Abstract
The deleterious effects of adversity are likely intergenerational, such that one generation’s adverse experiences can affect the next. Epidemiological studies link maternal adversity to offspring depression and anxiety, possibly via transmission mechanisms that influence offspring fronto-limbic connectivity. However, studies have not thoroughly disassociated postnatal exposure effects nor considered the role of offspring sex. We utilized infant neuroimaging to test the hypothesis that maternal childhood maltreatment (CM) would be associated with increased fronto-limbic connectivity in infancy and tested brain-behavior associations in childhood. Ninety-two dyads participated (32 mothers with CM, 60 without; 52 infant females, 40 infant males). Women reported on their experiences of CM and non-sedated sleeping infants underwent MRIs at 2.44 ± 2.74 weeks. Brain volumes were estimated via structural MRI and white matter structural connectivity (fiber counts) via diffusion MRI with probabilistic tractography. A subset of parents (n = 36) reported on children’s behaviors at age 5.17 ± 1.73 years. Males in the maltreatment group demonstrated greater intra-hemispheric fronto-limbic connectivity (b = 0.96, p = 0.008, [95%CI 0.25, 1.66]), no differences emerged for females. Fronto-limbic connectivity was related to somatic complaints in childhood only for males (r = 0.673, p = 0.006). Our findings suggest that CM could have intergenerational associations to offspring brain development, yet mechanistic studies are needed.
Keywords: Adversity, intergenerational transmission, infant neuroimaging
The mechanisms underlying the deleterious effects of early life adversity – particularly childhood maltreatment (CM) – continue to be investigated, yet long-lasting impacts on physical and mental health are well-documented.1 Effects may also be intergenerational, such that the experiences of adversity in one generation could affect the next.2–5 Understanding the potential intergenerational effects of adversity may open new avenues for intervention with broad reaching influence in deterring psychiatric illness in subsequent generations.
Offspring of women with a history of childhood adversity are at a higher risk for externalizing, depressive, and anxiety disorders.6,7 Although findings are equivocal, effects often vary depending on offspring sex, such that male offspring may be more susceptible to intergenerational adversity early in life.8–10 Several transmission mechanisms are hypothesized, including physiological changes that lead to alterations to the intrauterine environment. For example, childhood adversity may influence the hypothalamic–pituitary–adrenal (HPA) axis and immune functioning in women and in turn, these alterations influence fetal brain development via increased exposure to glucocorticoids or cytokines in utero.2,11,12 Preclinical studies provide strong support for these mechanisms,13 yet significant questions about intergenerational transmission in humans remain.
Identifying intergenerational transmission effects independent of influences exerted by the postnatal environment has proven difficult in human research. Postnatal influences include factors like social learning, parenting, and the shared parent–infant environment. These factors can have downstream effects, complicating the disassociation of maternal adversity effects from those of postnatal exposures – both of which may impact offspring. For example, maternal CM may affect offspring brain and behavior via parenting, as data suggest individuals with a history of CM may parent differently.14,15 Infant neuroimaging provides a unique opportunity to index neurodevelopmental effects of maternal childhood stress while greatly minimizing postnatal influences.
Three infant neuroimaging studies of maternal childhood adversity exist. The first documented reduced intracranial volume, particularly within gray matter in offspring of mothers who self-reported experiences of CM (abuse and neglect).16 The second also documented decreased gray matter volume, as well as reduced amygdala volumes.17 The third showed that childhood neglect was associated with increased resting state functional connectivity between the bilateral amygdala and the dorsal anterior cingulate cortex (dACC) and ventral medial orbitofrontal cortex.18 This last study may comport with the hypothesis that the maternal HPA axis has a role in adversity transmission, as limbic and prefrontal regions are rich in glucocorticoid receptors and sensitive to prenatal glucocorticoid exposure.19
Prior work has been limited by inconsistently considering the critical role of offspring sex. This is particularly important if intergenerational effects are mediated through in utero glucocorticoid exposure. Preclinical and human research suggest offspring sex may impact susceptibility to glucocorticoid exposure-mediated neurodevelopmental changes, yet studies have been inconsistent in whether males or females are more susceptible.19–21 Despite the strong support for sex effects in preclinical research on intergenerational adversity, small sample sizes have often limited the exploration of sex effects. In the three, extant infant studies, the MRI sample sizes were 80, 57, and 48. In the latter two, important sex-specific effects might have been missed because of limited statistical power to detect interactions. Studies are further limited by not including longitudinal assessments of child symptomatology, without which the clinical or behavioral significance of the documented neural/MRI differences remain unknown.
We thus aim to extend our understanding of the associations between maternal CM and infant offspring brain development, while accounting for other factors important to brain development like socioeconomic status (SES) and prenatal distress.22 The present study represents a significant expansion of the literature by examining structural connectivity (i.e., white matter connectivity) and by exploring associations to parent-reported childhood behaviors. Neonatal white matter connectivity predicts risk for socioemotional problems in childhood23 and is sensitive to the effects of early life adversity,24 signifying a potential role in the intergenerational transmission of adversity. We hypothesized maternal CM would be related to increased fronto-limbic connectivity; we did not make directional hypotheses on offspring sex effects because of the previously mixed results. To maximize our sample size, we combine two infant MRI datasets using state-of-the-art harmonization techniques. One cohort is from mother–infant dyads in New York City and the other from São Paulo, Brazil – both are populations with high rates of childhood adversity. We examine white matter connectivity and regional gray matter volumes. To control for possible confounds, we employ propensity weighting, a technique used when randomization is not possible to minimize bias introduced by confounders that may be associated to both the exposure (maternal CM) and the outcome (offspring neurodevelopment).25,26 This technique has been highlighted as particularly helpful in prenatal programing work, where randomization is impossible.27 Finally, in a subset up participants, we explore relations between white matter connectivity and behaviors in childhood, up to age 8, aiming to elucidate the potential impact of maternal childhood adversity on the next generation.
Methods
Procedures
The study combined data from two cohorts: Cohort 1 was based at the Universidade Federal de São Paulo, Brazil and Cohort 2 was a study on prenatal selective serotonin reuptake inhibitor (SSRI) exposure effects in New York City.28,29 In both studies, pregnant women were recruited through obstetricians, midwives, and psychiatrists and were invited to participate in prenatal interviews, biospecimen collections, and infant MRIs. All study procedures were approved by appropriate Institutional Review Boards. Interested participants were screened for eligibility and consented. Prenatal interviews were conducted in the third trimester of gestation and included reports of CM and perinatal stressors, clinical interviews, and demographic questionnaires. Infants were scanned at 2.44 ± 2.74 weeks post birth and a subset of parents reported on children’s behaviors at age 5.17 ± 1.73 years.
