Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a potentially life-threatening reaction characterized by fever, rash, lymphadenopathy, eosinophilia, and organ injury.1 DRESS is strongly associated with human leukocyte antigens (HLA) class I (e.g., carbamazepine to HLA-B15:02), has an estimated incidence of 1 in 1,000–10,000 drug exposures, and carries a 5–10% fatality rate.1
The high morbidity and mortality associated with DRESS makes prompt recognition and withdrawal of all potentially causal drugs critical. Although no specific rash morphology is pathognomonic for DRESS, a rash often serves as an early indication of DRESS. There is an overrepresentation of severe cutaneous adverse reactions (SCARs) associated with known HLA class I risk alleles in people of color (POC).1–3 However, the underrepresentation of skin color images in medical education may lead to cognitive biases and delayed diagnoses.4,5 This study aimed to characterize DRESS syndrome differences in POC.
We used informatics searches on large electronic health record (EHR) data from Mass General Brigham (MGB) from 2013–2021, followed by manual EHR review to identify specialist-diagnosed DRESS cases.6 We considered cases with known race and ethnicity, categorized from self-identified EHR demographics into White (non-Hispanic), Black, Asian, or Hispanic. Non-white or Hispanic-identifying patients were considered POC. We used descriptive statistics and univariable tests to compare demographics, causal drugs, clinical presentation, European registry on severe cutaneous adverse drug reactions (RegiSCAR) score, treatment, and outcomes. Analyses were performed using SAS® software version 9.4. The MGB Human Research Committee approved this study.
Of 252 patients with DRESS, 79 (31%) were POC: 27 Black (11%), 28 Asian (11%), and 24 Hispanic (10%) patients (Table 1). Female sex comprised most POC (56% Black, 68% Asian, and 46% Hispanic). POC were younger than White patients (46 vs. 55 years; p=0.003). Vancomycin was the most common culprit, particularly in White and Hispanic patients. Cephalosporins were also a common culprit in White, patients. Anticonvulsants were a common a cause of DRESS in POC, particularly in Black patients, who were overrepresented for phenytoin-induced DRESS (5 of 10 cases).
Table.
Characteristics of DRESS syndrome according to people of color status
| Characteristics | White n (%) |
Patients of Color (POC) n (%)*; p-value** |
||||
|---|---|---|---|---|---|---|
| n = 173 (69) |
Blacka n = 27 (11) |
Asian n = 28 (11) |
Hispanicb n = 24 (10) |
POC n = 79 (31) |
P-value*** | |
| Female, n(%) | 107 (62) | 15 (56) | 19 (68) | 11 (46) | 45 (57) | 0.46 |
| Age, μ(SD) (years) | 55 (17) | 48 (22) | 44 (16); 0.04 | 45 (22) | 46 (20) | 0.0003 |
| Causal drugs, n(%) | ||||||
| Vancomycin | 68 (39) | 4 (15); 0.02 | 4 (14); 0.01 | 9 (38) | 17 (22) | 0.006 |
| Cephalosporin | 42 (24) | 3 (11) | 4 (14) | 4 (17) | 11 (14) | 0.06 |
| Anticonvulsantsc | 19 (11) | 9 (33); 0.005 | 4 (14) | 4 (17) | 17 (22) | 0.01 |
| TMP-SMX | 20 (12) | 1 (4) | 6 (21) | 1 (4) | 8 (10) | 0.74 |
| Allopurinol | 9 (5) | 3 (11) | 3 (11) | 0 (0) | 6 (8) | 0.46 |
| Clinical features, n(%) | ||||||
