Abstract
Anticancer therapy is frequently efficient in early stages of the disease, whereas advanced tumors are usually resistant to the same treatments. The molecular basis for this change is not entirely understood. Many anticancer agents are DNA- or cytoskeleton-damaging drugs that show some specificity towards dividing cells. However, recent studies show that these agents also activate stress-signaling cascades that may play a role in eliciting the observed therapeutic effects. We discuss recent findings that suggest that induction of stress signaling in oncogenically transformed cells is integrated into apoptotic pathways. Reactive oxygen species (ROS) and stress-activated protein kinases (SAPKs), which are potentiated in recently transformed cells, emerge as key effectors of cell death. In advanced tumors, however, these agents are downregulated and, consequently, death signaling is suppressed. Such changes in ROS and SAPK activity levels during the course of tumor development may underlie the changes in responsiveness to anticancer therapy.
Introduction
Mortality from cancer has not changed significantly since the ‘War on cancer’ was declared by an act of the US Congress 30 years ago. Billions of dollars have been invested in cancer research, but progress toward a cure has been painfully slow. It is well known that, in the early stages of the disease, cancer cells are more vulnerable to chemotherapy and radiotherapy than normal cells, but, as the disease progresses, they lose their preferential sensitivity to the same treatments. Understanding this shift in cellular behavior is essential if we wish to eliminate metastatic cancer cells by dismantling the shield that they acquire.
When cytotoxic drugs were originally generated, they were believed to target the cancer cell quite selectively, since they mainly damage rapidly dividing cells. Today, we understand that this simplistic view is inaccurate. We now recognize that cytotoxic agents, as well as ionizing radiation, are often effective treatments not only because they cause cellular injury directly, but also because they induce stress responses as a consequence of damage to the DNA or the cytoskeleton. In many cases, induction of the cellular stress response results in apoptotic cell death, a feature not appreciated until a few years ago. This linkage between cytotoxic therapy, stress signaling and apoptosis may hold the key to a deeper understanding of the cancer cell and may unravel its Achilles’ heel.
Cancer cells are sensitized to stress
An emerging view is that, upon oncogenic transformation, cells activate a stress response primarily as a protective measure. Indeed, oncogenes are well known for their ability to drive cells to senescence or cell death (Evan and Vousden, 2001). In order to survive, transformed cells need to suppress these stress signals. However, this suppression is not complete, since, in the early stages of oncogenesis, the surviving transformed cells may still be sensitized to stress from other sources, including the DNA damage induced by anticancer agents (Benhar et al., 2001; Figure 1). Cells with these properties are referred to as being in the ‘potentiated state’. The cells that ultimately survive to become tumors are further selected to survive adverse environmental conditions such as hypoxia, lack of growth and survival factors or reduced substratum adhesion. Thus, the potentiated state is gradually lost or shielded by anti-apoptotic robustness (Brown and Wouters, 1999). Understanding the molecular events that underlie these phenomena is a great challenge in cancer research. Here, we discuss recent discoveries related to stress signaling in cancer. Specifically, findings on the functions of reactive oxygen species (ROS) and stress-activated kinases are discussed in the framework of the cellular stress response during the evolution of the malignant state (Figure 1). It should be noted that the idea that drugs and radiation kill cancer cells by triggering apoptosis has been recently challenged by the suggestion that, in solid tumors, anticancer treatment induces primarily non-apoptotic cell death (Brown and Wouters, 1999). Nevertheless, the evidence linking apoptosis and therapy (Kaufmann and Earnshaw, 2000; Lowe and Lin, 2000) is sufficient to warrant discussion of the modulation of apoptosis-related signaling pathways in connection with anticancer therapy responsiveness.