Participants
Participants included 92 infant–mother dyads with usable structural (T2w and diffusion-weighted images (DWIs)) infant MRI data (29 Cohort 1, 63 Cohort 2). Women were 29.04 ± 6.01 years old at the time of recruitment, and the infants included 40 males and 52 females. Forty percent of participants identified as Hispanic or Latine (19.6% not Hispanic or Latine, 40.2% missing). Twenty-three percent identified as Other race, followed by 16.3% who identified as White and 12% who identified as Black or African American, 5.4% who identified as biracial, and 1.1% who identified as American Indian (40.2% missing). Exclusion criteria included maternal prenatal psychotropic medication use, offspring MRI contraindications (e.g., irremovable metal), and gestational complications that resulted in a neonatal intensive care unit stay. Supplemental methods detail recruitment procedures and exclusion criteria across cohorts. For Cohort 2, this meant that dyads were excluded from the present study if the woman had taken a serotonin-based antidepressant (SSRI) during pregnancy (n = 16). SSRIs may impact prenatal brain development due to the critical role of serotonin in neurodevelopment.30 In fact, our prior work documented significant differences in brain structure and connectivity in SSRI exposed offspring, which would have resulted in large confounds in the current analysis.28 While it is possible that this exclusion introduced sampling bias, excluded mother–infant dyads did not differ in the exposures of interest: CM, prenatal distress, or substance use (see Table S1).
Measures
Demographic characteristics
Demographic characteristics included: maternal age, pre-pregnancy body mass index (BMI; based on medical record or self-report), prenatal medication use (e.g., over-the-counter allergy medications), infant sex, weight at MRI scan, gestational age at delivery and at scan, birth type, and SES. In Cohort 1, SES was indexed using the Brazilian Socioeconomic Scale (ABEP), a widely used and official categorization system for SES stratification in Brazil. In Cohort 2, mothers reported on household income. Data was harmonized by creating a three-level categorical variable; see Table 1.
Table 1.
Demographic characteristics
| Name | Cases CM+ (n = 32) | Controls CM−(n = 60) | Test Statistic (df) | p value |
|---|---|---|---|---|
| Infant sex | X2 (1) = 0.230 | 0.631 | ||
| Male | n = 15; 16% | n = 25; 27% | ||
| Female | n = 17; 19% | n = 35; 38% | ||
| Infant weight at scan (grams) | 3850.093 ± 776.953 | 3771.167 ± 6000.043 | F (1,90) = 0.288 | 0.593 |
| Infant gestational age at birth (weeks) | 39.574 ± 1.383 | 39.356 ± 1.309 | F (1,90) = 0.715 | 0.400 |
| Postmenstrual age at scan (weeks) | 42.641 ± 1.743 | 42.413 ± 2.916 | F (1,90) = 0.164 | 0.687 |
| Maternal age | 28.653 ± 6.243 | 29.236 ± 5.936 | F (1,90) = 0.190 | 0.664 |
| Birth type | X2 (2) = 2.692 | 0.260 | ||
| C-section | n = 10; 11% | n = 24; 26% | ||
| Vaginal delivery | n = 22; 24% | n = 33; 36% | ||
| Other (forceps, induced, etc.) | n = 0; 0% | n = 3; 3% | ||
| Socioeconomic class | X2 (2) = 0.303 | 0.859 | ||
| 1: $0–25,000 or D-E | n = 9; 10% | n = 15; 16% | ||
| 2: $25,001–100,000 or C2, C1, B2, B1 | n = 19; 21% | n = 39; 42% | ||
| 3: $100,001+ or A | n = 4; 4% | n = 6; 7% | ||
| Cohort | X2 (1) = 0.002 | 0.967 | ||
| Cohort 1 | n = 10; 11% | n = 19; 21% | ||
| Cohort 2 | n = 22; 24% | n = 41; 44% | ||
| Maternal prenatal BMI | 26.402 ± 5.691 | 25.366 ± 5.356 | F (1,90) = 0.740 | 0.329 |
| Prenatal medication use 1 | X2 (1) = 0.021 | 0.886 | ||
| Yes | n = 6; 7% | n = 12; 13% | ||
| No | n = 26; 28% | n = 48; 52% | ||
| Prenatal alcohol, substance, and tobacco use | X2 (1) = 4.257 | 0.039 | ||
| Yes | n = 10; 11% | n = 8; 9% | ||
| No | n = 22; 24% | n = 52; 56% | ||
| Prenatal maternal distress | X2 (1) = 11.932 | 0.001 | ||
| Yes | n = 17; 19% | n = 11; 12% | ||
| No | n = 15; 16% | n = 49; 53% | ||
| CBCL somatic complaints 2 | 55.714 ± 6.12 | 54.000 ± 6.532 | F (1,34) = 0.618 | 0.437 |
| CBCL attention problems 2 | 55.429 ± 6.969 | 52.955 ± 6.004 | F (1,34) = 1.282 | 0.265 |
| CBCL aggressive behaviors 2 | 55.857 ± 5.628 | 53.909 ± 7.412 | F (1,34) = 0.705 | 0.407 |
| CBCL internalizing problems 2 | 54.214 ± 10.055 | 48.182 ± 12.435 | F (1,34) = 2.231 | 0.137 |
| CBCL externalizing problems 2 | 51.786 ± 10.693 | 48.955 ± 10.943 | F (1,34) = 0.583 | 0.451 |
| Child age at CBCL assessment 2 | 5.396 ± 1.182 | 5.0339 ± 1.709 | F (1,34) = 0.366 | 0.549 |
BMI, body mass index; CM, childhood maltreatment.
Prenatal SSRI users were excluded. Medications included allergy medications, etc.
Total CBCL n = 36.
Maternal Childhood Trauma Questionnaire
The Childhood Traumatic Questionnaire (CTQ), a widely used 28-item self-report of experiences of CM,31 was used to index CM in both cohorts. It yields 5 subscales: physical abuse, physical neglect, sexual abuse, emotional abuse, and emotional neglect. Higher scores indicate greater maltreatment. In Cohort 1, a version of the CTQ (QUESI) validated for use with Brazilian populations32 was used. Dichotomous variables were used to indicate presence and absence of abuse or neglect for each subscale (following publication manual cutoffs).33 Women who endorsed the presence of abuse or neglect on any subscale were placed in the positive history of CM group (CM+); all others were placed in the negative history group (CM−). Although it has been documented that experiences of abuse and neglect can have different sequalae,34 the present sample demonstrated too much overlap between the two domains to examine the unique contributions of both types of maltreatment. For example, only 3 women endorsed having only experienced abuse and not having simultaneously experienced neglect. This same issue limited us from examining the unique contribution of each of the five maltreatment domains.
Prenatal maternal distress
A dichotomous prenatal maternal distress variable was created to harmonize the different indicators assessed across both studies. Given the different measures and different scales of the measures used, it was not possible to create a continuous variable. In Cohort 1, mothers were characterized as experiencing prenatal distress if they either endorsed 3 or more symptoms of depression and/or anxiety on the MINI International Neuropsychiatric Interview (MINI)35 or endorsed two or more items on the Abuse Assessment Screen.36 In Cohort 2, mothers who had scores higher or equal to 27 on the Perceived Stress Scale,37 18 on the Hamilton Anxiety Rating Scale,38 or 16 on the Center for Epidemiological Studies-Depression scale39 were characterized as having experienced prenatal distress. Because prior prenatal programing literature suggests that symptoms of prenatal depression, anxiety, and stress26,40–42 are related to child emotional outcomes, even when symptomatology does not meet full diagnostic criteria, cutoffs were chosen to index distress not limited to disorders meeting diagnostic criteria. However, to examine whether these admittedly arbitrary cutoffs inflated estimates, we conducted sensitivity analyses where we only considered mothers with a depressive or anxiety disorder as meeting criteria for prenatal maternal distress and results did not change substantially. See Supplemental Analyses and Table S2.
