Abstract
Biologics are unaffordable to a large majority of the global population because of prohibitively expensive fermentation systems, purification and the requirement for cold chain for storage and transportation. Limitations of current production and delivery systems of biologics were evident during the recent pandemic when <2.5% of vaccines produced were available to low-income countries and ~19 million doses were discarded in Africa due to lack of cold-chain infrastructure. Among FDA-approved biologics since 2015, >90% are delivered using invasive methods. While oral or topical drugs are highly preferred by patients because of their affordability and convenience, only two oral drugs have been approved by FDA since 2015. A newly launched oral biologic costs only ~3% of the average cost of injectable biologics because of the simplified regulatory approval process by elimination of prohibitively expensive fermentation, purification, cold storage/transportation. In addition, the cost of developing a new biologic injectable product (~$2.5 billion) has been dramatically reduced through oral or topical delivery. Topical delivery has the unique advantage of targeted delivery of high concentration protein drugs, without getting diluted in circulating blood. However, only very few topical drugs have been approved by the FDA. Therefore, this review highlights recent advances in oral or topical delivery of proteins at early or advanced stages of human clinical trials using chewing gums, patches or sprays, or nucleic acid drugs directly, or in combination with, nanoparticles and offers future directions.
1. Introduction:
Despite the availability of therapeutic proteins for treatment of various metabolic, inherited, or infectious diseases for several decades, they remain mostly unaffordable for large populations of low-income or developing countries. This became quite evident during the recent pandemic. The cost of production of biologics is very high due to their production in prohibitively expensive fermentation systems, high cost of purification, and cold storage/transportation. Limited cold storage/transportation infrastructure in developing countries was quite evident during the pandemic when African countries administered <50% of all coronavirus disease 2019 (COVID-19) vaccines they received, leading to disposal of ~19 million doses [1,2]. Therefore, the global rollout of COVID-19 vaccines progressed at two alarmingly different rates; among 7.51 billion administered doses, 52.1% were in developed countries but only 4.5% in low-income countries have received their first vaccine [3]. Only 2% of the African continent was vaccinated, even though it has >17% of the global population, when developed countries offered a third or even a fourth booster shot.
In this review, we compiled comprehensive data on biological drugs approved by the FDA since 2015, cost and their modes of delivery. Although intravenous, intramuscular, and subcutaneous injections are bioavailable methods to quickly deliver drug products, they are prohibitively expensive, limiting their availability to a large majority of global population. Limitations of current production and delivery of biologics were quite evident during the recent pandemic. In contrast, topical delivery of drugs offers a more affordable, less invasive approach. In this review, we evaluate advantages and disadvantages of different delivery methods, including intravenous, subcutaneous, intramuscular, and topical delivery. Recent advances in human clinical trials on topical delivery include the chewing gum with viral trap proteins to decrease self-infection and transmission of Corona or other viruses in the oral cavity [4–6], oral [7,8,9] and topical delivery of peanut protein allergens to prevent peanut allergy [10,11]. Among different drug production systems, expression of protein drugs in plant cells offers unique advantages by elimination of prohibitively expensive fermentation, purification processes, and the need for cold storage/transportation. Therefore, we evaluate recent advances in this field. In addition, we review recent advances in oral topical delivery of nucleic acid therapeutics, including the use of nanoparticles. Since oral delivery of nanoparticles for gastrointestinal and systemic applications has been covered by other excellent reviews recently [12–15], our emphasis will be on delivery to the oral cavity to match the theme of this review.
2.
2.1. Biological drugs approved by FDA since 2015
In order to gain an understanding of recent trends in biological drugs approved by the United States (U.S.) Food and Drug Administration (FDA) since 2015 and their mode of delivery, we utilized publicly available and reliable databases at FDA, Center for Drug Evaluation Research (CEDER), and Center for Biologics Evaluation and Research (CEBER). Therefore, the chemical name, brand name, applications, and date of licensure were obtained from the Purple Book [16,17], an online database that lists all licensed biological products monitored by the FDA (CEDER). The Biological Product Patent Transparency (BPPT) requires that the FDA update the list every 30 days to add any biologics approved under section 351(k) or 351(a) of the Public Health Service (PHS) Act [16]. The National Institutes of Health (NIH) National Library of Medicine (NLM) RxNorm database was utilized as confirmation of the biological product reaching the market [18]. In line with the focus of this review, all vaccines, monoclonal antibodies and their fragments, and diagnostics were excluded. As the Purple Book in the portable document format (pdf) is from April 2020, the Purple Book Database of Licensed Biological Products (accessed January 8, 2023) was used to access products approved from May 2020 through December 2022 [16]. Therefore, the data obtained for this review is current. Biological products that did not have a proprietary name and those voluntarily revoked were excluded from the analysis. Similarly, newly approved biological products regulated by the CEBER that received approval for their Biological License Application (BLA) in or after 2015 were also evaluated [19]. Information regarding the clinical trials mentioned in the BLAs was identified from ClinicalTrials.gov, a database regulated by the NIH U.S. National Library of Medicine [20]. The route of delivery of the biologics was gathered from the drugs@FDA website [21]. Additional descriptive qualities were obtained from both the NIH NLM Drug Information Portal and NIH NLM DailyMed database [22,23].
Since 2015, 89 biological products were approved by the FDA and met the inclusion criteria. In terms of the main routes of delivery that these FDA-approved biologics utilize, ~45% and 36% follow a pathway of intravenous or subcutaneous injection, respectively. The remaining 19% is split among various other routes, with less than 10% being non-invasive (Fig. 1–2, Table 1) [16,21]. Although injection is the dominant mode of protein drug delivery and saves millions of lives, there are several limitations, as discussed below. Beyond these safety concerns and lower patient compliance than other modes of drug delivery, injectable drugs are very expensive and pose a greater total (pharmacy and medical) economic burden than drugs that utilize an oral delivery pathway [24]. The loss of ~50% of vaccines during the recent pandemic in African countries due to failure of the cold chain [2] is yet another challenge for injectable drug delivery. Among many other factors, equity barriers to accessing life-saving drugs persist, especially in low-income and lower-middle-income countries (LMICS). Furthermore, those in LMICS are often living in near proximity to others, making infectious diseases more dangerous. Therefore, creating protein therapeutics that do not rely on the cold chain is an urgent priority [25], especially when considering how social inequities and social determinants of health also influence disease susceptibility and distribution [26].
Figure 1:
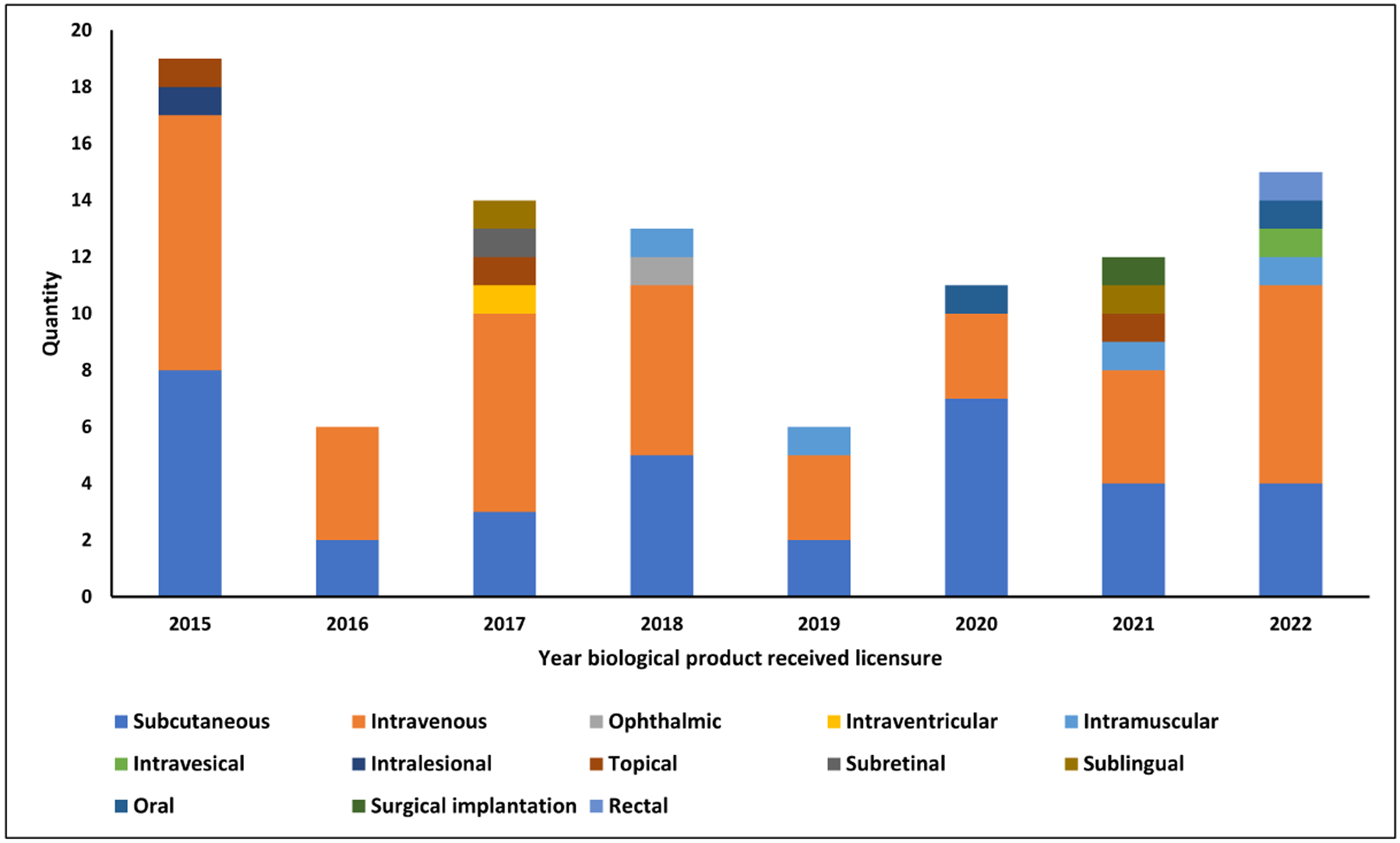
Drug route of administration trends from biological products approved by the FDA between 2015 and 2022 that met the inclusion criteria [16, 19].
Figure 2:
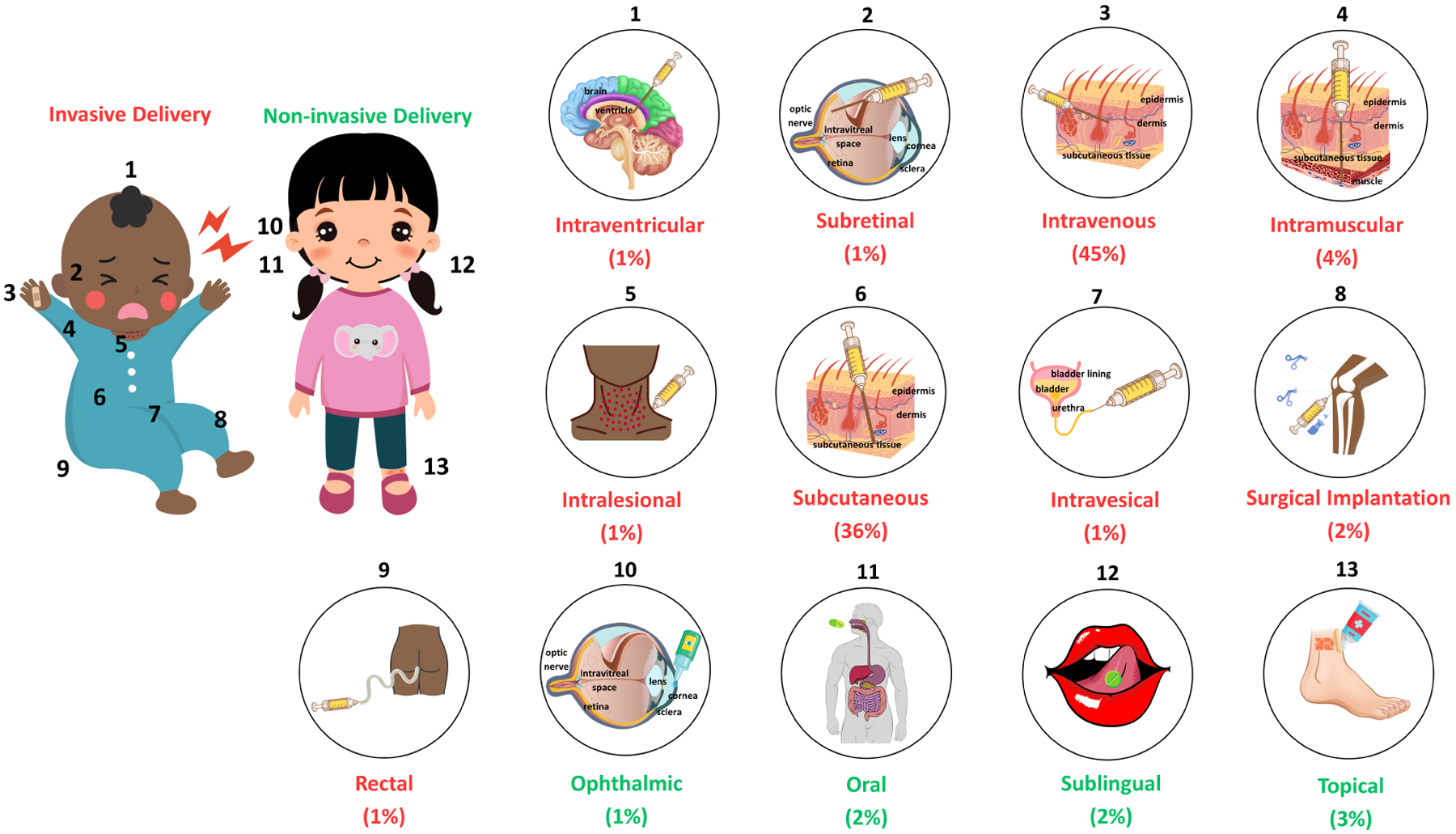
Visualization of the drug delivery pathways that the 89 biologics approved by the FDA between 2015 and 2022 follow. The numbers on the children portray typical sites of administration for each respective delivery method, and all numbers are associated with a more in-depth depiction of the delivery type. The percentage below each delivery method highlights the proportion of FDA-approved biologics that can be administered through that specific pathway. The red percentage (>90% total) indicates an invasive delivery method, and the green percentage corresponds to administration techniques that do not require invasive means. Monoclonal antibodies and their fragments, diagnostics, vaccines, and biologics that were voluntarily revoked and/or did not have a proprietary name were excluded [16, 19].
Table 1:
Drug delivery methods of all FDA-approved biological products regulated by CEDER and CEBER since 2015 that met the review’s inclusion criteria [16,21].
