Abstract
Re-transplant is an option for those who develop end-stage lung disease due to rejection however little data exists following re-transplantation in cystic fibrosis (CF). Data from the Canadian CF Registry and US CF Foundation Patient Registry supplemented with data from United Network for Organ Sharing were used. Individuals who underwent a 2nd lung transplant between 2005–2019 were included. Kaplan-Meier method was used to estimate the probability of survival post-second transplant at 1, 3, and 5- years.. Of those people who were waitlisted for a second transplant (N=818), a total of 254 (31%) died waiting, 395 (48%) were transplanted and 169 (21%) people were alive on the waitlist. Median survival time after 2nd lung transplant was 3.3 years (95% CI 2.8–4.1). The 1-, 3- and 5-year survival rates were 77.4% (95% CI 73.1–82%), 52% (95% CI 46.7–58%) and 39.4% (95% CI 34.1–45.6%). Survival following second lung transplant in CF patients is lower than estimates following first transplant. Over half of subjects who are potentially eligible for a second transplant die without receiving a second organ. This warrants further investigation.
INTRODUCTION
Although survival in cystic fibrosis (CF) has improved dramatically over the last several decades, pulmonary disease remains the primary cause of morbidity and mortality.1 Published guidelines outline factors to consider to ensure timely referral for lung transplant as it has been shown to improve quality of life and extend survival in CF.2–4 Despite the positive impact that lung transplant can have on an individual’s health outcomes, chronic lung allograft dysfunction (CLAD) is associated with progressive loss of lung function and ultimately results in recurrence of end-stage lung disease. CLAD can present either as a predominantly obstructive ventilatory pattern, a restrictive pattern, or a mixed picture that is not explained by other conditions and is the primary obstacle to better outcomes post-lung transplant. The incidence of CLAD is approximately 50% five-years post-lung transplant.5 Once CLAD is diagnosed, 3-year survival is only 50%, and drops to 30–40% at 5 years. Given lack of therapeutic options once CLAD occurs, re-transplant is considered in carefully selected individuals based on extra-pulmonary organ dysfunction, anatomy, indication for re-transplant, and social supports.6 The volume of re-transplants represents between 3–5% of the annual lung transplant volume; however, with the availability of highly effective modulator therapy, it is anticipated that a larger proportion of CF transplants will be re-transplants moving forward, rather than first transplants, making it even more important to understand health outcomes in this population. This is supported by the fact that the number of first lung transplants for CF has dramatically decreased since the availability of elexacaftor/tezacaftor/ivacaftor (ETI) to treat the underlying protein defect in CF. Currently, ETI in not indicated post-transplant as the lungs no longer have CF thus there will be a relative increase in the number of re-transplants relative to primary lung transplantation moving forward.
Pre-transplant factors known to be associated with mortality following first lung transplant include B. cepacia complex, year of transplant, and CFTR functional class while male sex and age at transplant were of borderline significance.7,8 Although risk factors for worse survival following re-transplant have been identified, the results are often reported overall and not by underlying disease state which limits our understanding of how CF-specific factors influence mortality in this population.9 The CF population may differ from the non-CF population given their young age, the impact of persistent CF transmembrane conductance regulatory (CFTR) dysfunction in other key organs like gut and liver, and their increased risk of complications such as CF-related diabetes and renal dysfunction from chronic aminoglycoside use.10,11 A recent study by Chan et al. utilized United Network for Organ Sharing (UNOS) data to examine factors associated with outcomes following second lung transplant specifically in individuals with CF (n=277) and found that only mechanical ventilatory support as a bridge to re-transplant was significantly associated with graft failure at 3 years.12 This study lacked CF-specific clinical variables as only UNOS data were available.
The objectives of this study were (1) to quantify re-transplant survival probability in a large CF cohort and (2) to identify CF-specific demographic and clinical predictors impact of survival following re-transplantation.
METHOD
This population-based study used data from the Organ Procurement and Transplantation Network (OPTN), US CF Foundation (CFF) Patient Registry (US CFFPR) and the Canadian CF Registry (CCFR) from 1984 to 2019 inclusive. The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN. The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. A probabilistic linkage was done between CFFPR and OPTN to update the CFFPR with transplant date and to ensure complete vital statistics. Additional details concerning the record linkage between the CFFPR and OPTN are published.1,13–15 A unified Canada-US-OPTN data set was created after harmonizing data definitions and data collection methods within each registry.1 As the lung allocation score (LAS) for triaging transplant recipients was implemented in the US in 2005, we focused on the post-LAS period of 2005 to 2019.
