Abstract
INTRODUCTION
Post‐operative delirium (POD) is associated with increased morbidity and mortality but is bereft of treatments, largely due to our limited understanding of the underlying pathophysiology. We hypothesized that delirium reflects a disturbance in cortical connectivity that leads to altered predictions of the sensory environment.
METHODS
High‐density electroencephalogram recordings during an oddball auditory roving paradigm were collected from 131 patients. Dynamic causal modeling (DCM) analysis facilitated inference about the neuronal connectivity and inhibition–excitation dynamics underlying auditory‐evoked responses.
RESULTS
Mismatch negativity amplitudes were smaller in patients with POD. DCM showed that delirium was associated with decreased left‐sided superior temporal gyrus (l‐STG) to auditory cortex feedback connectivity. Feedback connectivity also negatively correlated with delirium severity and systemic inflammation. Increased inhibition of l‐STG, with consequent decreases in feed‐forward and feed‐back connectivity, occurred for oddball tones during delirium.
DISCUSSION
Delirium is associated with decreased feedback cortical connectivity, possibly resulting from increased intrinsic inhibitory tone.
Highlights
Mismatch negativity amplitude was reduced in patients with delirium.
Patients with postoperative delirium had increased feedforward connectivity before surgery.
Feedback connectivity was diminished from left‐side superior temporal gyrus to left primary auditory sensory area during delirium.
Feedback connectivity inversely correlated with inflammation and delirium severity.
Keywords: auditory roving oddball paradigm, delirium, dynamic causal modeling, event‐related potentials, evoked response potentials, high‐density electroencephalogram, mismatch negativity, postoperative delirium, predictive coding framework
1. BACKGROUND
Delirium is defined as a disturbance in attention and awareness (representing an acute change from baseline) and a disturbance in either cognition or arousal driven by a pathophysiological stressor. Delirium is associated with a doubling in the rate of cognitive decline, 1 a 12‐fold increased risk of dementia, 2 increased costs, 3 and increased mortality 4 .
Cohort studies have shown that postoperative delirium (POD) affects up to 53% of patients undergoing major surgery. 4 Modifiable risk factors for POD include infection, inflammation, metabolite disturbances, medication, pain, and sleep disruption. 4 Non‐modifiable factors include older age, cognitive impairment, dementia, and comorbid diseases. 4 The pathophysiology of delirium is poorly understood, with various theories incorporating disrupted neurotransmission in conjunction with altered network connectivity and neuroinflammation. 4 , 5 , 6
The scalp electroencephalogram (EEG) is a useful, non‐invasive neurophysiological tool that has been used to provide insights into the pathophysiology of delirium. In previous studies, EEG slowing, reduced complexity, and reduced functional connectivity differed between those with and without delirium. 7 , 8 , 9 However, an understanding of the network dynamics underlying functional changes in neuronal processing remains a critical knowledge gap. 7 , 8
One framework for cognitive processing focuses on the “Bayesian brain,” which proposes that the brain predicts current and future events based on prior experience. As stated more fully, the brain makes sense of the sensory environment by estimating the statistical probabilities of future events based on prior accrued information. A prominent implementation of this concept is predictive coding (PC). The PC model incorporates a hierarchical neural network in which perception results from the interaction between bottom‐up sensory‐driven inputs and top‐down expectations. The network produces prediction errors when there is discordance in the information (i.e., a mismatch between the prediction and the “observed” sensory world). This information is then fed up the cortical hierarchy, and the higher order prediction is updated. 10 , 11 The PC may explain information processing across a wide range of systems such as sensory, motor, and higher order cognitive networks. 12 The PC model and related analyses have been applied previously to neurocognitive disorders such as schizophrenia and psychotic features, 13 and to detecting abnormalities during the preclinical phase of Alzheimer's disease (AD). 14
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the delirium literature using traditional (e.g., PubMed, Scopus) sources and related meeting abstracts and presentations. Various theories incorporating a deranged neurotransmission and an altered network connectivity in the brain, as well as neuroinflammation, have been proposed.
Interpretation: Our findings suggest that the predictive coding framework may provide a useful construct for understanding delirium pathogenesis and those changes in sensory processing occur with delirium. The postulated underlying mechanism is that increased cortical inhibition leads to decreased feedback connectivity, supporting the cognitive disintegration model.
Future directions: The manuscript proposes a framework for the generation of new hypotheses and the conduct of additional studies. Examples include: (a) identifying the neural correlates of different delirium sub‐phenotypes and symptoms, (b) examining the link between vulnerability to delirium and Alzheimer's disease, (c) investigating longitudinal changes in hierarchical connectivity over time in persons who develop delirium.
Patients with delirium become disoriented in space and time, demonstrating significant impairments of cognitive processing similar to late‐stage dementia. Application of a PC framework to delirium may help explain the profound disturbances of cognition, attention, and orientation that characterize this state. According to PC, the mechanism for updating predictions is through feedback signaling to lower levels of the cortical hierarchy. We hypothesize that POD would be characterized by reduced feedback connectivity, with impaired updating of predictions about the sensory environment, making processing of new information slow and inefficient and leading to disorientation as well as deficits in attention. We further hypothesize that decreased feedback connectivity would correlate with increased systemic inflammation (consistent with inflammatory models of POD). 15 This represents an extension of our cognitive disintegration model, which proposes that delirium results when increased inhibitory tone leads to impaired cortical connectivity. 5
In the present study, we apply an established method for studying PC—the auditory roving oddball paradigm—to patients with delirium. In this paradigm, tones are divided into “standards,” which are repeated tones of unchanging frequency, and “oddballs,” which are tones that generate surprise by being of a different frequency from the prior tones. Oddballs generate larger evoked responses, and this response is conceptualized to reflect prediction error (surprise). This prediction error (surprise) is then communicated down the cortical hierarchy through feedback connections. By modeling a network of generators of auditory cortical activity that have been established previously as key sources of the auditory evoked response, we sought to measure the impact of delirium on hierarchical brain dynamics.
2. METHODS
The data are derived from a perioperative cohort study (IPOD‐B3) registered with ClinicalTrials.gov (NCT03124303) and approved by the University of Wisconsin–Madison Institutional Review Board (2015‐0374). Patients over the age of 65 undergoing elective, non‐intracranial surgery with an anticipated hospital stay of at least 2 days were recruited to participate. Participants were excluded if they had a documented history of dementia, resided in a nursing home, or could not complete neurocognitive testing. The work was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Resting state 7 and roving oddball auditory stimulation EEG data were collected from participants before and after surgery. A delirium assessment was done immediately prior to the EEG assessments (both preoperatively and postoperatively). Participants were classified as delirious or non‐delirious at the time of the EEG recording using the Confusion Assessment Method (CAM+/–, 16 , 17 or CAM‐ICU+/– if ventilated 18 ). The Delirium Rating Scale‐Revised‐98 (DRS‐R‐98) was administered at the same time points to measure delirium severity. The DRS‐R‐98 provides a valid measure of delirium severity in a Delirium Rating Scale total score (DRS‐TS) over a broad range of symptoms and is a useful diagnostic and assessment tool. 19 A Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) diagram of the study design is shown in Figure S1 in supporting information.