Fig. 1. Modulation of apoptosis sensitivity during oncogenesis. Most normal cells are relatively resistant to stress caused by DNA- or cytoskeleton-damaging agents. Following moderate levels of damage, these cells usually initiate cell-cycle arrest, which is succeeded by damage repair. During the process of oncogenic transformation, both proliferative and stress signals are enhanced, leading to sensitization to apoptotic stimuli. This is the basis for the selectivity of anticancer agents towards neoplastic cells. As tumor cells evolve, they undergo stress-driven selection, which finally endows them with anti-apoptotic armor. Thus, advanced tumor cells usually display a high degree of resistance to stress signals.
ROS and cancer
In metazoan cells, ROS are generated by mitochondria (during respiration) and by distinct enzyme systems (Kamata and Hirata, 1999). Low levels of ROS regulate cellular signaling and play an important role in normal cell proliferation (Burdon, 1995; Kamata and Hirata, 1999), and it has been appreciated for a number of years that ROS production is increased in cancer cells (Szatrowski and Nathan, 1991; Burdon, 1995). Constant activation of transcription factors (NF-κB and AP-1) appears to be one functional role of elevated ROS levels during tumor progression (Gupta et al., 1999). Oxidative stress can also induce DNA damage that leads to genomic instability, which may contribute to cancer progression (Jackson and Loeb, 2001). Thus, ROS are thought to play multiple roles in tumor initiation, progression and maintenance. Recently, it was shown that ROS are produced in cells stimulated with growth factors such as EGF and PDGF (Bae et al., 1997, 2000). Moreover, scavenging of extracellular H2O2 by catalase inhibits the proliferation of Her-2/neu-transformed Rat-1 fibroblasts (Preston et al., 2001). ROS generation during mitogenic stimulation may inhibit phosphatases and, in particular, tyrosine phosphatases, facilitating the activation of associated receptor tyrosine kinases (Kamata and Hirata, 1999). Also, Ras mitogenic activity is, in part, superoxide dependent (Irani et al., 1997). Compelling evidence for the transforming capacity of ROS is the finding that overexpression of Mox1 (the catalytic subunit of NADPH oxidase) induces superoxide generation and transforms NIH 3T3 cells. Furthermore, Mox1-transfected cells produce aggressive tumors in athymic mice similar in size to those produced by Ras-transformed NIH 3T3 cells (Suh et al., 1999), illustrating the critical role of this ROS regulator in vivo.
In contrast to their role in promoting cell growth under non-stress conditions, ROS appear to activate and modulate apoptosis when cells are under stress. ROS levels are increased in cells exposed to various stress agents, including anticancer drugs (Jabs, 1999), and they promote apoptosis by stimulating pro-apoptotic signaling molecules, such as ASK1, JNK and p38 (Benhar et al., 2001; Davis et al., 2001; Tobiume et al., 2001). ROS also play a pivotal role in p53-induced apoptosis (Polyak et al., 1997). In addition, ROS can act directly on the apoptotic machinery, by accelerating mitochondrial depolarization and dysfunction during the effector phase of apoptosis (Jabs, 1999).
ROS consist of various radicals, which might exert different effects on cellular signaling. The available data, obtained mostly using pharmacological tools, indicate that mitochondria are a major source of ROS during the course of apoptosis (Cai and Jones, 1999). However, additional research is needed to rigorously determine the sources and identities of ROS produced during cellular stress and to establish the respective contribution of all these in distinct signaling pathways.
Stress-activated MAP kinases and cancer
Mitogen-activated protein kinases (MAPKs) are components of kinase cascades that connect extracellular stimuli to specific transcription factors, thereby converting these signals into cellular responses. In mammalian systems, there are three subgroups of MAPKs: ERKs (extracellular signal-regulated kinases), JNKs (c-Jun N-terminal kinases) and p38 MAPKs. Extensive research has documented the pivotal roles of the ERK subgroup in proliferative responses and of the stress-activated protein kinases (SAPKs) JNK and p38 subgroups in stress responses and in programmed cell death (Lewis et al., 1998; Davis, 2000; Kyriakis and Avruch, 2001).
