Abstract
G-protein-coupled receptors (GPCRs) form one of the largest superfamilies of membrane proteins. Obtaining high yields of GPCRs remains one of the major factors limiting a detailed understanding of their structure and function. Photoreceptor cells (PRCs) contain extensive stacks of specialized membranes where high levels of rhodopsins are naturally present, which makes them ideal for the overexpression of GPCRs. We have generated transgenic flies expressing a number of GPCRs in the PRCs. Drosophila melanogaster metabotropic glutamate receptor (DmGluRA) expressed by this novel strategy was purified to homogeneity, giving at least 3-fold higher yields than conventional baculovirus expression systems due to the higher membrane content of the PRCs. Pure DmGluRA was then reconstituted into liposomes of varying composition. Interestingly, glutamate binding was strictly dependent on the presence of ergosterol.
INTRODUCTION
G-protein-coupled receptors (GPCRs) form one of the largest superfamilies of membrane proteins and are central to many signal transduction events. The only high-resolution structure available to date for a member of this family is rhodopsin (Palczewski et al., 2000). Rhodopsin is an exception among GPCRs, not only in its regulation, but also in its abundance in the eye. However, other GPCRs are present in membranes only at very low levels, and their functional overexpression is the major bottleneck for further studies.
Conventional overexpression systems for production of many GPCRs have been reported previously (Grisshammer and Tate, 1995). However, because of their specific limitations, there is still a need for effective expression systems for GPCRs.
Photoreceptor cells (PRCs) contain extensive stacks of specialized membranes and dedicated machinery for the expression, folding and targeting of high levels of rhodopsin. These membranes, from bovine and frog eyes, have been successfully used for the isolation of rhodopsin in structural studies (Schertler and Hargrave, 1995; Okada et al., 1998, 2000). Therefore, these cells, with their unique architecture, present an attractive site for the expression of membrane proteins. PRCs from Drosophila melanogaster are also equipped with these membrane systems, called rhabdomeres (Kumar and Ready, 1995). Since it is possible to engineer transgenic flies that can direct the overexpression of proteins to specific tissues at a desired stage of development, it is possible to overexpress GPCRs in the fly PRCs. Here we demonstrate the use of flies for the production of a number of GPCRs in PRCs. We report the purification and functional reconstitution of D. melanogaster metabotropic glutamate receptor (DmGluRA) produced by this novel strategy.
RESULTS AND DISCUSSION
Fly genetics offer well-characterized drivers, which are synthetic or natural promoters that are activated by intrinsic, cell-specific transcription factors at certain stages of development, driving the expression of GAL4. Transgenic flies bearing these drivers have been extensively used for developmental studies (D’Avino and Thummel, 1999). We utilized PRC-specific drivers (gmr-GAL4 and rh1-GAL4) to produce transgenic D. melanogaster lines overexpressing GPCRs in these cells; gmr (glass minimum response element) is a sequence to which the PRC-specific transcriptional activator glass binds, and rh1 is the promotor for rhodopsin 1 from Drosophila.
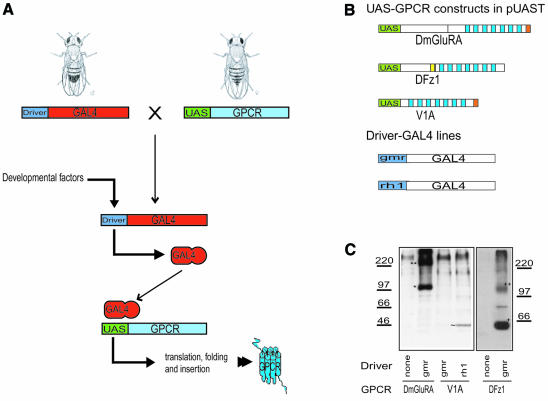
The general expression strategy is illustrated in Figure 1A. In order to test this expression strategy, three receptors belonging to different subclasses of GPCRs were chosen: human vasopressin 1A (V1A), D. melanogaster Frizzled 1 receptor (DFz1) and DmGluRA (Thibonnier et al., 1994; Chini et al., 1995; Parmentier et al., 1996; Boutros et al., 2000). The UAS (upstream activating sequence for GAL4) GPCR constructs and the driver lines used are shown in Figure 1B.
