Abstract
Aurora-A kinase is a mitotic spindle-pole-associated protein that has been implicated in duplication and separation of centrosomes and in spindle assembly. The proper timing and amplitude of Aurora-A expression seems to be important, as elevated levels of this protein have been associated with centrosome abnormalities and aneuploidy in mammalian cells. We show that Aurora-A increases at the G2–M transistion and disappears completely at G1 in XL2 cells. Using Xenopus oocyte extracts, we demonstrate that degradation of Aurora-A is mediated by the anaphase-promoting complex (APC) and is regulated by Fizzy-Related but not by Fizzy. Degradation of Aurora-A depends on a D-Box, but not on its KEN-Box motif, as mutation of its C-terminal D-Box sequence induces stabilization of the protein. Accordingly, addition into the extracts of a cyclin B-type D-Box-motif-containing peptide completely suppresses its degradation. Furthermore, APC/Fizzy-Related ubiquitylates the wild type but not a D-Box mutant form of Aurora-A in vitro. Consistent with these data, ectopic expression of Fizzy-Related in Xenopus oocytes induces complete degradation of endogenous Aurora-A. Aurora-A is thus the first protein, at least in our assay system, that undergoes a D-Box-dependent degradation mediated by APC/Fizzy-Related but not by APC/Fizzy.
INTRODUCTION
In vertebrates, the Aurora kinase family contains three different members, Aurora-A, -B and -C, that are associated with mitotic structures, such as spindle poles, centrosomes, chromosomes and the mid-body. Aurora-A is implicated in centrosome duplication/separation and spindle assembly. Interestingly, this kinase is amplified in human cancers, and its overexpression induces cell transformation (Bischoff et al., 1998; Zhou et al., 1998), indicating that the control of Aurora-A levels is important in modulating cell division. In HeLa cells, Aurora-A is degraded and re-synthesized during cell-cycle transit (Honda et al., 2000). Despite the fact that Aurora-A proteolysis seems to be essential for preventing cell transformation, the pathway that mediates its degradation is not known.
The ubiquitin-dependent pathway is a precise and rapid mechanism used by the cell to induce proteolysis. At the metaphase–anaphase and M–G1 transitions, degradation is initiated by the E3/ubiquitin-ligase known as the anaphase-promoting complex (APC) (for a review, see Zachariae and Nasmyth, 1999). This E3 is regulated by the Fizzy/Cdc20 and Fizzy-Related/Cdh1 proteins (Sigrist and Lehner, 1997; Fang et al., 1998; Lorca et al., 1998; Kramer et al., 2000), two distinct activators that transiently interact with this ubiquitin ligase in a cell-cycle-specific manner. APC/Fizzy complex mediates securin and cyclin B degradation, a prerequisite for progression through mitosis (Cohen-Fix et al., 1996; Lorca et al., 1998). Fizzy-Related mainly controls progression through G1 by ensuring the complete proteolysis of cyclin B and Fizzy (Visintin et al., 1997; Pfleger and Kirschner, 2000).
Here, we establish a further role for APC/Fizzy-Related at the M–G1 transition, namely the ubiquitylation and thus degradation of Aurora-A in a D-Box-dependent manner.
RESULTS AND DISCUSSION
Aurora-A proteolysis is not mediated by the APC/Fizzy complex
Similarly to human Aurora-A (Kimura et al., 1997; Bischoff et al., 1998), Xenopus Aurora-A increases at the G2–M transition and then disappears at the M–G1 transition of the cell cycle (see Supplementary figure 1 available at EMBO reports Online). Previously, Aurora-A was described to be degraded at metaphase II exit in Xenopus oocytes (Frank-Vaillant et al., 2000), a transition regulated by APC/Fizzy (Lorca et al., 1998). We investigated whether this reported decrease of Aurora-A is, in fact, mediated by this ubiquitin ligase. Using western blotting, we measured endogenous Aurora-A levels at different times following in vitro activation of APC/Fizzy by calcium addition to extracts prepared from Xenopus metaphase-II-arrested oocytes (CSF extracts). As expected, both Aurora-A and cyclin B2 were stable in the absence of calcium (Figure 1A). Surprisingly, the addition of Ca2+ did not trigger Aurora-A proteolysis, whereas cyclin B2 was completely degraded. To confirm these results, we investigated whether endogenous Aurora-A was degraded in metaphase-II-arrested oocytes activated by the calcium ionophore A32187. Oocytes were homogenized at different times following ionophore treatment, and the levels of endogenous Aurora-A and cyclin B2 were analysed by immunoblotting. In contradiction to Frank-Vaillant et al. (2000), endogenous Aurora-A levels were stable during the entire time course (Figure 1B). On the other hand, cyclin B2 was degraded 20 min post-activation and accumulated afterwards. Similar results were obtained when the oocytes were activated by electric shock or fertilization (data not shown). Taken together, these results demonstrate that Aurora-A is not degraded at metaphase II exit in Xenopus oocytes under conditions where APC/Fizzy is active. Thus, Aurora-A degradation observed at the M–G1 transition in somatic XL2 cells may not be mediated by this ubiquitin ligase.