SAPKs have been studied primarily in the context of stress responses and apoptosis. However, accumulating genetic and biochemical data suggest that SAPKs, and especially the JNK pathway, contribute to proliferative responses in a non-stress setting. For example, JNK1–/– mouse embryonic fibroblasts (MEFs), as well as JNK1–/–JNK2–/– MEFs, proliferate more slowly than do wild-type MEFs and reach a lower saturation density, establishing that JNK is required for normal MEF proliferation (Tournier et al., 2000). JNK and p38 also have been shown to cooperate with ERK in pp60(v-src)-induced cyclin D1 expression in breast cancer cells (Lee et al., 1999). A role for JNK in cancer development is supported by recent studies. In one, it was shown that skin tumorigenesis is suppressed in JNK2-deficient mice (Chen et al., 2001a), and, in another, expression of an inactive variant of the JNK substrate c-Jun (JunAA, lacking JNK phosphorylation sites) in immortalized fibroblasts expressing v-src and v-fos was shown to reduce tumorigenicity in nude mice (Behrens et al., 2000). These and other data (Table I) suggest that signaling through the JNK pathway mediates oncogenic signals and supports cell proliferation in the absence of stress.
Table I. The role of SAPKs in cell proliferation and tumorigenesis.
| Cell type or cancer model | SAPK effect on proliferative response | Reference |
|---|---|---|
| Skin tumors (Papillomas) | Skin tumorigenesis is suppressed in JNK2–/– mice | Chen et al. (2001a) |
| NIH 3T3 | JNK and p38 participate in Cot-induced transformation | Chiariello et al. (2000) |
| NCI-H82 lung carcinoma | JNK mediates Ras-induced transformation | Xiao and Lang (2000) |
| Murine primary embryonic fibroblasts | JNK1–/– and JNK1–/–JNK2–/– MEFs have reduced proliferation potential | Tournier et al. (2000) |
| MCF7 breast cancer | p38 and JNK are involved in pp60(v-src) induction of cyclin D1 | Lee et al. (1999) |
| A549 lung carcinoma | JNK2 is required for EGF-induced transformation | Bost et al. (1999) |
| Fischer rat 3T3 | JNK activity is essential for Met-induced transformation | Rodrigues et al. (1997) |
| HUVEC (human endothelial), 293 and NIH 3T3 | JNK pathway mediates integrin-induced cell-cycle progression | Oktay et al. (1999) |
| T89G human glioblastoma | JNK is essential for cell growth | Potapova et al. (2000) |
| Mast cells | JNK pathway is critical for Kit receptor-induced proliferation | Timokhina et al. (1998) |
| NIH 3T3 | JNK pathway mediates v-Crk-induced transformation | Tanaka et al. (1997) |
| Murine myeloid cells | JNK pathways mediates Bcr-Abl-induced transformation | Raitano et al. (1995) |
Under stress, on the other hand, activation of SAPKs appears to be important in promoting apoptosis in many cell types, including tumor cells and transformed cells in culture. [A detailed discussion of the downstream signaling of SAPKs leading to cell death can be found in Davis (2000).] Current data indicate that signaling through the JNK pathway is important in the activation of the mitochondria-dependent apoptotic pathway (also known as the intrinsic pathway) but dispensable for apoptosis induced by the activation of death receptors (the extrinsic pathway) (Tournier et al., 2000). JNK- and p38-mediated phosphorylation of p53, which augments the p53 response, may also play a role in their pro-apoptotic actions (Fuchs et al., 1998; Sanchez-Prieto et al., 2000).
Recent data have demonstrated that oncogenic transformation can significantly affect SAPK signaling. Comparison of stress signaling between non-transformed NIH 3T3 cells and NIH 3T3 cells that overexpress the epidermal growth factor receptor (EGFR), the Her-2 kinase or oncogenic Ras revealed that SAPK activation was greatly augmented in the transformed cells relative to non-transformed cells in response to various genotoxic agents (Benhar et al., 2001). These findings, together with other studies (Chen et al., 1997; Yu et al., 1997), reveal a state of potentiated stress signaling in oncogenically transformed cells.
