Introduction
Regulated transit of macromolecules across the nuclear envelope (NE) is a hallmark of eukaryotic cells, and in the last 10 years basic transport mechanisms have been elucidated. Transport factors of the importin/karyopherin-β (Impβ) superfamily decode nuclear localization signals (NLS) or nuclear export signals (NES) in the amino acid sequence of proteins and chaperone them through the nuclear pore complex (NPC). Vectorial transport of proteins and some ribonucleoproteins (RNPs) is achieved due to two principal features of the transport machinery: the asymmetric localization of NPC proteins on the nuclear and the cytoplasmic faces of the pore; and the nucleo-cytoplasmic gradient of the small GTPase Ran, which controls the stability of Impβ interactions with particular cargo molecules.
This meeting emphasized the important links between nucleo-cytoplasmic transport and signal transduction pathways involving small GTPases, kinases and phosphatases. Among the highlights were reports on the regulation of the subcellular localization of signal transducers and transcription factors and the suggestion that the nucleus itself is endowed with key components that are regulated during signal transduction, in particular Ca2+. Also exciting was the discussion of how large nucleic acids traffic to and across the NE. This holds the key to understanding the complexity of mRNA biogenesis and function and of viral trafficking. It emerged that nuclear export of bulk mRNAs follows somewhat different rules than the well-characterized Imp-mediated export of proteins, tRNAs or small nuclear (sn) RNAs and that certain viruses use different import mechanisms than do generic NLS-containing proteins. These findings suggest that the regulatory elements controlling housekeeping functions are distinct from those controlling atypical processes.
Nuclear and mitotic roles of Ran
The NPC is the element that ultimately governs macromolecular transit across the NE. Short NPC fibrils extend towards the cytoplasm and consist of RanBP2 (also called Nup358), CAN/Nup214 and four other proteins (for a recent review, see Vasu and Forbes, 2001). Cargo interacts with Impβ in the cytoplasm due to low concentrations of Ran and the Ran GTPase activating protein (GAP) and, after transport through the NPC into the nucleus, the complexes dissociate in response to the relatively high levels of Ran–GTP. H. Pickersill (Manchester, UK) used field emission in-lens scanning electron microscopy to investigate the mechanism behind the transport step. By microinjecting a constitutively activated GTP-bound form of Ran into Xenopus oocytes, she was able to demonstrate that Ran–GTP causes conformational changes in the NPC. These results support previous notions that, despite having mainly what could be called a ‘housekeeping’ role, the NPC is a dynamic structure that is able to adapt to different physiological conditions, such as active transport or stress.
It is now emerging that Ran is not only a key factor in interphase cells, but that it also controls mitotic processes, including spindle assembly during metaphase and the reformation of the NE during telophase. O. Gruss from I. Mattaj’s laboratory (Heidelberg, Germany) showed that Ran–GTP is generated on M-phase chromatin and activates the microtubule (MT)-assembly factor TPX2, thus stabilizing the plus ends of MTs and contributing to chromosome segregation in M phase. Therefore, rather than simply effecting traffic across the NE, Ran also appears to coordinate chromatin-dependent reactions in the cell, thus ensuring the maintenance and transmission of the cellular genome.
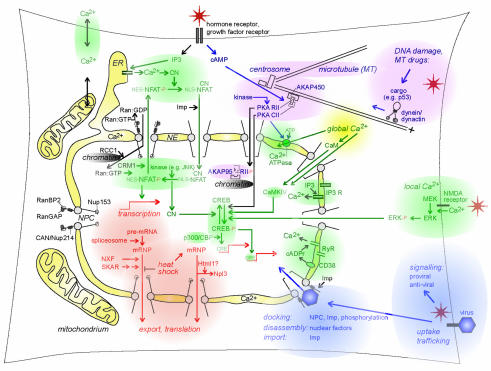
The subcellular specificity of Ran action is determined by RanGAP, which, during interphase, is attached to the NPC fibrils via RanBP2 (Figure 1, middle left). F. Melchior (Munich, Germany) underscored the previous notion that SUMO-1 modification of RanGAP1 is important for this attachment. She then used in vitro assays to show that RanBP2 is itself sumoylated and also suggested that RanBP2 may act as an E3 SUMO-ligase for certain import cargo, although not for RanGAP1. The identity of the RanGAP1 SUMO-ligase thus remains enigmatic. Also mysterious is the role of cargo sumoylation: earlier experiments had shown that this modification is not required for nuclear import and that translocation is not required for sumoylation. However, R. Hay (St Andrews, UK) illustrated that sumoylation of soluble proteins requires a functional NLS and identified a transferable consensus peptide sequence for sumoylation. It is possible that, at least for a subset of SUMO-1 targets, RanBP2 couples nuclear import with SUMO-conjugation and that this coupling is mediated, in part, by the import receptors docking to RanBP2. Theoretically, the SUMO modification of cargo in transit could also serve to increase its protection from ubiquitin-dependent proteasomal degradation, and this may be particularly relevant to the control of transcription factor activities at steady state and under stress conditions.