Preoperatively, there were 16 participants who subsequently developed POD with available evoked response potential (ERP) data (mean age ± standard deviation [SD]: 72.0 ± 3.56 years; 7 females) and 106 preoperative “non‐delirious” participants (i.e., participants who did not develop delirium postoperatively while being monitored with EEG) with available ERP data (mean age ± SD: 72.25 ± 4.67 years; 40 females). Postoperatively, there were 19 “delirium” participants with available ERP data (mean age ± SD: 72.16 ± 3.42 years; 8 females, and 3 patients with missing preoperative data) and 91 “non‐delirious” participants with available ERP data (mean age ± SD: 72.0 ± 4.64 years; 28 females). We included 11 hypoactive, 2 hyperactive, and 6 mixed delirium phenotypes in our dataset. Postoperatively, the mean (SD) number of trials included per participant were similar across phenotypes (90.23 [29.51] hypoactive, 107 [12.06] hyperactive, and 76.58 [22.43] mixed). There were 16 “delirium” participants with both pre‐ and postoperative paired data available and 86 “non‐delirious” participants with both pre‐ and postoperative paired data available. Data are described as follows:
Delirium PRE: Preoperative data from patients who experienced delirium postoperatively
Delirium POST: Postoperative data from patients who experienced delirium postoperatively
Non‐Delirious PRE: Preoperative data from patients who did not experience delirium postoperatively
Non‐Delirious POST: Postoperative data from patients who did not experience delirium postoperatively
3. THE AUDITORY PARADIGM
The auditory roving oddball paradigm (adapted from Garrido et al., 19 , 20 as applied in our previous work; 14 also in Figure S2 in supporting information) consisted of the presentation of a sequence of pure sinusoidal tones belonging to seven frequencies varying from 500 to 800 Hz in steps of 50 Hz, with a roving, or sporadically changing, frequency. Each stimulus train was comprised of tones of one frequency followed by a train of tones of a different frequency. The number of times the same tone was presented in the same train varied pseudo‐randomly between one and eleven. The duration of each tone was 70 ms (with 5 ms rise and fall times), and the interstimulus interval was set to 500 ms. The oddball was the first tone in a train of a different frequency from the preceding train, and the standard stimulus was the sixth tone in the same frequency train. As standards and oddball were selected as pairs from the same stimulus train, it ensured that the physical characteristics of the stimuli were identical (Figure S2). As the tones are played in a randomized fashion, there is no learning effect in this paradigm. The sequence of the auditory stimuli was delivered via stimulus presentation software (E‐Prime, Psychology Software Tools, Inc.). Headphone tone volume was adjusted until rated as comfortable. The auditory paradigm was delivered in two 6‐minute blocks interspersed with resting state EEG collection in one session preoperatively and one session postoperatively, typically on the morning of postoperative day 1 unless the patient was unable to undergo delirium assessment (e.g., coma) or the patient refused the EEG (and was not delirious). The median (interquartile range [IQR]) of the day of postoperative assessment was 1 (IQR 1‐1).
3.1. Cognitive testing
Preoperative cognitive tests are reported in the demographics table (Table S1 in supporting information). The Trail‐Making Test Part B (TMT‐B) measures mental flexibility, executive function, speed, and attention by requiring the participants to connect a series of numbers alternating with letters and is also scored by the number of seconds taken for completion, with a cut‐off time of 300 seconds.
3.2. Blood samples and cytokine analysis
Blood samples were collected for biomarker analyses at the time of the preoperative and postoperative EEG. Plasma samples were collected in ethylenediamine tetraacetic acid (EDTA)‐containing tubes and stored at −80°C. A multiplex assay was conducted at Eve Technologies (Montreal, Canada), and the cytokines measured were interleukin (IL)‐1 beta, IL‐1 receptor antagonist, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐12, monocyte chemoattractant protein 1 (MCP‐1), and tumor necrosis factor alpha (TNF‐α).
3.3. EEG data acquisition, preprocessing, and extraction of ERPs
High‐density EEG data were acquired at a 250 Hz sampling rate using a 256‐channel system (Electrical Geodesics, Inc.), capable of accepting 8‐bit digital trigger input. EEG data were processed in EEGLAB, 21 a toolbox running in MATLAB (The MathWorks Inc.). EEG signals were bandpass filtered between 1 and 40 Hz. Data segments heavily compromised by artifacts were detected by the EEGLAB function “clean_artifacts” and removed. The detected noisy channels by the same function were also removed (then later replaced by interpolated data before the final epoch “averaging”). Non‐neuronal artifactual components (due to eye movements, muscle activity, and cardiac electric field) were detected and rejected using independent component analysis (ICA). After pre‐processing, EEG data were segmented to epochs of interest ([−100 to 400 ms] relative to stimulus onset at 0 ms) separately for oddball and standard stimuli, and further bad epochs exceeding a threshold of ± 150μV were rejected. Finally, in each individual subject, the auditory ERPs for standard and oddball stimuli were obtained by the method of averaging of the previously obtained epochs followed by baseline correction (i.e., subtraction of the mean amplitude during the baseline [−100 to 0 ms] interval). Difference waves were calculated by subtracting the averaged ERP of standard stimuli from the averaged ERP to oddball stimuli. Mismatch negativity (MMN) amplitudes were obtained from the difference waves at each time point as well as mean scores at an interval of interest post‐stimulus onset (124–176 ms), based on the prior literature for the known timings of the MMN effect . 22 Grand averages for ERPs of standard, oddball, and MMN waveforms in Delirium PRE (N = 16), Delirium POST (N = 19), Non‐Delirious PRE (N = 106), and Non‐Delirious POST (N = 91) conditions were obtained by averaging respective individual subject ERPs. No significant differences were found in the accepted number of trials for standard and oddball stimuli between all conditions data compared (t tests: P > 0.05).
3.4. Statistics on scalp ERP data
Prior to performing DCM, we conducted scalp‐based analyses to confirm we could detect relevant changes in the EEG. Non‐parametric permutation‐based t statistics were used to compare the MMN amplitudes between subgroups or conditions (primary comparison: Delirium POST vs. Non‐Delirious POST, as well as secondary comparisons: Delirium POST vs. Delirium PRE, Delirium PRE vs. Non‐Delirious PRE) at each sensor of the 256‐channel array for individual time points and averages from the time interval of interest (124–176 ms) for MMN (Fieldtrip toolbox function ft_statistics_montecarlo operated from function ft_timelockstatistics). 23 , 24 The correction for multiple comparisons and for the primary comparison was performed using the threshold‐free cluster enhancement (TFCE) method implemented in FieldTrip toolbox, 23 , 25 running in MATLAB. TFCE overcomes the need to define the cluster‐forming threshold. For the secondary comparisons, the resulting Monte Carlo significance for the mean signal from frontocentral sensors (a typical spatial exploration for MMN on scalp space) was corrected for multiple comparisons based on t values for the actual comparisons within a null distribution generated from storing extreme t values across all time points in the time interval of interest on a large number (5000) of data label permutations. The significance at was set at P < 0.05 for a two‐tailed test (i.e., the actual comparison statistics > 95% of t statistics in the permutation‐based null distribution).
