Abstract
The heterotetrameric AP-1A adaptor complex of clathrin-coated vesicles is ubiquitously expressed. The µ1-adaptin subunit of the complex exists as the ubiquitous µ1A and the polarized epithelia-specific µ1B, which are 80% identical. In polarized epithelia, µ1B is incorporated into the AP-1B complex, which is required for basolateral plasma membrane sorting of the low-density lipoprotein receptor. Binding of AP-1B to subdomains of the trans-Golgi network (TGN) appears to be part of the mechanism by which protein sorting is mediated. We expressed µ1B in µ1A-deficient fibroblasts to test for µ1B function in non-polarized cells. AP-1B complexes were formed and bound to the TGN and to endosomes. Moreover, AP-1B restored the AP-1A-dependent sorting of mannose 6-phosphate receptors between endosomes and the TGN. This demonstrates that µ1A and µ1B do have overlapping sorting functions and indicates that AP-1A and AP-1B mediate protein sorting along parallel pathways between the TGN and endosomes in polarized epithelia.
INTRODUCTION
The clathrin adaptor protein complex AP-1A has been localized to the perinuclear trans-Golgi network (TGN). Microscopic and biochemical analysis indicates that it mediates vesicular protein transport from the TGN to endosomes. Mouse ‘knock-outs’ of AP-1A subunits revealed that AP-1A is indispensable for embryonic development and that it is also required for endosome-to-TGN transport (Zizioli et al., 1999; Meyer et al., 2000). The AP-1A complex consists of four adaptin subunits, which are ubiquitously expressed: the two ≥100 kDa adaptins γ1 and β1, the 47 kDa µ1A-adaptin and the 19 kDa σ1-adaptin (Hirst and Robinson, 1998; Kirchhausen, 1999). A polarized epithelia-specific µ1B isoform exists that is 80% identical to µ1A (Ohno et al., 1999). µ1 and β1 bind tyrosine and leucine containing sorting signal sequences within the cytoplasmic tails of vesicle cargo proteins (Rapoport et al., 1998; Rodionov and Bakke, 1998; Heilker et al., 1999). Binding of the YxxQ type protein sorting motif to µ2 of the homologous plasma membrane AP-2 complex has been analysed by co-crystallization and X-ray analysis (Owen and Evans, 1998). The region in µ2 binding the YxxQ motif is conserved in µ-adaptins and is identical in µ1A and µ1B. The µ1B-containing complex is named AP-1B and appears to mediate basolateral TGN to plasma membrane sorting of the low-density lipoprotein receptor (LDL-R) (Fölsch et al., 1999). Basolateral sorting of LDL-R is due to AP-1B binding to its non-canonical Y-based sorting signal motifs (Fölsch et al., 2001).
It is not known by which mechanisms AP-1B mediates basolateral sorting. It appears that AP-1A and AP-1B bind to specific regions of the TGN, and it could be that this subdomain specificity is part of the mechanism by which polarized sorting is mediated (Fölsch et al., 1999). We wanted to test whether µ1B function is restricted to polarized epithelia as indicated by its expression pattern and transformed µ1B cDNA into µ1A-deficient fibroblasts.
In the µ1A–/– fibroblasts, sorting of mannose 6-phosphate receptors MPR46 and MPR300 was analysed. They recycle between the TGN and endosomes mediating intracellular sorting of lysosomal enzymes. MPR300 also mediates endocytosis of ligands such as IGF-II. In various cell types, ∼50% of receptors are concentrated in the perinuclear TGN (Klumperman et al., 1993). In µ1A–/– cells, they are redistributed from the TGN into early endosomes at the expense of the TGN. They recycle between these endosomes and the plasma membrane, but transport to the TGN is blocked, indicating that AP-1A also mediates endosome-to-TGN transport (Meyer et al., 2000, 2001). MPRs contain multiple AP-1A binding motifs, whose precise functions in sorting are not known (Höning et al., 1997; Heilker et al., 1999). Sorting between the TGN and endosomes involves a dileucine motif present at the C-termini of MPR300 and MPR46 (Johnson and Kornfeld, 1992; Tikkanen et al., 2000). The dileucine motif binds to β1, but binding to µ-adaptins has also been demonstrated (Rapoport et al., 1998; Storch and Braulke, 2001).