Fig. 1. (A) Schematic representation of the expression strategy. Two transgenic fly lines, one containing a PRC-specific driver and the other containing the open reading frame of the GPCR of interest downstream of a GAL4 UAS, are crossed, and a stable line, containing at least one copy of both transgenes in every generation, is produced. In these flies, developmental factors drive the expression of GAL4, which then activates the expression of the GPCR via the UAS. (B) Schematic presentation of UAS-GPCR and driver-GAL4 lines used in this study. Each receptor was cloned after a GAL4 UAS (green). C-terminal His6 tag (orange) was added to DmGluRA and V1A. DFz1 contained a myc tag (yellow) in the linker region between the extracellular cysteine-rich domain (CRD) and the seven transmembrane region (7TM, blue) (Boutros et al., 2000). Driver-GAL4 lines used in this study were gmr-GAL4 and rh1-GAL4. (C) Western blot analysis of fly head samples run on a 10% SDS–PAGE. Anti-His5 antibody (Qiagen) was used to detect DmGluRA and V1A. DFz1 was detected by anti-myc antibody (9E5, Santa-Cruz). DmGluRA run on an SDS–PAGE gel in the presence of 2-mercaptoethanol (βME, 0.7 M) as two different bands corresponding to the sizes of a monomer (*) and a dimer (**). In the absence of βME, only the dimeric form was detected (data not shown). V1A expression was detected in rh1-GAL4; UAS-V1A flies only (∼). Both monomer (+) and dimer (++) DFz1 were detected in gmr-GAL4/UAS-DFz1 flies. Molecular weight markers (kDa) are shown for both blots.
Expression in Drosophila PRCs is receptor- and driver-dependent
Expression of GPCRs was tested by western blotting of membrane fractions from fly heads (Figure 1C). DmGluRA and DFz1 were highly expressed under the control of the gmr-GAL4 driver, but no expression was detected in the absence of a driver. No V1A expression was detected under the control of the gmr-GAL4 driver, but the eyes of these flies were significantly reduced in size and disordered. The overexpression of V1A by the gmr-GAL4 driver was most likely toxic and induced PRC death. Using a later onset driver (rh1-GAL4) did not affect the ‘normal’ eye phenotype, and its expression was detectable in a western blot (Figure 1C). This shows that the selection of driver enables the control of expression induction time and intensity. Even though the expression level of V1A is low, it is an improvement compared with our previous attempts to express this receptor in Escherichia coli and Sf9 cells (data not shown). This illustrates that the fly eye can be a useful alternative, especially when the more conventional expression strategies fail.
The gmr-GAL4/UAS-DmGluRA flies were chosen for further characterization of this expression system based on the high expression level of DmGluRA. The endogenous DmGluRA levels in UAS-DmGluRA/+ flies were negligible, since they were below the detection limits of western blot analysis using a DmGluRA-specific polyclonal antibody (data not shown).
DmGluRA expression localizes to PRCs
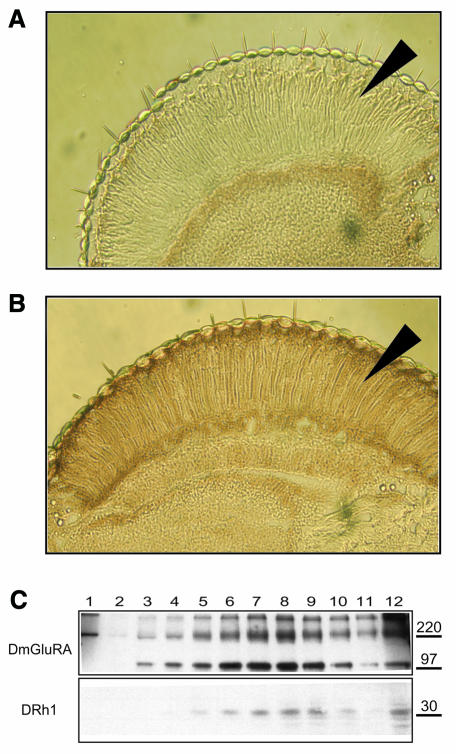
In order to confirm the localization of ectopically expressed DmGluRA, frozen head sections from UAS-DmGluRA/+ and gmr-GAL4/UAS-DmGluRA flies were immunostained with a DmGluRA-specific polyclonal antibody. No staining was detected in UAS-DmGluR/+ flies, which served as a negative control (Figure 2A). The strong staining in the PRCs of gmr-GAL4/UAS-DmGluRA flies indicated a high level of DmGluRA expression in these cells (Figure 2B).
