Abstract
Objective:
The objective of the review was to synthesize the evidence on the effectiveness of lifestyle-based interventions in mitigating absolute cardiovascular disease (CVD) risk.
Introduction:
Evidence-based guidelines recommend employing an absolute CVD risk score to inform the selection and intensity of preventive interventions. However, studies employing this strategy have reported mixed results, hence the need for a systematic review of the current evidence.
Inclusion criteria:
Studies published in English including a lifestyle-based intervention to mitigate CVD risk that enrolled individuals aged ≥18 years, with no history of CVD at baseline were considered. The primary outcome was change in absolute CVD risk score post-intervention.
Methods:
PubMed, Embase, and CINAHL searches were conducted from database inception to February 2022. The trial registers searched included Cochrane Central Register of Controlled Trials (CENTRAL) and ClinicalTrials.gov. Searches for unpublished studies/gray literature were conducted in ProQuest Dissertations and Theses Global, GreyLit Report, and OCLC First Search Proceedings. Two independent reviewers selected the studies and critically appraised them for methodological quality using JBI tools. Data extraction was performed for main outcome variables. Data were presented using separate pooled statistical meta-analysis for quasi-experimental and randomized clinical trials. Random effects models were employed in the analyses. Effect sizes (Cohen’s d) were expressed as standardized mean difference at 95% CI. Heterogeneity was assessed via Cochran’s Q statistic, and the inconsistency index (I2) was used to describe variability in effect estimates due to heterogeneity rather than sampling error.
Results:
Twenty-nine studies with a total sample of 5490 adults free of CVD at baseline were included: Fifteen were RCTs (n=3605), and 14 quasi-experimental studies (n=1885). The studies were conducted in the United States (n=5), Canada (n=1), Europe (n=18), Asia (n=3), Mexico (n=1), and Australia (n=1) and included the following lifestyle interventions: diet, physical activity, motivational interviewing, problemsolving, psychological counseling, cardiovascular risk assessment and feedback, health self-management education, and peer support. Six validated absolute CVD risk assessment tools were used to measure the study outcomes including Framingham, SCORE, Heart Health Risk Assessment Score, Dundee, ASSIGN, and The UK Prospective Diabetes Study risk score. Overall, the methodological rigor of the RCTs and quasi-experimental studies was high. Of the 15 RCTs included in the meta-analysis, lifestyle intervention was favored over control in reducing absolute CVD risk score (p=0.032; Cohen’s d = −0.39; Z= −2.14; I2 = 96). Similarly, in the 14 quasi-experimental studies, the absolute CVD risk score after lifestyle intervention was significantly lower compared to baseline (p<0.001; Cohen’s d = −0.39; Z= −3.54; I2 = 88). RCTs that combined diet and physical activity reported no significant impact on absolute CVD risk score, but those that used either intervention independently reported significant improvement in the absolute CVD risk score.
Conclusions:
There is evidence supporting the positive impact of lifestyle modification on absolute CVD risk score in adult populations free of CVD. Our analysis further suggests that diet and physical activity had significant impact on absolute CVD risk, and a variety of validated screening tools can be used to monitor, evaluate, and communicate changes in absolute risk score after lifestyle modification.
Keywords: absolute CVD risk assessment, cardiovascular disease, cardiovascular risk reduction, lifestyle modification
Summary of findings
| The impact of lifestyle-based interventions on absolute cardiovascular disease risk |
| Should lifestyle-based interventions vs. usual care be used to mitigate absolute CVD risk in adults? |
|
Bibliography: Kariuki, J. K., Imes, C. C., Engberg, S. J, Scott, P., Klem, M.L., Yamnia, C. I. The impact of lifestyle-based interventions on absolute cardiovascular disease risk |
| Outcomes | Anticipated absolute effects* (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
| Standardized mean difference with lifestyle-based interventions | |||
| Absolute CVD risk score assessed with validated risk assessment algorithms Scale from: 0 to 100 follow up: range 12 weeks to 18 months |
SMD 0.39 SD lower (0.74 lower to 0.03 lower) | 3605 (15 RCTs) | ⨁⨁⨁O Moderatea |
| Absolute CVD risk score assessed with validated risk assessment algorithms Scale from: 0 to 100 follow up: range 3 weeks to 16 years |
SMD 0.39 SD lower (0.60 lower to 0.17 lower) |
1885 (14 Quasi-experimental studies) | ⨁⨁OO Lowb,c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; SMD: Standardized mean difference |
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: True effect may be substantially different from the estimate the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect Explanations
|
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability globally.1,2 In 2019, there were 18.6 million deaths and 34.4 million years lived with disability attributable to CVD globally.3 In the United States, CVD is responsible for over 860,000 deaths annually and about $360 billion in direct and indirect costs.4 Although significant gains in reducing CVD mortality have been made in the last 5 decades,5 the rate of decline has been low among racial/ethnic minorities,1 and individuals under 65 years.6 To address the disparities in CVD outcomes, evidence based primary prevention strategies are needed. Current guidelines recommend screening for CVD risk in asymptomatic individuals and initiating risk mitigation interventions among those at high-risk of incident CVD.7,8 Lifestyle interventions such as physical activity and a healthy diet play a pivotal role in primary prevention of CVD, and are efficacious in managing multiple CVD risk factors including hypertension, dyslipidemia, and obesity.7
In recent years, major professional associations including the American Heart Association, American College of Cardiology, European Society of Cardiology, and the World Health Organization have issued CVD prevention guidelines recommending screening for CVD and tailoring preventive interventions based on absolute risk metrics rather than the traditional focus on individual risk factors.9–11 The absolute CVD risk assesses the likelihood of an individual developing CVD within a given time frame considering the impact of co-occuring risk factors.10 To estimate the absolute CVD risk, various risk assessment algorithms are used to compute absolute CVD risk scores, which aggregates the total impact of the present risk factors.12 The scores (0–100%) estimate the cumulative impact of multiple CVD risk factors that are detected during screening.10,12 High absolute CVD risk scores are associated with high odds of a CVD event occuring.9 The absolute CVD risk score concept is increasingly being used in clinical setings for early detection and for tailoring the choice and intensity of preventive lifestyle interventions.11 In some populations bearing a high burden of CVD (eg, African Americans), about 90% of all CVD events are predicted by elevated or borderline risk factors.1 The cumulative impact of co-occuring risk factors can be easily missed when the focus is on individual risk factor cut points rather than absolute risk.10
The clinical value of the absolute CVD risk approach is demonstrated by the clinical case study featuring Mr. Hue, a 64-year-old African American male. He is a nonsmoker who is not on any medications, and with no history of hypertension or diabetes. He presents with a blood pressure of 128/78 mmHg, body mass index (BMI) of 29.5 kg/m2, high-density lipoprotein (HDL) cholesterol of 1.04 mmol/L, low-density lipoprotein (LDL) cholesterol of 3.88 mmol/L, and total cholesterol of 5.62 mmol/L. Based on individual CVD risk factors (eg, lipids, blood pressure), Mr. Hue does not meet the typical cut points for initiating the respective treatments. However, his 10-year absolute CVD risk, calculated via the non-laboratory based Framingham risk algorithm,13 is 21.7%, while the Pooled Cohort Equations9 depicts a 10.4% risk profile. The Framingham algorithm predicts incident general CVD while the Pooled Cohort Equations focus is on hard atherosclerotic CVD (ASCVD) events.9,13 Both scores are over the low risk threshold (<7.5) and qualify him for risk reduction interventions.9,11,14
Over the years, various risk assessment tools have been developed to estimate absolute CVD risk scores. The risk scores have been used to evaluate the impact of various CVD risk reduction strategies. Lifestyle-based interventions targeting major CVD risk factors, including physical inactivity, poor diet, smoking, and stress, have, in some studies, shown to be effective in reducing absolute CVD risk score in adults at high-risk for CVD.15–17 Other studies have reported no significant improvements or between-group differences after the intervention.18,19
The heterogeneous findings on the impact of lifestyle modification on absolute CVD risk call for a systematic review to examine the current state and strength of evidence. The review should take into account that there are ≥360 algorithms designed to estimate absolute CVD risk, with significant differences in the number and type of risk factors/covariates used to compute the risk scores.20 Also, the algorithms focus on predicting different CVD outcomes (eg, general vs hard atherosclerotic cardiovascular disease [ASCVD] events) with varying time horizons (eg, 10 or 30 years).12 The multiplicity of these tools can be overwhelming, thus only algorithms with high sensitivity/specificity and external validity are recommended for clinical use.20
This systematic review synthesizes the current evidence on the impact of lifestyle-based CVD risk reduction interventions on absolute CVD risk score in studies that employed validated risk assessment algorithms. Studies that employed comparable designs (eg, randomized clinical trials), interventions (eg, physical activity), and absolute CVD risk assessment tools (eg, Framingham) were analyzed and pooled together. These pooled data inform our discussion on the strength of evidence regarding the effectiveness of lifestyle-based interventions in reducing absolute CVD risk in adult populations. The search for existing systematic reviews on the topic was conducted in November 2016 and updated in October 2018 and February 2022. The databases searched included Cochrane Library: Cochrane Reviews, JBI Database of Systematic Reviews and Implementation Reports (now JBI Evidence Synthesis), PubMed, Excerpta Medica Database (EMBASE), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and PROSPERO. No published or in-progress systematic reviews on the topic were found.
Review question
What impact does lifestyle modification have on absolute CVD risk in adult populations with no history of CVD?
Inclusion criteria
Participants
Studies that enrolled individuals who were ≥18 years of age, with no history of CVD at baseline were considered. No considerations were made for gender, ethnicity, or socioeconomic status.
We acknowledge a minor deviation from our published protocol. We intended to include only studies that enrolled individuals at high risk of CVD. However, it was not possible to identify a uniform absolute CVD risk score threshold for the high-risk status due to inherent variations in the absolute CVD risk screening tools that were employed in different studies.
Interventions
This review considered studies where lifestyle modification was used as a strategy to reduce CVD risk and no pharmacotherapeutics were used as part of the intervention. The lifestyle modification strategies employed included: diet, physical activity, motivational interviewing, problemsolving, psychological counseling, cardiovascular risk assessment and feedback, health education on self-management, and peer support. These interventions were used independently or as a combination of multiple strategies in one intervention.
Comparators
This review considered studies that compared lifestyle-based interventions to usual care or no intervention, and quasi-experimental studies that examined absolute CVD risk after a lifestyle-based intervention. All lifestyle-based interventions were considered for this review with no eligibility requirements on frequency, intensity, duration, or delivery method. Usual care was delivered based on prevailing clinical practices at the time each study was conducted and patient education materials (eg, handouts focusing on diet and physical activity with no educational support from the investigators).
Outcomes
This review considered studies that measured absolute CVD risk using validated algorithms. The validated risk assessment algorithms used in the included studies were Framingham,21 SCORE,22 Heart Health Risk Assessment Score,23 Dundee,24 ASSIGN,25 and the UK Prospective Diabetes Study (UKPDS) risk score.26 The outcomes were categorized by study methodology, with RCTs and quasi-experimental studies pooled separately.
Types of studies
This review considered both experimental and quasi-experimental study designs, including RCTs, non-RCTs, before and after studies, and interrupted time-series studies.
Methods
The systematic review was conducted following the JBI methodology for systematic reviews of effectiveness.27 The review was registered in PROSPERO (CRD42017073543) and was conducted in accordance with an a priori protocol.28
Search strategy
A 3-step search strategy was employed to identify published and unpublished studies. An initial search was performed in PubMed and Embase, followed by a review of the keywords used in the title and abstract, and of the index terms used to describe the articles. These data were used to inform the second search strategy that included the identified keywords and index terms tailored for each database included in the review. The full search strategies are provided in Appendix I. The search terms used in all databases were broad to allow for an inclusive list of results to review. In the final step, the bibliographies of the studies selected for critical appraisal were reviewed to identify more studies.
Searches in PubMed, Embase, and CINAHL (EBSCO) were conducted from the inception to February 2022. The search strategies included natural language and standardized terms for each database: EMTREE for Embase, Medical Subject Headings (MeSH) for PubMed, and CINAHL headings. The trial registers searched included: Cochrane Central Register of Controlled Trials (CENTRAL) and ClinicalTrials.gov. Searches for unpublished studies/gray literature were conducted in ProQuest Dissertations and Theses Global, Grey Literature Report, OCLC First Search Proceedings (Proceedings database), Web of Science, and BIOSIS Previews.
Only studies published in English were included due to lack of resources to translate and process articles written in other languages. No date limits were employed to maximize the scope of the review, but since the first absolute CVD risk score was derived from the Framingham database in 1976,29 the studies included in this analysis reflect this timeline.
Study selection
The citations identified during the literature search were collated and uploaded into DistillerSR (DistillerSR. Version 2.35. DistillerSR Inc.; 2023. Accessed May-June 2023. https://www.distillersr.com), where duplicates were removed. Titles and abstracts were screened by 2 independent reviewers for suitability guided by the inclusion criteria. The full text of studies meeting the inclusion criteria were retrieved and imported into the JBI System for the Unified Management, Assessment and Review of Information (JBI SUMARI; JBI, Adelaide, Australia). The full texts of articles were further reiviewed by 2 independent reviewers and those that did not meet the inclusion criteria were excluded (reasons are provided in Appendix II). Disagreements between reviewers were resolved through discussion or with a third reviewer. The search and selction process is outlined in a Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow driagram (Figure 1).30
Figure 1:
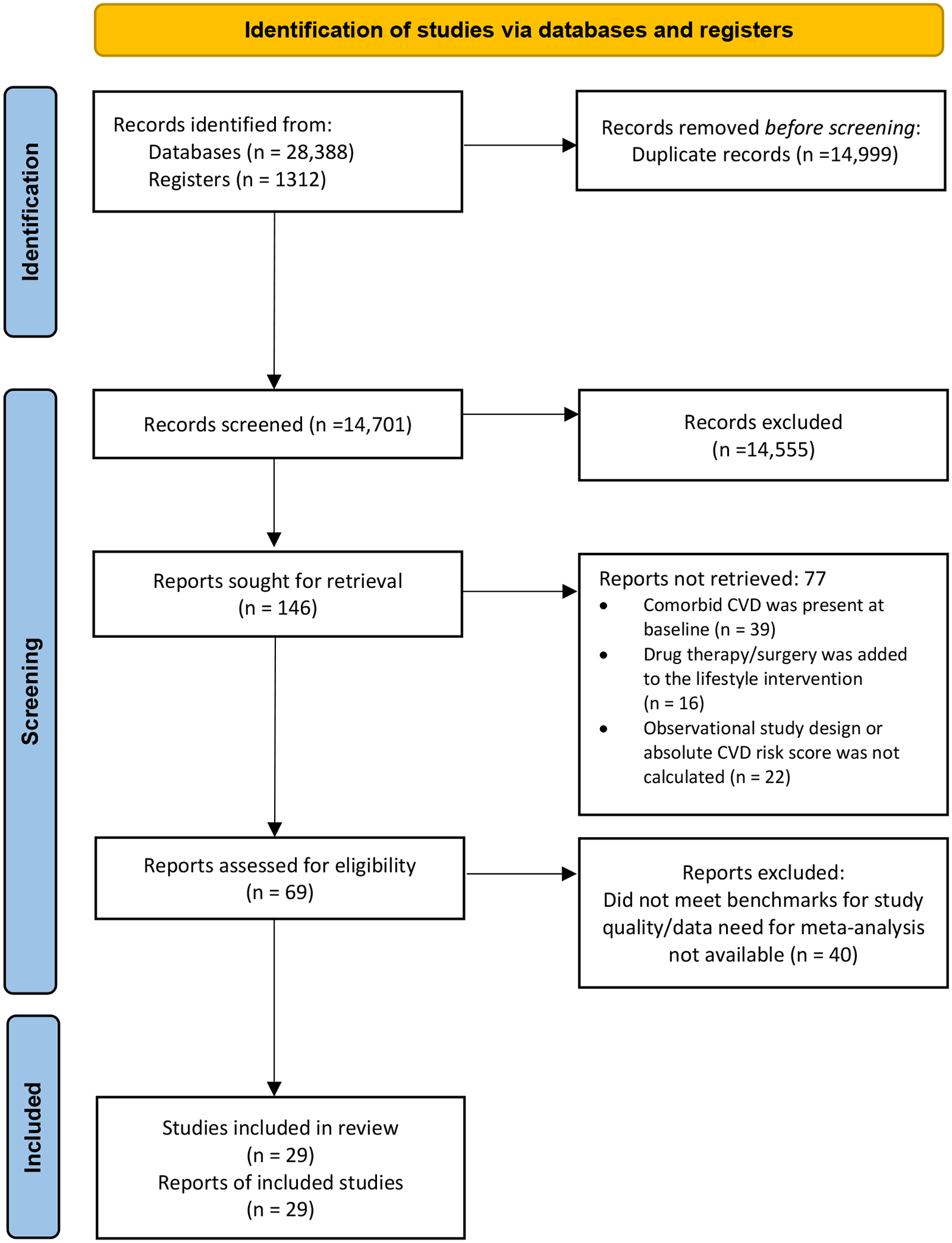
Search results and and study selection process38
CVD, cardiovascular disease
Assessment of methodological quality
Two independent reviewers critically appraised the studies that met the inclusion criteria for methodological quality using the appropriate JBI tools for quasi-experimental studies and randomized controlled trials (RCT).31 The RCT criteria 3, 6, 7, 9, 10, and 11 and quasi-experimental criteria 1, 2, 3, 5, 7, and 8 were considered a priori to be essential for methodological rigor of their respective studies.28 Therefore, only studies that met these criteria were included in the final analyses. Disagreements between reviewers on methodological quality were resolved through discussion, or with the help of a third reviewer. In instances where the data or study findings were not clear, the corresponding author was contacted for clarification.
Data extraction
Data extraction was performed by 2 independent reviewers using the standardized data extraction tool available in JBI SUMARI (JBI SUMARI; JBI, Adelaide, Australia). The extracted data included the type of interventions, target population, study design, and primary outcome (absolute CVD risk score). Any disagreements between the reviewers on data extraction were resolved through discussion or with a third reviewer.
Data synthesis
Studies were pooled in statistical meta-analysis using JBI SUMARI (JBI SUMARI; JBI, Adelaide, Australia) for the primary outcome (absolute CVD risk score). Data were presented using separate pooled statistical meta-analysis for quasi-experimental and randomized clinical trials. In RCTs, the synthesis entailed pooling and comparing the end-of-study mean absolute CVD risk scores of experimental groups that received lifestyle interventions vs control groups that received usual care or no interventon. In instances where a study included more than 2 comparative groups in a trial (eg, a 3-arm trial with diet, exercise, and control groups), we selected the intervention that was associated with the greatest impact on absolute CVD risk score. This was done to avoid double counting of control group participants, which could introduce a unit of analysis error in our analysis. In subanalysis that pooled RCTs that employed comparable lifestyle interventions, each intervention arm was included in its respective category without any impact on data independence. For quasi-experimental studies, the synthesis included pooling and comparing the absolute CVD risk score means before and after the lifestyle interventions. Further analyses were conducted to pool studies that employed similar interventions and study design (eg, RCTs focusing on diet), and similar absolute CVD risk assessment tools (eg, Framingham algorithm) together. Since we had a priori knowledge about the heterogeneity of the CVD risk assessment algorithms employed in the studies that were included in this review, random effects models were employed in the analysis as recommended by Haidich.32 Effect sizes (Cohen’s d) were expressed as standardized mean difference at 95% CI when different CVD risk assessment algorithms were used, and as mean differences when similar algorithms were employed in the pooled studies. Heterogeneity was assessed via Cochran’s Q statistic,32,33 and an inconsistency index (I2) was used to describe variability in effect estimates due to heterogeneity rather than sampling error.34
We acknowledge a deviation from our published protocol by not including secondary outcomes (ie, changes in individual CVD risk factors) in our results. As we conducted the review, we found a wide variation in individual CVD risk factors that were examined across the studies. Additionally, a significant number of studies did not report changes in individual CVD risk factors. These limitations made it difficult for us to aggregate the secondary outcomes.
Assessing certainty in the findings
A Summary of Findings was created using GRADEPro GDT software 2015 (McMaster University, ON, Canada).35 We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach36,37 to evaluate the quality of evidence. GRADE assesses certainty using 5 domains: risk of bias, imprecision, inconsistency, imprecision, and publication bias. The included Summary of Findings presents absolute risks for treatment and control groups, and ranks the quality of the evidence based on the GRADE domains. The outcomes included in Summary of Findings were the impact of lifestyle-based interventions on absolute CVD risk score.
Results
Study inclusion
The total number of records identified through database searching and other sources included 13,241 articles in PubMed, 14,450 articles in Embase, 401 articles in CINAHL, 296 articles in Cochrane Library, 100 articles in ProQuest Dissertations and Theses, 474 articles in ClinicalTrials.gov, 367 articles in Grey Literature Report, and 371 articles in OCLC First Search Proceedings (Figure 1). No relevant articles were identified in the Web of Science and BIOSIS Previews. After the removal of duplicates, 14,701 articles were screened by title according to the inclusion criteria, which resulted in exclusion of an additional 14,555 articles. The remaining 146 articles were subjected to abstract screening, where 77 articles were excluded. This was followed by full-text screening, which resulted in the exclusion of an additional 41 articles based on eligibility requirements and critical appraisal for methodological rigor (Appendix II) using the criteria outlined in the protocol.28 The final 29 articles were eligible for data extraction.
Methodological quality
The 29 studies included in the review met the quality benchmarks outlined in the criteria selected from the JBI critical appraisal checklists. Fourteen studies39–52 met quasi-experimental criteria 1, 2, 3, 5, 7, and 8 (Table 1), while the remaining 15 studies53–67 met RCT criteria 3, 6, 7, 9, 10, and 11 (Table 2). In the study protocol,28 these criteria were deemed to be critical in ensuring the methodological rigor of the studies included in the review. In instances where adequate data to evaluate the criteria was not included in the article, we contacted the corresponding author for more information. In instances where we did not hear back from the authors, the RCT and quasi-experimental criteria are marked as unclear. Only the criteria that were applicable to all studies in a specific category were employed as a benchmark of quality. Overall, the methodological rigor of the RCTs and quasi-experimental studies was high.
Table 1:
Critical appraisal of quasi-experimental studies
| Citation | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 |
|---|---|---|---|---|---|---|---|---|---|
| Ahn and Kim,48 2020 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| al Mheid et al.,39 2016 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Baldwin,52 2015 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Bernocchi et al.,49 2011 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Chan et al.,47 2012 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Kemmler et al.,44 2016 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Kim et al.,42 2011 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Lazarevic et al.,43 2008 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Price et al.,40 2000 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Richardson et al.,50 2008 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Ródenas et al.,45 2005 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Sartorio et al.,46 2001 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Widmer et al.,41 2014 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Siren et al.,51 2016 | Y | Y | Y | N/A | Y | Y | Y | Y | Y |
| Total % | 100 | 100 | 100 | N/A | 100 | 100 | 100 | 100 | 100 |
Y, yes; N, no; U, unclear; N/A, not applicable.
JBI critical appraisal checklist for quasi-experimental studies
Q1 Is it clear in the study what is the ‘cause’ and what is the ‘effect’ (i.e., there is no confusion about which variable comes first)?
Q6 Was follow-up complete and if not, were differences between groups in terms of their follow-up adequately described and analyzed?
Q2 Were the participants included in any comparisons similar?
Q7 Were the outcomes of participants included in any comparisons measured in the same way?
Q3 Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
Q8 Were outcomes measured in a reliable way?
Q4 Was there a control group?
Q9 Was appropriate statistical analysis used?
Q5 Were there multiple measurements of the outcome both pre and post the intervention/exposure?
Table 2:
Critical appraisal for randomized controlled trials
| Citation | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand, et al.,53 2016 | Y | Y | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y |
| Balducci, et al.,61 2012 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Bebenek et al.,57 2010 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Brotons et al.,67 2021 | Y | Y | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y |
| Curtis et al.,59 2012 | Y | Y | Y | U | U | Y | Y | Y | Y | Y | Y | Y | Y |
| Elramli,55 2017 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Fontana et al.,60 2007 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Hanlon et al.,54 1995 | Y | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y |
| Kemmler et al.,56 2010. | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Khanji et al.,66 2019 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Lakerveld et al.,64 2013 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Lukaczer et al.,58 2006 | Y | Y | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y |
| Márquez-celedonio et al.,65 2009 | Y | Y | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y |
| Riddell et al.,63 2016 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Tuthill et al.,62 2007 | Y | U | Y | N | N | U | Y | Y | Y | Y | Y | Y | Y |
| Total % | 100 | 100 | 100 | 27 | 7 | 60 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Y, yes; N, no; U, unclear; N/A, not applicable.
JBI critical appraisal checklist for quasi-experimental studies
Q1 Was true randomization used for assignment of participants to treatment groups?
Q7 Were treatment groups treated identically other than the intervention of interest?
Q2 Was allocation to treatment groups concealed?
Q8 Was follow-up complete, and if not, were strategies to address incomplete follow-up utilized?
Q3 Were treatment groups similar at baseline?
Q9 Were participants analyzed in the groups to which they were randomized?
Q4 Were participants blind to treatment assignment?
Q10 Were outcomes measured in the same way for treatment groups?
Q5 Were those delivering treatment blind to treatment assignment?
Q11 Were outcomes measured in a reliable way?
Q6 Were outcome assessors blind to treatment assignment?
Q12 Was appropriate statistical analysis used?
Q13 Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial?
Characteristics of included studies
Twenty-nine studies with a total sample of 5490 adults who were free of CVD at baseline were included: 15 were RCTs (n=3605), and 14 quasi-experimental studies (n=1885). The studies were conducted in the United States,39,41,52,58,60 Canada,53 Europe,40,43–46,49–51,54–57,59,61,62,64,66,67 Asia,42,47,48 Mexico,65 and Australia.63
The main lifestyle interventions employed in these studies included: diet, physical activity, motivational interviewing, problemsolving, psychological counseling, cardiovascular risk assessment and feedback, health education on self-management, and peer support. The duration of the interventions ranged from 3 weeks to 192 months. Table 3 outlines the key characteristics of the interventions included in this review.