| Proprietary Name* | Proper Name | Licensure Y ear | Indicated Usage |
|---|---|---|---|
| Non-invasive | |||
| Sublingual | |||
| Odactra | House dust mite (HDM) (Dermatophagoides farinae and Dermatophagoides pteronyssinus) allergen extract | 2017 | Treats allergic rhinitis induced by HDM |
| Ragwitek | Short ragweed pollen (SRP) allergen extract | 2021 | Allergic rhinitis induced by SRP |
| Oral | |||
| Palforzia [243] | Peanut (Arachis hypogaea) allergen powder-dnfp | 2020 | Mitigates allergic reaction to peanuts |
| Sucraid [244] | Sacrosidase | 2022 | Treats genetically determined sucrase deficiency |
| Topical | |||
| Raplixa [245] | Fibrin sealant (Human) | 2015 | Controls bleeding during surgery |
| Vistaseal [246] | Fibrin sealant (Human) | 2017 | Controls bleeding during surgery |
| StrataGraft [247] | Allogeneic cultured keratinocytes and dermal fibroblasts in murine collagendsat | 2021 | Treats thermal burns |
| Ophthalmic | |||
| Oxervate** [248] | cenegermin-bkbj | 2018 | Treats neurotrophic keratitis |
| Invasive | |||
| Intralesional | |||
| Imlygic | talimogene laherparepvec | 2015 | Treats lesions |
| Intraventricular | |||
| Brineura | cerliponase alfa | 2017 | Slows the loss of ambulation |
| Subretinal | |||
| Luxturna | voretigene neparvovec-rzyl | 2017 | Treats biallelic RPE65 mutationassociated retinal dystrophy |
| Implantation | |||
| Rethymic | Allogeneic processed thymus tissue-agdc | 2021 | Immune reconstitution |
| Rectal | |||
| Rebyota | Fecal microbiota, live - jslm | 2022 | Prevents the recurrence of Clostridioides difficile infection |
| Intravesical | |||
| Adstiladrin | nadofaragene firadenovec-vncg | 2022 | Treats high-risk Bacillus Calmette- Guerin-unresponsive non-muscle invasive bladder cancer |
| Intramuscular | |||
| Revcovi | elapegademase-lvlr | 2018 | Treats adenosine deaminase severe combined immune deficiency |
| Jeuveau | prabotulinumtoxinA-xvfs | 2019 | Temporarily improves appearance of glabellar lines |
| Rylaze | asparaginase erwinia chrysanthemi (recombinant)- rywn | 2021 | Treats acute lymphoblastic leukemia and lymphoblastic lymphoma |
| Daxxify | daxibotulinumtoxinA-lanm | 2022 | Temporarily improves appearance of glabellar lines |
| Subcutaneous & Intravenous | |||
| Humalog | Insulin lispro injection | 2015 | Improves glycemic control |
| Zarxio | filgrastim-sndz | 2015 | Decreases the incidence of infection and expedites recovery |
| Fiasp | Insulin aspart injection | 2017 | Improves glycemic control |
| Admelog | Insulin lispro injection | 2017 | Improves glycemic control |
| Retacrit | epoetin alfa-epbx | 2018 | Treats anemia |
| Nivestym | filgrastim-aafi | 2018 | Decreases the incidence of infection and expedites recovery |
| Lyumjev | Insulin lispro-aabc | 2020 | Improves glycemic control |
| Subcutaneous | |||
| Strensiq | asfotase alfa | 2015 | Treats hypophosphatasia |
| Tresiba | Insulin degludec injection | 2015 | Improves glycemic control |
| Ryzodeg 70/30 | Insulin degludec and insulin aspart injection | 2015 | Improves glycemic control |
| Basaglar | Insulin glargine injection | 2015 | Improves glycemic control |
| Toujeo | Insulin glargine injection | 2015 | Improves glycemic control |
| Natpara | Parathyroid hormone | 2015 | Controls hypocalcemia |
| Xultophy 100/3.6 | Insulin degludec and liraglutide injection | 2016 | Improves glycemic control |
| Soliqua 100/33 | Insulin glargine and lixisenatide injection | 2016 | Improves glycemic control |
| Haegarda | C1 Esterase Inhibitor Subcutaneous [Human] | 2017 | Prevents Hereditary Angioedema attacks |
| Udenyca | pegfilgrastim-cbqv | 2018 | Decreases the incidence of infection |
| Fulphila | pegfilgrastim-jmdb | 2018 | Decreases the incidence of infection |
| Palynziq | pegvaliase-pqpz | 2018 | Decreases blood phenylalanine concentrations |
| Reblozyl | luspatercept-aamt | 2019 | Treats anemia |
| Ziextenzo | pegfilgrastim-bmez | 2019 | Decreases the incidence of infection |
| Nyvepria | pegfilgrastim-apgf | 2020 | Decreases incidence of infection |
| Semglee | Insulin glargine injection | 2020 | Improves glycemic control |
| Qwo | collagenase clostridiumhisto lyticum-aaes | 2020 | Treats cellulite in the buttocks |
| Sogroya | somapacitan-beco | 2020 | Treats growth hormone deficiency |
| Trulicity | dulaglutide | 2020 | Improves glycemic control and reduces risk of adverse cardiovascular events |
| Sogroya | somapacitan-beco | 2020 | Treats growth hormone deficiency |
| Skytrofa | lonapegsomatropin-tcgd | 2021 | Treats growth hormone failure |
| Besremi | ropeginterferon alfa-2b-njft | 2021 | Treats polycythemia vera |
| Rezvoglar | Insulin glargine-aglr | 2021 | Improves glycemic control |
| Semglee | Insulin glargine-yfgn | 2021 | Improve glycemic control |
| Fylnetra | pegfilgrastim-pbbk | 2022 | Reduces incidence of infection |
| Stimufend | pegfilgrastim-fpgk | 2022 | Reduces incidence of infection |
| Rolvedon | eflapegrastim-xnst | 2022 | Reduces incidence of infection |
| Erelzi | etanercept-szzs | 2022 | Treats arthritis, plaque psoriasis, and ankylosing spondylitis |
| Intravenous | |||
| Kanuma | sebelipase alfa | 2015 | Treats Lysomal Acid Lipase deficiency |
| Vonvendi | von Willebrand factor (Recombinant) | 2015 | Treats and controls bleeding episodes |
| Adynovate | Antihemophilic Factor (Recombinant), PEGylated | 2015 | Treats and controls bleeding episodes |
| Coagadex | Coagulation Factor X (Human) | 2015 | Treats and controls bleeding episodes |
| Nuwiq | Antihemophilic Factor (Recombinant) | 2015 | Treats and controls bleeding episodes |
| Anavip | Crotalidae Immune F(ab’)2 (Equine) | 2015 | Manages North American rattlesnake envenomation |
| Ixinity | Coagulation factor IX (Recombinant) | 2015 | Treats and controls bleeding episodes |
| Clevecord | HPC, Cord Blood | 2016 | Procedures involving hematopoietic progenitor cell transplantation |
| Afstyla | Antihemophilic Factor (Recombinant) | 2016 | Treats and controls bleeding episodes |
| Kovaltry | Antihemophilic Factor (Recombinant) | 2016 | Treats and controls bleeding episodes |
| Idelvion | Coagulation Factor IX (Recombinant), Albumin Fusion Protein | 2016 | Treats and controls bleeding episodes |
| Mepsevii | vestronidase alfa-vjbk | 2017 | Treats Mucopolysaccharidosis VII |
| Yescarta | axicabtagene ciloleucel | 2017 | Treats refractory or relapsed large B-cell lymphoma |
| Kymriah | tisagenlecleucel | 2017 | Treats refractory or relapsed large B-cell lymphoma |
| Fibryna | Fibrinogen (Human) | 2017 | Treats and controls bleeding episodes |
| Rebinyn | Coagulation Factor IX (Recombinant), GlycoPEGylated | 2017 | Treats and controls bleeding episodes |
| Asparlas | calaspargase pegol - mknl | 2018 | Treats acute lymphoblastic leukemia |
| Elzonris | tagraxofusp-erzs | 2018 | Treats blastic plasmacytoid dendritic cell neoplasm |
| Albuminex 25% | Albumin (Human) | 2018 | Maintains and restores circulating blood volume |
| Andexxa | Coagulation factor Xa (recombinant), inactivatedzhzo | 2018 | Reverses apixaban and rivaroxaban |
| Myxredlin | Insulin human in sodium chloride injection | 2019 | Improves glycemic control |
| Zolgensma | onasemnogene abeparvovecxioi | 2019 | Treats spinal muscular atrophy |
| Esperoct | Antihemophilic factor (Recombinant), glycopegylated-exei | 2019 | Treats and controls bleeding episodes |
| Sevenfact | Coagulation Factor VIIa (Recombinant)-jncw | 2020 | Treats and controls bleeding episodes |
| Tecartus | brexucabtagene autoleucel | 2020 | Treats refractory or relapsed acute lymphoblastic leukemia and mantle cell lymphoma |
| Nexviazyme | avalglucosidase alfa-ngpt | 2021 | Treats Pompe disease |
| Abecma | idecabtagene vicleucel | 2021 | Treats refractory or relapsed multiple myeloma |
| Ryplazim | plasminogen, human-tvmh | 2021 | Treats plasminogen deficiency type 1 |
| Breyanzi | lisocabtagene maraleucel | 2021 | Treats large B-cell lymphoma |
| Kimmtrak | tebentafusp-tebn | 2022 | Treats HLA-A*02:01 positive patients |
| Nuwiq | Antihemophilic Factor (Recombinant), rAHF | 2022 | Treats and controls bleeding episodes |
| Carvykti | ciltacabtagene autoleucel | 2022 | Treats refractory or relapsed multiple myeloma |
| Zynteglo | betibeglogene autotemcel | 2022 | Treats patients with β-thalassemia |
| Xenpozyme | olipudase alfa-rpcp | 2022 | Treats acid sphingomyelinase deficiency |
| Skysona | elivaldogene autotemcel | 2022 | Slows neurological dysfunction progression |
| Hemgenix | etranacogene dezaparvovecdrlb | 2022 | Treats Hemophilia B |
Vaccines, monoclonal antibodies and their fragments, diagnostics, biologics voluntarily revoked, and products that did not have a proprietary name were excluded.
Oxervate is an ophthalmic biologic medication for topical ophthalmic usage.
2.2. Unaffordability of protein drugs
Strikingly, the cost of per capita prescription of drugs in the U.S. is the highest in the world [27]. From 2008 to 2021, the median launch prices of drugs first marketed increased by $177,892, or by 8,411%. The interquartile range of biological product prices ranged from $18,861 to $288,759 during this same timeframe [28]. This median price does not include prohibitively expensive gene therapy drugs. For example, hemophilia A drug Roctavian costs $2.9 million per patient [29] and hemophilia B Hemgenix costs $3.5 million per patient [30]. Additionally, clinical trials can cost hundreds of millions of dollars and have an inherent risk component [31,32], which acts as a barrier to drug commercialization and contributes to the high cost of drug products. The estimated cost to develop a new biological product is ~$2.6 billion [31,33]. As drug prices continue to rise, many countries have sought to implement a drug price transparency initiative to better maintain affordability and control prices. However, these initiatives have not guaranteed a reduction in the price of the drug [34].
The Patient Protection and Affordable Care Act of 2010 facilitates the development of biological products that are “biosimilar” to FDA-approved biologics and are given a shorter drug review and licensure process [35]. Compared to the reference biologic, biosimilar products generally are priced around 28%, 30%, and 60% lower in the European Union, Japan, and China, respectively [36]. Unfortunately, the complexity of patent disputes, regulations, and litigations has resulted in biosimilar products being withheld from the market [37,38]. The lack of availability of these more affordable drug options in the U.S. has prevented market competition [38]. This competition is necessary to lead to less expensive drug options. For example, as multiple manufacturers create generic medications, the drug is listed at a fraction of the price of the brand-name product. More specifically, the price of a drug product decreases to around 55%, 33%, and 13% of the reference product when the medications are created by two, five, and 15 generic manufacturers, respectively. When this was done for the drug metformin (small molecule), approximately a 92% decrease in drug price was observed, leading to a much more affordable and accessible product [39]. However, such significant price declines have not been achieved with protein products. When Semglee, the first FDA-approved biosimilar insulin product, was approved, it was listed at 95% of the price of Lantus [40]. Despite a predicted savings of $44.2 billion in the U.S. from 2014 to 2024, the introduction of biosimilar products has almost had the opposite effect. For example, a biosimilar product to adalimumab resulted in a sudden and drastic 73% increase in the price of this drug [41]. Therefore, many factors have accounted for the high cost of drug products in the U.S., and urgent attention and action are required from policymakers and scientists to ensure more equitable and accessible medical treatment. The technological revolution has dramatically lowered the price of communication via phone or Skype or Internet in the past two decades, with dramatic improvements in speed and service quality. In order to achieve such affordability for biologics, new modes of protein drug production and delivery are urgently needed.
2.3. Delivery Methods
During the last eight years, only eight approved biological products that met this review’s inclusion criteria were non-invasive, and these non-invasive drugs could be administered in one of three ways: topical (includes ophthalmic), sublingual, or oral. Topical delivery was the most common (50%) of these types, followed by both sublingual (25%) and oral drug delivery pathways (25%). Of the four topically delivered biologics, only one followed the ophthalmic pathway. Furthermore, although drug delivery through the oral cavity has been a popular noninvasive method to deliver drugs since 2015, it only entered the drug delivery space in 2020. The proportion of non-invasive biologics approved to total biologics approved each year was around 6%, 0%, 17%, 8%, 0%, 10%, 20%, and 7% in 2015, 2016, 2017, 2018, 2019, 2020, 2021, and 2022, respectively. Therefore, a trend in proportionality cannot be established. Similarly, despite being safer overall and leading to higher patient compliance and satisfaction rates, the actual number of non-invasive biologics approved does not have a clear or increasing trend by year. Strikingly, since 2015, there has not been a year that more than two non-invasive biological products were approved, and the maximum proportion of non-invasive to total biologics approved in a year peaked at less than 20% in 2021. Additionally, over the past eight years, biological products approved in 2017, 2021, and 2022 showed the most variation in delivery methods, with six different delivery pathways existing among the 41 approved biologics.
Moreover, all insulin products in this review require an injection for administration, with subcutaneous injection being the most popular way to deliver the drug and intravenous being the only other delivery method. All coagulation factor complexes, drugs designed to treat cancer, and many other biologics also require an injection for drug delivery. Overall, >90% of approved biologics are invasive. Interestingly, the non-invasive biological products approved since 2015 have been centered around preventing allergies and controlling excessive bleeding during surgery. For example, of the four biologics that are administered topically, two control bleeding (Raplixa and Fibrin Sealant) and one (StratGraft) treat thermal burns. Only one biologic can be administered ophthalmically (Oxervate), and it is used to treat neurotrophic keratitis. Similarly, both biologics delivered through sublingual tablets either control allergies induced by house dust mites (Odactra) or ragweed pollen (Ragwitek). Finally, two biologics can be administered orally, with one mitigating the effects of peanut allergies (Palforzia) and the other being an oral replacement therapy to treat sucrase deficiency (Sucraid). Strikingly, 360 capsules with peanut cells (annual dose) are < 3% (~$2500) of the median annual price of biologics that were newly marketed between 2008 and 2021 ($84,508) [28,42]. Therefore, as seen over the past eight years, a non-invasive method to deliver proteins has not been achieved with great success and remains highly elusive (Fig. 1–2, Table 1).
Every year, it is estimated that >16 billion injections are administered globally [43–45], with ~95% for therapeutic reasons [43]. As shown in Fig. 1–2, Table 1 [16,21], intravenous infusion (IV) is the most bioavailable and quick method [44,46] to deliver large doses of drugs [47,48] into the systemic circulation [46]. IV infusion facilitates immediate and continuous drug delivery into the circulatory system [47–49], but the dose should be titrated very carefully. In the case of insulin, recent clinical trials reveal that insulin pens (used by >95% of diabetic patients) cause hypoglycemia and related consequences, but precise delivery of insulin doses using pumps based on blood glucose levels overcomes this problem [50,51]. Unfortunately, pumps are not affordable for most diabetic patients. In the U.S., insulin pumps cost approximately $6,000 and require an additional $3,000 to $6,000 every year for supplies [52]. With one-third of the global population earning <$2 per day, insulin pumps pose high barriers to the global population at large. Insulin delivery to the peripheral circulation by injections is a major reason for hypoglycemia [53–55] but delivering insulin to the liver overcomes this challenge. Indeed, oral insulin bioencapsulated in plant cells or enteric capsules does not lead to hypoglycemia, similar to that of natural insulin released from pancreas [56,57]. Nasal delivery of insulin failed in the clinic largely due to a limited absorption surface of around 180 centimeters2 [58]. Moreover, intranasal insulin has a low bioavailability of 15–25%, has an onset of action of around 20 minutes (mins), and can cause blistering, irritation to the nasal cavity, and redness [59–61]. Due to its low bioavailability, larger insulin doses are required compared to subcutaneous methods, leading to higher patient costs [62,63]. In contrast, the large mucosal area of the human small intestine (30 meters2) offers the greatest surface area for protein drug absorption and delivery [64].
The medications that are administered subcutaneously use a bolus and are delivered into the subcutis [44]. Subcutaneous injections (SC) result in a rapid onset of action and high bioavailability (80% for insulin) [58,65] but are constrained by a limited volume of delivery (maximum of 1.5 milliliters (mL)) [66,67] and intra-individual and inter-individual variation [68–70]. The rate of insulin absorption varies depending on the site of injection. A barrier to subcutaneous insulin absorption is passing through the extracellular matrix [70] without binding to matrix proteins, like type V collagen [71].
Intramuscular delivery is most commonly used [72], comprising >75% of all injections performed around the world [45,73]. In injecting a medication intramuscularly, the drug is delivered within a person’s highly vascularized muscles, allowing for uniform and rapid absorption by the bloodstream and incorporation into the systemic circulation [44,72]. The onset of action for intramuscular injection (IM) delivery ranges from 5 to 10 mins [74], faster than SC, can deliver larger volumes, and avoids first-pass metabolism [72]. However, some disadvantages of IMs include (i) constriction due to small drug volumes (1–2 mL for the deltoid site and 5 mL for the quadriceps site), (ii) periostitis, (iii) bleeding, (iv) haematoma, (v) tissue necrosis, (vi) infection, (vii) contractures, (viii) abscess, (ix) nerve and vascular injury, (x) muscle fibrosis, (xi) gangrene, (xii) skin slough, and (xiii) pain [72,75–77].
Oral delivery of biologics is one of the most challenging drug delivery methods to develop but is highly preferred by patients. The first challenge is to protect protein drug digestion from acids and enzymes in the stomach. Therefore, protein drugs should be bioencapsulated in materials that could protect them from acids and enzymes. One of the drugs approved by FDA (Palforzia) (Table 1), the therapeutic protein (Ara h), was protected via bioencapsulation within peanut plant cells. Biologics bioencapsulated in plant cells are protected from the human digestive system because digestive enzymes cleave alpha linkages but plant cell wall polymers are linked by beta 1,4 −1,6 bonds [78–80]. The next challenge is the lysis of plant cells to release protein drugs in the gut lumen. This is naturally done by gut microbes because they release enzymes that cleave beta linkages of plant cell wall polymers [81,82]. The final challenge is the delivery of therapeutic proteins across the gut epithelium into the immune or circulatory system. While a few proteins like Ara h may recognize human cell receptors, most proteins require tags to cross the gut epithelium. Several such tags are developed and engineered to be cleaved off using proteases present ubiquitously during transport and confer desired therapeutic goals upon oral delivery of protein drugs [80,82–85].
3. Topical drug delivery
The non-invasive drug delivery methods that are compliant and safe for the patients are getting more attention recently. Topical delivery has the unique advantage of delivering higher doses to the target site, without getting diluted by the large volume of blood in circulation and degraded by several proteases. The topical delivery method facilitates the delivery of a mixture of proteins, which is not feasible for systemic delivery due to unfavorable immune response or potential toxicity. Proteins or peptides are known for their high specificity and potency [86] and are therefore preferred over non-specific small molecules or chemical agents. When small molecules are unable to penetrate extracellular polymeric substances (EPS) in the dental biofilm/plaque, topical delivery of enzymes is ideal to disrupt EPS and increase access to antimicrobials and kill pathogenic microbes [87,88]. Topical drug delivery interventions such as prophylaxis measures of infections in oral cancer patients undergoing radio or chemotherapy become more important [89]. These immunocompromised patients are more prone to oral cavity infections [90] because of hyposecretion of saliva [89]. Mucositis (tissue swelling and irritation in mouth) and neutropenia (too few neutrophils) further facilitate bacterial and fungal pathogen infection in the oral cavity of these patients. Bacterial infections predominate during the early phases of a neutropenic episode, while fungal infections are more common with prolonged neutropenia [91,92]. Among the bacterial pathogens, gram-positive are more common [90], while among the fungal pathogens, Candida albicans (C. albicans) are more prominent [89]. Although small molecules are used in the clinic [89,93], lack of specificity or inability to penetrate biofilm are major challenges. Furthermore, the duration of oral rinse or mouthwash is very short and ineffective in killing pathogens protected by EPS secreted by pathogens.
Saliva contains diverse pathogenic viruses, including severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and salivary droplets, and aerosols are the prime agents for the transmission of these viruses. Pathogens in the aerosolized state can persist for a prolonged time period and could be infectious to nearby patients or healthcare providers. The use of mouth rinses before the dental procedure is recommended to reduce the viral load and possible spread of infections [94]. Unfortunately, clinical evaluation of mouth rinses did not reduce the viral load at statistically significant levels [95]. The chewing gum delivery method has been recently explored for the targeted delivery of anti-viral proteins/peptides and to enhance the duration of their delivery to neutralize oral viruses.
3.1. Chewing gum
Chewing gum production has flourished and is expected to continue growing, with many Americans chewing gum on a weekly basis [96]. Chewing gums can contain various nutrients and active pharmaceutical ingredients [97,98]; while chewing these gums, the nutrients and active drug ingredients can be absorbed by both the oral mucosa and gastrointestinal tract, providing local and systemic delivery pathways [99]. Chewing gums containing small molecules are a highly attractive therapeutic and preventative treatment option as they conveniently offer a way for patients to discreetly consume medication, have an agreeable taste, promote swift drug absorption, and support patient compliance even among young children and those with difficulties swallowing [99,100]. Moreover, chewing a piece of gum can increase a person’s alertness [101] and memory [101,102], prevent dental caries [103] and dry mouth [104], improve a person’s mood [101,102,105], and reduce hunger cravings [106]. To name a few, there are commercially available plant-produced small molecules, such as aspirin [107], nicotine [108], vitamin C [109], and Xylitol [110]. However, there has yet to be an effective chewing gum impregnated with target proteins for drug delivery until recently [4–6,88].