Fig. 2. Localization of ectopically expressed DmGluRA. Frozen head sections from UAS-DmGluRA (A) and gmr-GAL4/UAS-DmGluRA (B) flies were immunostained with DmGluRA-specific polyclonal antibody. UAS-DmGluRA flies do not express DmGluRA in PRCs. In contrast, gmr-GAL4/UAS-DmGluRA fly head sections give a strong staining on the PRCs (marked by arrows) due to overexpression of DmGluRA. (C) Western blot analysis of sucrose gradient fractions of fly head membranes. DmGluRA was detected by polyclonal anti-DmGluRA antibody and DRh1 was detected by 4C5 monoclonal anti-DRh1 antibody.
Drosophila melanogaster rhodopsin 1 (DRh1) is highly expressed in fly PRCs and is inserted into the rhabdomeres (Kumar and Ready, 1995), making DRh1 a suitable marker for these membranes. Total membranes prepared from gmr-GAL4/UAS-DmGluRA fly heads were fractionated in a linear sucrose density gradient and analyzed by immunoblotting. Figure 2C shows that DmGluRA and DRh1 co-fractionated, indicating that both receptors are localized in the same membranes. These results show that DmGluRA can be specifically overexpressed in Drosophila PRCs.
DmGluRA can be purified from fly heads with high yields
In order to compare the expression level and the yield of receptor obtained from fly heads with a ‘conventional’ expression system, DmGluRA was also expressed in insect (Sf9) cells using a recombinant baculovirus (RBV).
Glutamate binding assays were performed on membranes from both sources. Although the specific binding per milligram of membrane protein was similar, at least four times more binding sites were detected in the fly head membranes, taking 1 g starting material (fly heads and Sf9 cells) as a basis (Table I). This can be attributed to the fact that fly heads contain much more membranes than the Sf9 cells.
Table I. Glutamate binding on membranes from fly heads and RBV-infected Sf9 cells.
| Source | Specific bindinga |
|---|---|
| D. melanogaster heads | |
| Total specific binding (pmol/g heads) | 211 |
| Specific binding (pmol/mg MP)b | 9.6 ± 1.5 |
| RBV-infected Sf9 cells | |
| Total specific binding (pmol/g cells) | 47 |
| Specific binding (pmol/mg MP)b | 10.6 ± 2.7 |
aAverage specific bindings (± SEM) were calculated from three independent experiments performed in triplicate.
bMP, membrane protein.
Binding counts obtained without any cold agonist were 8041 ± 628 d.p.m. for fly head membranes and 8329 ± 1434 d.p.m. for RBV-infected insect cell membranes.
The receptor was then purified from fly heads (Figure 3) and insect cells (data not shown) to compare the yields. After solubilization of the membranes by lyso-phosphocholine-12 (FC-12), DmGluRA was purified to homogeneity in a single step using the anti-DmGluRA monoclonal antibody 7G11 coupled to Sepharose. In agreement with the binding data from the membranes, the yield of purified receptor from fly heads was at least 3-fold higher than from insect cells (170 µg = 1600 pmol/g fly heads and 50 µg = 470 pmol/g insect cells).