This study was approved at St. Michael’s Hospital, Toronto, Ontario (Research Ethics Board #14–148), Seattle Children’s Hospital (Institutional Review Board (IRB) #PIROSTUDY15294) and University of Washington (IRB #STUDY2270).
Demographic characteristics of the CF subjects obtained from the CF registries included sex, genotype, race, pancreatic status and age at re-transplant. Race was categorized as white vs. non-white. Genotype was classified as homozygous deltaF508, heterozygous deltaF508, and other, as well as by functional class (Class I-V). The milder of the two alleles determined the functional class category. An ‘early’ re-transplant was defined as those transplanted in less than 365 days from their first transplant while ‘late’ re-transplant is defined as those transplanted beyond 365 days from their first transplant. As very few early transplants were done in Canada (< 5), the results were combined with the US. The number of lung transplants done per year was calculated for each center. High volume transplant centres were defined by a centre doing at least 26 lung transplants per year based on prior published literature.16
Defining the potentially eligible pool of subjects for second transplant
We started with all individuals who had received a first lung transplant. Using cause of death data available in UNOS (for the US cohort only), we excluded those individuals who died without a second transplant if they died of non-cardiopulmonary causes under the assumption that if they had other organ dysfunction, this would make them ineligible for a 2nd lung transplant (N=1163). If someone died of cardiorespiratory causes without evidence of graft organ dysfunction (based on the variable GRF_STAT) in the UNOS data set, these individuals were also excluded from the eligible re-transplant pool under the assumption that the cardiorespiratory death was due to something other than CLAD (for example, myocardial infarction etc.) and thus they would not be a candidate for a 2nd lung transplant (N=283). Finally, we excluded anyone who remained alive without listing for re-transplant as we have no data to suggest they had progressive lung disease that would warrant a re-transplant (N=2565). Using these criteria, a total of 1226 people were potentially eligible for re-lung transplantation.
Statistical Analyses
Continuous variables were summarized by reporting the median and interquartile range (IQR) while categorical variables were summarized by reporting the frequency and proportion. Demographic and clinical differences between countries were compared using the Mann-Whitney test for continuous variables and the Fisher’s exact test for categorical variables. To study the survival after re-transplant in the contemporary LAS period, we considered only those patients who received a second transplant during the period 2005 (the year that LAS was implemented in the US) to 2019. Subjects were considered “potentially eligible” for a second lung transplant if they met the following criteria: they had received a first lung transplant at any time point and they were alive in 2005 and they had not received a second transplant by 2005. Survival post-second transplant was calculated from the date of the second lung transplant to the date of death or December 31st of the last year of follow-up. A sensitivity analysis was done censoring at the date of third transplant. Subgroup analyses were done by sex, genotype, timing of re-transplant (early or late), excluding pediatric transplants. The Kaplan-Meier method was used to estimate the probability of survival post-second transplant at 1, 3, and 5- years. The log-rank test was used to compare survival curves between subgroups. Associations between static demographic (sex, race, genotype) and clinical factors (CFRD, pancreatic status) and survival were assessed using univariable Cox proportional hazards model. Lung transplant centre volume was examined as a covariate both as a categorical variable as well as a continuous variable. Schoenfeld residuals were graphically assessed to determine if the proportional hazards assumption was satisfied.
All p-values were two-sided and assessed at p<0.05. All analyses were conducted using the open-source software R version 4.0.3.
RESULTS
The study cohort flow diagram is shown in Figure 1. Between 2005 and 2019, there were 395 out of 1226 (32.2%) individuals with CF who underwent second lung transplant. A total of 662/1226 (54.0%) individuals died without a second lung transplant. Of those people who were listed for a second transplant (N=818), a total of 254 (31%) died waiting during the study period, 395 (48.3%) were ultimately transplanted. The majority (95%) of re-transplants were in adults over the age of 18 years. Median age at re-transplant was 30.1 years (interquartile range (IQR) 23.9–37.3). A total of 27 (6.8%) individuals were re-transplanted early (within 1 year of the first transplant). Demographic and clinical characteristics of the lung transplant recipients and those who did not receive a second transplant are presented in Table 1. Information on reasons for delisting for those who died on the waitlist can be found in Table S1.
Figure 1: Flow diagram of study population.