3.5. Dynamic causal modeling and Bayesian model comparison
The DCM module for ERPs in SPM12 (https://www.fil.ion.ucl.ac.uk/spm) was used to estimate effective connectivity between brain regions and test the effect of experimental perturbations on coupling among the involved sources generating the acquired ERP signals. The DCM general methodology, when applied to ERP data, supplements the conventional electromagnetic forward models with a model explaining how source activity is generated by neuronal dynamics, and enables inference about both the spatial involvement of sources and the underlying neuronal architecture generating the signals. 26 We used the “ERP” neural mass model, which emulates the activity of a source using three neural subpopulations (pyramidal cells, and excitatory and inhibitory local interneurons), each assigned to one of three cortical layers. 27 It describes coupled extrinsic connections among multiple sources in the form of forward, backward, and lateral connections. The forward model used the “IMG” option in the DCM module for SPM12 in which each node/source was treated as a patch on the cortical surface. The selection of the involved brain areas as part of a given task are based on previous studies reported in the literature. 20 , 28 , 29
Twenty DCMs were used for Bayesian model comparison. Each model receives (parameterized) subcortical input at the A1 sources, which elicit transient perturbations in the remaining sources. M1 to M11 were identical to those run in Boly et al. 28 Further extensions in models M12 to M18* were based on the network‐level work on generation of auditory mismatch negativity. 29 The 20 different models (Figure S3 in supporting information) were fitted to the data to obtain estimates of the parameters. Different DCM models included different numbers of sources (i.e., two, four, five, and six). The models were generated using the entire poststimulus window (0 to 400 ms) setting the prior for when the auditory cue is supposed to arrive at cortex to 60 ms (onset [ms] and duration [SD] parameters: onset = 60, duration = 25) based on literature recommendations from previous DCM papers involving auditory oddball paradigms, confirmed by further Bayesian model comparison that we conducted (i.e., comparing onsets of 50, 60, and 64 ms).
Random‐effects Bayesian model selection was used to test which population‐level model was the most likely among a set of competing models. The winning model (M17*) was selected for subsequent quantitative analysis of effective connectivity between the subgroups and conditions studied (Delirium POST vs. Non‐Delirious POST as a primary comparison, as well as Delirium POST vs. Delirium PRE, and Delirium PRE vs. Non‐Delirious PRE).
The output of the DCM includes observed connectivity changes, either extrinsic (between regions/sources) or intrinsic (self‐connections in a region or source). The intrinsic connections can be considered the inhibitory or excitatory influences within the region; an increase in intrinsic connectivity is a modeled response consistent with increased inhibition. DCM outputs are divided into two matrices. The so‐called “Matrix A” denotes the (rate of) change in neural response due to neural activity elicited by the standard tone, that is, the effective connectivity. The so‐called “Matrix B” denotes the (rate of) change in the effective connectivity due to the modulatory inputs (i.e., oddball stimulus relative to standard stimulus). Within “Matrix B,” the self‐connections in the model represent a change in inhibition. The more positive the self‐connection parameter, the more inhibited the region. Within DCM, this inhibition is modeled through increased interneuron signaling.
3.6. Parametric empirical Bayes
Parametric empirical Bayes (PEB) analyses (focused on the “B” modulation matrix, which evaluates the relative effects of the oddball stimulus compared to the standard across conditions in the DCM module of SPM12) were done only with DCM data from the winning model (M17*). The PEB model has parameters encoding the deviation from the mean due to the group difference. For the group difference, negative estimated parameters indicate weaker connectivity, and positive parameters indicate the opposite. The obtained posterior probabilities > 95% correspond to a strong evidence level for the effect of interest. Different from taking the expected values of the estimated connectivity parameters from all subjects and running classical statistics, the PEB framework takes both the expected values and the covariance of the parameters to the group level. 30 As a result, more precise parameter estimates will have a greater influence on the group‐level result, so subjects with noisy data and uncertain parameter estimates will be down‐weighted. 30 However, for the sake of comparison, we also computed Cohen d values of effect sizes for the independent samples t tests.
The primary outcome of the study was the PEB analysis of the “B” matrix, which represents the difference between the standard and oddball. This analysis tests both cortical inhibition and connectivity parameters with reference to different levels of surprise (the predictability of a stimulus). Additional PEB analyses were conducted on the “A” matrix, which evaluates differences in the standard stimulus effects across conditions in internodal connectivity parameters (but not intrinsic inhibition due to the lack of variation in surprise).
3.7. Associations among connectivity changes, delirium severity, and inflammatory markers
Associations between changes in extrinsic internodal connectivity and intrinsic nodal excitability with cytokine levels and delirium rating scale scores were tested with Pearson correlations in MATLAB (two‐tailed tests with significance level set at P < 0.05, uncorrected for multiple testing given the exploratory nature of these analyses). The cytokines tested (the ones with limited missing data) were: IL‐1 beta, IL‐1 receptor antagonist, IL‐6, IL‐8, IL‐10, IL‐12, and TNF‐α.
3.8. Sample size
An a priori sample size power calculation was not conducted, as there are limited ways to perform this for DCM analyses. Given that resting state EEG changes were evident in a smaller sample from our study (n = 70), we considered that 100 subjects would provide sufficient power to define relevant differences between postoperative patients with delirium versus without (primary outcome). We would note that this study is significantly larger than most studies using DCM. 14 , 28 All available data from the cohort study were included in this work (until the start of analyses on August 12, 2021). Our primary contrast was set as postoperative delirious participants versus postoperative non‐delirious participants to maximize the sample size. This contrast also takes into account some non‐specific effects of incurring surgery that are not associated with delirium.
4. RESULTS
4.1. Subjects
Of 167 participants enrolled, 131 underwent surgery and completed at least one EEG assessment; 102 participants had paired preoperative and postoperative EEG assessments (see STROBE diagram; Figure S1) and 19 participants were delirious according to their POD assessment (see Table S1 for demographic data).
4.2. Sensor ERP data
Figure 1 displays grand average ERPs for each condition and subgroup (overlaying the standard, oddball, and difference waves) from a representative sensor combination for MMN on the scalp (signal average of E8/FCz and four other neighboring mid‐frontocentral sensors), as well as topographical plots for the mean MMN amplitudes during the time interval of interest (124–176 ms) from stimulus presentation onset.
FIGURE 1.