RESULTS
µ1B expression restores membrane binding of γ1 and clathrin
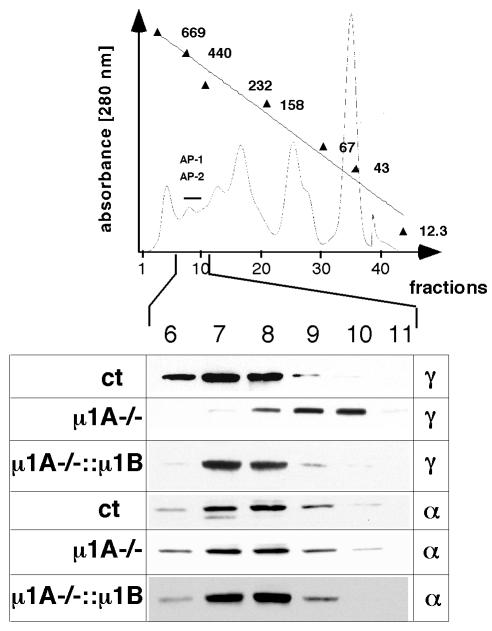
Murine µ1B cDNA was stably expressed in µ1A–/– cells. The anti-µ1A peptide serum does not recognize µ1B, and thus µ1B mRNA levels were determined. µ1B mRNA was expressed in clone 3 to levels ∼2-fold higher than those of µ1A (data not shown). Clone 3 was used for all further experiments. Protein expression was determined indirectly by analysis of AP-1 complex assembly. In µ1A-deficient fibroblasts, γ1, β1 and σ1 form a trimeric complex, which, in gel filtration experiments, elutes two fractions later than AP-1A (Meyer et al., 2000). AP-1B complex assembly was analysed by gel filtration of cytosolic proteins, and the distribution of γ1 in the fractions was analysed by western blotting. Fractionation of AP-2 served as a control and was followed by anti-α-adaptin western blotting. In µ1B-expressing cells, γ1 fractionated as in AP-1A-expressing control cells, demonstrating the formation of the AP-1B complex (Figure 1). Adaptins not incorporated into a complex are degraded (Zizioli et al., 1999).
Fig. 1. AP-1B complex formation in µ1A–/– fibroblasts. AP-1 and AP-2 cytoplasmic adaptor complexes were separated by gel filtration and detected by western blotting using anti-γ1 or anti-α antibodies.
In control cells, γ1 and clathrin are concentrated at the perinuclear TGN and clathrin is also found on vesicles throughout the cell, presumably AP-2 endocytic vesicles. The trimeric γ1–β1–σ1 complex of µ1A–/– cells does not bind to membranes. Membrane staining by γ1 in confocal immunofluorescence microscopy is lost, as is the perinuclear concentration of clathrin, whereas clathrin labelling of vesicles throughout the cytoplasm is not affected (Meyer et al., 2000). µ1B expression in the µ1A–/– cells restored the perinuclear concentration of γ1 and of clathrin, indicating the formation of functional AP-1B complexes (Figure 2).
Fig. 2. Distribution of γ1 and clathrin heavy-chain (chc) in control (ct), µ1A–/– and µ1A–/–::µ1B fibroblasts. µ1A–/– cells do not show γ1 and clathrin-heavy-chain binding to the TGN, whereas membrane binding of these proteins is observed in µ1A–/–::µ1B cells as in control cells. MPR46 subcellular distribution in these cell lines was detected by MPR46 and EEA1 confocal double-immunofluorescence microscopy. Control cells show perinuclear TGN concentration of MPR46 (red) and colocalization with EEA1 (green) is limited (four vesicles), whereas extensive colocalization of MPR46 and EEA1 is observed in µ1A–/– cells. In µ1A–/–::µ1B cells, perinuclear TGN localization of MPR46 is restored and colocalization with EEA1 is lost.
AP-1B relocalizes MPRs to the TGN
In control cells, the majority of MPR46 and MPR300 is concentrated in the perinuclear TGN, and only 5–10% of receptors are found in early endosomes (Klumperman et al., 1993; Meyer et al., 2001). In µ1A–/– cells, MPR46 and MPR300 are redistributed at the expense of the TGN into endosomes, which are labelled for the early endosomal antigen EEA1. MPR46 and MPR300 recycle between the endosomes and the plasma membrane but are not transported out of endosomes back to the TGN (Meyer et al., 2000, 2001). Formation of AP-1B in the µ1A–/– cells caused the redistribution of the MPR46 from EEA1-positive endosomes back to the TGN, indicating restoration of endosome-to-TGN transport. Colocalization of MPR46 with EEA1 in the AP-1B-expressing fibroblasts is comparable with control cells (Figure 2).