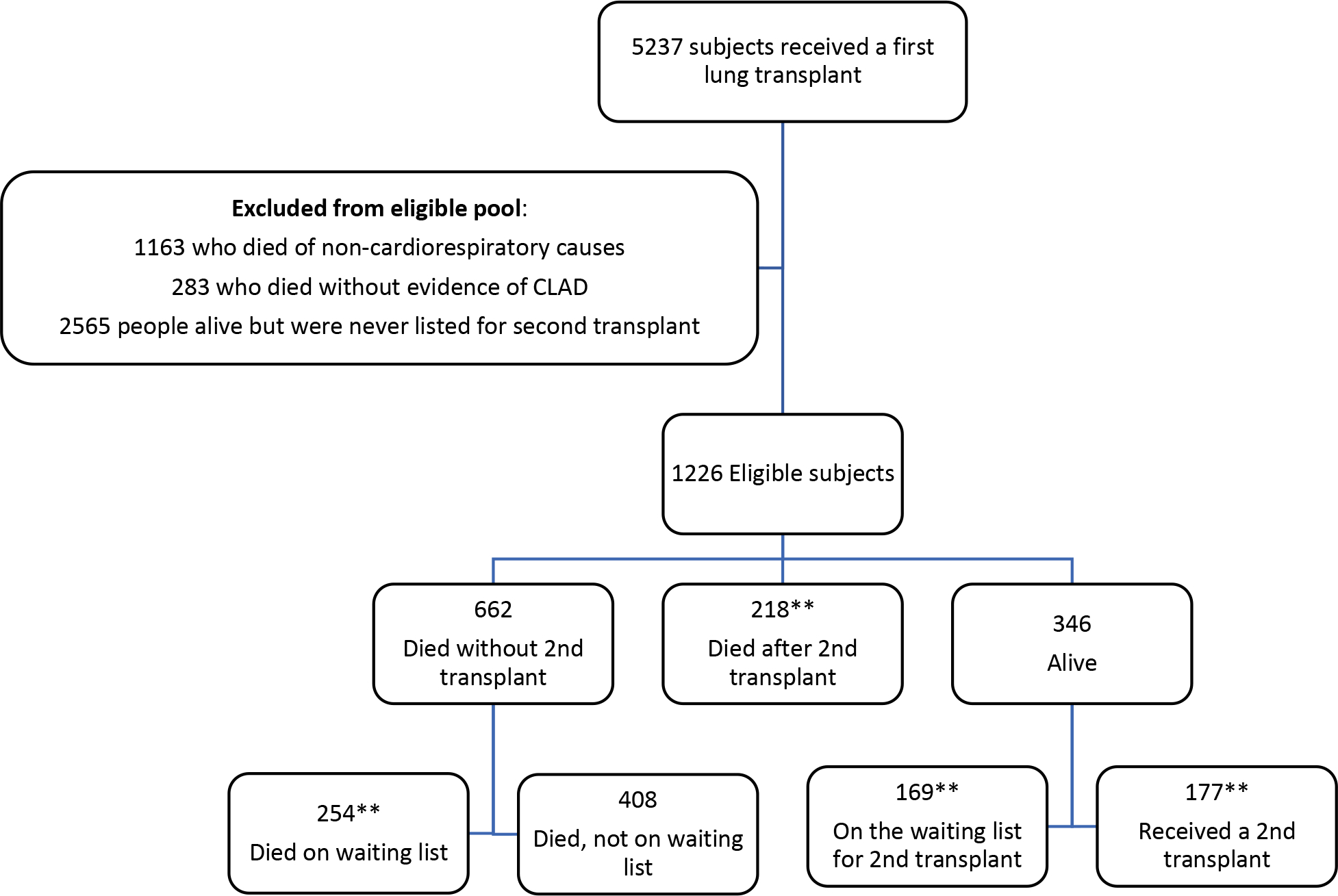
**The sum of these patients equals the number of people who were on the waiting list for a second lung transplant (N=818). 254 out of 818 (31%) patients died while waiting for re-transplant. Note: it is possible that some of the 408 individuals who died without a transplant may have been missing a waiting list date thus the deaths on the waiting list may be underestimated.
The sum of the individuals in the orange boxes (218+177=395) equals the total number of re-transplant recipients.
Table 1:
Demographic and clinical characteristics, 2005 to 2019
| Variable | Re-transplants (N=395) | Individuals who did not receive a second transplant (N=831) |
|---|---|---|
|
| ||
| Females | 208 (52.7) | 409(49.2) |
|
| ||
| White | 385 (97.5) | 797 (95.9) |
|
| ||
| Age at 2nd lung transplant (median, interquartile range) | 30.1 (23.9–37.3) | N/A |
|
| ||
| Genotype | ||
| Homozygous F508 | 187 (47.3) | 367 (44.2) |
| Heterozygous F508 | 120 (30.4) | 267 (32.1) |
| Other | 87 (22) | 191 (23.0) |
| Missing | <5 | 6 (0.70) |
|
| ||
| Mutation Class | ||
| I-III | 289 (73.2) | N/A* |
| IV-V | 17 (4.3) | |
| Unclassified | 89 (22.5) | |
|
| ||
| Pancreatic Insufficiency | 388 (98.2) | 805 (96.9) |
|
| ||
| CFRD | 269 (90.3) | 574 (69.1) |
All values are number (percentage) unless otherwise stated.