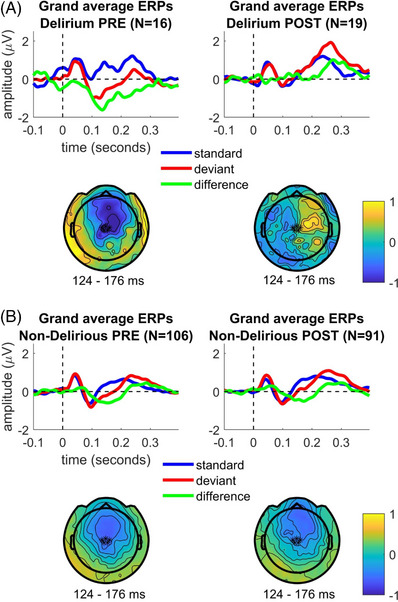
Sensor space evoked response data from patients who experienced postoperative delirium collected preoperatively (Delirium PRE) and postoperatively (Delirium POST). A, Topographical plots of MMN grand averages from Delirium PRE and Delirium POST conditions, as well as MMN grand‐averaged waveforms from a representative sensor combination for MMN on scalp (signal average of E8/FCz and four other neighboring mid‐frontocentral sensors) highlighted with asterisks (*) in the displayed topoplots. B, The same is shown for Non‐Delirious PRE and Non‐Delirious POST participants’ data. ERP, evoked response potential; MMN, mismatch negativity
Our primary outcome was the difference between the Delirium POST subgroup compared to the Non‐Delirious POST subgroup in the DCM, but we report scalp‐based statistics for this contrast for completeness. At the sensor level, MMN amplitudes were found to be significantly smaller in the Delirium POST subgroup compared to the Non‐Delirious POST subgroup. The sensors showing significant differences (P < 0.05, TFCE‐corrected for multiple comparisons) in mean MMN amplitudes between Delirium POST and Non‐Delirious POST participants for each time point (Figure 2) as well as average (124–176 ms) response in the evaluated time interval (Figure S4 in supporting information) are highlighted with red asterisks.
FIGURE 2.
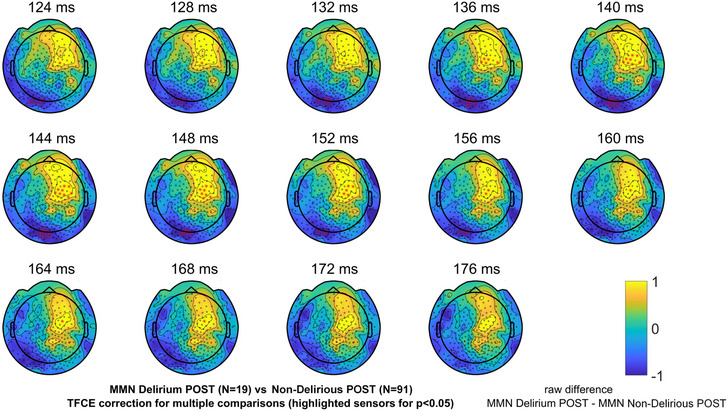
Sensor space evoked response data from patients who experienced delirium postoperatively (Delirium POST) and those who did not (Non‐Delirious POST). Topographical plots of raw differences in MMN amplitudes between Delirium POST and Non‐Delirious POST participants’ data for each time point in the evaluated time interval (124–176 ms). Significant differences (P < 0.05, TFCE‐corrected for multiple comparisons) of MMN amplitudes being smaller in Delirium POST compared to Non‐Delirious POST participants for each time point in the evaluated time interval (124–176 ms) are highlighted with red asterisks. MMN, mismatch negativity; TFCE, threshold‐free cluster enhancement
As secondary comparisons, we conducted analyses for MMN amplitude differences between Delirium PRE and Delirium POST, as well as Delirium PRE and Non‐Delirious PRE, conditions (Figure S5 in supporting information). Figure S5 displays the same MMN amplitude comparison for a mean signal of selected frontocentral sensors statistically corrected over all time points in the time interval of interest. In both of these contrasts, significant differences were noted around the time of the classic MMN (124–176 ms) response, showing that Delirium PRE had larger MMN responses than Delirium POST or Non‐Delirious PRE.
4.3. DCM, Bayesian model comparison, and PEB analysis
The random effects of the Bayesian model selection method showed that the fully connected model (M17*: including modulation of intrinsic/self‐connection at each node and lacking the lateral connections between the left and right inferior frontal gyrus [IFG] sources) had the greatest evidence, and this model was selected for subsequent quantitative analysis of effective connectivity between the four respective data subgroups and condition combinations (Figure 3, and Figures S6A and S6B in supporting information). Figures S7A and S7B in supporting information display the grand‐averaged source waveforms during the interval (0–400 ms) for standard, oddball, and difference waves from data belonging to Delirium PRE, Delirium POST, Non‐Delirious PRE, and Non‐Delirious POST conditions, respectively.
FIGURE 3.
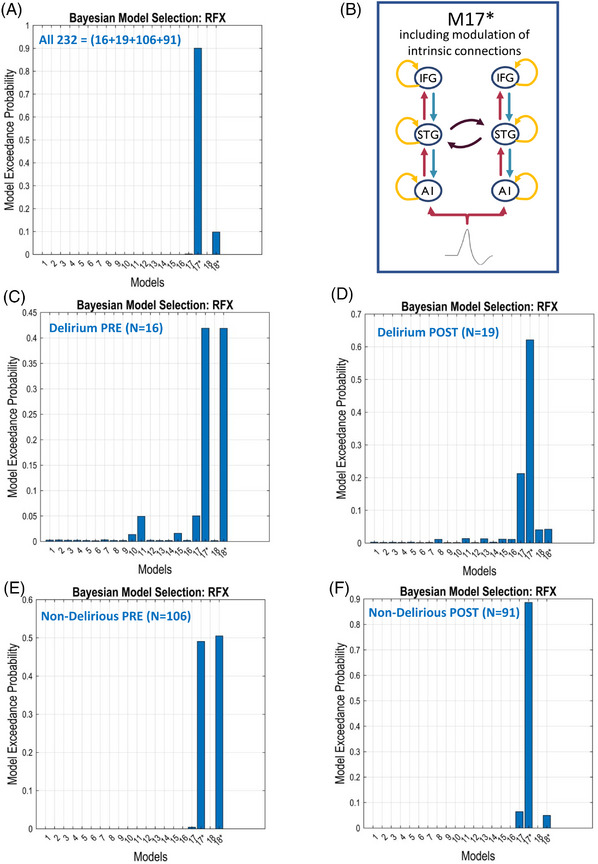
Population‐level best model resulting from a Bayesian model comparison. Random effects Bayesian model selection showed that labeled Model #17* (representing here a fully connected original M17 model with modulation of intrinsic/self‐connection at each node, with exception of lateral IFG connections [B]) had greater evidence compared to the other models, and was selected for subsequent quantitative analysis of effective connectivity between the subgroups (Delirium, Non‐Delirious) or conditions (PRE, POST). Here, Bayesian model comparison was conducted for all data (A) and separately for (C) Delirium PRE, (D) Delirium POST, (E) Non‐Delirious PRE, and (F) Non‐Delirious POST. Note: Exceedance probability is the probability of each model being better than any other model. The advantage of using exceedance probabilities is that they are sensitive to the confidence in the posterior probability and easily interpretable (because they sum to unity over all models tested). The best model is the one with highest exceedance probability (equivalently, the highest expected posterior probability; the ranking is the same). A1, primary auditory sensory area; IFG, inferior frontal gyrus; RFX, random effect analysis; STG, superior temporal gyrus
4.3.1. Secondary outcome: preoperative differences in connectivity
Analysis of the preoperative data showed that in response to the standard tone, there was increased feedforward cortical connectivity in participants who subsequently became delirious postoperatively compared to those that did not (Figure 4A). Similarly, across both the standard tone analyses as well as the differences in evoked responses between the oddball and standard tone, there was reduced interhemispheric connectivity between the left and right superior temporal gyrus (STG; Figure 4B).