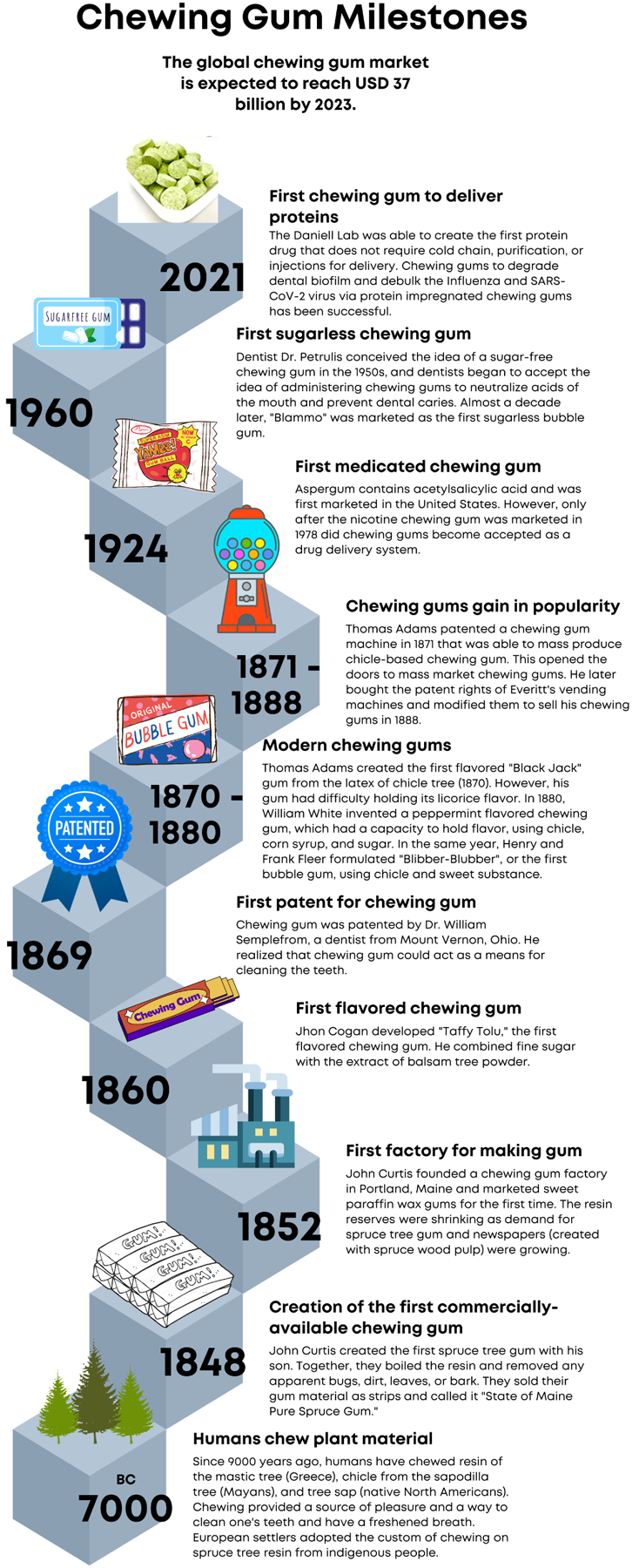
3.2. Chewing Gum History
The expanding success of the $25 billion global chewing gum market [111] has involved over 9000 years of history and experimentation. Transitioning from chewing resin and sap directly from the tree [111–115], chewing gum was first commercialized in the mid-1800s [111,113,115] and quickly gained traction. This growing popularity [116,117] can be attributed to many factors, including (i) the creation of a chewing gum factory [113,118], (ii) the introduction of flavored chewing gums [111], (iii) the desire to patent the chewing gum [115,119,120], and eventually, (iv) the invention of the modern chewing gum [111,117]. Almost a century after chewing gum was first commercialized by John Curtis, small molecules were incorporated into chewing gums as a method of topical delivery [98]. A few decades later, sugarless chewing gums were invented to curb the onset of cavities [115]. More recently, many have sought to develop chewing gums with proteins, such as insulin, to deliver protein drugs orally. Even so, most of the protein drug has been lost due to degradation by the acids and enzymes of the stomach [121]. However, when insulin is bioencapsulated in plant cells, protein drug delivery is effective [57,122] as plant cell wall ꞵ 1,4 and ꞵ 1,6 linkages protect protein drugs from digestion [82]. The Daniell Lab has been able to successfully incorporate plant cell proteins into chewing gums to prevent dental caries and debulk the SARS-CoV-2 and Influenza viruses alike [4–6,88]. On May 31, 2022, the Daniell Lab received FDA/Investigational New Drug (IND) approval (IND 154807) and became the first to engineer a plant-based protein drug that does not require purification, can be delivered orally, and is free of the restraints posed by cold chain products. A timeline representing the milestones of chewing gum is highlighted in Figure 3 [4,5,14,88,98,111–120].
Figure 3:
3.3. Chewing gum delivery for prevention and treatment of diseases and beyond
Plant-derived protein therapeutics offer one possible sustainable solution to combat biologic unaffordability due to its low production cost, ease to scaleup, and elimination of cold chain for storage and transportation. Most recently, vaccines against the current pandemic were produced in tobacco (Phase I- [123]; Phase III – [124]). Although this addressed cost at the early stages of production, the purification of vaccine antigen and cold chain required for storage and transportation did not significantly lower the cost of this vaccine. However, alternate approaches are currently advanced to the clinic for complete elimination of the cost of fermentation, purification, cold storage, and transportation. Several biologics expressed in chloroplasts are now advancing to the clinic: angiotensin converting enzyme 2 (ACE2) to prevent SARS-CoV-2 infection and transmission is currently in Phase I/II clinical trials [4–6] for topical drug delivery using the chewing gum containing freeze-dried plant cells. Preclinical studies are in progress for oral drug delivery through the protection of therapeutic proteins in the digestive system via bioencapsulation in plant cells [80,82,84].
Topical delivery of plant-derived pharmaceuticals is believed to be a promising candidate for anaphylactic and pre-prophylaxis or post-prophylaxis measures against food allergens and infectious diseases that are particularly uncurable. Developments in these perspectives are compiled in Table 2. Recent successful completion of Phase III trials of peanut proteins to manage anaphylactic reactions in toddlers of one to three years through the topical delivery route [10] is one of the most promising efforts. The patches loaded with peanut proteins were safe and efficacious in toddlers in inducing tolerance against peanut allergens. Peanut proteins delivered through the topical route in patches were safe and effective in preventing allergy in Phase III clinical trials [11]. It is envisaged that similar technologies could be developed against other food allergens (egg and milk allergens). Development of topical delivery interventions is highly relevant to induce tolerance against food allergens because the delivery of allergens at an early stage of life is more efficacious in tolerance inductions against food allergens, and therefore, the topical delivery method is ideal for infants. Significant efforts in topical delivery interventions have also been recognized for the prevention of sexually transmitted diseases like the acquired immunodeficiency syndrome (AIDS) and Herpes Simplex Virus (HSV) mediated diseases. Several promising anti-viral lectin molecules (carbohydrate or glycoprotein binding) like PC-6500 (Griffithsin lectin of red algae expressed in tobacco), scytovirin and cyanovirin lectins (expressed in Oryza sativa), engineered banana lectin, etc. are expressed recombinantly in an intension to be delivered topically through the vaginal route as an important component of the microbicides [125–129]. The safety, pharmacokinetics, and pharmacodynamics of the plant lectin griffithsin formulated in carrageenan vaginal gel have been evaluated in healthy women in a Phase I clinical trial (NCT02875119).
Table 2:
Plant-made or synthetic peptides used as biologics for topical delivery.
| Candidate molecule/active ingredient | Class of molecule | Mode of action | Plant host | Type of expression (heterologous) | Disease/target | Product development stage (Phase I/II/III/date/status) | Treatment regimen | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Peanut allergy | ||||||||
| AR101 | Peanut-protein | Tolerance induction | Peanut | NA | Peanutallergy | Phase III (NCT03211247) | Viaskin peanut-protein-containing patches | [10] |
| COVID-19 | ||||||||
| CTB-ACE2 | Angiotensin converting enzyme-2 | Virus trap | Lactuca sativa | Stable | SARS-CoV-2 | Phase I/II (NCT05433181) | Chewing gum | [4,5] |
| FRIL | Lectin | Virus trap | Lablab purpureus | NA (Natural plant source) | SARS-CoV-2 | -- | Chewing gum | [5] |
| Q-GRFT | EngineeredGriffithsi n (lectin) | Inhibitory activity against SARS-CoV-2 | N. benthamiana | Transient | SARS-CoV-2 | Phase I (NCT05180500; NCT05122260; NCT05437029) | Nasal spray | https://clinicaltrials.gov/study/NCT05180500?term=NCT05180500&rank=1; [242] |
| AIDS | ||||||||
| PC-6500 | Griffithsi n (lectin) | Inhibitory activity against HIV | N. benthamiana | Transient | HIV | Phase I (NCT02875119) | Griffithsin formulatedin carrageena n vaginal gel | [126] |
| Anti-biofilm/plaque/gingivitis | ||||||||
| KSL-W | Antimicr obial peptide | Reducing plaque and gingivitis | NA | NA | Plaque and gingivitis | Phase I/II (NCT01877421; NCT02864901) | Topical oral formulation/Chewing gum | httos://classic.clinicaltrials.sov/ct2/show/NCT01877421; [150] |
| GFP-PG1 | Antimicr obial peptide | Killing of S. mutans | N. tabacum | Stable | S. mutans | Topical oral formulation/Chewing gum | [87] | |
| GFP-RC101 | Antimicr obial peptide | Killing of S. mutans | N. tabacum | Stable | S. mutans | Topical oral formulation/Chewing gum | [87] | |
| Lipase | Esterase enzyme | Inhibit transition of C. albicance yeast to filamento us form | Lactuca sativa | Stable | C. albicance | Topical oral formulation/Chewing gum | [88] | |
| Dextranase | Glucanase enzyme | Degrade dental biofilm matrix | Lactuca sativa | Stable | Biofilm matrix | Topical oral formulation/Chewing gum | [88] | |
| Mutanase | Glucanase enzyme | Degrade dental biofilm matrix | Lactuca sativa | Stable | Biofilm matrix | Topical oral formulation/Chewing gum | [88] | |
Several recombinantly expressed monoclonal antibodies (mAb) expressed in plants, including 2G12, Anti-α-C-C chemokine receptor type 5 (anti-α-CCR5), bNVRC01, and 2F5 (expressed in Zea mays) have been evaluated for passive immunization against the Human Immunodeficiency Virus (HIV) infection [130–132]. Among all explored monoclonal antibodies, Anti-αCCR5 and 2G12 are at the drug development stage of preclinical and clinical trial Phase I, respectively. A double-blind, placebo-controlled, randomized, dose-escalation Phase I Safety Study of protein drug product 2G12 as a single vaginal administration in healthy female subjects was evaluated in 2011 (NCT01403792) but has not advanced further into the clinic. Griffithsin expressed in tobacco and anti-HSV-2 mAb expressed in soybean have been demonstrated for anti-HSV activity [129,133]. Recent developments in the topical delivery approach using chewing gums containing biologics expressed in plant cells to prevent and treat oral diseases received significant attention from the scientific community, as discussed in this section.
3.3.1. Oral diseases
Worldwide, dental caries is the most prevalent human disease [134,135], afflicting upwards of the vast majority of both adults and children [136,137] and disproportionality affecting those living in poverty [138] and people of color [134,136]. Dental caries is a microbial disease caused by a bacterial infection [135] from the transmissible bacteria Streptococcus mutans (S. mutans) [134], with the word caries deriving from the Latin word “decay” or “rot” [139]. Tooth decay typically originates at the proximal or occlusal surfaces of a tooth [134] after consumption of carbohydrates and sugars. In this, the bacterium in plaque produces acids and leads to repeated cycles of demineralization of the inorganic component of the tooth and the simultaneous breakdown of the organic part of the calcified dental tissue [140]. Moreover, in addition to dental caries, the accumulation of dental biofilm leads to periodontal and gingival disease [141–144]. The most popular method to mitigate these effects and remove plaque is by manually brushing one’s teeth, however, human brushing habits are often highly variable and ineffective [145–147]. Therefore, additional measures to prevent dental caries are needed.
Treatment or prevention of oral diseases becomes necessary because these conditions are not only restricted to the oral cavity, but dental biofilm in advanced stages, like in the periodontal pocket, is sometimes responsible for bacteremia. It accumulates in the endothelial cells and causes cardiovascular diseases. Inflammatory responses against dental caries pathogen transfers to other organs; as a result, dental caries is a risk factor for other auto immune or inflammatory diseases, including Diabetes mellites. It is reported that C. albicans prevalence in children with childhood caries is related to the prevalence of C. albicans in mothers; this suggests that oral hygiene matters not only for the present generation, but also for the next generation. Taking into consideration topical delivery interventions of plant-derived molecules, like monoclonal antibodies, Guy’s 13 SIgA/G (Secretory IgA -antibody against cell surface adhesion (antigen I/II) molecule of S. mutans expressed in tobacco) and Anti-FimA (fimbrial protein fimbrillin A of Porphyromonas gingivalis), and antimicrobial peptides (Protegrin and Retrocyclin expressed in tobacco effective against S. mutans) have been developed and demonstrated for efficacy [87,148,149]. The developed monoclonal antibodies and microbial peptides are targeted for single microbial species while dental biofilm or other oral complications are derived from multispecies or multi-kingdom microbial partners. Moreover, dental biofilm is a complex structure in which microbes are embedded inside the EPS matrix and are protected from the exposure of applied antimicrobial components. For the efficacy of the antimicrobial agent, the matrix needs to be first degraded.
For example, Singh et al. [88] developed recombinant enzymes dextranase, mutanase and lipase in lettuce for topical delivery as chewing gum formulations and demonstrated anti-biofilm efficacy of plant-produced molecules in vitro against cross-kingdom microbes (S. mutans and C. albicans). The dextranase/mutanase enzyme produced in lettuce chloroplasts efficiently degrades the EPS matrix that resulted in a dispersed bacterial colony of S. mutans in the biofilm. Interestingly, lipase produced in lettuce was able to inhibit the transition of C. albicans yeast form to filamentous form in the biofilm. Interestingly, dextranase/mutanase and lipase topical application together were able to kill both bacterial and fungal partners (killed pathogens are visualized as magenta color in the treatment while alive bacteria in green and fungus in cyan color in the untreated sample after staining with suitable dye) in the bacterial-fungal mixed kingdom biofilm (Fig. 4). In this study, authors demonstrated the suitability of freeze-dried plant powder as chewing gum formulations. The plant powder impregnated into the chewing gum released the protein in a time-dependent manner in a mechanical simulator device in conditions simulated as an oral cavity. Most importantly, proteins in the chewing gum remained stable when stored at ambient temperature for several years. Chewing gum formulations using antimicrobial peptides have also been explored for better oral health or anti-plaque potential. Two clinical trials investigated the safety of antimicrobial gum formulation (NCT02864901; NCT01877421). Efficacy was not the primary endpoint of NCT01877421 and therefore, the study was not powered for determination of a statistically significant efficacy outcome but suggested that the antimicrobial peptide could be effective but with limited sample size or short duration of 4 days [150]. The trial NCT02864901 was designed for longer durations (14, 28, 34 days) but antimicrobial gum did not meet the anticipated efficacy. One of the key limitations of these studies would be the inability of antimicrobial peptides to penetrate the EPS secreted by colonizing microbes. Therefore, therapeutic delivery of proteins is still elusive. Pedersen et al. [151] examined the efficacy of Lactobacillus reuteri containing lozenges for severity of recurrent aphthous ulcer in another pilot study (NCT02976922). Although reduced severity of aphthous ulcer after daily administration of L. reuteri up to 90 days was observed, the improvement was not significantly better than the placebo.
Figure 4: Demonstration of plant-made (Pm) protein therapeutics as chewing gum formulations and topical delivery to control growth of human pathogens responsible for various infectious diseases.
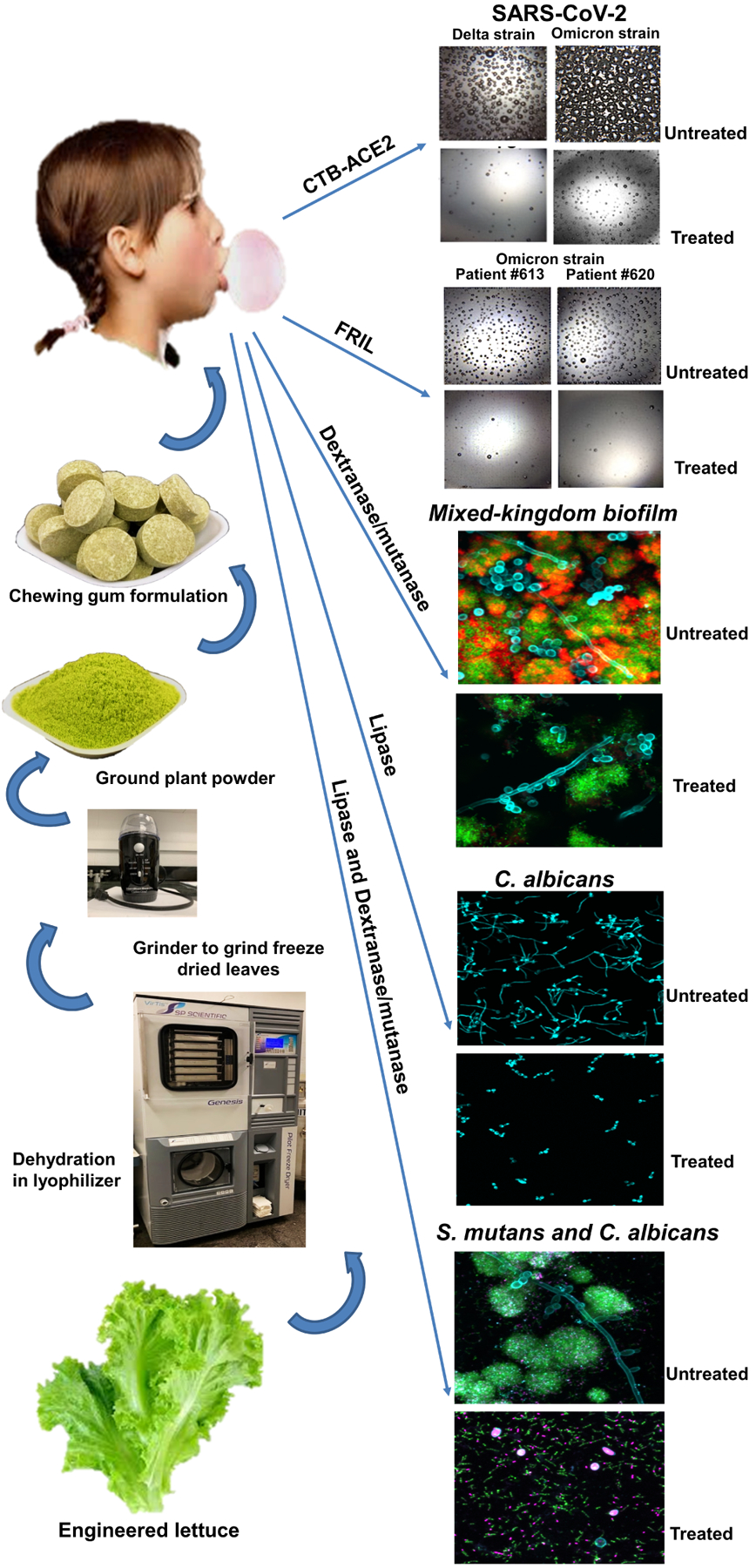
The engineered lettuce producing protein therapeutics is grown in a contained environment where the leaves are harvested, dehydrated in lyophilizer, and ground into plant powder. This powder is then incorporated into chewing gums for topical drug delivery (left panel). The Pm protein therapeutics impregnated in chewing gums are able to control the growth of human pathogens of different domains, such as viruses (SARS-CoV-2 Delta and Omicron strains), bacteria (S. mutans), and fungi (C. albicans) (right panel) [4,5,88].
3.3.2. COVID-19 pandemic
On March 11, 2020, the World Health Organization (WHO) declared COVID-19 to be a global pandemic [152], with unprecedented health and economic burdens following [153–155]. As of early June 2022, the WHO has reported over 529 million cases of COVID-19 and more than 6.5 million deaths globally. By early June of 2023, more than 238 million more cases of COVID-19 were reported, increasing the number of deaths by nearly half a million people [155]. Moreover, poor and worsening psychiatric conditions, burnout, and substance use disorder among the public and healthcare professionals alike have been exacerbated by the pandemic [156–159]. The pandemic has also induced immense economic strain, affecting globalization, trade, travel, food, agriculture, academia, healthcare, business, and tourism, ultimately instigating the crash of many financial markets [154]. The total estimated cost of the pandemic is $16 trillion and growing [153], which is more than the 2020 gross domestic product for the entire European Union [160]. Among underserved racial, ethnic, and socioeconomic populations, these effects are even more pronounced, leading to severe global health disparities [161].
