 196 × 104 mm (600 × 600 DPI).
Oklahoma TTP Registry serum validation samples
Serum samples collected from the Oklahoma TTP Registry (n=118 samples from 78 patients) previously analyzed using FRETS-VWF73 and quantitative immunoblotting assay were assayed using Cattle-FRETS71 following the modified Cattle-FRETS71 protocol described in the Methods section. When the results of the Cattle-FRETS71 assay were correlated with previously recorded FRETS-VWF73 assay results, an r value of 0.81, p<0.0001 was obtained (Fig. 4A). Cattle-FRETS71 accurately classified 101 of 118 samples as having low ADAMTS13 activity (<10%), or greater than the TTP cutoff threshold (≥10%) (Kappa = 0.60). Of the 17 discordantly classified samples, 15 were identified as having ADAMTS13 activity <10% using Cattle-FRETS71 but not with FRETS-VWF73, while 2 samples were identified as having ADAMTS13 activity <10% using FRETS-VWF73 but not with Cattle-FRETS71 (Table S2). When analyzing only patient samples collected during an acute TTP hospitalization (42 samples, 42 patients), an r value of 0.71, p<0.0001 was obtained (Fig. 4B). Cattle-FRETS71 accurately classified 29 out of 42 samples as either low ADAMTS13 (<10%) or above the TTP cutoff (≥10%) (Kappa= 0.30). Out of the 13 discordantly classified samples, 11 were identified as having low activity using Cattle-FRETS71 but not with FRETS-VWF73, and 2 samples were identified as low activity using FRETS-VWF73 but not with Cattle-FRETS71 (Table S2).
FIGURE 4.