Table 3:
Intervention characteristics
| Study | Intervention/sample frame | Follow-up | Intervention strategy and intensity | Comparator |
|---|---|---|---|---|
| Ahn and Kim,48 2020 | Quasi-experimental study including older women recruited from an Elderly Health Promotion Center in South Korea | 6 months |
Physical activity Exercise program that was made up of combined workouts of elastic band resistance exercise and aerobics with dance music, 3 times/week for 6 months with each session lasting 60 minutes |
N/A |
| al Mheid I et al.,39 2016 | Quasi-experimental study including participants recruited from university-sponsored health insurance plans in Atlanta | 24 months |
Health risk assessment and lifestyle counseling Trained health partners supported participants via weekly to monthly email or phone contacts and met with them during 6-, 12-, and 24-months follow-up. Counseling focused on healthier lifestyle (physical activity, body weight, cholesterol, glucose, stress, diet, and smoking habits). |
N/A |
| Anand et al.,53 2016 | RCT including participants recruited from Toronto and Vancouver, Canada | 6 months |
Diet and physical activity Participants received 2 types of messages: i) stages of change–oriented motivational messages, sent by email every 2 weeks; and ii) health tips focused on diet and physical activity sent by email/text messages (participant’s choice) every week. Access SAHARA website for prevention advice. |
Attention control Visit SAHARA website |
| Balducci et al.,61 2012 | RCT including participants with diabetes recruited from outpatient diabetes clinics in Italy | 12 months |
Physical activity Twice-a-week supervised aerobic and resistance training plus exercise |
Usual care
Counseling only |
| Baldwin,52 2015 | Quasi-experimental study including community-dwelling adult Hispanic women in Minnesota, USA | 3 months |
Diet, physical activity, and motivational interviewing A combination of physical activity classes and walking, assignments, wellness education classes, cooking, motivational interviewing groups, and health coaching. Lifestyle modification, education classes, and individualized health coaching were scripted and delivered by nursing students. A total of 14 active-phase classes were conducted. |
N/A |
| Bebenek et al.,57 2010 | RCT including early post-menopausal women living independently in Erlangen-Nuremberg, Germany | 12 months |
Physical activity Group 1 (exercise program): High-intensity resistance/high-impact exercise interspersed by blocks of 10 weeks of training focusing on cardiovascular parameters Group 2 (Exercise + CR): Exercise program in addition to 40mg/day CR. |
Wellness program Activities: coordination, relaxation, walk, dances, games, breath, balance, endurance, muscle strength, and flexibility |
| Bernocchi et al.,49 2011 | Quasi-experimental study including participants recruited from a clinic in Italy | 6 months |
Health risk assessment and lifestyle counseling using telemedicine Multidisciplinary approach that included scheduled home visits, telehealth counseling to evaluate weight management and physical activity, smoking, dietary habits, and stress. Participants had to undergo at ≥3 sessions a week of bicycle exercise training or fast walking for at least 30 minutes and calisthenics |
N/A |
| Chan et al.,47 2012. | Quasi-experimental study including participants recruited from a rehabilitation hospital in Hong Kong | 10 weeks |
Diet and physical activity The intervention included 14 sessions of empowerment workshops on cardiovascular health, diet, and community exercise classes. During the empowerment sessions, a physiotherapist and a nurse facilitated subjects to adopt healthy behaviors and lifestyle through active and mutual participation, goals setting, action planning, self-reflection, and peer-support. |
N/A |
| Curtis et al.,59 2012 | RCT including post-menopausal women with type 2 DM recruited from a Clinic in the UK | 12 months |
Diet 27 g/day (split dose) of flavonoid-enriched chocolate (850 mg flavan-3-ols and 100 mg isoflavones/day) |
Placebo chocolate
Taken daily, twice a day for a year like the flavonoid-enriched chocolate |
| Elramli,55 2017 | RCT including participants recruited from rheumatology outpatient clinics in Glasgow, UK | 6 months |
Physical activity 6 interactive weekly sessions lasting about 1 hour. In addition, a physiotherapist contacted the participants at the end of weeks 7, 9, and 11 to discuss their step counts for the past month, their step goals for the following month, any barriers to PA they faced and how they planned to overcome them. Participants also received 2 booster sessions, at 3 and 6 months after starting the program. |
Education handout on diet and exercise One education session regarding the importance of exercise and a healthy diet. Participants were given written education material and encouraged to read it. |
| Fontana, et al.60 2007 | RCT including participants recruited from the St. Louis metropolitan area, USA | 12 months |
Physical activity The exercise prescription started with about 16% increase in energy expenditure over baseline expenditure for 3 months followed by a 20% increase for the final 9 months. Exercise trainers worked with participants individually to establish and monitor their exercise routines, provide advice, encouragement, and update exercise prescriptions weekly. Diet The goal was to decrease energy intake without changing energy expenditure. The CR prescription started with a 16% decrease below the participants’ baseline energy intake for 3 months and then increased to 20% for the remaining 9 months. For 5 days during the first month participants received all meals from the research study. |
Yoga classes and healthy diet handouts The control group received general information about a healthy diet and were offered free yoga classes. |
| Hanlon et al.,54 1995 | RCT including participants recruited from 2 work sites in Glasgow | 12 months |
Health risk assessment and lifestyle counseling 5 intervention groups: Group 1 received health education without feedback on cholesterol or risk score. Group 2 received health education with feedback on cholesterol but without feedback on risk score. Group 3 received health education with feedback on risk score but not on cholesterol. Group 4 received a full health check: health education with feedback on cholesterol and risk score. Group 5 acted as an internal control group, intervention was delayed, but was administered after 5 months instead of the end of the study to promote participation. |
Delayed health education package
Participants from the control site were recruited as the external control group for the study. Their intervention was delayed but was administered after 5 months instead of the end of the study to promote participation. |
| Kemmler et al.,56 2010 | RCT including women 65 years or older living independently in Erlangen-Nuremberg, Germany | 18 months |
Physical activity The weekly exercise program consisted of 2 60-minute supervised group classes and 2 20-minute home training sessions. Group classes were structured into 4 sequences. |
Wellness program Activities: coordination, relaxation, walking, dances, games, breath, balance, endurance, muscle strength, and flexibility |
| Kemmler et al.,44 2016. | Quasi-experimental study including post-menopausal women with osteopenia living in Erlangen- Nuremberg | 192 months |
Physical activity Two group classes of 60 to 65 minutes and 2 home training sessions of 20 to 25 minutes for up to 50 weeks a year, supervised by certified instructors. After a conditioning phase (6–9 months), the exercise intensity was adapted to performance. |
Status quo Asked to maintain their present lifestyle and physical activity level. |
| Khanji et al.,66 2019 | RCT including participants with a 10-year absolute CVD risk of ≥10%, recruited from primary care databases in London, UK | 6 months |
Health risk assessment and personalized e-coaching Electronic (e-)coaching, using personalized web-based lifestyle and risk factor counseling in addition to standard care |
Usual care Personalized face-to-face counseling on cardiovascular risk factors during the baseline visit. |
| Kim et al.,42 2011 | Quasi-experimental study including adults with type 2 DM and metabolic syndrome recruited at a university hospital in Korea | 16 weeks |
Diet and physical activity Reduced caloric intake (200 to 300 kcal) for weight control; individual counseling sessions, and 150 minutes of moderate exercise per week |
Usual care Booklet and basic education on diabetes as part of routine care |
| Lakerveld et al.,64 2013 | RCT including participants recruited from a diabetes research center in West-Friesland, the Netherlands | 12 months |
Motivational interviewing and problem-solving Six face-to-face. 30-minute counseling sessions followed by 3 monthly telephone sessions with practice nurses |
Usual care Brochures containing health guidelines regarding PA and a healthy diet |
| Lazarevic et al.,43 2008 | Quasi-experimental study including participants with obesity and diabetes, recruited from outpatient clinics in Serbia | 6 months |
Physical activity Aerobic exercise program consisting of 3–5 sessions of moderate aerobic exercise weekly with an average duration of 45–60 minutes and a workout intensity corresponding to 50–75% of maximal heart rate |
N/A |
| Lukaczer et al.,58 2006 | RCT including post-menopausal women recruited in Washington State, USA | 3 months |
Diet Dietary program combining a low glycemic index diet with food providing 30 g of soy protein and 4 g of phytosterols per day. |
Standard dietary program
American Heart Association Step 1 diet |
| Márquez-celedonio et al.,65 2009 | RCT including participants recruited from primary health care clinics in Mexico | 6 months |
Diet and physical activity Lifestyle modification program including a low-sodium, DASH diet with energy content determined using the Harris-Benedict formula. Also 3–5 sessions of aerobic exercise complemented by group sport sessions (45 min per session). Smokers: 6 educational classes. |
Handouts on diet and exercise Received education on the exercises they should undertake, and dietetic recommendation |
| Price et al.,40 2000 | Quasi-experimental study including participants recruited from an inner-city general practice in Stoke-on-Trent, North Staffordshire, UK | 24 months |
Diet A session with a nurse focusing on baseline diet with the aim of reducing the fat content by substituting saturated fats with polyunsaturated fats. A supplementary diet sheet, devised by the Family Heart Association, was also provided. |
N/A |
| Richardson et al.,50 2008 | Quasi-experimental study including participants recruited from Wales general practitioner clinics | 12 months |
Health risk assessment and lifestyle counseling Baseline assessment of CVD risk and referral to a general practitioner, dietician, an exercise referral scheme, or to local smoking cessation services. |
N/A |
| Riddell et al.,63 2016 | RCT including participants recruited from Australia’s National Diabetes Services Scheme Registry | 12 months |
Health education and peer support
A diabetes education session at baseline. Monthly community-based group meetings led by trained peer supporters and active encouragement to use primary care, community resources, and supports related to diabetes. |
Usual care A diabetes education session at baseline. Feedback on clinical measures collected as part of assessments |
| Ródenas et al.,45 2005 | Quasi-experimental study including post-menopausal nuns recruited from a convent in Spain | 28 days |
Diet The culinary oil used for years in the convent (a blend of sunflower and olive oils) were substituted for extra virgin olive oil for 28 days |
N/A |
| Sartorio et al.,46 2001 | Quasi-experimental study including participants with obesity recruited from an inpatient setting in Italy | 3 weeks |
Diet, physical activity, and psychological counseling 5 days of moderate physical activity per week, energy-restricted diet (1200 ± 1800 kcal/day), 2–3 sessions of psychological counseling per week, and daily educational lectures |
N/A |
| Siren et al.,51 2016 | Quasi-experimental study including male participants recruited from Helsinki, Finland | 60 months |
Health risk assessment and lifestyle counseling Trained nurses reviewed participants’ lifestyle and evaluated their absolute CVD risk score. Those at risk received counseling based upon their individual risk profile as recommended by the Finnish guidelines for preventing CVD. |
N/A |
| Tuthill et al.,62 2007 | RCT including participants with obesity recruited from outpatient clinics in Dublin, Ireland | 6 months |
Diet and physical activity Monthly evening group sessions focusing on dietary advice from a dietician and exercise advise from a physiotherapist |
No information on the control condition is provided |
| Widmer et al.,41 2014 | Quasi-experimental study with participants from employer-sponsored health program in Tennessee, USA | 3 months |
Health risk assessment and lifestyle counseling Participants were assessed for CVD risk factors and referral was made for those who did not meet all healthy benchmarks (body mass index, blood pressure, glucose, total cholesterol). |
N/A |
CR, cimicifuga racemosa; CVD, cardiovascular disease; DB, diabetes mellitus; PA, physical activity; RCT, randomized controlled trial
Six validated absolute CVD risk assessment tools were used to measure the study outcomes, including Framingham (7 RCTs56–58,60,65–67 and 10 quasi-experimental studies39–41,44–50), SCORE (1 RCT64 and 2 quasi-experimental studies43,51), Heart Health Risk Assessment Score (1 quasi-experimental study52), Dundee (1 RCT54), ASSIGN (2 RCTs55,67), and The UK Prospective Diabetes Study (UKPDS) risk score (4 RCTs59,61–63 and 1 quasi-experimental study42). Total sample sizes per study ranged from 12 to 711 participants. The characteristics and main outcomes of the 29 studies included in this review are detailed in Appendix III.
Review findings
The quantitative findings for the primary outcome variable (absolute CVD risk score) are presented with their meta-analysis data organized by study design. Although different screening tools were employed in the studies, all studies that employed the same design were pooled together. This was possible because our primary outcome focused on general absolute CVD risk rather than specific CVD events or time to incident CVD where the screening tools differ. We conducted additional analyses to examine studies that used similar screening tools, and the impact of specific lifestyle interventions on absolute CVD risk. Table 3 outlines the specific interventions, as well as each study’s sampling frame, duration, intervention strategy, and intensity. Heterogeneity was explored using the I2 statistic. To mitigate the anticipated heterogeneity of the risk assessment tools, random effects models were employed in the analysis as recommended by Haidich.32
For all studies that employed RCT design, between-group comparisons were made to evaluate any difference between the experimental and control group absolute CVD risk status at the end of the study. In the studies employing a quasi-experimental design, the comparisons were made between baseline and after intervention for the absolute CVD risk status. The effect sizes are reported as the standard mean difference where different screening tools were used, and as mean differences where similar screening tools were used.
Among the studies that employed RCT design, the magnitude of the average reduction in absolute CVD risk varied across the studies, but the intervention group was consistently favored over the control group. Of the 15 RCTs included in the comparative meta-analysis, the lifestyle intervention groups had an overall average absolute CVD risk score that was 0.39 standard deviations below that of the control (standardized mean difference = −0.39, 95% CI: −0.74,−0.03; I2 = 96; Figure 2).
Figure 2:
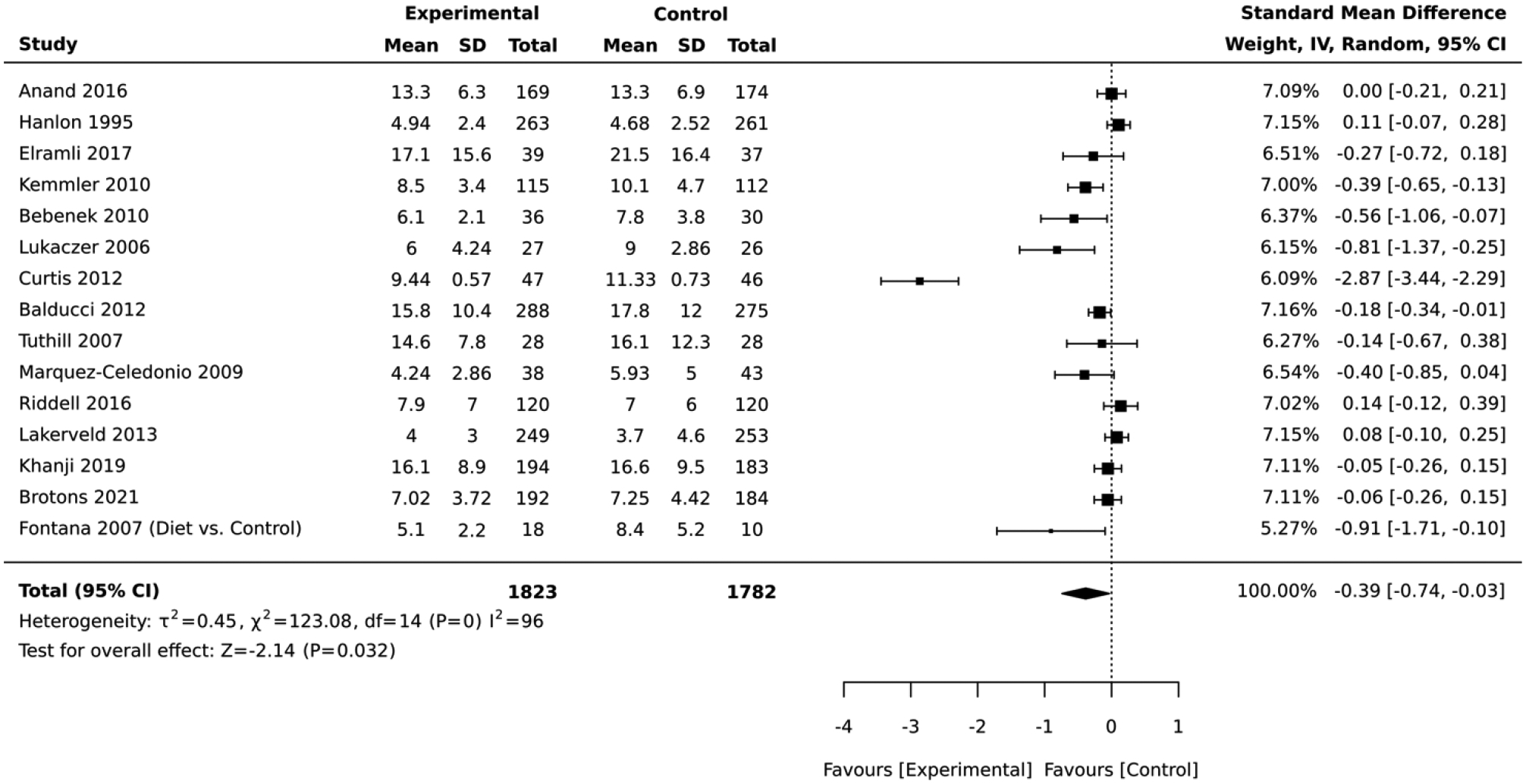
Forest plot of the comparative meta-analysis including studies that employed RCT design
In the quasi-experimental studies, the magnitude and direction of the average change in absolute CVD risk varied across the studies, but the post-intervention group tended to have a lower absolute CVD risk than the pre-intervention group. In the meta-analysis of 14 studies, the overall average for the absolute CVD risk score measured immediately after lifestyle intervention was 0.39 standard deviations lower than the baseline values (Standardized Mean Difference=−0.39, 95% CI:−0.60, −0.17; I2 = 88; Figure 3).
Figure 3:
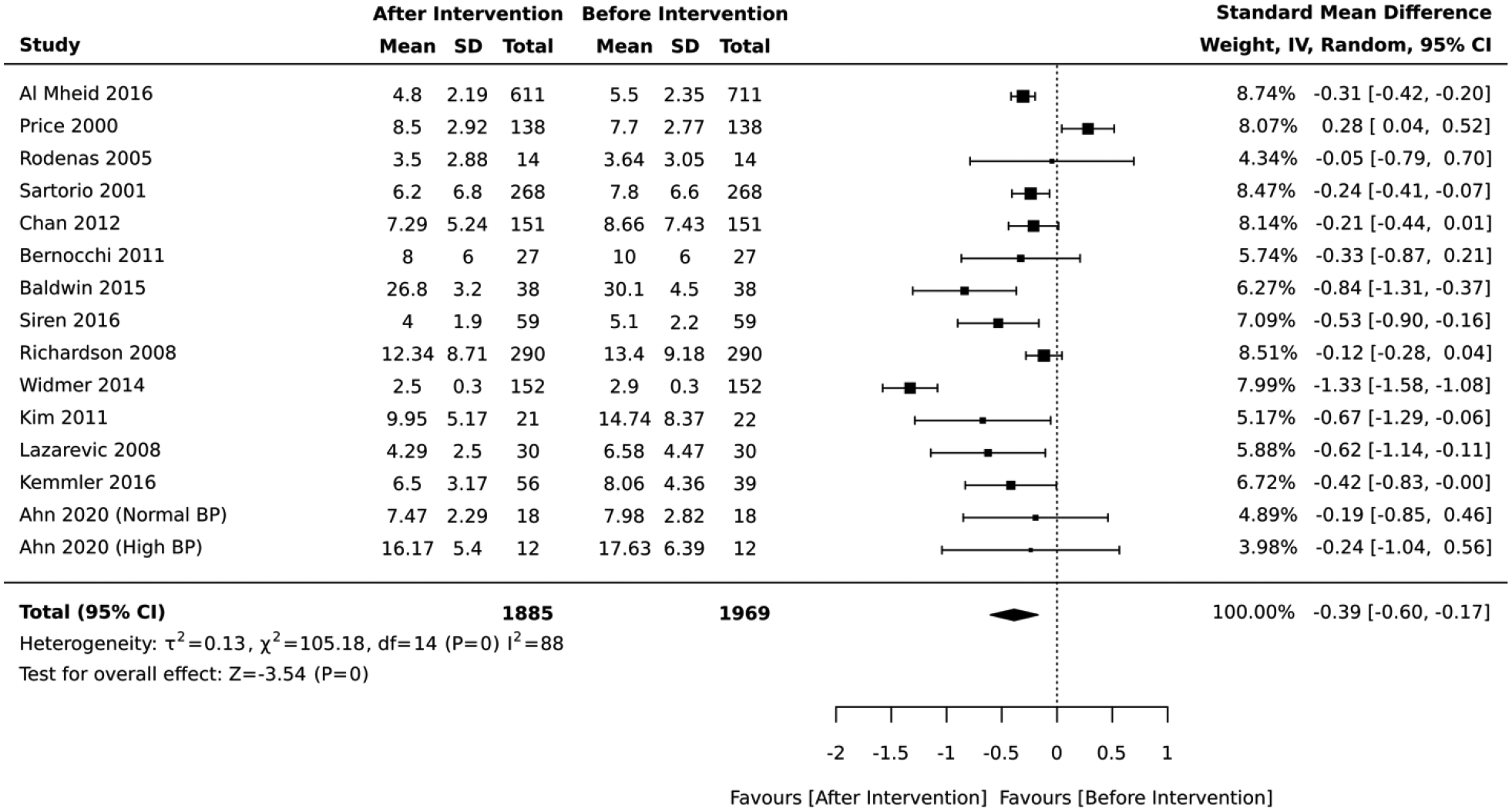
Forest plot of the meta-analysis of quasi-experimental design studies
Five studies included interventions that combined diet and physical activity. In the 3 studies that employed a RCT design, the impact on absolute CVD risk was equivocal across the studies and there was no difference between the intervention vs. control group (standardized mean difference = −0.13, 95% CI: −0.38, 0.13; I2 = 32; Figure 4). The duration of these RCTs was 6 months. Similarly, the 2 studies that employed a quasi-experimental design did not report any significant change in the average absolute CVD risk score after lifestyle intervention (standardized mean difference = −0.35, 95% CI: −0.76, 0.06; I2 = 47; Figure 5). The duration of these quasi-experimental studies was 10 and 16 weeks.
Figure 4:
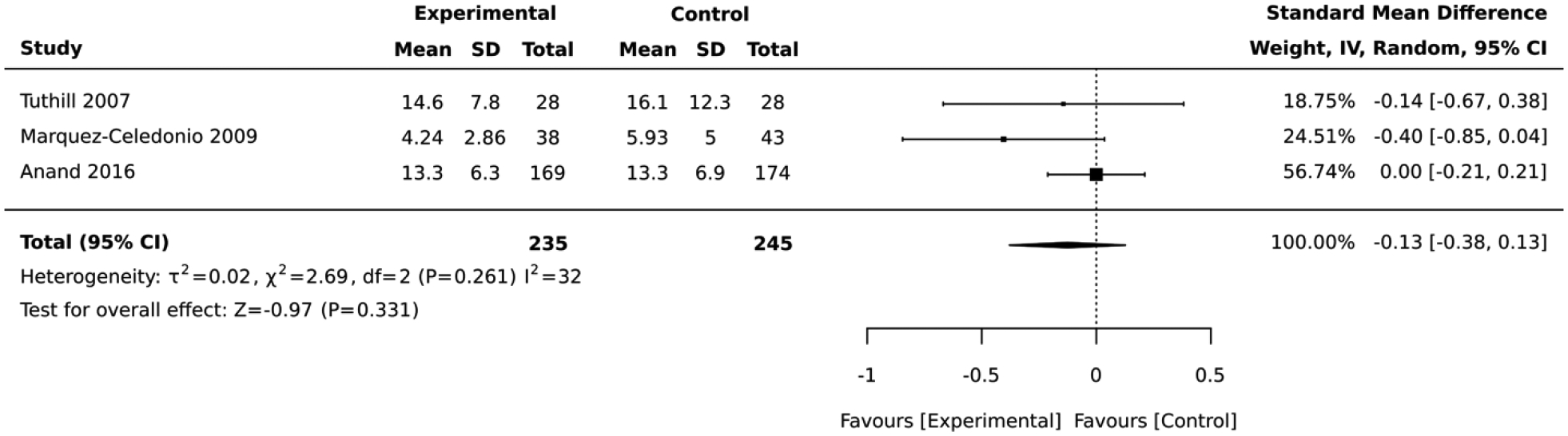
Forest plot of the comparative meta-analysis including RCT studies that employed a combination of diet and physical activity in their intervention
Figure 5:
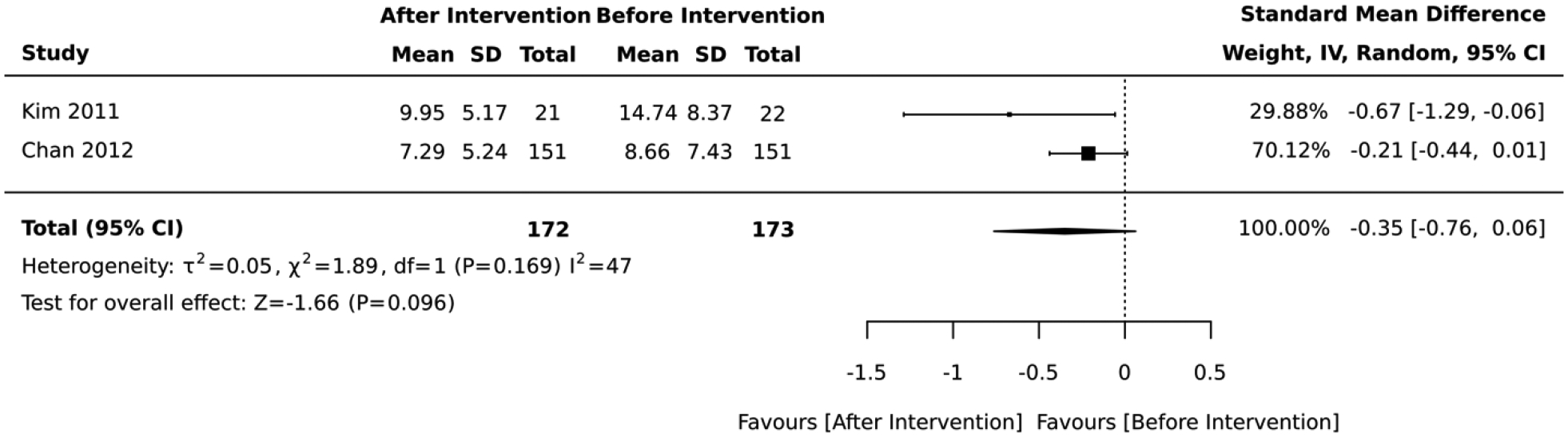
Forest plot of the meta-analysis including quasi-experimental studies that employed a combination of diet and physical activity in their intervention
Five studies included interventions that focused on diet. In the 3 studies that employed a RCT design, the magnitude of the average reduction in absolute CVD risk varied across studies, but the intervention group was consistently favored over the control group. The diet lifestyle intervention groups had an overall average absolute CVD risk score that was 1.54 standard deviations below that of the control (standardized mean difference=−1.54, 95% CI: −2.87,−0.21; I2 = 92; Figure 6). Two of the RCTs had a 12-month follow-up while 1 lasted for 3 months. In contrast, the 2 studies that employed a quasi-experimental design reported a significant deteroriation in the average absolute CVD risk score after lifestyle intervention (mean difference= 0.72, 95% CI: 0.08, 1.36; I2 = 0; Figure 7). The duration of these quasi-experimental studies was 28 days and 24 months.