To further compare AP-1A and AP-1B distribution on the TGN, we determined colocalization of γ1 in AP-1A- and AP-1B-expressing cells with MPR300 by double-immunofluorescence microscopy. AP-1A and AP-1B colocalized to a comparable extent with MPR300 (Figure 3).
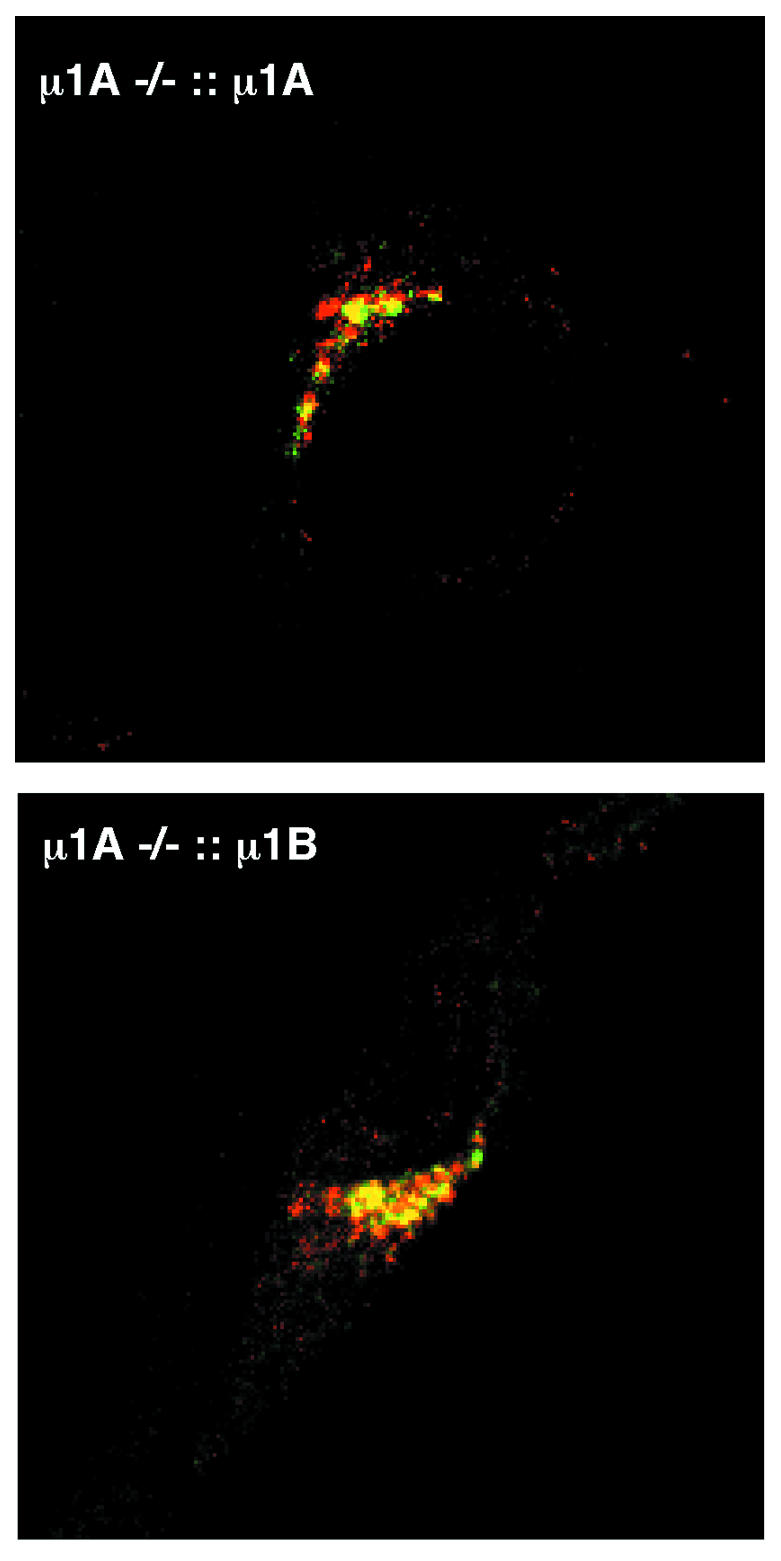
Fig. 3. Colocalization of MPR300, AP-1A and AP-1B at the TGN analysed by anti-γ1 and anti-MPR300 double-immunofluorescence confocal microscopy. Both cell lines show colocalization of γ1 (green) and MPR300 (red).