| Fever ≥ 100.6F (38 °C) | 122 (71) | 21 (78) | 15 (54) | 18 (75) | 54 (68) | 0.73 |
| Rash | 172 (99) | 27 (100) | 28 (100) | 23 (96) | 78 (99) | 0.57 |
| AEC > 500 cells/mcL | 122 (71) | 20 (74) | 20 (71) | 19 (79) | 59 (75) | 0.50 |
| Hypotension | 28 (16) | 6 (22) | 2 (7) | 0 (0); 0.03 | 8 (10) | 0.20 |
| Itching | 93 (54) | 13 (48) | 13 (46) | 9 (38) | 35 (44) | 0.16 |
| Myalgias | 11 (6) | 4 (15) | 0 (0) | 4 (17) | 8 (10) | 0.29 |
| Edema | 51 (29) | 17 (63); 0.006 | 8 (29) | 7 (29) | 32 (41) | 0.08 |
| Nausea | 30 (17) | 4 (15) | 7 (25) | 3 (13) | 14 (18) | 0.94 |
| Diarrhea | 15 (9) | 1 (4) | 4 (14) | 2 (8) | 7 (9) | 0.96 |
| Atypical lymphocytes | 41 (24) | 11 (41) | 8 (29) | 9 (38) | 28 (35) | 0.05 |
| Lymphadenopathyd | 28 (16) | 10 (37); 0.01 | 5 (18) | 5 (21) | 20 (25) | 0.09 |
| Liver injurye | 98 (57) | 21 (78); 0.04 | 19 (68) | 13 (54) | 53 (67) | 0.12 |
| Kidney injuryf | 73 (42) | 12 (44) | 4 (14); 0.005 | 9 (38) | 25 (32) | 0.11 |
| Mucosa involvement | ||||||
| Ocular mucosa | 12 (7) | 1 (4) | 0 (0) | 1 (4) | 2 (3) | 0.15 |
| Oral mucosa | 21 (12) | 3 (11) | 6 (21) | 3 (13) | 12 (15) | 0.5 |
| Genital mucosa | 8 (5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.05 |
| Rash morphology | ||||||
| Erythematous | 126 (73) | 15 (56) | 19 (68) | 14 (58) | 48 (61) | 0.05 |
| Morbilliform | 101 (58) | 13 (48) | 14 (50) | 15 (63) | 42 (53) | 0.52 |
| Macules | 29 (17) | 5 (19) | 6 (21) | 8 (33) | 19 (24) | 0.17 |
| Urticarial | 11 (6) | 3 (11) | 2 (7) | 1 (4) | 6 (8) | 0.93 |
| Bullous | 5 (3) | 1 (4) | 0 (0) | 1 (4) | 2 (3) | 0.87 |
| Pustular | 0 (0) | 1 (4); 0.01 | 2 (7); 0.02 | 0 (0) | 3 (4) | 0.01 |
| Rash descriptors | ||||||
| Erythema | 31 (18) | 6 (22) | 5 (18) | 2 (8); 0.24 | 13 (16); 0.78 | 0.78 |
| Dusky | 1 (1) | 3 (11); 0.0008 | 3 (11); 0.009 | 2 (8); 0.04 | 8 (10) | 0.0002 |
| Treatment, n(%) | ||||||
| Systemic steroids | 133 (77) | 20 (74) | 23 (82) | 18 (75) | 61 (77) | 0.95 |
| Topical steroids | 87 (50) | 16 (59) | 18 (64) | 12 (50) | 46 (58) | 0.24 |
| Antihistamine | 71 (41) | 11 (41) | 14 (50) | 8 (33) | 33 (42) | 0.91 |
| Cyclosporine | 6 (3) | 2 (7) | 2 (7) | 2 (8) | 6 (8) | 0.15 |
| IVIG | 1 (1) | 0 (0) | 1 (4) | 1 (4) | 2 (3) | 0.18 |
| Epinephrine | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.33 |
| RegiSCAR, n(%) | ||||||
| >5 | 34 (20) | 9 (33) | 5 (18) | 4 (17) | 18 (23) | 0.69 |
| 4–5 | 57 (33) | 9 (33) | 7 (25) | 7 (29) | 23 (29) | 0.64 |
| 2–3 | 44 (25) | 6 (22) | 7 (25) | 8 (33) | 21 (27) | 0.97 |
| <2 | 38 (22) | 3 (11) | 9 (32) | 5 (21) | 17 (21) | 0.93 |
| Suspected causal drug withdrawal, μ days (SD)g | −0.5 (7.8) | 3.1 (10.6); 0.04 | 2.2 (5.9) | 1.3 (6.3) | 2.2 (7.9); 0.01 | 0.01 |
| Time to systemic steroid, μ days (SD) | 6.9 (9.6) | 6.2 (6.2) | 8.7 (6.1) | 7.2 (7.2) | 7.4 (6.5) | 0.67 |
| Length of Stay, μ days (SD)h | 19 (23.5) | 14 (16.5) | 21 (21.0) | 25 (22.8) | 19 (20.6) | 0.91 |
| Resolution, μ days (SD) | 31 (42) | 25 (22) | 41 (37) | 19 (15); 0.04 | 29 (28) | 0.75 |
| Hospital mortality, n(%) | 5 (3) | 1 (4) | 1 (4) | 2 (8) | 4 (5) | 0.62 |
Percentage is the prevalence for non-continuous characteristics among each subgroup; columns may add up to greater than 100%.