COVID-19 is a highly transmissible respiratory illness, with a transmission rate of around 2.2 [162]. Moreover, with almost all the latest COVID-19 diagnostic targets facing mutations [163], it is estimated that the annual rate of nucleotide mutation in the SARS-CoV-2 genome is approximately 6.7 × 10−4 substitutions per site [164]. Furthermore, the targets of the SARS-CoV2 nucleocapsid (N) gene probes and primers undergo most mutations [163,165,166]. Heightened rates of transmission have been observed among close-contact communities while singing [167], speaking, breathing, sneezing, and coughing [168–170], highlighting the intimate role the oral and nasal cavity play in the transmission, replication, and infection of the SARS-CoV-2 virus [171–173]. Additionally, the urgency to debulk pathogens in the oral cavity is further highlighted as there are more ACE2 receptors in the salivary glands than that in the lungs, the most common transmission pathway comes from salivary droplets, and the virus is abundant in the oral cavity during the first 10 days of infection [174].
As of May 2022, the WHO recommends social distancing, mask-wearing, hand washing, and quarantining to prevent the onset and mitigate the effects of COVID-19 [175]. However, an affordable, complete, safe, and convenient measure to decrease both the rate of transmission and reinfection is not yet available. The Daniell Laboratory has sought to engineer a novel treatment to prevent viral transmission and reinfection reliably and effectively by decreasing the viral load of the SARS-CoV-2 virus in the oral cavity and throat area, the primary sites of viral replication and infection [174,176]. To target the oral cavity, chewing gum containing anti-viral proteins expressed in plant cells served as a novel topical delivery system [4–6].
3.3.3. Recombinant CTB-ACE2 and FRIL chewing gum for topical delivery:
Recombinant therapeutic protein CTB-ACE2 (Cholera toxin B subunit – Angiotensin Converting Enzyme 2) produced in lettuce chloroplast is considered a potential drug candidate to prevent infection or reinfection of the SARS-CoV-2 virus through the oral cavity when used as a chewing gum formulation. Moreover, CTB-ACE2 administration is also helping patients to prevent secondary complications as an outcome of renin-angiotensin aldosterone dysregulation. In a study, Daniell et al. [4] found that the lettuce produced CTB-ACE2 as chewing gum formulation debulked SARS-CoV-2 in swab/saliva samples of COVID-19 patients. The debulking potential of CTB-ACE2 was confirmed experimentally by microbubble SARS-CoV-2 antigen assay (Fig. 4) and quantitative polymerase chain reaction (qPCR). When CTB-ACE2 was incubated with vesicular stomatitis virus spike (VSV-S) pseudo typed virus particle, the authors found spike-mediated entry into the Vero E6 cells was inhibited. This means that CTBACE2 formulated as chewing gum can prevent reinfection in patients through the oral route and in general transmission to others by acting as a trapping agent and inhibiting viral entry into the host cells. Interestingly, during chewing gum formulation, the CTB-ACE2 protein remained functionally active. Moreover, CTB-ACE2 chewing gum can be stored at ambient temperature for several years. As an advancement of the study, authors demonstrated the efficacy of CTBACE2 chewing gum in another SARS-CoV-2 strain Omicron [5] that is considered even more contagious. The chewing gum formulated CTB-ACE2 debulked SARS-CoV-2 strain Omicron in the nasopharyngeal (NP)/oropharyngeal (OP) swab samples. The debulking potential was confirmed by microbubble SARS-CoV-2 antigen assay (Fig. 4) and Real-time Accurate Portable Impedimetric Detection (RAPID) assay. The FDA has approved the evaluation of CTB-ACE2 chewing gum to decrease SARS-CoV-2 infection and transmission in Phase I/II clinical trials (IND 154807, NCT05433181). In addition, the authors showed the anti-viral efficacy of the Flt3 Receptor Interacting Lectin (FRIL) from lablab bean through neutralization of different strains of SARS-CoV-2 (omicron, delta strains). FRIL chewing gum also neutralized potent Influenza virus strains H1N1 and H3N2 in plaque reduction assay. Recently, another plant-derived lectin, i.e. engineered griffithsin (Q-Griffithsin, single substituted amino acid increased resistance to oxidation and enhanced stability), was evaluated for topical application as a nasal spray to prevent SARS-CoV-2 infection. The safety, acceptability, pharmacokinetics, and tolerability of Q-Griffithsin have been successfully evaluated in Phase I clinical trial (NCT05180500).
Another promising topical delivery formulation against SARS-CoV-2 is the nasal spray. One of the limitations of currently approved mAbs delivered through the parenteral route is suboptimal delivery due to the poor transportation of these large biologics across the respiratory epithelium to the airways. Therefore, monoclonal antibodies have been formulated as a nasal spray for local neutralization action when delivered topically. Monoclonal antibody SA58, identified from a large collection of broad sarbecovirus neutralizing antibodies isolated from SARS-CoV-2-vaccinated SARS convalescents [177] has been explored for post-exposure prophylaxis against COVID-19 [178,179]. In a randomized, single-blind, placebo-controlled clinical study (NCT05667714), nasal spray of SA58 was efficacious and safe in preventing symptomatic COVID-19 infection in adults who had exposure to SARS-CoV-2 within 72 hours [178]. The safety and effectiveness of the SA58 nasal spray were evaluated in healthcare workers at high risk of SARS-CoV-2 infection (NCT05664919) [179]. Nasal spray formulated with 35B5 monoclonal antibody provided protection against different variants of concern, including Alpha, Beta, Delta, or Omicron variants in a clinical trial not registered in the federal website (ClinicalTrials.gov), but registered locally (2022-005-02-KY) [180]. Nasal spray formulated with a cocktail of two monoclonal antibodies 55A8 and 58G6 neutralized SARS-CoV-2 Omicron variant BA.4/5 in a clinical trial not registered in the federal website (ClinicalTrials.gov) (ChiCTR2200066525) [181].
4. Nanoparticle-based topical delivery in the oral cavity
4.1. Overview of Nanoparticles
4.1.1. Importance of nanoparticles in drug delivery
The basic principles and features described for these non-protein drugs are also applicable to proteins. Nanoparticles have garnered considerable attention in the field of drug delivery with a particular emphasis on their potential for topical administration in the oral cavity. For example, nanoparticles loaded with antifungal drugs, such as fluconazole [182] and miconazole [183,184], have been used for the topical treatment of oral candidiasis, a fungal infection of the oral cavity most associated with immunosuppression. The unique physicochemical properties of nanoparticles, characterized by their minute size and large surface area-to-volume ratio, make them highly desirable for trafficking to specific tissues and facilitating efficient drug delivery. In this section, we provide an overview of the use of nanoparticles for topical drug delivery in the oral cavity, emphasizing their pivotal role in enhancing drug stability, improving drug bioavailability, enabling targeted delivery of drugs to specific tissues, and mitigating systemic toxicity.
4.1.2. Enhanced drug stability
The significance of nanoparticles in drug delivery stems from their ability to ameliorate drug stability and augment drug bioavailability within the oral cavity, a critical factor that ensures the potency of pharmaceutical formulations. Drugs encapsulated within nanoparticles can be safeguarded against degradation from factors that complicate the oral cavity environment, such as fluctuations in pH, enzymatic degradation, and mechanical stresses. Consequently, nanoparticles can function as protective carriers that shield drugs from these deleterious factors, thereby enhancing drug stability, prolonging shelf-life, and ensuring optimal drug performance [185]. For example, although spearmint oil, which contains terpene derivatives, has antitumor activities, its in vivo efficacy is very low due to poor water solubility. The use of polyoxyethylene castor oil derivatives to form oil-containing nanoemulsion droplets in water resulted in significantly improved drug stability and increased cytotoxicity against oral cancer cells [186]. Chitosan nanoparticles surface-modified with alginate and poly(ethylene glycol) (PEG) nanoparticles showed enhanced adhesion to the mucosal tissue and sustained release of ovalbumin in intestinal fluid, demonstrating their potential for vaccine delivery through the oral mucosa [187]. Nanoparticles loaded with antibiotics, such as metronidazole [188] and doxycycline [189,190], have been explored for topical treatment of periodontal disease, a chronic inflammatory condition affecting the gums and supporting tissues of the teeth. These nanoparticles can target the periodontal pockets and release antibiotics over an extended period, improving their local bioavailability and reducing the need for frequent dosing.
4.1.3. Targeted delivery of drugs to specific tissues
Nanoparticles allow for precise and targeted delivery of drugs to specific tissues in the oral cavity. Size tuning and surface modification of nanoparticles enable specific interactions with the target tissues, minimize off-target effects, and maximize therapeutic efficacy [185]. This is particularly important for the treatment of oral cancers, and nanoparticles loaded with anticancer drugs, such as paclitaxel [191], have been investigated as potential therapeutic agents. These nanoparticles can selectively accumulate in the tumor tissues and deliver the drugs directly to the cancer cells while minimizing systemic toxicity. Nanoparticles can be functionalized with ligands, such as anti-clusters of differentiation 44 (CD44), anti-epidermal growth factor receptor (EGFR), anti-αvβ6, and folate, to actively target tumor cells [192]. By delivering drugs directly to the site of action, nanoparticles can significantly reduce systemic exposure to drugs, thereby lowering the risk of systemically induced adverse effects. The ability of nanoparticles to provide localized drug delivery with minimal systemic toxicity is particularly relevant for drugs with narrow therapeutic windows or high toxicity profiles [193].
4.1.4. Alternatives to nanoparticles for drug delivery to the oral cavity
Although we have focused on the analysis of nanoparticle-based topical drug delivery in the oral cavity, there are alternative approaches that are worth mentioning, particularly microscale biomaterials such as patches, films, or microdevices. Even though these systems are not likely to penetrate the oral mucosa, they present other strengths that can still make delivery possible, such as strong adhesiveness and high loading capacity.
The use of microscale biomaterials for drug delivery to the oral cavity is another burgeoning area of research in pharmaceutical technologies. These novel delivery systems offer advantages such as controlled release, enhanced bioavailability, and targeted administration. Oral patches and films, composed of biocompatible polymers, adhere to the oral mucosa, enabling drug delivery into the bloodstream following transport across the mucosal layers, circumventing hepatic first-pass metabolism. While many engineering advances have been made to improve tolerance of these films and patches, biocompatibility issues continue to arise that lead to inflammatory immune responses and can reduce passage of active ingredients across the mucosa [194].
Various pharmacological drugs have been incorporated into oral transmucosal formations, including cardiovascular agents, sedatives, and analgesics [195]. A typical oral fastdissolving film consists of a thin adhesive strip that is placed on the patient’s tongue or any oral mucosal tissue. The strip rapidly hydrates and attaches to the site of application, which is wet by saliva [196]. The biodegradable oral films facilitate the drug delivery to the systemic circulation [195,196], which is highly beneficial for those suffering from dysphagia, repeated vomiting, hypertension, heart attack, asthma, nausea, paralysis, and mental disorders [197].
Microdevices, or microfabricated devices, can release drugs responsively, exploiting factors like pH shifts. A widely studied type of microdevices is microneedles (MNs) made of three-dimensional microstructures with microscale length (<1500 μm), designed to bypass the barrier of transdermal drug delivery. MNs can generate transient microchannels by piercing the stratum corneum, allowing for the effective delivery of drugs that are 10 to 100 times less permeable than the mucosa [198]. Another example is microcontainers (MCs), which are small devices that can be loaded with drugs and then adhered to the buccal mucosa. MCs with a chitosan coating have been shown to have good adhesion to the mucosa [199]. They can be loaded with antimicrobial peptides, which can be released over time to provide a sustained effect on the oral microbiome. These microdevice-based drug delivery systems have the potential to offer several advantages over traditional methods, such as localized and systemic drug administration, reduced side effects, and heightened therapeutic efficacy. While each of these devices may find use in specific applications, biocompatibility hurdles and patient compliance pose risks to the long-term effectiveness of these devices in many oral pharmaceutical applications [200]. Challenges encompass formulation stability, adhesion optimization, and regulatory considerations. Continued progress in materials and biotechnology will hopefully propel the advancement of these drug delivery strategies and lead to greater adoption.
4.2. Different materials and formulations of nanoparticles
Nanoparticles for topical drug delivery in the oral cavity can be prepared using various materials and formulations, each with their unique properties and advantages. In this section, we provide an overview of different types of nanoparticles commonly used for topical drug delivery in the oral cavity, including polymeric nanoparticles, lipid-based nanoparticles, dendrimers, and inorganic nanoparticles.
4.2.1. Polymeric nanoparticles
Polymeric nanoparticles are among the most extensively studied nanoparticles for topical drug delivery in the oral cavity. They include biocompatible polymers such as poly (lactic-coglycolic acid (PLGA), polyethylene glycol (PEG), and chitosan, among others. Polymeric nanoparticles offer numerous advantages, including controlled drug release, excellent biocompatibility, and the ability to encapsulate a wide range of drugs with varying physicochemical properties. Additionally, their size and surface properties can be easily tailored to achieve desired drug delivery characteristics. For example, various polymeric nanoparticles, such as alginate, polylactic acid (PLA), PLGA, and chitosan, have been used for oral topical delivery of nystatin, an antifungal drug [201–207]. When compared with the direct administration of nystatin, polymeric nanoparticles have demonstrated high encapsulation efficiency (>70%), prolonged release, and high adhesion capacity to the oral mucosa [203]. Hydrogel nanoparticles were also developed to deliver nystatin. When tested in a rat model of oral infection, nystatinencapsulated nanocapsular hydrogels resulted in a significantly reduced fungal count and eradication of infection [208]. Methotrexate (MTX), which is used to treat oral inflammatory conditions such as oral lichen planus, is another drug that has been investigated for oral topical delivery via nanoparticles. MTX-loaded PLGA nanoparticles showed a sustained release and improved therapeutic outcome in both rats and mice when compared with free MTX [209,210]. Chitosan microspheres were also associated with promising outcomes in terms of their ability to provide sustained delivery of MTX [204]. Chitosan, with its positive charge, can temporarily disrupt the mucosa in the oral cavity and enhance the transport of drugs across the epithelium barrier [205].
In most cases, the bioactivity of a drug delivery system is due to the drug itself. The polymers used in these systems are typically chosen for their benign effects on non-target tissues. However, some polymers and other nanoparticle systems can be used specifically for their inherent bioactivity in situ, which can promote synergistic effects with the drug being delivered. This synergistic activity is most notable in nanoparticle-mediated cancer immunotherapy treatments. For example, anti-PD1 and anti-PD-L1 cancer immunotherapies exhibit improved outcomes when delivered in polymeric nanoparticle systems that trigger an immune response. The more rapid and elevated immune response leads to a greater influx of lymphocytes and macrophages at the target tumor site, as well as a better overall treatment response [206].
4.2.2. Lipid-based nanoparticles
Lipid-based nanoparticles (LNPs), including liposomes and solid lipid nanoparticles (SLNs), are another prominent class of nanoparticles for topical drug delivery in the oral cavity. Lipids have hydrophobic tails and hydrophilic heads, which can form monolayer micelles, bilayer vesicles, or solid matrices to encapsulate drugs. These nanoparticles offer several advantages such as high biocompatibility and biodegradability, high drug loading capacity, protection of drugs from degradation, as well as enhanced drug absorption due to their lipid nature, and controlled drug release. LNPs are associated with improved retention at the targeted sites, permeability, and enhanced antifungal activities [211]. Liposomes have also been used to deliver various therapeutics for the treatment of oral cancers, including chemotherapeutics, radiotherapeutics agents, and photosensitizers for photodynamic therapy [192]. In addition to drug delivery, LNPs can also deliver nucleic acids [212,213] making them one of the most promising nano-formulations for employing gene therapy to treat oral cancers [214]. Given the recent success of LNPs in delivering messenger ribonucleic acid (mRNA) for COVID-19 vaccines, it is likely that researchers will give them a high priority in contemplating gene therapy for oral cancers in the future. Although there are no clear gene targets for oral cancers, a recent study suggested that PE38KDEL may be used as a suicidal gene for treating oral squamous cell carcinoma [215].
4.2.3. Dendrimers
Dendrimers are highly branched macromolecules with a defined structure that have also emerged as a promising class of carriers for topical drug delivery in the oral cavity. Dendrimer nanoparticles are formed as unimolecular micelles with a hydrophobic interior and hydrophilic exterior. An important characterization of dendrimers is their generation, which is defined as the number of radial branching units. Dendrimers offer unique advantages because of their precisely controlled composition, size, shape, potential biodegradability, and surface functionalities, which can be customized to achieve desired drug delivery properties. Dendrimers can encapsulate hydrophobic cargoes within their branches or conjugate drugs to their surface, thus enabling controlled drug release, targeted delivery, and improved drug stability. The use of dendrimers for topical application has greatly expanded in dentistry, as studies demonstrated their ability to stimulate remineralization in damaged enamel and dentin [216]. Based on this discovery, drugloaded dendrimers have been developed to enhance therapeutic effects. For example, dendrimers loaded with alendronate, a medication used to treat osteoporosis, can help restore tooth enamel by inducing remineralization [217]. Similarly, a triclosan-loaded dendrimer was shown to provide extended release of the antimicrobial while promoting the repair of damaged human dentin [218]. Thiolated dendrimers have also been developed to enhance mucoadhesion by forming covalent bonds with the cysteine domains of the mucin [219,220]. Cationic polyamidoamine (PAMAM) dendrimers have been shown to decrease inflammation by scavenging negatively charged cell-free deoxyribonucleic acid (DNA) [221] and consequently reduce inflammatory bone loss in cases of periodontitis [222]. This last example deviates from conventional drug delivery in that the nanoparticle exerts its therapeutic effect by removing a negative factor but not delivering a therapeutic agent. The future might be to incorporate a drug delivery function into these nanoparticles to achieve a synergistic push-pull effect for maximal therapeutic benefits.
4.2.4. Inorganic nanoparticles
Inorganic nanoparticles, such as gold, silver, and silica nanoparticles, have also gained attention for use in topical drug delivery in the oral cavity. Inorganic nanoparticles offer distinct properties, such as high stability, unique shapes, and flexibility for surface functionalization. Both gold (Au) and silver (Ag) nanoparticles have antimicrobial effects, making them potential anticaries agents. They can also serve as a synergistic enhancer to other antimicrobial drugs and biomolecules [223,224]. In addition, both Au and Ag nanoparticles have been reported to promote osteogenesis [225], which adds great therapeutic value when integrated into titanium dental implants [226] – another good example of synergistic therapeutics. Silica nanoparticles, particularly mesoporous silica nanoparticles (MSNs), have high surface areas and thus are advantageous for drug loading and enhancing therapeutic benefits. For example, although curcumin has anti-inflammatory and antitumor activities, it exhibits poor aqueous solubility and low permeability [227,228]. MSNs can serve as an effective vehicle for loading curcumin and providing a controlled and responsive release [229]. MSNs are also a versatile platform that can be functionalized for multiplexing, such as loading curcumin and silver nanoparticles simultaneously [230]. Another advantage of MSNs is their biodegradability when they contain disulfide and diselenide bonds in the silicate network [231,232].
In summary, nanoparticle-based topical delivery in the oral cavity holds great promise for the treatment of various diseases. The unique advantages of nanoparticles, namely enhanced drug stability, improved drug bioavailability, targeted drug delivery, and reduced systemic toxicity, make them attractive for this application. When comparing different types of nanoparticles, several factors should be considered, including their physicochemical properties, drug loading capacity, release kinetics, biocompatibility, and ease of fabrication. The choice of nanoparticle formulation will depend on the specific drug and therapeutic application as well as the desired drug delivery characteristics. Proper selection of nanoparticles can greatly influence the success of topical drug delivery in the oral cavity. Additional research and development in this area are necessary to optimize nanoparticle formulations for specific oral healthcare applications [186,233–235] and may lead to novel and effective therapeutic approaches for oral diseases.