Association of AP-1A and AP-1B to endosomes
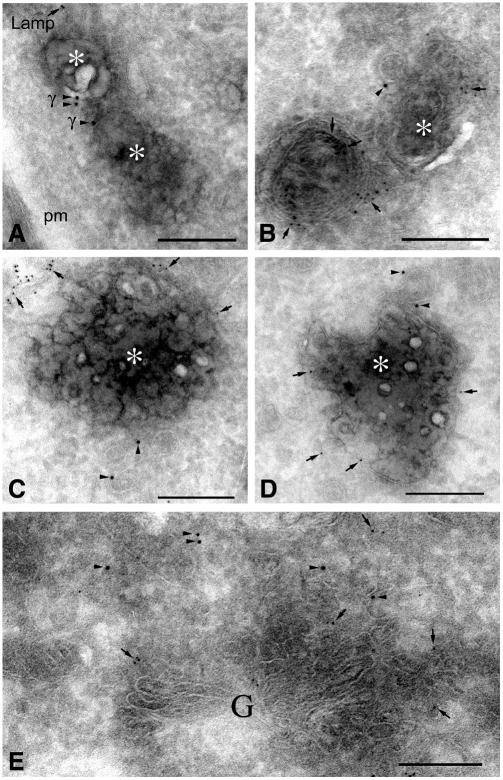
AP-1A function is required for endosome-to-TGN transport of MPRs (Meyer et al., 2000, 2001). Therefore, we asked whether AP-1A and AP-1B (labelled by γ1 antibodies) can be detected on early endosomal compartments by immunogold electron microscopy. Endosomes were labelled by horseradish peroxidase (HRP) fluid phase endocytosis over 20 min. HRP was visualized by diaminobenzidine (DAB). To identify late endosomal compartments, the thin cryosections were also labelled for the lysosomal membrane protein LAMP-1. DAB-positive endosomes, containing no or only low amounts of LAMP-1, are likely to be early endosomes or endosomal carrier vesicles (Griffiths et al., 1989), whereas strongly LAMP-1-positive vesicles (such as the structure in the lower left corner of Figure 4B) are late endosomes or lysosomes. AP-1A and AP-1B complexes were detected by anti-γ1 antibodies and 10 nm gold. γ1 was detected on the limiting membrane of endosomes in AP-1A-expressing wild-type cells (data not shown), in agreement with earlier reports in other cell types (Futter et al., 1998; Mallard et al., 1998). γ1 was also detected on limiting membranes of early endosomes in AP-1B-expressing cells (Figure 4A–C). Occasionally, γ1 was seen associated with a coated bud (Figure 4B). Quantitation of γ1 labelling showed that 3.45 ± 0.18 and 5.57 ± 1.31% of total labelling was associated with limiting membranes of DAB-positive endosomes in wild-type and AP-1B-expressing cells, respectively. γ1 was also seen associated with small vesicles located next to early endosomes in wild-type cells (data not shown) and AP-1B-expressing cells (Figure 4C and D). In addition, γ1 was located to the TGN in both cell lines (shown for AP-1B, Figure 4E). AP-1B has also been detected at the TGN in polarized cells (Fölsch et al., 2001).
Fig. 4. γ1 localization to early endosomes and TGN in µ1A–/–::µ1B fibroblasts. The cells were fed with HRP for 20 min and fixed, and HRP was then visualized by DAB reaction. Thin cryosections were labelled with antibodies against γ1 (10 nm gold) and LAMP-1 (5 nm gold). Arrowheads indicate all gold particles localizing γ1, whereas arrows indicate some of the LAMP-1-associated gold particles. Asterisks indicate the DAB reaction product. (A–D) Endosomes, (E) TGN. Plasma membrane (pm) is indicated in (A). The γ1-positive coated bud in (B) is 64 nm in diameter. TGN was identified by the proximity of a Golgi stack (G) in (E). Scale bars = 200 nm.