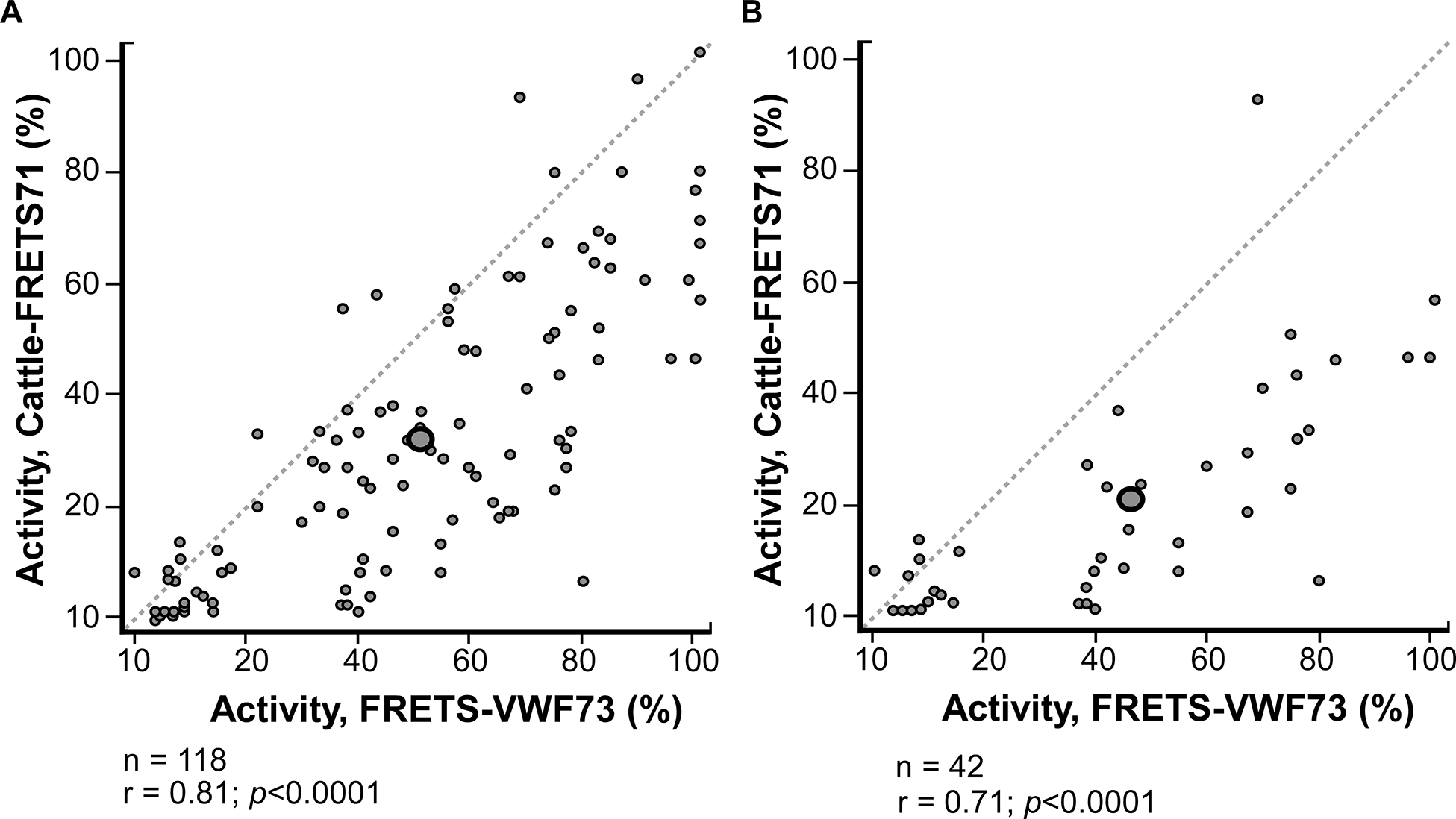
Assays of Oklahoma Thrombotic Thrombocytopenic Purpura (TTP) Registry serum samples. (A) Correlation between Cattle-FRETS71 and FRETS-VWF-73 for all serum samples collected from patients enrolled in the Oklahoma TTP Registry (n = 118; r =.81; P <.0001). (B) Correlation between Cattle-FRETS71 and FRETS-VWF73 for serum samples collected during acute hospitalization from patients enrolled in the Oklahoma TTP Registry (n = 42; r =.71; P <.0001). Size = 194 × 109 mm (600 × 600 DPI).
Switzerland citrated plasma validation samples
Citrated plasma samples of Bern University Hospital, University of Bern (n=32 samples of 21 patients) previously analyzed using FRETS-VWF73 assay were assayed using Cattle-FRETS71 following the modified protocol used for citrated samples described previously [14]. Notably, all citrated plasma samples were recalcified before assaying ADAMTS13 activity as described previously. Comparing the data from Cattle-FRETS71 versus FRETS-VWF73 by simple linear regression, an r value of 0.85, p<0.0001, was obtained (Fig. 5). Cattle-FRETS71 accurately classified 27 of the 32 samples as having low ADAMTS13 activity (<10%) or above the TTP cutoff (≥10%) (Kappa = 0.69). Out of the discordantly classified samples, 2 were identified as having low activity using Cattle-FRETS71 but not with FRETS-VWF73, while 3 samples were identified as having low activity using FRETS-VWF73 but not with Cattle-FRETS71 (Table S3).
FIGURE 5.