ROS and stress kinase signaling
Potentiation of SAPKs in transformed murine cells was shown to be independent of the particular overexpressed proto-oncogene but dependent on ROS, whose production is elevated in these cells (Benhar et al., 2001). These observations were extended to the situation in human cells, where higher ROS levels and SAPK activity were measured in tumor cells that were sensitive to anticancer agents than in those that were drug-resistant (Benhar et al., 2001). Other data further implicate ROS-dependent MAPK activation in regulating the behavior of the transformed cell. In one study, increased ROS levels stimulated MAPK activity in a mouse keratinocyte cell line that had progressed to malignancy (Gupta et al., 1999). Overexpression of manganese superoxide dismutase (MnSOD), in another case, reduced oxidative stress, inhibited the JNK/AP-1 pathway and suppressed tumor formation in a multistage skin carcinogenesis model (Zhao et al., 2001). Redox regulation also appears to be important in SAPK activation under stress (Adler et al., 1999; Davis et al., 2001). For example, it has been demonstrated that ROS function as intermediates in SAPK activation in response to stress agents such as ceramide and anticancer drugs (Mansat-de Mas et al., 1999; Shiah et al., 1999).
Recent studies offer some mechanisms linking ROS and SAPKs. ASK1, an upstream regulator of SAPKs, is inhibited in non-stressed cells through its association with thioredoxin (Saitoh et al., 1998). Increased ROS levels lead to the dissociation of this complex and thereby enable the activation of ASK1 and downstream SAPKs (Liu et al., 2000). A similar redox ‘switch’ has been documented for JNK, with ROS triggering the detachment of JNK associated glutathione-S-transferase-π (GSTp) and thereby facilitating JNK activation (Adler et al., 1999). ROS-dependent activation of JNK may also involve downregulation of a JNK phosphatase (Chen et al., 2001b), and additional, as yet unknown, mechanisms linking ROS and SAPKs undoubtedly exist. For instance, scaffold proteins have been gaining increasing interest as a mechanism for SAPK regulation (Davis, 2000), and the possibility that ROS-induced SAPK activation involves modulation of protein scaffolds merits investigation. All this evidence indicates that ROS do not act within the cell solely to induce random damage, as thought previously. Rather, ROS levels fluctuate in response to intracellular as well as extracellular signals and, in turn, stimulate specific signaling cascades (such as those involving MAPKs) that regulate cell growth and cell death.
ROS and MAP kinases: complex roles in tumor cell behavior
The diverse, and even opposing, effects of ROS on cell behavior outlined above could be explained by the notion that growth is maximally promoted when cells are protected from excessive toxicity but maintain an oxidant signal sufficient for the induction of growth-competence genes. Thus, under optimal growth conditions, elevated ROS levels confer a growth advantage to tumor cells. However, exposure of these cells to damaging agents induces a prolonged increase in ROS levels resulting in potentiation of apoptosis. Hence, these tumor cells are hypersensitive to stress signals. Conversely, in drug-resistant tumors, glutathione or other antioxidant defenses are often upregulated (Shen et al., 1997), shielding cells from apoptosis. As in the case of ROS, the ability of stress kinases to stimulate cell growth or cell death most likely depends on signal intensity and signal duration. This concept has been invoked to explain complex functions of ERK in cell-cycle regulation (Roovers and Assoian, 2000) and in SAPK signaling (Chen et al., 1996). Thus, transient, low-level activity of SAPK promotes cell proliferation, whereas persistent, high-level activity results in cell death.
