Abstract
Purpose:
To assess the role of static and dynamic ocular biometric parameters measured in the dark and light for predicting progression of primary angle closure suspect (PACS) to primary angle closure (PAC).
Design:
Retrospective cohort study using prospective RCT data from untreated, control eyes.
Methods:
Zhongshan Angle Closure Prevention Trial subjects underwent anterior segment OCT imaging in the dark and light. Static biometric parameters were measured, consisting of angle, iris, lens, and anterior chamber parameters. Dynamic change parameters were calculated by subtracting light measurements from dark measurements. Cox proportional hazards regression models were developed to assess risk factors for PACD progression.
Results:
861 eyes of 861 participants were analyzed (36 progressors). On univariable analysis, TISA500 measurements in the light and dark were associated with progression (p<0.001), whereas dynamic change parameters were not (p≥0.08). In the primary multivariable model, older age (HR=1.09 per year), higher IOP (HR = 1.13 per mmHg), and smaller TISA500 in the light (HR=1.28 per 0.01mm2) were significantly associated with greater risk of progression (p≤0.04). Dark TISA500 had similar significance (HR=1.28, p=0.002) when replacing light TISA500. Risk of progression was more predictive among eyes in the lowest quartile of light TISA500 measurements (HR=4.56, p<0.001) compared to dark measurements (HR=2.89, p=0.003).
Conclusion:
Static parameters measured in the light are as predictive, and possibly more so, of angle closure progression as those measured in the dark. Ocular biometrics measured under light and dark conditions may provide additional information for risk-stratifying patients for angle closure progression.
Table of Contents Statement
This study utilized ocular biometric data from the ZAP Trial to assess static and dynamic risk factors for angle closure progression. Our findings suggest that static angle width measured in the light using OCT is independently associated with progression and may provide unique information about progression risk. Biometric data acquired in the light may help identify individuals at higher risk of angle closure progression who would benefit from closer monitoring or earlier treatment.
Introduction
Primary angle closure glaucoma (PACG) is a leading cause of irreversible vision loss, affecting 20 million people worldwide.1,2 PACG is caused by appositional or synechial closure of the anterior chamber angle, which impedes outflow of aqueous humor through the trabecular meshwork and can lead to elevated intraocular pressure (IOP) and glaucomatous optic neuropathy.3 Angle closure eyes can be categorized as primary angle closure suspects (PACS), primary angle closure (PAC), or PACG along a continuous spectrum of disease severity.4 The Zhongshan Angle-Closure Prevention (ZAP) Trial found that the risk of progression from PACS to PAC is less than 1% per eye year even among untreated eyes. Therefore, current challenges of clinical practice include identifying which patients with evidence of early angle closure will develop more severe disease and could benefit from laser or surgical treatment.5,6
Ocular biometric parameters are well established risk factors for more severe PACD.7–11 By convention, clinical assessments of parameters related to angle closure using AS-OCT and gonioscopy are conducted in the dark where angles tend to be the narrowest.12,13 Static parameters that describe angle width in the dark are associated with elevated IOP and angle closure severity and progression.7,14–16 Other static parameters, such as iris curvature (IC), which reflects degree of pupillary block, and lens vault (LV), which reflects the phacomorphic component of angle closure, are also associated with PACD severity when measured in the dark.17–20 However, despite significant associations, ocular biometrics measured under dark conditions alone appear incompletely predictive of clinical outcomes.7,21,22 Biometric data obtained under other lighting conditions could provide additional information about angle closure progression risk. For example, recent studies identified an association between dynamic anatomical changes, specifically dark-to-light change in iris area, and PACD severity.13,23,24 However, these studies could not assess if static measurements in the light or dynamic dark-to-light changes in measurements are predictive of progression risk due to the cross-sectional nature of their data.
In this study, we use longitudinal data from the ZAP Trial to assess and compare static and dynamic ocular biometric risk factors for angle closure progression. We hypothesize that biometrics measured in the light may provide more information about progression risk than biometrics measured in the dark as typically more time is spent in lit environments and partially miotic states than in dark environments and fully mydriatic states. Furthermore, angle parameters may vary more in the light than in the dark, allowing for greater power to differentiate true risk. In addition, we hypothesize that dynamic change parameters that are predictive of PACD severity, such as light-to-dark change in iris area, are also predictive of angle closure progression.24–26
Methods
The ZAP Trial was approved by the Ethical Review Board of Sun Yat Sen University, the Ethical Committee of Zhongshan Ophthalmic Center, and the Institutional Review Boards of Moorfields Eye Hospital and Johns Hopkins University. The University of Southern California Institutional Review Board approved the current study. All study procedures adhered to the Declaration of Helsinki, and all study participants provided informed consent.
Data for the current study were derived from the ZAP Trial, a single-center randomized controlled trial conducted in Guangzhou, China. In brief, the ZAP Trial recruited participants aged 50 to 70 years with bilateral PACS, defined as eyes with 2 or more quadrants of non-visible pigmented TM on manual gonioscopy, in the absence of PAS, IOP above 21 mmHg, and glaucomatous optic neuropathy. One eye per participant was randomized to treatment with LPI. The other eye was monitored without treatment and served as the control eye. Participants underwent complete baseline eye examinations prior to LPI treatment, including AS-OCT imaging and gonioscopy. Data used in the current study were derived solely from untreated eyes at the baseline visit to avoid the confounding effect of LPI treatment on progression risk. Study endpoints included the development of PAC, which was defined as development of IOP >24 mmHg at 2 separate visits, 1 or more clock hours of PAS, or an attack of AAC.
Static gonioscopy was performed under dark ambient lighting standardized at less than 1 lux illumination (EA30 EasyView Light Meter; Extech Instruments; Waltham, MA, USA) with a 1-mm light beam and a Goldmann-type 1-mirror goniolens (Haag-Streit AG; Köniz, Switzerland) before pharmacologic pupillary dilation. Gonioscopy was performed by one of two fellowship- trained glaucoma specialists with high intergrader agreement (weighted k > 0.80).27 Care was taken to avoid light falling on the pupil, inadvertent indentation of the globe, and tilting of the lens of more than 10°. The angle was graded in each quadrant according to the modified Shaffer classification system: grade 0, no structures visible; grade 1, nonpigmented TM visible; grade 2, pigmented TM visible; grade 3, scleral spur visible; and grade 4, ciliary body visible.