FIGURE 4.
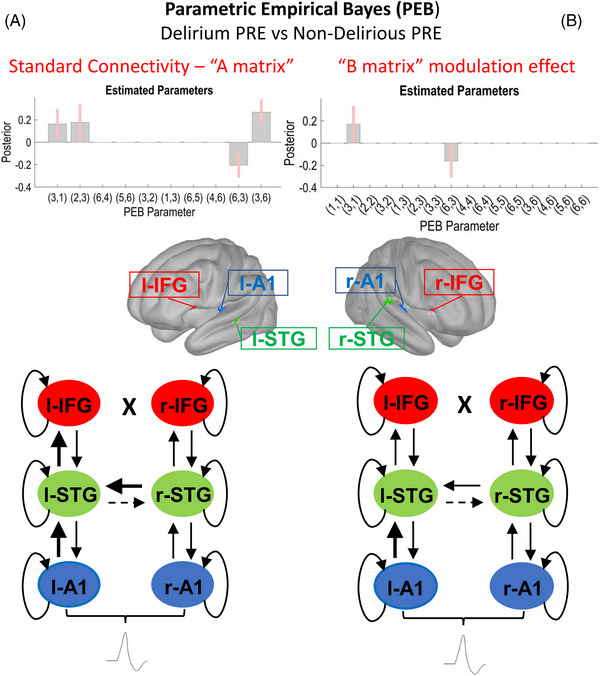
Secondary outcome: comparison of preoperative data from patients who experienced postoperative delirium (Delirium PRE, N = 16) and those who did not experience postoperative delirium (Non‐Delirious PRE, N = 106). Schematic display of the results of the parametric empirical Bayes (PEB) analysis following the dynamic causal modeling (DCM) based estimation (i.e., fitting the winning model to the individual data, to get estimates of the parameters). The PEB model has parameters encoding the deviation from the mean due to the group difference (covariate 2). For the two‐group difference, positive estimated parameters indicate stronger connectivity in first group than second group and negative parameters indicate the opposite. Posterior probabilities > 95% (corresponding to a strong evidence level) for the deviance detection effect of interest are shown. Sources #: (1) left A1; (2) left IFG; (3) left STG; (4) right A1; (5) right IFG; (6) right STG. In the diagrams, dashed and thicker lines for internodal connections mean decreased and increased connectivity, respectively. In case of curved lines for intrinsic inhibition, thicker and dashed lines mean increased and decreased inhibition, respectively. (A) Left section: Standard tone connectivity analyses (“A matrix”)—comparison of preoperative data from patients who experienced postoperative delirium (Delirium PRE, N = 16) and those who did not experience postoperative delirium (Non‐Delirious PRE, N = 106). PEB results interpretation (increase/decrease in connection strength): left A1 → l‐STG (increased in Delirium PRE), left STG → left IFG (increased in Delirium PRE), left STG → right STG (decreased in Delirium PRE), right STG → left STG (increased in Delirium PRE). (B) Right section: Difference between oddballs and standards (“B matrix”) modulation effect—comparison of preoperative data from patients who experienced postoperative delirium (Delirium PRE, N = 16) and those who did not experience postoperative delirium (Non‐Delirious PRE, N = 106). PEB results interpretation (increase/decrease in connection strength): left A1 → l‐STG (increased in Delirium PRE), left STG → right STG (decreased in Delirium PRE). A1, primary auditory sensory area; IFG, inferior frontal gyrus; STG, superior temporal gyrus
4.3.2. Secondary outcome: postoperative differences in connectivity related to the standard tone
On review of the postoperative contrasts across the standard tone analyses, we found several differences in cortical connectivity. These results indicated that, in delirium, feedback connectivity is diminished from left STG (l‐STG) to left primary auditory sensory area (l‐A1) as well as reduced interhemispheric connectivity between left and right STG (Figure 5A). We report here also Cohen d (i.e., effect size) for the main significant finding: a decrease in extrinsic feedback connectivity related to standard tone between l‐STG and l‐A1 (d = 0.52).
FIGURE 5.
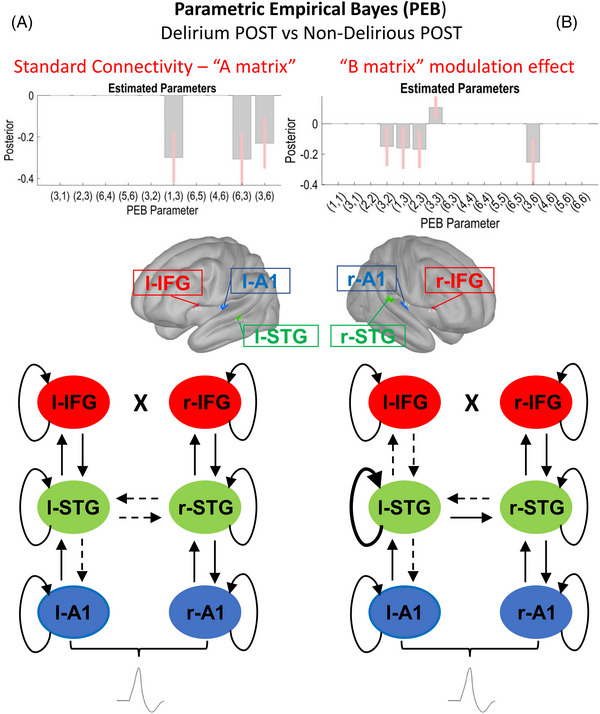
Primary outcome: comparison of postoperative data from delirious (Delirium POST, N = 19) and non‐delirious (Non‐Delirious POST, N = 91) patients. Schematic display of the results of the parametric empirical Bayes (PEB) analysis following the dynamic causal modeling (DCM) based estimation (i.e., fitting the winning model to the individual data, to get estimates of the parameters). The PEB model has parameters encoding the deviation from the mean due to the group difference (covariate 2). For the two‐group difference, positive estimated parameters indicate stronger connectivity in first group than second group and negative parameters indicate the opposite. Posterior probabilities > 95% (corresponding to a strong evidence level) for the deviance detection effect of interest are shown. Sources #: (1) left A1; (2) left IFG; (3) left STG; (4) right A1; (5) right IFG; (6) right STG. In the diagrams, dashed and thicker lines for internodal connections mean decreased and increased connectivity, respectively. In case of curved lines for intrinsic inhibition, thicker and dashed lines mean increased and decreased inhibition, respectively. (A) Left section: Standard tone connectivity analyses (“A matrix”)—comparison of postoperative data from delirious (Delirium POST, N = 19) and non‐delirious (Non‐Delirious POST, N = 91) patients. PEB results interpretation (increase/decrease in connection strength): left STG → left A1 (decreased in Delirium), left STG → right STG (decreased in Delirium), right STG → left STG (decreased in Delirium). (B) Right section: Difference between oddballs and standards (“B matrix”) modulation effect—comparison of postoperative data from delirious (Delirium POST, N = 19) and non‐delirious (Non‐Delirious POST, N = 91) patients. PEB results interpretation (increase/decrease in connection strength): left IFG → left STG (decreased in Delirium), left STG → left A1 (decreased in Delirium), left STG → left IFG (decreased in Delirium), left STG (increased self‐Inhibition in Delirium), right STG → left STG (decreased in Delirium). A1, primary auditory sensory area; IFG, inferior frontal gyrus; STG, superior temporal gyrus
Comparison of the postoperative delirious participants with their preoperative data showed that this connection, and feedforward and feedback connections between each level of the cortical hierarchy, may be involved in delirium (though this contrast includes non‐specific effects related to surgery as well; Figure 6A).