4.3. Challenges and Limitations of Nanoparticle-based Delivery
Despite the promising potential of nanoparticle-based topical delivery in the oral cavity, there remain considerable challenges and limitations for translation into clinical practice. An additional burden for biologics is their delicate nature, which requires special effort to retain their bioactivity in formulation, storage, and shipping.
4.3.1. Regulatory challenges
While there are many nanoparticle-based drug delivery systems on the market, regulatory guidelines specific to nanoparticles and their use in the oral cavity may not be well-established, which can add complexity to the development process which is already time-consuming and resource-intensive [236].
4.3.2. Manufacturing challenges
The manufacturing of nanoparticles with reproducible quality and scalable production can pose challenges. The formulation and synthesis of nanoparticles need to be carefully controlled to achieve desired properties, such as size, drug loading capacity, and drug release kinetics. The scalability of nanoparticle production can also be challenging, as the methods used for laboratory-scale production may not be feasible for large-scale manufacturing. The costeffectiveness of nanoparticle production and the stability of nanoparticles during storage also need to be considered, along with process optimization and quality control measures to ensure consistent and reliable nanoparticle production.
4.3.3. Delivery challenges
The delivery of nanoparticles to the target tissues in the oral cavity can be challenging due to the complex anatomy and physiology of the oral cavity. The oral mucosa, which serves as the primary barrier for drug penetration, can vary in thickness and permeability in different regions of the oral cavity. The presence of saliva, which can dilute and clear nanoparticles, can also affect their delivery. Therefore, optimizing the formulation and delivery methods to achieve efficient and controlled delivery of nanoparticles to the target tissues in the oral cavity is a critical challenge. Strategies such as the use of penetration enhancers, and mucoadhesive nanoparticles or encapsulation of nanoparticles in carrier systems, such as gels or films, may be employed to improve the delivery efficiency in the oral cavity. Additionally, the selection of appropriate administration techniques, such as swabbing, rinsing, or spraying, can also impact the efficacy of nanoparticle-based topical delivery and requires careful consideration.
A more focused examination of the anatomy of the physiological barriers reveals the challenges of achieving effective drug delivery across the barrier. When a drug carrier enters the oral cavity, the first major barrier is the oral mucosa layer, which includes both the sublingual and buccal mucosa. Unlike the gastrointestinal (GI) mucosa, the oral mucosa does not have the micro-villa structure, which means the adsorptive surface area is small. Also, the administration time or retention time in the oral cavity is highly limited, due to patients’ behaviors, such as eating or drinking. A typical time window for drug administration at the oral mucosa is up to 5–6 hr [205]. These limitations require the drug to be highly potent. Furthermore, the tissue in the oral mucosa is much thicker than that in the GI tract. The top layer is the oral epithelium, which is mainly made of keratin. This keratinized layer provides mechanical protection, similar to the stratum corneum of the skin. Deeper down, there is the epithelial tight junctions (ETJ), a barrier formed by epithelial cells connected by tight junctions. This barrier can block the penetration of nanoparticles [237,238]. However, studies have shown that nanoparticles with the appropriate size and surface properties can exploit paracellular routes by modulating the tight junction permeability [237,239]. The deeper layer is known as the lamina propria, which is a fibrous connective tissue layer that consists of a network of collagen and elastin fibers. This layer also contains an array of extracellular matrix proteins that rapidly opsonize foreign nanoparticles [240]. For effective passage across these two layers, nanoparticle systems must be designed with the appropriate charge, architecture, and composition to achieve both non-interactional and diffusive capabilities.
Additionally, saliva is a very dynamic environment for nanoparticle-drug systems. It has a multifaceted composition and several functions. Saliva houses several enzymes, including amylases and proteases, which are capable of enzymatic degradation of certain nanoparticles. Recent research underscores the significance of enzyme-nanoparticle interactions, which can impact nanoparticle stability and functionality. Rheologically, saliva’s lubricating properties can both enhance and impede nanoparticle mobility within the oral cavity, complicating passage across oral mucosa layers. Salivary mucins, high-molecular weight glycoproteins, contribute to the formation of the mucosal barrier and can influence both nanoparticle adhesion to the mucosal surface and subsequent translocation [241].
In summary, several challenges and limitations, namely regulatory, manufacturing, and delivery challenges, need to be addressed for successful translation. Overcoming these challenges and limitations will contribute to the development of safe, effective, and reliable nanoparticle-based drug delivery systems for oral diseases, ultimately benefiting patients and advancing the field of oral healthcare. Further research and innovation in this area are warranted to unlock the full potential of nanoparticle-based topical delivery in the oral cavity and translate these technological advances into clinical practice.
5. Conclusions:
Vaccines use surface proteins of pathogenic bacteria or viruses, along with adjuvants to stimulate human immune response. Such vaccine therapeutics have been used for almost a century and they have saved millions of lives. However, the recent COVID-19 pandemic brought to light limitations of vaccine production and delivery methods. Although breakthrough innovations through mRNA vaccines saved millions of lives, they are beyond the reach of low or middle-income countries. The reason for this is their production in prohibitively expensive cell culture systems and the cost of regulatory requirements, including the number of exchanges of sterile air in fermenters and other measures to eliminate contamination. Furthermore, the requirement of cold-chain for storage and transportation is a major challenge – ~19 million mRNA vaccine doses were discarded in Africa due to lack of cold chain. Most importantly, all SARS-CoV-2 vaccines induced systemic immunity (IgG – immunoglobulin G) that conferred protection after infection but not mucosal immunity (IgA – secretory immunoglobulin A) that would have prevented transmission. This review addresses this important gap, by debulking viruses in the oral or nasal cavity using chewing gum or nasal sprays containing viral trap proteins, or monoclonal antibodies, to prevent self-infection and transmission. This approach is not limited to oral viruses but could be used to kill pathogenic bacteria or fungi in the oral cavity.
In addition, this review examines the reasons behind the high cost of biologics production and delivery methods. Among FDA-approved biologics, >90% are invasive injectable drugs. These biologics are produced in cell cultures requiring prohibitively expensive fermentation systems and associated regulatory cost. In addition, removal of host proteins to purify biologics to >99% purity is the most expensive aspect of biologics production. After such heavy investment, biologics are highly unstable and have short shelf-life despite requirement for cold chain for their storage and transportation. Oral or topical drugs are highly preferred by patients because of their affordability and convenience, but only two oral drugs has been approved by FDA since 2015. Oral delivery of biologics reduces their cost >90% by elimination of complex and expensive production, purification and delivery systems [42]. In addition to immunotherapy to reduce antidrug antibodies from injected proteins, the oral delivery of antigens bioencapsulated in plant cells could reduce antibodies generated by gene therapy [249]. This is even more important considering the high cost of gene therapy per patient – $2.9–3.5 million [30,42]. This also addresses the major healthcare crisis – greed in healthcare [250]. Oral delivery of functional therapeutic proteins bioencapsulated in plant cells has been reviewed recently [251,252]. Recent advances include oral delivery of ACE2 bioencapsulated in plant cells treated cardiopulmonary diseases [82], oral insulin regulated blood sugar levels like natural insulin [57] and oral growth hormones accelerated diabetic bone fracture healing [80].
Topical delivery has the unique advantage of targeted delivery of high concentrations of protein drugs, without getting diluted in circulating blood. Proteins/enzymes in chewing gum are stable for many years at ambient temperature and could be transported to remote villages around the globe. However, any new platform for drug delivery would face regulatory challenges. Surprisingly, the chewing gum containing viral trap protein was rapidly approved by the FDA (IND 154807; NCT05433181) because of extensive toxicology studies conducted and minimal systemic exposure of this drug. Topical delivery of peanut proteins in patches delivered to babies is successful in preventing allergy in Phase III clinical trials (NCT03211247). Similarly, several nasal spray formulations were evaluated in the clinic to minimize SARS-CoV-2 infection (NCT05180500; NCT05122260; NCT05437029). Completion of these trials will reveal future challenges in large scale production and global distribution. Elimination of cell culture, purification, and cold chain could significantly reduce the cost of protein drugs, thereby driving forward health equity. Therefore, this review highlights recent advances in topical delivery of protein or nucleic acid drugs directly, or in combination, with nanoparticles and offers future directions.
Funding
Research performed in the Daniell Lab on oral or topical delivery was supported NIH grant R01 HL 107904 and Commonwealth of Pennsylvania, Department of Community and Economic Development grants. “COVID-19 Pennsylvania Discoveries: Responding to SARS-CoV-2 Through Innovation & Commercialization.”
Declaration of competing interest
HD is a patentee in plant-based oral drug delivery and was previously funded by Novo Nordisk, Bayer, Shire and Takeda. Topical drug delivery reported here is funded by federal and state agencies. However, several industry discussions for advancing clinical trials in oral or topical delivery are in progress, including PhylloPharma LLC. Complete list of patents is available in the Google Scholar link: http://scholar.google.com/citations?user=7sow4jwAAAAJ&hl=en All other authors declare no conflict of interest.
Footnotes
Declaration of interests
Henry Daniell reports a relationship with PhylloPharma LLC that includes: equity or stocks. Henry Daniell has patent issued to University of Pennsylvania. None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Loembé MM; Tshangela A; Salyer SJ; Varma JK; Ouma AEO; Nkengasong JN COVID-19 in Africa: The Spread and Response. Nat. Med 2020, 26, 999–1003, doi: 10.1038/s41591-020-0961-x. [DOI] [PubMed] [Google Scholar]
- [2].Loembé MM; Nkengasong JN COVID-19 Vaccine Access in Africa: Global Distribution, Vaccine Platforms, and Challenges Ahead. Immunity 2021, 54, 1353–1362, doi: 10.1016/j.immuni.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ritchie H; Ortiz-Ospina E; Beltekian D; Methieu E; Hasell J; Macdonald B; Giattino C; Appel C; Rodes-Guirao L; Roser M Our World in Data, 2021, “Coronavirus (COVID-19) Vaccinations - Statistics and Research”. [Google Scholar]
- [4].Daniell H; Nair SK; Esmaeili N; Wakade G; Shahid N; Ganesan PK; Islam MR; Shepley-McTaggart A; Feng S; Gary EN; et al. Debulking SARS-CoV-2 in Saliva Using Angiotensin Converting Enzyme 2 in Chewing Gum to Decrease Oral Virus Transmission and Infection. Mol. Ther 2022A, S1525001621005797, doi: 10.1016/j.ymthe.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Daniell H; Nair SK; Guan H; Guo Y; Kulchar RJ; Torres MD; Shahed-Al-Mahmud M; Wakade G; Liu YM; Marques AD; Graham-Wooten J, et al. Debulking different Corona (SARS-CoV-2 delta, omicron, OC43) and Influenza (H1N1, H3N2) Virus Strains by Plant Viral Trap Proteins in Chewing Gums to Decrease Infection and Transmission. Biomaterials 2022B, 288, 121671, doi: 10.1016/j.biomaterials.2022.121671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ganesan PK; Kulchar RJ; Kaznica P; Montoya‐Lopez R; Green BJ; Streatfield SJ; Daniell H Optimization of Biomass and Target Protein Yield for Phase III Clinical Trial to Evaluate Angiotensin Converting Enzyme 2 Expressed in Lettuce Chloroplasts to Reduce SARS‐COV ‐2 Infection and Transmission. Plant Biotechnol. J 2023, 21, 244–246, doi: 10.1111/pbi.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bird JA; Spergel JM; Jones SM; Rachid R; Assa’ad AH; Wang J; Leonard SA; Laubach SS; Kim EH; Vickery BP; et al. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double-blind, placebocontrolled phase 2 clinical trial. J. Allergy Clin. Immunol. Prac 2018, 6(2), 476–485. [DOI] [PubMed] [Google Scholar]
- [8].Vickery BP; Vereda A; Casale TB; Beyer K; Toit G. du; Hourihane JO’B; Jones SM; Shreffler WG; Marcantonio A; Zawadzki R; et al. AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med 2018, 379, 1991–2001, DOI: 10.1056/NEJMoa1812856 [DOI] [PubMed] [Google Scholar]
- [9].Hourihane JOB; Beyer K; Abbas A; Fernández-Rivas M; Turner PJ; Blumchen K; Nilsson C; Ibáñez MD; Deschildre A; Muraro A; et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Adolesc. Health 2020, 4(10), 728–739. [DOI] [PubMed] [Google Scholar]
- [10].Greenhawt M; Sindher SB; Wang J; O’Sullivan M; du Toit G; Kim EH; Albright D; Anvari S; Arends N; Arkwright PD; Bégin P, et al. Phase 3 Trial of Epicutaneous Immunotherapy in Toddlers with Peanut Allergy. N. Engl. J. Med 2023, 388(19), 1755–1766, doi: 10.1056/NEJMoa2212895. [DOI] [PubMed] [Google Scholar]
- [11].Togias A Good News for Toddlers with Peanut Allergy. N. Engl. J. Med 2023, 388(19), 1814–1815, doi: 10.1056/NEJMe2301157. [DOI] [PubMed] [Google Scholar]
- [12].Chen G; Kang W; Li W; Chen S; Gao Y Oral Delivery of Protein and Peptide Drugs: From Non-specific Formulation Approaches to Intestinal Cell Targeting Strategies. Theranostics 2022, 12(3), 1419–1439. doi: 10.7150/thno.61747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Haddadzadegan S; Dorkoosh F; Bernkop-Schnürch A Oral Delivery of Therapeutic Peptides and Proteins: Technology Landscape of Lipid-based Nanocarriers. Adv. Drug Deliv. Rev 2022, 182, 114097, doi: 10.1016/j.addr.2021.114097. [DOI] [PubMed] [Google Scholar]
- [14].Kumar R; Islam T; Nurunnabi M Mucoadhesive Carriers for Oral Drug Delivery. J. Control. Release 2022, 351, 504–559, doi: 10.1016/j.jconrel.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kumari N; Siddhanta K; Panja S; Joshi V; Jogdeo C; Kapoor E; Khan R; Kollala SS; Kumar B; Sil D; et al. Oral Delivery of Nucleic Acid Therapies for Local and Systemic Action. Pharm. Res 2023, 40(1), 107–122, doi: 10.1007/s11095-022-03415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Purple Book Database of Licensed Biological Products. (2020). Retrieved June 20, 2022, from https://purplebooksearch.fda.gov/faqs
- [17].US Food and Drug Administration, Center for Drug Evaluation and Research. The Purple Book: list of licensed biological products with (1) reference product exclusivity and (2) biosimilarity or interchangeability evaluations to date. Accessed June 20, 2022. https://www.fda.gov/media/89589/download
- [18].National Library of Medicine. Unified Medical Language System. RxNorm. Published July 9, 2021. Accessed June 20, 2022. https://www.nlm.nih.gov/research/umls/rxnorm/index.html [Google Scholar]
- [19].Biological Approvals by Year; U.S. Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biologicalhttps://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biological-approvals-yearapprovals-year.
- [20].ClinicalTrials.gov. (n.d.). https://www.clinicaltrials.gov/ct2/home.
- [21].Drugs@FDA: FDA-Approved Drugs. (n.d.). https://www.accessdata.fda.gov/scripts/cder/daf/
- [22].Drug Information Portal. (n.d.). National Institutes of Health. https://druginfo.nlm.nih.gov/drugportal/name/cyltezo.
- [23].DailyMed. (n.d.). National Institutes of Health National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/index.cfm
- [24].Bhattacharya R; Herren K; Poonawalla I; Bunniran S; Bloomfield A; Schwab P Comparing Medical Utilization and Cost Outcomes in Oral Versus Injectable Immunotherapy Users with Chronic Inflammatory Joint and Skin Diseases. JMCP 2020, 26(10), 1246–1256, doi: 10.18553/jmcp.2020.26.10.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].The Lancet Infectious Diseases COVID-19 Vaccine Equity and Booster Doses. Lancet Infect. Dis 2021, 21(9), 1193, doi: 10.1016/S1473-3099(21)00486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Farmer P Social Inequalities and Emerging Infectious Diseases. Emerg. Infect. Dis 1996, 2(4), 259–269, doi: 10.3201/eid0204.960402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kesselheim AS; Avorn J; Sarpatwari A The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform. JAMA 2016, 316, 858, doi: 10.1001/jama.2016.11237. [DOI] [PubMed] [Google Scholar]
- [28].Rome BN; Egilman AC; Kesselheim AS Trends in Prescription Drug Launch Prices, 2008–2021. JAMA 2022, 327, 2145, doi: 10.1001/jama.2022.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hopkins J New $2.9 Million Gene Therapy Promises to Remake Hemophilia Treatment. The Wall Street Journal. 2023, June 29. https://www.wsj.com/articles/new-2-9-million-genehttps://www.wsj.com/articles/new-2-9-million-gene-therapy-promises-to-remake-hemophilia-treatment-da254ef4therapy-promises-to-remake-hemophilia-treatment-da254ef4. [Google Scholar]
- [30].Naddaf M Researchers Welcome $3.5-million Haemophilia Gene Therapy—But Questions Remain. Nature 2022, 612, 388–389, doi: 10.1038/d41586-022-04327-7. [DOI] [PubMed] [Google Scholar]
- [31].DiMasi JA; Grabowski HG; Hansen RW Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ 2016, 47, 20–33, doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- [32].Sertkaya A; Birkenbach A; Berlind A; Eyraud J Examination of Clinical Trial Costs and Barriers for Drug Development; Office of the Assistant Secretary for Planning and Evaluation, https://aspe.hhs.gov/reports/examination-clinical-trial-costs-barriers-drug-development-0.