Anterior segment OCT imaging was performed with the Visante AS-OCT system (Carl Zeiss Meditec, Inc; Dublin, CA, USA) in the dark (< 1 lux) and in the light (350–400 lux) before pupillary dilation. Eyelids were gently retracted during imaging, and care was taken to avoid inadvertent pressure on the globe. At the start of the ZAP Trial, only scans along the horizontal (temporal-nasal) meridian were performed in the dark and light. Partway through the ZAP Trial, scans along the vertical (superior-inferior) meridian were also performed in the dark. However, only horizontal scans were included in the current study as no vertical scans were performed in the light.
AS-OCT Image Analysis
One AS-OCT image per eye oriented along the horizontal meridian was analyzed using the custom Zhongshan Angle Assessment Program, which automatically segmented anterior segment structures and produced biometric measurements after the scleral spurs were marked. Image analysis was performed by 5 certified graders who were masked to examination results and intervention assignments. Each image was analyzed by a single grader aside from a set of 20 images that was analyzed by all 5 graders to establish intergrader agreement. Graders confirmed the segmentation and marked the scleral spurs in each image.28
In total, 11 biometric parameters describing the anterior segment were measured in each AS-OCT image obtained at the initial visit. These included angle opening distance 500 and 750 um anterior to the scleral spur (AOD500 and AOD750, respectively) trabecular iris space area bounded by AOD500 or AOD750 (TISA500 and TISA750, respectively), posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris, superiorly by the inner corneoscleral wall, and inferiorly by the iris surface; iris thickness at 750 um from the scleral spur (IT750); iris area (IA); iris curvature (IC); lens vault; anterior chamber depth (ACD); anterior chamber width (ACW); and pupillary diameter (PD). A set of 20 images from 20 eyes was selected randomly and graded independently by all 5 graders. Intergrader agreement in the form of intraclass correlation coefficients were excellent for all AS-OCT parameters (ICC > 0.83). Horizontal measurements of AOD500, AOD750, TISA500, TISA750, IT750, IA, and IC were calculated by averaging corresponding measurements from left and right sides of images. Dynamic change parameters, denoted with a “Δ”, were calculated by subtracting light measurements from corresponding dark measurements.
Statistical Analysis
Due to the relatively small sample size of progressors (N=36), multiple imputation using predictive mean matching was performed to fill in missing data rather than exclude these individuals from the analysis. Predictive mean matching utilizes regression statistics to impute appropriate predictions based on the available observed values of included variables.29 Variables used in the multiple imputation algorithm included age, sex, IOP, and all static ocular biometrics measured in the light and dark. This method was used to create 10 discrete datasets and statistical analyses were pooled across datasets using Rubin’s rules.30 Fewer angle width measurements were missing (<4.0%) than measurements of IC, ACD, and ACW (10.5 to 11.5%) (Supplementary Table 1). Normality of data was assessed using the Shapiro-Wilk test and by plotting histograms of measurement distributions. Spearman’s correlation coefficients were calculated between light and dark variables to identify collinearity. Differences between means of continuous variables were compared between progressors and non-progressors using the unpaired t-test or the Wilcoxon rank sum test. Proportions of categorical variables were compared using the chi-squared test.
Univariable and multivariable Cox proportional hazards regression models were developed to assess the relationship between baseline clinical and biometric characteristics of untreated eyes and progression risk in a time dependent manner. Multivariable models were limited to four variables due to the number of progressors (N = 36). The best subset selection method, which balances a higher R-squared statistic (indicator of predictive performance) with a lower Akaike information criterion (AIC) statistic (indicator of model overfitting), was used to automate variable selection for the primary multivariable model. Variables were included in the best subset selection based on a p-value < 0.1 in the univariable analysis. Biometric parameters that were significant in both the light and dark were separated in the selection process due to relatedness of the data. Concordance indices (C-index) were calculated to estimate model performance. Z-scores were calculated to interpret the units of variables included in multivariable regression models. Dichotomized representations of age, IOP, and TISA500 were created to develop secondary multivariable models. Categorical cutoffs were established based on the top quartile of age (age ≥ 62 years), top quartile of IOP (IOP ≥ 17.0 mmHg), lowest quartile of TISA500 in the dark (TISA500 ≤ 0.03 mm2), and TISA500 in the light (TISA500 ≤ 0.06 mm2). All analyses were performing using the R software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were conducted using a significance level of 0.05.
Results
889 untreated eyes of 889 ZAP Trial participants underwent baseline clinical examinations. 28 eyes were excluded due to missing baseline AS-OCT data in the dark and/or light. Among the remaining 861 participants and eyes (825 non-progressors, 36 progressors), mean age was 58.68 ± 5.01, mean IOP was 15.29 ± 2.90, and 717 (76.7%) of the participants were female.
There were significant differences (p < 0.05) between non-progressors and progressors (Table 1) in IOP (15.25 ± 2.93 and 16.37 ± 2.97, respectively), dark TISA500 (0.055 ± 0.034 and 0.033 ± 0.022, respectively), dark IA (1.56 ± 0.25 and 1.47 ± 0.20, respectively), dark IC (0.38 ± 0.09 and 0.34 ± 0.09, respectively), dark ACD (2.21 ± 0.21 and 2.15 ± 0.24, respectively), light TISA500 (0.08 ± 0.04 and 0.06 ± 0.04, respectively), and light ACD (2.21 ± 0.21 and 2.14 ± 0.25, respectively). There were no significant differences among measurements of the dynamic change parameters (p ≥ 0.05), including ΔIA and ΔIA/ΔPD. Correlation between light and dark parameters ranged from 0.4 to 0.7 except for ACD, which had a strong correlation at 0.94 (Supplementary Table 2).
Table 1.