FIGURE 6.
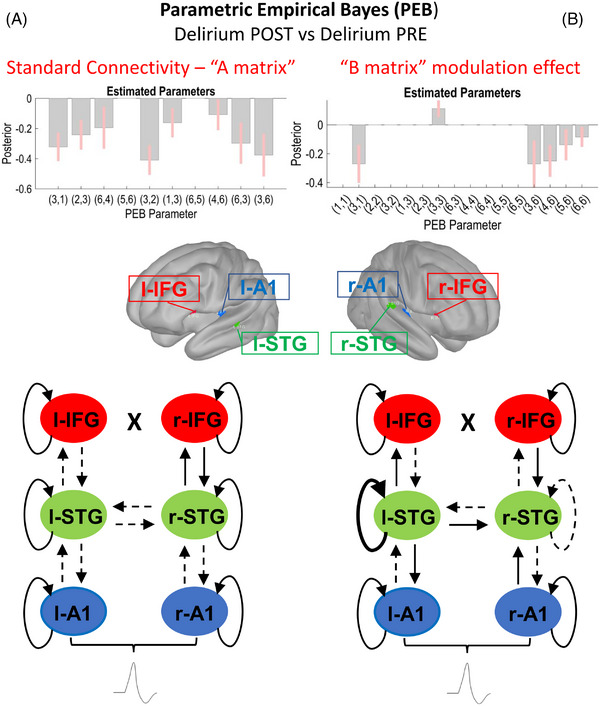
Secondary outcome: comparison of postoperative (Delirium POST, N = 19) and preoperative data (Delirium PRE, N = 16). Schematic display of the results of the parametric empirical Bayes (PEB) analysis following the dynamic causal modeling (DCM) based estimation (i.e., fitting the winning model to the individual data, to get estimates of the parameters). The PEB model has parameters encoding the deviation from the mean due to the group difference (covariate 2). For the two‐condition difference, positive estimated parameters indicate stronger connectivity in first group than second group and negative parameters indicate the opposite. Posterior probabilities > 95% (corresponding to a strong evidence level) for the deviance detection effect of interest are shown. Sources #: (1) left A1; (2) left IFG; (3) left STG; (4) right A1; (5) right IFG; (6) right STG. In the diagrams, dashed and thicker lines for internodal connections mean decreased and increased connectivity, respectively. In case of curved lines for intrinsic nodal inhibition, thicker and dashed lines mean increased and decreased inhibition, respectively. (A) Left section: Standard tone connectivity analyses (“A matrix”) for the difference between postoperative (Delirium POST, N = 19) and preoperative data (Delirium PRE, N = 16). PEB results interpretation (increase/decrease in connection strength): left A1 → left STG (decreased in Delirium), left STG → left IFG (decreased in Delirium), right A1 → right STG (decreased in Delirium), left IFG → left STG (decreased in Delirium), left STG → left A1 (decreased in Delirium), right STG → right A1 (decreased in Delirium), left STG → right STG (decreased in Delirium). right STG → left STG (decreased in Delirium). (B) Right section: Difference between oddballs and standards (“B matrix”) modulation effect for the difference between postoperative (Delirium POST, N = 19) and preoperative data (Delirium PRE, N = 16). PEB results interpretation (increase/decrease in connection strength): left A1 → left STG (decreased in Delirium), left STG (increased self‐Inhibition in Delirium), right STG → left STG (decreased in Delirium), right STG → right A1 (decreased in Delirium), right STG → right IFG (decreased in Delirium), left STG (decreased self‐Inhibition in Delirium). A1, primary auditory sensory area; IFG, inferior frontal gyrus; STG, superior temporal gyrus
4.3.3. Primary outcome: differential changes in connectivity and excitability with the oddball relative to standard tone with delirium
Results from PEB analysis for the study's primary outcome, the modulatory effect of the oddball compared to the standard tone, showed decreases in connectivity strength between Delirium POST and Non‐Delirious POST subgroups (Figure 5B), including decreased l‐STG to l‐A1, decreased feedforward connectivity from l‐STG to left IFG (l‐IFG), and decreased feedback connectivity from l‐IFG to l‐STG. The analysis also demonstrated increased inhibition of l‐STG. We report here also Cohen d (i.e., effect size) for the main significant findings: increase in intrinsic self‐inhibition for l‐STG related to modulatory effects of the deviant tone (d = 0.46).
4.3.3.1 Secondary outcome: differential changes in connectivity and excitability with the oddball relative to standard tone with delirium compared to baseline
In related analyses, we showed similar significant decreases in connectivity between Delirium POST and Delirium PRE conditions (Figure 6B). Notably, while l‐STG showed a similar increased inhibition to the contrast of the Delirium POST and Non‐Delirious POST subgroups, right‐STG showed disinhibition (increased excitability).
4.3.3.2 Secondary outcome: differential changes in connectivity and excitability with the oddball relative to standard tone for non‐delirious participants compared to baseline
For completeness, we also included analyses of the comparison of non‐delirious participants’ preoperative data compared to their postoperative data. Multiple changes were noted for the response to standard tones, with diverse changes in feedforward and feedback connectivity compared to the preoperative state (Figure S8 in supporting information). Interestingly, rather than the increased inhibition noted in delirium, participants who did not have delirium had greater cortical excitability postoperatively.
4.3.4. Exploratory analysis: correlations between connectivity changes and delirium severity
Next, we investigated whether the l‐STG to l‐A1 feedback connectivity also correlated with delirium severity (Figure 7A). The standard tone analyses of postoperative data demonstrated a correlation in the change in connectivity from l‐STG to l‐A1 with delirium severity (r = −0.24; P = 0.02). However, correlations for the differential effects of the oddball and standard were not significant (Figures S9A and S10A in supporting information).
FIGURE 7.