Prenatal maternal alcohol, substance, and tobacco use
Mothers self-reported use via the MINI International Neuropsychiatric Interview (Cohort 1) or the Schedule for Affective Disorders and Schizophrenia (SADS1; Cohort 2). A dichotomous variable was created, where any endorsement of use alcohol, tobacco, or substance (e.g., cannabis) during pregnancy resulted in classification as positive for use.
Child Behavior Checklist (CBCL)
A subset of dyads (n = 36) were re-contacted and mothers completed the CBCL, a widely used parental report of childhood behaviors. Children were 3–7 years old at the time of the assessment, thus both the preschool (1.5–5 years)43 and school-age (6–18 years) 44 versions of the CBCL were used. Mothers who completed CBCL reports did not differ on CM group status from those that did not complete the CBCL (CBCL CM+: n = 14, CM−: n = 22; no CBCL: CM+: n = 18, CM-: n = 38; X2(1) = 0.440, p = .507), yet they had lower prenatal BMI and their children had greater weight at birth (Table S8). Child age at CBCL completion did not differ between CM groups, see Table 1.
MRI acquisition, harmonization, and processing
Structural and diffusion MRIs were acquired on 3T whole-body scanners. Non-sedated, sleeping infants were scanned. Supplemental methods provide scanning protocols for each site. The Developing Human Connectome Project pipeline45 and a customized approach to yield finer parcellations of the frontal lobe46 were used to segment T2 images and estimate cortical and sub-cortical volumes. The dHCP pipeline is a fully automated cortical surface-based processing pipeline developed specifically for segmenting the developing neonatal brain. Further details about the pipeline are provided in the supplement.
After preprocessing (denoising and distortion correction), DWIs underwent probabilistic tractography using the MRtrix pipeline. Briefly, preprocessing included denoising, de-Gibbs ringing, and motion/eddy current correction with eddy_cuda9.1.47 We estimated fiber orientation distributions (FODs) from the preprocessed data using multi-shell multi-tissue constrained spherical deconvolution, and log-domain intensity normalizing. Probabilistic tractography was performed by taking the second-order integration over the FODs (iFOD2), using the anatomically constrained tractography48 framework. Streamline counts were the connectivity metric chosen as our outcome variable.
A total of 92 participants had usable fiber count estimate data (DTI); 3 participants were dropped from the volumetric analyses due to failure to properly complete the subcortical segmentation pipeline (1 CM+, 2 CM-). Next, ComBat49 was used to model and remove unwanted inter-site variability in volumetric and fiber count estimates. ComBat is a harmonization tool originally developed for the removal of batch-effect in genomics, which has become a standard tool in multi-site MRI research due to its success in removing unwanted variation introduced by MR scanners while preserving biological variability.49,50 To ensure no systematic differences attributable to site, scanner, or pulse sequence remained in the data, site differences in connectivity, volumetric, and principal component data were tested. Analyses (Table S3) revealed only one white connectivity variable differed between the cohorts, yet this difference was not significant when we controlled for multiple comparisons (via FDR correction). Supplemental methods provide details.Regions of Interest (ROI). Analyses examined differences in fronto-limbic circuitry, consistent with prior studies.16,18,19 Volumetric ROIs included the bilateral amygdala, hippocampus, caudate, and anterior cingulate cortex (ACC). White matter connectivity analyses examined connections within these regions and the frontal lobe. Further details on the processes presented in the Supplemental methods. Total intracranial volume, white matter, and cortical and subcortical gray matter volumes were examined to maximize overlap with prior work.16
Statistical analysis
Propensity weighting
To account for potential confounders, inverse probability of treatment weighting using propensity scores was implemented via the WeightIt R package.26,51 WeightIt generates inverse probability weights from propensity scores through logistic regression to equally distribute confounders across exposed and unexposed groups.25,52 Weights were generated using the following variables, all of which were selected as could impact offspring brain development and confound results: maternal age, pre-pregnancy BMI, prenatal medication use, infant weight at scan, infant gestational age at delivery and at scan, birth type, and SES. Standardized mean differences were smaller than 0.05 for all variables, coefficients of variation were less than 0.47, and the effective sample sizes were 56.91 for the CM- group and 27.89 for CM + group.52,53
Dimension reduction
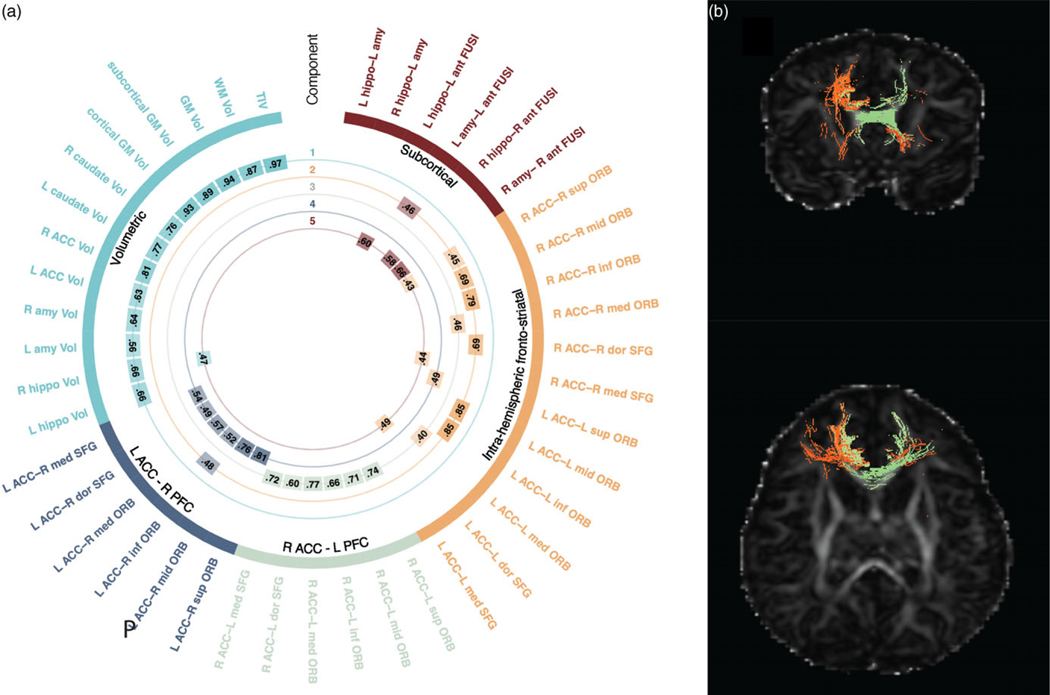
Dimension reduction of MRI data was conducted using principal components analysis (PCA). Varimax rotation was applied to obtain parsimonious components, and a scree plot (Fig. S1) was used to determine the optimal number of components. Five components, explaining 55% of the variance across all examined connections (n = 30) and volumetric estimates (n = 13) were selected (Fig. 1). Based on inspection of the loadings, we henceforth refer to the five components as volumetric, intra-hemispheric fronto-limbic connectivity, right ACC–left prefrontal cortex (PFC) connectivity, left ACC–right PFC connectivity, and subcortical connectivity. See Fig. 1a and Table S4 for component loadings. Dimension reduction was employed for two reasons. First, based on prior work on infant neuroimaging that suggests that the microstructure of individuals tracts is highly correlated, and that using a common factor approach (specifically PCA) is an appropriate way to examine infant brain development.54,55 Low-dimensional components from PCA analyses may account for almost half of the variance of white matter tract microstructure in newborn brains54 and correlated with concurrent and future cognitive functioning.55 Second, we employed PCA to minimize the number of statistical comparisons, reducing our dependent variables from the 43 original ROIs to 5 components. Individual variables are listed in Table S4, and the ROI selection process is detailed in the Supplemental Methods.