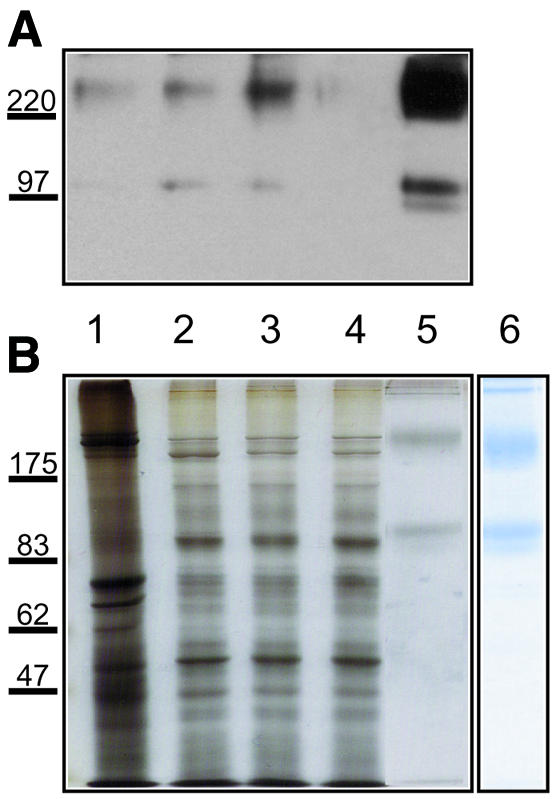
Fig. 3. Purification of DmGluRA by 7G11 affinity column. Total protein samples (5 µg) from (1) crude extract, (2) membrane fraction, (3) FC-12 solubilized fraction, (4) 7G11 affinity column flow-through and (5) 10 µl (0.2 µg) of column eluate were subjected to 8% SDS–PAGE and analyzed by (A) western blotting (with polyclonal anti-DmGluRA antibody) and (B) silver staining. (6) Coomassie Blue staining of 2 µg column eluate shows that DmGluRA is purified to homogeneity. The receptor migrates in two major forms: as a dimer (∼200 kDa) and as a monomer (∼100 kDa). The faint band below the monomer is most likely a differently glycosylated form of the receptor. Molecular weight markers (kDa) are shown on the left.
The high yield of receptor from the fly heads can be attributed mainly to the high membrane content of the PRCs. In these specialized cells, internal membranes are reduced to a minimum and extensive stacks of plasma membrane (the rhabdomeres) are present. This seems to decrease the heterogeneity of the purified receptor due to incomplete maturation caused by mislocalization to intracellular membranes. When using the fly heads, a single-step membrane preparation with minimal contaminations from other membranes is possible, whereas, in insect cells, a differential centrifugation step is necessary to remove the nuclei and other intracellular membranes.
Another advantage of expression in PRCs is the low proteolytic activity of the fly head lysate due to the stable expression and the location of the protein in rhabdomeres, which are naturally shielded from protease action. In contrast, insect cells have elevated levels of proteolytic activity due to RBV infection, and additional protease inhibitors are required during purification.
Today, the methods for generation of transgenic flies are well established. In addition, many well-characterized cell-specific driver lines are readily available; once a stable transgenic fly stock is generated, scaling-up does not require any further optimization. Unlike the cell culture-based expression systems, no decrease in expression levels during scaling-up was observed. Once a fly production cycle is established, material is continuously available and large-scale rearing of flies is far more economical compared with any cell culture-based techniques.
Glutamate binding to purified DmGluRA is strictly dependent on ergosterol
Since the novel expression strategy presented here gave access to high amounts of DmGluRA, a member of the mGluR family was purified and reconstituted into liposomes for the first time. Different lipid compositions were tested in order to investigate the requirements for receptor activity (Table II). When the receptor was analyzed in a detergent solubilized state after purification, no specific binding was detected. This can be attributed to the delipidation of the receptor during purification, as it has been observed for many membrane proteins, including GPCRs (Balen et al., 1994; Klein and Fahrenholz, 1994; Fahrenholz et al., 1995; Heimpel et al., 2001).
Table II. Specific binding of purified DmGluRA reconstituted into liposomes of different lipid compositions.
| Lipids (%)a | Specific binding | |||
|---|---|---|---|---|
| PE | PC | PA | Ergosterol | (pmol/mg)b |
| 70 | 20 | 10 | — | — |
| 65 | 20 | 10 | 5 | 43.8 ± 8.5c |
| 55 | 20 | 10 | 15 | 52.7 ± 26.3c |
| 208.1 ± 49.9d | ||||
| 45 | 20 | 10 | 25 | 25.7 ± 16.6c |
aPE, phosphatidylethanolamine; PC, phosphatidylcholine; PA, phosphatidic acid.
bAverage data (± SEM) given are from experiments performed in triplicate.
