Abstract
Background:
Few studies have measured ventilation during early cardiopulmonary resuscitation (CPR) before advanced airway placement. Resuscitation guidelines recommend pauses after every 30 chest compressions to deliver ventilations. The effectiveness of bag-valve-mask (BVM) ventilation delivered during the pause in chest compressions is unknown. We sought to determine (1) the incidence of lung inflation with BVM ventilation during 30:2 CPR and (2) the association of ventilation with outcomes after out-of-hospital cardiac arrest (OHCA).
Methods:
We studied OHCA patients from six sites of the Resuscitation Outcomes Consortium Trial of Continuous or Interrupted Chest Compressions during CPR. We analyzed patients assigned to the 30:2 CPR arm with ≥ two minutes of thoracic bioimpedance signal recorded with a cardiac defibrillator/monitor. Detectable ventilation waveforms were defined as having a bioimpedance amplitude ≥0.5 Ohm (corresponding to ≥ 250 ml VT) and duration ≥1 sec. We defined a chest compression pause as a break in chest compressions of 3 – 15 sec duration. We compared the incidence of ventilation and outcomes in two groups: patients with ventilation waveforms in <50% of pauses (Group 1) versus those with waveforms in ≥50% of pauses (Group 2).
Results:
Among 1,976 patients, mean age was 65 years, 66% were male. From the start of chest compressions until advanced airway placement, mean (SD) duration of 30:2 CPR was 9.8 ± 4.9 min. During this period, we identified 26,861 pauses in chest compressions; 60% of patients had ventilation waveforms in <50% of pauses (Group 1, N=1177), and 40% had waveforms in ≥50% of pauses (Group 2, N=799); Group 1 had a median 12 pauses and 2 ventilations per patient vs. Group 2 had 12 pauses and 12 ventilations per patient. Group 2 had higher rates of prehospital ROSC (40.7% vs. 25.2%, p<0.0001), survival to hospital discharge (13.5% vs. 4.1%, p <0.0001), and survival with favorable neurological outcome (10.6% vs. 2.4%, p <0.0001). These associations persisted after adjustment for confounders.
Conclusions:
In this study, lung inflation occurred infrequently with BVM ventilation during 30:2 CPR. Lung inflation in ≥50% of pauses was associated with improved ROSC, survival, and survival with favorable neurological outcome.
Keywords: Heart arrest, cardiopulmonary resuscitation, ventilation detection, outcomes, bioimpedance
INTRODUCTION
Over 400,000 out-of-hospital cardiac arrests (OHCA) occur annually in the United States.1,2 Multiple, large-scale observational and experimental studies have informed cardiopulmonary resuscitation (CPR) guidelines on best practices of delivering chest compressions, including rate, depth, and fraction (the proportion of time spent doing chest compressions).3–7 The findings have improved OHCA patient care and clinical outcome.1 In contrast to reporting chest compression metrics, ventilation metrics have not been investigated because there have been no readily available methods to routinely measure ventilation in the out-of-hospital setting, especially during the initial and most important stages of resuscitation. Thus, it is uncertain whether the quality of ventilations or specific ventilation metrics are factors affecting patient outcomes.
Chest compression alone does not generate sufficient tidal volume for adequate gas exchange. 8–10 Therefore, professional responders usually give some form of rescue ventilation during CPR, often using a bag-valve-mask (BVM) device with the goal of delivering enough volume to achieve visible chest rise as an indication of successful lung inflation. The tidal volume associated with detectable chest wall movement is estimated to be between 300 to 500 mL.11 Capnography can be used to determine if ventilation is present, however, capnography is usually measured only after placement of an advanced airway, which typically occurs later in the advanced life support stage of CPR. There are no widely available technologies for detecting ventilations during BVM ventilation, the most crucial initial phase of resuscitation.
Thoracic bioimpedance recordings have been shown and validated to measure ventilation frequency during CPR.12–15 When a person inhales and exhales, the chest wall expands and contracts, and thoracic electrical resistance oscillates, which is detected by changes in thoracic bioimpedance. During CPR, thoracic bioimpedance is captured through the defibrillator pads placed on the chest and recorded by the defibrillator. During 30:2 CPR, chest compressions are paused, which prevents their concurrent confounding of the ventilation impedance signal. One recent single-site pilot study used thoracic bioimpedance to count ventilations during OHCA and emergency medical services (EMS) 30:2 CPR and found that the number of ventilations was associated with outcomes.16
The objectives of this multicenter study were to determine the incidence of bioimpedance-detected ventilation waveforms during BVM ventilation in 30:2 CPR and to assess the association of detectable ventilation (lung inflation) with outcomes from OHCA.
METHODS
Design and Setting
We conducted a secondary analysis of clinical and continuous cardiac monitor data from six sites (Birmingham, AL; British Columbia, Canada; Dallas-Fort Worth, TX; King County, WA; Ottawa/OPALS,ON, Canada; and Pittsburgh, PA) participating in the 30:2 arm of the Resuscitation Outcomes Consortium (ROC) Trial of Continuous or Interrupted Chest Compressions during CPR (CCC) clinical trial.17 Institutional Review Boards (IRBs) at participating sites approved the parent study under Exception from Informed Consent. These IRBs later approved the present study.
Two ROC sites did not participate in the ROC CCC study and two sites used only ZOLL defibrillators, which were excluded from our study because the defibrillator recordings do not include a useable bioimpedance signal.