Abbreviations: CFRD, cystic fibrosis-related diabetes; DF508, delta F508 genotype
Mutation class data were not provided in the US CFF data for those lung transplant recipients who did not go on to receive a second transplant
Post-transplant survival: 2005–2019
Of those who were re-transplanted, a total of 218/395 (55.2%) post-transplant deaths were recorded. Median survival following re-transplantation was 3.3 years (95% confidence interval (CI) 2.8–4.1) (Figure 2). The 1-, 3- and 5-year survival rates were 77.4% (95% CI 73.1–82%), 52% (95% CI 46.7–58%) and 39.4% (95% CI 34.1–45.6%). The median survival and 1-, 3-, and 5-year survival probabilities were unchanged when the data were censored at the 3rd transplant (Figure S1, Table S2). There was insufficient evidence to suggest that there is a difference in survival between the two countries. Median survival times were similar between re- early transplant vs. late re-transplant (3.3 years (95% CI 2-N/A) compared to 3.2 years (95% CI 2.8–4.2) respectively, log rank test p value 0.70). The 1-, 3-, and 5-year survival probabilities were similar comparing early versus late re-transplantation (Figure 3). Univariable analyses revealed a statistically significant quadratic relationship (p=0.02) between age at re-transplant and better survival amongst the youngest and oldest ages compared to those in middle age. Mutation class showed a borderline statistically significant association with individuals who are Class I-III at higher risk of death after re-transplantation compared to Class IV-V (HR 2.76, 95% CI 1.03–7.43; p=0.045). Higher lung transplant center volume was associated with lower mortality although this was not statistically significant (HR 0.997, 95% CI 0.99–1.0; p=0.07). However, when transplant center volume was categorized into low vs. high volume, there was a significant relationship with higher mortality at low volume centers (HR 1.43, 95% CI 1.06–1.94, p=0.02). There were no significant associations found between sex, race, CFRD, or pancreatic status and survival after re-transplant.
Figure 2: Kaplan-Meier survival curve post-2nd lung transplant, 2005–2019.
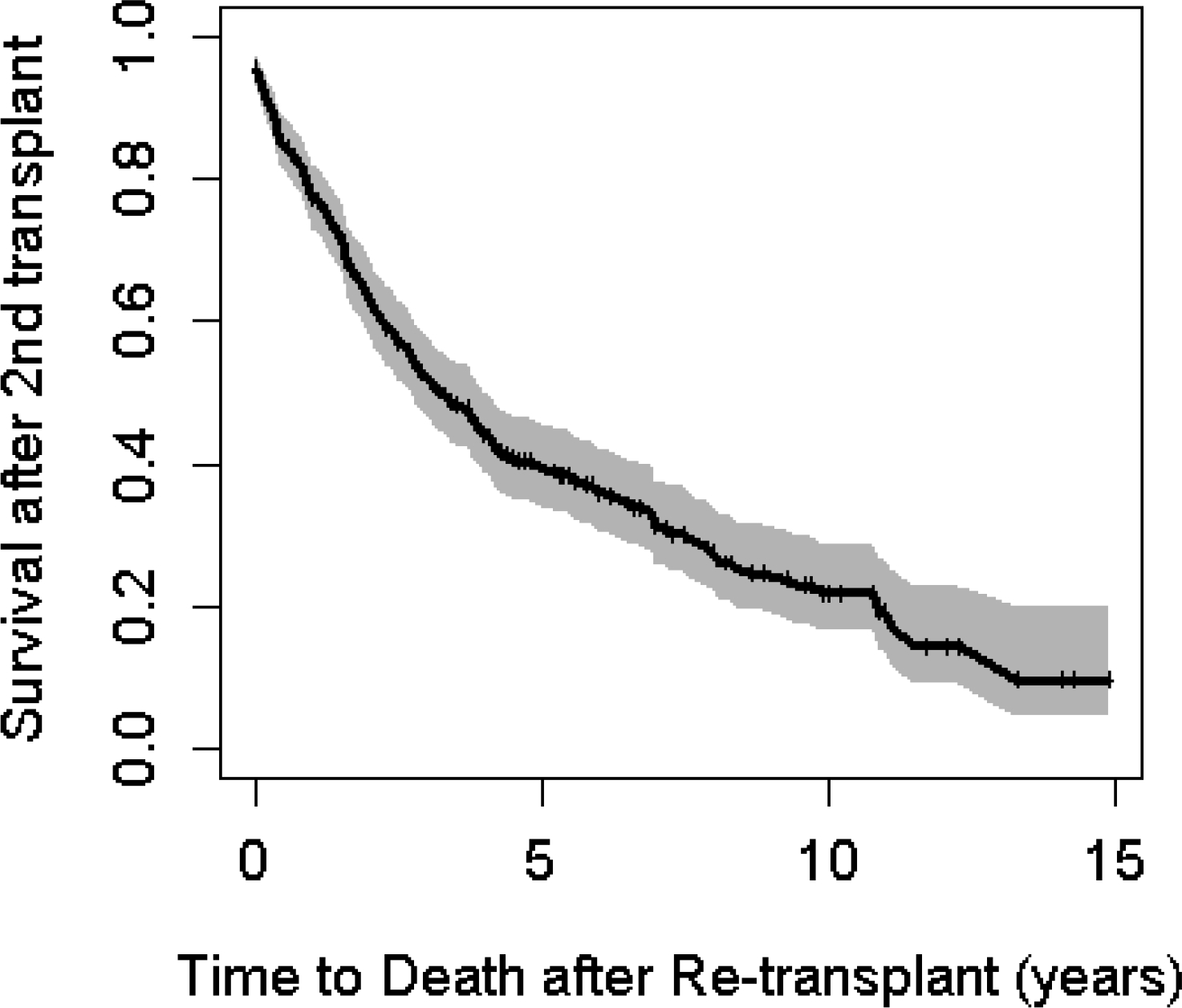
Figure 3:
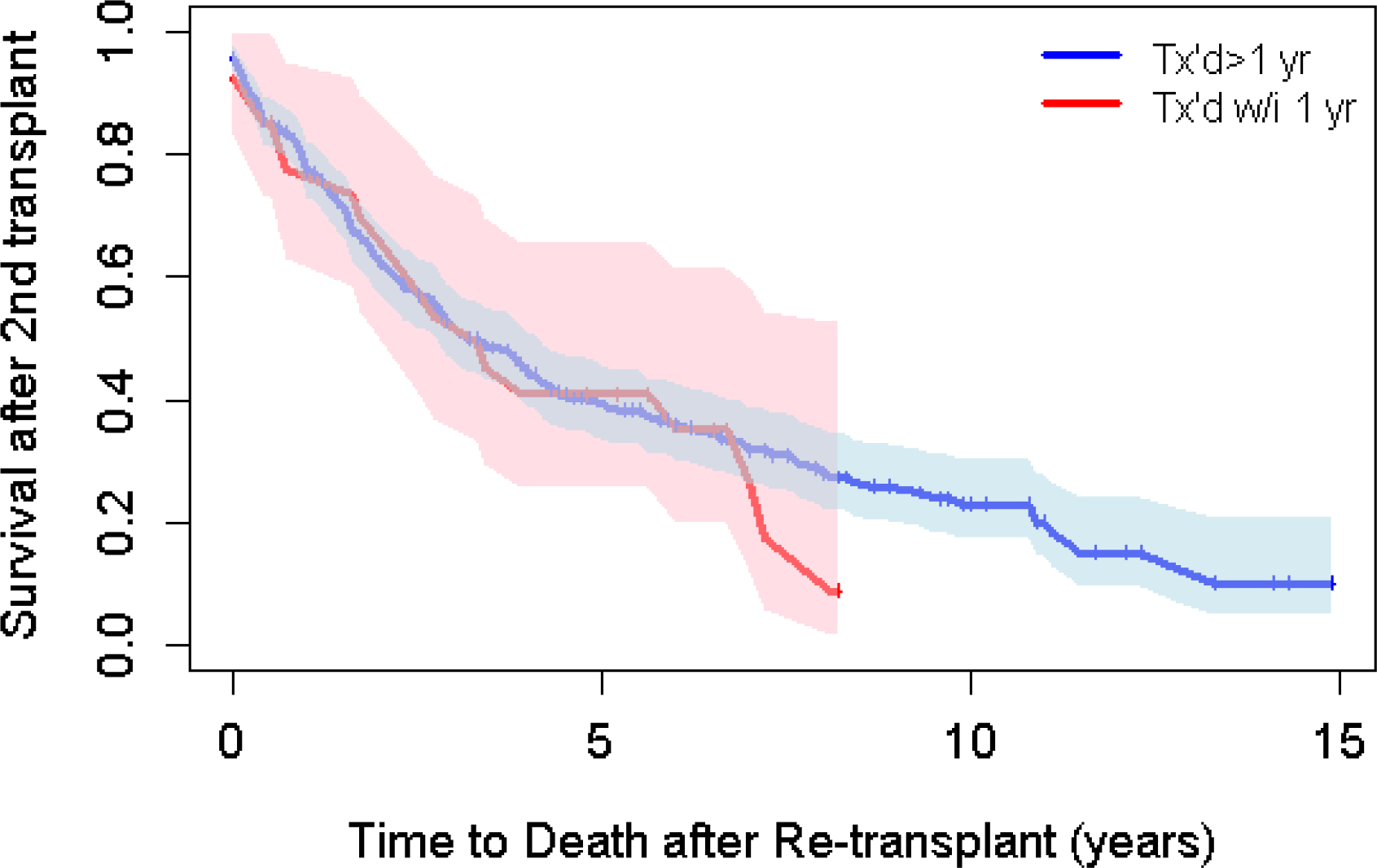
Survival by timing of re-transplant.