Binding counts obtained without any cold agonist were between 10 943 ± 1215 and 5922 ± 1103 d.p.m.
cReconstituted by dialysis.
dReconstituted by detergent absorption column.
The receptor was found inserted into liposomes in all cases, independent of the lipid composition. Surprisingly, recovery of specific glutamate binding was strictly dependent on the presence of ergosterol, which is the main sterol in yeast-fed flies (69%; Rietveld et al., 1999). The highest specific binding was obtained with 15% ergosterol (Table II). The only other similar example for sterol requirement in GPCR activity is for oxytocin receptor, which has no apparent homology to mGluRs (Klein and Fahrenholz, 1994).
mGluRs have large extracellular domains, which are responsible for glutamate binding. The ligand binding domains of several mGluRs have been expressed (Okamoto et al., 1998; Peltekova et al., 2000) and characterized; however, no lipid requirements for glutamate binding were observed. The lipid dependence observed here for the full-length receptor may point to a structural constraint imposed by the transmembrane domain on the extracellular part. This might have a regulatory role in ligand binding. Further studies are needed to understand the role of sterols in mGluR function.
In addition, the technique for detergent removal had a significant influence on the recovery of binding. A 4-fold increase in specific binding was obtained using a detergent absorption column instead of dialysis (Table II). This may be due to faster removal of detergent and rapid insertion of the receptor into a lipid environment.
In summary, our data show that expression in the fly eye is a powerful, accessible method for the production of GPCRs up to the level required for structural studies. The method described here may certainly be extended to other membrane proteins. In addition, the protocols for the expression, purification and reconstitution of DmGluRA developed here allow for detailed biochemical and structural studies in the future.
METHODS
Cloning and transgenic flies. DmGluRA cDNA was a kind gift from Dr J.P. Pin (CNRS, Montpellier, France). Human V1A cDNA was a kind gift from Dr B. Mouillac (CNRS). DmGluRA and V1A were subcloned into pUAST after a 3′ His6 tag was added by PCR. The pUAST-receptor constructs were injected into Drosophila embryos, and transgenic flies were selected and balanced by standard procedures. The myc-tagged UAS-DFz1 flies were a kind gift from Dr M. Mlodzik (EMBL, Heidelberg, Germany). Driver lines gmr-GAL4 and rh1-GAL4 were kind gifts from Dr C. Desplan (New York University, NY). Stable D. melanogaster lines expressing DmGluRA were amplified in population cages as described by Quivy and Becker (1997). Frozen fly heads were separated from other body parts as described previously (Oliver and Philips, 1970). The heads were kept at –80°C until use.
Expression of DmGluRA in insect cells. DmGluRA was expressed in monolayer cultures of Sf9 cells at 27°C by infection with an RBV expressing DmGluRA with a C-terminal His6 tag (V. Panneels, C. Eroglu, P. Cronet and I. Sinning, in preparation). The cells were harvested 84 h after infection.
Membrane preparation from fly heads and insect cells. Total membranes were prepared from fly heads after homogenizing in ice-cold homogenization buffer (HB; 20 mM Tris–HCl pH 7.4, 250 mM sucrose, 2 mM MgCl2, 500 mM NaCl) containing protease inhibitors (Complete EDTA free, Roche). The membrane fractions from insect cells were prepared by differential centrifugation (Grunewald et al., 1996). The membranes were resuspended in solubilization buffer (SB; HB with 20% glycerol). The total membrane protein concentration was estimated by Bradford assay.
SDS–PAGE, immunoblotting and antibody staining of frozen fly head sections. SDS–PAGE analysis and immunoblotting of the fly head proteins were performed as described previously (Ozaki et al., 1993). The polyclonal antibody was raised against a peptide corresponding to the sequence of DmGluRA from amino acids 445–459 (Genosys, Sigma). Horseradish peroxidase-conjugated secondary antibodies were used and the blots were developed using an ECL-Kit (Amersham). The antibody staining on frozen eye sections was performed as described previously (Mollereau et al., 2000).