In order to minimize the possibility of unintentionally sharing information that can be used to re-identify private information, a subset of the data generated for this study are available at NIH BioLINCC and can be accessed at https://biolincc.nhlbi.nih.gov/studies/roc_ccc/
Patient Population
The ROC CCC study used cluster-crossover randomization to assign EMS agencies to use either continuous chest compression CPR or 30:2 CPR (cycles of chest compressions interrupted by ventilation in a ratio of 30 chest compressions to 2 ventilations) prior to placement of an advanced airway. EMS crews (Basic and Advanced Life Support) used the assigned protocol for adults requiring CPR unless they had an obvious exclusion. EMS agencies were crossed over to the other arm twice per year. Details about cluster-crossover randomization are given in the parent trial publication and in the accompanying supplementary appendix.17 We limited the analysis to patients with OHCA assigned to the 30:2 arm of the study from June 2011 to May 2015 at the six ROC sites with files available from the LIFEPAK 12 (LP12) and LIFEPAK 15 (LP15) defibrillators (Physio-Control/Stryker), Redmond, WA, USA) or the MRx defibrillator (Philips, Andover, MA). Inclusion criteria were non-traumatic cardiac arrest patients at least 18 years old who had defibrillator recordings with a minimum of two minutes of 30:2 CPR by EMS providers. Of the patients assigned to the 30:2 CPR arm from the six participating sites, we excluded patients who had a defibrillator other than the Lifepak 12 or 15 or the Philips MRx defibrillator applied during CPR. In addition, recordings sometimes could not be downloaded from the defibrillator or the file could not be identified in the site database (Fig. 1). In addition, we excluded patients who received continuous chest compressions instead of the assigned 30:2 CPR or did not have obvious 30:2 CPR. We also excluded LP12, LP15, and MRx defibrillator files that lacked a usable recorded impedance signal or had less than two minutes of recorded 30:2 CPR. Occasionally, an automated external defibrillator (AED) was applied first before the arrival of a manual monitor-defibrillator. When an AED file was available, we included such data as chest compression metrics, initial cardiac rhythm, shocks or no shock advised, and start times. We combined the AED recording with the monitor-defibrillator recording for the same patient to create one continuous recording for analysis. Unfortunately, AEDs were unable to record bioimpedance ventilation waveforms and the first few minutes of ventilation data may be missing for some patients.
Figure 1.

CONSORT Diagram: Study cohort and exclusions. ROC indicates Resuscitation Outcomes Consortium; CCC, Trial of Continuous or Interrupted Chest Compressions during CPR; CPR, cardiopulmonary resuscitation; LP, Physio-Control/Stryker LIFEPAK defibrillator (models LP12 and LP15); MRx, Philips MRx defibrillator; 30:2 refers to cycles of 30 chest compressions to 2 ventilations during CPR.
Reviewers identified 1,976 files that met our inclusion criteria (Fig. 1). We included ventilation data from the start of chest compressions until placement of an advanced airway. If an advanced airway was not placed (N = 326), we analyzed the first 15 minutes of the recording from the start of chest compressions.
Ventilation Waveform Analysis
We previously developed and validated criteria specifying ventilation bioimpedance waveform characteristics associated with lung inflation from patients with OHCA who received 30:2 CPR and attempted ventilation with a BVM device.15 We then developed computer software that incorporated the criteria for bioimpedance ventilation waveforms that could automatically identify ventilation waveforms from defibrillator recordings. A detectable bioimpedance ventilation waveform has a bioimpedance amplitude of ≥0.5 Ohm with a duration ≥1 sec. Our ventilation detection method has a sensitivity >90% and a positive predictive value >90% compared with capnography.18–20
Prior studies showed that >250 mL is a reasonable minimum tidal volume that can result in gas exchange.21 The threshold of 250 mL approximates the minimum amount of tidal volume needed to overcome anatomic and physiologic dead space and produce clinically meaningful gas exchange. Accordingly, we set the minimum bioimpedance waveform amplitude threshold to ≥0.5 Ohm, which corresponds to ≥250 mL of tidal volume. We determined the bioimpedance amplitude/tidal volume relationship in a laboratory setting in human volunteers16 and it has also been validated in patients.22 In addition, the threshold of 0.5 Ohm is large enough to be distinguished reliably from artifact.15
We reviewed eligible defibrillator files manually to identify chest compression pauses with a duration of 3 sec to 15 sec. We excluded pauses that were marked for special events, such as attempted defibrillation and rhythm and pulse checks.
Outcomes
We determined data elements about patient demographics, arrest circumstances, clinical care, and outcomes from dispatch, EMS, and hospital records using a standard data dictionary consistent with the Utstein template.
The primary outcome was survival to hospital discharge. Secondary outcomes were return of spontaneous circulation (ROSC) at any time, pre-hospital ROSC, ROSC on arrival at the emergency department (ED ROSC), survival to hospital admission, and survival with favorable neurological outcome [modified Rankin Scale (mRS) ≤3] at hospital discharge.
Statistical Analysis
We chose to perform a dichotomous analysis by pre-specifying two groups a priori for comparison: patients with at least one lung inflation waveform in < 50% of chest compression pauses (Group1) vs. patients who had at least one lung inflation waveform in ≥ 50% of chest compression pauses (Group 2).
We determined descriptive statistics for witnessed status, initial cardiac rhythm, chest compression rate and fraction over the first 6 minutes of CPR, ventilation quality metrics, prehospital ROSC, ED ROSC, ROSC at any time, survival at discharge, and survival with favorable neurological outcome. We present summary results as mean (SD) or median with 25th percentile (Q1) and 75th percentile (Q3). For those with available data, we categorized the cases into two pre-specified groups and compared them using unadjusted Poisson regression with significance level of 0.05. We used a multiple Poisson regression model with robust standard errors to calculate unadjusted and adjusted relative risk (RR) and 95% confidence intervals (CI) of the association between those with ventilation waveforms in < 50% of pauses vs. those with ventilation waveforms in ≥ 50% of pauses for prehospital ROSC, ROSC at any time, ED ROSC, admission to hospital, survival to hospital discharge, and survival with favorable neurological outcome. The model contained potential confounding variables identified a priori including age, sex, bystander-witnessed cardiac arrest, attempted bystander CPR, public location, first known EMS rhythm, and ROC site.