Significant p-values (<0.05) comparing specific race or ethnicity to white, non-Hispanic patients.
P values compare people of color to the reference group (white, non-Hispanic).
Patients who identified their race as Black and ethnicity as Hispanic (n=2) or as non-Hispanic (n=20) were considered Black.
Patients who identified their ethnicity as Hispanic and race as white (n=4), other (n=6), or unknown (n=4) were considered Hispanic.
Carbamazepine (white, n=1; Asian n=2), lamotrigine (white, n=12; Black, n=3; Asian, n=1; Hispanic, n=4), levetiracetam (white, n=1; Black, n=1), phenytoin (white, n=4; Black, n=5; Asian, n=1), sodium valproate (white, n=1).
Lymphadenopathy was defined as having at least two sites with lymph nodes 1 cm or greater.
Liver Injury was defined as alanine transaminase ≥ 100 U/L.
Kidney injury is a creatinine increase by 0.5 mg/dL or 50% above baseline.
Time to suspected causal drug withdrawal was defined as the number of days between the start of the first symptom of DRESS and the withdrawal of the suspected offending drug.
Length of stay was calculated as the number of days between the admission date and discharge date.
Abbreviations: TMP-SMX, trimethoprim-sulfamethoxazole; AEC, absolute eosinophil count; IVIG, intravenous immune globulin; RegiSCAR, European registry on severe cutaneous adverse drug reactions; LOS,
Compared to White patients, Black patients were more likely to have documented edema (63% vs. 29%; p=0.006), liver injury (78% vs. 57%; p=0.04), and lymphadenopathy (37% vs. 16%; p= 0.01); and Asian patients were less likely to have kidney injury (14% vs. 42%; p=0.005). Rashes were mostly described as erythematous and/or morbilliform (Table 1). Compared to White patients, POC had documented pustules more frequently (4% vs. 0%; p=0.01) and duskiness (10% vs. 1%; p=0.0002; Figure 1).
Figure 1.
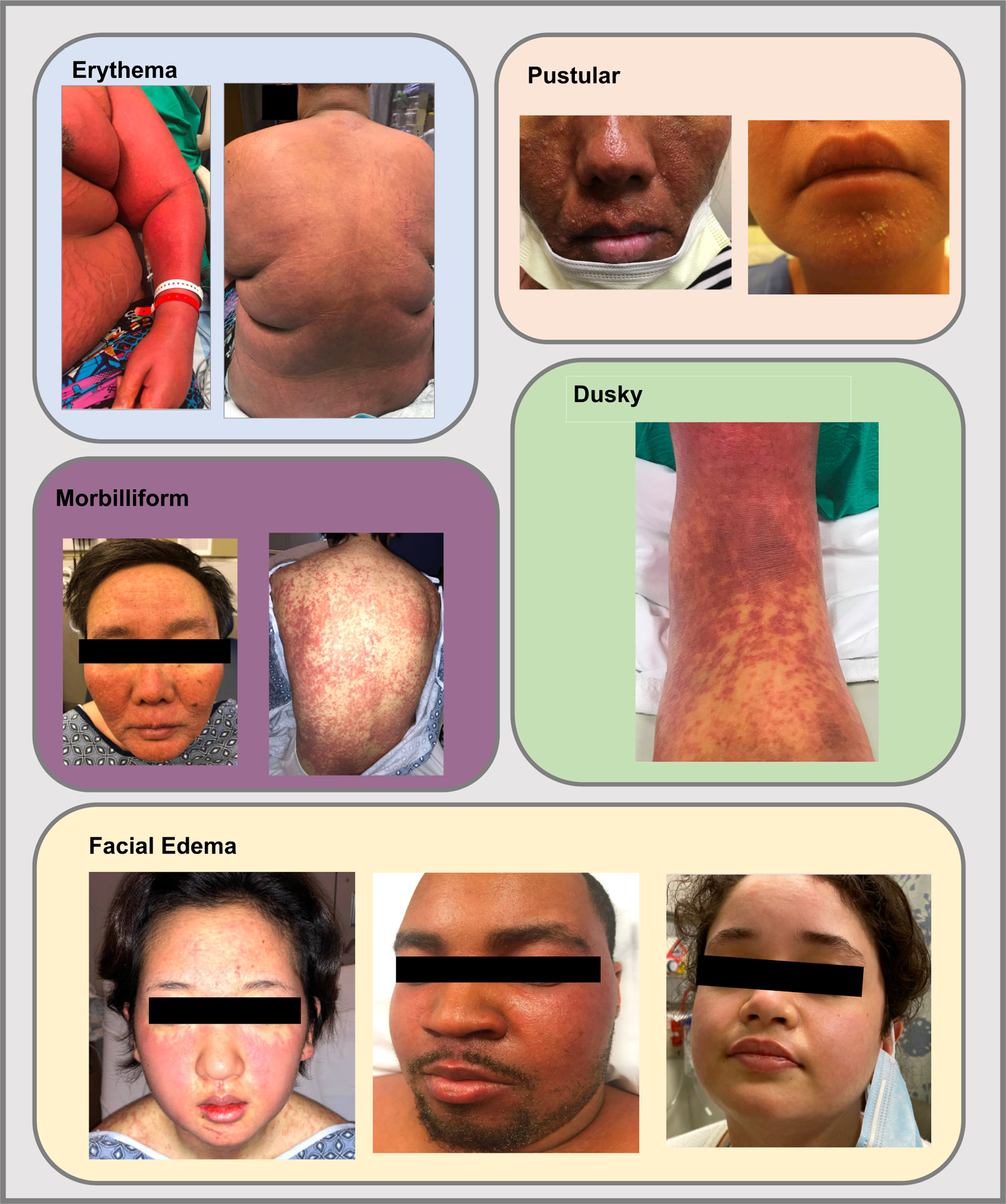