AP-1B restores MPR-mediated intracellular protein sorting
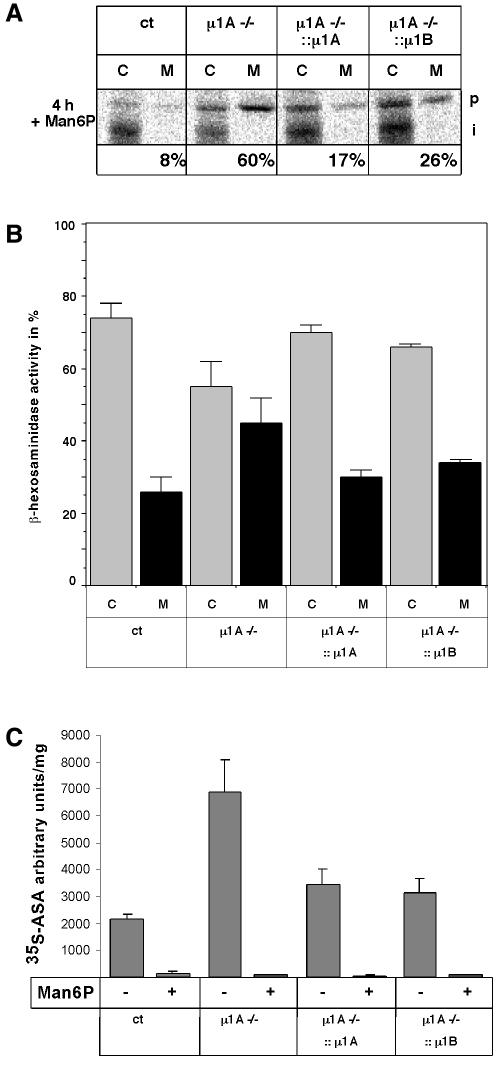
The microscopic analysis indicated that AP-1B mediates MPR sorting between endosomes and TGN. This was analysed by determining intracellular lysosomal protein sorting. Receptor– ligand complexes are transported from the TGN to endosomes, where they dissociate. MPRs recycle back to the TGN, and their ligands are transported to lysosomes. Control cells secrete only ∼10% of the Golgi precursor cathepsin D (Meyer et al., 2000). This fraction does not increase by the addition of 5 mM mannose 6-phosphate (Man6P) to the medium (Figure 5A, p). Thus, uptake of cathepsin D does not contribute to its lysosomal sorting. µ1A–/– cells secrete 33% of cathepsin D into the culture medium. This fraction increases to 60% in the presence of Man6P (Meyer et al., 2000). Sorting of cathepsin D was analysed by metabolic labelling with [35S]methionine for 1 h followed by a 4 h chase in the presence of Man6P. Expression of µ1A and µ1B restored intracellular sorting of cathepsin D. µ1A::µ1A cDNA cells secreted 17% of cathepsin D and µ1A::µ1B cDNA cells secreted 26% in the presence of Man6P (Figure 5A).
Fig. 5. Cathepsin D and β-hexosaminidase intracellular sorting in control (ct), µ1A–/–, µ1A–/–::µ1A and µ1A–/–::µ1B cells. (A) Man6P was added to the medium to prevent endocytosis of secreted cathepsin D. Metabolically labelled cathepsin D was immunoprecipitated from cell extracts (C) and from the medium (M). The Golgi precursor form (p) and the endosomal intermediate processed form (i) are indicated. (B) Secretion of β-hexosaminidase to the medium. Shown here is the distribution of the enzymatic activity between the cells and the medium as a percentage of the total (n = 2). (C) Endocytosis of arylsulfatase A (ASA) by control (ct), µ1A–/–, µ1A–/–::µ1A and µ1A–/–::µ1B cells (n = 2). Arbitrary units correspond to pixel numbers on phosphoimager screens (Fuji BAS1000).
We further determined the secretion of the lysosomal enzyme β-hexosaminidase. Cells were washed with phosphate-buffered saline, and medium was added containing heat-inactivated and dialysed fetal calf serum (FCS). After 24 h, the medium was collected and cells were harvested. Enzymatic activity was determined in cell extracts and in the media (Figure 5B) (von Figura, 1978). Cultures of control cells contained 25% of the total β-hexosaminidase activity in the medium, and those of µ1A–/– cells contained 45%. Expression of µ1A or µ1B in the latter reduced β-hexosaminidase activity in the medium to 30 and 33%, respectively (n = 2).