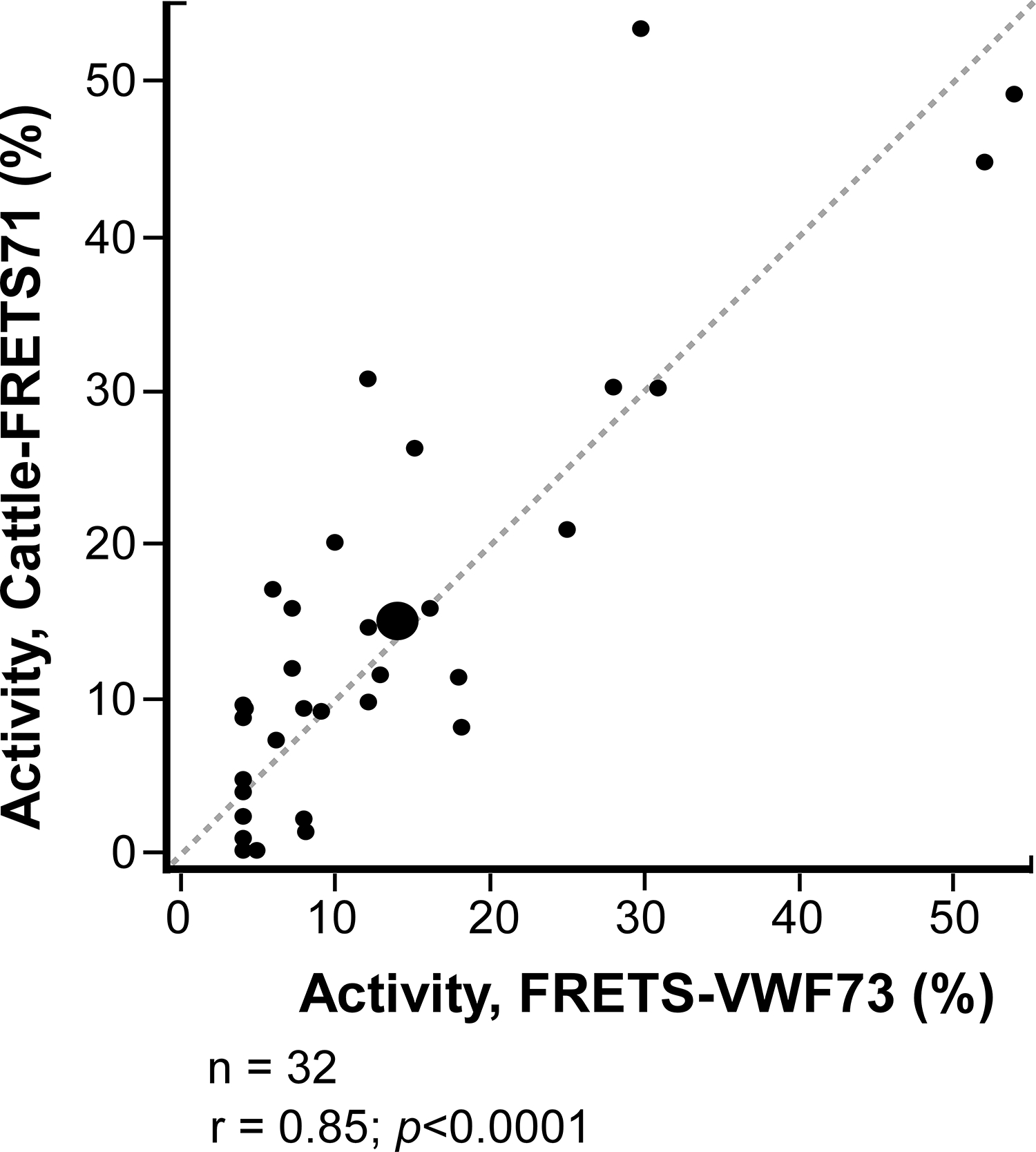
Assays of citrated plasma samples. Correlation between Cattle-FRETS71 and the commonly used FRETS-VWF73 for serum samples collected at the University Hospital Bern, University of Bern, Bern, Switzerland (n = 32; r = .85: P <.0001). Size = 94 × 104 mm (1200 × 1200 DPI).