Figure 1.
Principal components representing volumetric and connectivity variables. Visual representation of volumetric and structural connectivity variable loadings onto the 5 principal components. a. The outermost labels indicate the individual connectivity and volumetric variables included in analyses; color arcs represent the five components yielded via Varimax. The numbers in the inner rings represent the loadings for each variable onto the component, thresholded at 0.5 for interpretability. b. Exampleof an individual infant’s white matter connectivity. White matter tracts colored in orange loaded into the intra-hemispheric fronto-limbic connectivity component, tracts colored in green loaded into the right ACC–left PFC connectivity component.
Hypothesis testing
Weighted linear regressions were used to assess relations between maternal CM and individual brain components. Primary regressions included prenatal maternal distress, alcohol, substance, and tobacco and the variables used to generate the weights as covariates. All regressions were also conducted without controlling for prenatal distress and substance use; these two sets of analyses allowed us to examine CM effects with and without adjusting for these two factors, which could conceivably arise from downstream effects of our exposure of interest.1 All regressions included infant sex by CM interaction terms. Supplemental linear regressions between CM and the individual volumetric and connectivity measures show individual associations in Table S5.
Exploratory analysis
Bivariate partial correlations assessed associations between the brain volume and connectivity components and maternal reports of children’s behaviors and symptoms on the CBCL. See Supplemental Methods for details. These exploratory analyses were explicitly hypothesis generating.
Sensitivity analyses
Although ComBat was used, sensitivity analyses tested study site effects in our main models. We re-ran any significant models including study site as an interaction term. Similarly, given concerns that retrospective reports of emotional abuse or emotional or physical neglect may show the lowest concordance with prospectively assessed ACEs,56 primary models were re-ran including in the CM + group only the participants who endorsed childhood sexual or physical abuse. Participants who were previously categorized in the CM + group but only endorsed emotional abuse, neglect, and physical neglect were dropped from these analyses.
Results
Demographics
The sample consisted of 92 infant–mother dyads. Thirty-two self-reported having experienced significant CM (CM+), 60 did not (CM-). Groups did not differ significantly on infant weight or gestational age, maternal age, prenatal BMI, birth type, SES, prenatal medication use, cohort, or CBCL. Mothers in the CM + group showed higher levels of prenatal maternal distress and prenatal alcohol, substance, and tobacco use (Table 1).
The cohorts did not differ on the exposures of interests: CM group status (Cohort 1: CM+: n = 19, CM−: n = 10; Cohort 2: CM+: n = 41, CM−: n = 22; X2(1) = 0.002, p = 0.967), maternal distress (Cohort 1: Distress: n = 8, No Distress -: n = 21; Cohort 2: Distress: n = 20, No Distress: n = 43; X2(1) = 0.162, p = 0.687), or alcohol, substance, and tobacco use (Cohort 1: Substance +: n = 9, Substance -: n = 20; Cohort 2: Substance +: n = 9, Substance -: n = 54; X2(1) = 3.540, p = 0.060). In Cohort 1 infants were slightly younger at the time of the MRI scan, mother had lower SES, less medication use, and lower BMI and children’s CBCL scores were higher on the somatic, aggressive, internalizing, and externalizing problems subscales, compared to Cohort 2. See Table S6. Male and female offspring did not differ on any demographic variables, but males showed greater values in the volumetric component compared to females. Female offspring’ CBCL scores were higher on the somatic and aggression subscales, compared to males, see Table S7.
Associations between maternal CM and offspring brain
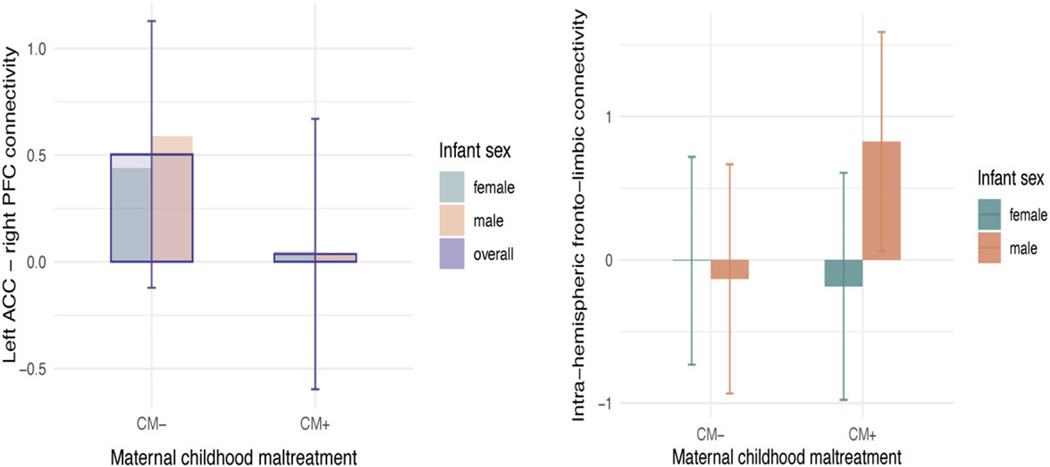
Analyses revealed significant associations between maternal CM and two of the five offspring brain components. First, there was a male-specific association between maternal CM and the component indexing intra-hemispheric fronto-limbic connectivity, with males in the CM + group demonstrating greater values in this component compared to males in the CM− group, (b = 0.96, p = 0.008, [95%CI 0.25, 1.66]). No maternal CM−related differences were detected for females on this component, and the interaction between maternal CM and offspring sex was significant (b =−1.14, p = 0.017, [95%CI −2.06, −0.21]), suggesting meaningful differential effects by sex (Table 2). No significant main effects of CM emerged (b = 0.35, p = ns, [−0.16, 0.87]). Results were comparable in a model run without maternal distress and substance use; both the above-described interaction (b =−1.17, p = 0.014, [95%CI −2.10, −0.25]) and the CM−related increase in intra-hemispheric fronto-limbic connectivity within males (b = 0.96, p= 0.006, [95%CI 0.29, 1.63]), remained significant (Fig. 2a). When an interaction term for study site was added to the model, the interaction between maternal CM and offspring sex continued to be significant (b =−1.19, p = 0.014, [95%CI −2.13, −0.25]), but the interaction between CM and study type was not: (b = 0.171; p = 0.88; [95%CI −2.09, 2.43]). Further, when analyses were conducted only including participants with a history of physical or sexual abuse in the CM + group the interaction between maternal CM and offspring sex continued to be significant (n = 83 b =−1.32, p = 0.007, [95%CI −2.27, −0.37]).