Fig. 1. An integrated view of signals and trafficking in nucleo-cytoplasmic communications. For details and abbreviations, please refer to the text.
Signalling-dependent nuclear and cytoplasmic residence
Nuclear and cytoplasmic activities of the kinases and phosphatases involved in signal transduction are effectively segregated by the cytoplasmic anchoring of these proteins; only when released from these bonds can they directly relay signals from the membrane to the nucleus. For example, the cAMP-dependent kinase (PKA) is targeted to cytoplasmic MTs through the interaction of its regulatory subunit RII (in its dephosphorylated form) with the A-kinase anchoring protein AKAP450 (Figure 1, upper right, in purple), a member of a larger family of AKAPs that form multivalent signalling complexes by binding several enzymes. The related chromatin-associated AKAP95 preferentially binds phosphorylated RII, thus acting as a nuclear sensor of cytoplasmic phosphorylation.
MTs also serve as tracks for directional transport of proteins towards the nucleus. P. Giannakakou (Atlanta, GA) provided evidence for a novel link between signals regulating the dynamics of MTs and nuclear import. She showed that the transcription factor p53 normally binds to interphase MTs but, upon DNA damage, is transported into the nucleus by the MT motor dynein/dynactin to effect cell-cycle arrest and apoptosis. Suppression of MT dynamics by exposure to low concentrations of MT active drugs, which preferentially stabilize MTs, further increased nuclear targeting of p53, an effect whose underlying mechanism is not yet clear. The fact that MT dynamics are regulated by a diverse array of signals raises the question of how the signals that respond specifically to DNA damage are transmitted to the MT-resident p53.
Anchoring proteins themselves are subject to dynamic regulation. The yeast scaffold protein Ste5p provides multiple binding sites for the regulators of both G-protein-mediated pheromone and MAP kinase (MAPK)-mediated signalling pathways that control cell proliferation. A. Flotho of the group of E. Elion (Boston, MA) showed that the assembly of active cytoplasmic Ste5p oligomers requires continuous shuttling of Ste5p into and out of the nucleus. Pheromone stimulation enhanced nuclear export of Ste5p and its translocation to the cell periphery and boosted the activation of MAPK. MAPK activation also required the oligomerization of Ste5p. In addition, both nuclear import and recruitment of Ste5p to the plasma membrane required cortical actin regulators, demonstrating a link between the cell cortex and the nucleus. The model suggests that nuclear shuttling somehow regulates the access of Ste5p to the plasma membrane and facilitates the building of a multimeric scaffold complex on which MAPK activation occurs.
A reverse signalling mode, from nucleus to cytoplasm, was demonstrated by A. Kotlyarov from the group of M. Gaestel (Hannover, Germany). He showed that nuclear export of a p38/MAPK target, MAPKAP kinase 2 (MK2), is regulated by phosphorylation of a single Thr residue in its hinge region, which also relieves autoinhibition by the C-terminal loop (Neininger et al., 2001). Activated MK2 was exported to the cytoplasm by the Imp family member CRM1, where it then targeted the small heat-shock protein Hsp27, which controls actin polymerization. This suggests that the actin and MT cytoskeletons not only provide scaffolds for the assembly of signalling complexes, but are themselves subject to dynamic feedback regulation upon cell signalling, even from the nucleus.