Comparison of demographic and ocular biometric factors between Non-progressors and Progressors
| Lighting | Mean Parameters (Mean ± SD) | Non-progressors | Progressors | P-valuea |
|---|---|---|---|---|
| N = 825 | N = 36 | |||
| Age (years) | 58.61 ± 4.97 | 60.08 ± 5.76 | 0.10 | |
| Sex (F:M) | 687:138 | 30:6 | > 0.99 | |
| IOP (mmHg) | 15.25 ± 2.93 | 16.37 ± 2.97 | 0.02 | |
| Dark | AOD500 (mm) | 0.088 ± 0.054 | 0.055 ± 0.049 | < 0.001 |
| AOD750 (mm) | 0.126 ± 0.062 | 0.097 ± 0.065 | 0.01 | |
| TISA500 (mm2) | 0.055 ± 0.034 | 0.033 ± 0.022 | < 0.001 | |
| TISA750 (mm2) | 0.104 ± 0.071 | 0.097 ± 0.087 | 0.05 | |
| IA (mm2) | 1.561 ± 0.248 | 1.472 ± 0.201 | 0.03 | |
| IT750 (mm) | 0.489 ± 0.087 | 0.495 ± 0.094 | 0.71 | |
| IC (mm) | 0.384 ± 0.093 | 0.336 ± 0.092 | 0.002 | |
| LV (mm) | 0.711 ± 0.237 | 0.703 ± 0.274 | 0.87 | |
| ACD (mm) | 2.211 ± 0.206 | 2.149 ± 0.235 | 0.03 | |
| ACW (mm) | 11.444 ± 0.448 | 11.359 ± 0.505 | 0.22 | |
| PD (mm) | 4.437 ± 0.696 | 4.461 ± 0.731 | 0.77 | |
| Light | AOD500 (mm) | 0.150 ± 0.053 | 0.113 ± 0.056 | < 0.001 |
| AOD750 (mm) | 0.203 ± 0.67 | 0.175 ± 0.070 | 0.01 | |
| TISA500 (mm2) | 0.083 ± 0.038 | 0.059 ± 0.035 | < 0.001 | |
| TISA750 (mm2) | 0.143 ± 0.063 | 0.111 ± 0.059 | < 0.001 | |
| IA (mm2) | 1.833 ± 0.326 | 1.796 ± 0.316 | 0.20 | |
| IT750 (mm) | 0.438 ± 0.088 | 0.422 ± 0.075 | 0.26 | |
| IC (mm) | 0.385 ± 0.098 | 0.370 ± 0.102 | 0.63 | |
| LV (mm) | 0.732 ± 0.259 | 0.780 ± 0.267 | 0.16 | |
| ACD (mm) | 2.214 ± 0.212 | 2.137 ± 0.246 | 0.01 | |
| ACW (mm) | 11.487 ± 0.441 | 11.513 ± 0.454 | 0.69 | |
| PD (mm) | 2.743 ± 0.532 | 2.804 ± 0.584 | 0.64 | |
| Dynamic | ΔAOD500 (mm) | −0.062 ± 0.046 | −0.058 ± 0.043 | 0.87 |
| ΔAOD750 (mm) | −0.078 ± 0.055 | −0.078 ± 0.052 | 0.99 | |
| ΔTISA500 (mm2) | −0.028 ± 0.032 | −0.025 ± 0.029 | 0.48 | |
| ΔTISA750 (mm2) | −0.039 ± 0.080 | −0.014 ± 0.104 | 0.12 | |
| ΔIA (mm2) | −0.273 ± 0.321 | −0.324 ± 0.346 | 0.35 | |
| ΔIT750 (mm) | 0.052 ± 0.088 | 0.072 ± 0.090 | 0.07 | |
| ΔIC (mm) | −0.001 ± 0.097 | −0.035 ± 0.099 | 0.05 | |
| ΔLV (mm) | −0.021 ± 0.251 | −0.077 ± 0.330 | 0.57 | |
| ΔACD (mm) | −0.003 ± 0.080 | 0.012 ± 0.097 | 0.96 | |
| ΔACW (mm) | −0.042 ± 0.363 | −0.154 ± 0.408 | 0.08 | |
| ΔPD | 1.694 ± 0.661 | 1.658 ± 0.743 | 0.93 | |
| ΔIA/ΔPD | −0.141 ± 0.670 | −0.178 ± 0.335 | 0.82 |
ACD: Anterior chamber depth; ACW: Anterior chamber width; AOD: Angle opening distance; IA: Iris area; IC: Iris curvature; IOP: Intraocular pressure; IT: Iris thickness; LV: Lens vault; PD: Pupillary diameter; TISA: Trabecular iris surface area
Statistical significance tested by Wilcoxon ranked sum test/Unpaired t-test or Chi-squared test
On univariable Cox regression analysis, greater IOP, smaller AOD500/AOD750/TISA500 in the light and dark, smaller TISA750 in the light, smaller IA and flatter IC in the dark, and smaller ACD in the light were significantly associated with higher risk of progression (p < 0.05). None of the dynamic change parameters were significantly associated (p ≥ 0.08). (Supplementary Table 3).
In the primary multivariable Cox regression model (model A, Table 2), narrower TISA500 in the light (HR = 1.28 per 0.01 mm2 or 0.26 standard deviations (SD), 95% confidence interval [CI] = 1.11–1.47; p = 0.001), older age (HR = 1.09 per year, 95% CI = 1.02–1.17; p = 0.02), and higher IOP (HR = 1.13 per mmHg, 95% CI = 1.01–1.26; p = 0.03) were associated with greater risk of progression (C-index = 0.76, 95% CI = 0.65–0.84). In the secondary multivariable Cox regression model (model B, Table 2), narrower TISA500 in the dark (HR = 1.28 per 0.01 mm2 or 0.30 SD, 95% CI = 1.09–1.49; p = 0.002), older age (HR = 1.09 per year, 95% CI = 1.02–1.17; p = 0.01), and higher IOP (HR = 1.12 per mmHg, 95% CI = 1.00–1.26; p = 0.03) were associated with greater risk of progression (C-index = 0.76, 95% CI = 0.68–0.83). There was a borderline significant association between flatter IC and progression in both models A (HR = 1.59 per 0.1 mm, 95% CI = 1.00–2.50; p = 0.05) and B (HR = 1.56 per 0.1 mm, 95% CI = 0.96–2.50, p = 0.06).
Table 2.
Multivariable cox regression analysis of demographic and ocular biometric factors associated with angle closure progression.
| Lighting | Variable (Unit) | Z-score | Model A | Model B | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| - | Age (1 year older) | 0.20 | 1.09 (1.02, 1.17) | 0.02 | 1.09 (1.02, 1.17) | 0.01 |
| - | IOP (1 mmHg higher) | 0.34 | 1.13 (1.01, 1.26) | 0.04 | 1.12 (1.00, 1.26) | 0.04 |
| Dark | TISA500 (0.01 mm2 smaller) | 0.30 | - | - | 1.28 (1.09, 1.49) | 0.002 |
| Dark | IC (0.1 mm smaller) | 1.07 | 1.59 (1.00, 2.50) | 0.05 | 1.56 (0.96, 2.50) | 0.06 |
| Light | TISA500 (0.01 mm2 smaller) | 0.26 | 1.28 (1.11, 1.47) | 0.001 | - | - |
Statistically significant p-values and hazard ratios are bolded; HR = hazards ratio; CI = Confidence Interval; IC: Iris curvature; IOP: Intraocular pressure; PAC: Primary angle closure; PACS: Primary angle closure suspect; PACG: Primary angle closure glaucoma; TISA: Trabecular iris surface area
TISA500 in the dark and light had similar predictive performance (C-index = 0.71, 95% CI = 0.63–0.78 and 0.72, 95% CI = 0.61–0.81, respectively) in separate univariable models. Predictive performance increased modestly when both were combined in the same model (C-index = 0.73, 95% CI = 0.63–0.81).