- [33].Mohs RC; Greig NH Drug Discovery and Development: Role of Basic Biological Research. Alzheimers Dement. Transl. Res. Clin. Interv 2017, 3, 651–657, doi: 10.1016/j.trci.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ahmad NS; Makmor-Bakry M; Hatah E Drug Price Transparency Initiative: A Scoping Review. Res. Soc. Adm. Pharm 2020, 16, 1359–1369, doi: 10.1016/j.sapharm.2020.01.002. [DOI] [PubMed] [Google Scholar]
- [35].Sections 7001–7003 (Biologics Price Competition and Innovation Act of 2009) of the Patient Protection and Affordable Care Act (Public Law No. 111–148); Food and Drug Administration, https://www.fda.gov/media/78946/download. [Google Scholar]
- [36].Bennett CL; Chen B; Hermanson T; Wyatt MD; Schulz RM; Georgantopoulos P; Kessler S; Raisch DW; Qureshi ZP; Lu ZK; et al. Regulatory and Clinical Considerations for Biosimilar Oncology Drugs. Lancet Oncol. 2014, 15, e594–e605, doi: 10.1016/S1470-2045(14)70365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen B; Yang YT; Bennett CL Challenges to Biosimilar Substitution. JAMA 2017, 318(12), 1186, doi: 10.1001/jama.2017.11930. [DOI] [PubMed] [Google Scholar]
- [38].Chen BK; Yang YT; Bennett CL Why Biologics and Biosimilars Remain So Expensive: Despite Two Wins for Biosimilars, the Supreme Court’s Recent Rulings Do Not Solve Fundamental Barriers to Competition. Drugs 2018, 78, 1777–1781, doi: 10.1007/s40265-018-1009-0. [DOI] [PubMed] [Google Scholar]
- [39].McEwen LN; Casagrande SS; Kuo S; Herman WH Why Are Diabetes Medications So Expensive and What Can Be Done to Control Their Cost? Curr. Diab. Rep 2017, 17, 71, doi: 10.1007/s11892-017-0893-0. [DOI] [PubMed] [Google Scholar]
- [40].Dafny LS A Radical Treatment for Insulin Pricing. N. Engl. J. Med 2022, 386, 2157–2159, doi: 10.1056/NEJMp2203001. [DOI] [PubMed] [Google Scholar]
- [41].Yang YT; Chen B; Bennett CL Biosimilars—Curb Your Enthusiasm. JAMA Oncol. 2017, 3, 1467, doi: 10.1001/jamaoncol.2017.1530. [DOI] [PubMed] [Google Scholar]
- [42].Daniell H; Kulchar RJ; Herzog RW; Kulis M; Leong KW; Plant Cell-based Drug Delivery Enhances Affordability of Biologics. Nature Biotechnology, 2023, 10.1038/s41587-023-01899-1. [DOI] [PubMed] [Google Scholar]
- [43].Dincer B; Yildirim D The Effect of Vibration Stimulation on Intramuscular Injection Pain and Patient Satisfaction: Single–Blind, Randomised Controlled Study. J. Clin. Nurs 2021, 30, 1615–1622, doi: 10.1111/jocn.15715. [DOI] [PubMed] [Google Scholar]
- [44].Jin J; Zhu L; Chen M; Xu H; Wang H; Feng X; Zhu X; Zhou Q The Optimal Choice of Medication Administration Route Regarding Intravenous, Intramuscular, and Subcutaneous Injection. PPA 2015, 923, doi: 10.2147/PPA.S87271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raddadi Y; Adib-Hajbaghery M; Ghadirzadeh Z; Kheirkhah D Comparing the Effects of Acupressure at LI4 and BL32 Points on Intramuscular Injection Pain. Eur. J. Integr. Med 2017, 11, 63–68, doi: 10.1016/j.eujim.2017.01.015. [DOI] [Google Scholar]
- [46].Yalkowsky SH; Krzyzaniak JF; Ward GH Formulation-Related Problems Associated with Intravenous Drug Delivery. J. Pharm. Sci 1998, 87, 787–796, doi: 10.1021/js980051i. [DOI] [PubMed] [Google Scholar]
- [47].Stoner KL; Harder H; Fallowfield LJ; Jenkins VA Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review. Patient 2015, 8, 145–153, doi: 10.1007/s40271-014-0075-y. [DOI] [PubMed] [Google Scholar]
- [48].Verma P; Thakur AS; Deshmukh K; Jha AK; Verma S Routes of Drug Administration. Int. J. Pharm. Studies Res 2010, 1(1), 54–59. [Google Scholar]
- [49].Zaoutis LB; Chiang VW Comprehensive Pediatric Hospital Medicine. Elsevier Health Sciences, 2007. [Google Scholar]
- [50].Flatt AJ; Peleckis AJ; Dalton-Bakes C; Nguyen H-L; Ilany S; Matus A; Malone SK; Goel N; Jang S; Weimer J; et al. Automated Insulin Delivery for Hypoglycemia Avoidance and Glucose Counter regulation in Long-Standing Type 1 Diabetes with Hypoglycemia Unawareness. Diabetes Technol. Ther 2023, 25(5), 302–314, doi: 10.1089/dia.2022.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaze AD; Yuyun MF; Ahima RS; Rickels MR; Echouffo-Tcheugui JB Autonomic Dysfunction and Risk of Severe Hypoglycemia Among Individuals with Type 2 Diabetes. JCI Insight 2022, 7(22), e156334, doi: 10.1172/jci.insight.156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McAdams B; Rizvi A An Overview of Insulin Pumps and Glucose Sensors for the Generalist. JCM 2016, 5, 5, doi: 10.3390/jcm5010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gregory JM; Kraft G; Scott MF; Neal DW; Farmer B; Smith MS; Hastings JR; Allen EJ; Donahue EP; Rivera N; et al. Insulin Delivery Into the Peripheral Circulation: A Key Contributor to Hypoglycemia in Type 1 Diabetes. Diabetes 2015, 64(10), 3439–3451, doi: 10.2337/db15-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Edgerton DS; Scott M; Farmer B; Williams PE; Madsen P; Kjeldsen T; Brand CL; Fledelius C; Nishimura E; Cherrington AD Targeting Insulin to the Liver Corrects Defects in Glucose Metabolism Caused by Peripheral Insulin Delivery. JCI Insight 2019, 4(7), e126974, doi: 10.1172/jci.insight.126974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Edgerton DS; Moore MC; Gregory JM; Kraft G; Cherrington AD Importance of the Route of Insulin Delivery to its Control of Glucose Metabolism. Am. J. Physiol. -Endocrinol. Metab 2021, 320(5), E891–E897, doi: 10.1152/ajpendo.00628.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li J; Wang Y; Han L; Sun X; Yu H; & Yu Y Time–Action Profile of an Oral Enteric Insulin Formulation in Healthy Chinese Volunteers. Clin. Ther 2012, 34(12), 2333–2338, doi: 10.1016/j.clinthera.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [57].Daniell H; Singh R; Mangu V; Nair SK; Wakade G; Balashova N Affordable Oral Proinsulin Bioencapsulated in Plant Cells Regulates Blood Sugar Levels Similar to Natural Insulin. Biomaterials, 2023, 298, 122142, doi: 10.1016/j.biomaterials.2023.122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Henkin RI Inhaled Insulin—Intrapulmonary, Intranasal, and Other Routes of Administration: Mechanisms of Action. Nutrition 2010, 26, 33–39, doi: 10.1016/j.nut.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [59].Illum L Nasal Drug Delivery — Recent Developments and Future Prospects. J. Control. Release 2012, 161(2), 254–263, doi: 10.1016/j.jconrel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- [60].Leary AC; Stote RM; Cussen K; O’brien J; Leary WP; Buckley B Pharmacokinetics and Pharmacodynamics of Intranasal Insulin Administered to Patients with Type 1 Diabetes: A Preliminary Study. Diabetes Technol. Ther 2006, 8(1), 81–88, doi: 10.1089/dia.2006.8.81. [DOI] [PubMed] [Google Scholar]
- [61].Shah R; Patel M; Maahs D; Shah V Insulin Delivery Methods: Past, Present and Future. Int. J. Pharma. Investig 2016, 6, 1, doi: 10.4103/2230-973X.176456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gaddam M; Singh A; Jain N; Avanthika C; Jhaveri S; De la Hoz I; Sanka S; Goli SR A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, doi: 10.7759/cureus.17219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hilsted J; Madsbad S; Hvidberg A; Rasmussen MH; Krarup T; Ipsen H; Hansen B; Pedersen M; Djurup R; Oxenbøll B Intranasal Insulin Therapy: The Clinical Realities. Diabetologia 1995, 38, 680–684, doi: 10.1007/BF00401839. [DOI] [PubMed] [Google Scholar]
- [64].Helander HF; Fändriks L Surface Area of the Digestive Tract – Revisited. Scand. J. Gastroenterol 2014, 49(6), 681–689, doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- [65].Usach I; Martinez R; Festini T; Peris J-E Subcutaneous Injection of Drugs: Literature Review of Factors Influencing Pain Sensation at the Injection Site. Adv. Ther 2019, 36, 2986–2996, doi: 10.1007/s12325-019-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bittner B; Richter W; Schmidt J Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs 2018, 32, 425–440, doi: 10.1007/s40259-018-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mathaes R; Koulov A; Joerg S; Mahler H-C Subcutaneous Injection Volume of Biopharmaceuticals—Pushing the Boundaries. J. Pharm. Sci 2016, 105, 2255–2259, doi: 10.1016/j.xphs.2016.05.029. [DOI] [PubMed] [Google Scholar]
- [68].Heinemann L Variability of Insulin Absorption and Insulin Action. Diabetes Technol. Ther 2002, 4, 673–682, doi: 10.1089/152091502320798312. [DOI] [PubMed] [Google Scholar]
- [69].Kim H; Park H; Lee SJ Effective Method for Drug Injection into Subcutaneous Tissue. Sci. Rep 2017, 7, 9613, doi: 10.1038/s41598-017-10110-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gradel AKJ; Porsgaard T; Lykkesfeldt J; Seested T; Gram-Nielsen S; Kristensen NR; Refsgaard HHF Factors Affecting the Absorption of Subcutaneously Administered Insulin: Effect on Variability. J. Diabetes Res 2018, 2018, 1–17, doi: 10.1155/2018/1205121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yaoi Y; Hashimoto K; Takahara K; Kato I Insulin Binds to Type V Collagen with Retention of Mitogenic Activity. Exp. Cell Res 1991, 194, 180–185, doi:10.1016/0014- 10.1016/0014-4827(91)90351-T4827(91)90351-T. [DOI] [PubMed] [Google Scholar]
- [72].Polania Gutierrez JJ; Munakomi S Intramuscular Injection; StatPearls Publishing, 2022, Available from: https://www.ncbi.nlm.nih.gov/books/NBK556121/ [PubMed] [Google Scholar]
- [73].Nicoll LH; Hesby A Intramuscular Injection: An Integrative Research Review and Guideline for Evidence-Based Practice. Appl. Nurs. Res 2002, 15, 149–162, doi: 10.1053/apnr.2002.34142. [DOI] [PubMed] [Google Scholar]
- [74].McDonald and Avery’s Dentistry for the Child and Adolescent; Dean JA, Avery DR, McDonald RE, Eds.; Tenth edition.; Elsevier: St. Louis, Missouri, 2016; ISBN 978-0-323-28745-6. [Google Scholar]
- [75].Altaf A Unsafe Injection Practices by Medical Practitioners in South Asia Associated with Hepatitis and HIV Outbreaks. J. Infectiology and Epidemiol 2018, 1, 1–3, doi:10.29245/2689- 10.29245/2689-9981/2018/2.11139981/2018/2.1113. [DOI] [Google Scholar]
- [76].Shaw H Intramuscular Injection: A CPD Article Enhanced Heather Shaw’s Knowledge of Appropriate Sites and Optimal Technique for Injections. Nursing Standard 2015, 30, 61–62, doi: 10.7748/ns.30.6.61.s48. [DOI] [PubMed] [Google Scholar]
- [77].Yilmaz DK; Dikmen Y; Kokturk F; Dedeoglu Y The Effect of Air-Lock Technique on Pain at the Site of Intramuscular Injection. SMJ 2016, 37, 304–308, doi: 10.15537/smj.2016.3.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Daniell H; Kulis M; Herzog RW Plant Cell-Made Protein Antigens for Induction of Oral Tolerance. Biotechnol. Adv 2019, 37(7), 107413, doi: 10.1016/j.biotechadv.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kwon K-C; Daniell H Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol. Ther 2016, 24(8), 1342–1350, doi: 10.1038/mt.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Park J; Yan G; Kwon K-C; Liu M; Gonnella PA; Yang S; Daniell H Oral Delivery of Novel Human IGF-1 Bioencapsulated in Lettuce Cells Promotes Musculoskeletal Cell Proliferation, Differentiation and Diabetic Fracture Healing. Biomaterials 2020, 233, 119591, doi: 10.1016/j.biomaterials.2019.119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kumar SRP; Wang X; Avuthu N; Bertolini TB; Terhorst C; Guda C; Daniell H; Herzog RW Role of Small Intestine and Gut Microbiome in Plant-Based Oral Tolerance for Hemophilia. Front. Immunol 2020, 11, 844, doi: 10.3389/fimmu.2020.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Daniell H; Mangu V; Yakubov B; Park J; Habibi P; Shi Y; Gonnella PA; Fisher A; Cook T; Zeng L; et al. Investigational New Drug Enabling Angiotensin Oral-Delivery Studies to Attenuate Pulmonary Hypertension. Biomaterials 2020, 233, 119750, doi: 10.1016/j.biomaterials.2019.119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Herzog RW; Nichols TC; Su J; Zhang B; Sherman A; Merricks EP; Raymer R; Perrin GQ; Häger M; Wiinberg B; et al. Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce. Mol. Ther 2017, 25, 512–522, doi: 10.1016/j.ymthe.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Srinivasan A; Herzog RW; Khan I; Sherman A; Bertolini T; Wynn T; Daniell H Preclinical Development of Plant‐based Oral Immune Modulatory Therapy for Haemophilia B. Plant Biotechnol. J 2021, 19(10), 1952–1966, doi: 10.1111/pbi.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xiao Y; Kwon K-C; Hoffman BE; Kamesh A; Jones NT; Herzog RW; Daniell H Low Cost Delivery of Proteins Bioencapsulated in Plant Cells to Human Non-Immune or Immune Modulatory Cells. Biomaterials 2016, 80, 68–79, doi: 10.1016/j.biomaterials.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Witting M; Obst K; Friess W; Hedtrich S Recent Advances in Topical Delivery of Proteins and Peptides Mediated by Soft Matter Nanocarriers. Biotechnol. Adv 2015, 33(6), 1355–1369, doi: 10.1016/j.biotechadv.2015.01.010. [DOI] [PubMed] [Google Scholar]
- [87].Liu Y; Kamesh AC; Xiao Y; Sun V; Hayes M; Daniell H; Koo H Topical Delivery of Low-cost Protein Drug Candidates Made in Chloroplasts for Biofilm Disruption and Uptake by Oral Epithelial Cells. Biomaterials, 2016, 105, 156–166, doi: 10.1016/j.biomaterials.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singh R; Ren Z; Shi Y; Lin S; Kwon K; Balamurugan S; Rai V; Mante F; Koo H; Daniell H Affordable Oral Health Care: Dental Biofilm Disruption Using Chloroplast Made Enzymes with Chewing Gum Delivery. Plant Biotechnol. J 2021, 19(10), 2113–2125, doi: 10.1111/pbi.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lalla RV; Latortue MC; Hong CH, Ariyawardana A; D’Amato-Palumbo S; Fischer DJ; Martof A; Nicolatou-Galitis O; Patton LL; Elting LS; Spijkervet FK; Brennan MT A Systematic Review of Oral Fungal Infections in Patients Receiving Cancer Therapy. Support. Care Cancer, 2010, 18(8), 985–992, doi: 10.1007/s00520-010-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Panghal M; Kaushal V; Kadayan S; Yadav JP Incidence and Risk Factors for Infection in Oral Cancer Patients Undergoing Different Treatments Protocols. BMC Oral Health, 2012, 12, doi: 10.1186/1472-6831-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chatzinikolaou I; Abi-Said D; Bodey GP, Rolston KV, Tarrand JJ; Samonis G Recent Experience with Pseudomonas Aeruginosa Bacteremia in Patients with Cancer: Retrospective Analysis of 245 Episodes. Arch. Intern. Med 2000, 160(4), 501–509, doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- [92].Rolston KV The Infectious Diseases Society of America 2002 Guidelines for the Use of Antimicrobial Agents in Patients with Cancer and Neutropenia: Salient Features and Comments. Clin. Infec. Dis 2004, 39(Supplement_1), S44–S48, doi: 10.1086/383053. [DOI] [PubMed] [Google Scholar]
- [93].Pappas PG; Kauffman CA; Andes D; Benjamin DK Jr; Calandra TF; Edwards JE Jr; Filler SG, Fisher JF; Kullberg BJ; Ostrosky-Zeichner L; Reboli AC, et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infec. Dis 2009, 48(5), 503–535, doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Imran E; Khurshid Z; Adanir N; Ashi H; Almarzouki N; Baeshen HA Dental Practitioners’ Knowledge, Attitude and Practices for Mouthwash Use Amidst the COVID-19 Pandemic. Risk Manag. Healthc. Policy 2021, 14, 605, doi: 10.2147/RMHP.S287547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ferrer MD; Barrueco ÁS; Martinez-Beneyto Y; Mateos-Moreno MV; Ausina-Márquez V; García-Vázquez E; Puche-Torres M; Giner MJF; González AC; Coello JMS; Rueda IA; Aubá JMV; Español CC; Velasco AL; Abad DS; García-Estebam S; Artacho A; López-Labrador X; Mira A Clinical Evaluation of Antiseptic Mouth Rinses to Reduce Salivary Load of SARS-CoV-2. Sci. Rep 2021, 11, doi: 10.1038/s41598-021-03461-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Martyn DM; Lau A Chewing Gum Consumption in the United States among Children, Adolescents and Adults. Food Addit. Contam. Part A 2019, 36, 350–358, doi: 10.1080/19440049.2019.1567944. [DOI] [PubMed] [Google Scholar]
- [97].Al Hagbani T; Nazzal S Medicated Chewing Gums (MCGs): Composition, Production, and Mechanical Testing. AAPS PharmSciTech 2018, 19, 2908–2920, doi: 10.1208/s12249-018-1123-z. [DOI] [PubMed] [Google Scholar]
- [98].Aslani A; Rostami F Medicated Chewing Gum, a Novel Drug Delivery System. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci 2015, 20(4), 403–411 [PMC free article] [PubMed] [Google Scholar]
- [99].Asija R.; Patel S; Asija S Oral Dosages Form: Medicine Containing Chewing Gum: A Review. J. Drug Deliv. Ther 2012, 2(6), doi: 10.22270/jddt.v2i6.333. [DOI] [Google Scholar]
- [100].Kaushik P; Kaushik D Medicated Chewing Gums: Recent Patents and Patented Technology Platforms. Recent Pat. Drug Deliv. Formul 2020, 13, 184–191, doi: 10.2174/1872211313666191010093148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chakraborty S Perceived Risk versus Empirically Founded Benefit of Masticating Gum. Eur. J. Risk Regul 2021, 12, 720–723, doi: 10.1017/err.2021.14. [DOI] [Google Scholar]
- [102].Yaman‐Sözbir Ş; Ayaz‐Alkaya S; Bayrak‐Kahraman B Effect of Chewing Gum on Stress, Anxiety, Depression, Self‐focused Attention, and Academic Success: A Randomized Controlled Study. Stress Health 2019, 35, 441–446, doi: 10.1002/smi.2872. [DOI] [PubMed] [Google Scholar]