Sucrose gradient with Drosophila head membranes. A 4 ml linear sucrose gradient (20–60%) was prepared in 20 mM Tris–HCl pH 7.4. Five-hundred microliters of membrane preparation (5 mg membrane protein/ml) was laid on the top and the sample was run on an SW-60 rotor (Beckman) for 16 h at 100 000 g. Fractions (350 µl) were taken from the top and analyzed by western blotting. Anti-DRh1 antibody was obtained from the Developmental Studies Hybridoma Bank developed under NICHD and maintained by The University of Iowa (Iowa City, IA).
Solubilization and purification of DmGluRA. The protein concentration of the membranes was set to 3 mg/ml in SB. FC-12 (Anatrace) was used for solubilization at a final concentration of 7.5 mM for 1 h at 4°C. The insoluble fraction was removed by centrifugation at 100 000 g for 30 min. The solubilized receptor was purified by immunoaffinity chromatography using the monoclonal antibody 7G11 raised against DmGluRA by DNA immunization (Costagliola et al., 1998). Five milligrams of 7G11 were purified from 2 ml ascites fluid by a MabTrapGII column (Amersham Pharmacia Biotech) and coupled to 1 ml CNBr-activated Sepharose beads Pharmacia) as described by the manufacturer. The bound receptor was eluted by pH change with 50 mM Glycine–HCl buffer pH 4.0 containing 20% glycerol, 500 mM NaCl and 3 mM FC-12. The eluate was immediately neutralized by 1:10 vol. 1 M Tris pH 8.0.
Reconstitution of DmGluRA into liposomes. For reconstitution, the detergent was changed to 60 mM β-octylglucoside (βOG) (Calbiochem) on the affinity column. Lipid mixes for reconstitution were prepared from egg phospholipids and ergosterol (Sigma) in CHCl3 and mixed with 60 mM βOG. Mixed micelles were added to 1 ml of 800 pmol/ml DmGluRA (1:5000, protein:lipid molar ratio). Detergent was removed by dialysis or by using a detergent-absorbing matrix (Calbisorb, Calbiochem). After detergent removal, the proteoliposomes were mixed 1:2 with OptiPrep (Nycomed) and were floated in a step gradient of 40, 30 and 5%. The distribution of DmGluRA in the fractions was checked by western blotting to verify the insertion into liposomes.
Glutamate binding assay. Rapid filtration binding assays (Okamoto et al., 1998; V. Panneels, in preparation) were performed on 20 µg Drosophila head and insect cell membranes or on 1 µg reconstituted receptor with 1.5 µM tritiated glutamate (49.0 Ci/mmol, Amersham Pharmacia). Non-specific binding was measured in the presence of 1 mM l-glutamate (Sigma).
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. Mlodzik and his group for fruitful discussions and hosting our flies at EMBL, C. Desplan for advice, materials and support, A. Giraldez and M. Bagnat for help and advice, and P. Becker for introducing us to large-scale fly production. We thank A. Kuhn and U. Schüssler for excellent technical assistance and A. Hoenger for critical reading of the manuscript. C.E. is supported by the EMBL PhD programme, P.C. and V.P. were supported by EU grants and P.B. by an HFSP grant.