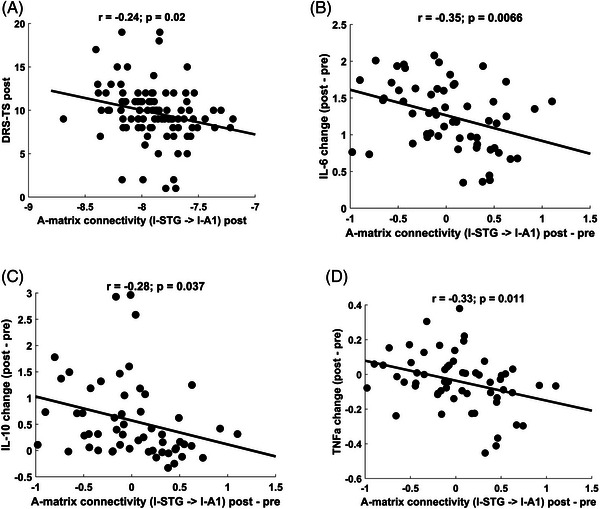
Secondary outcome: correlations between “A matrix” connectivity changes with DRS‐TS and cytokine levels. Decreased feedback connectivity from l‐STG to l‐A1 (due to standard stimulus effects) significantly correlated with larger DRS‐TS scores postoperatively and increased change in systemic inflammation represented by measured cytokine levels: (A) DRS‐TS (r = −0.24; P = 0.02), (B) IL‐6 (r = −0.35; P = 0.007), (C) IL‐10 (r = −0.28; P = 0.04), (D) TNF‐α (r = −0.33; P = 0.01), supporting the association of loss of feedback connectivity and delirium severity. Cytokine levels were log10‐transformed. A1, primary auditory sensory area; DRS‐TS, Delirium Rating Scale total score; IL, interleukin, STG, superior temporal gyrus; TNF‐α, tumor necrosis factor alpha
4.3.5. Exploratory analysis: correlations between connectivity changes and cytokine levels
Decreased feedback connectivity from l‐STG to l‐A1 (change post–pre, due to standard stimulus effects) significantly correlated with increased systemic inflammation, represented by postoperative change in levels of 3 of 10 cytokines from preoperative values (IL‐6 [r = −0.35; P = 0.007], TNF‐α [r = −0.33; P = 0.01], and IL‐10 [r = −0.28; P = 0.04]), supporting the association of loss of feedback connectivity and increased systemic inflammation (Figure 7B,C,D). Note that these exploratory analyses were not corrected for multiple comparisons. Correlations with differences between oddballs and standards were not found (Figures S9B,C,D and S10B,C,D).
4.3.6. Exploratory analysis: correlations between connectivity changes and TMT‐B cognitive test scores
No significant associations were found between the TMT‐B cognitive test scores and changes in connectivity (Figure S11A,B,C in supporting information).
5. DISCUSSION
These data suggest that POD is associated with changes in hierarchical connectivity and regional excitability. Specifically, decreased feedback connectivity was found in delirium, and the magnitude of the changes correlated with the severity of delirium and levels of systemic inflammatory markers. In sensitivity analyses, other changes in connectivity were noted. However, our a priori choice of primary analysis here is key: the contrast between the participants with delirium and those without after surgery (to avoid the confound of non‐specific changes that may occur in the perioperative period). Our results are consistent with our prior hypothesis that delirium is associated with increased inhibitory tone and changes in connectivity. 5 Namely, we showed that delirium was associated with reduced excitability (increased inhibition) of STG as well as changes in feedback connectivity. Our findings indicate that the predictive coding framework, with associated modeling of cortical effects, may provide a useful construct for understanding delirium pathogenesis.
Our data suggest a specific mechanism of POD: increases in inhibitory tone leading to decreased connectivity. The DCM analysis suggests that increased interneuron activity may underpin dampened responses in STG, though we are unsure why the STG was the only region determined to be affected in our modeling. This may be due to the augmented sensitivity of this region, difficulties in assessing upstream regions once STG is suppressed, altered folding of the cortex making unilateral responses easier to detect (left vs. right), and/or the importance of STG in hierarchical processing in the auditory system. Future, larger studies should examine whether more diverse effects on inhibition can be identified. Nonetheless the data are consistent with our prior published hypothesis, 5 and with analyses showing diminished functional connectivity in the scalp EEG. 7 , 31 Similar to our findings with DCM, functional connectivity changes were associated with slow‐wave activity in the resting EEG and plasma cytokines. 7 , 14 We have found similar changes in a mouse model of systemic inflammation with intraperitoneal lipopolysaccharide. 32 Our current working model is that inflammation‐driven prostaglandin release leads to adenosine signaling, 14 , 32 and activation of GABAergic‐interneuron signaling pathways. 33 We recently showed that caffeine, an adenosine antagonist, could reverse the EEG effects of inflammation in mice. 32 It will be fascinating to test whether caffeine can reverse the EEG changes identified herein and alter delirium symptoms (as suggested by a secondary outcome in a recent study in patients 34 ).
Prior to surgery, our effective connectivity analyses suggest that patients who become delirious after surgery had increased feedforward connectivity. This is consistent with our resting state EEG analyses suggesting that vulnerable patients have increased functional connectivity prior to surgery. 7 This also parallels our recent findings that preclinical AD, detected on positron emission tomography (PET) with MK6240 (tau) or Pittsburgh compound B (amyloid), was associated with increased feedforward connectivity. 14 Given the data linking AD pathology to vulnerability to delirium from plasma biomarkers, the DCM findings provide an intriguing pathophysiological link between vulnerability to delirium and AD. 35 Further work should investigate the auditory evoked response as a translational biomarker to understand both pathophysiological states and their relative overlap. It is important to note that increased feedforward effective connectivity was not part of our original hypothesis explaining the pathogenesis of delirium. Whether this increased feedforward connectivity represents a functional compensation for structural changes (on diffusion tensor imaging as suggested by a prior analysis) 7 will require future study.
Of interest, our work also highlights an interesting contrast between delirium and dementia pathogenesis. In delirium, our data suggest STG was suppressed. This contrasts with our recent report that patients with tau disease on PET imaging have increased excitability of the STG. 14 This discordance (both predicted a priori) suggests critical differences in the pathogenesis of the two conditions, with the potential need for opposing therapies.
Our work will spur the development of animal models that can directly probe these pathways in the setting of inflammation or other stimuli that contribute to delirium. At the same time, electrophysiology offers a translational pathway for understanding delirium in humans that bypasses the complexities of recapitulating the complicated nature of delirium symptoms in animals.
We note some important limitations of our work. These data are observational, and although this is a relatively large patient study for the DCM field, it is still a small enough sample size that it should not be considered definitive. Future validation in a larger cohort is warranted, including cases of delirium from a diverse patient and surgical population. Nevertheless, in exploratory analyses, we found that there were proportional changes in feedback connectivity, inflammation, and delirium severity. This adds increased plausibility for our findings. It is worth emphasizing that we have modeled a network that we can both stimulate through auditory stimuli and measure the response (EEG). Nonetheless, we are constrained by the model and, while we have identified important changes in feedback connectivity within the model, we cannot be certain that all feedback connections (i.e., those that were not modeled) would be similarly affected. Indeed, we modeled a very discrete network of the brain that does not fully recapitulate all brain dynamics. Despite modeling a restricted network, our results are consistent with our conceptual model of delirium and recent observations from PET data that show suppressed thalamic metabolism in delirium. 36 Given the critical role in thalamocortical neurons in feedback connectivity, our DCM analyses may reflect impairments at subcortical levels leading to modulation in the cortex. In our analyses, we chose not to model the thalamus as it is such a deep source, but future exploratory studies could attempt this. One surprising feature was that inflammation did not correlate with “B matrix” effects (those associated with the difference between the oddball and standard tone). This likely reflects the fact that the response to all tones was diminished by inflammation, not the relative difference between the standard and oddball, manifesting as increased “environmental surprise” to each stimulus.