- [103].Newton JT; Awojobi O; Nasseripour M; Warburton F; Di Giorgio S; Gallagher JE; Banerjee A A Systematic Review and Meta-Analysis of the Role of Sugar-Free Chewing Gum in Dental Caries. JDR Clin. Transl. Res 2020, 5, 214–223, doi: 10.1177/2380084419887178. [DOI] [PubMed] [Google Scholar]
- [104].Ozen N; Aydin Sayilan A; Mut D; Sayilan S; Avcioglu Z; Kulakac N; Ecder T; Akyolcu N The Effect of Chewing Gum on Dry Mouth, Interdialytic Weight Gain, and Intradialytic Symptoms: A Prospective, Randomized Controlled Trial. Hemodial. Int 2021, 25, 94–103, doi: 10.1111/hdi.12878. [DOI] [PubMed] [Google Scholar]
- [105].Smith AP; Clayton H The Effects of Chewing Gum on Perceived Stress and Wellbeing in Students Under a High and Low Workload. In Human Mental Workload: Models and Applications; Longo L, Leva MC, Eds.; Communications in Computer and Information Science; Springer International Publishing: Cham: 2020, 1318, 124–137 ISBN 978-3-030-62301-2. [Google Scholar]
- [106].Bobillo C; Finlayson G; Martínez A; Fischman D; Beneitez A; Ferrero AJ; Fernández BE; Mayer MA Short-Term Effects of a Green Coffee Extract-, Garcinia c Ambogia- and lCarnitine-Containing Chewing Gum on Snack Intake and Appetite Regulation. Eur. J. Nutr 2018, 57, 607–615, doi: 10.1007/s00394-016-1347-1. [DOI] [PubMed] [Google Scholar]
- [107].Greene T; Rogers S; Franzen A; Gentry R A Critical Review of the Literature to Conduct a Toxicity Assessment for Oral Exposure to Methyl Salicylate. Crit. Rev. Toxicol 2017, 47, 98–120, doi: 10.1080/10408444.2016.1236071. [DOI] [PubMed] [Google Scholar]
- [108].Round EK; Chen P; Taylor AK; Schmidt E Biomarkers of Tobacco Exposure Decrease After Smokers Switch to an E-Cigarette or Nicotine Gum. Nicotine Tob. Res 2019, 21, 1239–1247, doi: 10.1093/ntr/nty140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Banakar M; Moayedi S; Shamsoddin E; Vahedi Z; Banakar MH; Mousavi SM; Rokaya D; Bagheri Lankarani K Chewing Gums as a Drug Delivery Approach for Oral Health. Int. J. Dent 2022, 2022, 1–10, doi: 10.1155/2022/9430988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Söderling E; Pienihäkkinen K Effects of Xylitol Chewing Gum and Candies on the Accumulation of Dental Plaque: A Systematic Review. Clin. Oral Investig 2022, 26, 119–129, doi: 10.1007/s00784-021-04225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Thivya P; Durgadevi M; Sinija VRN Biodegradable Medicated Chewing Gum: A Modernized System for Delivering Bioactive Compounds. Future Foods 2021, 4, 100054, doi: 10.1016/j.fufo.2021.100054. [DOI] [Google Scholar]
- [112].Hartel RW; von Elbe JH; Hofberger R Chewing and Bubble Gum. In: Confectionery Science and Technology 2018, 393–420. Springer International Publishing, 10.1007/978-3-319-61742-8_14 [DOI] [Google Scholar]
- [113].Mathews JP, & Schultz GP Chicle: The chewing gum of the Americas, from the ancient Maya to William Wrigley 2009. University of Arizona Press. [Google Scholar]
- [114].Maya Produce. In S. D. Coe, America’s First Cuisines 1994. (pp. 161–168). University of Texas Press. 10.7560/711556-014 [DOI] [Google Scholar]
- [115].Roy AS Improper Disposal of Non-biodegradable Chewing Gum is One of the Biggest Threats to Our Ecology: A Review. Curr. World Environ 2021, 16(3), 916–927, 10.12944/CWE.16.3.22 [DOI] [Google Scholar]
- [116].Blery EK; Antoniades L Marketing Chewing Gum: A Case Study of a Cypriot Company. J. Food Prod. Mark 2010, 16(4), 337–349, 10.1080/10454446.2010.509227 [DOI] [Google Scholar]
- [117].Huppatz DJ Robot salesmen: Automated food retailing in the United States, 1925–39. His. Retail. Consum 2022, 7(3), 1–16, 10.1080/2373518X.2022.2044196 [DOI] [Google Scholar]
- [118].Redclift M Chewing Gum in the United States and Mexico: The Everyday and the Iconic. Sociol. Rural 2002, 42(4), 391–403, 10.1111/1467-9523.00223. [DOI] [Google Scholar]
- [119].Khatun S; Sutradhar KB Medicated chewing gum: An unconventional drug delivery system. Int. Curr. Pharm. J 1970, 1(4), 86–91, 10.3329/icpj.v1i4.10064 [DOI] [Google Scholar]
- [120].Rømer Rassing M Chewing gum as a drug delivery system. Adv. Drug Deliv. Rev 1994, 13(1–2), 89–121, 10.1016/0169-409X(94)90028-0. [DOI] [Google Scholar]
- [121].Freitas AAR; Ribeiro AJ; Santos AC; Veiga F; Nunes LCC; Silva DA, SoaresSobrinho JL, Silva-Filho EC Sterculia Striata Gum as a Potential Oral Delivery System for Protein Drugs. Int. J. Bio. Macromol 2020, 164, 1683–1692, doi: 10.1016/j.ijbiomac.2020.07.276. [DOI] [PubMed] [Google Scholar]
- [122].Boyhan D; Daniell H Low-cost Production of Proinsulin in Tobacco and Lettuce Chloroplasts for Injectable or Oral delivery of Functional Insulin and C-peptide. Plant Biotechnol. J 2011, 9(5), 585–598, doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ward BJ; Gobeil P; Séguin A; Atkins J; Boulay I; Charbonneau P-Y; Couture M; D’Aoust M-A; Dhaliwall J; Finkle C; et al. Phase 1 Randomized Trial of a Plant-Derived Virus-like Particle Vaccine for COVID-19. Nat. Med 2021, 27, 1071–1078, doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hager KJ; Pérez Marc G; Gobeil P; Diaz RS; Heizer G; Llapur C; Makarkov AI; Vasconcellos E; Pillet S; Riera F; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine. N. Engl. J. Med 2022, 386, 2084–2096, doi: 10.1056/NEJMoa2201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Armario-Najera V; Blanco-Perera A; Shenoy SR; Sun Y; Marfil S; Muñoz-Basagoiti J; Perez-Zsolt D; Blanco J; Izquierdo-Useros N; Capell T; et al. Physicochemical characterization of the recombinant lectin scytovirin and microbicidal activity of the SD1 domain produced in rice against HIV-1. Plant Cell Rep. 2022, 41, 1013–1023, 10.1007/s00299-022-02834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Teleshova N; Keller MJ; Fernández Romero JA; Friedland BA; Creasy GW, Plagianos MG; Ray L; Barnable P; Kizima L; Rodriguez A; Cornejal N, et al. Results of a Phase 1, Randomized, Placebo-controlled First-in-human trial of Griffithsin Formulated in a Carrageenan Vaginal Gel. PLoS One 2022, 17(1), e0261775, doi: 10.1371/journal.pone.0261775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Tyo KM; Lasnik AB; Zhang L; Jenson AB; Fuqua JL; Palmer KE; SteinbachRankins JM Rapid-release griffithsin fibers for dual prevention of HSV-2 and HIV-1 infections. Antimicrob. Agents Chemother 2020, 64(6), e02139–19, 10.1128/AAC.02139-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Vamvaka E; Farré G; Molinos-Albert LM; Evans A; Canela-Xandri A; Twyman RM; Carrillo J; Ordóñez RA; Shattock RJ; O’Keefe BR; et al. Unexpected synergistic HIV neutralization by a triple microbicide produced in rice endosperm. Proc. Natl. Acad. Sci. U.S.A 2018, 115(33), E7854–E7862, 10.1073/pnas.1806022115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zeitlin L; Olmsted SS; Moench TR; Co MS; Martinell BJ; Paradkar VM; Russell DR; Queen C; Cone RA; Whaley KJ A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat. Biotechnol 1998, 16(13), 1361–1364. [DOI] [PubMed] [Google Scholar]
- [130].Ma JKC; Drossard J; Lewis D; Altmann F; Boyle J; Christou P; Cole T; Dale P; van Dolleweerd CJ; Isitt V; et al. Regulatory approval and a first‐in‐human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J 2015, 13(8), 1106–1120, 10.1111/pbi.12416 [DOI] [PubMed] [Google Scholar]
- [131].Pogue GP; Vojdani F; Palmer KE; Hiatt E; Hume S; Phelps J; Long L; Bohorova N; Kim D; Pauly M; et al. Production of pharmaceutical‐grade recombinant aprotinin and a monoclonal antibody product using plant‐based transient expression systems. Plant Biotechnol. J 2010, 8(5), 638–654, 10.1111/j.1467-7652.2009.00495.x [DOI] [PubMed] [Google Scholar]
- [132].Sabalza M; Madeira L; Van Dolleweerd C; Ma JK; Capell T; Christou P Functional characterization of the recombinant HIV-neutralizing monoclonal antibody 2F5 produced in maize seeds. Plant Mol. Biol 2012, 80, 477–488, 10.1007/s11103-012-9962-6 [DOI] [PubMed] [Google Scholar]
- [133].Derby N; Lal M; Aravantinou M; Kizima L; Barnable P; Rodriguez A; Lai M; Wesenberg A; Ugaonkar S; Levendosky K; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nature Commun. 2018, 9(1), 3881, 10.1038/s41467-018-06349-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Heng C Tooth Decay Is the Most Prevalent Disease. Fed. Pract, 2016, 33(10), 31–33 [PMC free article] [PubMed] [Google Scholar]
- [135].Bin C; Al-Dhabi NA; Esmail GA; Arokiyaraj S; Arasu MV Potential Effect of Allium Sativum Bulb for the Treatment of Biofilm Forming Clinical Pathogens Recovered from Periodontal and Dental Caries. Saudi J. Biol. Sci 2020, 27(6), 1428–1434, doi: 10.1016/j.sjbs.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Dye BA; Thornton-Evans G; Li X; Iafolla TJ Dental Caries and Tooth Loss in Adults in the United States, 2011–2012; NCHS Data Brief, 2015, 1–8 [PubMed]
- [137].Park AH; Kulchar RJ; Susarla SM; Turton B; Sokal-Gutierrez K Fewer Children in Families Associated with Lower Odds of Early Childhood Caries: A Sample from Three Countries. Int. J. Environ. Res. Public Health 2023, 20(3), 2195, doi: 10.3390/ijerph20032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Selwitz RH; Ismail AI; Pitts NB Dental Caries. Lancet 2007, 369(9562), 51–59, doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- [139].Shafer AW; Hine MK; Levy BM; Rajendran R; Sivapathasundharam B Shafer’s Textbook of Oral Pathology 2015; ISBN 978-81-312-3097-8.
- [140].Buzalaf MAR; Pessan JP; Honório HM; Ten Cate JM Mechanisms of Action of Fluoride for Caries Control. Monogr. Oral Sci 2011, 22, 97–114, doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- [141].Goswami D Dental Bioflim and Periodontal Disease. Int. J. Health Res. Medico-Leg. Pract 2021, 7, doi: 10.31741/ijhrmlp.v7.i1.2021.18. [DOI] [Google Scholar]
- [142].Larsen T; Fiehn N-E Dental Biofilm Infections - an Update. APMIS 2017, 125(4), 376–384, doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- [143].Pitts NB; Zero DT; Marsh PD; Ekstrand K; Weintraub JA; Ramos-Gomez F; Tagami J; Twetman S; Tsakos G; Ismail A Dental Caries. Nat. Rev. Dis. Primer 2017, 3, 17030, doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- [144].Takenaka S; Ohsumi T; Noiri Y Evidence-Based Strategy for Dental Biofilms: Current Evidence of Mouthwashes on Dental Biofilm and Gingivitis. Jpn. Dent. Sci. Rev 2019, 55, 33–40, doi: 10.1016/j.jdsr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Essalat M; Morrison D; Kak S; Chang EJ; Penso IR; Kulchar RJ; Padilla OHM; Shetty V A Naturalistic Study of Brushing Patterns Using Powered Toothbrushes. Plos One 2022, 17, e0263638, doi: 10.1371/journal.pone.0263638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Deinzer R; Cordes O; Weber J; Hassebrauck L; Weik U; Krämer N; Pieper K; Margraf-Stiksrud J Toothbrushing Behavior in Children – an Observational Study of Toothbrushing Performance in 12 Year Olds. BMC Oral Health 2019, 19, 68, doi: 10.1186/s12903-019-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Deinzer R; Ebel S; Blättermann H; Weik U; Margraf-Stiksrud J Toothbrushing: To the Best of One’s Abilities Is Possibly Not Good Enough. BMC Oral Health 2018, 18, 167, doi: 10.1186/s12903-018-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ma JKC; Hikmat BY; Wycoff K; Vine ND; Chargelegue D; Yu L; Hein MB; Lehner T Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med 1998, 4(5), 601–606. [DOI] [PubMed] [Google Scholar]
- [149].Choi YS; Moon JH; Kim TG; Lee JY Potent in vitro and in vivo activity of plantibody specific for Porphyromonas gingivalis FimA. Clin. Vaccine Immunol 2016, 23(4), 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kirkwood B; Miller M; Milleman J; Milleman K; Leung K Four-day plaque regrowth evaluation of a peptide chewing gum in a double-blind randomized clinical trial. Clin. Exp. Dent. Res 2020, 6(3), 318–327, 10.1002/cre2.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Pedersen AML; Bukkehave KH; Bennett EP; Twetman S Effect of lozenges containing Lactobacillus reuteri on the severity of recurrent aphthous ulcers: a pilot study. Probiotics Antimicrob. Proteins, 2020, 12, 819–823. [DOI] [PubMed] [Google Scholar]
- [152].Cucinotta D; Vanelli M WHO Declares COVID-19 a Pandemic. Acta Bio Medica: Atenei Parmensis 2020, 91(1), 157–160, doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cutler DM; Summers LH The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324(15), 1495, 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Shrestha N; Shad MY; Ulvi O; Khan MH; Karamehic-Muratovic A; Nguyen U-SDT; Baghbanzadeh M; Wardrup R; Aghamohammadi N; Cervantes D; et al. The impact of COVID-19 on globalization. One Health 2020, 11, 100180. doi: 10.1016/j.onehlt.2020.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].WHO Coronavirus (COVID-19) Dashboard. (n.d.). https://covid19.who.int/
- [156].Amanullah S, & Ramesh Shankar R The Impact of COVID-19 on Physician Burnout Globally: A Review. Healthcare 2020, 8(4), 421, 10.3390/healthcare8040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Avena NM; Simkus J; Lewandowski A; Gold MS; Potenza MN Substance Use Disorders and Behavioral Addictions During the COVID-19 Pandemic and COVID-19-Related Restrictions. Front. Psychiatry 2021, 12, 653674, 10.3389/fpsyt.2021.653674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kulchar RJ; Haddad M-B Preventing Burnout and Substance Use Disorder Among Healthcare Professionals Through Breathing Exercises, Emotion Identification, and Writing Activities. J. Interprofessional Educ. Pract 2022, 29, 100570, 10.1016/j.xjep.2022.100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Vindegaard N, & Benros ME COVID-19 Pandemic and Mental Health Consequences: Systematic review of the current evidence. Brain Behav. Immun 2020, 89, 531–542, 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].World Bank national accounts data, and OECD National Accounts data files. (n.d.). https://data.worldbank.org/indicator/NY.GDP.MKTP.CD.
- [161].Alcendor DJ Racial Disparities-Associated COVID-19 Mortality among Minority Populations in the US. J. Clin. Med 2020, 9(8), 2442, 10.3390/jcm9082442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Li Q; Guan X; Wu P; Wang X; Zhou L; Tong Y; Ren R; Leung KS; Lau EH; Wong JY; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med 2020, 382(13), 1199–1207, doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Wang R; Hozumi Y; Yin C; Wei G-W Mutations on COVID-19 diagnostic targets. Genomics 2020, 112(6), 5204–5213, doi: 10.1016/j.ygeno.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Wang S; Xu X; Wei C; Li S; Zhao J; Zheng Y; Liu X; Zeng X; Yuan W; Peng S Molecular evolutionary characteristics of SARS‐CoV‐2 emerging in the United States. J. Med. Virol 2021, 94(1), 310–317, doi: 10.1002/jmv.27331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Justo Arevalo S; Zapata Sifuentes D; J. Huallpa C; Landa Bianchi G; Castillo Chávez A; Garavito-Salini Casas R; Uribe Calampa CS; Uceda-Campos G; Pineda Chavarría R Dynamics of SARS-CoV-2 mutations reveals regional-specificity and similar trends of N501 and high-frequency mutation N501Y in different levels of control measures. Sci. Rep 2021, 11(1), 17755, doi: 10.1038/s41598-021-97267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang R; Chen J; Gao K; Wei G-W Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics 2021, 113(4), 2158–2170, doi: 10.1016/j.ygeno.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Charlotte N High Rate of SARS-CoV-2 Transmission Due to Choir Practice in France at the Beginning of the COVID-19 Pandemic. J. Voice 2020, S0892199720304525, 10.1016/j.jvoice.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Asadi S; Bouvier N; Wexler AS; Ristenpart WD The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol 2020, 54(6), 635–638, doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Bourouiba L Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA, 2020, 323(18), 1837–1838, doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- [170].Eiche T; Kuster M Aerosol Release by Healthy People during Speaking: Possible Contribution to the Transmission of SARS-CoV-2. Int. J. Environ. Res. Public Health 2020, 17(23), 9088, doi: 10.3390/ijerph17239088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Baghizadeh Fini M Oral saliva and COVID-19. Oral Oncol. 2020, 108, 104821, doi: 10.1016/j.oraloncology.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Huang N; Pérez P; Kato T; Mikami Y; Okuda K; Gilmore RC; Conde CD; Gasmi B; Stein S; Beach M, et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nature Med. 2021, 27, 892–903, doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Xu J; Li Y; Gan F; Du Y; Yao Y Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Den. Res 2020, 99(8), 989–989, doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- [174].Herrera D; Serrano J; Roldán S; Sanz M Is the Oral Cavity Relevant in SARS-CoV-2 Pandemic? Clin. Oral Investig 2020, 24(8), 2925–2930, doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Advice for the Public: Coronavirus Disease (COVID-19). 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-forhttps://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public-:~:text=Keep%20physical%20distance%20of%20at,rub%20or%20soap%20and%20waterpublic#:~:text=Keep%20physical%20distance%20of%20at,rub%20or%20soap%20and%20water.