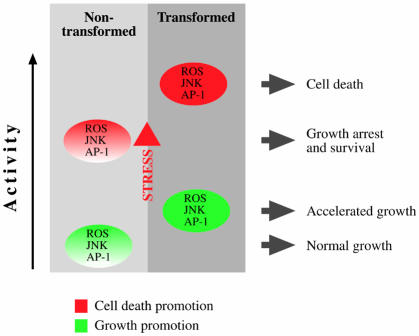
Based on the studies reviewed above, we propose the following model that explains the sensitized state of tumor cells (Figure 2). In non-transformed cells, ROS levels and SAPK activity are relatively low. The process of oncogenic transformation leads to an elevation in the basal levels of ROS (under non-stress conditions) and to a potentiation of SAPK activity. This intermediate state facilitates mitogenic signaling through the activation of AP-1 or through other mechanisms discussed above. Upon exposure of cells to stress stimuli such as anticancer agents, ROS levels and SAPK activity are elevated. In the transformed cells, where basal stress signaling is the potentiated state, additional stress stimuli result in substantially higher levels of ROS and SAPK activity, which, in turn, augment the apoptotic response of these cells. This model can be extended to the circumstances of the advanced cancer, where the situation is reversed. In other words, following many generations of selection, ROS levels decrease (due to elevated antioxidant activity) and SAPK activity is suppressed, conferring greater stress resistance upon these cells. This notion implies that active SAPKs can suppress cancer progression to advanced stages. Indeed, SEK1 (MKK4), the upstream activator of JNK, has been implicated as a prostate cancer metastasis suppressor gene (Yoshida et al., 1999). Furthermore, the expression of MKP-1, a JNK phosphatase, is elevated in prostate cancer (Magi-Galluzzi et al., 1997), and the hypoxic conditions found in the environment of solid tumors also induce MKP-1 expression, thereby inhibiting JNK activity (Laderoute et al., 1999). These data indicate that the progression of tumor cells towards the metastatic state is characterized by suppression of the JNK pathway.
Fig. 2. A model that illustrates how the dysregulation of ROS and SAPKs in transformed cells may set the cellular response to anticancer agents. In non-neoplastic cells, ROS and SAPK activities are usually maintained at low levels (green). Following exposure to stress stimuli, ROS and SAPK activities are induced (red), leading to cell-cycle arrest, repair and survival. During oncogenic transformation, ROS and SAPK activities rise to levels that support cell growth (green). In these rapidly proliferating cells, exposure to stress results in an amplified stress response (further increase in ROS and SAPKs) that induces cell death (red). In advanced cancer (not shown), ROS/SAPK signaling is suppressed to confer protection from death signals (see text for details). Consequently, advanced tumor survival is maintained even when cells experience stress and damage.
Anti-apoptotic signaling and stress: therapeutic implications
Whereas the potentiated stress state is readily observed in some tumor cells, in other cases it is masked by the activation of anti-apoptotic signaling. If this shield could be removed, the potentiated stress state might be regained, with the cells becoming re-sensitized to death signals. A number of examples imply that this can be achieved. For example, non-small-cell lung carcinoma cells become more resistant to cytotoxic agents such as cisplatin, doxorubicin and etoposide as the level of Her-2/neu expression is elevated, but upon blockage of Her-2/neu signaling the cells become re-sensitized to these agents (Tsai et al., 1996). Similarly, the overexpression of a mutated EGFR in advanced glioma correlates with resistance to cisplatin due to reduced apoptosis, and blocking EGFR signaling in these cells restores their cisplatin sensitivity (Nagane et al., 1998). Blocking Her-2 with Herceptin (Trastuzumab) sensitizes highly drug-resistant breast cancer cells to cytotoxic drugs (Pegram et al., 2000). In fact, the approach of ‘chemo-signal therapy’, namely, the combination of chemotherapy with cellular response modifiers, has shown promising results in clinical trials (Pusztai et al., 1999). How these combined therapies modulate ROS and stress kinase pathways at the cellular level has not been determined. Yet, in prostate cancer cells, inhibition of HER-2/neu signaling triggers p38 activation and apoptosis (Murillo et al., 2001).
In summary, both ROS metabolism and MAP kinase signaling are dysregulated in cancer. In this review, we have discussed recent findings illustrating that ROS and SAPKs affect each other and together play an important role in determining the cells’ responsiveness to apoptotic signals. The study of stress signaling has proved to be instructive in explaining recent advances in cancer therapy and should facilitate the development of improved anticancer strategies.