This work uses established modeling techniques to estimate changes in neuronal function. However, interventional studies, perhaps targeting interneurons that critically regulate local excitation and feedforward and feedback connectivity, are needed to confirm the underlying mechanisms. Designing those studies and developing therapies targeting interneurons is a significant challenge, and data from human studies is critical to supporting such an ambitious task. Establishing whether similar changes occur in non‐surgical populations and in those with more extensive vulnerability to disease will be a next step. We propose that the link between vulnerability to delirium and AD, that is, exaggerated feedforward connectivity, may be a good biomarker. Furthermore, the decrease in feedback connectivity associated with delirium may well map to the changes in established dementia. Further work is needed to understand the longitudinal changes in hierarchical connectivity over time in persons who develop dementia.
In sum, these findings suggest that the predictive coding framework may provide a useful construct for understanding delirium pathogenesis, and those changes in sensory processing occur with delirium that include increased cortical inhibition and decreased feedback connectivity. This work supports the cognitive disintegration model, though it questions the role of impaired connectivity as a vulnerability factor to delirium, mandating reappraisal of that hypothesis. Therapies that are targeted to interneurons, as mediators of increased inhibition in delirium, may be indicated. This work will be translational in nature and, we hope, will stimulate precision medicine initiatives to reduce the burden of delirium.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest pertinent to this manuscript. Author disclosures are available in the supporting information.
CONSENT STATEMENT
The study was approved by the University of Wisconsin–Madison Institutional Review Board and all participants provided informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study was supported by NIH R01 AG063849. The funding source had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Gjini K, Casey C, Kunkel D, et al. Delirium is associated with loss of feedback cortical connectivity. Alzheimer's Dement. 2024;20:511–524. 10.1002/alz.13471
Contributor Information
Klevest Gjini, Email: gjini@neurology.wisc.edu.
Robert D. Sanders, Email: robert.sanders@sydney.edu.au.
DATA AVAILABILITY STATEMENT
Data are available from the authors on reasonable request and in adherence to the requirements of the local ethics committee on data sharing.
REFERENCES
- 1. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long‐term cognitive decline: A meta‐analysis. JAMA Neurol. 2020;77:1373‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pereira JV, Aung Thein MZ, Nitchingham A, Caplan GA. Delirium in older adults is associated with development of new dementia: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36(7):993‐1003. [DOI] [PubMed] [Google Scholar]
- 3. Gou RY, Hshieh TT, Marcantonio ER, et al. One‐year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg. 2021;156:430‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. 2011;343:d4331. [DOI] [PubMed] [Google Scholar]
- 5. Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140‐143. [DOI] [PubMed] [Google Scholar]
- 6. Wilson JE, Mart MF, Cunningham C, et al. Delirium Nat Rev Dis Primers. 2020;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanabe S, Mohanty R, Lindroth H, et al. Cohort study into the neural correlates of postoperative delirium: The role of connectivity and slow‐wave activity. Br J Anaesth. 2020;125:55‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boord MS, Moezzi B, Davis D, et al. Investigating how electroencephalogram measures associate with delirium: A systematic review. Clin Neurophysiol. 2021;132:246‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanabe S, Parker M, Lennertz R, Pearce RA, Banks MI, Sanders RD. Reduced electroencephalogram complexity in postoperative delirium. J Gerontol A Biol Sci Med Sci. 2022;77:502‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mumford D. On the computational architecture of the neocortex. II. The role of cortico‐cortical loops. Biol Cybern. 1992;66:241‐251. [DOI] [PubMed] [Google Scholar]
- 11. Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360:815‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shipp S. Neural elements for predictive coding. Front Psychol. 2016;7:1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gjini K, Casey C, Tanabe S, et al. Greater tau pathology is associated with altered predictive coding. Brain Commun. 2022;4:fcac209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor J, Parker M, Casey CP, et al. Postoperative delirium and changes in the blood‐brain barrier, neuroinflammation, and cerebrospinal fluid lactate: A prospective cohort study. Br J Anaesth. 2022;129:219‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941‐948. [DOI] [PubMed] [Google Scholar]
- 17. Marcantonio ER, Ngo LH, O'Connor M, et al. 3D‐CAM: Derivation and validation of a 3‐minute diagnostic interview for CAM‐defined delirium: A cross‐sectional diagnostic test study. Ann Intern Med. 2014;161:554‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286:2703‐2710. [DOI] [PubMed] [Google Scholar]
- 19. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the delirium rating scale‐revised‐98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229‐242. [DOI] [PubMed] [Google Scholar]
- 20. Garrido MI, Friston KJ, Kiebel SJ, Stephan KE, Baldeweg T, Kilner JM. The functional anatomy of the MMN: A DCM study of the roving paradigm. Neuroimage. 2008;42:936‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9‐21. [DOI] [PubMed] [Google Scholar]
- 22. Moran RJ, Campo P, Symmonds M, Stephan KE, Dolan RJ, Friston KJ. Free energy, precision and learning: the role of cholinergic neuromodulation. J Neurosci. 2013;33:8227‐8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maris E, Oostenveld R. Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods. 2007;164:177‐190. [DOI] [PubMed] [Google Scholar]
- 24. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83‐98. [DOI] [PubMed] [Google Scholar]
- 26. Chen CC, Kiebel SJ, Kilner JM, et al. A dynamic causal model for evoked and induced responses. Neuroimage. 2012;59:340‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. David O, Harrison L, Friston KJ. Modelling event‐related responses in the brain. Neuroimage. 2005;25:756‐770. [DOI] [PubMed] [Google Scholar]
- 28. Boly M, Garrido MI, Gosseries O, et al. Preserved feedforward but impaired top‐down processes in the vegetative state. Science. 2011;332:858‐862. [DOI] [PubMed] [Google Scholar]
- 29. Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeidman P, Jafarian A, Seghier ML, et al. A guide to group effective connectivity analysis, part 2: Second level analysis with PEB. Neuroimage. 2019;200:12‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Dellen E, van der Kooi AW, Numan T, et al. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121:328‐335. [DOI] [PubMed] [Google Scholar]
- 32. Sultan ZW, Jaeckel ER, Krause BM, et al. Electrophysiological signatures of acute systemic lipopolysaccharide‐induced inflammation: potential implications for delirium science. Br J Anaesth. 2021;126:996‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qi G, van Aerde K, Abel T, Feldmeyer D. Adenosine differentially modulates synaptic transmission of excitatory and inhibitory microcircuits in layer 4 of rat barrel cortex. Cereb Cortex. 2017;27:4411‐4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlisides PE, Li D, McKinney A, et al. The effects of intraoperative caffeine on postoperative opioid consumption and related outcomes after laparoscopic surgery: A randomized controlled trial. Anesth Analg. 2021;133:233‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nitchingham A, Pereira JV, Wegner EA, Oxenham V, Close J, Caplan GA. Regional cerebral hypometabolism on 18F‐FDG PET/CT scan in delirium is independent of acute illness and dementia. Alzheimers Dement. 2023;19:97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data are available from the authors on reasonable request and in adherence to the requirements of the local ethics committee on data sharing.