Table 2.
Associations between maternal childhood maltreatment and newborn brain volume and white matter connectivity
| β [95% CI] | β [95% CI] | β [95% CI] | |
|---|---|---|---|
| Models controlling for perinatal distress and alcohol/substance use | |||
| Volumetric | 0.15 [−0.23, 0.53] | −0.72 [−1.08, −0.35]*** | −0.35 [−1.07, 0.36] |
| Intra-hemispheric fronto-limbic connectivity | 0.35 [−0.16, 0.87] | −0.40 [−0.90, 0.09] | −1.14 [−2.06, −0.21]* |
| Right ACC connectivity | −0.47 [−0.92, −0.01]* | −0.07 [−0.51, 0.36] | 0.16 [−0.68, 1.01] |
| Left ACC connectivity | 0.19 [−0.29, 0.67] | 0.07 [−0.39, 0.53] | 0.29 [−0.60, 1.18] |
| Subcortical connectivity | −0.07 [−0.49, 0.36] | 0.44 [0.04, 0.85]* | −0.30 [−1.09, 0.49] |
| Models not controlling for perinatal distress and alcohol/substance use | |||
| Volumetric | 0.10 [−0.24, 0.44] | −0.75 [−1.10, −0.40]*** | −0.35 [−1.05, 0.36] |
| Intra-hemispheric fronto-limbic connectivity | 0.33 [−0.14, 0.79] | −0.39 [−0.87, 0.09] | −1.17 [−2.10, −0.25]* |
| Right ACC connectivity | −0.27 [−0.68, 0.14] | 0.02 [−0.41, 0.45] | 0.17 [−0.69, 1.02] |
| Left ACC connectivity | 0.01 [−0.43, 0.44] | −0.01 [−0.46, 0.44] | 0.27 [−0.63, 1.16] |
| Subcortical connectivity | −0.21 [0.59, 0.18] | 0.36 [−0.04, 0.76] | −0.28 [−1.08, 0.52] |
ACC, anterior cingulate cortex; β, standardized coefficient; CI, confidence interval; CM, childhood maltreatment.
p < 0.001.
p < 0.01.
p < 0.05.
Figure 2.
Associations between maternal childhood maltreatment, infant offspring brain structure, and depressive symptoms in early childhood. a. Significant infant sex by maternal childhood maltreatment (CM; n = 92) interactions were observed across the intra-hemispheric fronto-limbic connectivity component. Error bars are shown. Males with a maternal history of CM (n = 15) showed increased connectivity compared to males without a history of CM (n = 25). No differences were found within females. Units on y-axis represent the principal component scores, higher values indicate higher connectivity and or volumetric scores on the variables represented by the principal component. b. A significant main effect of CM was observed on the right ACC-left PFC connectivity component. Infants of mothers with a history of CM showed decreased connectivity, but only when controlling for prenatal distress and substance use.
Second, an effect of CM was detected in right ACC–left PFC connectivity, such that infants in the CM + group showed lower values in connectivity relative to the CM− group (b =−0.47, p = 0.044, [95%CI −0.92, −0.01]; Fig. 2b). No significant sex interaction emerged. Interestingly, in a model without prenatal distress and alcohol, substance, and tobacco use, the effect of CM was no longer significant. This may be a result of opposing mediational processes. The direct effect of CM on right ACC–left PFC connectivity described above is negative, while the indirect effect through prenatal maternal distress is marginally positive (Fig. S2); thus, when prenatal maternal distress is not included in the model the opposing processes may lead to a diminished overall effect. Larger samples are needed to understand counteracting effects. Sensitivity analyses showed that when an interaction term for study site was added to the model, the main effect of maternal CM continued to be significant (b = 0.47, p = 0.044, [95%CI 0.926, 0.013]), but the interaction between CM and study site was not: (b = 0.355; p = 0.45; [95%CI −1.29, 0.58]). Finally, analyses only including participants with a history of physical or sexual abuse in the CM + group showed that the main effect of CM was now marginally significant, yet in the same direction even with a reduced sample size (n = 83 b =−0.43, p = 0.97, [95%CI [−0.945, −0.080]).
No main effects of CM or significant CM X infant sex effects were detected in the volumetrics, left ACC–right PFC connectivity, or subcortical connectivity components.
Associations between infant brain connectivity and childhood anxiety and depression
Exploratory analyses (n = 36) revealed that only for male offspring, there was a significant association between intra-hemispheric fronto-limbic connectivity and somatic complaints on the CBCL (rmale = 0.673, pmale = 0.006, nmale = 13; Table 3). This association was not significant for female offspring (rfemale = 0.031, pfemale = 0.901, nfemale = 17) or when analyses combined both sexes. No associations with attention problems, aggressive behaviors, externalizing, or internalizing problems were detected.
Table 3.
Associations between newborn brain volume and white matter connectivity and depressive and anxiety symptoms on the CBCL
| Volumetric | Intrahemispheric | R ACC connectivity | L ACC connectivity | Subcortical | CBCL somatic | CBCL attention | CBCL aggressive | CBCL internalizing | |
|---|---|---|---|---|---|---|---|---|---|
| Complete Sample a | |||||||||
| Volumetric | |||||||||
| Intra-hemispheric | −0.351* | ||||||||
| R ACC connectivity | −0.162 | −0.101 | |||||||
| L ACC connectivity | 0.033 | −0.315 | 0.193 | ||||||
| Subcortical | −0.033 | −0.036 | 0.019 | −0.204 | |||||
| CBCL somatic | −0.164 | 0.180 | 0.279 | 0.099 | 0.101 | ||||
| CBCL attention | −0.251 | 0.047 | 0.257 | 0.280 | 0.090 | 0.581*** | |||
| CBCL aggressive | −0.027 | 0.023 | 0.287 | 0.238 | −0.002 | 0.568*** | 0.793*** | ||
| CBCL internalizing | −0.220 | 0.167 | 0.283 | 0.124 | 0.088 | 0.710*** | 0.618*** | 0.764*** | |
| CBCL externalizing | −0.110 | 0.154 | 0.103 | 0.233 | −0.086 | 0.508** | 0.657*** | 0.861*** | 0.808*** |
| Males b | |||||||||
| Volumetric | – | ||||||||
| Intra-hemispheric | −0.412 | ||||||||
| R ACC connectivity | −0.030 | 0.087 | |||||||
| L ACC connectivity | −0.059 | −0.120 | −0.012 | ||||||
| Subcortical | −0.030 | 0.097 | −0.388 | −0.125 | |||||
| CBCL somatic | −0.131 | 0.673** | 0.464 | −0.045 | −0.142 | ||||
| CBCL attention | −0.402 | −0.146 | −0.316 | 0.430 | −0.268 | −0.158 | |||
| CBCL aggressive | 0.047 | −0.287 | 0.117 | 0.123 | −0.073 | 0.102 | 0.021 | ||
| CBCL internalizing | −0.273 | 0.088 | 0.380 | 0.031 | 0.099 | 0.414 | −0.256 | 0.672** | |
| CBCL externalizing | −0.304 | −0.305 | 0.077 | 0.248 | −0.010 | −0.025 | 0.284 | 0.864*** | 0.628* |
| Females c | |||||||||
| Volumetric | |||||||||
| Intra-hemispheric | −0.279 | ||||||||
| R ACC connectivity | −0.320 | −0.134 | |||||||
| L ACC connectivity | −0.045 | −0.453 | 0.385 | ||||||
| Subcortical | 0.212 | −0.165 | 0.368 | −0.302 | |||||
| CBCL somatic | 0.178 | 0.031 | 0.188 | 0.305 | 0.090 | ||||
| CBCL attention | −0.047 | 0.068 | 0.369 | 0.455 | 0.088 | 0.572* | |||
| CBCL aggressive | 0.050 | 0.156 | 0.345 | 0.373 | −0.022 | 0.669 ** | 0.911*** | ||
| CBCL internalizing | −0.004 | 0.206 | 0.221 | 0.272 | −0.010 | 0.756*** | 0.709 ** | 0.787*** | |
| CBCL externalizing | 0.021 | 0.352 | 0.111 | 0.275 | −0.175 | 0.671** | 0.756 *** | 0.864*** | 0.883*** |
ACC, anterior cingulate cortex; CBCL, child behavior checklist; L, left; R, right.