Figure 6:
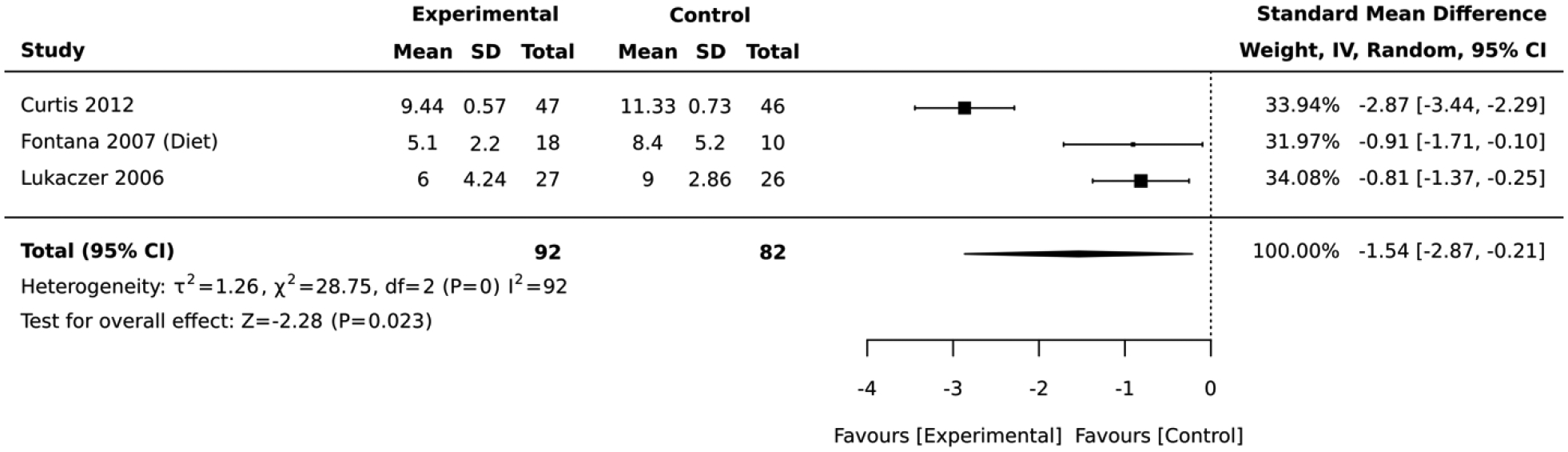
Forest plot of the comparative meta-analysis including RCT studies that employed dietary interventions
Figure 7:
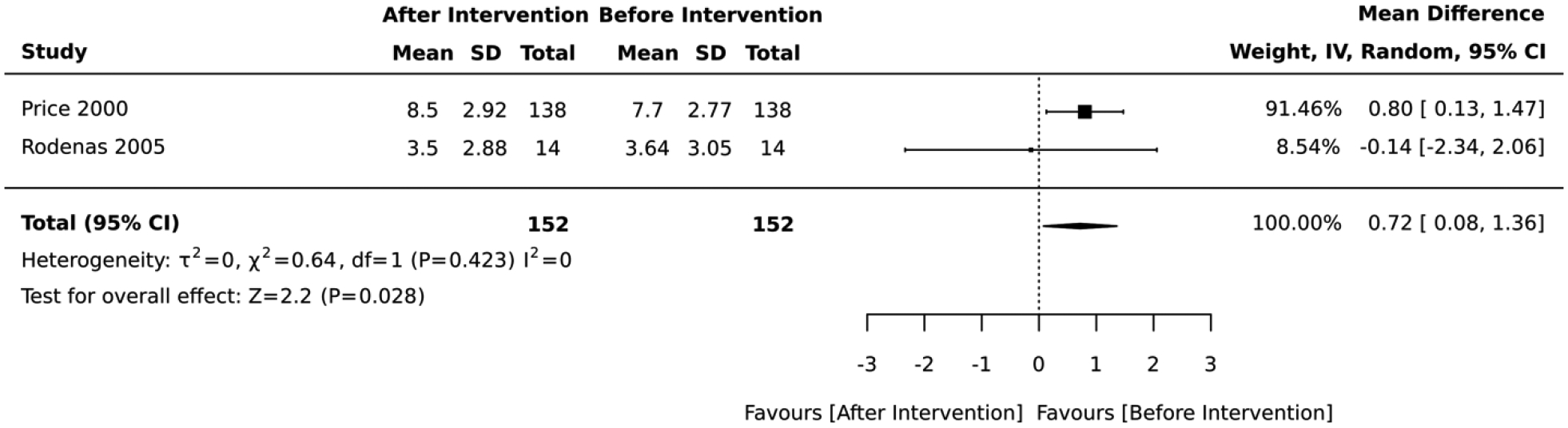
Forest plot of the meta-analysis including quasi-experimental studies that employed dietary interventions
Eight studies included interventions that focused on physical activity. In the 5 studies that employed a RCT design, the magnitude of the average reduction in absolute CVD risk varied across studies, but the physical activity intervention group was consistently favored over the control group. The physical activity lifestyle intervention groups had an overall average absolute CVD risk score that was 0.30 standard deviations below that of the control (standardized mean difference=−0.30, 95% CI: −0.46,−0.13; I2 = 20; Figure 8). The duration of these RCTs ranged from 6 to 18 months. Similary, the 3 studies that employed a quasi-experimental design reported a significant improvement in the average absolute CVD risk score after lifestyle intervention (standardized mean difference= −0.42, 95% CI: −0.69,−0.14; I2 = 0; Figure 9). The duration of these quasi-experimental studies was 6 to 192 months.
Figure 8:
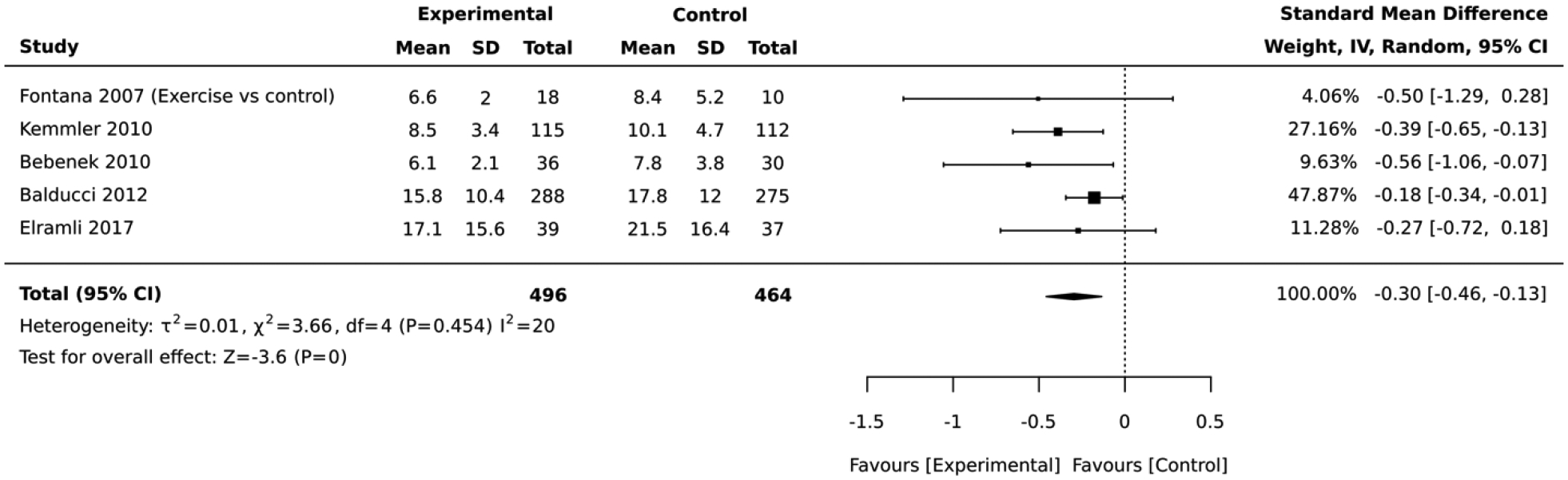
Forest plot of the comparative meta-analysis including RCT studies that employed physical activity interventions
Figure 9:
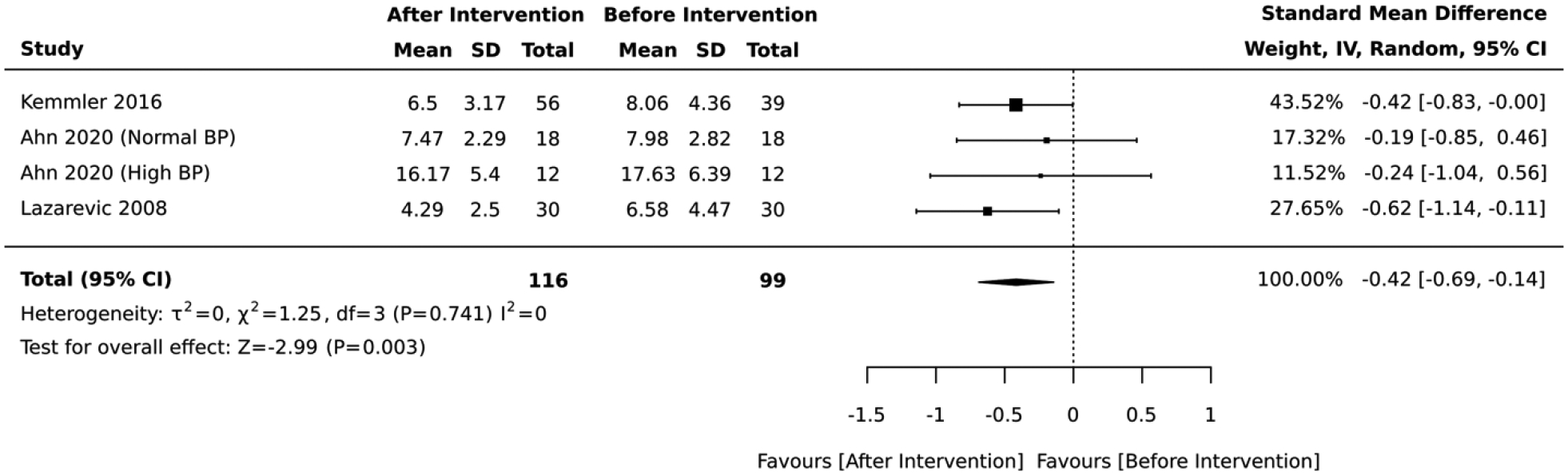
Forest plot of the meta-analysis including quasi-experimental studies that employed physical activity interventions
Two quasi-experimental studies that focused on diet, physical activity, and psychological counseling reported no significant change in the average absolute CVD risk score after lifestyle intervention (standardized mean difference= −0.50, 95% CI: −1.08, 0.08; I2 = 82; Figure 10). The duration of these quasi-experimental studies was 21 days and 3 months.
Figure 10:
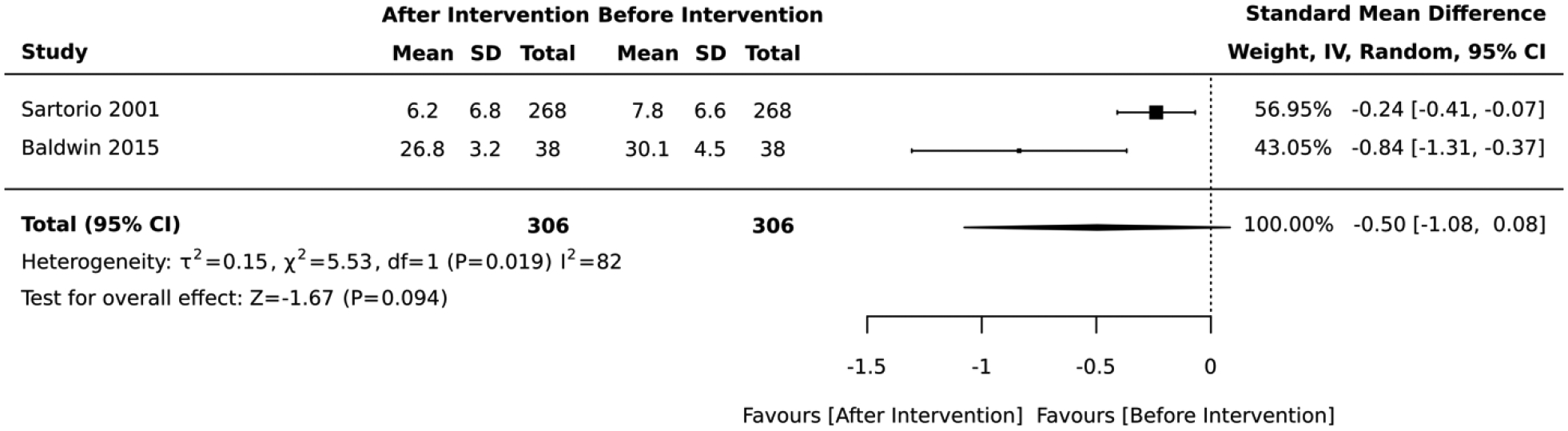
Forest plot of the meta-analysis including quasi-experimental studies that employed diet, physical activity, and psychological counseling interventions
Seven studies included interventions that focused on health risk assessment and lifestyle counseling. In the 2 studies that employed a RCT design, there was no significant difference in absolute CVD risk between the intervention vs. control group (standardized mean difference= 0.03, 95% CI: −0.12, 0.19; I2 = 28; Figure 11). The duration of these RCTs was 6 and 12 months. The 5 studies that employed a quasi-experimental design reported a significant improvement in the average absolute CVD risk score after lifestyle intervention (standardized mean difference= −0.53, 95% CI: −0.96,−0.09; I2 = 95; Figure 12). The duration of these quasi-experimental studies was 3 to 60 months.
Figure 11:
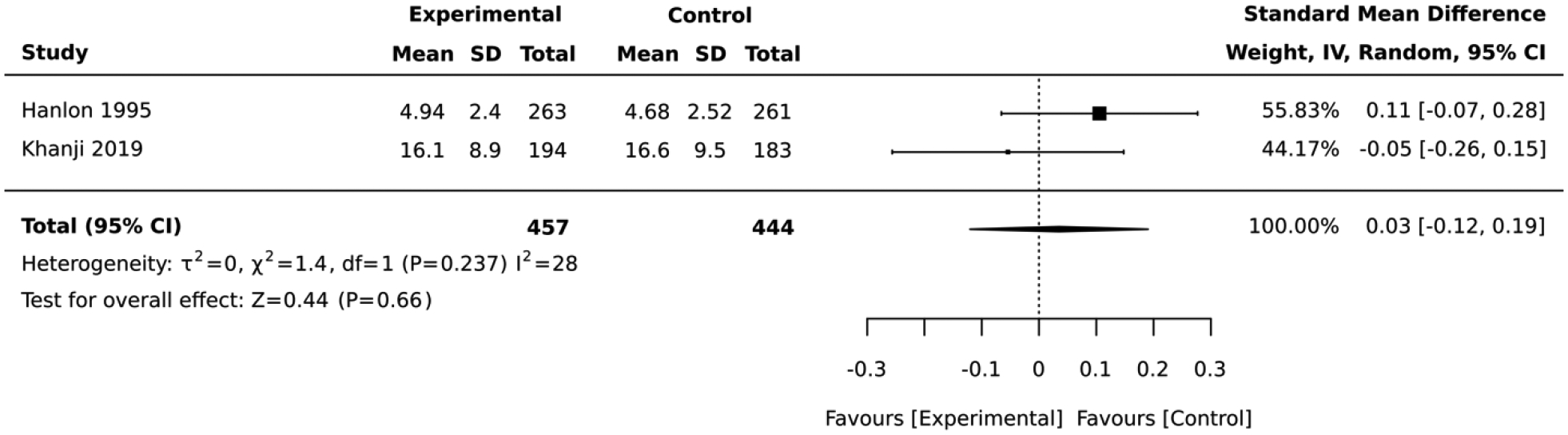
Forest plot of the comparative meta-analysis including RCT studies that employed health risk assessment and lifestyle counseling interventions
Figure 12:
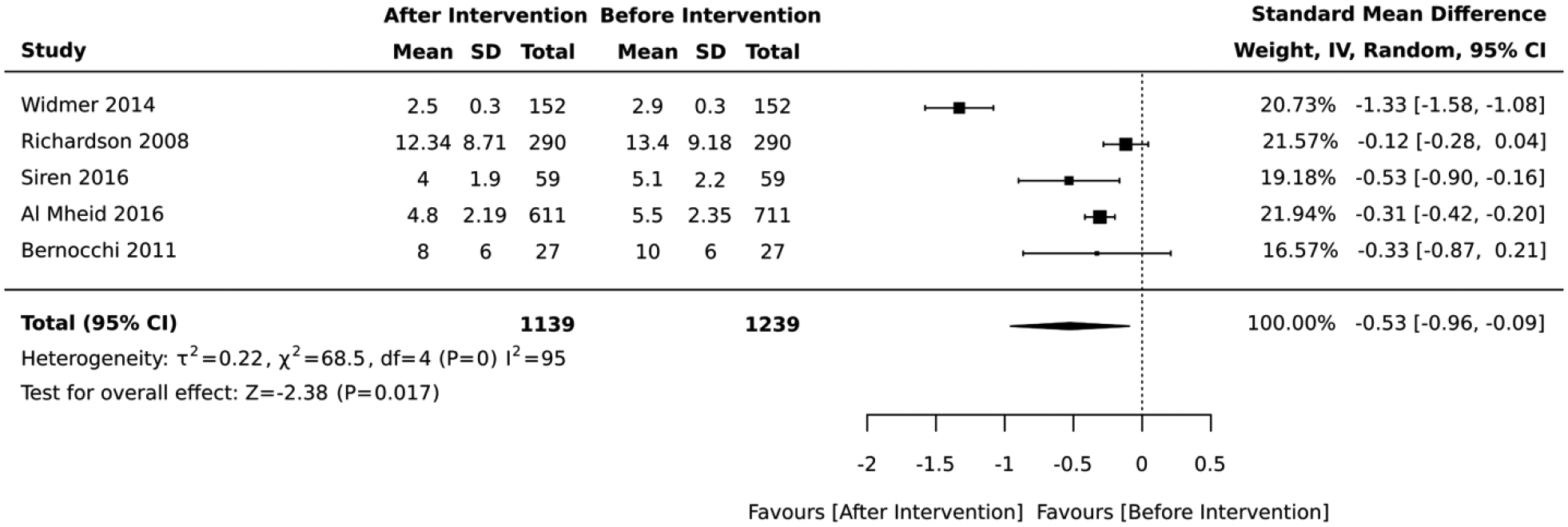
Forest plot of the meta-analysis including quasi-experimental studies that employed health risk assessment and lifestyle counseling interventions
Lastly, there were 2 RCTs that did not fit in either of the aforementioned categories. One was focused on motivational interviewing and problem solving, while the other focused on health education and peer support. Both had 12 months of follow-up and reported no significant difference in absolute CVD risk between the intervention vs. control group as outlined in Table 3.
In the final analysis, we pooled studies that employed similar tools to assess the absolute CVD risk status. Only the Framingham algorithms were consistently used in studies with comparable design. Of the 7 RCTs that employed the Framingham algorithms, the lifestyle intervention groups had an overall average absolute CVD risk score that was 1.40 lower that of the control (mean difference= −1.40, 95% CI: −2.19,−0.61; I2 = 47; Figure 13).
Figure 13:
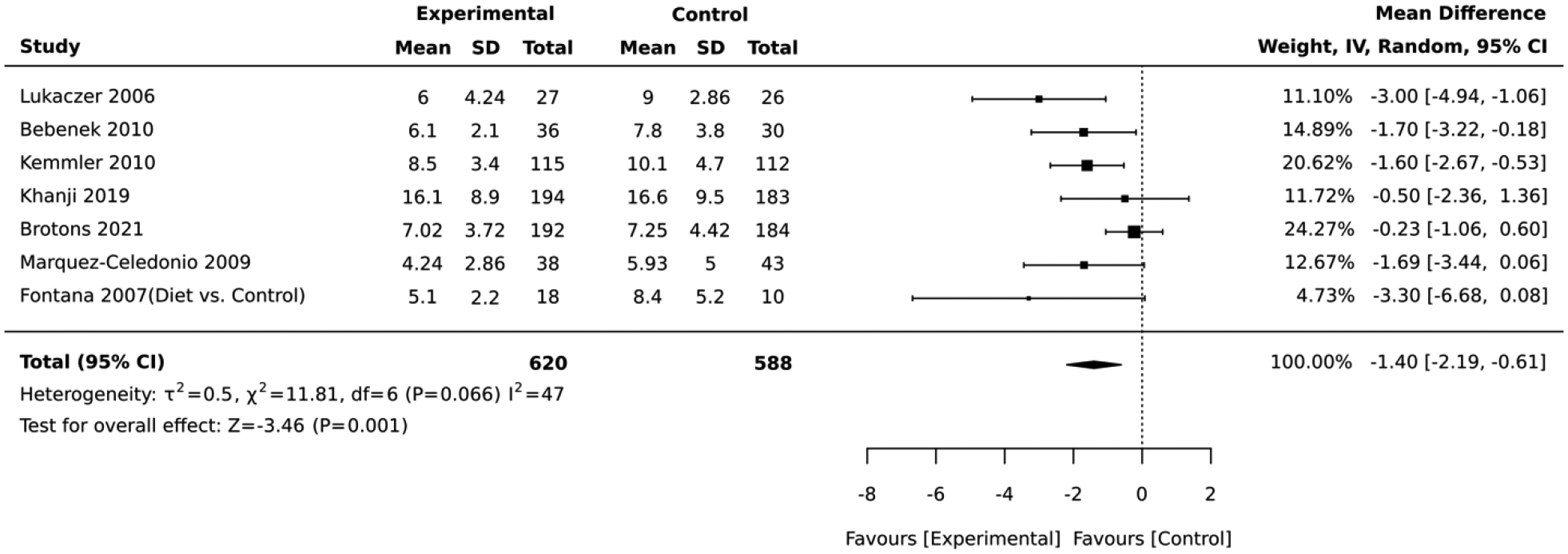
Forest plot of the comparative meta-analysis including all studies that used the Framingham algorithm and RCT design
Similarly, in the quasi-experimental studies that employed the Framigham algorithms, the post-intervention absolute CVD risk was lower than the baseline values. In the meta-analysis of 10 studies, the overall average for the absolute CVD risk score measured immediately after lifestyle intervention was 0.67 lower than the baseline values (mean difference= −0.67, 95% CI:−1.21, −0.12; I2 = 86; Figure 14).
Figure 14:
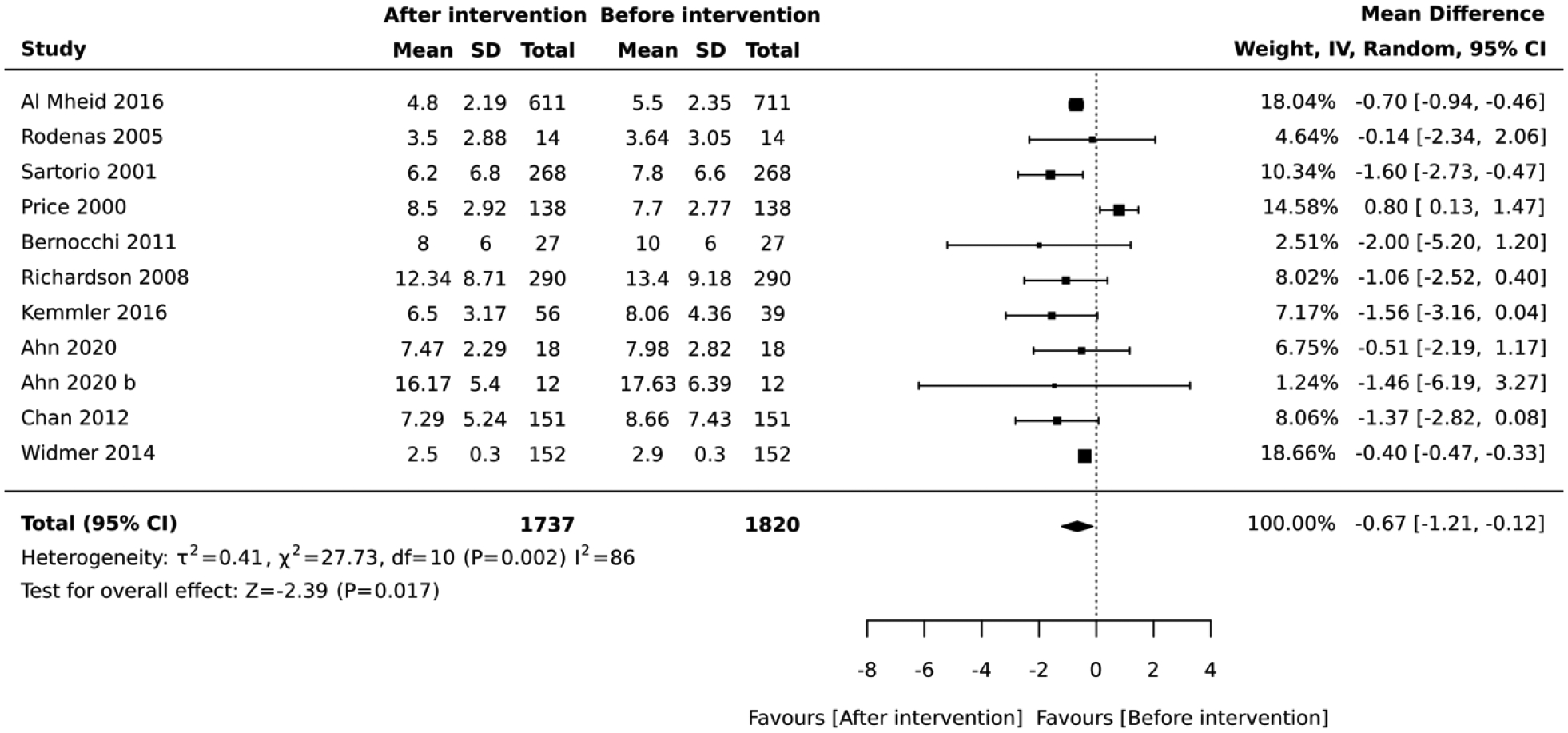
Forest plot of the meta-analysis including all studies that used the Framingham algorithm and quasi-experimental design
Discussion
The meta-analysis of 29 RCTs and quasi-experimental studies using lifestyle interventions suggest that lifestyle modification is effective in reducing absolute CVD risk score in adult populations with no history of CVD. Dietary and physical activity interventions had the greatest impact in reducing absolute CVD risk. Although 6 different risk assessment algorithms were employed to measure absolute CVD risk, all of them are externally validated and are widely used in research and clinical settings21,22,24–26,68 which increases the clinical utility of the findings.