DISCUSSION
Our results suggest that about one-third of potentially eligible CF lung transplant recipients in our study underwent a second lung transplant and less than 5% of re-transplants recipients are in the pediatric population. We confirmed that 1-, 3- and 5-year survival after re-transplant is lower than survival after the initial transplant for CF. Re-transplantation is surgically more complex than the initial transplant and our data show that undergoing re-transplant at a high-volume transplant center may decrease the risk of mortality after re-transplantation. The Organ Procurement Transplantation Network (OPTN) report using data from 2008–2015 states that for lung re-transplants (all-comers) the 1-, 3- and 5-year survival estimates are 76% (95% CI 71, 80.2), 48.9% (95% CI 43.4–54.2) and 32.9% (95% CI 27.7–38.2) respectively (based on OPTN data as of May 6, 2023). In comparison, the 1-, 3- and 5-year CF survival rates were 77.4% (95% CI 73.1–82%), 52% (95% CI 46.7–58%) and 39.4% (95% CI 34.1–45.6%). Further targeted research is needed to confirm if the 3- and 5-year probabilities are significantly higher. Furthermore, we found that 33.3% (408/1226) of potentially eligible individuals died without evidence of being listed for or receiving a 2nd lung transplant. Unfortunately, we were unable to tease out whether or not the individuals who died without transplant in our cohort missed the opportunity or whether re-transplant was considered and deemed not to be an acceptable therapeutic option.
As would be anticipated, survival following second transplant is lower than what has been reported following the first transplant in the CF population.9,17,18 Previous published literature reported the 1-, 3- and 5-year survival rates were 88.3%, 71.8% and 60.3% in the US and 90.5%, 79.9% and 69.7% in Canada for individuals who underwent a first lung transplantation for CF.4 Our results confirm lower survival after re-transplant at each of these time points. Kawut et al. showed that although survival rates post lung re-transplant have improved in the modern era, re-transplant still carries with it an increased risk compared to the initial transplant.18 More recently, a study by Chan et al. looked at 5-year survival in CF patients and found similar results in that survival after second transplant was lower than the first transplant survival estimate. These authors also found that CF patients experienced significant clinical decline in renal, cardiac, and pulmonary function at the time of lung re-transplantation.12 This may indicate that an earlier evaluation and rehabilitation process may be necessary to identify patients earlier for lung re-transplantation prior to significant deterioration. Further, the authors stated that the second lung transplant cohort experienced re-intubation more frequently, required longer ventilatory support and were in hospital longer than those who had a first lung transplant. Interestingly, Chan et al. reported the 5-year survival for the re-transplant group was 47.9% compared to 39.4% in our current analysis. This could be due to the fact that our analysis included pediatric transplants and we did not censor at 3rd transplant both of which could result in lower survival.
Previous literature has suggested that early re-transplant recipients have worse outcomes than those requiring a re-transplant farther out from the initial transplant.19 Osho et al. found worse survival in those who underwent re-transplant within 90 days of the initial transplant. Kawut et al. found that early re-transplant within 30 days of the first transplant was associated with significantly higher mortality (HR 2.6, 95% CI 1.4–4.9, p=0.003). In our study, people re-transplanted early, within the 1st year, had similar survival compared to those who were re-transplanted after the 1st year. It is difficult to compare our results to the published literature as prior papers typically include all-comers and were not specific to individuals with CF. Furthermore, in our study only 14 individuals were re-transplanted within 90 days of the initial transplant making is difficult to make a direct comparison to prior published literature. It is possible that the individuals with CF fare better due to their younger age; however, this needs to be further investigated. Furthermore, our sample size of early re-transplants was small and may have been underpowered to detect a significant difference.