n = 36.
n = 16.
n = 20.
p < 0.001.
p < 0.01.
p < 0.05.
Discussion
This paper adds to a growing body of literature suggesting that CM may influence neurodevelopment in the next generation, potentially perpetuating cycles of hardship and adversity. The present study represents a significant advance documenting associations between maternal CM and intra-hemispheric fronto-limbic white matter connectivity in newborn males. It is the first to examine white matter connectivity, moving beyond examination of regional and global volumes or functional activity, thus growing our understanding on the circuits involved in the intergenerational transmission of adversity. This study not only suggests that intergenerational adversity may be relayed to male infant’s white matter connectivity, but also suggests (via exploratory analyses) that these increases in fronto-limbic connectivity may herald later childhood somatic problems. Our findings underscore the critical need for more research on the intergenerational effects of CM that can inform prevention and early intervention.
Intra-hemispheric fronto-limbic structural connectivity near birth was increased in male offspring of mothers with a history of CM. These findings align with the one existing study that has examined, and documented, increased functional connectivity between the amygdala and vmPFC and dACC in infants of mothers with a history of emotional neglect.18 Although the possible mechanisms underlying this association remain unknown, early life adversity has been associated to long-term effects on HPA axis functioning, including epigenetic changes such as increased methylation of the 11β-HSD-2 gene that are associated to increased intrauterine cortisol exposure.22 It is thus possible that the effects of maternal CM on offspring risk for psychiatric illness are mediated by alterations in fronto-limbic connectivity related to excess prenatal glucocorticoid exposure, yet studies that directly test this hypothesis are needed. Interestingly, maternal adversity was associated with alterations in intra-hemispheric connectivity. Because local (intra-hemispheric) connectivity57 is believed to develop first, it may be that alterations due to intrauterine exposures may have a greater effect on the development of intra-hemispheric, rather than cross-hemispheric connectivity, yet this conjuncture requires empirical testing.
Our analyses indicated that maltreatment was related to increased fronto-limbic connectivity only in males. Consistent with epidemiological and preclinical studies documenting increased susceptibility in males, our study suggests that sex-specific effects on brain development may be present in humans at birth. Only one of the three existing studies of maternal CM on newborn infant brain development (based on MRI) examined interactions between maltreatment and sex, the other two only controlled for offspring sex in their analyses. The only study to look at infant sex did not find significant interactions with maternal maltreatment, but it only examined global gray and white matter volumes, complicating comparisons of the present study with prior work.16 The mechanisms underlying sex-dependent effects require elucidation, but sexual dimorphism in the placental response to glucocorticoids58 may play a role. Further, rodent models suggest perinatal stress exposure may lead to the demasculinization of the developing fetal brain, documenting decreased testosterone and increased estradiol levels in exposed males, but not females, further suggesting a role for endocrine disruptions following adversity exposure.59 However, our understanding of these sex-specific processes remains limited. It is important to note whereas in the broader (i.e., non-infant neuroimaging) literature of intergenerational adversity there are a number of studies that have documented increased susceptibility in males, there are exceptions.60 Studies have assessed sex effects inconsistently, and offspring outcomes have been assessed at varying timepoints from early childhood until adulthood. Such variability in methods and outcome measures may account for disparate findings, as the age at which symptoms (and brain structure) are examined, as well as the types of symptoms or behaviors, may matter.8,10 Longitudinal studies that carefully consider offspring sex effects will be required to fully understand risk.
Fronto-limbic connectivity is critical to efficient emotion regulation and fear learning, and both of these domains are related to susceptibility for mood disorders.61 Further, alterations in fronto-limbic connectivity have been documented in individuals exposed to early life adversity.62,63 Because fronto-limbic connectivity increases as a part of normative development, increased connectivity in adversity-exposed individuals has been hypothesized to reflect accelerated maturation.64 Accelerated maturation is in turn believed to prepare the individual for an immediate challenging rearing environment, yet at the cost of shortening periods of brain plasticity and behavioral exploration, and in the long run increasing risk for later psychiatric disorders.64 Our exploratory analyses related fronto-limbic connectivity to increased parent-reported somatic complaints in children. Somatic complaints have been associated with future risk for psychiatric disorders in a number of studies65 including large, representative longitudinal cohorts.66 Although our analyses were exploratory and only hypotheses generating due to the small sample size, it may be that somatic complains at this age (~5 years) signal increased susceptibility for future psychiatric disorders in children of mothers with a history of adversity. Longitudinal samples that follow children through developmental periods when psychopathology becomes more pronounced (e.g., adolescence) are needed to test this hypothesis, as results will potentially help identify a group of children that could benefit from early interventions. Ours is the first study of maternal CM and infant neuroimaging to include longitudinal assessments of offspring symptomatology and offers a testable hypothesis in need of further elucidation.
The present study represents an important step in the advancement of our understanding of the potential intergenerational effects of adversity on the brain, yet significant questions remain. The interplay between early life adversity and perinatal distress in shaping offspring neurodevelopment, as was documented in the case of right ACC–left PFC connectivity, is poorly understood. Uncovering mediating, independent, and interactive effects will be critical to developing timely interventions. Additionally, due to the substantial overlap between experiences of abuse and neglect in our sample (i.e., only 3 women endorsed having exclusively experienced abuse and not additionally neglect) the present study broadly examined child maltreatment, encompassing both abuse and neglect, which may have differential sequelae.34 Importantly, maltreatment is one of the several domains of childhood adversity implicated in negative psychiatric outcomes. Studies that examine the intergenerational effects of a comprehensive set of adverse childhood experiences, including discrimination, on offspring brain and behavior are needed. Thus, to address the nuanced ways in which different types of maternal childhood adversity interact with other perinatal exposures (e.g., perinatal distress), large studies will be needed, highlighting the importance of including maternal childhood assessments in large initiatives like the HEALthy Brain and Child Development Study. Further, studies of early adversity often find contradictory results (e.g., increased versus decreased fronto-striatal connectivity),63 highlighting the need for large samples and replication studies.