RCT and quasi-experimental studies demonstrated a significant effect of lifestyle intervention in reducing absolute CVD risk by about 0.4 standard deviations—implying that lifestyle interventions can bring the average absolute CVD risk 15.5% below that of the untreated population. The 15.5% is a probability unit from a standard normal distribution (ie, Pr[−0.4<SMD<0]=0.1554), implying that moving from the mean to 0.4 deviations below the mean has an associated probability of 15.5%. A person with Z=−0.4 has a probability of CVD 15.5% lower than that of a person at the mean Z=0. Aside from improving comparability across studies, using standardized mean differences allows us to translate back to the original risk score with probabilities on the cumulative standard normal distribution.69
The included studies had a high score in methodologic appraisal, but high uncertainty index and risk of bias (quasi-experimental studies only) moderated the rigor of the evidence as outlined in the Summary of Findings. To our knowledge, this is the first systematic review and meta-analysis to assess the impact of lifestyle modification on absolute CVD risk. Previous meta-analyses have focused on individual CVD risk factors, which are a less reliable metric of overall CVD risk.70 An absolute CVD risk–based approach has been shown to facilitate decision-making and improve individualized care71 by quantifying the cumulative effect of multiple CVD risk factors, as demonstrated by the Mr. Hue clinical case study. In this analysis, we identified lifestyle behaviors that have been effective in mitigating the absolute CVD risk. Unfortunately, these factors have not been widely adopted by high risk populations. According to the American Heart Association, only about 5% of Americans have adopted the lifestyle factors needed to achieve “ideal” cardiovascular health.72 More effort is needed to encourage patients to make the lifestyle changes during routine clinical visits.
Eight categories of lifestyle interventions were implemented across the studies. RCTs that employed dietary interventions were associated with the largest effect size in mitigating absolute CVD risk, albeit with a high degree of heterogeinity (Figure 5).58–60 Of these, 1 study focused on caloric restriction without altering energy expenditure,60 another prescribed daily intake of flavonoid-enriched chocolate,59 while another encouraged the intake of low-calorie, high-protein diet with plant sterols.58 However, in quasi-experimental studies, dietary interventions had a negative impact on absolute CVD risk profile (Figure 6). The strategies associated with the negative outcomes included providing participants with extra virgin oil45 and a session with a nurse to evaluate baseline diet coupled with a dietary handout.40 The duration of the diet interventions included in the RCTs ranged from 3 to 12 months, while those included in the quasi-experimental studies ranged from 3 weeks to 192 months.
Prior reviews have demonstrated that lifestyle interventions consisting of particular nutrients or food groups (ie, high intake of legumes, nuts, and chocolate) significantly reduce the risk of coronary heart disease.73 In this meta-analysis, RCT interventions that included a low glycemic index diet,58,60 soy protein and phytosterols,58 or flavonoid-enriched chocolate59 reported a significant reduction in absolute CVD risk,58,59 suggesting a potential impact of these dietary strategies on multiple CVD risk components, such as blood pressure, total cholesterol, and high-density lipoprotein levels.74,75 Although RCTs present better quality of evidence, the contradicting results from quasi-experimental studies necessitate further evaluation of the association between changes in diet and absolute CVD risk score in studies of comparable design and duration.
Other interventions that improved the absolute CVD risk profile included physical activity (Figures 8 and 9) and health risk assessment with lifestyle counseling (Figure 12). It is noteworthy that only physical activity interventions were consistent in mitigating absolute CVD risk in both RCT and quasi-experimental studies, and their comparative meta-analysis results did not have a high degree of heterogeneity compared with other strategies that improved the absolute CVD risk profile. The remaining ifestlyle modification strategies, including motivational interviewing, problem solving, health education, and peer support, did not impact the absolute CVD risk score. It is worth noting that there was a wide variation in the duration of follow-up employed even for comparable interventions. For instance, the quasi-experimental studies focusing on diet ranged in duration from 28 days to 24 months. Although we are not aware of any specific intervention duration associated with changes in CVD risk, we contend that studies lasting for a few days are unlikely to yield any significant changes in absolute CVD risk score. Future studies may need to focus on studies that attain a specific intervention duration threshold.
In this systematic review and meta-analysis, we note that multiple lifestyle interventions and CVD risk assessment tools were used across studies. This may partially explain the high heterogeneity observed in RCTs (I2 = 96) and quasi-experimental (I2 = 88) meta-analysis results combining interventions by study design. When the meta-analysis was organized by intervention category and study design, the heterogeneity was low in some intervention strategies, such as physical activity RCTs (I2 = 20), but high in others (eg, health risk assessment and lifestyle counseling quasi-experimental studies [I2 = 95]). Various factors, including differences in sampling frame, study protocols and the risk assessment tools employed, may explain some of the heterogeneity observed in the meta-analysis.
The most commonly used CVD risk assessment tool was the 10-year Framingham Risk Score,21 which was developed to predict incident risk of absolute CVD risk using covariates that include age, diabetes status, smoking, blood pressure, total cholesterol, and high-density lipoprotein cholesterol levels. A simplified version using body mass index instead of lipids is also available.76 In the pooled analysis, studies that employed the Framigham Risk Score demonstrated significant improvement in total CVD risk score after lifestyle intervention. The pooled data from 7 RCTs clearly demonstrated a significant impact of the lifestyle interventions in the context of low heterogeneity. Given that prior studies have shown the Framingham Risk Score may overestimate CVD risk in the general European population,77 several studies implemented the SCORE, Dundee, and the UKPDS risk scores. Differences between these metrics include the individual CVD risk factors included in the risk prediction models and the populations used to generate and validate these tools. We did not have adequate studies to do a meta-analysis focusing on these tools. However, the pooled analysis by study design indicates that, regardless of the CVD risk assessment tool used, lifestyle modifications were shown to reduce CVD risk in this meta-analysis. These findings highlight the importance of valid, reliable, and consistent CVD risk assessment tools to guide decision-making in primary prevention of CVD, and to compare lifestyle modification interventions across adult populations.
Limitations of the review
It is important to note several limitations in this review. In an attempt to be inclusive in assessing the impact of lifestyle modifications on absolute CVD risk, we collected data on various lifestyle modification modalities across different populations and using multiple CVD risk assessment tools. Therefore, as noted previously, heterogeneity in study results was present secondary to variation in study populations, intervention modalities, lengths of follow-up, and outcome assessment. To mitigate the impact of these differences in our analysis, we employed random effects models in the meta-analysis as recommended by Haidich.32 Notwithstanding the heterogeneity, our findings demonstrated an overall CVD risk reduction following lifestyle modification.
Another limitation is that only articles that were written in English were included in this meta-analysis. In addition, a significant number of studies were excluded because of quality issues or lack of data needed for meta-analysis (as outlined in Apendix II). These exclusions limit the scope of the meta-analysis and the inferences that can be drawn from our results.
While we included 29 studies in this review, we did not have enough studies or sample diversity to perform additional subgroup analyses based on sex, age groups, race, or geographic location. However, we had a sufficient number of articles to separate our meta-analyses by study design and intervention components. Small sample sizes across multiple studies may also attenuate the precision of the effect sizes, although the direction of the observed effects was consistent. Moreover, the follow-up period of many studies was relatively short (<12 months) and reassessment of CVD risk at a longer follow-up may be warranted. However, all other critical components scored high in both RCTs and quasi-experimental studies.
Conclusions
Our systematic review and meta-analysis results provide evidence to support a modest positive impact of lifestyle modification on absolute CVD risk score in adult populations with no history of CVD. Lifestyle intervention programs with multiple group or individual sessions and involving diet, physical activity, or health risk assessment with lifestyle counseling were effective for primary prevention of CVD. These results suggest that lifestyle modification programs need repeated exposure and reinforcement to be beneficial for cardiovascular health. Our analysis further revealed that a variety of validated absolute CVD risk screening tools are being used in different geographical regions to monitor, evaluate, and communicate changes in absolute risk score after lifestyle modification.
Recommendations for research
In this analysis, there were many high quality studies that were excluded because the absolute CVD risk score was not included in the outcomes. Since all evidence-based guidelines recommend the use of absolute CVD risk score to guide CVD prevention efforts, it is important for future studies to include the score as part of study outcomes. The availability of multiple studies reporting absolute CVD risk score would make it possible to conduct meta-analysis focusing on studies with comparable samples, study protocols, and the risk assessment tools, which will possibly reduce the degree of heterogeneity observed in the analysis. Additional studies with a longer follow-up are necessary to determine the long-term effect of lifestyle modification on CVD risk.
Recommendations for practice
The following recommendations for practice are as follows:
Assess total CVD risk score in clinical settings to capture the cumulative impact of co-occurring CVD risk factors. This will facilitate early risk detection and timely prevention. (Grade A).
Lifestyle-based interventions, including diet, physical activity or health risk assessment with lifestyle counselling, could reduce total CVD risk score in adults. (Grade B)
Use the total CVD risk score to inform the choice and intensity of preventive interventions prescribed to patients as recommended by the guidelines. (Grade A)
Demonstrate to patients how the CVD risk score changes in response to specific lifestyle changes. This could improve risk communication and adherence to preventive therapies. (Grade B)
Acknowledgments
Timothy Kilkelly and Adrian Bermudez for their help in extracting the full text of the articles included in this review.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI HL107370S) via a grant awarded to JKK. The funders played no role in the review process.
Appendix I: Search strategy
PubMed
Search conducted: November 16, 2016; updated in October 2018 and February 2022
| Search | Query |
|---|---|
| #1 | Cardiovascular Diseases[mh] OR cardiovascular disease*[tiab] OR cardiovascular disease*[ot] OR CVD[tiab] OR CVD[ot] OR coronary disease[tiab] OR coronary disease[ot] OR coronary heart disease[tiab] OR coronary heart disease[ot] OR MI[tiab] OR MI[ot] OR myocardial infarction[tiab] OR myocardial infarction[ot] OR myocardial ischemia[tiab] OR myocardial ischemia[ot] OR myocardial ischaemia[tiab] OR myocardial ischaemia[ot] |
| #2 | Risk[mh] OR risk[tiab] OR risk[ot] |
| #3 | absolute[tiab] OR absolute[ot] OR global[tiab] OR global[ot] OR total[tiab] OR total[ot] OR Framingham[tiab] OR Framingham[ot] OR office based[tiab] OR office based[ot] OR office-based[tiab] OR office-based[ot] OR non-laboratory[tiab] OR non-laboratory[ot] OR non-laboratory[tiab] OR non-laboratory[ot] OR IDEAL[tiab] OR IDEAL[ot] OR SCORE[tiab] OR SCORE[ot] |
| #4 | #2 AND #3 |
| #5 | FR-10[tiab] OR FR-10[ot] OR FRS[tiab] OR FRS[ot] OR ACC/AHA [tiab] OR ACC/AHA [ot] OR American College of Cardiology/American Heart Association[tiab] OR American College of Cardiology/American Heart Association[ot] OR QRISK[tiab] OR QRISK[ot] OR PROCAM[tiab] OR PROCAM[ot] OR REYNOLDS[tiab] OR REYNOLDS[ot] OR WHO/ISH[tiab] OR WHO/ISH[ot] |
| #6 | (American College of Cardiology[tiab] OR American College of Cardiology[ot]) AND (American Heart Association[tiab] OR American Heart Association[ot]) |
| #7 | #5 OR #6 |
| #8 | #4 OR #7 |
| #9 | Primary Health Care[mh] OR primary care[tiab] OR primary care[ot] OR Primary Prevention[mh] OR prevention and control[sh] OR prevent*[tiab] OR prevent*[ot] OR Health Promotion[mh] OR Health Education[mh] OR Urban Health Services[mh] OR Community Networks[mh] OR Community Medicine[mh] OR community[tiab] OR community[ot] OR Mass Screening[mh] OR screening[tiab] OR screening[ot] OR neighborhood[tiab] OR neighborhood[ot] OR program[tiab] OR program[ot] |
| #10 | Risk Assessment[mh] OR Risk Management[mh] OR Risk Reduction Behavior[mh] OR risk appraisal[tiab] OR risk appraisal[ot] OR Exercise[mh] OR exercise[tiab] OR exercise[ot] OR physical activit*[tiab] OR physical activit*[ot] OR Walking[mh] OR walking[tiab] OR walking[ot] OR Smoking Cessation[mh] OR Smoking[mh] OR smoking[tiab] OR smoking[ot] OR Weight Loss[mh] OR weight loss[tiab] OR weight loss[ot] OR Body Weight[mh] OR Diet[mh] OR Diet Therapy[mh] OR diet therapy[sh] OR diet[tiab] OR diet[ot] OR dietary[tiab] OR dietary[ot] OR Health Behavior[mh] OR behavior[tiab] OR behavior[ot] OR behavioral[tiab] OR behavioral[ot] OR behaviour[tiab] OR behaviour[ot] OR behavioural[tiab] OR behavioural[ot] OR Life Style[mh] OR life style[tiab] OR life style[ot] OR lifestyle[tiab] OR lifestyle[ot] |
| #11 | Outcome Assessment[mh] OR Patient Outcome Assessment[mh] OR outcome*[tiab] OR outcome*[ot] OR Exercise[mh] OR exercise[tiab] OR exercise[ot] OR physical activit*[tiab] OR physical activit*[ot] OR Walking[mh] OR walking[tiab] OR walking[ot] OR Smoking Cessation[mh] OR Smoking[mh] OR smoking[tiab] OR smoking[ot] OR Weight Loss[mh] OR weight loss[tiab] OR weight loss[ot] OR Body Weight[mh] OR Diet[mh] OR Diet Therapy[mh] OR diet therapy[sh] OR diet[tiab] OR diet[ot] OR dietary[tiab] OR dietary[ot] OR Life Style[mh] OR life style[tiab] OR life style[ot] OR lifestyle[tiab] OR lifestyle[ot] |
| #12 | #1 AND #8 AND #9 AND #10 AND #11 |
| #13 | ((“Infant”[Mesh] OR “Child”[Mesh] OR “Adolescent”[Mesh]) NOT “Adult”[Mesh]) |
| #14 | #12 NOT #13 |
| #15 | Animals[mh] NOT Humans[mh] |
| #16 | #14 NOT #15 |
| #17 | rat[tiab] OR rats[tiab] OR mouse[tiab] OR mice[tiab] OR murine[tiab] OR monkey[tiab] OR monkeys[tiab] OR primate[tiab] OR primates[tiab] OR rabbit[tiab] OR rabbits[tiab] OR pig[tiab] OR pigs[tiab] OR swine[tiab] |
| #18 | #16 NOT #17 |
| Results retrieved: 13,241 | |
Embase
Search conducted: November 30, 2016; updated in October 2018 and February 2022
| Search | Query |
|---|---|
| #1 | ‘cardiovascular disease’/exp OR ‘cardiovascular disease*’:ti,ab OR ‘coronary disease’:ti,ab OR ‘coronary heart disease’:ti,ab OR ‘CVD’:ti,ab OR ‘MI’:ti,ab OR ‘myocardial infarction’:ti,ab OR ‘myocardial ischaemia’:ti,ab OR ‘myocardial ischemia’:ti,ab |
| #2 | ‘Framingham risk score’/de OR ‘IDEAL score’/de |
| #3 | ‘risk’/de OR ‘risk’:ti,ab |
| #4 | ‘absolute’:ti,ab OR ‘global’:ti,ab OR ‘total’:ti,ab OR ‘Framingham’:ti,ab OR ‘office based’:ti,ab OR ‘office-based’:ti,ab OR ‘non-laboratory’:ti,ab OR ‘non-laboratory’:ti,ab OR ‘IDEAL’:ti,ab OR ‘SCORE’:ti,ab |
| #5 | ‘American College of Cardiology’:ti,ab AND ‘American Heart Association’:ti,ab |
| #6 | #4 OR #5 |
| #7 | #3 AND #6 |
| #8 | ‘FR-10’:ti,ab OR ‘FRS’:ti,ab OR ‘ACC/AHA’:ti,ab OR ‘QRISK’:ti,ab OR ‘PROCAM’:ti,ab OR ‘REYNOLDS’:ti,ab OR ‘WHO/ISH’:ti,ab |
| #9 | #2 OR #7 OR #8 |
| #10 | ‘community program’/de OR ‘health promotion’/de OR ‘health service’/de OR ‘primary medical care’/de OR ‘primary prevention’/de OR ‘screening’/de OR ‘community’:ti,ab OR ‘primary care’:ti,ab OR ‘prevent*’:ti,ab OR ‘screening’:ti,ab OR ‘neighborhood’:ti,ab OR ‘program’:ti,ab OR ‘intervention’:ti,ab |
| #11 | ‘aerobic exercise’/de OR ‘behavior modification’/de OR ‘caloric intake’/de OR ‘diet restriction’/de OR ‘exercise’/de OR ‘exercise’:ti,ab OR ‘feeding behavior’/de OR ‘group therapy’/de OR ‘lifestyle’/de OR ‘lifestyle’:ti,ab OR ‘life style’:ti,ab OR ‘lifestyle’:ti,ab OR ‘lifestyle modification’/de OR ‘Mediterranean diet’/de OR ‘patient counseling’/de OR ‘patient education’/de OR ‘personalized medicine’/de OR ‘physical activity’/exp OR ‘risk assessment’/de OR ‘screening’:ti,ab OR ‘smoking’:ti,ab OR ‘smoking cessation’/de OR ‘smoking cessation program’/de OR ‘smoking’:ti,ab OR ‘smoking ‘/de OR ‘walking’/de OR ‘walking’:ti,ab OR ‘weight reduction’/de OR ‘weight loss’:ti,ab OR ‘weight loss program’/de OR ‘diet’:ti,ab OR ‘dietary’:ti,ab OR ‘yoga’/de OR ‘yoga’:ti,ab |
| #12 | ‘outcome assessment’/de OR ‘outcome*’:ti,ab OR ‘cardiorespiratory fitness’/de OR ‘smoking cessation’/de OR ‘smoking cessation’:ti,ab OR ‘smoking’:ti,ab OR ‘smoking ‘/de OR ‘weight reduction’/de OR ‘weight loss’:ti,ab OR ‘risk reduction’/de OR ‘risk management’/de |
| #13 | #1 AND #9 AND #10 AND #11 AND #12 |
| #14 | #13 AND ([adolescent]/lim OR [child]/lim OR [embryo]/lim OR [fetus]/lim OR [infant]/lim OR [newborn]/lim OR [preschool]/lim OR [school]/lim) |
| #15 | #13 AND ([adult]/lim OR [aged]/lim OR [middle aged]/lim OR [very elderly]/lim OR [young adult]/lim) |
| #16 | #14 NOT #15 |
| #17 | #13 NOT #16 |
| #18 | #17 AND [animals]/lim |
| #19 | #17 AND [humans]/lim |
| #20 | #18 NOT #19 |
| #21 | #17 NOT #20 |
| #22 | ‘rat’:ti,ab OR ‘rats’:ti,ab OR ‘mouse’:ti,ab OR ‘mice’:ti,ab OR ‘murine’:ti,ab OR ‘monkey’:ti,ab OR ‘monkeys’:ti,ab OR ‘primate’:ti,ab OR ‘primates’:ti,ab OR ‘rabbit’:ti,ab OR ‘rabbits’:ti,ab OR ‘pig’:ti,ab OR ‘pigs’:ti,ab OR ‘swine’:ti,ab |
| #23 | #21 NOT #22 |
| Results retrieved: 14,450 | |
CINAHL (EBSCO)
Search conducted: December 1, 2016; updated in October 2018 and February 2022
| Search | Query |
|---|---|
| #1 | (MH “Cardiovascular Diseases+”) OR “cardiovascular disease*” OR “coronary disease” OR “coronary heart disease” OR “CVD” OR “MI” OR “myocardial infarction” OR (MH “Myocardial Ischemia”) OR “myocardial ischemia” OR “myocardial ischaemia” |
| #2 | “risk” |
| #3 | “absolute” OR “global” OR “total” OR “Framingham” OR “office based” OR “office-based” OR “non-laboratory” OR “non-laboratory” OR “IDEAL” OR “SCORE” |
| #4 | “American College of Cardiology” AND “American Heart Association” |
| #5 | #3 OR #4 |
| #6 | #2 AND #5 |
| #7 | “FR-10” OR “FRS” OR “ACC/AHA “ OR “QRISK” OR “PROCAM” OR “REYNOLDS” OR “WHO/ISH” |
| #8 | #6 OR #7 |
| #9 | (MH “Community Health Services”) OR (MH “Preventive Health Care”) OR “prevent*” OR (MH “Primary Health Care”) OR “primary care” OR (MH “Health Screening”) OR “screening” OR “community” OR “neighborhood” OR “program*” |
| #10 | (MH “Behavioral Changes”) OR (MH “Body Mass Index”) OR (MH “Cardiovascular Risk Factors”) OR (MH “Coronary Prone Behavior”) OR (MH “Counseling”) OR “diet” OR “dietary” OR “Exercise” OR “exercise” OR (MH “Health Behavior”) OR (MH “Health Screening”) OR (MH “Life Style”) OR “life style” OR (MH “Life Style Changes”) OR “lifestyle” OR (MH “Physical Activity”) OR “physical activity” OR “risk appraisal” OR (MH “Risk Assessment”) OR (MH “Risk Factors”) OR (MH “Health Screening”) OR “screening” OR (MH “Smoking”) OR (MH “Smoking Cessation”) OR (MH “Smoking Cessation Programs”) OR “smoking “ OR “smoking cessation” OR (MH “Walking”) OR “walking” OR (MH “Weight Control “) OR (MH “Weight Loss”) OR “weight loss” OR (MH “Weight Reduction Programs “) OR (MH “Yoga”) OR “yoga” |
| #11 | (MH “Outcome Assessment”) OR (MH “Outcomes (Health Care)”) OR “risk management” OR “risk reduction” OR (MH “Smoking”) OR (MH “Smoking Cessation”) OR “Weight Loss” OR “weight loss” OR “weight reduction” |
| #12 | #1 AND #8 AND #9 AND #10 AND #11 |
| Results retrieved: 401 | |
Cochrane Central Register of Controlled Trials (CENTRAL)
Search conducted: December 2, 2016; updated in October 2018 and February 2022
| Search | Query |
|---|---|
| #1 | MeSH descriptor: [Cardiovascular Diseases] explode all trees |
| #2 | MeSH descriptor: [Risk] this term only |
| #3 | risk in Trials |
| #4 | absolute or “global” or “total” or “Framingham” or “office based” or “office-based” or “non-laboratory” or “non-laboratory” or “IDEAL” or “SCORE” in Trials |
| #5 | American College of Cardiology and “American Heart Association” in Trials |
| #6 | #2 or #3 |
| #7 | #6 and (#4 or #5) |
| #8 | FR-10 or “FRS” or “ACC/AHA “ or “QRISK” or “PROCAM” or “REYNOLDS” or “WHO/ISH” in Trials |
| #9 | #7 or #8 |
| #10 | community or “neighborhood” or “prevent” or “prevents” or “prevention” or “primary care” or “program” or “screening” in Trials |
| #11 | MeSH descriptor: [Mass Screening] explode all trees |
| #12 | MeSH descriptor: [Primary Health Care] explode all trees |
| #13 | MeSH descriptor: [Primary Prevention] explode all trees |
| #14 | #10 or #11 or #12 or #13 in Trials |
| #15 | [mh “Body Weight”] or [mh Exercise] or [mh “Health Behavior”] or [mh “Life Style”] or [mh “Risk Assessment”] or [mh “Risk Management”] or [mh “Risk Reduction Behavior”] or [mh Smoking] or [mh “Smoking Cessation”] or [mh Walking] or [mh “Weight Loss”] or [mh “Mass Screening”] or [mh “Risk Assessment”] in Trials |
| #16 | behavior or behavioral or behaviour or behavioural or diet or dietary or exercise or life style or lifestyle or physical activit* or risk appraisal or smoking or smoking cessation or walking or weight loss or screening in Trials |
| #17 | #15 or #16 in Trials |
| #18 | [mh “Outcome Assessment”] or [mh “Patient Outcome Assessment”] or [mh Smoking] or [mh “Smoking Cessation”] or [mh Walking] or [mh “Weight Loss”] or [mh “Risk Management”] in Trials |
| #19 | outcome or “outcomes” or “smoking” or “smoking cessation” or “walking” or “weight loss” or “weight reduction” in Trials |
| #20 | #18 or #19 in Trials |
| #21 | #1 and #9 and #14 and #17 and #20 in Trials |
| #22 | [mh Child] or [mh infant] in Trials |
| #23 | [mh Adult] in Trials |
| #24 | #22 not #23 in Trials |
| #25 | #21 not #24 |
| Results retrieved: 296 | |
ProQuest Dissertations and Theses
Search conducted: April 13, 2018; updated in February 2022
| Search | Query |
|---|---|
| #1 | TI(cardiovascular disease OR coronary OR myocardial) OR AB(cardiovascular disease OR coronary OR myocardial) |
| #2 | TI(risk) OR AB(risk) |
| #3 | AB(absolute OR global OR total OR Framingham OR office based OR office-based OR non-laboratory OR IDEAL OR SCORE) |
| #4 | AB(American College of Cardiology AND American Heart Association) |
| #5 | #3 OR #4 |
| #6 | #2 AND# 5 |
| #7 | AB(FR-10 OR FRS OR ACC/AHA OR QRISK OR PROCAM OR REYNOLDS OR WHO/ISH) |
| #8 | #6 OR #7 |
| #9 | TI(community OR neighborhood OR prevent OR prevents OR prevention OR primary care OR screening) OR AB(community OR neighborhood OR prevent OR prevents OR prevention OR primary care OR screening) |
| #10 | TI(behavior OR behavioral OR behaviour OR behavioural OR diet OR dietary OR exercise OR life style OR lifestyle OR physical activity OR risk appraisal OR smoking OR smoking cessation OR walking OR weight loss OR weight reduction) OR AB(behavior OR behavioral OR behaviour OR behavioural OR diet OR dietary OR exercise OR life style OR lifestyle OR physical activity OR risk appraisal OR smoking OR smoking cessation OR walking OR weight loss OR weight reduction) |
| #11 | TI(outcome OR outcomes OR smoking cessation OR weight loss OR weight reduction) OR AB(outcome OR outcomes OR smoking cessation OR weight loss OR weight reduction) |
| #12 | #1 AND #8 AND# 9 AND #10 AND #11 |
| #13 | English only |
| Results retrieved: 100 | |
OCLC First Search Proceedings
Search conducted: April 25, 2018; updated in February 2022
| Search | Query |
|---|---|
| #1 | ti: cardiovascular or ti: coronary or ti: myocardial and ln= “english” |
| #2 | ti: risk and ln= “english” |
| #3 | (ti: cardiovascular or ti: coronary or ti: myocardial and ln= “english”) and (ti: risk and ln= “english”) |
| #4 | kw: absolute or kw: global or kw: total or kw: Framingham or (kw: office and kw: based) or kw: office-based or kw: non-laboratory or kw: IDEAL or kw: SCORE and ln= “english” |
| #5 | kw: American w College w1 Cardiology OR kw: American w Heart w Association and ln= “english” |
| #6 | kw: FR-10 or kw: FRS or kw: ACC/AHA or kw: QRISK or kw: PROCAM or kw: REYNOLDS or kw: WHO/ISH and ln= “english” |
| #7 | (kw: absolute or kw: global or kw: total or kw: Framingham or (kw: office and kw: based) or kw: office-based or kw: non-laboratory or kw: IDEAL or kw: SCORE and ln= “english”) or (kw: American w College w1 Cardiology OR kw: American w Heart w Association and ln= “english”) or (kw: FR-10 or kw: FRS or kw: ACC/AHA or kw: QRISK or kw: PROCAM or kw: REYNOLDS or kw: WHO/ISH and ln= “english”) |
| #8 | kw: community or kw: neighborhood or kw: prevent or kw: prevents or kw: prevention or kw: primary w care or kw: screening and ln= “english” |
| #9 | kw: behavior or kw: behavioral or kw: behaviour or kw: behavioural or kw: diet or kw: dietary or kw: exercise or kw: life w style or kw: lifestyle or kw: physical w activity or kw: risk w appraisal or kw: smoking or kw: smoking w cessation or kw: walking or kw: weight w loss or kw: weight w reduction and ln= “english” |
| #10 | kw: outcome or kw: outcomes or kw: smoking w cessation or kw: weight w loss or kw: weight w reduction and ln= “english” |
| #11 | (kw: cardiovascular or kw: coronary or kw: myocardial and ln= “english”) and (kw: risk and ln= “english”) and ((kw: absolute or kw: global or kw: total or kw: Framingham or (kw: office and kw: based) or kw: office-based or kw: non-laboratory or kw: IDEAL or kw: SCORE and ln= “english”) or (kw: American w College w1 Cardiology OR kw: American w Heart w Association and ln= “english”) or (kw: FR-10 or kw: FRS or kw: ACC/AHA or kw: QRISK or kw: PROCAM or kw: REYNOLDS or kw: WHO/ISH and ln= “english”)) and (kw: community or kw: neighborhood or kw: prevent or kw: prevents or kw: prevention or kw: primary w care or kw: screening and ln= “english”) and (kw: behavior or kw: behavioral or kw: behaviour or kw: behavioural or kw: diet or kw: dietary or kw: exercise or kw: life w style or kw: lifestyle or kw: physical w activity or kw: risk w appraisal or kw: smoking or kw: smoking w cessation or kw: walking or kw: weight w loss or kw: weight w reduction and ln= “english”) and (kw: outcome or kw: outcomes or kw: smoking w cessation or kw: weight w loss or kw: weight w reduction and ln= “english”) |
| #12 | #3 and #7 and #8 and #9 and #10 |
| Results retrieved: 371 | |
Clinicaltrials.gov search string (Classic website)
Search conducted: April, 2018; updated in February 2022
| In Advanced Search interface: | |
| Search # | Query |
| Condition or disease: | Cardiovascular diseases |
| Other terms: | Risk AND prevention AND behavior |
| Eligibility criteria: | Age Group = Adult 18–65 and Senior 66+ |
| Results retrieved: 474 | |
Grey Literature Report search string (Classic website)
Search conducted: Nov, 2016; search updates were not run because updates to the website and database ceased as of January 2017
| In Advanced Search interface: | |
|---|---|
| Search # | Query |
| Condition or disease: | Cardiovascular disease |
| Other terms: | Prevention |
| Eligibility criteria: | English |
| Results retrieved: 367 | |
Appendix II: Studies excluded on full text
Reason for exclusion: Studies included participants with a history of cardiovascular disease at baseline
-
1
Amin-Shokravi F, Rajabi R, Ziaee N. exercise effects on risk of cardiovascular disease among iranian women. Asian J Sports Med. 2011;2:37–43.