Findings associated with drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in people of color.
Compared to White patients, POC had a longer mean interval between documented symptoms onset and suspect drug withdrawal (2.2 vs. −0.5 days; p=0.01). The longest mean time from documented symptom onset to drug withdrawal was found in Black patients (3.1 days [SD 10.6]). Compared to White patients, the mean time to resolution was shorter for Hispanic patients (19 vs. 31 days, p=0.04). The delay between symptoms onset and corticosteroid initiation, hospital length of stay, and in-hospital mortality were similar across groups.
Although severe infections requiring broad-spectrum antibiotics can exhibit signs/symptoms like those observed in DRESS (e.g., fever, lymphadenopathy, organ involvement), we noted a significant difference in the prevalence of vancomycin- and cephalosporin-induced DRESS between White patients and POC. Anticonvulsant-induced DRESS was more prevalent in POC than in White patients. Recent studies suggest that the Black race may be a risk factor for DRESS associated with first-generation anticonvulsants (e.g., carbamazepine and phenytoin). This could be due to underlying prescribing bias based on race, with first-generation anticonvulsants being over-prescribed to Black patients.7
The initial skin presentation of DRESS syndrome was broadly similar across demographic groups. However, pustules were observed in 6 POC but none of the 173 White patients. The rash descriptor, ‘dusky,’ was more frequently recorded in Black and Hispanic patients than in White patients. Edema was more commonly documented in Black than White patients, a difference potentially related to visual limitations of the skin examination.8 We also found that compared to White patients, liver injury and lymphadenopathy were reported at significantly higher rates in Black patients, while kidney injury was less common in Asian patients.