Not all children of women who experience CM will develop psychiatric symptomatology. As our understanding of risk develops, so should our understanding of resilience. Whereas research on intergenerational resilience is strikingly underdeveloped, a handful of studies point to parenting as critical to building resilience.67 Further, studies on the intra-individual effects of early childhood adversity suggest supportive caregiving and school and community-based support and connectedness may be important sources of resilience.68 Identifying the mechanisms underlying resilience will be crucial when developing early intervention and policy.
The principal limitations of the present study are sample size and retrospective reporting of maternal adversity. Although ours is the largest study to date of infant MRI and maternal adversity, larger samples are needed to fully understand the documented effects. This limitation is particularly true of the analyses of childhood psychiatric symptomatology that should be considered hypotheses generating and require replication. Given the small sample and differences between children with and without this follow-up, findings should be viewed as hypothesis generating and interpreted with caution until replicated. Limited sample sizes across both cohorts did not allow us to treat one as a replication cohort, yet this strategy is important for replicability. The use of retrospective reports of CM may represent another limitation, as these do not allow examination of maternal age (e.g., early versus late childhood) at the moment of exposure, and prior work has documented disagreement between prospective and retrospective reports.69 Although both kinds of reports are related to negative outcomes, research is needed to understand the correlates and determinants of each. Similarly, future research should include assessments of child outcomes that do not rely on maternal report to reduce potential reporting bias. Because childhood adversity has been related to more negative pregnancy and birth outcomes, exclusion criteria related to obstetrical and delivery complications may have introduced bias. However, our results also highlight that intergenerational adversity effects are present even in the context of a healthy pregnancy and delivery. Exclusion due to gestational SSRI use could introduce bias. Whereas the group of mothers using SSRI in pregnancy did not demonstrate higher rates of CM or prenatal distress, study covariates important to white matter development (e.g., gestational age at birth) differed in mothers using SSRIs, complicating differentiation of potential bias due to SSRI use versus other demographic factors. Finally, although the geographic and SES diverse nature of our sample is a strength, differences between the two cohorts in demographic variables related to brain development (e.g., age at scan, medication use), and data acquisition protocols (specifically DTI protocols) may have introduced bias. Findings require replication in other cohorts.
The present study joins a growing body of work possibly suggesting the existence of intergenerational effects of maternal CM on infant brain development. We document alterations in white matter connectivity, specifically in the male brain. Our findings, although limited by sample size, suggest that maternal childhood adversity may have intergenerational effects on brain development, which in turn may be associated to increased risk for subsequent somatic complaints in male offspring. Although our understanding of intergenerational mechanisms is underdeveloped, the present study supports the need for more research on childhood adversity and its subsequent effects to help generations of families to come.
Supplementary Material
Acknowledgments.
We thank all the families for their participation.
Financial support.
This work was partly funded by National Institute of Mental Health grants P05-MH090966 (Gingrich), K08-MH117452 (Lugo-Candelas), and R01-MH121070 (Posner) and Fundação de Amparo à Pesquisa do Estado São Paulo Grant 2019/21612-0 (Jackowski).
Footnotes
Supplementary material.
The supplementary material for this article can be found at https://doi.org/10.1017/S2040174423000247.
Competing interests. None.
Ethical standard.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees at the Universidade Federal de São Paulo and New York State Psychiatric Institute.
Data availability statement.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Conselho Nacional de Ética em Pesquisa (CONEP) e Comitê de Ética em Pesquisa (CEP) da Universidade Federal de Sao Paulo (UNIFESP) 1200/2017 and The New York State Psychiatric Institute Institutional Review Boards.
References
- 1.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017; 2(8), e356–e366. [DOI] [PubMed] [Google Scholar]
- 2.Yehuda R, Lehrner A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry. 2018; 17(3), 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scorza P, Duarte CS, Hipwell AE, et al. Research review: intergenerational transmission of disadvantage: epigenetics and parents’ childhoods as the first exposure. J Child Psychol Psychiatry. 2019; 60(2), 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooke JE, Racine N, Pador P, Madigan S. Maternal adverse childhood experiences and child behavior problems: a systematic review. Pediatrics. 2021; 148(3), e2020044131. [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Biaggi A, Sacchi C, et al. Mediators and moderators in the relationship between maternal childhood adversity and children’s emotional and behavioural development: a systematic review and meta-analysis. Psychol Med. 2022; 52(10), 1817–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betancourt TS, McBain RK, Newnham EA, Brennan RT. The intergenerational impact of war: longitudinal relationships between caregiver and child mental health in postconflict Sierra Leone. J Child Psychol Psychiatry. 2015; 56(10), 1101–1107. [DOI] [PubMed] [Google Scholar]
- 7.Yehuda R, Bell A, Bierer LM, Maternal Schmeidler J, not paternal. PTSD is related to increased risk for PTSD in offspring of holocaust survivors. J Psychiatr Res. 2008; 42(13), 1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letourneau N, Dewey D, Kaplan BJ, et al. Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. J Dev Orig Health Dis. 2019; 10(1), 88–99. [DOI] [PubMed] [Google Scholar]
- 9.Bierer LM, Bader HN, Daskalakis NP, et al. Elevation of 11β-hydroxysteroid dehydrogenase type 2 activity in holocaust survivor offspring: evidence for an intergenerational effect of maternal trauma exposure. Psychoneuroendocrinology. 2014; 48, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 2019; 86(6), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowers ME, Yehuda R. Intergenerational transmission of stress in humans. Neuropsychopharmacology. 2016; 41(1), 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosnowski DW, Booth C, York TP, Amstadter AB, Kliewer W. Maternal prenatal stress and infant DNA methylation: a systematic review. Dev Psychobiol. 2018; 60(2), 127–139. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Fontaine A, MacCallum PE, Drover J, Blundell J. Stress across generations: DNA methylation as a potential mechanism underlying intergenerational effects of stress in both post-traumatic stress disorder and pre-clinical predator stress rodent models. Front Behav Neurosci. 2019; 13(113). 10.3389/fnbeh.2019.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage L-É., Tarabulsy GM, Pearson J, Collin-Vézina D, Gagné L-M . Maternal history of childhood maltreatment and later parenting behavior: a meta-analysis. Dev Psychopathol. 2019; 31(1), 9–21. [DOI] [PubMed] [Google Scholar]
- 15.Stenz L, Schechter DS, Serpa SR, Paoloni-Giacobino A. Intergenerational transmission of DNA methylation signatures associated with early life stress. Curr Genomics. 2018; 19(8), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moog NK, Entringer S, Rasmussen JM, et al. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol Psychiatry. 2018; 83(2), 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury JE, Ahtam B, Sisitsky M, et al. Maternal childhood maltreatment is associated with lower infant gray matter volume and amygdala volume during the first two years of life. Biol Psychiatry Glob Open Sci. 2021; 2(4), 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix CL, Dilks DD, McKenna BG, Dunlop AL, Corwin EJ, Brennan PA. Maternal childhood adversity associates with frontoamygdala connectivity in neonates. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020; 6(4), 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 2013; 74(9), 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland S, Brunwasser SM. Sex differences in vulnerability to prenatal stress: a review of the recent literature. Curr Psychiat Rep. 2018; 20(11), 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J PhysiolENDOC M. 2001; 280(5), E729–E739. [DOI] [PubMed] [Google Scholar]