-
2
Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med. 2010;170:1794–803.
-
3
Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet FA, Lefrandt JD, et al. Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha-linolenic Enriched Groningen Dietary Intervention (MARGARIN) study. Am J Clin Nutr. 2002;75:221–7.
-
4
Bruckert E, Giral P, Paillard F, Ferrieres J, Schlienger JL, Renucci JF, et al. Effect of an educational program (PEGASE) on cardiovascular risk in hypercholesterolaemic patients. Cardiovasc Drugs Ther. 2008;22:495–505.
-
5
Burke V, Mansour J, Beilin LJ, Mori TA. Long-term follow-up of participants in a health promotion program for treated hypertensives (ADAPT). Nutr Metab Cardiovasc Dis. 2008;18:198–206.
-
6
Carrington MJ, Stewart S. Cardiovascular disease prevention via a nurse-facilitated intervention clinic in a regional setting: the Protecting Healthy Hearts Program. Eur J Cardiovasc Nurs. 2015;14:352–61.
-
7
Claes N, Jacobs N, Clays E, Schrooten W, De Bourdeaudhuij I. Comparing the effectiveness of two cardiovascular prevention programmes for highly educated professionals in general practice: a randomised clinical trial. BMC Cardiovasc Disord. 2013;13:38.
-
8
Colkesen EB, Ferket BS, Tijssen JG, Kraaijenhagen RA, van Kalken CK, Peters RJ. Effects on cardiovascular disease risk of a web-based health risk assessment with tailored health advice: a follow-up study. Vasc Health Risk Manag. 2011;7:67–74.
-
9
El Fakiri F, Bruijnzeels MA, Uitewaal PJ, Frenken RA, Berg M, Hoes AW. Intensified preventive care to reduce cardiovascular risk in healthcare centres located in deprived neighbourhoods: a randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2008;15:488–93.
-
10
Elley CR, Kerse N, Arroll B, Robinson E. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793.
-
11
Ellsworth DL, O’Dowd SC, Salami B, Hochberg A, Vernalis MN, Marshall D. Intensive lifestyle modification: impact on cardiovascular disease risk factors in subjects with and without clinical cardiovascular disease. Prev Cardiol. 2004;7:168–75.
-
12
Family Heart Study Group. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. Family Heart Study Group. BMJ. 1994;308:313–20.
-
13
Gomel MK, Oldenburg B, Simpson JM, Chilvers M, Owen N. Composite cardiovascular risk outcomes of a work-site intervention trial. Am J Public Health. 1997;87:673–6.
-
14
Kranjcevic K, Bergman Markovic B, Ivezic Lalic D, Vrdoljak D, Vucak J. Is a targeted and planned GP intervention effective in cardiovascular disease prevention? A randomized controlled trial. Med Sci Monit. 2014;20:1180–7.
-
15
Krantz MJ, Coronel SM, Whitley EM, Dale R, Yost J, Estacio RO. Effectiveness of a community health worker cardiovascular risk reduction program in public health and health care settings. Am J Public Health. 2013;103:e19–27.
-
16
Lauritzen T, Jensen MS, Thomsen JL, Christensen B, Engberg M. Health tests and health consultations reduced cardiovascular risk without psychological strain, increased healthcare utilization or increased costs. An overview of the results from a 5-year randomized trial in primary care. The Ebeltoft Health Promotion Project (EHPP). Scand J Public Health. 2008;36:650–61
-
17
Lindholm LH, Ekbom T, Dash C, Eriksson M, Tibblin G, Schersten B. The impact of health care advice given in primary care on cardiovascular risk. CELL Study Group. BMJ. 1995;310:1105–9.
-
18
Maron DJ, Forbes BL, Groves JR, Dietrich MS, Sells P, DiGenio AG. Health-risk appraisal with or without disease management for worksite cardiovascular risk reduction. J Cardiovasc Nurs. 2008;23:513–18.
-
19
Meland E, Laerum E, Ulvik RJ. Effectiveness of two preventive interventions for coronary heart disease in primary care. Scand J Prim Health Care. 1997;15:57–64.
-
20
Mendivil CO, Cortes E, Sierra ID, Ramirez A, Molano LM, Tovar LE, et al. Reduction of global cardiovascular risk with nutritional versus nutritional plus physical activity intervention in Colombian adults. Eur J Cardiovasc Prev Rehabil. 2006;13:947–55.
-
21
Onat A, Soydan I, Tokgozoglu L, Sansoy V, Koylan N, Domanic N, et al. Guideline implementation in a multicenter study with an estimated 44% relative cardiovascular event risk reduction. Clin Cardiol. 2003;26:243–9.
-
22
Painter PL, Hector L, Ray K, Lynes L, Paul SM, Dodd M, et al. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am J Kidney Dis. 2003;42:362–9
-
23
Pirraglia PA, Taveira TH, Cohen LB, Wu WC. Effectiveness of a multifactorial cardiovascular risk reduction clinic for diabetes patients with depression. Prev Chronic Dis. 2008;5:A127.
-
24
Racette SB, Deusinger SS, Inman CL, Burlis TL, Highstein GR, Buskirk TD, et al. Worksite Opportunities for Wellness (WOW): effects on cardiovascular disease risk factors after 1 year. Prev Med. 2009;49:108–14.
-
25
Rankin P, Morton DP, Diehl H, Gobble J, Morey P, Chang E. Effectiveness of a volunteer-delivered lifestyle modification program for reducing cardiovascular disease risk factors. Am J Cardiol. 2012;109:82–6.
-
26
Rautio N, Jokelainen J, Oksa H, Saaristo T, Peltonen M, Puolijoki H, et al. Family history of diabetes and effectiveness of lifestyle counselling on the cardio-metabolic risk profile in individuals at high risk of Type 2 diabetes: 1-year follow-up of the FIN-D2D project. Diabet Med. 2012;29:207–11.
-
27
Rautio N, Jokelainen J, Polonen A, Oksa H, Peltonen M, Vanhala M, et al. Changes in lifestyle modestly reduce the estimated cardiovascular disease risk in one-year follow-up of the Finnish diabetes prevention program (FIN-D2D). Eur J Cardiovasc Nurs. 2015;14:145–52.
-
28
Seely D, Szczurko O, Cooley K, Fritz H, Aberdour S, Herrington C, et al. Naturopathic medicine for the prevention of cardiovascular disease: a randomized clinical trial. CMAJ. 2013;185:E409–16.
-
29
Singh RB, Rastogi SS, Ghosh S, Niaz MA, SinghNK. The diet and moderate exercise trial (DAMET): results after 24 weeks. Acta Cardiol. 1992;47:543–57.
-
30
Reason for exclusion: Studies where participants received drug therapy or surgery in addition to lifestyle modification
-
31
Abbas SZ, Pollard TM, Wynn P, Learmonth A, Joyce K, Bambra C. The effectiveness of using the workplace to identify and address modifiable health risk factors in deprived populations. Occup Environ Med. 2015;72:664–9.
-
32
Becker DM, Yanek LR, Johnson Jr WR, Garrett D, Moy TF, Reynolds SS, et al. Impact of a community-based multiple risk factor intervention on cardiovascular risk in black families with a history of premature coronary disease. Circulation. 2005;111:1298–304.
-
33
Brett T, Arnold-Reed D, Phan C, Cadden F, Walker W, Manea-Walley W, et al. The Fremantle Primary Prevention Study: a multicentre randomised trial of absolute cardiovascular risk reduction. Br J Gen Pract. 2012;62:e22–8.
-
34
Cioe PA, Merrill JE, Gordon REF, Guthrie KM, Freiberg M, Williams DM, Risica PM, et al. Personalized feedback improves cardiovascular risk perception and physical activity levels in persons with HIV: results of a pilot randomized clinical trial. AIDS Care. 2021;33(6):786–94.
-
35
Cochrane T, Davey R, Gidlow C. Contribution of individual risk factor changes to reductions in population absolute cardiovascular risk. J Epidemiol Community Health. 2014;2014:626205.
-
36
Cramer H, Michalsen A, Steckhan N, Lauche R, Hohmann C, Choi KE, et al. Comprehensive lifestyle modification and fasting in patients with the metabolic syndrome: A randomized controlled trial. Glob Adv Health Med. 2020;9:30–1.
-
37
Devaraj S, Rockette-Wagner B, Arena V, Miller RG, Napoleone J, Conroy M, et al. The impact of a yearlong diabetes prevention program-based lifestyle intervention on cardiovascular health metrics. Circulation. 2020;141(SUPPL 1).
-
38
Di Renzo L, Cinelli G, Dri M, Gualtieri P, Attinà A, Leggeri C, et al. Mediterranean personalized diet combined with physical activity therapy for the prevention of cardiovascular diseases in Italian women Nutrients. 2020;12(11):1–16.
-
39
Dos Santos L, Ribeiro AS, Nunes JP, Tomeleri CM, Nabuco HCG, Nascimento MA, et al. Effects of pyramid resistance-training system with different repetition zones on cardiovascular risk factors in older women: a randomized controlled trial. Int J Environ Res Public Health. 2020;17(17).
-
40
Green BB, Anderson ML, Cook AJ, Catz S, Fishman PA, McClure JB, et al. e-Care for heart wellness: a feasibility trial to decrease blood pressure and cardiovascular risk. Am J Prev Med. 2014;46:368–77.
-
41
He Y, Shen J, He X, Dong X. Effects of community intervention and management on preventing and treating cardiovascular diseases among patients with dyslipidemia. Int J Clin Experiment Med. 2021;14(2):1283–91.
-
42
Henritze J, Brammell HL, McGloin J. LIFECHECK: a successful, low touch, low tech, in-plant, cardiovascular disease risk identification and modification program. Am J Health Promot. 1992;7(2):129–36.
-
43
Izzo R, de Simone G, Giudice R, Chinali M, Trimarco V, De Luca N, et al. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J Hypertens. 2010;28:1482–7.
-
44
Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, et al. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76(2):365–72.
-
45
Ketola E, Makela M, Klockars M. Individualised multifactorial lifestyle intervention trial for high-risk cardiovascular patients in primary care. Br J Gen Pract. 2001;51:291–4.
-
46
Keyserling TC, Sheridan SL, Draeger LB, Finkelstein EA, Gizlice Z, Kruger E, et al. A comparison of live counseling with a web-based lifestyle and medication intervention to reduce coronary heart disease risk: a randomized clinical trial. JAMA Intern Med. 2014;174:1144–57.
-
47
Kornitzer M, De Backer G, Dramaix M, Thilly C. The Belgian heart disease prevention project. Modification of the coronary risk profile in an industrial population. Circulation. 1980;61:18–25.
-
48
Lerman RH, Minich DM, Darland G, Lamb JJ, Schiltz B, Babish JG, et al. Enhancement of a modified Mediterranean-style, low glycemic load diet with specific phytochemicals improves cardiometabolic risk factors in subjects with metabolic syndrome and hypercholesterolemia in a randomized trial. Nutr Metab (Lond). 2008;5:29.
-
49
Lewis M, Chondros P, Mihalopoulos C, Lee YY, Gunn JM, Harvey C, et al. The assertive cardiac care trial: a randomised controlled trial of a coproduced assertive cardiac care intervention to reduce absolute cardiovascular disease risk in people with severe mental illness in the primary care setting. Contemp Clin Trials. 2020;97.
-
50
Lima EM, Gualandro DM, Yu PC, Giuliano Ide C, Marques AC, Calderaro D, et al. Cardiovascular prevention in HIV patients: results from a successful intervention program. Atherosclerosis. 2009;204:229–32.
-
51
Mor A, Omotosho P, Torquati A. Cardiovascular risk in obese diabetic patients is significantly reduced one year after gastric bypass compared to one year of diabetes support and education. Surg Endosc. 2014;28(10):2815–20.
-
52
Rossouw JE, Jooste PL, Chalton DO, Jordaan ER, Langenhoven ML, Jordaan PC, et al. Community-based intervention: the Coronary Risk Factor Study (CORIS). Int J Epidemiol. 1993;22:428–38.
-
53
Tiessen AH, Smit AJ, Broer J, Groenier KH, van der Meer K. Randomized controlled trial on cardiovascular risk management by practice nurses supported by self-monitoring in primary care. BMC Fam Pract. 2012;13:90.
-
54
Willaing I, Ladelund S, Jorgensen T, Simonsen T, Nielsen LM. Nutritional counselling in primary health care: a randomized comparison of an intervention by general practitioner or dietician. Eur J Cardiovasc Prev Rehabil. 2004;11:513–20.
Reason for exclusion: Studies were observational or absolute CVD risk score was not calculated
-
55
[Risk factor changes in four years in the Rome Project of Coronary Heart Disease Prevention (PPCC)]. G Ital Cardiol. 1980;10:204–15. [Italian]
-
56
Alavi R, Appel L, Maruthur N. The impact of achieving recommended lifestyle goals on CHD risk: Results from the premier trial. J Gen Intern Med. 2010. 25:S404-S405.
-
57
Alkhouli M, Homko CJ, Kashem A, Santimore WP, Memon N, Gonzalez J, et al. Impact of telemedicine system on CVD risk reduction and adoption of healthy life style behaviors. J Am College Cardiol. 2010;55:A55.E524.
-
58
Alkhouli M, Homko CF, Nabeel M, Kashem A, Bove AA. Behavioral comparison between rural and urban populations in cardiovascular disease risk reduction. J Am College Cardiol. 2010;55:A55.E523.
-
59
Alkhouli M, Kashem A, Homko CJ, Gonzalez J, Gupta A, Santimore WP, et al. Patient-centered care of stage I and II hypertension in underserved communities. Circulation. 2011;4.
-
60
Armah CN, Traka MH, Dainty JR, Doleman JF, Potter JF, Mithen RF. Effect of a high glucoraphanin broccoli diet on blood pressure and the cardiovascular risk profile of an at risk group: a randomised controlled study. J Hypertens. 2012;30:e123.
-
61
Benson G, Sidebottom A, Sillah A, Boucher J, Knickelbine T, Van Wormer J. Primary cardiovascular disease prevention is leaving the office: early results from the heartbeat connections integrated telemedicine program. J Am College Cardiol. 2014;63:A1432.
-
62
Bünger J, Lanzerath I, Ruhnau P, Görlitz A, Fischer C, Kott J, et al.. Company health care: evaluation of concepts for reducing cardiovascular risks. Arbeitsmed Sozialmed Praventivmed. 2003;38:421–5.
-
63
Davey R, Cochrane T, Iqbal Z, Rajaratnam G, Chambers R, Mawby Y, et al. Randomised controlled trial of additional lifestyle support for the reduction of cardiovascular disease risk through primary care in Stoke-on-Trent, UK. Contemp Clin Trials. 2010;31:345–54.
-
64
Engberg M, Christensen B, Karlsmose B, Lous J, Lauritzen T. [Can systematic general health screening and patient-physician health discussions improve the cardiovascular profile of the population? A randomized controlled trial in general practice with a 5-year follow-up]. Ugeskr Laeger. 2002;164:3354–60.
-
65
Fanghanel G, Sanchez-Reyes L, Felix-Garcia L, Violante-Ortiz R, Campos-Franco E, Alcocer LA. Impact of waist circumference reduction on cardiovascular risk in treated obese subjects. Cir Cir. 2011;79:175–81.
-
66
Gruninger U, Weidmann P, Abelin T, Bally C, Howald H, Mordasini R, et al. [Cardiovascular risk factors: an intervention program for general practice]. Schweiz Med Wochenschr. 1984;114:1744–6.
-
67
Hjermann I. Intervention of smoking and eating habits in healthy men carrying high risk for coronary heart disease. The Oslo Study. Acta Med Scand Suppl. 1981;651:281–4.
-
68
Marcon ER, Gus I, Neumann CR. [Impact of a minimum program of supervised exercises in the cardiometabolic risk in patients with morbid obesity]. Arq Bras Endocrinol Metabol. 2011;55:331–8.
-
69
Rywik S, Szostak WB, Charzewska J, Wagrowska H, Chabros E, Chotkowska E. [The changes in risk factors levels after two years of implementation of the coronary diseases prevention in Poland. I. The dynamics of changes in total population (author’s transl)]. Przegl Lek. 1981;38:769–77.
-
70
Sierra MC, Bonacho EC, Garcia AG, Moraga MR, Gutierrez JC, Barrientos AC, et al. [Effectiveness of a preventive intervention strategy based on structured telephone interviews in a working population with a moderate to high cardiovascular risk]. Aten Primaria. 2010;42:498–505.
-
71
Song R, Ahn S, So HY, Park IS, Kim HL, Joo KO, et al. [Effects of Tai Chi exercise on cardiovascular risk factors and quality of life in post-menopausal women]. J Korean Acad Nurs. 2009;39:136–44.
-
72
Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72(11):1819–25.
-
73
Weinehall L, Hellsten G, Boman K, Hallmans G, Asplund K, Wall S. Can a sustainable community intervention reduce the health gap?--10-year evaluation of a Swedish community intervention program for the prevention of cardiovascular disease. Scand J Public Health Suppl. 2001;56:59–68.
-
74
Weinehall L, Lewis C, Nafziger AN, Jenkins PL, Erb TA, Pearson TA, et al. Different outcomes for different interventions with different focus!--A cross-country comparison of community interventions in rural Swedish and US populations. Scand J Public Health Suppl. 2001;56:46–58.
-
75
Weinehall L, Westman G, Hellsten G, Boman K, Hallmans G, Pearson TA, et al. Shifting the distribution of risk: results of a community intervention in a Swedish programme for the prevention of cardiovascular disease. J Epidemiol Community Health. 1999;53:243–50.
-
76
Winkleby MA, Feldman HA, Murray DM. Joint analysis of three U.S. community intervention trials for reduction of cardiovascular disease risk. J Clin Epidemiol. 1997;50:645–58.
-
77
Yang SY, Li XJ, Zhang W, Liu CQ, Zhang HJ, Lin JR, et al. Chinese lacto-vegetarian diet exerts favorable effects on metabolic parameters, intima-media thickness, and cardiovascular risks in healthy men. Nutr Clin Pract. 2012;27:392–8.
Studies where data needed for meta-analysis not available/study quality problems
-
78
Athyros VG, Kakafika AI, Papageorgiou AA, Tziomalos K, Peletidou A, Vosikis C, et al. Effect of a plant stanol ester-containing spread, placebo spread, or Mediterranean diet on estimated cardiovascular risk and lipid, inflammatory and haemostatic factors. Nutr Metab Cardiovasc Dis. 2011;21(3):213–21.
-
79
Balducci S, Zanuso S, Cardelli P, Salvi L, Mazzitelli G, Bazuro A, et al. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care. 2012;35(6):1347–54.
-
80
Barranco-Ruiz Y, Ramírez-Vélez R, Martínez-Amat A, Villa-González E. Effect of two choreographed fitness group-workouts on the body composition, cardiovascular and metabolic health of sedentary female workers. Int J Environ Res Public Health. 2019;16(24).
-
81
Bartunkova S. An attempt to intervene combined risk score by increased physical activity. Act Nerv Super (Praha). 1982;Suppl 3(Pt 2):466–9.
-
82
Bedard A, Dodin S, Corneau L, Lemieux S. Impact of the traditional Mediterranean diet on the Framingham risk score and the metabolic syndrome according to sex. Metab Syndr Relat Disord. 2014;12(2):95–101.
-
83
Benner JS, Erhardt L, Flammer M, Moller RA, Rajicic N, Changela K, et al. A novel programme to evaluate and communicate 10-year risk of CHD reduces predicted risk and improves patients’ modifiable risk factor profile. Int J Clin Pract. 2008;62(10):1484–98.
-
84
Dutheil F, Lac G, Lesourd B, Chapier R, Walther G, Vinet A, et al. Different modalities of exercise to reduce visceral fat mass and cardiovascular risk in metabolic syndrome: the RESOLVE randomized trial. Int J Cardiol. 2013;168(4):3634–42.
-
85
Edelman D, Oddone EZ, Liebowitz RS, Yancy WS, Olsen MK, Jeffreys AS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21(7):728–34.
-
86
Garcia-Ortiz L, Grandes G, Sanchez-Perez A, Montoya I, Iglesias-Valiente JA, Recio-Rodriguez JI, et al. Effect on cardiovascular risk of an intervention by family physicians to promote physical exercise among sedentary individuals. Rev Esp Cardiol. 2010;63(11):1244–52.
-
87
Ghadimi Nouran M, Kimiagar M, Abadi A, Mirzazadeh M, Harrison G. Peanut consumption and cardiovascular risk. Public Health Nutr. 2010;13(10):1581–6.
-
88
Gibbs BB, King WC, Belle SH, Jakicic JM. Six-month changes in ideal cardiovascular health vs. Framingham 10-year coronary heart disease risk among young adults enrolled in a weight loss intervention. Prev Med. 2016;86:123–9.
-
89
Harrington DM, Champagne CM, Broyles ST, Johnson WD, Tudor-Locke C, Katzmarzyk PT. Cardiometabolic risk factor response to a lifestyle intervention: a randomized trial. Metab Syndr Relat Disord. 2015;13(3):125–31.
-
90
Haruyama Y, Muto T, Nakade M, Kobayashi E, Ishisaki K, Yamasaki A. Fifteen-month lifestyle intervention program to improve cardiovascular risk factors in a community population in Japan. Tohoku J Exp Med. 2009;217(4):259–69.
-
91
Hellenius ML, e Faire U, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis. 1993;103(1):81–91.
-
92
Jones PJ, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, et al. DHA-enriched high-oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am J Clin Nutr. 2014;100(1):88–97.
-
93
Lara JJ, Economou M, Wallace AM, Rumley A, Lowe G, Slater C, et al. Benefits of salmon eating on traditional and novel vascular risk factors in young, non-obese healthy subjects. Atherosclerosis. 2007;193(1):213–21.
-
94
Liu Z, Chen S, Zhang G, Lin A. Mobile phone-based lifestyle intervention for reducing overall cardiovascular disease risk in Guangzhou, China: a pilot study. Int J Environ Res Public Health. 2015;12(12):15993–6004.
-
95
Lopez-Gonzalez AA, Aguilo A, Frontera M, Bennasar-Veny M, Campos I, Vicente-Herrero T, et al. Effectiveness of the Heart Age tool for improving modifiable cardiovascular risk factors in a Southern European population: a randomized trial. Eur J Prev Cardiol. 2015;22(3):389–96.
-
96
Lotfaliany M, Sathish T, Shaw J, Thomas E, Tapp RJ, Kapoor N, et al. Effects of a lifestyle intervention on cardiovascular risk among high-risk individuals for diabetes in a low- and middle-income setting: Secondary analysis of the Kerala Diabetes Prevention Program. Prevent Med. 2020;139:106068.
-
97
Lukaczer D, Liska DJ, Lerman RH, Darland G, Schiltz B, Tripp M, et al. Effect of a low glycemic index diet with soy protein and phytosterols on CVD risk factors in postmenopausal women. Nutrition. 2006;22(2):104–13.
-
98
Maccoby N, Farquhar JW, Wood PD, Alexander J. Reducing the risk of cardiovascular disease: effects of a community-based campaign on knowledge and behavior. J Community Health. 1977;3(2):100–14.