LOWESS plots of probability of progression predicted by models A and B (Figure 1) showed a steeper rise in probability for smaller measurements of TISA500 in the light (model A) than in the dark (model B). Histograms of the distribution of TISA500 measurements in the light and dark showed a leftward shift of measurement values in the dark compared to the light, consistent with narrower angles in the dark than light (Figure 2).
Figure 1:
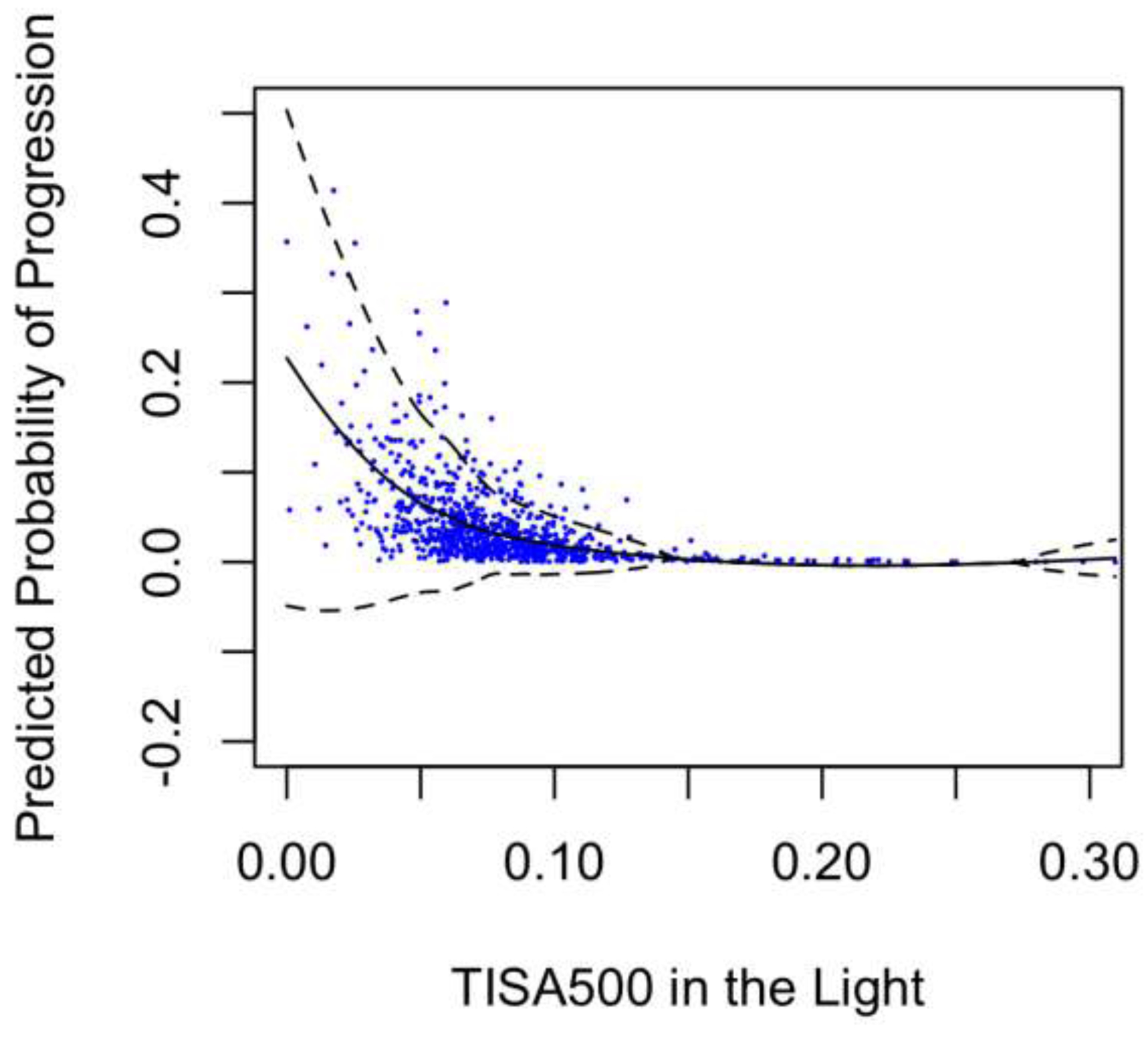
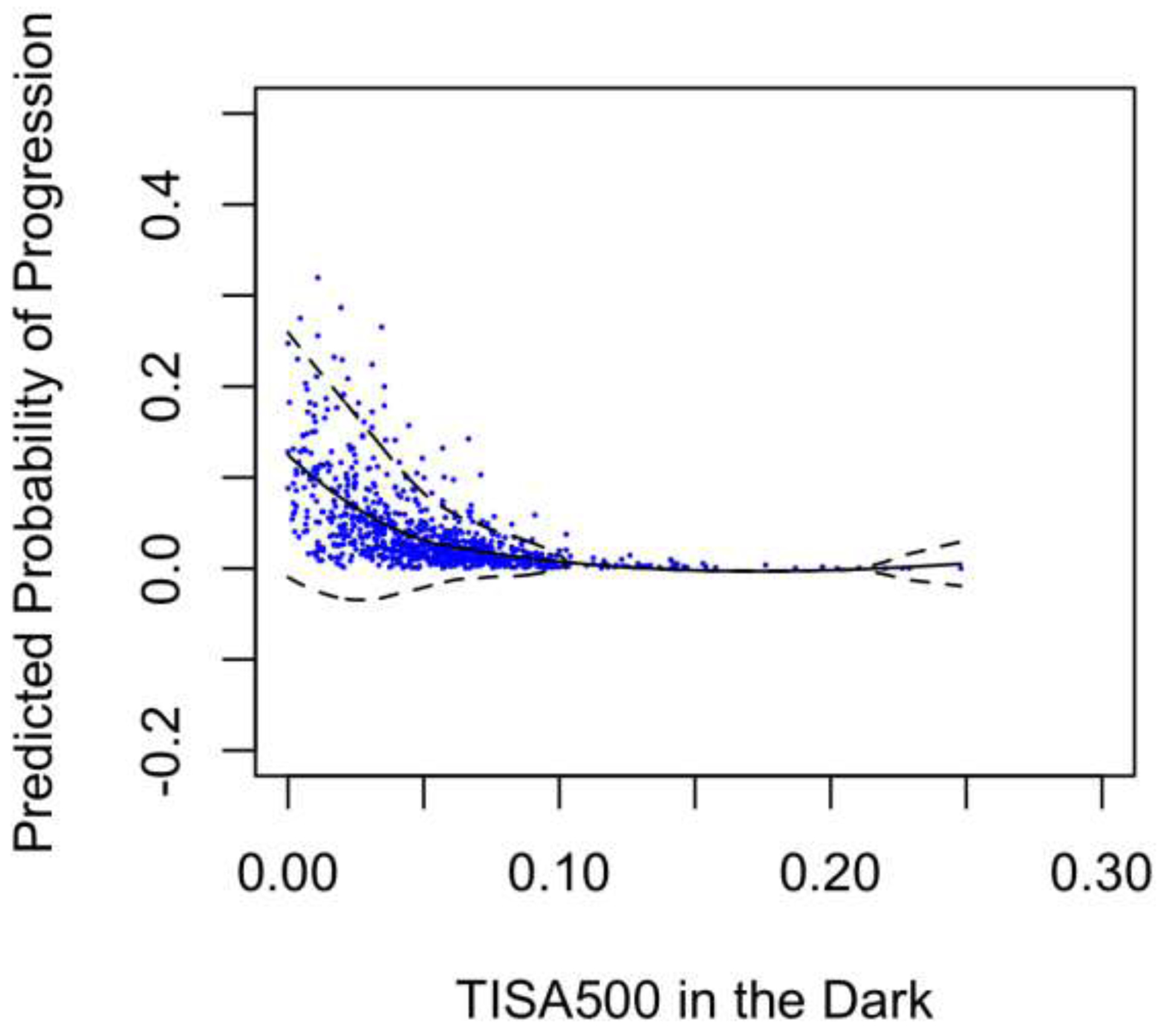
Predicted Probability of Progression over TISA500 in the light or dark
Figure 2:
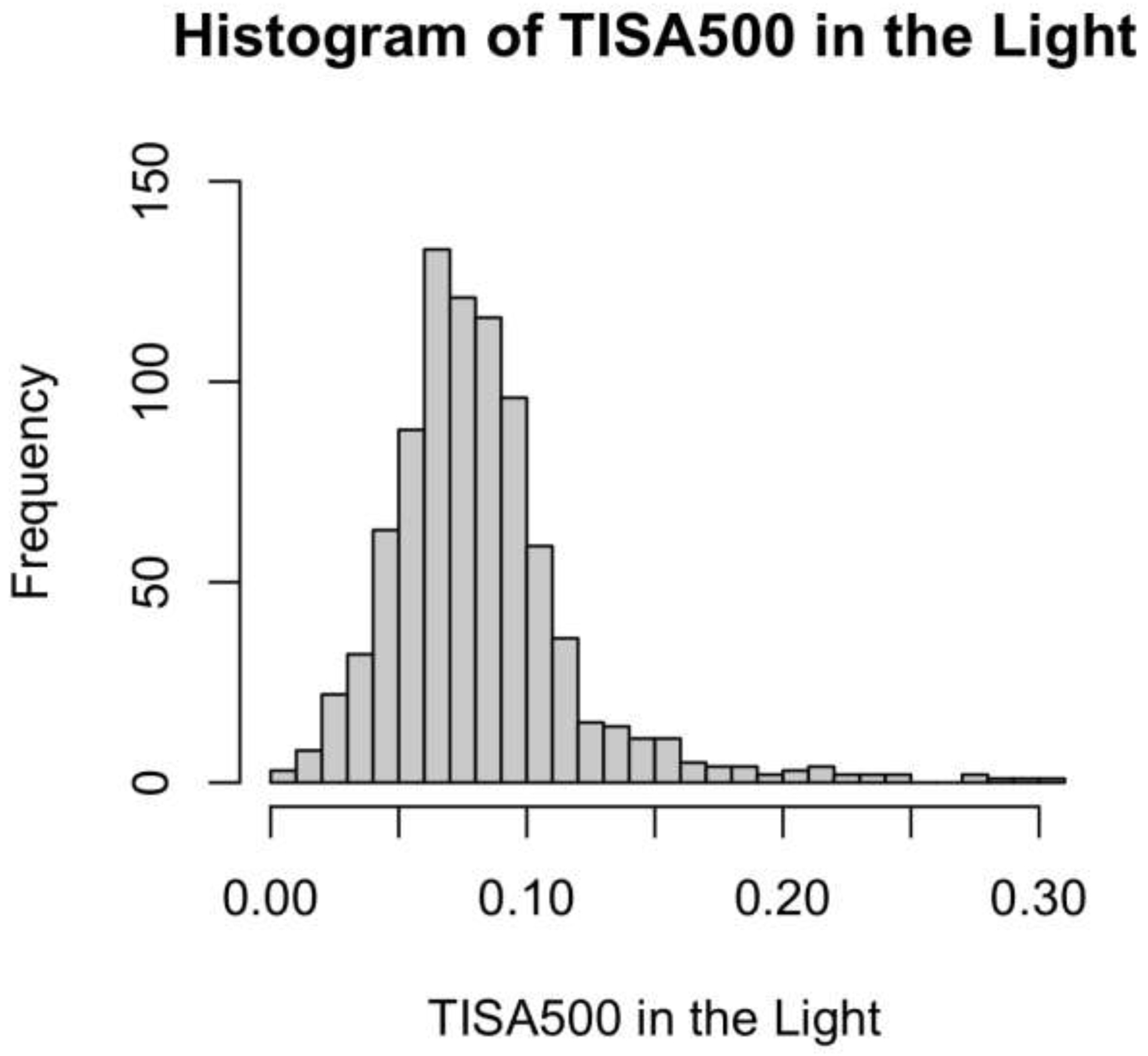
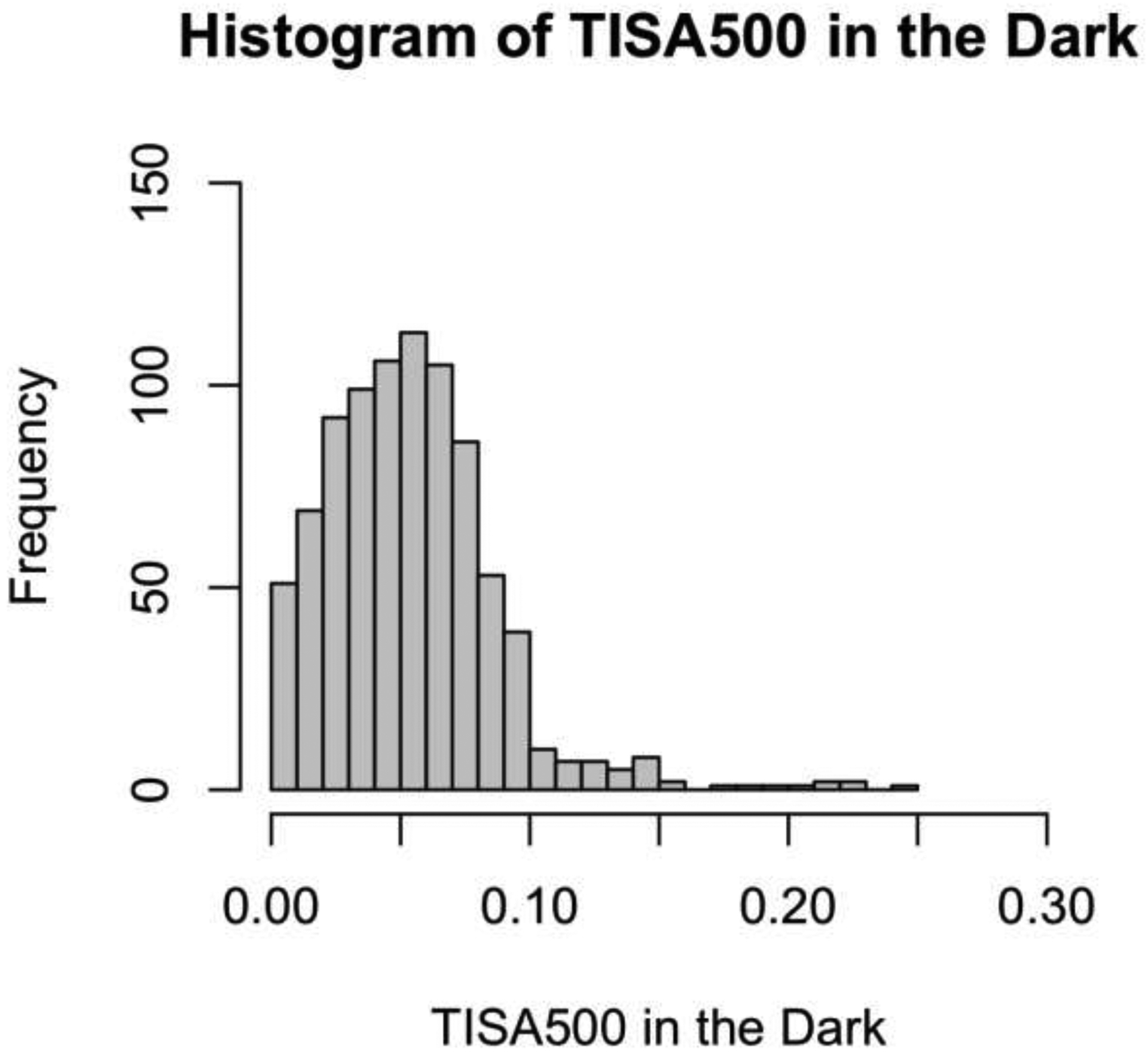
Distribution of TISA500 Measurements in the Light and Dark
In multivariable Cox regression model C (Table 3) with dichotomized representations of significant risk factors for progression from model A, eyes in the top quartile of age (HR = 2.20, 95% CI = 1.10–4.37; p = 0.03), top quartile of IOP (HR = 2.21, 95% CI = 1.12– 4.38; p = 0.02), and bottom quartile of TISA500 in the light (HR = 4.52, 95% CI = 2.27–9.01; p < 0.001) were at greater risk of progression (C-index = 0.76, 95% CI = 0.69–0.82). In multivariable Cox regression model D with categorical representations of significant risk factors for progression from model B (Table 3), eyes within the top quartile of age (HR = 2.22, 95% CI = 1.12–4.41; p = 0.02), top quartile of IOP (HR = 2.13, 95% CI = 1.08–4.21; p = 0.03), and bottom quartile of TISA500 in the dark (HR = 2.89, 95% CI = 1.46–5.71; p = 0.003) were at greater risk of progression (C-index = 0.71, 95% CI = 0.62–0.78).