Moran Benhar, Moran Benhar & Alexander Levitzki
References
- Adler V., Yin, Z., Tew, K.D. and Ronai, Z. (1999) Role of redox potential and reactive oxygen species in stress signaling. Oncogene, 18, 6104–6111. [DOI] [PubMed] [Google Scholar]
- Bae Y.S., Kang, S.W., Seo, M.S., Baines, I.C., Tekle, E., Chock, P.B. and Rhee, S.G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem., 272, 217–221. [PubMed] [Google Scholar]
- Bae Y.S., Sung, J.Y., Kim, O.S., Kim, Y.J., Hur, K.C., Kazlauskas, A. and Rhee, S.G. (2000) Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem., 275, 10527–10531. [DOI] [PubMed] [Google Scholar]
- Behrens A., Jochum, W., Sibilia, M. and Wagner, E.F. (2000) Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene, 19, 2657–2663. [DOI] [PubMed] [Google Scholar]
- Benhar M., Dalyot, I., Engelberg, D. and Levitzki, A. (2001) Enhanced ROS production in oncogenically transformed cells potentiates c-jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol. Cell. Biol., 21, 6913–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F., McKay, R., Bost, M., Potapova, O., Dean, N.M. and Mercola, D. (1999) The Jun kinase 2 isoform is preferentially required for epidermal growth factor-induced transformation of human A549 lung carcinoma cells. Mol. Cell. Biol., 19, 1938–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M. and Wouters, B.G. (1999) Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res., 59, 1391–1399. [PubMed] [Google Scholar]
- Burdon R.H. (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med., 18, 775–794. [DOI] [PubMed] [Google Scholar]
- Cai J. and Jones, D.P. (1999) Mitochondrial redox signaling during apoptosis. J. Bioenerg. Biomembr., 31, 327–334. [DOI] [PubMed] [Google Scholar]
- Chen G., Shu, J. and Stacey, D.W. (1997) Oncogenic transformation potentiates apoptosis, S-phase arrest and stress-kinase activation by etoposide. Oncogene, 15, 1643–1651. [DOI] [PubMed] [Google Scholar]
- Chen N., Nomura, M., She, Q.B., Ma, W.Y., Bode, A.M., Wang, L., Flavell, R.A. and Dong, Z. (2001a) Suppression of skin tumorigenesis in c-Jun NH2-terminal kinase-2-deficient mice. Cancer Res., 61, 3908–3912. [PubMed] [Google Scholar]
- Chen Y.R., Wang, X., Templeton, D., Davis, R.J. and Tan, T.H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem., 271, 31929–31936. [DOI] [PubMed] [Google Scholar]
- Chen Y.R., Shrivastava, A. and Tan, T.H. (2001b) Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene, 20, 367–374. [DOI] [PubMed] [Google Scholar]
- Chiariello M., Marinissen, M.J. and Gutkind, J.S. (2000) Multiple mitogen-activated protein kinase signaling pathways connect the cot oncoprotein to the c-jun promoter and to cellular transformation. Mol. Cell. Biol., 20, 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Davis W. Jr, Ronai, Z. and Tew, K.D. (2001) Cellular thiols and reactive oxygen species in drug-induced apoptosis. J. Pharmacol. Exp. Ther., 296, 1–6. [PubMed] [Google Scholar]
- Evan G.I. and Vousden, K.H. (2001) Proliferation, cell cycle and apoptosis in cancer. Nature, 411, 342–348. [DOI] [PubMed] [Google Scholar]
- Fuchs S.Y., Adler, V., Pincus, M.R. and Ronai, Z. (1998) MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl Acad. Sci. USA, 95, 10541–10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Rosenberger, S.F. and Bowden, G.T. (1999) Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis, 20, 2063–2073. [DOI] [PubMed] [Google Scholar]
- Irani K., Xia, Y., Zweier, J.L., Sollott, S.J., Der, C.J., Fearon, E.R., Sundaresan, M., Finkel, T. and Goldschmidt-Clermont, P.J. (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science, 275, 1649–1652. [DOI] [PubMed] [Google Scholar]