µ1A–/– cells have an enhanced capacity to endocytose lysosomal proteins due to increased MPR300 recycling between the plasma membrane and endosomes. This is determined by measuring the endocytosis of metabolically 35S-labelled arylsulfatase A (ASA) added to the cell culture medium (Meyer et al., 2000, 2001). Over 4 h, µ1A–/– cells endocytosed four times more ASA than control cells (Figure 5C). Expression of µ1A or µ1B in the latter cells reduced endocytosis of ASA to 160 and 140%, respectively, of control cells (n = 2).
DISCUSSION
Expression of µ1B is restricted to certain polarized epithelia, indicating the presence of a unique basolateral sorting machinery in these cell types (Ohno et al., 1999; Meyer et al., 2000). Expression of µ1B in µ1A–/– fibroblasts, however, led to the formation of the polarized epithelia-specific AP-1B complex. AP-1B binds to the perinuclear TGN and endosomes of fibroblasts as AP-1A. Moreover, AP-1B expression restored perinuclear TGN concentration of MPRs and their intracellular sorting between the TGN and endosomes demonstrating complementation of AP-1A function.
However, AP-1B does not restore all AP-1A functions. Prohormone convertase furin recycling between the TGN and endosomes also depends on AP-1A (Molloy et al., 1999). Perinuclear concentration of furin is not restored by AP-1B expression (Fölsch et al., 2001). Furin is mislocalized to endosomes not labelled by EEA1 (Meyer et al., 2001). AP-1B and furin do not colocalize at the TGN, whereas AP-1A colocalizes with furin (Fölsch et al., 2001). It appears that AP-1A and AP-1B bind to different TGN domains and endosomes. It is not clear whether cargo proteins, like the MPRs, are required for high-affinity binding of AP-1A to the TGN (Le Borgne and Hoflack, 1997; Zhu et al., 1999). It is more likely that compartment-specific adaptor–receptor structures exist, which contribute to the compartment-specific binding of adaptors (Mallet and Brodsky, 1996; Seaman et al., 1996). In addition, adaptors do have overlapping sorting signal recognition specificities, and thus binding to the cargo proteins cannot be the mechanism mediating compartment-specific binding (Heilker et al., 1999). However, compartment receptor structures required for AP-1B-mediated sorting are not exclusively expressed in polarized epithelial cells in contrast to µ1B itself. Thus, only expression of µ1B in polarized epithelia would be required for basolateral sorting of certain cargo proteins such as the LDL-R (Meyer et al., 2000; Fölsch et al., 2001).
Why did the new µ1B develop and why is the basolateral sorting of the LDL-R not mediated by µ1A? It could be that proteins like the endocytic LDL-R follow additional trafficking pathways, which are incompatible with binding to the ubiquitous AP-1A. The normally basolateral sorted LDL-R also mediates apical-to-basolateral transcytosis of LDL at the blood–brain barrier. The mechanism of this pathway is not known (Dehouck et al., 1997). In contrast to LDL-R, the MPRs have only been detected in basolateral endosomes and plasma membranes (Bresciani et al., 1997). Evolution of polarized epithelia and of specific proteins such as the LDL-R might have required the evolution of new sorting signal sequence motifs and new sorting adaptins such as the µ1B.
LDL-R with a point mutation in the cytoplasmic domain fails to be basolaterally sorted in hepatocytes and in polarized MDCK cells (Koivisto et al., 2001). µ1B is not expressed in hepatocytes but in MDCK cells (Fölsch et al., 1999; Ohno et al., 1999; Meyer et al., 2000). Thus, there must be additional adaptins to µ1B required for basolateral sorting of LDL-R. The AP-1 homologous and ubiquitously expressed AP-4 complex is also required for basolateral sorting in polarized cell lines such as MDCK cells. MDCK cells expressing µ4 antisense-RNA mis-sort LDL-R and MPR46 to the apical site, whereas µ1B-deficient cells sort MPR46 basolaterally (Simmen et al., 2002). Thus, polarized sorting appears to be mediated by AP-1B and AP-4 complexes, indicating that polarized sorting decisions are made at two different compartments: the TGN and endosomes. Thus, AP-1A and AP-1B appear to mediate protein sorting along parallel pathways between the TGN and endosomes and that additional sorting decisions are made in endosomes.