-
99
Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119(15):2026–31.
-
100
Nishi SK, Kendall CW, Bazinet RP, Bashyam B, Ireland CA, Augustin LS, et al. Nut consumption, serum fatty acid profile and estimated coronary heart disease risk in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2014;24(8):845–52.
-
101
White K, Jacques PH. Combined diet and exercise intervention in the workplace: effect on cardiovascular disease risk factors. AAOHN J. 2007;55(3):109–14.
-
102
Will JC, Massoudi B, Mokdad A, Ford ES, Rosamond W, Stoddard AM, et al. Reducing risk for cardiovascular disease in uninsured women: combined results from two WISEWOMAN projects. J Am Med Womens Assoc. 2001;56(4):161–5.
-
103
Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. CMAJ. 2007;177(8):859–65.
-
104
van den Brekel-Dijkstra K, Rengers AH, Niessen MA, e Wit NJ, Kraaijenhagen RA. Personalized prevention approach with use of a web-based cardiovascular risk assessment with tailored lifestyle follow-up in primary care practice--a pilot study. Eur J Prev Cardiol. 2016;23(5):544–51.
-
105
Henritze J, Brammell HL, McGloin J. LIFECHECK: a successful, low touch, low tech, in-plant, cardiovascular disease risk identification and modification program. Am J Health Promot. 1992;7(2):129–36.
-
106
Hjermann I. Intervention of smoking and eating habits in healthy men carrying high risk for coronary heart disease. The Oslo Study. Acta Med Scand Suppl. 1981;651:281–4.
-
107
Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, et al. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76(2):365–72.
-
108
Mor A, Omotosho P, Torquati A. Cardiovascular risk in obese diabetic patients is significantly reduced one year after gastric bypass compared to one year of diabetes support and education. Surg Endosc. 2014;28(10):2815–20.
-
109
Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72(11):1819–25.
-
110
Pereira MA, Mullane SL, Toledo MJL, Larouche ML, Rydell SA, Vuong B, et al. Efficacy of the ‘stand and move at work’ multicomponent workplace intervention to reduce sedentary time and improve cardiometabolic risk: a group randomized clinical trial. Int J Behav Nutr Phys Act. 2020;17(1):133.
-
111
Seguin-Fowler RA, Strogatz D, Graham ML, Eldridge GD, Marshall GA, Folta SC, et al. The Strong Hearts, Healthy Communities Program 2.0: an RCT examining effects on Simple 7. Am J Prev Med. 2020;59(1):32–40.
-
112
Yousuf H, Reintjens R, Slipszenko E, Blok S, Tulevski II, Hofstra L. Effectiveness of web-based personalised e-Coaching lifestyle interventions. Netherlands Hear J. 2019;27(1):24–9.
-
113
Klimis H, Thiagalingam A, McIntyre D, Marschner S, Von Huben A, Chow CK. Text messages for primary prevention of cardiovascular disease: The TextMe2 randomized clinical trial. Am Heart J. 2021;242:33–44.
-
114
Schouw D, Mash R, Kolbe-Alexander T. Changes in risk factors for non-communicable diseases associated with the ‘Healthy choices at work’ programme, South Africa. Glob Health Action. 2020;13(1).
-
115
Pesis-Katz I, Smith JA, Norsen L, Devoe J, Singh R. Reducing cardiovascular disease risk for employees through participation in a wellness program. Popul Health Manag. 2020;23(3):212–19.
-
116
Vuksan V, Sievenpiper JL, Jovanovski E, Jenkins AL, Komishon A, Au-Yueng F, et al. Effect of soluble-viscous dietary fibre on coronary heart disease risk score across 3 population health categories: Data from randomized, double-blind, placebo-controlled trials. Appl Physiol Nutr Metab. 2020;45(7):801–4.
Appendix III: Characteristics of included studies
| Study | Country | Setting/context | Participant characteristics | Groups | Outcomes measured | Description of main results |
|---|---|---|---|---|---|---|
| Ahn and Kim,48 2020 | South Korea | Elderly Health Promotion Center | Elderly women (>65 years) who were members of the Elderly Health Promotion Center in South Korea. The participants did not have any comorbid chronic conditions except hypertension | Normal blood pressure group (120–129/80–84) included n=18 participants High-normal blood pressure group (130–139/85–89) included 12 participants. The groups’ categories were based on the European Guidelines for the Management of Arterial Hypertension. Both groups used an exercise program that was made up of combined workouts of elastic band resistance exercise and aerobics with dance music, 3 times/week for 6 months |
Framingham coronary heart disease risk score | In the normal blood pressure group, the Framingham risk score reduced from 7.98 at baseline to 7.47 post-intervention. In the high-normal blood pressure group, the Framingham risk score reduced from 17.63 at baseline to 16.17 post-intervention. |
| Al Mheid et al.,39 2016 | United States | University | University employees for at least 2 years and covered by university-sponsored health insurance plans Participants were recruited through human resources department 711 participants were enrolled in the study, but 14% were lost to follow-up. Mean age 48.5 (11.1) years |
Intervention: Counseling provided by a trained health partner focusing on promoting clinical self-knowledge and adoption of a healthier lifestyle Sampling frame: 10,000 university employees who had worked for ≥2 years and were covered by the university-sponsored health insurance plans Sampling method: Random (every 10th employee was sent an invitation to participate) Baseline characteristics: Median age 48.5 (11.1) Sex: 35% male Race: 72% White; 23% Black; 6% other Education: 82% college degree Smokers: 5% Weight: 64% overweight or obese History of high blood pressure: 34% History of diabetes: 11% Median total CVD risk score: 5.5% No control group was included |
The American Heart Association “Life’s Simple 7” ideal cardiovascular health score was used to assess total cardiovascular health; Framingham risk score (FRS CHD) was used to estimate 10-year risks for coronary death or nonfatal myocardial infarction | The ideal cardiovascular health score increased by 0.28, 0.40, and 0.33 at 6-months, 1 year, and 2 years, respectively, compared with baseline visit (mean LS7 score 7.93) There was a significant mean reduction in total CVD risk score from baseline to 24 months: −12% (<0.05) |
| Anand et al.,53 2016 | Canada | Community: Health messages sent via email Single-blind randomized clinical trial |
Single-blind randomized clinical trial intervention Intervention: n=169 (n=169 -> n=164; 3% attrition) Age: 50.6 ±12.0 Sex: 79 (46.7%) M Current or former smoker: 14 (8.3%) BMI (mean, SD): men 26.8 (3.2); women 26.7 (3.9) Baseline MI risk score: 13.3 (6.3); 152 of the 169 participants randomized to the intervention were included in the primary analysis. The median number of motivational messages sent to the DHI group was 26 (interquartile range, 24–28) and the median number of health tips was 54 (interquartile range, 53–60) compared with 0 sent to the control group during 12 months. Control: n=174 (n=174 -> n=173; 0.6% attrition) Age: 50.6 ±10.9 Sex: male 99 (56.9%) Current or former smoker: 20 (11.5%) Vegetarian: 67 (38.5%) BMI (mean, SD): men 27.3 (3.6); women 26.7 (4.3) Baseline MI risk score (mean, SD): 13.3 (6.9); 159 of the 174 participants randomized to the control were included the primary analysis |
DHI: Dietary intake and physical activity were targeted each for 6 months. Participants randomized to a DHI received the following types of messages: i) stages of change: oriented motivational messages, which supported confidence in behavior change, sent by email every 2 weeks; and ii) health tips focused on diet and physical activity sent by email or text messages (participant’s choice) every week. Participants were also encouraged to access the SAHARA website for South Asian–specific prevention advice Control participants randomized to the control condition were encouraged to access the SAHARA website |
Clinical assessments at 6 months and 1 year after randomization MI risk score: age, sex, brief dietary and physical activity questions, tobacco exposure, psychosocial stress, blood pressure, waist and hip circumference, and levels of apolipoprotein A and B and hemoglobin A1C Ratio of ApoB: ApoA1 A1C level Self-reported type 2 diabetes mellitus Self-reported hypertension BP Waist-to-hip ratio Current or former smoker; exposure to second-hand smoke Stress during past year Depression ≥2 wk in past year No. of servings per day of fruits and vegetables, deep-fried foods, salty snacks, meat, or poultry Moderate or very active in leisure time |
The MI risk score decreased from 13.3 to 12.3 in the intervention group and from 13.3 to 12.6 in the control group. The relative change between intervention participants and controls was not significant (−0.27; 95% CI, −1.12 to 0.58; p=0.53) and remained nonsignificant in the adjusted model (−0.39; 95% CI, −1.24 to 0.45; p=0.36). No difference between the intervention and control participants was observed in the sensitivity analysis among participants with high adherence (−0.02; 95% CI, −1.05 to 1.01; p=0.97). Furthermore, no changes in the measured components of the risk score occurred between baseline and the end of the study. |
| Balducci et al.,61 2012 | Italy | Hospital/clinic | Recruitment/sampling: Sedentary patients with type 2 diabetes Randomization: stratified by center and, within each center, by age (<60 vs. >=60 years) and type of diabetes treatment (diet with or without oral agents vs. insulin) using permuted-block randomization software. |
RCT with 2 study groups: intervention and standard care Group 1 (intervention, n=303 baseline; n= 288 12 months): Structured, individualized counseling aimed at achieving the currently recommended amount of PA by encouraging any type of PA. Age (mean, SD): 58.8 (8.5) Gender: 58% M, 42% F History of hypertension: 67.4% History of diabetes: 6.6% Total CVD risk score (UKPDS) at baseline (mean, SD): 19.5% (13.3) Protocol adherence: mean attendance 80.3%; attrition rates 5.0%. Group 2 (standard care, n= 303 baseline; n= 275 12 months) Standard care consisted of a treatment regimen aimed at achieving optimal glycemic, lipid, BP, and body weight targets, as established by current guidelines. Age (mean, SD): 58.8 (8.5) Gender: 58% M, 42% F History of hypertension: 60.7% History of diabetes: 8.0% Total CVD risk score (UKPDS) at baseline (mean, SD): 18.5 (12.2) Attrition rate: 9.25% |
Global CHD 10-year risk (UKPDS) A1C Blood glucose BMI BP Triglyceride Total cholesterol HDL |
PA/exercise-induced improvements in in UKPDS (−0.152, p=0.027). Control group 10-year CHD UKPDS risk score baseline to 12 months: 18.5 (12.2) vs 17.8 (12.0); p=0.08 Exercise group 10-year CHD UKPDS risk score baseline to 12 months: 19.5 (13.3) vs. 15.8 (10.4); p <0.001 Exercise vs. control p value <0.001. There was also improvement in physical fitness: higher VO2 max associated with decrease in A1C from baseline to end of study (−0.023, p=0.03); decrease in waist circumference (−0.206, p<0.0001); increase in HDL (0.206, p=0.038) |
| Baldwin,52 2015 | USA | Community | n=38 Age (mean, SD): 58 years (32) 45% were widowed, divorced, or single; 20% were uninsured |
The pilot study used a non-randomized, pretest–post-test, 1-group design without comparators. The program was adapted from several programs with established efficacy that used the Small Steps, Big Rewards Program (National Diabetes Education Program, 2010). The program encompassed multilevel interventions, such as a combination of PA classes and walking, assignments, wellness education classes, cooking, motivational interviewing groups, and health coaching. Individual health coaching and motivational interviewing group sessions helped participants to select and attain goals and plans of action. Lifestyle modification, education classes, and individualized health coaching were scripted and delivered by students. Program adherence was defined as completion of the orientation session, at least 11 of the 14 active-phase classes, and post-program measurements. |
Total risk score: American Heart Association guidelines (2012) and 4 clinical outcomes (A1C, BMI, waist-to-hip ratio, and BP) Systolic BP Diastolic BP BMI A1C Questionnaires assessed: Health behavior total (unclear how it was determined) Physical activity (unclear how it was determined) Nutritional behavior (unclear how it was determined) |
Baseline vs. 12-week, paired t-test, significance Total risk score: 30.1 (4.5) vs. 26.8 (3.2), 3.14, p ≤ 0.05 Systolic BP: 146.8 (11.2) vs. 135.7 (8.3), 2.57, not significant Diastolic BP: 84.6 (6.2) vs. 83.9 (5.1), 1.44, not significant BMI: 38.7 (3.0) vs. 35.48 (2.0), 3.44, p ≤ 0.05 A1C: 6.8 (3.0) vs. 6.0 (1.5), 3.04, p ≤ 0.05 Health behavior total: 66.3 (8.1) vs. 69.7 (5.0), −2.02, p ≤ 0.05 Physical activity: 1.88 (0.2) vs. 2.58 (0.3), −6.75, p ≤ 0.05 Nutritional behavior: 3.41 (0.3) vs. 3.47 (0.2), −0.93; not significant |
| Bebenek et al.,57 2010 | Germany | University | Recruitment/sampling: 128 women Women 48 to 55 years old, 1 to 3 years after menopuase Simple random sampling: mailings Computer-generated block randomization stratified for menopause age |
RCT including 3 study groups: exercise program, exercise plus CR program, wellness control. Group 1 (exercise program, n=43 baseline; n= 36 12-month) Age (mean, SD): 52.3 (2.3) Gender: 100% F Smokers, current: 11.6% Weight (mean, SD): 69.5 (9.6) kg History of diabetes: 0% Total CVD risk score at baseline (mean, SD): 6% (2.5) Protocol adherence: attendance rate 76.3% group session; 42.2% home training Attrition rates: 16.3%. Group 2 (exercise + CR, n= 43 baseline; n= 37 12-month) Exercise program in addition to 40mg/day Cimicifuga racemosa (CR) Age (mean, SD): 51.8 (2.7) Gender: 100% F Smokers: 9.3% current Weight (mean, SD): 72.0 (16.8) kg History of diabetes: 0% Total CVD risk score at baseline (mean, SD): 6.2% (2.5) Attrition rate: 14%. Group 3 (Wellness Program, n= 42 baseline; n= 30 12-month) Mean (SD): 52.4 (2.7) Gender: 100% F Smokers: 14.3% current Weight (mean, SD): 70.9 (16.8) kg History of diabetes: 2.4% Total CVD risk score at baseline (mean, SD): 6.7% (3.5) Attrition rates: 28.6%. |
10-year CHD risk (FRS CHD) Not considered primary or secondary endpoint, but used to calculate FRS: fasting glucose total cholesterol HDL triglyceride blood pressure |
Control group CHD risk score baseline, 12 month, Diff (95% CI): 6.7 (3.5); 7.8 (3.8); 1.10 (2.09) p=0.007 Exercise group CHD risk score baseline, 12 month, Diff (95% CI): 6.0 (2.5); 6.1 (2.1); 0.16 (1.89) p=0.603 Exercise + CR group CHD risk score baseline, 12 month, Diff (95%CI): 6.2 (2.5); 7.0 (3.1); 0.78 (1.98) p=0.018 No significant changes were observed among the groups. |
| Bernocchi et al.,49 2011 | Italy | Hospital/clinical | Recruitment/Sampling: 27 men and women age <75 years with 3+ risk factors (smoking, HTN, DM, obesity, hyperchol) Sampling not mentioned |
Quasi-experimental pre- and post-test with Group 1 (n= 27 baseline; n= 27 6-month): At least 3 sessions a week of bicycle exercise training or fast walking for at least 30 minutes. Age (mean, SD): 54.9 (9.0) Gender: 37% F Smokers: 14.8% Weight (mean, SD): 92 (18) kg Waist circumference (SD): 110 (4) cm History of hypertension: 100% History of diabetes: 30% Total CVD risk score at baseline (mean, SD): FRS 10 (6) Total CVD risk score at baseline (mean, SD): Progetto 10 (8) Protocol adherence: attendance rate 64 sessions (3.7%), 19% with 3+ sessions per week Attrition rates: 0% |
10-year CVD risk (FRS CHD) 10-year Progetto CUORE, BMI, weight, blood glucose, total cholesterol, HDL, Triglyceride, BP, activity Level | Baseline vs. 6-month, 10-year CVD risk (FRS CHD): 10 (6) vs 8 (6) p=0.05 10-year BMI: 2 (6) vs 31 (5) p=0.01 Total cholesterol: 230 (43) vs 222 (46) NS HDL: 50 (9.6) vs 47 (8.3) NS Triglyceride: 193 (17) vs 151 (76) NS BP: 132 (12)/78 (7) vs 130 (11)/77 (6) NS Sedentary (%): 76.2 (8.7) vs 71.5 (9.3) p=0.01 |
| Brotons et al.,67 2021 | Spain | Primary health care centers in urban and semi-urban areas | 464 subjects were randomly assigned to intervention or control groups Gender: 59.3% M Age (mean, SD): 61.0 (8.0) years |
Intervention group (n=228) provided tailored education about the meaning of absolute CVD risk, relative risk, and vascular age. The control group (n= 236) were visited at the beginning of the study and 1 year after the baseline visit. There were no significant differences between the 2 groups at baseline. |
Primary outcome was total CVD risk, estimated using the REGICOR score, which was modified from Framingham-Wilson score for the Girona region in Spain. Framingham-Wilson risk score estimates 10-year risk of coronary morbidity and mortality |
The REGICOR score reduced from 7.65% to 7.02% (p = 0.005) in the intervention group. The control group did not have any significant reduction in total CVD risk score (7.70% to 7.25%, p=0.059). There were no statistically significant differences between intervention and control groups. |
| Chan et al.,47 2012 | Hong Kong | Local community and a rehabilitation hospital in Hong Kong | Cantonese-speaking Chinese adults aged ≥18 years old with no history of CVD were enrolled in a single group pre- and post-intervention study 215 participants were recruited from the community on the voluntary basis Age (mean, SD): 51.1 (9.5) years Attrition rate: 30% |
Sampling frame: 250 community-dwelling individuals were invited to participate in the study. To be eligible, the participants had to be free of CVD, uncontrolled CVD risk factors, and cognitive impairment 35 of those invited were ineligible, and 215 were enrolled in the study Sampling strategy: convenient sample of those who met eligibility criteria The intervention included 14 sessions of empowerment workshops on cardiovascular health, diet, and community exercise classes. During the empowerment sessions, a physiotherapist and a nurse facilitated subjects to adopt healthy behaviors and lifestyle through active and mutual participation, goals setting, action planning, self-reflection, and peer-support. Assessment of different health domains, self-efficacy, and risks were also conducted. Baseline characteristics: Age (mean, SD): 51.1 (9.5) years Gender: 59% F SmokersL 7% Weight: 40% obese Race: 100% Chinese History of diabetes: 5.6% History of HTN: 33%. The baseline total CVD risk score was 8.73% (8.29) |
Total CVD risk estimated using FRS to estimate 10-year risk of general CVD (CHD, stroke, PVD, CHF, cardiac death) Lab-based measures, including fasting blood glucose, total cholesterol, HDL cholesterol, and triglycerides were assessed after overnight fast (method not discussed). LDL cholesterol formula not specified. BP measurements taken according to the recommendation of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7). The protocols for anthropometric measurements are not described. |
Baseline to post-intervention (14 sessions) changes (mean unless otherwise noted) Significant changes were reported for: total CVD risk (FRS) −3.4% (15.3 vs 11.93, p<0.001) for men; 0.6% (6.18 vs 5.56, p=0.01) for women. The overall change in total CVD risk score was −1.4% (8.66 vs 7.29, p<0.001); SBP: 1% (127.80 vs 126.53, p>0.05); DBP: 2.1% (76.25 vs 74.63, p=0.01); total cholesterol −0.7% (5.30 mmol/L (204.95mg/dL) vs 5.26 mmol/L (203.4mg/dL), p>0.05); HDL 3.9% (1.36 mmol/L [52.59 mg/dL] vs 1.42 mmol/L [54.91 mg/dL], p<0.001); LDL −1% (3.34 mmol/L [129.16mg/dL] vs 3.31 mmol/L [128mg/dL], p>0.05); and fasting blood glucose −0.3 (5.18 mmol/L [93.33 mg/dL] vs 5.16 mmol/L [92.97 mg/dL] p>0.05). No other outcomes of interest are reported. |
| Curtis et al.,59 2012 | UK | Hospital/clinic | Recruitment/sampling: 118 postmenopausal women aged 51–74, with T2DM Simple random sampling: general practitioners, specialists, advertisements Randomization: simple randomization balanced by age, BMI, years since menopause, and insulin use |
RCT with 2 study groups Group 1 (Flavonoid-enriched, n=59 baseline; n= 47 12-month) Daily intake of 27g flavonoid-enriched chocolate/1 year, 90mg epicatechin, 100mg isoflavones Age (mean, SD): 62.1 (0.73) years Gender: 100% F Smokers: 30% past Weight (mean, SD): BMI 32.69 (1.09) History of HTN: 60% (medicated for HTN) History of diabetes: 19% (medicated with insulin) Total CVD risk score at baseline (mean, SD): 9.35% (0.56) UKPDS CHD RISK Total CVD risk score at baseline (mean, SD): 6.14% (0.49) Attrition rates: 20.3% Group 2 (Placebo, n= 59 baseline; n= 46 12-month) Placebo chocolate twice a day for a year Age (mean, SD): 62.98 (0.8) years Gender: 100% F Race/ethnicity: not provided Smokers: 41% BMI (mean, SD): 31.85 (0.87) History of HTN: 54% History of diabetes: 20% (medicated with insulin) Total CVD risk score at baseline (mean, SD): 10.21% (0.65) UKPDS CHD RISK Total CVD risk score at baseline (mean, SD): 6.79% (0.56) Attrition rates: 22% |
10-year UKPDS CHD RISK; 10-year UKPDS FATAL CHD RISK; 10-year UKPDS STROKE RISK HOMA-IR; glucose; insulin; triglycerides; LDL; total cholesterol; HDL; SBP A1C | Flavonoid-enriched group vs placebo group at 12 months: 10-year UKPDS CHD RISK 9.44 (0.57) vs 11.33 (0.73) (p<0.05). |
| Elramli,55 2017 | United Kingdom | Hospitals (ie, rheumatology outpatient clinics at Gartnavel General Hospital, Glasgow Royal Infirmary Hospital and Stobhill Hospital in Glasgow, UK) | Patients ≥18 years old with rheumatoid arthritis who were within 5 years of diagnosis and free from severe HTN, joint replacement in the previous 6 months, unstable cardiac conditions or other serious pathology affecting their ability to take part in physical activity 76 participants were recruited Mean age: 56 years Attrition rate: intervention group 4.1%; control group 40.6% |
Sampling frame: 320 patients with rheumatoid arthritis were invited to participate in the study; 244 (76%) did not meet the eligibility criteria 76 participants were enrolled in the study, which lasted for 6 months There were no significant difference in baseline characteristics for the intervention and control groups in regards to: mean age 58.2 vs 54.5 years; 74.4 vs 91,1% female, 25.6 vs 24.3% smokers, 28.1 vs 26.7 mean BMI, 25.6 vs 24.3% tobacco use; 124 vs 125 mean BP. No data on race or diabetes are provided. Mean total CVD risk score at baseline: intervention group 19.4% (16.3); control group 19.6% (15.0). Intervention: 6 interactive weekly sessions lasting about 1 hour. In addition, a physiotherapist contacted the participants at the end of weeks 7, 9, and 11 to discuss their step counts for the past month, their step goals for the following month, any barriers to PA they faced, and how they planned to overcome them. Participants also received 2 booster sessions: 3 and 6 months after starting the program. The control group received 1 education session regarding the importance of exercise and healthy diet, were given written education material and encouraged to read it. |
The outcomes included total CVD risk, estimated using ASSIGN score that estimates 10-year risks of CVD Lab-based measures, including blood glucose levels, total cholesterol, and HDL cholesterol, were evaluated following the standard operating procedures. Blood glucose was measured immediately using YSI 2300 STAT plus Glucose and Lactate Analyzer. BP was measured with the participants in a sitting position from the right arm and a mean of the 3 readings recorded. Height was measured to the nearest 0.5cm via stadiometer. PA was assessed using step count via activPALTM. |
There were significant differences in total CVD risk score, systolic blood pressure, and step count between the intervention vs control groups at 6 months. Difference in total CVD risk was −7.8 mmHg (16.1 vs 24, p<0.001) Difference in systolic BP was −13.9 mmHg (116.9 vs 130.7, p<0.001) Difference in step count was 3599 (9820 vs 6221, p<0.001) No significant between group differences were evident in glucose, weight, BMI, total cholesterol, HDL and LDL |
| Fontana et al.,60 2007 | US | Community | 48 men and women, age 50–60 years with BMI in the 23.5–29.9 range, who were weight stable for >3 months, non-smokers. If female, post-menopausal were recruited to participate. |
Exercise group: the intervention was a 12-month exercise program with a goal of inducing an energy deficit comparable to the diet intervention through exercise and no change in caloric intake. The exercise group prescription started with an approximately 16% increase in energy expenditure over baseline expenditure for 3 months, followed by a 20% increase for the final 9 months. Exercise trainers worked with participants individually to establish and monitor their exercise routines, provide advice and encouragement, and update exercise prescriptions weekly. The method used to recruit participants was not reported. 379 volunteers were assessed for eligibility with 321 excluded and 63 declining to participate. Of the 58 who were eligible and willing to participate, 10 withdrew before the baseline assessment, leaving a final sample of 48. Participants were randomized 2:2:1 to the diet caloric restriction (CR), exercise (EX), or healthy lifestyle (HL) groups. There were 18 participants randomized to the exercise group. Age (mean, SD): 58.9 (2.70) years Gender: 67% (n=12) F; 33% (n=6) M Race/ethnicity: 89% White, 6% Black, 6% other Smokers: none (smokers were excluded) Weight (mean, SD): BMI not reported separately for each group History of hypertension: not reported Total CVD risk score at baseline (mean, SD): Framingham 10-year CHD risk score M=7.6 (2.7) Attrition rates: 2 participants (11.1%). Group 2 was the diet intervention group. The goal of this intervention was to decrease energy intake without changing energy expenditure. The diet intervention prescription started with a 16% decrease below the participants’ baseline energy intake for 3 months and then increased to 20% for the remaining 9 months. For 5 days during the first month participants received all meals from the research study. They attended weekly group meetings with a dietician and a behavioral psychologist and were encouraged to record their food and beverage consumption daily. Number of participants included in the group: 18 Age (mean, SD): 55.2 (3.4) years (significantly younger than the exercise group, which was controlled for during analysis) Gender: 61% (n=11) F; 39% (n=7) M Race/ethnicity: 94% White, 6% other Smokers: none (smokers were excluded) Weight (mean, SD): BMI not reported separately by group History of hypertension: not reported Total CVD risk score at baseline (mean, SD): Framingham 10-year CHD risk score M=6.8 (4.6) Protocol adherence: Based on 7-day food diaries, energy expenditure decreased ~ 300 kcal/day; energy expenditure did not change. Attrition rates (%): none The control group received general information about a healthy diet and were offered free yoga classes. Number of participants included in the group: 10 Age (mean, SD): 56.0 (2.7) years, significantly younger than the exercise group Gender: 60% (n=6) F; 40% (n=4) M Race/ethnicity: 70% White, 20% Black, 10% other Smokers: none (smokers were excluded) Weight (mean, SD): weight and BMI were not reported separately by group History of hypertension: not reported Total CVD risk score at baseline (mean, SD): Framingham 10-year CHD risk score M=7.7 (5.7) Attrition rates: 10% (n=1) Protocol adherence: neither energy intake nor expenditure changed significantly in the control group. |