Calcium signalling and nuclear transport
A major portion of the workshop dealt with the effect of Ca2+ on nuclear transport and with the regulation of the levels of nuclear Ca2+ itself. The latter is a subject of some controversy because, although the existence of a number of specific Ca2+-modulated processes within the nucleus would certainly indicate a degree of regulation, the large pores of the NE have been traditionally assumed to be freely permeable to Ca2+, implying a passive equilibrium between the two compartments. The suggestion that the pores could somehow be ‘gated’ has been put forward previously as a way out of the difficulty. However, another mechanism was discussed by O. Petersen (Liverpool, UK), who concentrated on the role of mitochondria as Ca2+ buffers. According to Petersen, these organelles can provide a ‘firewall’ in the perinuclear region. M. Zaidi (New York, NY) further suggested that Ca2+ concentration within the nucleoplasm can be controlled directly by Ca2+ release from the NE reservoir. He focused on CD38, the enzyme that synthesizes the second messenger cyclic ADP ribose (cADPr), which has been shown previously to be a plasma membrane ectoenzyme (its catalytic site would be outside the cell). Zaidi demonstrated that it is also located on the inner nuclear membrane, where its catalytic site faces the nucleoplasm. Upon stimulation of CD38, cADPr induces Ca2+ release into the nucleoplasm by opening a specific channel (the ryanodine receptor RyR), which is not only present in the endoplasmic reticulum (ER) but also in the NE. These findings could well explain the previous demonstration of cADPr-gated channel localization to the NE (Figure 1, lower right, in green).
In addition to harbouring Ca2+ channels sensitive to messengers, the NE has also been found to contain a Ca2+ pump (Ca2+ ATPase) that transports cytoplasmic Ca2+ into the NE lumen. A. Malviya (Strasbourg, France) showed that the pump is strongly phosphorylated by PKA and that this facilitates the transport of intermediate size molecules (e.g. 10 kDa fluorescent dextran) into the nucleus due to the increased NE Ca2+ levels (Figure 1, upper right, in green/purple). Other aspects of Ca2+ signalling in the nucleus were also discussed at length. For example, B. Lefevre (Clamart, France) suggested the involvment of nuclear phosphoinositides in Ca2+ mobilization, based on studies of phospholipase C β1. This protein translocates from the cytoplasm to the nucleoplasm prior to NE breakdown in mouse oocytes, and nuclear injections of anti-PLCβ1 antibodies inhibit concurrent Ca2+ oscillations. Furthermore, L. Santella (Napoli, Italy) provided evidence in support of NE localization of distinct pools of inositol tris-phosphate receptors (IP3R), another calcium release channel, during maturation of starfish oocytes. Activated IP3R might boost the nuclear Ca2+ levels by releasing the NE stores, thus triggering a myriad of nuclear events.
A major Ca2+ target in the nucleus is the transcriptional machinery. The best-studied systems are the cAMP responsive element binding protein (CREB) and its cofactor CREB-binding protein (p300/CBP), the nuclear factor of activated T cells (NFAT), Ca2+-calmodulin (CaM)-dependent phosphatase calcineurin (CN) and the ubiquitous CaM kinase II and Cam kinase IV in T lymphocytes. E. Carafoli (Padova, Italy) surveyed the role of CN in the regulation of transcription: the activation of nuclear CN leads to the dephosphorylation of the transcription factor CREB; furthermore, cytoplasmic CN causes the NFAT1, NFAT2 and NFAT4 transcription factors to translocate into the nucleus, where they bind and activate cytokine gene promotors in cooperation with other transcription factors (AP-1, c-Maf and GATA-family proteins) and coactivators (p300 and CBP) (Figure 1, upper left, in green). He also described recent results that showed CN-mediated downregulation of the genes coding for membrane transporters of Ca2+ in cerebellar neurons.
A. Rao (Boston, MA) also focused on the NFAT proteins family of transcription factors. She reported on their phosphorylation-dependent retention in the cytoplasm of T lymphocytes and showed that CN-mediated dephosphorylation of all the phospho-serine residues in the regulatory domain of NFAT1 is required for maximal NFAT nuclear import and concomitant suppression of the NES. However, nuclear residence is not the sole factor controlling the activity of NFAT, since the latter is regulated by an inducible phosphorylation site in the transactivation domain. This idea was complemented by C.-W. Chow (New York, NY), who found that removal of the Jun N-terminal kinase (JNK) phosphorylation sites on NFAT4 (present in immature lymphocytes) induces constitutive nuclear localization and JNK activation in CN-stimulated cells.