Table 3.
Univariable and multivariable cox regression analysis of dichotomized variables associated with angle closure progression.
| Lighting | Variable | Cutoff | Univariable | Multivariable C | Multivariable D | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| - | Top Quartile Age | ≥ 62.0 years | 2.03 (1.03, 4.02) | 0.04 | 2.20 (1.10, 4.37) | 0.03 | 2.22 (1.12, 4.41) | 0.02 |
| - | Top Quartile IOP | ≥ 17.0 mmHg | 2.14 (1.08, 4.21) | 0.03 | 2.21 (1.12, 4.38) | 0.02 | 2.13 (1.08, 4.21) | 0.03 |
| Dark | Lowest Quartile TISA500 | ≤ 0.03 mm2 | 2.89 (1.47, 5.70) | 0.003 | - | - | 2.89 (1.46, 5.71) | 0.003 |
| Light | Lowest Quartile TISA500 | ≤ 0.06 mm2 | 4.56 (2.29, 9.08) | < 0.001 | 4.52 (2.27, 9.01) | < 0.001 | - | - |
Statistically significant p-values and hazard ratios are bolded; HR = hazards ratio; CI = Confidence Interval; IOP: Intraocular pressure; TISA: Trabecular iris surface area.
Discussion
In this longitudinal study, we demonstrated that static biometric parameters measured in the light and dark, including TISA500, are predictive of six-year progression from PACS to PAC. We also demonstrated that TISA500 measured in the light is equally predictive, if not more, of angle closure progression, especially when measurements are in the lowest quartile. Finally, our results suggest that dynamic biometric change parameters are poorly predictive of progression. These findings raise questions about the clinical convention of solely assessing angles in the dark and ideal lighting conditions to risk-stratify patients with PACS for more severe disease.
Our findings support the central role of static ocular biometrics in angle closure pathogenesis; specifically, narrower angle width measured by AS-OCT confers higher risk of angle closure progression.7 Previous studies found that angle width measured in the dark by AS-OCT is significantly associated with PACD severity and progression risk.7,31,32 However, our study provides the first evidence that it is not solely biometric measurements obtained in the dark that are predictive of angle closure progression; biometric measurements obtained in the light appear equally predictive. Separate multivariable models with TISA500 measured in the light (model A) or dark (model B) yielded similar hazard ratios and concordance indices, suggesting that the strength of association and predictive performance is similar between the two sets of measurements. Angles tend to be narrowest in the dark on average; therefore, it is logical from an anatomical perspective to evaluate the angle in the dark using AS-OCT or gonioscopy. However, from a clinical perspective, it is important to recognize that the angle likely assumes this configuration for only a few brief moments throughout the day due to the miotic effects of external lighting and dark adaptation. While it is reasonable to speculate that progression risk reflects an aggregate effect of different anatomical configurations over time, this point requires additional longitudinal study using biometric data collected under a range of lighting conditions.