Outcomes assessed: Framingham 10yr CHD risk score, SBP, SBP, BMI, weight, % body fat, total cholesterol, HDL, LDL, total cholesterol/HDL ratio, triglycerides | Total CVD risk score With group: there as a significant decrease in the CR (from M=1.45 (0.73) at baseline to 1.10 (0.61) at 12 months, p=0.0007) and HL groups (from 1.82 (0.93) at baseline to 1.51 (0.77) at 12 months, p=0.008); no significant change (p=0.62) in the exercise group. There was a significant difference in the changes across the 3 groups (p=0.01), with the change in the dietary intervention group (−0.34 [0.34], p<0.001) significantly greater than the exercise group (+0.04 [0.33], p<0.05). Blood pressure: no significant within or between group differences in SBP or DBP. BMI: BMI decreased significantly in both the exercise group (M=27.1 [1.9] to 24.8 [2.6]) and the dietary intervention group (M=27.1 [2.5] to 24.2 [2.8]); p-value not reported. Weight: Weight loss averaged 6.6 (5.5) kg in the exercise group, 8.2 (4.8) kg in the dietary intevention group and 1.2 (2.1) kg in the control group; p value not reported. Total body fat also decreased significantly: 5.6 (4.9) kg in the exercise group, and 6.3 (3.8) kg in the dietary intervention group; p-value not reported. Total cholesterol: there was a significant decrease from 5.48 (0.77) to 4.91 (0.88) mmol/l in the dietary intervention group, p<0.0001; there were no significant within-group changes in the exercise or control groups. Across the groups, the differences in change scores were significant, with the change in the dietary intervention group (M=−0.57 [0.46] mmol/l) significantly different than the control group (M=−0.06 [0.52] mmol/l). HDL: No significant within- or between-group differences. LDL: significant within-group differences in the exercise (from M=2.15 [0.82] to 2.71 [0.77] mmol/l, p=0.01) and the dietary intervention (from 3.39 [0.62] to 2.87 [0.64], p<0.0001) groups. No significant change in the control group. Across groups, there were significant (p=0.004) differences in the change in LDL with reductions in both the exercise (M= −0.43 [0.59], p<0.05) and dietary intervention (M= −0.49 [0.64], p<0.05) groups significantly greater than in the control group (M=+0.18 [0.49]). Total cholesterol/HDL ratio: there were significant within-group differences in the exercise (from M=3.7 [0.70] to 3.2 [0.70], p=0.004) and dietary intervention (from M=3.9 [1.0] to 3.3 [0.80], p<0.0001) groups, but no significant change within the control group. There were significant differences across the 3 groups in the change in the total cholesterol/HDL ratio (p=0.002), with significantly larger changes in the exercise (M= −0.5 [0.6], p<0.05) and dietary intervention groups (M= −0.6 [0.5], p<0.05) compared to the control group (M=+0.1 [0.03]). |
| Hanlon et al.,54 1995 | United Kingdom | Participants were recruited from 2 work sites in Glasgow. The intervention site was a large engineering factory with over 2600 employees, while the control group site was an engineering and repair facility with 290 employees. | Blue collar workers who were not on permanent night shifts were recruited in a randomized clinical trial. Recruited participants were not participating in other cardiovascular studies and were not taking lipid-lowering agents. The age range was 20 to 65 years. The attrition rate data is not provided. | Intervention site sampling frame: 1600 subjects who were not on permanent night shifts and not taking lipid-lowering agents were randomly selected and invited to participate in the study. A total of 1381 subjects accepted the invitation and 1371 were enrolled in the study (10 were ineligible); 261 employees in the control group site were enrolled in the study. The intervention entailed a health education package that included an interview backed up by written information. Eligible participants at the intervention site were randomized to 5 groups. Participants recruited at the control site are considered group 6 of the study. There were no significant differences between study groups in key baseline measurements. The only baseline characteristics provided for the entire study included age range (20–65 years) and gender (11% female). The study lasted for 12 months. Group 1 received health education without feedback on cholesterol concentration or risk score. Group 2 received health education with feedback on cholesterol concentration but without feedback on risk score. Group 3 received health education with feedback on risk score but not on cholesterol cconcentration. Group 4 received a full health check: health education with feedback on cholesterol concentration and on risk score. Group 5 acted as an internal control group, their intervention being delayed, but was administered after 5 months instead of the end of the study to promote participation. Group 6 subjects from the control site were recruited as the external control group for the study. Their intervention was delayed but was administered after 5 months instead of the end of the study to promote participation. |
The outcomes included total CVD risk, estimated using Dundee risk score that estimates 5-year risks of coronary heart disease. Non-fasting plasma cholesterol measures were taken. No data is provided on assays used or LDL computation. Blood pressure was assessed using a random zero sphygmomanometer, measured twice after resting for 5 minutes). No data on anthropometric assessments (height, body mass, waist). | Internal control vs full intervention: There were no significant differences between the full health check intervention (group 4) and the internal control (group 5) in Dundee risk score. The between group difference in mean Dundee risk score at the end of the 5th month of the intervention was 0.19 (95% CI: −0.11 to 0.50, p=0.21). Between group 4 and 5, small but significant changes were reported in mean cholesterol concentration (0.16 vs 0.03 mmol/l) with a difference in change of 0.13 mmol/ (95% CI for difference in change 0.02 to 0.22), p=0.02). External control vs full intervention: There was a modest difference between the full health check intervention (group 4) and the external control (group 6) in Dundee risk score at the end of the intervention. The between group difference in mean Dundee risk score at the end of the 5th month of the intervention was 0.28 (95% CI: −0.01 to 0.58, p=0.05). Other comparisons of interest were not significant. |
| Kemmler et al.,56 2010 | Germany | Hospital/clinic (identified via health insurance) | Recruitment/sampling: 246 F, all members of Siemens Health Age: >65 years Computer-generated block randomization stratified for age Randomization: stratified randomization (by age) |
This was an RCT including 2 study groups: exercise program and wellness program Group 1 (exercise program, n= 123 baseline; n= 115 18-month): The weekly exercise program consisted of 2 60-minute supervised group classes and 2 20-minute home training sessions. Group classes were structured into 4 sequences. Age (mean, SD): 68.9 (3.9) years Gender: 100% F Smokers: 3.3% Weight (mean, SD): 68.1 (10.9) kg History of hypertension: 41.5% History of diabetes: 8.1% Total CVD risk score at baseline (mean, SD): 10.5 (4.2) Protocol adherence: attendance rate 76.3% group session, 42.2% home training Attrition rates: 6.5% Group 2 (wellness program, n= 123 baseline; n= 112 18-month): Program focused on well-being and was designed not to cause physical adaptations. These participants executed a low intensity, low frequency protocol for 60 minutes once a week for 10 weeks followed by 10 weeks of rest. The main topic changed from week to week. Within each of the 4 10-week blocks, the following activities were relaxation, games/interaction, general coordination, endurance, balance, dance, body sensitivity, muscle strength, breathing, and flexibility. Age (mean, SD): 69.2 (4.1) years Gender: 100% F Smokers: 3.3% Weight (mean, SD): 69.5 (12) kg History of hypertension: 48.8% History of diabetes: 8.9% Total CVD risk score at baseline (mean, SD): 11.2 (5) Protocol adherence: attendance rate 72.0% group session Attrition rates: 8% |
10-year CHD risk (Framingham risk score) Blood pressure HDL LDL DM Smoking |
Exercise vs control group CHD Risk Score Baseline: 10.5 (4.2) vs 11.2 (5.0) 18 month: 8.5 (3.4) vs 10.1 (4.7) CHD risk score: Absolute difference between groups: 0.8 (−0.08, 1.7); p 0.22 Control group CHD risk score: Difference between baseline and 18-month risk score: −1.96% (3.8) HDL: Difference between baseline and 18-month risk score: 1.8% (CI 3.1, 6.0) LDL: Difference between baseline and 18-month risk score: 3.1% (CI −0.1, 6.3) SBP: Difference between baseline and 18-month risk score: −4.8% (CI −7.1, −2.5) DBP: Difference between baseline and 18-month risk score: −7.6% (CI −9.9, −5.3) Exercise group CHD risk score: Difference between baseline and 18-month risk score: −1.15% (2.8) HDL: Difference between baseline and 18-month risk score: 6.5% (CI 4.3, 8.7) LDL: Difference between baseline and 18-month risk score: −1.9% (CI −4.5, 0.7) SBP: Difference between baseline and 18-month risk score: −3.5% (CI −5.8, −1.3) DBP: Difference between baseline and 18-month risk score: −8.7% (CI −10.9, −6.6) |
| Kemmler et al.,44 2016 | Germany | Community settings: females living in the area of in Erlangen-Nuremberg, Germany | Nonrandomized, semi-blinded (outcome assessors) exercise trial with parallel group design. Two group classes of 60 to 65 minutes and 2 home exercise training sessions of 20 to 25 minutes for 49 to 50 weeks a year Participants could join their preferred study group. |
Initial group (n=86); completers (n=59); 31.5% attrition Age: 55±3.3; 55.3±3.4 years BMI: 25.2±3.2; 25.7±3.4 kg/m2 Total body fat %: 36.0±5.0; 35.6±4 Physical activity: 4.1±1.3; 4.3±1.2; assessed by self-rated physical activity score (1 [very low] to 7 [very high]) Exercise volume, min/wk: 82±75; 85±79 Weekly attendance: supervised sessions stable (1.5–1.6 sessions/wk), whereas home training frequency decreased linearly from year 2 (0.96) to year 16 (0.61 sessions/wk). |
BMI waist circumference 10-year risk index of myocardial infarction or coronary death (Framingham) Total CVD risk Blood pressure Total cholesterol HDL LDL triglycerides Fasting blood sugar Diet (5-day protocol where food weighed precisely and documented using protocols provided) |
Only 10-year CVD risk reported: Exercise baseline vs. 16-y follow-up: 1.57±0.91 vs. 6.50±3.17; difference = 4.92±2.94; p <0.0001 Control baseline vs. 16-y follow-up: 1.36±0.80 vs. 8.06±4.36; difference = 6.69±3.98; p <.0001 Absolute difference (control–exercise): 1.77, p = 0.024; effect size = 0.51 |
| Khanji et al.,66 2019 | United Kingdom | A single-center, 2-arm randomized controlled trial with 1:1 allocation to e-coaching and standard of care (SOC) versus SOC alone. Participants with a 10-year QRISK2 CVD risk of ≥10% were enrolled. | Potential participants were identified from primary care database searches, were aged between 40 and 74 years, and had a 10-year CVD risk score of at least 10% based on the UK validated QRISK2 score. |
Intervention group (n=205, age 65.1 [6.3] years, 62% female, 88.8% White) The intervention included electronic coaching, using personalized web-based lifestyle and risk factor counseling on top of SOC. The SOC group (n=197, age 65.9 [4.8] years, 64% female, 87.3% White) SOC of care, which entailed personalized, face-to-face counseling on cardiovascular risk factors during the baseline visit. There were no significant differences between the 2 groups at baseline. |
The 10-year Framingham CVD risk score at 6 months. | Baseline to post-intervention (6 months) changes: Significant within-group changes in total CVD risk were reported for the treatment (−1.23) and control (−1.37) group. There were no between group differences in the Framingham risk scores. |
| Kim et al.,42 2011 | South Korea | Hospital/clinic | Recruitment/sampling:
|
Pre-test and post-test, quasi-experimental design with 2 study groups: intervention and control. Group 1 (intervention, n= 27 baseline; n= 21 16-week): Individual 60 to 90-minute initial counselling session and 30 to 40-minute follow-up every 2 months. 150 minutes of moderate exercise (3–5 days per week) Age (mean, SD): 56.6 (11.8) years Gender: 47.6% F Weight (mean, SD): 71.4 (14.2) kg BMI: 26.53 (3.49) History of diabetes: 100% Total CVD risk score at baseline (mean, SD): 11.93% (6.39) Attrition rates: 22.2% Group 2 (Control, n= 27 baseline; n= 22 16-week): Booklet and basic education on diabetes as part of routine care Age (mean, SD): 54.7 (9.2) years Weight (mean, SD): 73.8 (16.9) kg BMI: 31.85 (0.87) History of diabetes: 100% Total CVD risk score at baseline (mean, SD): 14.67% (8.16) Attrition rates: 18.5% |
10-year UKPDS CHD Waist circumference SBP DBP |
Between group differences 10-year UKPDS risk score −4.79% F=3.226 (p=0.080) Waist circumfrence −2.55 cm, F=0.587 (p=0.448) SBP −2.93mmHg F=1.008 (p=0.321) DBP −5.35 mmHg F=2.586 (p=0.116) |
| Lakerveld et al.,64 2013 | Netherlands | Hospital/ cinic (diabetes research center) |
Recruitment/sampling: Men and women aged 30–50 years, no diabetes Simple random sampling: mailings Randomization: computer-generated simple randomization, family members randomized to same group |
RCT with 2 study groups: intervention and control Group 1 (intervention, n= 314 baseline; n= 249 12-month): Lifestyle intervention provided by practice nurses, 6 face-to-face 30-minute counseling sessions followed by 3 monthly tele sessions. Age (mean, SD): 43.6 (5.1) years Gender: 56.7% F Smokers: 23.9% Weight (mean, SD): 90.2 (15.5) kg Total CVD risk score (ARIC) at baseline (mean, SD): 19.0% (7.8) Total CVD risk score (SCORE) at baseline (MEAN, SD): 4.0% (3.0) Protocol adherence: Median of 2 face-to-face and 2.3 telephone calls. Attrition: 20.7% Group 2 (control, n= 308 baseline; n= 253 6-month): Brochures containing health guidelines regarding physical activity and a healthy diet. Age (mean, SD): 43.4 (5.5) years Gender: 60.1% F Smokers: 17.6% Weight (mean, SD): 90.7 (15.4) kg Total CVD risk score (ARIC) at baseline (mean, SD): 18.8% (8.5) Total CVD risk score (SCORE) at baseline (mean, SD): 3.8% (2.9) Attrition rates: 17.9% |
10-year ARIC CHD risk score 10-year fatal CVD risk (SCORE) BP Weight Physical activity (light; moderate; vigorous; meeting recommendations) Dietary behavior (fruit; vegetables) Smoking Total cholesterol HDL Diabetes BP meds |
Control group:
ARIC: baseline 18.8%; 6 months 18.0%; 12 months 17.8%. SCORE: baseline 3.8%; 6 months 3.7%; 12 months 3.7 (4.6) Intervention group: ARIC: baseline 19.0%; 6 months 18.8%, 12 months 18.5% SCORE: baseline 4.0%; 6 months 4.0%; 12 months 4.0% No significant difference between groups at 6 or 12 months |
| Lazarevic et al.,43 2008 | Serbia | Community | 30 sedentary obese men with T2DM | Single group pre-post design, although the authors aim was to also determine if the effects of the intervention differed by SCORE risk. The intervention was a supervised 6-month aerobic exercise program consisting of 3–5 sessions of moderate aerobic exercise weekly, with an average duration of 45–60 minutes and a workout intensity corresponding to 50–75% of maximal heart rate. Study sample: obese men with T2DM were recruited from participating outpatient clinics. Age (mean, SD): 52.3 (7.4) years Gender: 100% M Race: not reported Smokers: not reported Weight (mean, SD): not reported; mean BMI 32.41 (2.44) History of HTN: not reported Total CVD risk score at baseline (SD) measured by SCORE risk median: 4% (range 1.00–17.00) Protocol adherence: not reported. Attrition rates: not reported. |
Outcomes assessed: SCORE, SBP, DBP, BMI, total cholesterol, HDL, LDL, A1C, waist circumference, physical activity index. There was no dietary measures (or intervention as described). |
Total CVD risk score: There was a significant decrease in SCORE risk from median=4% at baseline to median=3% (range 1–10%) at both 3 and 6 months (p<0.001 from baseline to 3 months and 3 to 6 months). Blood pressure: There was a significant reduction in SBP from m=139.93 (11.19) mm Hg at baseline to 128.13 (10.59) at 3 months and 122.66 (9.29) at 6 months (p<0.001 for both time points relative to baseline and at 3 months compared with 6 months). |
| Lukaczer et al.,58 2006 | USA | Research center | 12-week randomized, controlled trial This 12-week trial compared the effects of a dietary program combining a low glycemic index diet with a functional food delivering 30 g of soy protein and 4 g of phytosterols per day (LGID) with a standard dietary program (American Heart Association Step 1 diet; AHAD) in postmenopausal women. A community sample of menopausal women between 40 and 65 years with a blood LDL level of 3.36 to 5.17 mmol/L (130 to 200 mg/dL) and a BMI of 27 to 39 kg/m2 were recruited through newspaper, email, and radio advertisements. Randomization was also performed at visit 1 by using a standard randomization chart (Excel) Baseline characteristics: LGID (n = 30) AHAD (n = 29) Age: 55.6 ± 5.5; 54.8 ± 5.9 years Weight: 84.4 ± 2.7; 88.0 ± 2.3 kg BMI: 32.5 ± 0.6; 32.4 ± 0.7 kg/m2 Blood pressure (mm Hg) Systolic: 126 ± 1.6; 127 ± 1.8 Diastolic: 84 ± 0.8; 83 ± 0.9 LDL (mmol/L): 4.24 ± 0.13; 4.27 ± 0.10 Attrition: LGID group 27 -> 30 completed (90%) and 22/30 compliant (73%) AHAD group 26 - > 29 completed (89.7) and 20/29 compliant (69%) Compliance based on 3-day diet diary and labs at 2, 4, 8, and 12 weeks |
AHAD: low-fat, high-carbohydrate diets, specifically the AHA Step 1 diet (AHAD); n = 29 Low glycemic index diet with 30 g of soy protein and 4 g of phytosterols per day (LGID); n = 30 |
FRS Body weight Cholesterol Total LDL HDL TG Blood pressure |
The FRS for coronary heart disease was determined for subjects in each group. Subjects in both groups had similar scores at initiation of the trial (LGID: median 10.0, 95% CI 8.8 to 11.2; AHAD: median 10.0, 95% CI 8.6 to 11.5). After the intervention, however, subjects on the LGID program showed a much lower risk (median 6.0, 95% CI 4.4 to 7.6) compared with the AHAD group (median 9.0, 95% CI 7.9 to 10.1). LGID baseline vs. 12-wk; AHAD baseline vs. 12-week; p-value (repeated measures analysis of variance on log-transformed data) Body weight (kg): 84.5 ± 2.2 vs. 77.7 ± 2.0; 89.4 ± 2.5 vs. 86.0 ± 2.4; 0.0031 Total cholesterol (mmol/L): 7.10 ± 0.27 vs. 5.98 ± 0.18; 6.63 ± 0.25 vs. 6.56 ± 0.27; 0.0036 LDL (mmol/L): 4.79 ± 0.20 vs. 4.08 ± 0.14; 4.43 ± 0.20 vs. 4.58 ± 0.23; 0.0041 HDL (mmol/L): 1.25 ± 0.03 vs. 1.32 ± 0.04; 1.22 ± 0.07 vs. 1.23 ± 1.18; not significant TG (mmol/L): 2.39 ± 0.32 vs. 1.32 ± 0.13; 2.34 ± 0.41 vs. 1.79 ± 0.21; 0.006 Systolic BP: 130 ± 1.8 vs.124 ± 2.8; 128 ± 2.7 vs. 125 ± 2.1; not significant Diastolic BP: 84 ± 1.0 vs. 77 ± 1.5; 83 ± 1.5 vs. 78 ± 1.5; not significant |
| Márquez-Celedonio et al.,65 2009 | Mexico | Community | 92 prehypertensive adults (SBP 12–139 mmHg and DBP 80–89 mmHg) aged 30–55 years who agreed to make lifestyle changes. Had to attend at least 3 exercise sessions to be included. |
Intervention (eg, home-based exercise program, DASH diet weight loss program): 6-month lifestyle modification program including a low-sodium, DASH diet with energy content determined using the Harris-Benedict formula. Also 3–5 sessions of aerobic exercise complemented by group sport sessions (45 minutes per session). Smokers: 6 educational classes. Participants were randomly assigned to groups, n=38 completed. Age (mean, SD) =3.97 (7.65) years Smokers: 7.8% Weight (mean, SD) BMI: 30.9 (4.9) History of HTN: by definition (eligibility criteria), all had pre-HTN Mean SBP: 133.03 (4.36); mean DBP: 87.58 (2.84) Total CVD risk score at baseline (mean, SD): FRS 5 (−10–12) (median and range) RCE (risk of CV event within 10 years): mean 5.29 (3.88) Lifestyle score (measured by the FANTASTIC questionnaire): median=62.5 (range=43–83) Protocol adherence: not reported; participant had to attend at least 3 sessions to be included. Attrition rate: not reported Control (guidelines outlining exercises they should undertake, plus dietetic recommendations. The authors reported that 43 completed the study; the number randomized to the 2 groups was not reported) Age (mean, SD): 42.56 (7.98) years Gender: not reported Race/ethnicity: not reported Smokers: n=4 (9.3%) Weight (mean, SD): BMI mean: 31.42 (5.69) History of HTN: by definition (eligibility criteria), all had pre-HTN Mean SBP=132.72 (4.18), mean DBP= 85.6 (4.05) Total CVD risk score at baseline (mean, SD): FRS 4 (−10 – 11; median and range) RCE (mean, SD): 5.79 (5.72) Attrition rate: not reported |
Outcomes assessed: Total CVD score at 3 and 6 months: FRS, RCE, SBP, DBP, BMI, Total cholesterol, HDL, FBS, waist circumference, lifestyle score (FANTASTIC questionnaire) Physical and aerobic capacity was measured by the Cooper test and by determining VO2max. |
Total CVD risk score: FRS: significant decrease in the Rx group (from median of 5 at baseline to 3.5 at 3 and 6 months, p<0.001) and no significant change in the control group (median of 4 at each time point, p=0.869) RCE within 10 years mean scores decreased significantly in the Rx group (from M=5.29 [3.88] at baseline to M=4.45 [3.26] at 3 months and M=4.24 [2.86] at 6 months, p<0.001) compared with no significant change in control group (mean = 5.79 [5.72] at baseline, 5.77 [4.93] at 3 months an 5.93 [5] at 6 months, p=0.962). Significantly more participants in the treatment group (63.16%) experienced a reduction in CV risk compared to 25.58% of control group participants: RR=0.3 (95% CI 0.11–0.83), p<0.05), Blood pressure: significant reduction in SBP (from mean of 133.03 [4.36] at baseline to 124.68 [9.71] at 3 months and 119 [7.97] at 6 months, p=0.01) in the intervention group; no significant change in the control group (baseline: mean=132.72 (4.18), 3 months: mean=132 (7.72), 6 months: 129.53 (9.81), p=0.126). |
| Price et al.,40 2000 | United Kingdom | Participants were recruited from an inner-city general practice, but the intervention occurred in community settings | Patients from an inner-city general practice with at least 1 coronary risk factor and baseline cholesterol above 5.2 mmol/l (201.1 mg/dL) were recruited in a 1-group, pre-test post-test study. Participants had not received dietary advice before and were free of coronary disease and conditions or drugs likely to affect their lipid profile. Age range: 20 to 75 years Attrition rate: 6% |
Sampling frame: 210 patients at an inner-city general practice with 1 or more CVD risk factors were screened; 59 (28%) did not meet the eligibility criteria (total cholesterol was <5.2 mmol/l or 201.1 mg/dL). 143 participants were enrolled in the study. The intervention entailed 1-to-1 session with a nurse, who suggested changes to their existing diet with the aim of reducing the fat content by substituting saturated fats with polyunsaturated fats. A supplementary and commonly used diet sheet, devised by the Family Heart Association, was also provided. The study lasted for 2 years. Baseline characteristics: Median age 51 and 49 years for women and men, respectively (data not provided for the entire sample) Gender: 42% F Smokers: 16.7% F and 27.2% M (data not provided for the entire sample) HTN: 40% (BP>160/90) Mean BMI, race, diabetes: not reported Median total CVD risk score at baseline: 7.7% (IQR 14.9%). |
The outcomes included total CVD risk, estimated using FRS. Lab-based measures, including total cholesterol, and HDL cholesterol were assessed after 12 hours fast, and were measured from serum samples at the Department of Biochemistry, North Staffordshire Hospital. No data on LDL computation is provided. No data on anthropometric assessments (height, body mass, waist) or physiologic measures (BP) are provided. |
There were no significant differences between the baseline and post intervention measures of total CVD risk, and total cholesterol to HDL ratio. However, there was a non-significant increase in median total CVD risk score from 7.7% (IQR 14.9%) to 8.5% (IQR 3.76%); p>0.05. |
| Richardson et al.,50 2008 | UK | Community | Men and women between 45 and 65 years of age from 3 GP practices who responded to a self-screening survey without a prior history of CV disease but with risk factors. |
Intervention: Assessment clinic with advice on relevant risk factors identified and, if relevant, referral to individual’s GP, a dietician, exercise program, and/or smoking cessation program. Sample: men and women from 3 GP practices were identified and invited to self-screen for eligibility to attend a more in-depth assessment of their risk for heart disease. Single group design: Age (mean, SD): not reported Of the total 290 participants, 93 (32%) were between 60 and 64; 84 (29%) between 55 and 59; 70 (24%) between 50 and 54; and 43 (15%) between 45 and 49 years of age Gender: 49% (n=142) M; 51% (n=148) F Race: all Caucasian Smokers: not reported Weight (mean, SD): 78.95 (16.74) kg BMI (mean, SD): 28.13 (4.84) History of HTN: not reported Mean 10-yr risk of CHD (%): 13.14 (9.18%) Protocol adherence (eg, mean sessions attended by participants): not reported for the intervention sessions Attrition rates: not reported |
Outcomes assessed: % reduction in FRS (primary outcome), SBP, DBP, weight, BMI, waist circumference, total cholesterol, HDL, and glucose. No measure of physical activity or diet. | Total CVD risk score: The FRS was slightly lower at 1-year follow-up (M=0.876%, 95% CI 0.21–1.54%) The mean 10-year risk of CHD decreased from 13.14% (9.18) at baseline to 34% (8.71) at 1-year follow-up, a mean reduction of 6.7%. BP: Significant reduction in SBP at 12 months (M=138.65 [17.01]) compared with baseline (M=141.54 [18.68] mmHg), p<0.001 BMI: mean increased from M=28.13 (28.13) at baseline to 28.38 (4.72) Total cholesterol decreased from M=5.11 (1.04) to 5.35 (0.99) mmol/l, p=0.002 HDL increased from M=1.28 (0.38) to 1.38 (0.41) mmol/l, p<0.001 |