Discussion
Fluorogenic substrate assays have become the standard method for quantifying ADAMTS13 activity in the clinical management of TTP [4]. FRETS-VWF73 provided the foundation for fluorogenic ADAMTS13 assays, providing clinicians and scientists with a reliable means for accurately measuring ADAMTS13 activity that was not technically challenging or extremely time intensive [10]. FRETS-rVWF71 built upon the success of the FRETS-VWF73 assay and introduced new benefits, such as compatibility with minimally diluted plasma and resistance to interference by bilirubin and hemoglobin [14]. With the development and validation of Cattle-FRETS71, the progression of fluorogenic substrate assays further advances. The Cattle-FRETS71 has additional advantages compared to any of the previously reported ADAMTS13 assays. For example, Cattle-FRETS71 is the only substrate that plasma ADAMTS13 activity from various vertebrates, including humans, can be assayed within a single platform with excellent sensitivity and accuracy [15]. It is for these reasons that we see the variety of potential applications of Cattle-FRETS71 and related VWF peptide substrates in research and clinical diagnosis of TTP and therapy monitoring. Some benefits of Cattle-FRETS71 have already been realized in the use of animal models to study trauma-induced organ failure and acquired von Willebrand syndrome [21, 22].
To realize its usefulness and to fully document its potential, the Cattle-FRETS71 assay needed to be validated with clinically relevant human plasma samples. Additionally, we have reasoned that widespread testing of ADAMTS13 activity should not be limited to citrated plasma samples only. Although the citrate chelates the active site metal ions, recalcification and addition of other bivalent metal ions can restore full ADAMTS13 activity. Nevertheless, the recalcification process is tedious and increases the possibility of citrated plasma clotting. Heparinized plasma and serum samples would be ideal for assaying ADAMTS13 activity because the physiological active status of the enzyme is preserved by the lack of prosthetic metal ion chelation. Therefore, we sought to validate Cattle-FRETS71 with serum and plasma samples anticoagulated with heparin and citrate. For internal validation (in-house), every heparinized plasma sample that we assayed by Cattle-FRETS71 was correctly categorized as low, moderate, or high ADAMTS13 activity as previously predicted by FRETS-rVWF71.
Although the correlation between Cattle-FRETS71 and FRETS-VWF73 was not as strong as expected for the Oklahoma TTP Registry samples (external validation), it was moderate. In these blinded serum samples (n=118), results from Cattle-FRETS71 tended to underestimate ADAMTS13 activity when compared to previously measured values using FRETS-VWF73. This trend is likely a result of serum samples undergoing multiple rounds of freezing and thawing over many years, thus interrupting enzymatic structure and function, and resulting in lower ADAMTS13 values as measured by Cattle-FRETS71. Some of these samples had been stored at −20°C for more than 20 years, which is not the ideal temperature to store serum. Therefore, additional testing validating Cattle-FRETS71 and FRETS-VWF73 will be required to circumvent these issues and gather more reliable data. We also cannot rule out the discrepancy between the ADAMTS13 activity of pooled serum that was previously used with FRETS-VWF73 and the one used for Cattle-FRETS71. Nonetheless, these results do demonstrate serum samples are as good as plasma samples for assaying ADAMTS13 activity.
As we previously reported, we also sought validation of citrated plasma samples using Cattle-FRETS71. As a standard practice, all samples were recalcified before assaying [14]. For the 32 samples that were shipped in dry ice from Bern University Hospital, Switzerland, to Tulsa, Oklahoma, Cattle-FRETS71 values were comparable with FRETS-VWF73 values by linear regression, r= 0.85. There were a few discrepancies between the two assays. For example, 2 samples had low ADAMTS13 activity with Cattle-FRETS71 but not with FRETS-VWF73. Likewise, 3 samples had low ADAMTS13 activity by FRETS-VWF73 but not by Cattle-FRETS71. It is not unusual for ADAMTS13 assays to show discrepancies among different assay methods [13, 23]. Our future work will further characterize such samples to ascertain the source of the variation.
Concerning assay turnaround times, the initial rates of Cattle-FRETS71 cleavage by human ADAMTS13 was ~2.5 times faster than FRETS-rVWF71 as reported previously [15]. As a result of an increased cleavage rate, assays using Cattle-FRETS71 substrate can be completed in a shorter time than other assays, thus making Cattle-FRETS71 time and cost-efficient.