- 22.Monk C, Lugo-Candelas C, Trumpff C. Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annu Rev Clin Psychol. 2019; 15(1), 317–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanel D, Vanes LD, Pecheva D, et al. Neonatal white matter microstructure and emotional development during the preschool years in children who were born very preterm. eNeuro. 2021; 8(5), ENEURO.0546–20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eluvathingal TJ, Chugani HT, Behen ME, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006; 117(6), 2093–2100. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46(3), 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015; 34(28), 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017; 78(2), e162–e165. [DOI] [PubMed] [Google Scholar]
- 28.Lugo-Candelas C, Cha J, Hong S, et al. Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatr. 2018; 172(6), 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posner J, Cha J, Roy AK, et al. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 2016; 6(11), e935–e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiryanova V, McAllister BB, Dyck RH. Long-term outcomes of developmental exposure to fluoxetine: a review of the animal literature. Dev Neurosci. 2013; 35(6), 437–439. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994; 151(8), 1132–1136. [DOI] [PubMed] [Google Scholar]
- 32.Grassi-Oliveira R, Stein LM, Pezzi JC. Tradução e validação de conteúdo da versão em português do childhood trauma questionnaire. Revista de Saúde Pública. 2006; 40(2), 249–255. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein DP, Fink L, Handelsman L, Foote J. Childhood Trauma Questionnaire. 1998. Assessment of Family Violence: A Handbook for Researchers and Practitioners, San Antonio, TX. [Google Scholar]
- 34.McLaughlin KA, Sheridan MA. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016; 25(4), 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amorim P. Mini international neuropsychiatric interview (MINI): validação de entrevista breve para diagnóstico de transtornos mentais. Braz J Psychiat. 2000; 22(3), 106–115. [Google Scholar]
- 36.Soeken KL, McFarlane J, Parker B, Lominack MC. The Abuse Assessment Screen: a Clinical Instrument to Measure Frequency, Severity, and Perpetrator of Abuse Against Women, 1998. SAGE Publications, Inc., Thousand Oaks. [Google Scholar]
- 37.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24(4), 385–396. [PubMed] [Google Scholar]
- 38.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959; 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1(3), 385–401. [Google Scholar]
- 40.Accortt EE, Cheadle AC, Schetter CD. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015; 19(6), 1306–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingston D, Tough S. Prenatal and postnatal maternal mental health and school-age child development: a systematic review. Matern Child Health J. 2014; 18(7), 1728–1741. [DOI] [PubMed] [Google Scholar]
- 42.Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev. 2012; 43(5), 683–714. [DOI] [PubMed] [Google Scholar]
- 43.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Vol 30, 2000. University of Vermont, Research Center for Children, Youth, Burlington, VT. [Google Scholar]
- 44.Achenbach TM, Rescorla L. Manual for the ASEBA School-age Forms & Profiles: An Integrated System of Multi-informant Assessment, 2001. ASEBA is publisher Achenbach System of Empirically Based Assessment (ASEBA), Aseba Burlington, VT. [Google Scholar]
- 45.Makropoulos A, Robinson EC, Schuh A, et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 2018; 173, 88–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Hinds W, Duarte CS, et al. Intra-session test-retest reliability of functional connectivity in infants. Neuroimage. 2021; 239, 118284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003; 20(2), 870–888. [DOI] [PubMed] [Google Scholar]
- 48.Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012; 62(3), 1924–1938. [DOI] [PubMed] [Google Scholar]
- 49.Fortin J-P, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017; 161, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007; 8(1), 118–127. [DOI] [PubMed] [Google Scholar]
- 51.Greifer N. WeightIt: weighting for covariate balance in observational studies, 2017. R package version 01 0. https://cran.r-project.org/web/packages/WeightIt/WeightIt.pdf [Google Scholar]
- 52.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009; 28(25), 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoemmes F, Ong AD. A primer on inverse probability of treatment weighting and marginal structural models. Emerging Adulthood. 2015; 4(1), 40–59. [Google Scholar]
- 54.Telford EJ, Cox SR, Fletcher-Watson S, et al. A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct. 2017; 222(9), 4023–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SJ, Steiner RJ, Yu Y, et al. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc Nat Acad Sci. 2017; 114(1), 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldwin JR, Reuben A, Newbury JB, Danese A. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiatry. 2019; 76(6), 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomason ME, Dassanayake MT, Shen S, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013; 5(173), 173ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickinson H, O’Connell B, Walker D, Moritz K. Sex-specific effects of prenatal glucocorticoids on placental development, 2012. Glucocorticoids—New Recognition of Our Familiar Friend, Rijeka, Croatia: InTech. [Google Scholar]
- 59.Verhaeghe R, Gao V, Morley-Fletcher S, et al. Maternal stress programs a demasculinization of glutamatergic transmission in stress-related brain regions of aged rats. GeroScience. 2021; 44(2), 1047–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santos HP Jr., Bhattacharya A, Martin EM, et al. Epigenome-wide DNA methylation in placentas from preterm infants: association with maternal socioeconomic status. Epigenetics. 2019; 14(8), 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011; 21(7), 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, Essex MJ. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016; 1(4), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 2019; 1(1), 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callaghan BL, Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016; 7, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campo JV. Annual research review: functional somatic symptoms and associated anxiety and depression – developmental psychopathology in pediatric practice. J Child Psychol Psychiatr. 2012; 53(5), 575–592. [DOI] [PubMed] [Google Scholar]
- 66.Shanahan L, Zucker N, Copeland WE, Bondy CL, Egger HL, Costello EJ. Childhood somatic complaints predict generalized anxiety and depressive disorders during young adulthood in a community sample. Psychol Med. 2015; 45(8), 1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods-Jaeger BA, Cho B, Sexton CC, Slagel L, Goggin K. Promoting resilience: breaking the intergenerational cycle of adverse childhood experiences. Health Educ Behav. 2018; 45(5), 772–780. [DOI] [PubMed] [Google Scholar]
- 68.Gartland D, Riggs E, Muyeen S, et al. What factors are associated with resilient outcomes in children exposed to social adversity? A systematic review. BMJ Open. 2019; 9(4), e024870–e024870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reuben A, Moffitt TE, Caspi A, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016; 57(10), 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the Conselho Nacional de Ética em Pesquisa (CONEP) e Comitê de Ética em Pesquisa (CEP) da Universidade Federal de Sao Paulo (UNIFESP) 1200/2017 and The New York State Psychiatric Institute Institutional Review Boards.