Several publications have reported worse overall survival in females with CF pre-transplant, however the results on the survival gap post-lung transplantation are conflicting. Some studies have shown males are at higher risk for death post transplant, others have reported lower risk of death, while other studies do not report any significant difference in post transplant survival based on sex. We did not find a significant sex difference in post-transplant survival following second transplant with equal proportion of males and females in the cohort. When looking at survival by genotype, Clausen et al. recently published a study showing that individuals with high-risk genotypes (categorized by functional class I-III) had worse survival following first lung transplant compared to those with at least one Class IV or V mutation.8 Our findings were consistent with these results following re-transplant.
It is recognized that those who undergo a second lung transplant are a highly selected group and there are many physical, psychological and psychosocial reasons why an individual would not be considered for re-transplantation. The definition of those potentially eligible for re-transplant used in our study was broad and likely underestimates the true prevalence of re-transplant in the truly eligible CF population given we did not have granular data on eligibility for a second transplant. However, we suggest that it is possible a portion of these individuals may have benefited from a second lung transplant but instead were not given the opportunity. This would be an important area of future study especially since it is anticipated that a significant proportion of CF transplants in the era of highly effective modulators will likely be re-transplants rather than de-novo lung transplants.20 It is unknown whether modulator therapy use after initial lung transplant will have any impact on the need for or timing of a 2nd transplant for individuals with CF.
The strengths of our study include population-based longitudinal data captured within both US CFFPR and OPTN registries in the US with low rates of loss to follow up allowing for the largest CF sample of re-transplants to date. The conducted linkage to OPTN resulted in more accurate capture of key data such as transplant and vital status while the inclusion of CF Registry data allowed for examination of CF-specific baseline characteristics. Limitations of our study must also be addressed. We did not have complete data specifically on clinical parameters (for example lung function, nutritional markers, healthcare utilization etc) after the initial transplant on all patients which limited our ability to examine the impact of these factors on survival. The focus of this analysis was on isolated lung re-transplantation, therefore the results may not apply to multi-organ lung transplant recipients. Individuals who undergo a second lung transplantation are a highly selected group of individuals deemed to have the best chance of success following the second transplant which limits the generalizability of our findings to the entirety of the CF lung transplant population. By focusing on those who received a second transplant, this ignores those individuals who may have been potentially eligible for re-transplant but who did not have the opportunity. An additional limitation of our study is that it was performed during a period when the LAS was used for lung allocation in the United States. With the recent transition to the composite allocation system (CAS) in March 2023, it is unknown whether the high rates of death without re-transplant seen in our study will persist in the setting of a different lung allocation algorithm. Future investigations into referral and waitlist outcomes will be required within the new system. When dichotomizing transplant center volume, we chose to use the cut off of 26 based on the literature. However, we recognize that dichotomizing a continuous variable may correspond with a loss in statistical power and therefore, the results when transplant center volume is categorized should be interpreted with caution. Finally, the CCFR lacked markers of low socioeconomic status (SES) for CF recipients in Canada, therefore we did not examine the relationship between SES and health outcomes as a potential barrier to re-transplantation. Examining barriers to re-transplant is an important focus of future research.
In conclusion, re-transplantation is a treatment option for CF lung recipients however survival following second lung transplant in CF patients is lower than estimates following first transplant. Over half of potentially eligible patients die without a second lung transplant which warrants further investigation.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the financial support of the US CFF which made this study possible (Grant #STEPHE14A0). Also, we are grateful to Cystic Fibrosis Canada and the US CFF for providing registry data for this project. In addition, we would like to acknowledge and thank all of the CF patients and families in the US and Canada who consent to be part of their respective national CF patient registries as well as the CF clinic staff who spend many hours inputting the data. Part of the data reported here have been supplied by UNOS as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as official policy of or interpretation by the OPTN or the U.S. Government
Funding
This work was funded by a grant provided by the Cystic Fibrosis Foundation (Grant #STEPHE14A0). There are no conflicts of interest related to this work. Outside of this work, CHG receives funding from the Cystic Fibrosis Foundation, the NIH (P30DK089507) and the FDA (R01FD006848). KJR receives funding from the Cystic Fibrosis Foundation (RAMOS17A0) and the NIH (K23HL138154).