METHODS
Cellular and molecular biology. Mouse embryonic fibroblasts were cultivated in Dulbecco’s modified Eagle’s medium and 10% FCS. Mouse µ1B and µ1A cDNAs were cloned into the murine expression vector pMPSV. µ1B and µ1A cDNAs were stably transfected into µ1A–/– mouse embryonic fibroblasts by the effectene transfection kit (Qiagen). Clones were selected by hygromycin (500 µg/ml) (Meyer et al., 2000). Expression of µ-adaptins was analysed by northern blotting of 5 µg total RNA and AP-1 complex formation (see below). Signals were quantified by phosphoimager analysis (Fuji BAS1000).
Adaptor assembly and protein sorting. Analysis of AP-1 complex formation was performed as described previously (Meyer et al., 2000). Freshly prepared cytosolic adaptors were separated by gel chromatography on a Sephadex 200 column (SMART, Pharmacia). Column fractions were analysed by western blotting. Sorting of cathepsin D, β-hexosaminidase and [35S]ASA endocytosis were performed as described previously (von Figura, 1978; Meyer et al., 2000).
Microscopy and antisera. Cells were fixed with p-formaldehyde and permeabilized with saponin following standard protocols. Adaptins and clathrin were detected with anti-γ1, anti-α and anti-clathrin heavy-chain mouse monoclonal antibodies (Transduction Laboratories). Affinity-purified anti-σ1 serum was a generous gift from M. Robinson (Cambridge, UK). Anti-LAMP-1 rabbit serum was a gift from Masaru Himeno (Kyushu University, Japan). Anti-µ1A peptide rabbit antiserum was described previously (Meyer et al., 2000). The serum does not recognize µ1B. Anti-cathepsin D, anti-MPR46 and anti-MPR300 sera were generously provided by R. Pohlmann and A. Hille-Rehfeld from the Institut. Anti-EEA1 was a generous gift from H. Stenmark (Oslo, Norway). Fluorophore-coupled secondary antibodies were from Jackson Immunoresearch. Confocal microscopy was performed using a Zeiss LSM 200 Series equipped with a Zeiss Plan Apochromat 63¥/1.40 and Carl Zeiss LSM version 3.95 software.
For electron microscopy, cells were fed with HRP for 20 min, fixed in 4% PFA, 0.1 M HEPES pH 7.4 for 2 h at room temperature, followed by 2% PFA at 4°C overnight. The DAB reaction was performed according to Griffiths et al. (1989). Cells were embedded in gelatine, infiltrated in 20% polyvinylpyrrolidone, 1.8 M sucrose, and frozen in liquid nitrogen. Thawed sections were labelled with rabbit anti-rat LAMP-1 and mouse monoclonal anti-γ1. Secondary antibodies were goat Fab2 coupled to 5 or 10 nm gold (British BioCell, Cardiff, UK). For quantitation of γ1 labelling, sections were systematically screened at a magnification of 30 000¥, using 10¥ magnifying binoculars. Gold particles within a distance of 30 nm outside or 20 nm inside of endosomal limiting membrane were scored as endosome associated (arrowheads in Figure 4A and B, and the upper of the two 10 nm gold particles in Figure 4C). A minimum of 600 gold particles, from two or three separate grids, were counted for each cell line. Images were processed for presentation using Adobe Photoshop Version 5 and Deneba Canvas 5.0.
Acknowledgments
ACKNOWLEDGEMENTS
We thank M. Robinson, M. Himeno and H. Stenmark for antibodies. We also thank C. Hinners for excellent technical assistance. This work is supported by the Deutsche Forschungsgemeinschaft SFB 523/A6.