In addition, we determined dose-response relationships between the number of ventilations per pause and each of the outcomes (ROSC, hospital admission, survival at discharge, and survival with favorable neurological outcome (mRS ≤ 3) using natural cubic splines with a single knot point at ventilations per pause value of 1 using the library “splines” in the R statistical software package. All Investigators, except the statistician (BL) and the database manager (JCarson) were blinded to survival outcomes and other clinical data during the process of measuring ventilation. All statistical analyses were performed with commercially available statistical packages (SAS, version 9.1.3, Cary, NC; R, version 2.5.1, Vienna, Austria; Stata, version 11, College Station, TX).
RESULTS
Of the 7,190 patients assigned to the 30:2 CPR arm from the six participating sites, we excluded 1,562 patients who had a defibrillator applied other than the Lifepak 12 or 15 or the Philips MRx defibrillator; 1,117 patients were excluded if the recording could not be downloaded or could not be identified (Fig. 1). In addition, 1,070 patients were excluded because they received continuous chest compressions instead of the assigned 30:2 CPR or they did not have obvious 30:2 CPR. Another 1,122 defibrillator files were excluded that lacked a usable recorded impedance signal and 343 cases had less than two minutes of recorded CPR. The analyzable cohort was comprised of 1,976 patients.
The overall mean patient age was 65 years old with a 66% male predominance (Table 1). The mean (SD) duration of 30:2 CPR was 9.8 (4.9) min from the start of chest compressions until placement of an advanced airway. During this period, we identified a total of 26,861 pauses in compressions in the 1,976 individual patient files. Most patients (N = 1177, 60%) comprised the group with lung inflation waveforms in <50% of pauses (Group 1), while 799 (40%) had waveforms in ≥50% of pauses (Group 2) (Table 1). There were differences between the two groups generally favoring Group 2 regarding public location (18.0%vs. 14.4%), witnessed status (44.8% vs. 38.9%), bystander CPR (52.2% vs. 49.1%), and initial shockable cardiac rhythm (29.1% vs. 20.9%) (Table 1).
Table 1.
Baseline characteristics by ventilation group
| < 50% of Pauses with Ventilation N=1177 |
≥ 50% of Pauses with Ventilation N=799 |
|
|---|---|---|
|
| ||
| Age - mean (sd) | 65.8 (16.1) | 64.9 (17.7) |
|
| ||
| Male - n (%) | 68.3% | 61.9% |
|
| ||
| Obvious Cause of Arrest1 - n (%) | 3.6% | 4.0% |
|
| ||
| Public Location - n (%) | 14.4% | 18.0% |
|
| ||
| Bystander Witnessed - n (%) | 38.9% | 44.8% |
|
| ||
| Bystander CPR - n (%) | 49.1% | 52.2% |
|
| ||
| Dispatch to first EMS arrival in minutes- mean (SD) | 5.8 (2.5) | 5.8 (2.7) |
|
| ||
| Dispatch to first EMS arrival ≤ 4 min - n (%) | 21.1% | 22.6% |
|
| ||
| Dispatch to first ALS arrival in minutes2 - mean (SD) | 8.2 (4.4) | 8.5 (4.4) |
|
| ||
| Treated by ALS - n (%) | 97.4% | 98.5% |
|
| ||
| Initial Cardiac Rhythm | ||
| VT/VF | 20.9% | 29.1% |
| PEA | 15.9% | 19.1% |
| Asystole | 50.3% | 39.9% |
| No shock advised | 12.8% | 11.8% |
|
| ||
| Site - % | ||
| ARC | 4.8% | 4.1% |
| DAL | 22.7% | 20.2% |
| OTT | 26.6% | 24.5% |
| PGH | 20.3% | 13.0% |
| SKC | 13.4% | 16.0% |
| VAN | 12.2% | 22.2% |
Obvious causes include but not limited to: Drug Poisoning, Foreign Body Obstruction, Terminal Illness, Respiratory
For those cases with ALS on scene
CPR, cardiopulmonary resuscitation; EMS, emergency medical services; ALS, advanced life support; VT, ventricular tachycardia; VF, ventricular fibrillation; PEA, pulseless electrical activity.
For Group 1 and Group 2, respectively, the median (Q1, Q3) number of pauses per minute was 1.3 (0.8, 1.8) vs. 1.4 (0.9, 1.9 [non-significant (NS)], the median number of pauses over the time period assessed by the study was 12 (7, 19) vs. 12 (7, 17) (NS), while the median number of ventilations was 2 (1, 5) vs. 12 (6, 21) (p < 0.0001)(Table 2). Furthermore, for Group 1 vs. Group 2, respectively, the median duration of pauses was 5.8 sec (5.0, 6.7) vs. 5.8 sec (5.1, 6.8) (NS). For Group 1 vs. Group 2, respectively, the interval from the first recorded chest compression to the first recorded ventilation waveform was 170 sec vs. 56 sec; median chest compression fraction was 0.78 (0.71, 0.84) vs. 0.78 (0.70, 0.83) (NS); and median chest compression rate was 108 (102, 115) vs. 109 (103, 117) compressions per minute (NS). Advanced airway placement success for Group 1 vs. Group 2, respectively, was 73.1% vs. 79.5%.
Table 2.
Treatment characteristics by ventilation group
| < 50% of Pauses with Ventilation N=1177 |
≥ 50% of Pauses with Ventilation N=799 |
|
|---|---|---|
|
| ||
| Time interval from machine on to first compression (sec) - Median (Q1, Q3) | 47 (26, 77) | 50 (25, 78) |
| Time interval from machine on to first ventilation (sec) - Median (Q1, Q3) | 244 (136, 398) | 119 (71, 188) |
| Time interval from first compression to first ventilation (sec) - Median (Q1, Q3) | 170 (75, 324) | 56 (19, 107) |
| Advanced airway successful - n/N (%) | 857/1173 (73.1%) | 621/781 (79.5%) |
| Chest compression fraction - Median (Q1, Q3) | 0.78 (0.71, 0.84) | 0.78 (0.70, 0.83) |
| Chest compression rate - Median (Q1, Q3) | 108 (102, 115) | 109 (103, 117) |
| Number of defined pauses - Median (Q1, Q3) | 12 (7, 19) | 12 (7, 17) |
| Duration of defined pauses (sec) - Median (Q1, Q3) | 5.8 (5.0, 6.7) | 5.8 (5.1, 6.8) |
| Number of ventilations - Median (Q1, Q3) | 2 (1, 5) | 12 (6, 21) |
Q1, 25th percentile; Q3, 75th percentile
The two pre-specified groups were compared using unadjusted Poisson regression. Those with ventilation waveforms in ≥ 50% of pauses had associated improved ROSC on ED arrival (30.7% vs. 18.7%, p < 0.0001), admission to hospital (32.0% vs. 20.6%, p = 0.0005), survival to hospital discharge (13.5% vs. 4.1%, p < 0.0001), and survival with favorable neurological outcome (10.6% vs. 2.4%, p < 0.0001) (Table 3).