POC, particularly Black and Asian patients, had a longer observed time to drug withdrawal after first documentation of DRESS symptoms. Given that withdrawal of the culprit drug is the first and most critical step in DRESS treatment, this finding warrants additional investigation. We did not observe differences in DRESS outcomes, such as length of hospitalization or in-hospital mortality, between demographic groups.
Our study has notable limitations. Given lack of validated diagnostics for DRESS syndrome, DRESS diagnoses were clinical, reliant on specialists applying clinical criteria. It is important to note that our study considered all specialist-diagnosed DRESS cases, regardless of the RegiSCAR score. In the EHR, we relied on self-reported race and ethnicity, which may not capture all patients or be easily and correctly assigned (e.g., Hispanic patients included 6 individuals identified as White and 10 who identified as other or unknown). Additionally, without Fitzpatrick skin types available, we could not determine differences according to skin color. Data come from a single health system, which may limit generalizability. Retrospective data introduces information biases as some clinical details, such as the location of edema or lymphadenopathy, may not be reported. Lastly, the small sample size limited our analyses to univariable tests of association, which cannot capture the complexity of these data. Despite these limitations, our study provides valuable insights into the differential presentations, causal drugs, and outcomes of DRESS syndrome among different racial and ethnic groups.
Prospective clinical studies are needed to accurately capture differential clinical presentations and outcomes for POC with DRESS and other SCARs. Improved understanding of differences in DRESS in POC and dissemination of diverse images of dermatologic findings may mitigate cognitive biases and promote optimal clinical care for all DRESS patients.
Clinical Implications:
Common causal drugs of DRESS Syndrome differed by race and ethnicity. ‘Pustular’ and ‘dusky’ rashes were more common in people of color. Suspected causal drug withdrawal following symptom onset was longer for people of color and longest for Black patients.
Acknowledgments:
This study was funded by award NIH/NIAID R01 AI150295. Dr. Alvarez-Arango receives support from the NIH-NCATS (KL2TR003099). Dr. Blumenthal received support from the Massachusetts General Hospital Department of Medicine Transformative Scholar in Medicine Program. Dr. Ezeofor receives support from the MGH Next Generation Physician-Scientist Training Pathway in partnership with NIH (R25AI147393).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- 1.Hama N, Abe R, Gibson A, Phillips EJ. Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis. J Allergy Clin Immunol Pract. 2022;10(5):1155–67.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu N, Rai SK, Terkeltaub R, Kim SC, Menendez ME, Choi HK. Racial disparities in the risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis as urate-lowering drug adverse events in the United States. Semin Arthritis Rheum 2016;46(2):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller SF, Lu N, Blumenthal KG, Rai SK, Yokose C, Choi JWJ, et al. Racial/ethnic variation and risk factors for allopurinol-associated severe cutaneous adverse reactions: A cohort study. Ann Rheum Dis. 2018;77(8):1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones VA, Clark KA, Shobajo MT, Cordova A, Tsoukas MM. Skin of color representation in medical education: an analysis of popular preparatory materials used for United States Medical Licensing Examinations. Journal of the American Academy of Dermatology. 2021. Sep 1;85(3):773–5. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Arango S, Ogunwole SM, Sequist TD, Burk CM, Blumenthal KG. Vancomycin infusion reaction - Moving beyond “Red Man Syndrome”. N Engl J Med. 2021;384(14):1283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal KG, Alvarez-Arango S, Fu X, Kroshinsky D, Choi H, Phillips E, et al. Risk Factors for Vancomycin Drug Reaction With Eosinophilia and Systemic Symptoms Syndrome. JAMA Dermatol. 2022;158(12):1449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensken WP, Fernandez Baca Vaca G, Alberti PM, Khan OI, Ciesielski TH, Jobst BC, et al. Racial and ethnic differences in antiseizure medications among people with epilepsy on medicaid: a case of potential inequities. Neurol Clin Pract. 2023. Feb;13(1):e200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onyekaba G, Taiwò Née Ademide Adelekun D, Lipoff JB. Skin of color representation in dermatology must be intentionally rectified. J Am Acad Dermatol. 2022;87(1):e43–e4. [DOI] [PubMed] [Google Scholar]