- Jabs T. (1999) Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol., 57, 231–245. [DOI] [PubMed] [Google Scholar]
- Jackson A.L. and Loeb, L.A. (2001) The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat. Res., 477, 7–21. [DOI] [PubMed] [Google Scholar]
- Kamata H. and Hirata, H. (1999) Redox regulation of cellular signalling. Cell Signal., 11, 1–14. [DOI] [PubMed] [Google Scholar]
- Kaufmann S.H. and Earnshaw, W.C. (2000) Induction of apoptosis by cancer chemotherapy. Exp. Cell Res., 256, 42–49. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch, J. (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev., 81, 807–869. [DOI] [PubMed] [Google Scholar]
- Laderoute K.R., Mendonca, H.L., Calaoagan, J.M., Knapp, A.M., Giaccia, A.J. and Stork, P.J. (1999) Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is induced by low oxygen conditions found in solid tumor microenvironments. A candidate MKP for the inactivation of hypoxia-inducible stress-activated protein kinase/c-Jun N-terminal protein kinase activity. J. Biol. Chem., 274, 12890–12897. [DOI] [PubMed] [Google Scholar]
- Lee R.J. et al. (1999) pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J. Biol. Chem., 274, 7341–7350. [DOI] [PubMed] [Google Scholar]
- Lewis T.S., Shapiro, P.S. and Ahn, N.G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res., 74, 49–139. [DOI] [PubMed] [Google Scholar]
- Liu H., Nishitoh, H., Ichijo, H. and Kyriakis, J.M. (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol., 20, 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S.W. and Lin, A.W. (2000) Apoptosis in cancer. Carcinogenesis, 21, 485–495. [DOI] [PubMed] [Google Scholar]
- Magi-Galluzzi C. et al. (1997) Mitogen-activated protein kinase phosphatase 1 is overexpressed in prostate cancers and is inversely related to apoptosis. Lab. Invest., 76, 37–51. [PubMed] [Google Scholar]
- Mansat-de Mas V., Bezombes, C., Quillet-Mary, A., Bettaieb, A., D’Orgeix, A.D., Laurent, G. and Jaffrezou, J.P. (1999) Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol. Pharmacol., 56, 867–874. [DOI] [PubMed] [Google Scholar]
- Murillo H., Schmidt, L.J. and Tindall, D.J. (2001) Tyrphostin AG825 triggers p38 mitogen-activated protein kinase-dependent apoptosis in androgen-independent prostate cancer cells C4 and C4-2. Cancer Res., 61, 7408–7412. [PubMed] [Google Scholar]
- Nagane M., Levitzki, A., Gazit, A., Cavenee, W.K. and Huang, H.J. (1998) Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc. Natl Acad. Sci. USA, 95, 5724–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay M., Wary, K.K., Dans, M., Birge, R.B. and Giancotti, F.G. (1999) Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol., 145, 1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram M.D., Lopez, A., Konecny, G. and Slamon, D.J. (2000) Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin. Oncol., 27, 21–25; discussion 92–100. [PubMed] [Google Scholar]
- Polyak K., Xia, Y., Zweier, J.L., Kinzler, K.W. and Vogelstein, B. (1997) A model for p53-induced apoptosis. Nature, 389, 300–305. [DOI] [PubMed] [Google Scholar]
- Potapova O., Gorospe, M., Bost, F., Dean, N.M., Gaarde, W.A., Mercola, D. and Holbrook, N.J. (2000) c-Jun N-terminal kinase is essential for growth of human T98G glioblastoma cells. J. Biol. Chem., 275, 24767–24775. [DOI] [PubMed] [Google Scholar]
- Preston T.J., Muller, W.J. and Singh, G. (2001) Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol. Chem., 276, 9558–9564. [DOI] [PubMed] [Google Scholar]
- Pusztai L., Esteva, F.J., Cristofanilli, M., Hung, M.C. and Hortobagyi, G.N. (1999) Chemo-signal therapy, an emerging new approach to modify drug resistance in breast cancer. Cancer Treat. Rev., 25, 271–277. [DOI] [PubMed] [Google Scholar]