Table 3.
Outcomes by ventilation group
| < 50% of Pauses with Ventilation N=1177 |
≥ 50% of Pauses with Ventilation N=799 |
Difference (95% CI) | Unadjusted Risk-Ratio (95% CI)* | Adjusted Risk-Ratio (95% CI)† | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-hospital ROSC - n/N (%) | 297/1177 (25.2%) | 325/799 (40.7%) | 15.5% | 1.6 (1.4, 1.8) | 1.3 (1.2, 1.5) | < 0.0001 |
| ROSC at ED arrival - n/N (%) | 220/1176 (18.7%) | 244/794 (30.7%) | 12.0% | 1.6 (1.4, 1.9) | 1.4 (1.2, 1.6) | < 0.0001 |
| Any ROSC - n/N (%) | 345/1177 (29.3%) | 359/799 (44.9%) | 15.6% | 1.5 (1.4, 1.7) | 1.3 (1.2, 1.5) | < 0.0001 |
| Admitted to Hospital - n/N (%) | 243/1177 (20.6%) | 256/799 (32.0%) | 11.4% | 1.6 (1.3, 1.8) | 1.3 (1.1, 1.5) | 0.0005 |
| Survival to Hospital Discharge - n/N (%) | 48/1175 (4.1%) | 107/793 (13.5%) | 9.4% | 3.3 (2.4, 4.6) | 2.2 (1.6, 3.0) | < 0.0001 |
| Survival with mRS 3 or Less - n/N (%) | 28/1175 (2.4%) | 84/793 (10.6%) | 8.2% | 4.4 (2.9, 6.7) | 2.8 (1.8, 4.3) | < 0.0001 |
All p-values are < 0.0001.
Adjusted for age, sex, bystander-witnessed cardiac arrest, attempted bystander CPR, public location, first known EMS rhythm, and ROC site.
ROSC, return of spontaneous circulation; ED, emergency department; mRS, modified Rankin Scale (favorable neurological outcome).
Patient survival outcomes were also analyzed using unadjusted and adjusted multiple Poisson regression models with robust standard errors. Compared to patients with at least one lung inflation in < 50% of pauses, patients who had at least one lung inflation in ≥ 50% of pauses had improved ROSC at ED arrival unadjusted RR 1.6 (95% CI 1.4 – 1.9), survival to hospital admission unadjusted RR 1.6 (95% CI 1.3 – 1.8), survival to hospital discharge unadjusted RR 3.3 (95% CI 2.4 – 4.6), and survival with favorable neurological outcome (modified Rankin score ≤ 3) unadjusted RR 4.4 (95% CI 2.9 – 6.7) (Table 3). After adjustment, RR for ROSC at ED was 1.4, (95% CI 1.2 – 1.6), survival to hospital admission RR 1.6 (95% CI 1.1 – 1.5), survival to hospital discharge RR 2.2 (95% CI 1.6 – 3.0), and survival with favorable neurological outcome RR 2.8 (95% CI 1.8 – 4.3) (Table 3).
Unadjusted and adjusted dose-response relationships using natural cubic spline models demonstrate a positive dose response relationship whereby an increasing number of ventilations per pause (0–2) is associated with a greater likelihood of favorable outcomes. (Figures 2a–d, 2e–h).
Figure 2.

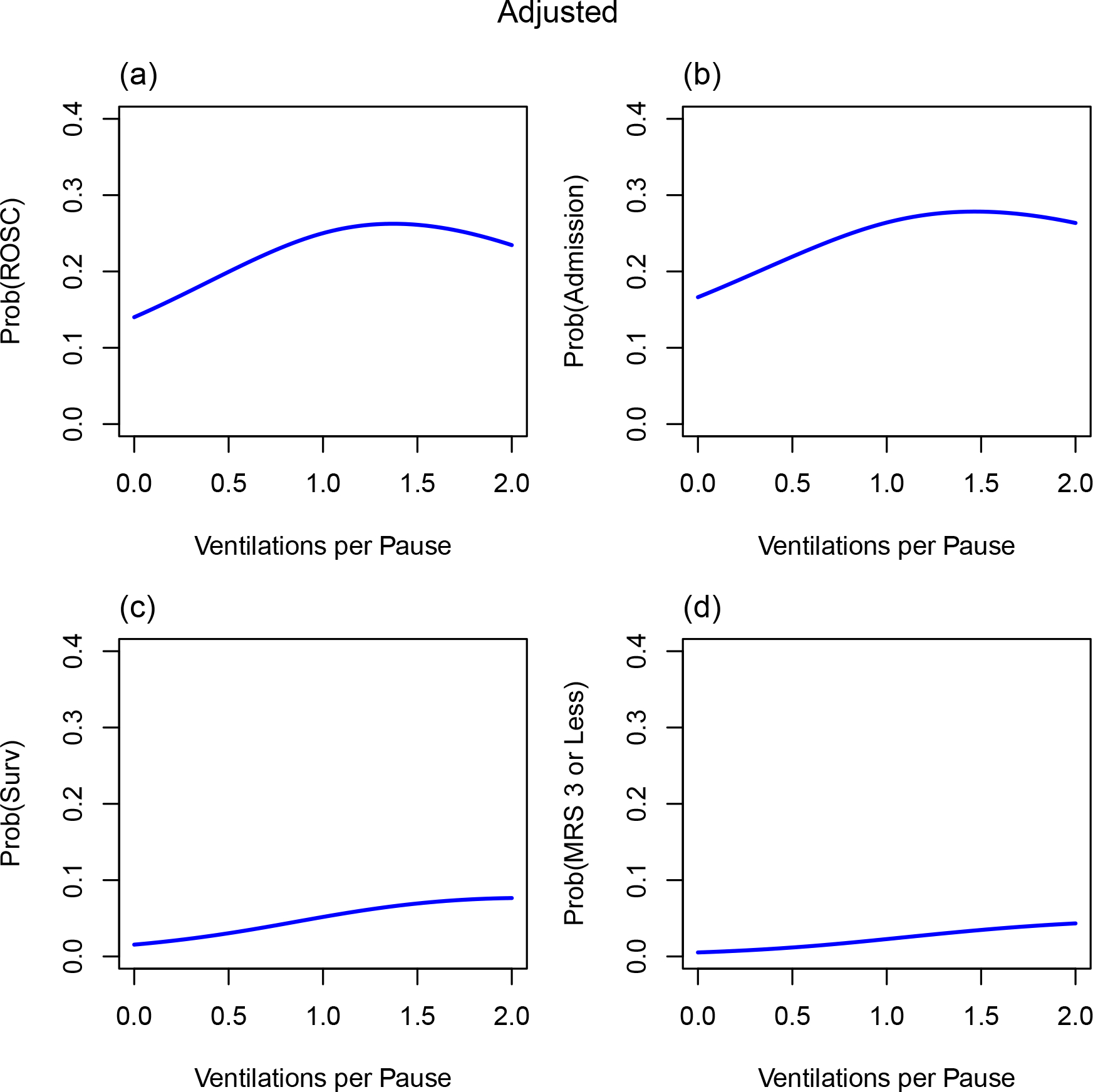
Ventilations per pause and outcomes. Association of the number of ventilations per pause vs. outcomes using natural cubic spline models (unadjusted and adjusted): (a, e) probability (prob) of return of spontaneous circulation (ROSC) on arrival at the emergency department (ED) vs. ventilations per pause (b, f) probability of hospital admission (admission) vs. ventilations per pause (c, g) probability of survival to hospital discharge vs. ventilations per pause (d, h) probability of mRS (modified Rankin Score) of 3 or less (favorable neurological outcome) vs. ventilations per pause. Figures e, f, g, and h were adjusted for age, sex, bystander-witnessed cardiac arrest, attempted bystander CPR, public location, first known EMS rhythm, and ROC site.
DISCUSSION
Ventilation during OHCA resuscitation has been difficult to study during early phases of CPR because a method for measurement had been lacking. This investigation is the first multicenter study that measured bioimpedance ventilation waveforms during 30:2 CPR for OHCA and its association with survival outcomes. In this novel investigation, we showed that detectable ventilation occurred in only 40% of pauses overall. In patients who had measurable ventilation in at least half of the pauses in 30:2 CPR, there was an associated improvement in ROSC, survival to hospital discharge, and survival with favorable neurological outcome.