Although our Cattle-FRETS71 validation results support the main objective of the current study, some limitations warrant further investigations. Additional widespread testing and validation in other laboratories, especially with a larger sample size, samples assayed using FRETS-VWF73 and other assays, additional serum samples, and plasma samples drawn closer to the time of assaying, are required to further solidify the accuracy and applicability of Cattle-FRETS71. Also, a comparison of ADAMTS13 antigen assays with Cattle-FRETS71 activity assays needs to be performed to ascertain if the activity is proportional to ADAMTS13 protein in the plasma or serum.
Conclusion
Many novel ADAMTS13 assays have been developed since the association between TTP and severely reduced ADAMTS13 activity was discovered in the late 1990s. However, none of these assays has been validated to show universal compatibility with plasma and serum samples from human and animal sources. The Cattle-FRETS71 assay fulfills this unmet need. We acknowledge that our data and experience are not yet sufficient to suggest the use of Cattle-FRETS71 in the context of medical care. That will require further clinical evaluations. Perhaps the most unique property of Cattle-FRETS71 is its versatility. In addition to the current study using clinically relevant human samples, Cattle-FRETS71 is compatible with plasma and serum samples from >25 other animal species [15], making it an important tool for standardizing ADAMTS13 activity across multiple mammals. This is a significant advancement because, for example, ADAMTS13 activity in TTP patients under an experimental therapy can be simultaneously standardized against ADAMTS13 activity in TTP animal models treated with the same therapy. Furthermore, Cattle-FRETS71 will likely find applications beyond TTP diagnosis and research in characterizing the activity, protein structure, and function of ADAMTS13 in other thrombotic and bleeding disorders.
Supplementary Material
Essentials.
An ADAMTS13 assay that can be seamlessly used for both TTP clinical diagnosis and animal research is lacking.
Cattle-FRETS71 is a substrate cleaved by plasma ADAMTS13 from diverse vertebrates, including humans.
Results of ADAMTS13 activity in serum, heparinized and citrated plasma samples using Cattle-FRETS71 were similar to routinely used assays.
The compatibility of Cattle-FRETS71 with samples from diverse vertebrate sources makes it possible to assay and standardize ADAMTS13 in a single platform.
Acknowledgments
This work is supported in part by the National Institutes of Health, National Institute of General Medical Sciences grant R35 GM142936 (J.M.), and Swiss National Science Foundation grant 310030-185233 (J.A.K.H.). J.C.B. was supported by the 2021 Hemostasis and Thrombosis Research Society (HTRS) Student Research Award; D.T. was supported by the National Institutes of Health, Heart, Lung, and Blood Institute grant K01 HL135466.