H. Bading (Cambridge, UK) discussed how the Ca2+ signal is transmitted from the synapse to the cytoplasm and the nucleus of neurons. Global Ca2+ waves are known to activate cytoplasmic CaM and, in some cell types, induce nuclear import of CaM (Figure 1, centre, yellow spot). Bading showed that in cultured hippocampal neurons, global Ca2+ waves may be propagated directly to the nucleus to induce CREB phosphorylation, independent of CaM nuclear import (Figure 1, centre, in green; Hardingham et al., 2001). This added a second possibility to the earlier notion that CaM ‘picks up’ Ca2+ at the mouth of Ca2+ influx channels and translocates to the nucleus to activate CREB phosphorylation via CaM kinase IV. Another mechanism of synapse–nucleus communication requires plasma membrane Ca2+ microdomains and leads to the activation of the extracellular signal regulated kinase (ERK), independent of global Ca2+ increases in the neuronal cytoplasm. Locally activated ERK leads to transcriptional responses of genes controlled by serum response element promotors and prolongs CREB phosphorylation at Ser133, which has been implicated in transcriptional activation. The fact that activated ERK proteins access the nucleoplasm by different mechanisms, including passive difffusion of monomers, Imp-mediated transport of dimers and direct attachment to the NPC protein CAN/Nup214 (Matsubayashi et al., 2001), raises the question of whether different nuclear import modes affect the transcriptional abilities of ERK.
A further level of complexity concerning nuclear Ca2+ was introduced by J. Hanover (Bethesda, MD). In a collaboration with B. Paschal (Charlottesville, VA), Hanover showed that calreticulin (CRT), a Ca2+-binding protein of the ER and other cell compartments, functions as a nuclear export factor of the glucocorticoid receptor (Holaska et al., 2001). Like CRM1, CRT requires Ran–GTP for cargo binding but apparently does not need an NES and may act on specific substrates, such as steroid hormone receptors. Indeed, CRT seems to antagonize DNA binding of steroid hormone receptors, thus providing a direct link between subcellular localization and function. It will be interesting to decipher how CRT is able to access the nucleus. Possibilities include an ER exclusion mechanism (e.g. the inactivation of the signal sequence), retrotranslocation from the ER to the cytosol and from there to the nucleus, or even non-vesicular trafficking across membranes, as has been shown for viral proteins and certain cellular protein domains (e.g. the human immunodeficiency virus TAT, human herpes simplex virus VP22 or the third helix of the homeodomain of the Drosophila antennapedia protein).
Nucleo-cytoplasmic trafficking of nucleic acids
The NPC is a formidable machine capable of translocating several thousand NLS proteins per second. But how does it achieve vectorial transport of large cargo? A number of presentations were dedicated to the question of how the cell exports spliced mRNA while retaining non-processed mRNA and intron sequences in the nucleus. Clearly, retaining incorrectly formed mRNAs and introns in the nucleus is crucial to avoid sequestration of limiting cytosolic factors or catalytic activities and to prevent the translation of potentially harmful dominant negative proteins. Therefore, nuclear export is coupled to the completion of the splicing reaction, and the presence of heterogeneous nuclear RNPs excludes the splicing machinery from introns. E. Izaurralde (Heidelberg, Germany) demonstrated that the export of bulk mRNA is independent of Impβ superfamily members: it relies on a novel class of evolutionarily conserved transport factors of the NXF family (Figure 1, lower left, in orange). Members of this family include the yeast mRNA export factor heterodimer Mex67p/Mtr2p and the mammalian TAP/p15 protein. Izaurralde showed that overexpression of TAP/p15 heterodimers stimulates nuclear export of viral mRNAs bearing a constitutive transport element. In addition, TAP preferentially binds to spliced cellular mRNA near exon–exon junctions, suggesting that TAP/p15 is recruited to mRNAs as a consequence of splicing (Le Hir et al., 2001). B. Cullen (Durham, NC) emphasized that TAP binds to the C-terminal domain of the NPC protein CAN/Nup214 and to FG-repeat domains (phenylalanine-glycine-rich domains in nucleoporins that are sites of interaction of specific transport factors) of both CG1 and the NPC filament protein Nup153, which are located on the nuclear side of the complex. Cullen further reported the identification of a TAP-related mRNA export factor, NXF3. Unlike TAP, NXF3 lacks the C-terminal ubiquitin-associated domain, which is involved in high affinity binding of TAP or Mex67p to nucleoporins but retains the ability to shuttle between the nucleus and the cytoplasm (Yang et al., 2001). This protein interacts directly with poly(A)+ RNA and binds the export factor CRM1 by virtue of an NES that is absent in TAP. In this respect, NXF3 is similar to the shuttling HIV adaptor protein Rev.