Bioimpedance as a surrogate for lung inflation
Various aspects of ventilation in OHCA patients have been studied such as the compression to ventilation ratio, ventilation frequency, and methods of ventilation.23 Most OHCA studies have not attempted to measure lung inflation. The tidal volume associated with visually detectable chest wall movement is between 300 to 500 mL.11 However, studies show that >250 mL is a reasonable minimum tidal volume that can result in gas exchange.18 Since bioimpedance can likely detect chest wall movement at lower volumes than visualization alone, we set a minimum bioimpedance waveform amplitude threshold to 0.5 Ω to detect lung inflation of ≥250 mL of tidal volume.16 We also found that reviewers could distinguish this bioimpedance waveform amplitude from artifact reliably.15
While some have used capnography to detect ventilation, this measurement typically becomes available only after advanced airway placement. In our study, the mean time interval for advanced airway placement was 9.8 minutes after the start of CPR. Thus, capnography measurement usually does not include this vital interval during initial phases of professional rescuer CPR. Most EMS protocols call for early placement of a defibrillator during CPR to detect the presence of a shockable rhythm. Hence, the advantage of bioimpedance for ventilation measurement is that it is recorded as soon as the defibrillator chest pads are applied.
Adequate ventilation by BVM
Our study showed a difference in the number of ventilations between Group 1 and Group 2 patients (2 vs. 12 ventilations) during more than nine minutes of 30:2 CPR, while the number of defined pauses between Group 1 and Group 2 patients (12 vs. 12 pauses) did not differ. This contrast between number of ventilations and pauses suggests that the rescuers in both Groups attempted ventilation about the same number of times per patient, but these attempts frequently did not result in lung inflation. Thus, lung inflation cannot be assumed to have occurred during CPR with a BVM device, but it must be measured to understand fully the impact of ventilation on outcomes.
In this study, only 40% of patients received lung inflation in more than half of the pauses in chest compressions, demonstrating that most pauses do not have detectable ventilation. This suggests overall poor oxygenation and ventilation during initial OHCA resuscitation using a BVM device. Adequate ventilation via BVM remains a difficult skill to perform properly and must be practiced to maintain proficiency.24–26 In order to maintain an open airway, the person performing ventilation must extend the neck, and/or perform a jaw thrust maneuver, or place an oral airway. In addition, a tight mask seal on the face must be maintained to prevent air from leaking around the mask, and the rescuer must then simultaneously squeeze the self-inflating bag over 1 to 1.5 seconds for each breath. In a simulation study, highly trained prehospital providers delivered breaths and ventilation that did not meet guidelines for rate or tidal volumes for the majority of patients, providing lower respiratory rates and lower volumes.27 BVM ventilation performance could be improved by having one rescuer maintain a tight facemask seal continuously while another rescuer squeezes the self-inflating bag.28 With 30:2 CPR, the chest compressor could squeeze the self-inflating bag during interruptions in chest compression. However, this is not possible during continuous chest compression CPR, and three rescuers may be required.