REFERENCES
- Bresciani R., Denzer, K., Pohlmann, R. and von Figura, K. (1997) The 46 kDa mannose-6-phosphate receptor contains a signal for basolateral sorting within the 19 juxtamembrane cytosolic residues. Biochem. J., 327, 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehouck B., Fenart, L., Dehouck, M.P., Pierce, A., Torpier, G. and Cecchelli, R. (1997) A new function for the LDL receptor: transcytosis of LDL across the blood–brain barrier. J. Cell Biol., 138, 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Ohno, H., Bonifacino, J.S. and Mellman, I. (1999) A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell, 99, 189–198. [DOI] [PubMed] [Google Scholar]
- Fölsch H., Pypaert, M., Schu, P. and Mellman, I. (2001) Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol., 152, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C.E., Gibson, A., Allchin, E.H., Maxwell, S., Ruddock, L.J., Odorizzi, G., Domingo, D., Trowbridge, I.S. and Hopkins, C.R. (1998) In polarized MDCK cells basolateral vesicles arise from clathrin-γ-adaptin-coated domains on endosomal tubules. J. Cell Biol., 141, 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Back, R. and Marsh, M. (1989) A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol., 109, 2703–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilker R., Spiess, M. and Crottet, P. (1999) Recognition of sorting signals by clathrin adaptors. BioEssays, 21, 558–567. [DOI] [PubMed] [Google Scholar]
- Hirst J. and Robinson, M. (1998) Clathrin and adaptors. Biochim. Biophys. Acta, 1404, 173–193. [DOI] [PubMed] [Google Scholar]
- Höning S., Sosa, M., Hille-Rehfeld, A. and von Figura, K. (1997) The 46 kDa mannose-6-phosphate receptor contains multiple binding sites for clathrin adaptors. J. Biol. Chem., 272, 19884–19890. [DOI] [PubMed] [Google Scholar]
- Johnson K.F. and Kornfeld, S. (1992) The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J. Cell Biol., 119, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell. Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Hille, A., Veenendaal, T., Oorschot, V., Stoorvogel, W., von Figura, K. and Geuze, H.J. (1993) Differences in the endosomal distributions of the two mannose-6-phosphate receptors. J. Cell Biol., 121, 997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto U.M., Hubbard, A.L. and Mellman, I. (2001) A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell, 105, 575–585. [DOI] [PubMed] [Google Scholar]
- Le Borgne R. and Hoflack, B. (1997) Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J. Cell Biol., 137, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Antony, C., Tenza, D., Salamero, J., Goud, B. and Johannes, L. (1998) Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol., 143, 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet W.G. and Brodsky, F.M. (1996) A membrane-associated protein complex with selective binding to the clathrin coat adaptor AP1. J. Cell Sci., 109, 3059–3068. [DOI] [PubMed] [Google Scholar]
- Meyer C., Zizioli, D., Lausmann, S., Eskelinen, E.L., Hamann, J., Saftig, P., von Figura, K. and Schu, P. (2000) µ1A adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J., 19, 2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C., Eskelinen, E.L., Guruprasad, R.M., von Figura, K. and Schu, P. (2001) µ1A-deficiency induces a profound increase of MPR300/IGF-II receptor internalization rate. J. Cell Sci., 114, 4469–4476. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Anderson, E.D., Jean, F. and Thomas, G. (1999) Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol., 9, 28–35. [DOI] [PubMed] [Google Scholar]
- Ohno H. et al. (1999) µ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett., 449, 215–220. [DOI] [PubMed] [Google Scholar]
- Owen D.J. and Evans, P.R. (1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science, 282, 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I., Chen, C.Y., Cupers, P., Shoelson, S.E. and Kirchhausen, T. (1998) Dileucine-based sorting signals bind to the β chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J., 17, 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D.G. and Bakke, O. (1998) Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J. Biol. Chem., 273, 6005–6008. [DOI] [PubMed] [Google Scholar]
- Seaman M.N.J., Sowerby, P.J. and Robinson, M.S. (1996) Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J. Biol. Chem., 271, 25446–25451. [DOI] [PubMed] [Google Scholar]
- Simmen T., Höning, S., Icking, A., Tikkanen, R. and Hunziker, W. (2002) AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nature Cell Biol., 4, 154–159. [DOI] [PubMed] [Google Scholar]
- Storch S. and Braulke, T. (2001) Multiple C-terminal motifs of the 46-kDa mannose 6-phosphate receptor tail contribute to efficient binding of medium chains of AP-2 and AP-3. J. Biol. Chem., 276, 4298–4303. [DOI] [PubMed] [Google Scholar]
- Tikkanen R., Obermüller, S., Denzer, K., Pungitore, R., Geuze, H.J., von Figura, K. and Höning, S. (2000) The dileucine motif within the tail of MPR46 is required for sorting of the receptor in endosomes. Traffic, 1, 631–640. [DOI] [PubMed] [Google Scholar]
- von Figura K. (1978) Secretion of β-hexosaminidase by cultured human skin fibroblasts. Exp. Cell Res., 111, 15–21. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Traub, L.M. and Kornfeld, S. (1999) High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell, 10, 537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizioli D., Meyer, C., Guhde, G., Saftig, P., von Figura, K. and Schu, P. (1999) Early embryonic death of mice deficient in γ-adaptin. J. Biol. Chem., 274, 5385–5390. [DOI] [PubMed] [Google Scholar]