A. Hatton from P. Silver’s laboratory (Boston, MA) presented data on regulated mRNA export under stress conditions. Heat-shocked yeast cells were found to stop exporting mRNPs other than those encoding heat-shock proteins. This correlated with a dissociation of the hnRNP Npl3 from the non-heat-shock mRNAs (Figure 1, bottom centre, in orange), but the trigger for Npl3 dissociation is unknown. Hatton showed that Npl3 is a substrate of the yeast arginine methyltransferase (Hmt1p), a predominantly nuclear RNA-binding protein. Interestingly, Hmt1p is non-essential, except in cells also lacking Npl3. Whether this is part of the stress sensor machinery and whether the arginine methylations are reversible is currently unknown. C. Richardson (Boston, MA) reported that mRNP export could be controlled by cell signalling. She identified SKAR as a novel target of the ribosomal protein p70/S6 kinase1 (S6K1) (Figure 1, lower left, in orange). SKAR interacts with S6K1 in vivo and in vitro, and Richardson speculated that it may have a role in mRNA export. If it turns out that SKAR has a specific role in the export of mRNA encoding ribosomal proteins and translation factors, it may be an important link between this housekeeping function and the control of cell size.
In his keynote address, G. Dreyfuss (Philadelphia, PA) highlighted a further link between nucleo-cytoplasmic shuttling of RNA-binding proteins and pre-mRNA maturation in the mammalian nucleus by describing the mechanism of nonsense-mediated decay of mRNA. Two proteins, Y14 and hUpf3, bind to mRNAs during the splicing process and remain associated with the mRNA in a position-dependent manner (Kim et al., 2001). These proteins form a large complex including TAP and the RNA-binding protein Aly/REF, a member of the RNA export factor family. However, whereas TAP and Aly/REF dissociate from the mRNP in the cytoplasm, Y14 and hUpf3 remain positioned on the mRNA, probably until the leading ribosome scans the mRNA and removes all of the Y14/hUpf3 complexes to yield a stable message ready for further rounds of translation. If a nonsense mutation is present in the mRNA upstream of the last exon–exon junction, at least one Y14/hUpf3 complex will remain and trigger mRNA degradation. The actual mechanism of mRNA export has been characterized further in yeast by F. Stutz (Lausanne, Switzerland), who investigated the function of Yra1p, the yeast homologue of Aly/REF. Genetic and biochemical experiments initially showed that Yra1p is essential for mRNA export and recruits Mex67p to the mRNP. These and results from other laboratories suggest a model in which, at least in higher eukaryotes, Aly/REF is recruited to spliced mRNAs by the spliceosome. The export factor TAP/p15 binds Aly/REF, thus targeting the mature mRNA to the NPC for export. The identification of all of the components of the exon junction complex will be required to illuminate how pre-mRNA splicing, nuclear export, cytoplasmic trafficking and translation are interconnected and contribute to post-splicing gene regulation. Furthermore, since yeast Yra1p and Mex67p and their metazoan homologues also mediate export of mRNAs that do not contain introns (e.g. heat shock mRNAs and most mRNAs in yeast), the mechanisms by which these proteins are recruited to intronless mRNAs need to be elucidated.