Footnotes
Declaration of competing interests
J. Muia holds a patent “Fluorogenic substrate for ADAMTS-13”, US 8663912, issued to Washington University. J.C.B, C.A, F.Z.M, R.K, J.A.K.H, D.T, S.K.V, and J.G. have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadler JE: Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 2008, 112(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai HM, Lian EC: Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998, 339(22):1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U et al. : von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med 1998, 339(22):1578–1584. [DOI] [PubMed] [Google Scholar]
- 4.Mackie I, Mancini I, Muia J, Kremer Hovinga J, Nair S, Machin S, Baker R: International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of ADAMTS13. Int J Lab Hematol 2020, 42:685–696. [DOI] [PubMed] [Google Scholar]
- 5.Furlan M, Robles R, Lämmle B: Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 1996, 87(10):4223–4234. [PubMed] [Google Scholar]
- 6.Tsai HM: Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood 1996, 87(10):4235–4244. [PubMed] [Google Scholar]
- 7.Gerritsen HE, Turecek PL, Schwarz HP, Lämmle B, Furlan M: Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP). Thromb Haemost 1999, 82(5):1386–1389. [PubMed] [Google Scholar]
- 8.Böhm M, Vigh T, Scharrer I: Evaluation and clinical application of a new method for measuring activity of von Willebrand factor-cleaving metalloprotease (ADAMTS13). Ann Hematol 2002, 81(8):430–435. [DOI] [PubMed] [Google Scholar]
- 9.Veyradier A, Obert B, Houllier A, Meyer D, Girma JP: Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood 2001, 98(6):1765–1772. [DOI] [PubMed] [Google Scholar]
- 10.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T: FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 2005, 129(1):93–100. [DOI] [PubMed] [Google Scholar]
- 11.Meyer SC, Sulzer I, Lämmle B, Kremer Hovinga JA: Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost 2007, 5(4):866–867. [DOI] [PubMed] [Google Scholar]
- 12.Studt JD, Kremer Hovinga JA, Antoine G, Hermann M, Rieger M, Scheiflinger F, Lämmle B: Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: in vitro inhibition of ADAMTS13 activity by hemoglobin. Blood 2005, 105(2):542–544. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich-Zahnd R, George JN, Vesely SK, Terrell DR, Aboulfatova K, Dong JF, Luken BM, Voorberg J, Budde U, Sulzer I et al. : Evidence for a role of anti-ADAMTS13 autoantibodies despite normal ADAMTS13 activity in recurrent thrombotic thrombocytopenic purpura. Haematologica 2012, 97(2):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muia J, Gao W, Haberichter SL, Dolatshahi L, Zhu J, Westfield LA, Covill SC, Friedman KD, Sadler JE: An optimized fluorogenic ADAMTS13 assay with increased sensitivity for the investigation of patients with thrombotic thrombocytopenic purpura. J Thromb Haemost 2013, 11(8):1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muia J, Zhu J, Greco SC, Vanhoorelbeke K, Gupta G, Westfield LA, Sadler JE: Phylogenetic and functional analysis of ADAMTS13 identifies highly conserved domains essential for allosteric regulation. Blood 2019, 133(17): 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslanidis C, de Jong PJ: Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 1990, 18(20):6069–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard AR, Heath AB, Kremer Hovinga JA: Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): communication from the SSC of the ISTH. J Thromb Haemost 2015, 13(6):1151–1153. [DOI] [PubMed] [Google Scholar]
- 18.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL: Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood 2009, 114(8):1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland KS, Reitsma SE, Shirai T, Zilberman-Rudenko J, Tucker EI, Gailani D, Gruber A, McCarty OJT, Puy C: Removal of the C-Terminal Domains of ADAMTS13 by Activated Coagulation Factor XI induces Platelet Adhesion on Endothelial Cells under Flow Conditions. Front Med (Lausanne) 2017, 4:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwicker JI, Muia J, Dolatshahi L, Westfield LA, Nieters P, Rodrigues A, Hamdan A, Antun AG, Metjian A, Sadler JE: Adjuvant low-dose rituximab and plasma exchange for acquired TTP. Blood 2019, 134(13):1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinveld DJB, Simons DDG, Dekimpe C, Deconinck SJ, Sloos PH, Maas MAW, Kers J, Muia J, Brohi K, Voorberg J et al. : Plasma and rhADAMTS13 reduce trauma-induced organ failure by restoring the ADAMTS13-VWF axis. Blood Advances 2021, 5(17):3478–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deconinck SJ, Nix C, Barth S, Bennek-Schopping E, Rauch A, Schelpe AS, Roose E, Feys HB, Pareyn I, Vandenbulcke A et al. : ADAMTS13 inhibition to treat acquired von Willebrand syndrome during mechanical circulatory support device implantation. J Thromb Haemost 2022, 20: 2797–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN: Thrombotic thrombocytopenic purpura: diagnostic criteria, clinical features, and long-term outcomes from 1995 through 2015. Blood Advances 2017, 1(10):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