ABBREVIATIONS
- CCFR
Canadian Cystic Fibrosis Registry
- CF
Cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- CFRD
cystic fibrosis-related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- CLAD
chronic lung allograft dysfunction
- ETI
elexacaftor/tezacaftor/ivacaftor
- HR
hazard ratio
- IQR
interquartile range
- IRB
Institutional Review Board
- LAS
lung allocation score
- OPTN
Organ Procurement and Transplantation Network
- SES
socioeconomic status
- UNOS
United Network Organ Sharing
- CFFPR
Cystic Fibrosis Foundation Patient Registry
- US
United States
Footnotes
Conflicts of interest: There are no conflicts of interest to disclose.
References
- 1.Stephenson AL, Sykes J, Stanojevic S, et al. Survival Comparison of Patients With Cystic Fibrosis in Canada and the United States: A Population-Based Cohort Study. Annals of internal medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos KJ, Smith PJ, McKone EF, et al. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros. 2019;18(3):321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busschbach JJ, Horikx PE, van den Bosch JM, Brutel de la Riviere A, de Charro FT. Measuring the quality of life before and after bilateral lung transplantation in patients with cystic fibrosis. Chest. 1994;105(3):911–917. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AL, Ramos KJ, Sykes J, et al. Bridging the survival gap in cystic fibrosis: An investigation of lung transplant outcomes in Canada and the United States. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2021;40(3):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos S, Vos R, Van Raemdonck DE, Verleden GM. Survival in adult lung transplantation: where are we in 2020? Curr Opin Organ Transplant. 2020;25(3):268–273. [DOI] [PubMed] [Google Scholar]
- 6.Michel E, Galen Hartwig M, Sommer W. Lung Retransplantation. Thoracic surgery clinics. 2022;32(2):259–268. [DOI] [PubMed] [Google Scholar]
- 7.Koutsokera A, Varughese RA, Sykes J, et al. Pre-transplant factors associated with mortality after lung transplantation in cystic fibrosis: A systematic review and meta-analysis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019;18(3):407–415. [DOI] [PubMed] [Google Scholar]
- 8.Clausen ES, Weber JM, Ramos KJ, Snyder LD. Survival difference between high-risk and low-risk CFTR genotypes after lung transplant. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rucker AJ, Nellis JR, Klapper JA, Hartwig MG. Lung retransplantation in the modern era. J Thorac Dis. 2021;13(11):6587–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quon BS, Mayer-Hamblett N, Aitken ML, Goss CH. Risk of post-lung transplant renal dysfunction in adults with cystic fibrosis. Chest. 2012;142(1):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quon BS, Mayer-Hamblett N, Aitken ML, Smyth AR, Goss CH. Risk factors for chronic kidney disease in adults with cystic fibrosis. American journal of respiratory and critical care medicine. 2011;184(10):1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan EG, Hyzny EJ, Ryan JP, Morrell MR, Pilewski J, Sanchez PG. Outcomes following lung re-transplantation in patients with cystic fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2022;21(3):482–488. [DOI] [PubMed] [Google Scholar]
- 13.Ramos KJ, Sykes J, Stanojevic S, et al. Survival and Lung Transplant Outcomes for Individuals With Advanced Cystic Fibrosis Lung Disease Living in the United States and Canada: An Analysis of National Registries. Chest. 2021;160(3):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pryor JB, Bradford MC, Jennerich AL, et al. Body Mass Index Recovery after Lung Transplant for Cystic Fibrosis. Annals of the American Thoracic Society. 2022;19(7):1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos KJ, Hee Wai T, Stephenson AL, et al. Development and Internal Validation of a Prognostic Model of the Probability of Death or Lung Transplantation Within 2 Years for Patients With Cystic Fibrosis and FEV(1) ≤ 50% Predicted. Chest. 2022;162(4):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilic A, Gleason TG, Kagawa H, Kilic A, Sultan I. Institutional volume affects long-term survival following lung transplantation in the USA. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Stephenson AL, Sykes J, Berthiaume Y, et al. Clinical and demographic factors associated with post-lung transplantation survival in individuals with cystic fibrosis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(9):1139–1145. [DOI] [PubMed] [Google Scholar]
- 18.Kawut SM, Lederer DJ, Keshavjee S, et al. Outcomes after lung retransplantation in the modern era. American journal of respiratory and critical care medicine. 2008;177(1):114–120. [DOI] [PubMed] [Google Scholar]
- 19.Osho AA, Castleberry AW, Snyder LD, et al. Differential outcomes with early and late repeat transplantation in the era of the lung allocation score. Ann Thorac Surg. 2014;98(6):1914–1920; discussion 1920–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanojevic S, Vukovojac K, Sykes J, Ratjen F, Tullis E, Stephenson AL. Projecting the impact of delayed access to elexacaftor/tezacaftor/ivacaftor for people with Cystic Fibrosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2021;20(2):243–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