| Riddell et al.,63 2016 | Australia | Community | Cluster randomized controlled trial Intervention (n=120) Age: 61.3 ± 9.3 years Sex: 60 M (50.0%) Ethnicity: Caucasian 92 (83.6%); South East Asian 9 (8.2%); Indian sub-continent 7 (6.4%); Other 2 (1.8%) Smokers: 12 (10.9%) current; 43 (39.1%) previous; 55 (50.0%) never At baseline, the mean UKPDS risk score was 11.5 % (SD 7.5 %) for M and 4.2 % (SD 2.8 %) for F Participants lost to follow-up from baseline to 12 months: n=22 Control (n=120) Age: 60.5 ± 8.7 years Sex: 62 (51.7%) M Ethnicity: Caucasian 98 (87.5%); South East Asian 6 (5.4%); Indian sub-continent 4 (3.6%); other 4 (3.6%) Smokers: 6 (5.5%) current; 42 (38.5%) previous; 61 (56%) never Participants lost to follow-up from baseline to 12 months: n=11 Baseline UKPDS risk score was higher in the intervention arm |
Peer support intervention: monthly community-based group meetings over 12 months led by trained peer supporters with active encouragement to use primary health care and other community resources and supports related to diabetes. Usual care was the comparison. | Weight; BMI; waist circumference; BP; total cholesterol; HDL; LDL; total cholesterol to HDL ratio; LDL to HSL ratio; triglycerides; A1C | Mean change between groups’ p-values: UKPDS risk scores reduced similarly in both groups over 12 months. The difference between arms was zero (95 % CI −0.011, 0.011, p=1.00) BMI: 31.9 ± 6.8 (17.6 – 54.1) vs. 31.9 ± 6.7 (21.1 – 49.7), −0.11 (−0.46, 0.24) 31.7 ± 5.9 (18.9 – 54.9) vs. 31.5 ± 6.2 (19.7–54.0), −0.10 (−0.38, 0.17) 1.0 SBP (mmHg) 137.4 ± 16.8 (95–192.7) vs. 128.1 ± 17.1 (96–189), −9.1 (−12.8, −5.4) 134.9 ± 15.5 (95–170) vs. 130.9 ± 16.3 (98–196.7), −5.1 (−9.1, −1.0) |
| Ródenas et al.,45 2005 | Spain | Convent | Postmenopausal nuns from an enclosed convent were enrolled in a 1 group pre-and post-intervention study. Age (mean, SD): 63 (11) years 14 participants were enrolled in the study No attrition data is provided, although it appears that all those enrolled completed the study |
Intervention: 28-day program where the culinary oil used for years in the convent (a blend of sunflower and olive oils) were substituted for extra virgin olive oil. Sampling frame: Nuns who were post-menopausal and shared a similar lifestyle and dietary habits. Sampling method: Not explicit but appears to be convenient. Baseline characteristics: Age (mean, SD): 63 (11) years Gender: 100% F BMI (mean, SD): 23.2 (3.4) History of diabetes: not reported Total CHD risk score (mean, SD): 3.64% (3.05) Smokers, race, level of education, history of HTN: not reported |
Primary outcome was total CVD risk, estimated using FRS that estimates 10-year risk of coronary heart disease in accordance with ATP III guidelines. Lab-based measures including total cholesterol, HDL, and LDL cholesterol were assessed after overnight fast using standard enzymatic methods | Baseline to post-intervention (28 days) changes (mean unless otherwise noted): No significant changes were reported for total CVD risk (FRS) −0.14 (3.64 vs 3.50, p>0.05) and HDL −4.6% (72.7 vs 68.3, p>0.05) Significant changes were reported for: total cholesterol −7.33% (247.87 vs 229.7, p<0.05); and LDL −10.39% (146.15 vs 131.48, p<0.05) No other outcomes of interest are reported. |
| Sartorio et al.,46 2001 | Italy | Hospital setting | Patients admitted between April 1999 and September 1999 to the 3rd Division of Metabolic Diseases of the Italian Institute for Auxology, Piancavallo, Italy, with a diagnosis of obesity were enrolled in a 1 group pre- and post-intervention test study. Age range (years): 19–81 (no mean or median age provided) No attrition data is provided, although it appears that all those enrolled completed the study |
The intervention consisted of a 3-week integrated energy-restricted diet (1200 ± 1800 kcal/day), moderate aerobic exercise (5 days per week training), psychological counseling (2 or 3 sessions per week), and daily educational lectures. Sampling frame: All patients admitted between April 1999 and September 1999 to the 3rd Division of Metabolic Diseases with a diagnosis of obesity. Sampling method: Not explicit but appears to be convenient (all eligible participants were asked to participate) Baseline characteristics: Mean age, smokers, race, level of education: not provided Gender: 84% F BMI (mean, SD): 42.1 (6.1) History of HTN: 15% (defined as taking BP-lowering meds) History of diabetes: 8.6% (defined as taking diabetes meds) Mean total CHD risk score: 7.8% |
Primary outcome was total CVD risk, estimated using FRS. Lab-based measures, including fasting blood glucose, total cholesterol, and HDL cholesterol, were assessed after overnight fast, using enzymatic-colorimetric methods (Hitachi Instrument, Japan). Two blood pressures were assessed after the participants had been sitting at least 5 minutes, and the mean value was used for analyses. |
Baseline to post-intervention (3-weeks) changes (mean unless otherwise noted): Significant changes were reported for: Total CVD risk (FRS):1.6% (7.8 vs 6.2) SBP: 16.4 (136.8 vs 120.4, p<0.05) DBP: 7.7 (84.2 vs 76.5, p<0.05) BMI −1.7 (42.1 vs 40.4, p<0.05) Total cholesterol −38.9 (216.1 vs 177.2, p<0.05) HDL −7.4 (45.6 vs 38.3, p<0.05) Fasting blood glucose −15.2(101.9 vs 87.4 p<0.05) No other outcomes of interest are reported. |
| Siren et al.,51 2016 | Finland | Hospital/local health care centre | Observational study Gender: 100% M Smokers: 55.3% Weight (mean, SD): BMI 29.5 (5.3) BP: 139.4 (16.7)/91.7 (11.1) Age, race/ethnicity, history of HTN: not provided Total CVD risk score at baseline: Modified North Karelia: 6.1 (1.6); SCORE low risk: 4.9 (2.1) Participation in 5-year follow-up rate: 159/389 |
Group 1 (n = 55, 34.6%) had visited no health care providers for CVD risk monitoring between baseline and follow-up. Group 2 (n = 59, 37.1%) had made visits to their primary health care centers. Group 3 (n = 45, 28.3%) had visited their occupational health care centers. |
Total CVD risk score: Modified North Karelia and SCORE |
The CVD risk score decreased the most in Group 2 (1.3% [95% CI: −1.6, −0.6]) compared to Group 3 (−0.6 [95% CI −1.3, 0.3]) and Group 1 (−0.1 [95% CI −0.5, 0.4]). |
| Tuthill et al.,62 2007 | Ireland | Community setting. Patients receiving care in outpatient clinics in 2 Dublin hospitals were eligible if their BMI was >30 and their weight was stable. The method of recruitment was not described. | 68 participants were recruited. Median age: 59 years 85.3% (n=58) were taking antihypertensives, 60.3% (n=41) were taking statins, and 91.2% (n=62) were taking aspirin. Baseline exercise habits ranged from none (n=29) to daily gym attendance (n=1). Characteristics were only reported for the total group. Subjects were randomized to the intervention or control group (method not reported). |
Intervention group: The number of participants randomized to this group was not reported but based on the results, there were 28 subjects at the 6-month data collection point. The intervention group attended monthly evening group sessions where they received dietary advice from a dietician and exercise advise from a physiotherapist. They were given Polar Heart Rate monitors to record heart rate during exercise and an individualized exercise prescription. No data on their baseline characteristics or the adherence in attending sessions. There was a control group but there was no information about it. The number of subjects randomized to the control group was not reported but based on the results, only 28 completed the 6-month exercise period. There was no information of the characteristics of this group. |
Cardiovascular risk scores using the United Kingdom Prospective Diabetes Study (UKPDS) risk engine. They only reported within-group changes from baseline to 6 months; there were no between group comparisons. Secondary outcomes reported: increase in exercise (did not report how this was measured), relationship between weight loss and exercise and SF-36 scores, and relationships between waist circumference reductions and UKPDS scores. |
UKPDS Scores: Active group - CHD score changed from 18.1 (10.1) at baseline to 14.6 (7.8) at 6 months, p<0.01 Fatal CHD score changed from 11.8 (8.6) to 9.2 (6.6), p<0.01 Stroke score changed from 7.3 (6.3) to 6.6 (5.2), p<0.0.5 Fatal Stroke scores changed from 1.3 (1.5) to 1.0 (1.0), not significant. In the control group none of the baseline to 6-month scores were statistically significant: CHD-15.6 (11.2) to 16.1 (12.3), Fatal CHD from 10.2 (9.5) to 10.4 (9.5), Stroke from 6.4 (5.1) to 6.6 (5.4), and Fatal Stroke from 1.0 (1.0) to 1.1 (1.2). |
| Widmer et al.,41 2014 | USA | Work health program; cohort study | Cohort study Online CareHere Connect Personal Health Assistant (PHA) designed and produced by Healarium, Inc (Dallas, TX). The PHA is an integrated and personalized interface that tracks, logs, educates, and forms actionable tasks for the user seeking to improve their current state of health in online and smartphone-based platforms. Reminders to complete tasks may be received via email or SMS text messaging. Participants were eligible for enrolment into the work health program and cohort if they met at least 1 of the 5 inclusion criteria: BMI (kg/m2) > 30; blood pressure (mm Hg) > 140/90; cholesterol level (mg/dL) > 220; blood glucose level (mg/dL) > 100; tobacco use | Single group demographics for the participants who were included and completed the PHA (n=508; 836 assigned to PHA and 508 completed program -> 60.8%) Age: 46.5 ± 11.1; M 126 (25%); F 382 (75%) years White: 389 (77%) Smokers: 3 (0.01%) Treated for HTN: 135 (27%) Treated for diabetes: 25 (5%) Treated for hyperlipidaemia: 39 (8%) Participants logged in on average 1.37 ±1.00 times per week. |
Baseline and 90-day assessments Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Glucose Systolic blood pressure Weight BMI FRS were only able to be calculated on 152 of the 508 patients |
There was a 10% reduction in FRS 10-year risk percentage (2.9% ± 0.3% to 2.5% ± 0.3%, p =0.003) after 90 days using the online PHA. Baseline vs. 90-day, and the resultant changes absolute values of CVD risk factors: Total cholesterol, mg/dL (n = 157): 191.9 ± 38.8 vs. 188.1 ± 37.7; −13.1 ± 28.7; p<0.0001 LDL cholesterol, mg/dL (n = 156): 120.1 ± 37.8 vs. 111.3 ± 31.9; −8.8 ± 12.2; p < 0.0001 HDL cholesterol, mg/dL (n = 160): 47.8 ± 12.2 vs. 48.4 ± 13.1; 0.52 ± 6.6; I = 0.15 Systolic blood pressure, mm Hg (n = 462): 121.1 ± 11.1 vs. 119.4 ± 12.5; −1.8 ± 13.1; p < 0.004 BMI, kg/m2 (n = 429): 34.1 ± 6.5 vs. 33.5 ± 6.5; −0.54 ± 3.5; p < 0.001. |
A1C, Glycated hemoglobin A1C; AHAD, American Heart Association Diet; ARIC, Atherosclerosis Risk In Communities; ATP III, Adult Treatment Panel III; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; CHF, cogestive heart failure; CR, Cimicifuga racemosa; CV, cardiovascular; CVD, cardiovascular disease; DASH, Dietary Approach to Stop Hypertension; DBP, diastolic blood pressure; DHI, digital health intervention; DM, diabetes mellitus; FBS, Fasting Blood Sugar; FRS, Framingham risk score; GP, general practitioner; HDL, high-density lipoprotein; HTN, hypertension; IQR, interquartile ratio; ITT, intention to treat; LDL, low-density lipoprotein; LGID, low glycemic index diet; MI, myocardial infarction; PA, physical activity; PVD, peripheral vascular disease; RCE, risk of cardiovascular event; RCT, randomized controlled trial; REGICOR, Registre Gironí del Cor (Girona Heart Registry); Rx, Treatment; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; SOC, standard of care; T2DM, type 2 diabetes mellitus; TG, triglycerides; UKPDS, UK Prospective Diabetes Study; VO2max, maximum (max) rate (V) of oxygen (O₂) used during exercise
Footnotes
The authors declare no conflicts of interest
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Sandeep RD, Deo R, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom DE DE, Cafiero EE, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The global economic burden of noncommunicable diseases. A report by the World Economic Forum and the Harvard School of Public Health [internet]. World Economic Forum; 2011. [cited 2020 Jul 19]. Available from: https://www3.weforum.org/docs/WEF_Harvard_HE_GlobalEconomicBurdenNonCommunicableDiseases_2011.pdf. [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:E254–E743. [DOI] [PubMed] [Google Scholar]
- 5.Pearson-Stuttard J, Guzman-Castillo M, Penalvo JL, Rehm CD, Afshin A, Danaei G, et al. Modelling future cardiovascular disease mortality in the united states: national trends and racial and ethnic disparities. Circulation. 2016;133(10):967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Preventable deaths from heart disease and stroke: improving care can save more lives [internet]. CDC; 2013. [cited 2018 Mar 27]. Available from: https://www.cdc.gov/vitalsigns/heartdisease-stroke/. [Google Scholar]
- 7.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124(8):967–90. [DOI] [PubMed] [Google Scholar]
- 8.Hennekens C, Lopez-Sendon J. Prevention of cardiovascular disease events in those with established disease or at high risk [internet]. UpToDate; 2017. [cited 2017 Mar 16]. Available from: https://medilib.ir/uptodate/show/1505. [Google Scholar]
- 9.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Prevention of cardiovascular disease: guidelines for assessment and management of cardiovascular risk [internet]. WHO; 2007. [cited 2019 Oct 26]. Available from: https://apps.who.int/iris/handle/10665/43685. [Google Scholar]
- 11.Piepoli MF, Hoes AW, Agewell S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Atherosclerosis. 2016;252:207–74. [DOI] [PubMed] [Google Scholar]
- 12.Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54(14):1209–27. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino R, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. Framingham Heart Study: Cardiovascular Disease (10-year risk) [internet]. Framinton Heart Study; 2015. [cited 2019 Mar 30]. Available from: https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/. [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares TS, Piovesan CH, Gustavo A da S, Macagnan FE, Bodanese LC, Feoli AMP. Alimentary habits, physical activity, and Framingham global risk score in metabolic syndrome. Arq Bras Cardiol. 2014;102(4):374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edelman D, Oddone EZ, Liebowitz RS, Yancy WS, Olsen MK, Jeffreys AS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med. 2006;21(7):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wister A, Loewen N, Kennedy-Symonds H, McGowan B, McCoy B, Singer J. One-year follow-up of a therapeutic lifestyle intervention targeting cardiovascular disease risk. Can Med Assoc J. 2007;177(8):859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketola E, Mäkelä M, Klockars M. Individualised multifactorial lifestyle intervention trial for high-risk cardiovascular patients in primary care. Br J Gen Pract. 2001;51(465):291–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Barone B, King WC, Belle SH, Jakicic JM. Six-month changes in ideal cardiovascular health vs. Framingham 10-year coronary heart disease risk among young adults enrolled in a weight loss intervention. Prev Med (Baltim). 2016;86:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damen AGJAAG, Hooft L, Schuit E, Debray TPA, Collins GS, Tzoulaki I, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. [DOI] [PubMed] [Google Scholar]
- 22.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. [DOI] [PubMed] [Google Scholar]
- 23.Medicom Health. Take the heart health risk assessment [internet]. SSM Health; 2022. [cited 2022 Feb 1. Available from: https://www.ssmhealth.com/heart-vascular-health/at-risk/heart-risk-assessment. [Google Scholar]
- 24.Tunstall-Pedoe H The Dundee coronary risk-disk for management of change in risk factors. BMJ. 1991;303(6805):744–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish heart health study: cohort study. Br Med J. 1997;315(7110):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens RJ, Kothari V, Adler AI, Stratton IM, Holman RR. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci. 2001;101(6):671–9. [PubMed] [Google Scholar]
- 27.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. JBI Manual of Evidence Synthesis [internet]. JBI, 2020. [cited 2021 Jan 16]. Available from: https://synthesismanual.jbi.global. [Google Scholar]
- 28.Kariuki JK, Yamnia CI, Imes CC, Weiss PM, Engberg SJ. Impact of lifestyle modification on absolute cardiovascular disease risk: a systematic review protocol. JBI Database System Rev Implement Rep. 2019;17(10):2106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular diseases: a historical perspective. Lancet. 2014;383(9921):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3(3):e123–30. [PMC free article] [PubMed] [Google Scholar]
- 31.Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [internet]. JBI; 2020. [cited 2021 Aug 4]. Available from: https://synthesismanual.jbi.global. [Google Scholar]
- 32.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane, 2022. [cited 2022 Sep 27]. Available from www.training.cochrane.org/handbook. [Google Scholar]
- 35.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guyatt G, Oxman AD, Akl EA, Kunz R, Bozek J, Norris S, et al. GRADE guidelines: 1. Introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 37.Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. [DOI] [PubMed] [Google Scholar]
- 38.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.al Mheid I, Kelli HM, Ko YA, Hammadah M, Ahmed H, Hayek S, et al. Effects of a health-partner intervention on cardiovascular risk. J Am Heart Assoc. 2016;5(10):e004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price D, Ramachandran S, Knight T, Jones PW, Neary RH. Observed changes in the lipid profile and calculated coronary risk in patients given dietary advice in primary care. Br J Gen Pract. 2000;50(458):712. [PMC free article] [PubMed] [Google Scholar]
- 41.Widmer RJ, Allison TG, Keane B, Dallas A, Lerman LO, Lerman A. Using an online, personalized program reduces cardiovascular risk factor profiles in a motivated, adherent population of participants. Am Heart J. 2014;167(1):93–100. [DOI] [PubMed] [Google Scholar]
- 42.Kim CJ, Kim DJ, Park HR. Effects of a cardiovascular risk reduction intervention with psychobehavioral strategies for Korean adults with type 2 diabetes and metabolic syndrome. J Cardiovasc Nurs. 2011;26(2):117–28. [DOI] [PubMed] [Google Scholar]
- 43.Lazarevic G, Antic S, Cvetkovic T, Djordjevic V, Vlahovic P, Stefanovic V. Effects of regular exercise on cardiovascular risk factors profile and oxidative stress in obese type 2 diabetic patients in regard to SCORE risk. Acta Cardiol. 2008;63(4):485–91. [DOI] [PubMed] [Google Scholar]
- 44.Kemmler W, Engelke K, von Stengel S. Long-term exercise and bone mineral density changes in postmenopausal women—are there periods of reduced effectiveness? J Bone Mineral Res. 2016;31(1):215–22. [DOI] [PubMed] [Google Scholar]
- 45.Ródenas S, Rodríguez-Gil S, Merinero MC, Sánchez-Muniz FJ. Dietary exchange of an olive oil and sunflower oil blend for extra virgin olive oil decreases the estimate cardiovascular risk and LDL and apolipoprotein aii concentrations in postmenopausal women. J Am Coll Nutr. 2005;24(5):361–9. [DOI] [PubMed] [Google Scholar]
- 46.Sartorio A, Lafortuna CL, Vangeli V, Tavani A, Bosetti C, la Vecchia C. Short-term changes of cardiovascular risk factors after a non-pharmacological body weight reduction program. Eur J Clin Nutr. 2001;55(10):865–9. [DOI] [PubMed] [Google Scholar]
- 47.Chan CKM, Leung KC, Chair SY. The effect of cardiac health promotion program among the general public in Hong Kong. J Hong Kong College Cardiol. 2012;20(1):21–30. [Google Scholar]
- 48.Ahn N, Kim K. Can active aerobic exercise reduce the risk of cardiovascular disease in prehypertensive elderly women by improving HDL cholesterol and inflammatory markers? Int J Environ Res Public Health. 2020;17(16):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernocchi P, Baratti D, Zanelli E, Rocchi S, Salvetti M, Paini A, et al. Six-month programme on lifestyle changes in primary cardiovascular prevention: a telemedicine pilot study. Eur J Cardiovasc Prev Rehabil. 2011;18(3):481–7. [DOI] [PubMed] [Google Scholar]
- 50.Richardson G, van Woerden HC, Morgan L, Edwards R, Harries M, Hancock E, et al. Healthy hearts - a community-based primary prevention programme to reduce coronary heart disease. BMC Cardiovasc Disord. 2008;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siren R, Eriksson JG, Vanhanen H. Observed changes in cardiovascular risk factors among high-risk middle-aged men who received lifestyle counselling: a 5-year follow-up. Scand J Prim Health Care. 2016;34(4):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldwin SA. A neighborhood-centered clinical project: improving diabetes and cardiovascular outcomes in Hispanic women. J Nurs Educ. 2015;54(3):159–63. [DOI] [PubMed] [Google Scholar]
- 53.Anand SS, Samaan Z, Middleton C, Irvine J, Desai D, Schulze KM, et al. A digital health intervention to lower cardiovascular risk: a randomized clinical trial. JAMA Cardiol. 2016;1(5):601–6. [DOI] [PubMed] [Google Scholar]
- 54.Hanlon P, Mcewen J, Carey L, Gilmour H, Tannahill C, Tannahill A, et al. Health checks and coronary risk: further evidence from a randomised controlled trial. BMJ. 1995;311(7020):1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elramli A Effectiveness of community based physical activity on step count and sedentary behaviour in people with rheumatoid arthritis within the first five years of diagnosis [thesis]. University of Glasgow; 2017. [Google Scholar]
- 56.Kemmler W, von Stengel S, Engelke K, Häberle L, Kalender WA. Exercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) study. Arch Intern Med. 2010;170(2):179–85. [DOI] [PubMed] [Google Scholar]
- 57.Bebenek M, Kemmler W, von Stengel S, Engelke K, Kalender WA. Effect of exercise and Cimicifuga racemosa (CR BNO 1055) on bone mineral density, 10-year coronary heart disease risk, and menopausal complaints: the randomized controlled Training and Cimicifuga racemosa Erlangen (TRACE) study. Menopause. 2010;17(4):791–800. [DOI] [PubMed] [Google Scholar]
- 58.Lukaczer D, Deann JL, Lerman RH, Darland G, Schiltz B, Tripp M, et al. Effect of a low glycemic index diet with soy protein and phytosterols on CVD risk factors in postmenopausal women. Nutrition. 2006;22(2):104–13. [DOI] [PubMed] [Google Scholar]
- 59.Curtis PJ, Dhatariya K, Sampson M, Kroon PA, Potter J, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized,controlled trial. Diabetes Care. 2012;35(2):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab. 2007;293(1):E197–202. [DOI] [PubMed] [Google Scholar]
- 61.Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS One. 2012;7(11):e49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuthill A, Quinn A, Mccolgan D, Mckenna M, O’shea D, Mckenna TJ. A prospective randomized controlled trial of lifestyle intervention on quality of life and cardiovascular risk score in patients with obesity and type 2 diabetes. Diabetes Obes Metab. 2007;9(6):917–19. [DOI] [PubMed] [Google Scholar]
- 63.Riddell MA, Dunbar JA, Absetz P, Wolfe R, Li H. Brand M, et al. Cardiovascular risk outcome and program evaluation of a cluster randomised controlled trial of a community-based, lay peer led program for people with diabetes. BMC Public Health. 2016;16(1):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakerveld J, Bot SD, Chinapaw MJ, van Tulder MW, Kostense PJ, Dekker JM, et al. Motivational interviewing and problem solving treatment to reduce type 2 diabetes and cardiovascular disease risk in real life: a randomized controlled trial. Int J Behav Nutr Phys Act. 2013;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Márquez-Celedonio FG, Téxon-Fernández O, Chávez-Negrete A, Hernéndez-López S, Marín-Rendón S, Berlín-Lascurain S. [Clinical effect of lifestyle modification on cardiovascular risk in prehypertensives: PREHIPER I study]. Rev Esp Cardiol. 2009;62(1):86–90. [Spanish] [PubMed] [Google Scholar]
- 66.Khanji MY, Balawon A, Boubertakh R, Hofstra L, Narula J, Hunink M, et al. Personalized e-coaching in cardiovascular risk reduction: a randomized controlled trial. Ann Glob Health. 2019;85(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brotons C, Moral I, Fernández D, Puig M, Vilella MT, Puig T, et al. Effectiveness of an intervention aimed at improving information for patients with high cardiovascular risk: INFORISK clinical trial. Int J Environ Res Public Health. 2021;18(7):3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brady W, De Souza K. The HEART score: a guide to its application in the emergency department. Turk J Emerg Med. 2018;18:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bliss CI. The method of probits. Science. 1934;79(2037):38–9. [DOI] [PubMed] [Google Scholar]
- 70.Gao L, Faller J, Majmudar I, Nguyen P, Moodie M, Health D. Are interventions to improve cardiovascular disease risk factors in premenopausal women effective? A systematic review and meta-analysis. BMJ Open. 2021;11:42103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SS, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139(25):E1162–E1177. [DOI] [PubMed] [Google Scholar]
- 72.Rippe JM. lifestyle strategies for risk factor reduction, prevention, and treatment of cardiovascular disease. Am J Lifestyle Med. 2019;13(2):204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chareonrungrueangchai K, Wongkawinwoot K, Anothaisintawee T, Reutrakul S. Dietary factors and risks of cardiovascular diseases: an umbrella review. Nutrients. 2020;12(4):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciumărnean L, Milaciu MV, Runcan O, Vesa SC, Răchișan AL, Negrean V, et al. The effects of flavonoids in cardiovascular diseases. Molecules. 2020;25(18):4320. doi: 10.3390/MOLECULES25184320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, et al. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012;33(4):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Framingham Heart Study. Cardiovascular Disease Risk based on D’Agostino, Vasan, Pencina, Wolf, Cobain, Massaro, Kannel. “A General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study.” Accessed August 21, 2017. https://www.framinghamheartstudy.org/fhs-risk-functions/cardiovascular-disease-10-year-risk/ [Google Scholar]
- 77.Thomsen TF, McGee D, Davidsen M, & Jørgensen T (2002). A cross-validation of risk-scores for coronary heart disease mortality based on data from the Glostrup Population Studies and Framingham Heart Study. International Journal of Epidemiology, 31(4), 817–822. 10.1093/ije/31.4.817 [DOI] [PubMed] [Google Scholar]