Ventilation and Clinical Outcomes
Our study reported detailed information regarding ventilation during 30:2 CPR for OHCA and associations with clinical outcomes. We showed that ventilation in more than half of the pauses in chest compressions during 30:2 CPR for OHCA was associated with better outcomes for ROSC, survival to hospital discharge, and survival with favorable neurological outcome. Further analysis demonstrated a dose-response relationship between the number of ventilations per pause and outcomes.
Oxygenation is important to sustaining vital organs, and in resuscitation the goal is to artificially provide such support until restoration of function. Although ventilation may be omitted during the first few minutes of CPR because there is sufficient oxygen stored on hemoglobin to last about four minutes since blood flow during CPR is only 20% to 25% of baseline, at best, ventilation should probably be delivered soon after EMS arrival.29 Most patients have not received oxygen for 10 to 20 minutes by the time EMS arrives (time intervals for rescuer to recognize an emergency and to call 911 plus EMS response time interval, plus time interval to assemble the airway equipment and to give a breath). Moreover, multiple prior studies have shown that passive ventilation associated with chest compressions is likely insufficient for gas exchange or oxygenation, because chest compressions alone provide negligible tidal volume compared to dead space.8–10
Additionally, multiple studies have indicated that early advanced airway management is associated with improved outcomes.30–32 This may be another reason Group 2 patients had improved outcomes in our study since they received more effective early BVM ventilation compared with Group 1 patients. Decreasing time to restore specific physiologic functions to the normal steady state is key to mitigate the effects of post-cardiac arrest syndrome (PCAS).33
Protocol Compliance
In the parent clinical trial, of those assigned to receive 30:2 CPR, only 48% had CPR that was compliant with the 30:2 CPR protocol as indicated by having at least 1.5 pauses/min in chest compressions.34 In the parent trial, the 30:2 CPR group had a mean of 1.3 (0.7) pauses/min. In our study, median pauses in chest compressions/min for Group 1 vs. Group 2, respectively, was 1.3 (0.8, 1.8) vs. 1.4 (0.9, 1.9) (NS). While some patients in our study received CPR that was not compliant (had fewer pauses than expected) with the 30:2 CPR protocol, there was no difference in compliance between the two groups.
We did a sensitivity analysis of those patients who had chest compressions with 1.5 pauses/min or more during 30:2 CPR (i.e., received CPR that was compliant with the 30:2 CPR protocol) (data not shown). The analysis showed an association of improvement for all outcomes with ventilation waveforms in ≥50% of pauses in chest compression and is comparable to the findings in Group 2 of the main cohort.
Future work
Our study reported detailed information regarding ventilation during 30:2 CPR for OHCA including ventilation incidence and frequency, and the number and duration of pauses. Future studies are needed to evaluate these metrics when continuous chest compression CPR is used and the effect of ventilation metrics on patient survival outcomes during out-of-hospital cardiac arrest. This is especially important because EMS frequently uses continuous chest compression CPR before and after advanced airway placement according to guideline recommendations. Clinical trials are urgently needed comparing ventilation strategies during early CPR and its effects on survival.
Recently, a portable spirometer has been developed that can be used in the EMS out-of-hospital setting and a manufacturer has developed a spirometer that can be coupled with its defibrillator. These devices make possible more detailed measurement of ventilation metrics than was previously possible during early CPR.
LIMITATIONS
The defibrillator files were from two device manufacturers. Several brands of defibrillator either did not record bioimpedance or the recording was of insufficient quality. We excluded patients who were randomized to the 30:2 CPR group if they received continuous chest compression CPR, the defibrillator could not record thoracic bioimpedance or the recording could not be located, and some patients had less than two minutes of recorded 30:2 CPR. Some patients received CPR that was not compliant with the 30:2 CPR protocol.
In addition, this study is a secondary observational analysis of data from a clinical trial that addresses a question that was not the purpose of the original trial. Associations between ventilation and outcomes may not represent causal effects. Given the retrospective and observational study design, we cannot account for residual confounding whereby undetected ventilation may be a marker for some other causal patient or care characteristics responsible for outcome differences. A high body-mass index may affect bioimpedance amplitude inversely and could affect detection of ventilation waveforms. Such patients may be more difficult to ventilate and the observation of poor initial ventilation and outcome may be reflective of such factors.
These limitations should be balanced against the study’s strengths, in having drawn data from a high-quality multicenter clinical trial, and whose outcomes were adjusted for EMS response interval, quality of CPR, bystander CPR rates, initial cardiac rhythm, and other confounders. In addition, we used an automated program to annotate ventilation waveforms. This increased consistency and reproducibility of the review and reduced potential bias. Finally, all Investigators, except the statistician (BL) and database manager (JCarson), were blinded to survival outcomes and other clinical data during the course of measuring ventilation metrics.
CONCLUSIONS
This novel multicenter study demonstrates that lung inflation occurs infrequently with bag-valve-mask ventilation during 30:2 CPR for out-of-hospital cardiac arrest. Ventilation with measurable lung inflation in ≥ 50% of pauses was associated with significantly increased rates of ROSC, survival, and increased likelihood of favorable neurological outcome.
Clinical Perspective.
What is new?
The 30:2 strategy of CPR pauses chest compressions (CC) to optimize bag-valve-mask (BVM) ventilation. No prior studies have verified if ventilations are effectively delivered during such CPR pauses.
In this analysis of data from the 30:2 arm of the ROC CCC trial, we applied novel signal processing techniques to identify ventilations during CC pauses.
In 60% of patients, lung inflation (ventilation) was detectable in <50% of pauses. Patients receiving detectable lung inflation in ≥50% of pauses had better clinical outcomes.