Our findings further suggest that biometrics measured in the light may provide additional information about angle closure progression compared to biometrics measured in the dark alone. Due to the angle-widening effect of pupillary constriction, imaging PACS eyes in the light produced a more even distribution of angle width measurements than in the dark. As a result, eyes within the lowest quartile of TISA500 measurements in the light had higher risk of progression (HR = 4.56) than in the dark (HR = 2.83). In addition, LOWESS plots of predicted progression probability rose higher for the narrowest angles in the light than the dark. This raises the possibility that different lighting conditions could induce different anatomical configurations that provide unique information about progression risk.
Our model with both TISA500 measured in the dark and light demonstrated modest gains in predictive performance compared to separate univariable models with the two parameters alone. While this finding is possibly related to the small sample of progressors, it suggests that dark and light measurements together may provide complementary information about progression risk that could be superior to analyzing one set of measurements alone. It is important to note that there was only moderate correlation between dark and light measurements of all parameters except ACD, ranging between 0.5 to 0.7. This finding rules out the possibility that static parameters, including TISA500 (R = 0.67), measured in the light were only associated with progression because of strong correlations with the same static parameters measured in the dark. The moderate correlation also suggests that each set of measurements carries unique information, which explains why a model with measurements from multiple lighting environments could be more predictive than a model with measurements from a single lighting environment. However, further work is needed to establish the clinical utility of combining multiple sets of biometric measurements and the optimal lighting conditions for obtaining these measurements.
While recent studies propose that dynamic anatomical changes, especially of the iris, are associated with PACD severity, we did not find an association between dynamic biometric change parameters and progression. The association between dark-to-light change in iris area and PACD severity is well-established; eyes with angle closure demonstrate smaller reductions in IA per millimeter of pupillary dilation, an effect that contributes to tissue congestion in the angle recess and iridotrabecular contact.24,25,33 In addition, Lifton et al. reported that dark-to-light increases in AOD750 and decreases in ACW were smaller and increases in LV were greater in eyes with PACD compared to eyes without.13 Our results did not show an association between dark-to-light ΔIA or ΔIA/ΔPD; rather, IA tended to decrease more among progressors than non-progressors, although the difference was not significant. These findings suggest that dynamic parameters are weakly associated with angle closure progression, if at all, especially compared to static parameters.
Our study has several limitations. First, the number of progressors in the ZAP Trial was relatively small, limiting the number of variables we could include in our multivariable models and this may have prevented us from identifying weaker risk factors, including some dynamic parameters. However, this is a general limitation of longitudinal studies on progression from PACS to PAC, which is a relatively rare event. Second, we used multiple imputation to fill in missing data, which helped preserve the overall sample size. While no variable had more than 11.5% missing values and angle width measurements all had fewer than 4.0% missing values, this approach may potentially limit our ability to identify real associations for variables with more missing values. However, it was reassuring that our results were entirely consistent with those by Xu et al., who did not use imputation to analyze the same dataset.7 Finally, the ZAP Trial database is comprised of only mainland Chinese participants between the ages of 50 to 70 years old with bilateral PACS. Therefore, our findings may not be generalizable to other demographic groups or patients with more severe angle closure.
In conclusion, static biometrics obtained from horizontal AS-OCT scans in the light are as predictive, if not more, of progression from PACS to PAC than biometrics obtained from similar scans in the dark, whereas dynamic biometrics are weakly predictive of progression, if at all. The ZAP Trial showed that the majority of PACS eyes do not progress, at least within a six-year time period.6 However, identifying and treating a small subset of eyes at higher risk of progression may help reduce future vision loss, especially in regions with lower access to eye care and cataract surgery.32,33 Integrating all available biometric data to identify individuals at highest risk of PAC and PACG could result in more personalized glaucoma care in the future.
Supplementary Material
Supplementary Table 1. Percentage of missing values of parameters used in analysis
Supplementary Table 2. Spearman correlation coefficients between parameters in the dark and light
Supplementary Table 3. Univariable cox regression analysis of demographic and ocular biometric factors associated with angle closure progression. Hazard ratios correspond to per unit increase in each independent variable.