- [176].Li Y; Ren B; Peng X; Hu T; Li J; Gong T; Tang B; Xu X; Zhou X Saliva is a Nonnegligible Factor in the Spread of COVID-19. Mol. Oral Microbiol 2020, 35, 141–145, doi: 10.1111/omi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cao Y; Jian F; Zhang Z; Yisimayi A; Hao X; Bao L; Yuan F; Yu Y; Du S; Wang J; et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Rep. 2022, 41(12), 10.1016/j.celrep.2022.111845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Song R; Zeng G; Yu J; Meng X; Chen X; Li J; Xie X; Lian X; Zhang Z; Cao Y Post-exposure prophylaxis with SA58 (anti-SARS-COV-2 monoclonal antibody) nasal spray for the prevention of symptomatic COVID-19 in healthy adult workers: a randomized, single-blind, placebo-controlled clinical study. Emerg. Microbes Infec 2023, 12(1), 2212806, 10.1080/22221751.2023.2212806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Si S; Jin C; Li J; Cao Y; Kan B; Xue F; Xie XS; Fang L; Zeng G; Zhang S; et al. Safety and Effectiveness of SA58 Nasal Spray Against COVID-19 Infection in Medical Personnel: An Open-Label, Blank-Controlled Study—Hohhot City, Inner Mongolia Autonomous Region, China, 2022. China CDC weekly 2023, 5(10), 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lin Y; Yue S; Yang Y; Yang S; Pan Z; Yang X; Gao L; Zhou J; Li Z; Hu L; et al. Nasal spray of neutralizing monoclonal antibody 35B5 confers potential prophylaxis against severe acute respiratory syndrome coronavirus 2 variants of concern: a small-scale clinical trial. Clin. Infect. Dis 2023, 76(3), e336–e341, 10.1093/cid/ciac448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zhang X; Luo F; Zhang H; Guo H; Zhou J; Li T; Chen S; Song S; Shen M; Wu Y A first-in-human clinical study of an intranasal spray of a cocktail containing two synergetic antibodies neutralizes Omicron BA. 4/5. MedRxiv, 2023, 2023–03, 10.1101/2023.03.17.23287398 [DOI] [Google Scholar]
- [182].Kraisit P; Hirun N;Mahadlek J; Limmatvapirat S Fluconazole-loaded Solid Lipid Nanoparticles (SLNs) as a Potential Carrier for Buccal Drug Delivery of Oral Candidiasis Treatment Using the Box-Behnken Design. J. Drug Deliv. Sci. Technol 2021, 63, 102437, doi: 10.1016/j.jddst.2021.102437. [DOI] [Google Scholar]
- [183].Aljaeid BM; Hosny KM Miconazole-loaded Solid Lipid Nanoparticles: Formulation and Evaluation of a Novel Formula with High Bioavailability and Antifungal Activity. Int. J. Nanomed 2016, 11, 441–447, doi: 10.2147/IJN.S100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Mendes AI; Silva AC; Catita JAM; Cerqueira F; Gabriel C; Lopes CM; Miconazoleloaded Nanostructured Lipid Carriers (NLC) for Local Delivery to the Oral Mucosa: Improving Antifungal Activity. Colloids Surf. B: Biointerfaces 2013, 111, 755–763, doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- [185].Makvandi P; Josic U; Delfi M; Pinelli F; Jahed V; Kaya E; Ashrafizadeh M; Zarepour A; Rossi F; Zarrabi A; Agarwal T; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv.Sci 2021, 8(8), doi: 10.1002/advs.202004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Pandey M; Choudhury H; Ying JNS; Ling JFS; Ting J; Ting JSS; Hwen IKZ; Suen HW; Kamar HSS; Gorain B; et al. Mucoadhesive Nanocarriers as a Promising Strategy to Enhance Intracellular Delivery against Oral Cavity Carcinoma. Pharmaceutics, 2022, 14(4), 795, doi: 10.3390/pharmaceutics14040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Amin MK; Boateng JS Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery. Mar. Drugs 2022, 20(3), 156, doi: 10.3390/md20030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Ho HN; Le HH; Le TG; Duong THA; Ngo VQT; Dang CT; Nguyen VM; Tran TH; Nguyen CN Formulation and Characterization of Hydroxyethyl Cellulose-based Gel Containing Metronidazole-loaded Solid Lipid Nanoparticles for Buccal Mucosal Drug Delivery. Int. J. Biol. Macromol 2022, 194, 1010–1018, doi: 10.1016/j.ijbiomac.2021.11.161. [DOI] [PubMed] [Google Scholar]
- [189].Hu F; Zhou Z; Xu Q; Fan C; Wang L; Ren H; Xu S; Ji Q; Chen X A Novel pHresponsive Quaternary Ammonium Chitosan-liposome Nanoparticles for Periodontal Treatment. Int. J. Biol. Macromol 2019, 129, 1113–1119. doi: 10.1016/j.ijbiomac.2018.09.057. [DOI] [PubMed] [Google Scholar]
- [190].Puri K; Puri N Local Drug Delivery Agents as Adjuncts to Endodontic and Periodontal Therapy. J. Med. Life 2013, 6(4), 414–419 [PMC free article] [PubMed] [Google Scholar]
- [191].Du X; Khan AR; Fu M; Ji J; Yu A; Zhai G Current Development in the Formulations of Non-injection Administration of Paclitaxel. Int. J. Pharm 2018, 542(1), 242–252, doi: 10.1016/j.ijpharm.2018.03.030. [DOI] [PubMed] [Google Scholar]
- [192].Zheng W; Zhou Q; Yuan C Nanoparticles for Oral Cancer Diagnosis and Therapy. Bioinorg. Chem. Appl 2021, 2021, 9977131, doi: 10.1155/2021/9977131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [193].Wang H; Yu J; Lu X; He X Nanoparticle Systems Reduce Systemic Toxicity in Cancer Treatment. Nanomedicine (Lond) 2016, 11(2), 103–106, doi: 10.2217/nnm.15.166. [DOI] [PubMed] [Google Scholar]
- [194].Jacob S; Nair AB; Boddu SHS; Gorain B; Sreeharsha N; Shah J An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics, 2021, 13(8), 1206, doi: 10.3390/pharmaceutics13081206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Remiro P.d.F.R.; Nagahara MHT; Azoubel RA; Franz-Montan M; d’Ávila MA; Moraes ÂM Polymeric Biomaterials for Topical Drug Delivery in the Oral Cavity: Advances on Devices and Manufacturing Technologies. Pharmaceutics, 2023, 15, 12, doi: 10.3390/pharmaceutics15010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Mostafa DAE Fast dissolving oral film: Overview. Eur. J. Biomed. Pharm. Sci 2018, 5, 86–101. [Google Scholar]
- [197].Gavaskar B; Vijaya Kumar S; Sharan G; Madhusudan Rao Y Overview on fast dissolving films. Int. J. Pharm. Pharm Sci 2010, 2, 0975–1491. [Google Scholar]
- [198].Wang M; Hu L; Xu C Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [DOI] [PubMed] [Google Scholar]
- [199].Birk SE; Mosgaard MD; Kjeldsen RB; Boisen A; Meyer RL; Nielsen LH Management of oral biofilms by nisin delivery in adhesive microdevices. Eur. J. Pharm. Biopharm 2021, 167, 83–88. doi: 10.1016/j.ejpb.2021.07.007. [DOI] [PubMed] [Google Scholar]
- [200].Mazzoni C; Nielsen LH Chapter 10 – Microdevices to successfully deliver orally administered drugs. Nanotechnology for Oral Drug Delivery, 2020, 285–315, doi: 10.1016/B978-0-12-818038-9.00012-0. [DOI] [Google Scholar]
- [201].Helal SH; Abdel-Aziz HMM; El-Zayat MM; Hasaneen MNA Preparation, Characterization and Properties of Three Different Nanomaterials Either Alone or Loaded with Nystatin or Fuconazole Antifungals. Sci. Rep 2022, 12(1), 22110, doi: 10.1038/s41598-022-26523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Humelnicu A-C; Samoilă P; Cojocaru C; Dumitriu R; Bostănaru A-C; Mareș M; Harabagiu V; SIMIONESCU BC Chitosan-Based Therapeutic Systems for Superficial Candidiasis Treatment. Synergetic Activity of Nystatin and Propolis. Polymers, 2022, 14(4), 689, doi: 10.3390/polym14040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Roque L; Alopaeus J; Reis C; Rijo P; Molpeceres J; Hagesaether E; Tho I; Reis C Mucoadhesive Assessment of Different Antifungal Nanoformulations. Bioinspir. Biomim. 2018, 13(5), 055001, doi: 10.1088/1748-3190/aad488. [DOI] [PubMed] [Google Scholar]
- [204].Yu W-J; Huang D-X; Liu S; Sha Y-L; Gao F-H; Liu H Polymeric Nanoscale Drug Carriers Mediate the Delivery of Methotrexate for Developing Therapeutic Interventions Against Cancer and Rheumatoid Arthritis. Front. Oncol 2020, 10, doi: 10.3389/fonc.2020.01734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Johnston TP Anatomy and Physiology of the Oral Mucosa, Oral Mucosal Drug Delivery and Therapy, 2015, 1–16, doi: 10.1007/978-1-4899-7558-4. [DOI] [Google Scholar]
- [206].Li Q; Liu Y; Huang Z; Guo Y; Li Q Triggering Immune System With Nanomaterials for Cancer Immunotherapy. Front. Bioeng. Biotechnol 2022, 10, 878524, doi: 10.3389/fbioe.2022.878524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Zhang Y; Jiang R; Lei L; Yang Y; Hu T Drug Delivery Systems for Oral Disease Applications. J. Appl. Oral. Sci 2022, 30, doi: 10.1590/1678-7757-2021-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].AbouSamra MM; Basha M; Awad GEA; Mansy SS A Promising Nystatin Nanocapsular Hydrogel as an Antifungal Polymeric Carrier for the Treatment of Topical Candidiasis. J. Drug Deliv. Sci. Technol 2019, 49, 365–374, doi: 10.1016/j.jddst.2018.12.014. [DOI] [Google Scholar]
- [209].Ha Y-J; Lee S-M; Mun CH; Kim HJ; Bae Y; Lim J-H; Park K-H; Lee S-K; Yoo K-H; Park Y-B Methotrexate-loaded Multifunctional Nanoparticles with Near-infrared Irradiation for the Treatment of Rheumatoid Arthritis. Arthritis Res. Ther 2020, 22(1), 146, doi: 10.1186/s13075-020-02230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Jang J-H; Jeong S-H; Lee Y-B Preparation and In Vitro/In Vivo Characterization of Polymeric Nanoparticles Containing Methotrexate to Improve Lymphatic Delivery. Int. J. Mol. Sci 2019, 20(13), doi: 10.3390/ijms20133312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Singh AK; Mukerjee A; Pandey H; Mishra SB Miconazole Nitrate–Loaded Solid Lipid Nanoparticle-Based Hydrogel Ameliorate Candida albicans Induced Mycoses in Experimental Animals. BioNanoScience 2022, 12, 512–526, doi: 10.1007/s12668-022-00948-4. [DOI] [Google Scholar]
- [212].Wang H-X; Li M; Lee CM; Chakraborty S; Kim H-W; Bao G; Leong KW CRISPR/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery. Chem. Rev 2017, 117(15), 9874–9906, doi: 10.1021/acs.chemrev.6b00799. [DOI] [PubMed] [Google Scholar]
- [213].Zylberberg C; Gaskill K; Pasley S; Matosevic S Engineering Liposomal Nanoparticles for Targeted Gene Therapy. Gene Ther. 2017, 24(8), 441–452, doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- [214].Young M; Overlid N; Konopka K; Düzgünes N Gene Therapy for Oral Cancer: Efficient Delivery of a ‘Suicide Gene’ to Murine Oral Cancer Cells in Physiological Milieu. J. Calif. Dent. Assoc, 2005, 33(12), 967–971 [PubMed] [Google Scholar]
- [215].Wu J; Guo Q; Zhang G; Zhao L; Lv Y; Wang J; Liu J; Shi W Study on the Targeted Therapy of Oral Squamous Cell Carcinoma with a Plasmid Expressing PE38KDEL Toxin Under Control of the SERPINB3 Promoter, Cancer Med. 2020, 9(6), 2213–2222, doi: 10.1002/cam4.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Bapat RA; Dharmadhikari S; Chaubal TV; Amin MCIM; Bapat P; Gorain B; Choudhury H; Vincent C; Kesharwani P The Potential of Dendrimer in Delivery of Therapeutics for Dentistry, Heliyon 2019, 5(10), doi: 10.1016/j.heliyon.2019.e02544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Wu D; Yang J; Li J; Chen L; Tang B; Chen X; Wu W; Li J Hydroxyapatite-anchored Dendrimer for in Situ Remineralization of Human Tooth Enamel, Biomaterials, 2013, 34(21), 5036–5047, doi: 10.1016/j.biomaterials.2013.03.053. [DOI] [PubMed] [Google Scholar]
- [218].Zhou Y; Yang J; Lin Z; Li J; Liang K; Yuan H; Li S; Li J Triclosan-loaded Poly(amido amine) Dendrimer for Simultaneous Treatment and Remineralization of Human Dentin. Colloids Surf. B: Biointerfaces 2014, 115, 237–243, doi: 10.1016/j.colsurfb.2013.11.045. [DOI] [PubMed] [Google Scholar]
- [219].Chauhan AS; Dendrimers for Drug Delivery. Molecules 2018, 23(4), 938, doi: 10.3390/molecules23040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Yandrapu SK; Kanujia P; Chalasani KB; Mangamoori L; Kolapalli RV; Chauhan A Development and Optimization of Thiolated Dendrimer as a Viable Mucoadhesive Excipient for the Controlled Drug Delivery: An Acyclovir Model Formulation, Nanomedicine: Nanotechnology. Biol. Med 2013, 9(4), 514–522, doi: 10.1016/j.nano.2012.10.005. [DOI] [PubMed] [Google Scholar]
- [221].Tu Z; Zhong Y; Hu H; Shao D; Haag R; Schirner M; Lee J; Sullenger B; Leong KW Design of Therapeutic Biomaterials to Control Inflammation. Nat. Rev. Mater 2022, 7(7), 557–574, doi: 10.1038/s41578-022-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Huang H; Pan W; Wang Y; Kim HS; Shao D; Huang B; Ho T-C; Lao Y-H; Quek CH; Shi J; et al. Nanoparticulate Cell-free DNA Scavenger for Treating Inflammatory Bone Loss in Periodontitis. Nat. Commun 2022, 13, doi: 10.1038/s41467-022-33492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Divakar DD; Jastaniyah NT; Altamimi HG; Alnakhli YO; Muzaheed; Alkheraif AA; Haleem S Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens, Int. J. Biol. Macromol, 2018, 108, 790–797, doi: 10.1016/j.ijbiomac.2017.10.166. [DOI] [PubMed] [Google Scholar]
- [224].Noronha VT; Paula AJ; Duran G; Galembeck A; Cogo-Muller K; Franz-Montan M; Duran N Silver nanoparticles in dentistry, 2017, 33(10), 1110–1126, doi: 10.1016/j.dental.2017.07.002. [DOI] [PubMed] [Google Scholar]
- [225].Bapat RA; Chaubal TV; Dharmadhikari S; Abdulla AM; Bapat P; Alexander A; Dubey SK; Kesharwani P Recent Advances of Gold Nanoparticles as Biomaterial in Dentistry. Int. J. Pharm 2020, 586, doi: 10.1016/j.ijpharm.2020.119596. [DOI] [PubMed] [Google Scholar]
- [226].Heo DN; Ko W-K; Lee HR; Lee SJ; Lee D; Um SH; Lee JH; Woo Y-H, Zhang LG; Lee D-W; Kwon IK Titanium Dental Implants Surface-immobilized with Gold Nanoparticles as Osteoinductive Agents for Rapid Asseointegration. J. Colloid Interface Sci 2016, 469, 129–137, doi: 10.1016/j.jcis.2016.02.022. [DOI] [PubMed] [Google Scholar]
- [227].Ipar VS; Dsouza A; Devarajan PV Enhancing Curcumin Oral Bioavailability Through Nanoformulations. Eur. J. Drug Metab. Pharmacokinet 2019, 44(4), 459–480, doi: 10.1007/s13318-019-00545-z. [DOI] [PubMed] [Google Scholar]
- [228].Meena J; Gupta A; Ahuja R; Singh M; Bhaskar S; Panda AK Inorganic Nanoparticles for Natural Product Delivery: A Review. Environ. Chem. Lett 2020, 18, 2107–2118, doi: 10.1007/s10311-020-01061-2. [DOI] [Google Scholar]
- [229].Fang L; Zhou H; Cheng L; Wang Y; Liu F; Wang S The Application of Mesoporous Silica Nanoparticles as a Drug Delivery Vehicle in Oral Disease Treatment. Front. Cell Infect. Microbiol 2023, 13, doi: 10.3389/fcimb.2023.1124411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Song Y; Cai L; Tian Z; Wu Y; Chen J Phytochemical Curcumin-Coformulated, Silver-Decorated Melanin-like Polydopamine/Mesoporous Silica Composites with Improved Antibacterial and Chemotherapeutic Effects against Drug-Resistant Cancer Cells. ACS Omega. 2020, 5(25), 15083–15094, doi: 10.1021/acsomega.0c00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Möller K; Bein T Degradable Drug Carriers: Vanishing Mesoporous Silica Nanoparticles. Chem. Mater 2019, 31(12), 4364–4378, doi: 10.1021/acs.chemmater.9b00221. [DOI] [Google Scholar]
- [232].Shao D; Li M; Wang Z; Zheng X; Lao Y-H; Chang Z; Zhang F; Lu M; Yue J; Hu H; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater 2018, 30(29), doi: 10.1002/adma.201801198. [DOI] [PubMed] [Google Scholar]
- [233].Patel A; Patel M; Yang X; Mitra AK Recent Advances in Protein and Peptide Drug Delivery: A Special Emphasis on Polymeric Nanoparticles. Protein Pept. Lett 2014, 21(11), 1102–1120, doi: 10.2174/0929866521666140807114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Patra JK; Das G; Fraceto LF; Campos EVR; Rodriguez-Torres MDP; Acosta-Torres LS; Diaz-Torres LA; grillo R; Swamy MK; Sharma S; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16(1), doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Şenel S; Özdoğan AI; Akca G Current Status and Future of Delivery Systems for Prevention and Treatment of Infections in the Oral Cavity. Drug Deliv. Transl. Res 2021, 11(4), 1703–1734, doi: 10.1007/s13346-021-00961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Nguyen OOT; Tran KD; Ha NT; Doan SM; Dinh TTH; Tran TH Oral Cavity: An Open Horizon for Nanopharmaceuticals. J. Pharm. Investig 2021, 51, 413–424, doi: 10.1007/s40005-021-00530-2. [DOI] [Google Scholar]
- [237].Lamson NG; Berger A; Fein KC; Whitehead KA Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat Biomed Eng. 2020. Jan;4(1):84–96. doi: 10.1038/s41551-019-0465-5. Epub 2019 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Gunzel D; Yu ASL Claudins and the Modulation of Tight Junction Permeability. Physiol Rev., 2013, 93(2), 525–569, doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Date AA; Hanes J; Ensign LM Nanoparticles for oral delivery: Design, evaluation and state-of-the-art. J Control Release. 2016. Oct 28;240:504–526. doi: 10.1016/j.jconrel.2016.06.016. Epub 2016 Jun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lundquist P; Arturrson P Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev 2016, 106(Part B), 256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- [241].Teubl BJ; Stojkovic B; Docter D; Pritz E; Leitinger G; Poberaj I; Prassl R; Stauber RH; Fröhlich E; Khinast JG, et al. The effect of saliva on the fate of nanoparticles. Clin Oral Investig. 2018. Mar;22(2):929–940. doi: 10.1007/s00784-017-2172-5. Epub 2017 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Nabeta HW; Zahin M; Fuqua JL; Cash ED; Leth I; Strauss M; Novak J; Wang L; Siegwald A; Sheppard RA; et al. A Phase 1a/1b Clinical Trial Design to Assess Safety, Acceptability, Pharmacokinetics and Tolerability of Intranasal Q-Griffithsin for COVID-19 Prophylaxis. ULJRI 2022, 6(1), 22. [Google Scholar]
- [243].Label: Palforzia Initital Dose Escalation- Peanut Kit. (n.d.). DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=17f5be03-6705-4ac9-b8f3-https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=17f5be03-6705-4ac9-b8f3-bc4993ebc0ebbc4993ebc0eb.
- [244].Label: Sucraid- sacrosidase solution (n.d.). DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d613bb7f-c3f4-462e-81a2-https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d613bb7f-c3f4-462e-81a2-da2347cc4b6bda2347cc4b6b.
- [245].Raplixa Package Insert. (n.d.). US Food and Drug Administration. https://www.fda.gov/media/91418/download.
- [246].Vistaseal Package Insert. (n.d.). US Food and Drug Administration. https://www.fda.gov/media/159369/download.
- [247].Label: StrataGraft- allogeneic cultured keratinocytes and dermal fibroblasts in murine collagendsat cellular sheet. (n.d.). DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9aa1103-ff31-4a93-b319-https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9aa1103-ff31-4a93-b319-1ccb604f0b0d1ccb604f0b0d.
- [248].Oxervate Package Insert. (n.d.). US Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761094s000lbl.pdf.
- [249].Butterfield JSS; Li X; Arisa S; Kwon K-C; Daniell H; Herzog RW Potential Role for Oral Tolerance in Gene Therapy. Cell. Immunol 2023, doi: 10.1016/j.cellimm.2023.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Berwick DM Salve Lucrum: The Existential Threat of Greed in US Health Care. JAMA. 2023, 329(8), 629–630. doi: 10.1001/jama.2023.0846. [DOI] [PubMed] [Google Scholar]
- [251].Daniell H; Jin S; Zhu X-G; Gitzendanner MA; Soltis DE; Soltis PS Green Giant-a Tiny Chloroplast Genome with Mighty Power to Produce High-value Proteins: History and Phylogeny. Plant Biotechnol. J 2021, 19(3), 430–447. doi: 10.1111/pbi.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].He W; Baysal C; Gómez ML; Huang X; Alvarez D; Zhu C; Armario-Najera V; Perera AB; Bennaser PC; Saba-Mayoral A; et al. Contributions of the International Plant Science Community to the Fight Against Infectious Diseases in Humans–Part 2: Affordable Drugs in Edible Plants for Endemic and Re‐emerging Diseases, Plant Biotechnol. J 2021, 19(10), 921–1936, doi: 10.1111/pbi.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]