Viruses take advantage of the superb organization of the cell nucleus and usurp nucleo-cytoplasmic transport for their own use. Attachment of the human hepatitis B virus (HBV) to NPCs was shown to require phosphorylation of capsid proteins, presumably by a capsid-associated protein kinase C (M. Kann, Giessen, Germany). HBV capsids bound to NPCs in permeablized cells provided that Impα and Impβ were supplied (Figure 1, lower right, in blue). Kann suggested that capsid disassembly occurred within the fishnet-like basket formed by the filaments of the NPC. This supported the notion that the NPC can accommodate particles as large as 39 nm. Adenovirus (Ad) particles are larger and therefore do not enter the nucleus. U. Greber (Zürich, Switzerland) showed that Ad directly binds to the NPC protein CAN/Nup214 (Trotman et al., 2001). This interaction is required for the viral deoxyribonucleoprotein (DNP) import that allows the virus to take over the cell and appears to be similar to the mechanism by which cellular uridine-rich snRNPs and yeast retrotransposons interact with the NPC. Key questions are how does the viral DNP translocate through the NPC, what is the molecular composition of the cargo in transit and does the NPC undergo dynamic changes during this process?
Perspectives
Extending and linking nuclear gene regulation to the plasma membrane and the cytoplasm holds the key to a unified view of the cell. An integrative role is attributed to Ca2+, a key switch with both nuclear and cytoplasmic effectors. Additionally, we will have to decipher the functionality and the regulatory potential of emerging nuclear receptors and specific import and export pathways. This will eventually solve the burning questions relating to how the NPC handles large cargo, such as RNPs and viruses, and how post-transcriptional and post-translational modifications regulate import and export reactions. It may eventually offer potential points of intervention for the therapy of diseased cells. We are looking forward to increasingly thorough cell biological analyses, which are bound to uncover new circuits between the nucleus and the other organelles.

An EMBO workshop on ‘Signal-Regulated Nuclear Transport’ in Strasbourg, France, August 11–14, 2001, and was organized by A.N. Malvia. The poster depicits the painting of Pierre Huet entitled ‘La situation du paradis’, showing Moses pointing to the ‘Happy Arabia’, i.e. the cell nucleus.
Acknowledgments
Acknowledgements
We thank Dr P. Rogue for the design of the meeting poster and the editors of the journal for their constructive comments on the manuscript.
REFERENCES
- Hardingham G.E., Arnold, F.J. and Bading, H. (2001) Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neurosci., 4, 261–267. [DOI] [PubMed] [Google Scholar]
- Holaska J.M., Black, B.E., Love, D.C., Hanover, J.A., Leszyk, J. and Paschal, B.M. (2001) Calreticulin is a receptor for nuclear export. J. Cell Biol., 152, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim V.N., Kataoka, N. and Dreyfuss, G. (2001) Role of the nonsense-mediated decay factor hUpf3 in the splicing-dependent exon–exon junction complex. Science, 293, 1832–1836. [DOI] [PubMed] [Google Scholar]
- Le Hir H., Gatfield, D., Braun, I.C., Forler, D. and Izaurralde, E. (2001) The protein Mago provides a link between splicing and mRNA localization. EMBO rep., 2, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y., Fukuda, M. and Nishida, E. (2001) Evidence for existence of a nuclear pore complex-mediated, cytosol-independent pathway of nuclear translocation of ERK MAP kinase in permeabilized cells. J. Biol. Chem., 276, 41755–41760. [DOI] [PubMed] [Google Scholar]
- Neininger A., Thielemann, H. and Gaestel, M. (2001) FRET-based detection of different conformations of MK2. EMBO rep., 2, 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman L.C., Mosberger, N., Fornerod, M., Stidwill, R.P. and Greber, U.F. (2001) Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nature Cell Biol., 3, 1092–1100. [DOI] [PubMed] [Google Scholar]
- Vasu S.K. and Forbes, D.J. (2001) Nuclear pores and nuclear assembly. Curr. Opin. Cell Biol., 13, 363–375. [DOI] [PubMed] [Google Scholar]
- Yang J., Bogerd, H.P., Wang, P.J., Page, D.C. and Cullen, B.R. (2001) Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol. Cell, 8, 397–406. [DOI] [PubMed] [Google Scholar]