Acknowledgements
This work was supported by grant K23 EY029763 from the National Eye Institute, National Institute of Health, Bethesda, Maryland and an unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY. The ZAP Trial was supported by the Fight for Sight (grant 1655; UK), the Sun Yat-sen University 5010 Project Fund (grant 2007033; China), the National Natural Science Foundation of China (grant 81420108008; China), Fundamental Research Funds of the State Key Laboratory in Ophthalmology (China), and Moorfields Eye Charity (previously Special Trustees of Moorfields Eye Hospital).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/J.OPHTHA.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. The British journal of ophthalmology. 2006;90(3):262–267. doi: 10.1136/BJO.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/JAMA.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baskaran M, Kumar RS, Friedman DS, et al. The Singapore Asymptomatic Narrow Angles Laser Iridotomy Study: Five-Year Results of a Randomized Controlled Trial. Ophthalmology. 2022;129(2):147–158. doi: 10.1016/j.ophtha.2021.08.017 [DOI] [PubMed] [Google Scholar]
- 6.He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393(10181):1609–1618. doi: 10.1016/S0140-6736(18)32607-2 [DOI] [PubMed] [Google Scholar]
- 7.Xu BY, Friedman DS, Foster PJ, et al. Ocular Biometric Risk Factors for Progression of Primary Angle Closure Disease: The Zhongshan Angle Closure Prevention Trial. Ophthalmology. 2022;129(3):267–275. doi: 10.1016/J.OPHTHA.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–479. doi: 10.1016/J.OPHTHA.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 9.Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117(10):1967–1973. doi: 10.1016/J.OPHTHA.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Aung T, Nolan WP, Machin D, et al. Anterior Chamber Depth and the Risk of Primary Angle Closure in 2 East Asian Populations. Archives of Ophthalmology. 2005;123(4):527–532. doi: 10.1001/archopht.123.4.527 [DOI] [PubMed] [Google Scholar]
- 11.Shan J, DeBoer C, Xu BY. Anterior Segment Optical Coherence Tomography: Applications for Clinical Care and Scientific Research. Asia Pac J Ophthalmol (Phila). Published online April 25, 2019:10.22608/APO.201910. doi: 10.22608/APO.201910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SD, Singh K, Lin SC, et al. Evaluation of the Anterior Chamber Angle in Glaucoma: A Report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(10):1985–1997. doi: 10.1016/j.ophtha.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 13.Lifton J, Burkemper B, Jiang X, et al. Ocular Biometric Determinants of Dark-to-Light Change in Angle Width: The Chinese American Eye Study. American journal of ophthalmology. 2022;237:183–192. doi: 10.1016/J.AJO.2021.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong RS, Sakata LM, Narayanaswamy AK, et al. Relationship between intraocular pressure and angle configuration: An anterior segment OCT study. Investigative Ophthalmology and Visual Science. 2013;54(3):1650–1655. doi: 10.1167/iovs.12-9986 [DOI] [PubMed] [Google Scholar]
- 15.Xu BY, Burkemper B, Lewinger JP, et al. Correlation between Intraocular Pressure and Angle Configuration Measured by OCT. Ophthalmology Glaucoma. 2018;1(3):158–166. doi: 10.1016/j.ogla.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu BY, Pardeshi AA, Shan J, et al. Effect of Angle Narrowing on Sectoral Variation of Anterior Chamber Angle Width. Ophthalmology Glaucoma. 2020;3(2):130–138. doi: 10.1016/j.ogla.2019.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabana N, Aquino MC, See J, et al. Quantitative evaluation of anterior chamber parameters using anterior segment optical coherence tomography in primary angle closure mechanisms. Clinical & experimental ophthalmology. 2012;40(8):792–801. doi: 10.1111/J.1442-9071.2012.02805.X [DOI] [PubMed] [Google Scholar]
- 18.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in chinese subjects with angle closure. Ophthalmology. 2011;118(3):474–479. doi: 10.1016/j.ophtha.2010.07.025 [DOI] [PubMed] [Google Scholar]
- 19.He M, Foster PJ, Johnson GJ, Khaw PT. Angle-closure glaucoma in East Asian and European people. Different diseases? Eye 2006 20:1. 2005;20(1):3–12. doi: 10.1038/sj.eye.6701797 [DOI] [PubMed] [Google Scholar]
- 20.Wang N, Wu H, Fan Z. Primary angle closure glaucoma in Chinese and Western populations. Chin Med J. 2002;115(11):1706–1715. [PubMed] [Google Scholar]
- 21.Quigley HA. The iris is a sponge: a cause of angle closure. Ophthalmology. 2010;117(1):1–2. doi: 10.1016/J.OPHTHA.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA. Angle-closure glaucoma-simpler answers to complex mechanisms: LXVI Edward Jackson Memorial Lecture. American journal of ophthalmology. 2009;148(5). doi: 10.1016/J.AJO.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 23.Soh ZD, Thakur S, Majithia S, Nongpiur ME, Cheng CY. Iris and its relevance to angle closure disease: a review. The British journal of ophthalmology. 2021;105(1):3–8. doi: 10.1136/BJOPHTHALMOL-2020-316075 [DOI] [PubMed] [Google Scholar]
- 24.Quigley HA, Silver DM, Friedman DS, et al. Iris cross-sectional area decreases with pupil dilation and its dynamic behavior is a risk factor in angle closure. J Glaucoma. 2009;18(3):173–179. doi: 10.1097/IJG.0b013e31818624ce [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Li SZ, Li L, He MG, Thomas R, Wang NL. Dynamic Iris Changes as a Risk Factor in Primary Angle Closure Disease. Investigative Ophthalmology & Visual Science. 2016;57(1):218–226. doi: 10.1167/iovs.15-17651 [DOI] [PubMed] [Google Scholar]
- 26.Aptel F, Denis P. Optical Coherence Tomography Quantitative Analysis of Iris Volume Changes after Pharmacologic Mydriasis. Ophthalmology. 2010;117(1):3–10. doi: 10.1016/j.ophtha.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Friedman DS, He M, Huang S, Kong X, Foster PJ. Design and methodology of a randomized controlled trial of laser iridotomy for the prevention of angle closure in southern China: the Zhongshan angle Closure Prevention trial. Ophthalmic Epidemiol. 2010;17(5):321–332. doi: 10.3109/09286586.2010.508353 [DOI] [PubMed] [Google Scholar]
- 28.Ho SW, Baskaran M, Zheng C, et al. Swept source optical coherence tomography measurement of the iris–trabecular contact (ITC) index: a new parameter for angle closure. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1205–1211. doi: 10.1007/s00417-012-2158-6 [DOI] [PubMed] [Google Scholar]
- 29.Schenker N, Taylor JMG. Partially parametric techniques for multiple imputation. Computational Statistics & Data Analysis. 1996;22(4):425–446. doi: 10.1016/0167-9473(95)00057-7 [DOI] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 31.Moghimi S, Vahedian Z, Fakhraie G, et al. Ocular Biometry in the Subtypes of Angle Closure: An Anterior Segment Optical Coherence Tomography Study. American Journal of Ophthalmology. 2013;155(4):664–673.e1. doi: 10.1016/j.ajo.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhang Q, Thomas R, Li SZ, Wang NL. Development of angle closure and associated risk factors: The Handan eye study. Acta Ophthalmol. 2022;100(1):e253–e261. doi: 10.1111/aos.14887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aptel F, Chiquet C, Beccat S, Denis P. Biometric evaluation of anterior chamber changes after physiologic pupil dilation using Pentacam and anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):4005–4010. doi: 10.1167/iovs.11-9387 [DOI] [PubMed] [Google Scholar]
- 34.George R, Panda S, Vijaya L. Blindness in glaucoma: primary open-angle glaucoma versus primary angle-closure glaucoma-a meta-analysis. Eye (Lond). 2022;36(11):2099–2105. doi: 10.1038/s41433-021-01802-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quek DTL, Koh VT, Tan GS, Perera SA, Wong TT, Aung T. Blindness and long-term progression of visual field defects in chinese patients with primary angle-closure glaucoma. Am J Ophthalmol. 2011;152(3):463–469. doi: 10.1016/j.ajo.2011.02.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Percentage of missing values of parameters used in analysis
Supplementary Table 2. Spearman correlation coefficients between parameters in the dark and light
Supplementary Table 3. Univariable cox regression analysis of demographic and ocular biometric factors associated with angle closure progression. Hazard ratios correspond to per unit increase in each independent variable.