What are the clinical implications?
BVM ventilation is often ineffective during CPR.
Improving ventilation may lead to improved clinical outcomes.
Acknowledgements
We are indebted to the firefighters and paramedics participating in the ROC for their hard work and dedication. We would also like to thank the data coordinators at each ROC site for their extraordinary diligence and focus in abstracting the data for this study.
Sources of Funding
This study was funded by NIH/NHLBI Grant # 1 R21 HL156196-02 (AHI) and multiple cooperative agreements (5U01 HL077863, University of Washington Data Coordinating Center; HL077867, University of Washington; HL077871, University of Pittsburgh; HL077881, University of Alabama at Birmingham; HL077885, Ottawa Hospital Research Institute; HL077887, University of Texas Southwestern Medical Center; HL077885 and FAS 06-2603 University of British Columbia), and PID2021-122727OB-I00 grant funded by MCIN/AEI/10.13039/501100011033 and ERDF A way of making Europe (EAE; XJ), and by Basque Government grant IT-1717-22 (EAE, XJ).
Disclosures
Dr. Idris receives grant support from the US National Institutes of Health (NIH) and the Centers for Disease Control and Prevention. He serves as an unpaid volunteer on the American Heart Association National Emergency Cardiovascular Care Committee and the Stryker, Inc. (Belfast) Clinical Advisory Board. Dr. Kudenchuk receives grant support from the NIH as institutional principal investigator for the SIREN Network. Dr. Wang received research funds from the NIH and DOD, site research funds from Marinus, Vasomune and Quidel, travel funds from Fisher-Paykel, Inc. and consulting fees from the American College of Emergency Physicians as the editor in chief of JACEP Open.
Contributor Information
Ahamed H. Idris, University of Texas Southwestern Medical Center.
Elisabete Aramendi Ecenarro, University of the Basque Country.
Brian Leroux, University of Washington.
Xabier Jaureguibeitia, University of the Basque Country.
Betty Y. Yang, University of Texas Southwestern Medical Center.
Sarah Shaver, University of Texas Southwestern Medical Center.
Mary P. Chang, University of Texas Southwestern Medical Center.
Tom Rea, University of Washington.
Peter Kudenchuk, University of Washington.
Jim Christenson, University of British Columbia.
Christian Vaillancourt, University of Ottawa.
Clifton Callaway, University of Pittsburgh.
David Salcido, University of Pittsburgh.
Jonas Carson, University of Washington.
Jennifer Blackwood, Public Health-Seattle&King County, Emergency Medical Services Division..
Henry E. Wang, The Ohio State University.
REFERENCES
- 1.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi D, Baernstein A, Nichol G. Cardiac Arrest: A Public Health Perspective. Emerg Med Clin North Am. 2012;30:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Idris AH, Guffey D, Pepe PE, Brown SP, Brooks SC, Callaway CW, Christenson J, Davis DP, Daya MR, Gray R, et al. ; Resuscitation Outcomes Consortium Investigators. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43:840–8.. [DOI] [PubMed] [Google Scholar]
- 4.Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, Callaway CW, Bishop D, Vaillancourt C, Davis Det al. Chest Compression Fraction Determines Survival in Patients With Out-of-Hospital Ventricular Fibrillation. Circulation. 2009;120:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiell IG, Brown SP, Nichol G, Cheskes S, Vaillancourt C, Callaway CW, Morrison LJ, Christenson J, Aufderheide TP, Davis DP, et al. What Is the Optimal Chest Compression Depth During Out-of-Hospital Cardiac Arrest Resuscitation of Adult Patients? Circulation. 2014;130:1962–1970. [DOI] [PubMed] [Google Scholar]
- 6.Vaillancourt C, Petersen A, Meier EN, Christenson J, Menegazzi JJ, Aufderheide TP, Nichol G, Berg R, Callaway CW, Idris AH, Davis D, Fowler R, Egan D, et al. The impact of increased chect compression fraction on survival for out-of-hospital cardiac arrest patients with a non-shockable initial rhythm. Resuscitation 2020;154:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RA, Hemphill R, Abella BS, Aufderheide TP, Cave DM, Hazinski MF, Lerner EB, Rea TD, Sayre MR, Swor RA. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S685–705. doi: 10.1161/CIRCULATIONAHA.110.970939. Erratum in: Circulation. 2011 Oct 11;124(15):e402. [DOI] [PubMed] [Google Scholar]
- 8.Idris AH, Banner MJ, Wenzel V, Fuerst RS, Becker LB, Melker RJ. Ventilation caused by external chest compression is unable to sustain effective gas exchange during {CPR}: a comparison with mechanical ventilation. Resuscitation. 1994;28:143–150. [DOI] [PubMed] [Google Scholar]
- 9.Deakin CD, O’Neill JF, Tabor T. Does compression-only cardiopulmonary resuscitation generate adequate passive ventilation during cardiac arrest? Resuscitation. 2007;75:53–59. [DOI] [PubMed] [Google Scholar]
- 10.McDannold R, Bobrow BJ, Chikani V, Silver A, Spaite DW, Vadeboncoeur T. Quantification of ventilation volumes produced by compressions during emergency department cardiopulmonary resuscitation. Am J Emerg Med. 2018;36:1640–1644. [DOI] [PubMed] [Google Scholar]
- 11.Baskett P, Nolan J, Parr M. Tidal volumes which are perceived to be adequate for resuscitation. Resuscitation. 1996;31:231–234. [DOI] [PubMed] [Google Scholar]