REFERENCES
- Balen P., Kimura, K. and Sidhu, A. (1994) Specific phospholipid requirements for the solubilization and reconstitution of D-1 dopamine receptors from striatal membranes. Biochemistry, 33, 1539–1544. [DOI] [PubMed] [Google Scholar]
- Boutros M., Mihaly, J., Bouwmeester, T. and Mlodzik, M. (2000) Signaling specificity by Frizzled receptors in Drosophila. Science, 288, 1825–1828. [DOI] [PubMed] [Google Scholar]
- Chini B. et al. (1995) Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J., 14, 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola S., Rodien, P., Many, M.C., Ludgate, M. and Vassart, G. (1998) Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J. Immunol., 160, 1458–1465. [PubMed] [Google Scholar]
- D’Avino P.P. and Thummel, C.S. (1999) Ectopic expression systems in Drosophila. Methods Enzymol., 306, 129–142. [DOI] [PubMed] [Google Scholar]
- Fahrenholz F., Klein, U. and Gimpl, G. (1995) Conversion of the myometrial oxytocin receptor from low to high affinity state by cholesterol. Adv. Exp. Med. Biol., 395, 311–319. [PubMed] [Google Scholar]
- Grisshammer R. and Tate, C.G. (1995) Overexpression of integral membrane proteins for structural studies. Q. Rev. Biophys., 28, 315–422. [DOI] [PubMed] [Google Scholar]
- Grunewald S., Haase, W., Reilander, H. and Michel, H. (1996) Glycosylation, palmitoylation, and localization of the human D2S receptor in baculovirus-infected insect cells. Biochemistry, 35, 15149–15161. [DOI] [PubMed] [Google Scholar]
- Heimpel S., Basset, G., Odoy, S. and Klingenberg, M. (2001) Expression of the mitochondrial ADP/ATP carrier in Escherichia coli. Renaturation, reconstitution, and the effect of mutations on 10 positive residues. J. Biol. Chem., 276, 11499–11506. [DOI] [PubMed] [Google Scholar]
- Klein U. and Fahrenholz, F. (1994) Reconstitution of the myometrial oxytocin receptor into proteoliposomes. Dependence of oxytocin binding on cholesterol. Eur. J. Biochem., 220, 559–567. [DOI] [PubMed] [Google Scholar]
- Kumar J.P. and Ready, D.F. (1995) Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development, 121, 4359–4370. [DOI] [PubMed] [Google Scholar]
- Mollereau B., Wernet, M.F., Beaufils, P., Killian, D., Pichaud, F., Kuhnlein, R. and Desplan, C. (2000) A green fluorescent protein enhancer trap screen in Drosophila photoreceptor cells. Mech. Dev., 93, 151–160. [DOI] [PubMed] [Google Scholar]
- Okada T., Takeda, K. and Kouyama, T. (1998) Highly selective separation of rhodopsin from bovine rod outer segment membranes using combination of divalent cation and alkyl(thio)glucoside. Photochem. Photobiol., 67, 495–499. [PubMed] [Google Scholar]
- Okada T., Le Trong, I., Fox, B.A., Behnke, C.A., Stenkamp, R.E. and Palczewski, K. (2000) X-ray diffraction analysis of three-dimensional crystals of bovine rhodopsin obtained from mixed micelles. J. Struct. Biol., 130, 73–80. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sekiyama, N., Otsu, M., Shimada, Y., Sato, A., Nakanishi, S. and Jingami, H. (1998) Expression and purification of the extracellular ligand binding region of metabotropic glutamate receptor subtype 1. J. Biol. Chem., 273, 13089–13096. [DOI] [PubMed] [Google Scholar]
- Oliver D.V. and Philips, J.P. (1970) Fruit fly fractionation. Drosophila Info. Serv., 45, 58. [Google Scholar]
- Ozaki K., Nagatani, H., Ozaki, M. and Tokunaga, F. (1993) Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron, 10, 1113–1119. [DOI] [PubMed] [Google Scholar]
- Palczewski K. et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science, 289, 739–745. [DOI] [PubMed] [Google Scholar]
- Parmentier M.L., Pin, J.P., Bockaert, J. and Grau, Y. (1996) Cloning and functional expression of a Drosophila metabotropic glutamate receptor expressed in the embryonic CNS. J. Neurosci., 16, 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltekova V., Han, G., Soleymanlou, N. and Hampson, D.R. (2000) Constraints on proper folding of the amino terminal domains of group III metabotropic glutamate receptors. Brain Res. Mol. Brain Res., 76, 180–190. [DOI] [PubMed] [Google Scholar]
- Quivy J.P. and Becker, P.B. (1997) Genomic footprinting of Drosophila embryo nuclei by linker tag selection LM-PCR. Methods, 11, 171–179. [DOI] [PubMed] [Google Scholar]
- Rietveld A., Neutz, S., Simons, K. and Eaton, S. (1999) Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem., 274, 12049–12054. [DOI] [PubMed] [Google Scholar]
- Schertler G.F. and Hargrave, P.A. (1995) Projection structure of frog rhodopsin in two crystal forms. Proc. Natl Acad. Sci. USA, 92, 11578–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M., Auzan, C., Madhun, Z., Wilkins, P., Berti-Mattera, L. and Clauser, E. (1994) Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem., 269, 3304–3310. [PubMed] [Google Scholar]