- Raitano A.B., Halpern, J.R., Hambuch, T.M. and Sawyers, C.L. (1995) The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc. Natl Acad. Sci. USA, 92, 11746–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G.A., Park, M. and Schlessinger, J. (1997) Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J., 16, 2634–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers K. and Assoian, R.K. (2000) Integrating the MAP kinase signal into the G1 phase cell cycle machinery. BioEssays, 22, 818–826. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono, K. and Ichijo, H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J., 17, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Prieto R., Rojas, J.M., Taya, Y. and Gutkind, J.S. (2000) A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res., 60, 2464–2472. [PubMed] [Google Scholar]
- Shen H., Kauvar, L. and Tew, K.D. (1997) Importance of glutathione and associated enzymes in drug response. Oncol. Res., 9, 295–302. [PubMed] [Google Scholar]
- Shiah S.G., Chuang, S.E., Chau, Y.P., Shen, S.C. and Kuo, M.L. (1999) Activation of c-Jun NH2-terminal kinase and subsequent CPP32/Yama during topoisomerase inhibitor β-lapachone-induced apoptosis through an oxidation-dependent pathway. Cancer Res., 59, 391–398. [PubMed] [Google Scholar]
- Suh Y.A., Arnold, R.S., Lassegue, B., Shi, J., Xu, X., Sorescu, D., Chung, A.B., Griendling, K.K. and Lambeth, J.D. (1999) Cell transformation by the superoxide-generating oxidase Mox1. Nature, 401, 79–82. [DOI] [PubMed] [Google Scholar]
- Szatrowski T.P. and Nathan, C.F. (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res., 51, 794–798. [PubMed] [Google Scholar]
- Tanaka S., Ouchi, T. and Hanafusa, H. (1997) Downstream of Crk adaptor signaling pathway: activation of Jun kinase by v-Crk through the guanine nucleotide exchange protein C3G. Proc. Natl Acad. Sci. USA, 94, 2356–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timokhina I., Kissel, H., Stella, G. and Besmer, P. (1998) Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J., 17, 6250–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K. et al. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO rep., 2, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier C. et al. (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science, 288, 870–874. [DOI] [PubMed] [Google Scholar]
- Tsai C.M., Levitzki, A., Wu, L.H., Chang, K.T., Cheng, C.C., Gazit, A. and Perng, R.P. (1996) Enhancement of chemosensitivity by tyrphostin AG825 in high-p185(neu) expressing non-small cell lung cancer cells. Cancer Res., 56, 1068–1074. [PubMed] [Google Scholar]
- Xiao L. and Lang, W. (2000) A dominant role for the c-Jun NH2-terminal kinase in oncogenic ras-induced morphologic transformation of human lung carcinoma cells. Cancer Res., 60, 400–408. [PubMed] [Google Scholar]
- Yoshida B.A., Dubauskas, Z., Chekmareva, M.A., Christiano, T.R., Stadler, W.M. and Rinker-Schaeffer, C.W. (1999) Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res., 59, 5483–5487. [PubMed] [Google Scholar]
- Yu K., Ravera, C.P., Chen, Y.N. and McMahon, G. (1997) Regulation of Myc-dependent apoptosis by p53, c-Jun N-terminal kinases/stress-activated protein kinases, and Mdm-2. Cell Growth Differ., 8, 731–742. [PubMed] [Google Scholar]
- Zhao Y., Xue, Y., Oberley, T.D., Kiningham, K.K., Lin, S.M., Yen, H.C., Majima, H., Hines, J. and St Clair, D. (2001) Overexpression of manganese superoxide dismutase suppresses tumor formation by modulation of activator protein-1 signaling in a multistage skin carcinogenesis model. Cancer Res., 61, 6082–6088. [PubMed] [Google Scholar]