- 12.Risdal M, Aase SO, Stavland M, Eftestøl T. Impedance-based ventilation detection during cardiopulmonaryresuscitation. IEEE transactions on bio-medical engineering 2007;54:2237–2245. [DOI] [PubMed] [Google Scholar]
- 13.Alonso E, Ruiz J, Aramendi E, González-Otero D, Ruiz de Gauna S, Ayala U, Russell JK, Daya M. Reliability and accuracy of the thoracic impedance signal for measuring cardiopulmonary resuscitation quality metrics. Resuscitation. 2015;88:28–34. [DOI] [PubMed] [Google Scholar]
- 14.Losert H, Risdal M, Sterz F, Nysaether J, Köhler K, Eftestøl T, Wandaller C, Myklebust H, Uray T, Aase SO, Laggner AN. Thoracic impedance changes measured via defibrillator pads can monitor ventilation in critically ill patients and during cardiopulmonary resuscitation. Crit Care Med. 2006;34:2399–2405. [DOI] [PubMed] [Google Scholar]
- 15.Aramendi E, Lu Y, Chang MP, Elola A, Irusta U, Owens P, Idris AH. A novel technique to assess the quality of ventilation during pre-hospital cardiopulmonary resuscitation. Resuscitation. 2018;132:41–46. [DOI] [PubMed] [Google Scholar]
- 16.Chang MP, Lu Y, Leroux B, Aramendi Ecenarro E, Owens P, Wang HE, Idris AH. Association of ventilation with outcomes from out-of-hospital cardiac arrest. Resuscitation. 2019;141:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015;373:2203–2214. [DOI] [PubMed] [Google Scholar]
- 18.Nassal MMJ, Jaureguibeitia X, Aramendi E, Irusta U, Panchal AR, Wang HE, Idris A. Novel application of thoracic impedance to characterize ventilations during cardiopulmonary resuscitation in the pragmatic airway resuscitation trial. Resuscitation. 2021;168:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaureguibeitia X, Aramendi E, Wang HE, Idris AH. Impedance-based Ventilation Detection and Signal Quality Control during Out-of-Hospital Cardiopulmonary Resuscitation. IEEE J Biomed Health Inform. 2023;PP. doi: 10.1109/JBHI.2023.3253780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaureguibeitia X, Irusta U, Aramendi E, Owens PC, Wang HE, Idris AH. Automatic Detection of Ventilations During Mechanical Cardiopulmonary Resuscitation. IEEE J Biomed Health Inform. 2020;24:2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651–1659. [DOI] [PubMed] [Google Scholar]
- 22.Berve PO, Irusta U, Kramer-Johansen J, Skålhegg T, Aramendi E, Wik L. Tidal volume measurements via transthoracic impedance waveform characteristics: The effect of age, body mass index and gender. A single centre interventional study. Resuscitation. 2021;167:218–224. [DOI] [PubMed] [Google Scholar]
- 23.Chang MP, Idris AH. The past, present, and future of ventilation during cardiopulmonary resuscitation. Curr Opin Crit Care. 2017;23:188–192. [DOI] [PubMed] [Google Scholar]
- 24.Bucher J, Vashisht R, Ladd M, Cooper J. Bag Mask Ventilation. StatPearls. Published online 2022. Accessed March 5, 2023. https://pubmed-ncbi-nlm-nih-gov.foyer.swmed.edu/28722953/. [Google Scholar]
- 25.Lyng J W, Guyette FX, Levy M, Bosson N. Prehospital Manual Ventilation: An NAEMSP Position Statement and Resource Document. Prehospital Emerg Care. 2022;26:23–31. [DOI] [PubMed] [Google Scholar]
- 26.Jabre P, Penaloza A, Pinero D, Duchateau FX, Borron SW, Javaudin F, Richard O, de Longueville D, Bouilleau G, Devaud ML, et al. Effect of Bag-Mask Ventilation vs Endotracheal Intubation During Cardiopulmonary Resuscitation on Neurological Outcome After Out-of-Hospital Cardiorespiratory Arrest: A Randomized Clinical Trial. JAMA. 2018;319:779–787. doi: 10.1001/jama.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neth MR, Benoit JL, Stolz U, McMullan J. Ventilation in Simulated Out-of-Hospital Cardiac Arrest Resuscitation Rarely Meets Guidelines. Prehospital Emerg Care. 2021;25:712–720. [DOI] [PubMed] [Google Scholar]
- 28.Gerber L, Botha M, Laher AE. Modified Two-Rescuer CPR With a Two-Handed Mask-Face Seal Technique Is Superior To Conventional Two-Rescuer CPR With a One-Handed Mask-Face Seal Technique. J Emerg Med. 2021;61:252–258. [DOI] [PubMed] [Google Scholar]
- 29.Turner I, Turner S, Armstrong V. Does the compression to ventilation ratio affect the quality of CPR: a simulation study. Resuscitation. 2002;52:55–62. [DOI] [PubMed] [Google Scholar]
- 30.Wang HE, Jaureguibeitia X, Aramendi E, Nichol G, Aufderheide T, Daya MR, Hansen M, Nassal M, Panchal AR, Nikolla DA, Alonso E, Carlson J, Schmicker RH. Airway strategy and ventilation rates in the pragmatic airway resuscitation trial. Resuscitation. 2022;176:80–87. [DOI] [PubMed] [Google Scholar]
- 31.Benoit JL, McMullan JT, Wang HE, Xie C, Xu P, Hart KW, Stolz U, Lindsell CJ. Timing of Advanced Airway Placement after Witnessed Out-of-Hospital Cardiac Arrest. Prehospital Emerg care. 2019;23:838–846. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda T, Ohashi-Fukuda N, Inokuchi R, Kondo Y, Sekiguchi H, Taira T, Kukita I. Association between time to advanced airway management and neurologically favourable survival during out-of-hospital cardiac arrest. Anaesth Crit Care Pain Med. 2021;40:100906. [DOI] [PubMed] [Google Scholar]
- 33.Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. Epub 2008 Oct 23. [DOI] [PubMed] [Google Scholar]
- 34.Schmicker RH, Nichol G, Kudenchuk P, Christenson J, Vaillancourt C, Wang HE, Aufderheide TP, Idris AH, Daya MR. CPR compression strategy 30:2 is difficult to adhere to, but has better survival than continuous chest compressions when done correctly. Resuscitation. 2021;165:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