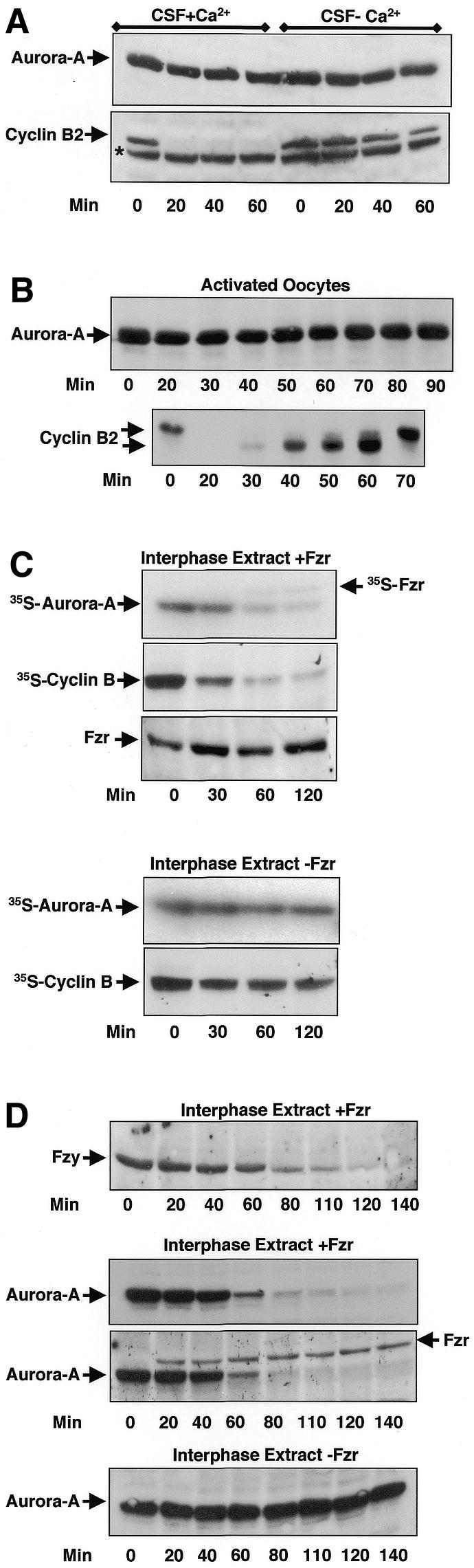
Fig. 1. Fizzy-Related but not Fizzy is required for Aurora-A degradation. (A) CSF extract (20 µl) was supplemented with 0.5 mM CaCl2 where indicated (CSF+Ca2+). Samples (2 µl) were taken at different times, and endogenous Aurora-A and cyclin B2 levels were analysed by western blotting. The asterisk represents an unspecific band recognized by the anti-cyclin B2 antibodies in the CSF extracts. (B) Metaphase-II-arrested oocytes were activated by the calcium ionophore A32187 (Activated Oocytes), homogenized individually and analysed at various times for the degradation of endogenous Aurora-A and cyclin B2. (C) Fizzy-Related mRNA was added to interphase extracts (50 µl) as indicated (Interphase Extract +Fzr). One hour later, 1 µl of either in vitro translated 35S-labelled Aurora-A or the same amount of 35S-labelled cyclin B was added. Samples (3 µl) were taken at different times and analysed by autoradiography. Fizzy-Related translation was verified by western blotting (Interphase Extract + Fzr and Fzr). (D) Interphase extracts (50 µl) were supplemented with Fizzy-Related mRNA as indicated (Interphase Extracts +Fzr). Endogenous Aurora-A and Fizzy degradation were analysed by western blotting. To verify Fizzy-Related translation, Aurora-A and Fizzy-Related were analysed on the same nitrocellulose membrane (Interphase Extract +Fzr, Aurora-A/Fzr).
Aurora-A proteolysis requires the APC/Fizzy-Related complex
We next investigated whether the APC/Fizzy-Related complex was involved in the degradation of Aurora-A associated with mitotic exit in somatic cells. To reconstitute a functional APC/Fizzy-Related complex, interphase Xenopus egg extracts, which are devoid of Fizzy-Related (Lorca et al., 1998), were supplied with an mRNA encoding this protein. One hour later, we added either Aurora-A or cyclin B, produced as [35S]methionine labelled proteins in reticulocyte lysates. The stability of these proteins was monitored at 30 min intervals. Fizzy-Related expression was visualized principally by western blotting (Figure 1C). A minor proportion was also 35S-labelled as a consequence of the free [35S]methionine present in the reticulocyte lysate. As expected, both Aurora-A and cyclin B were stable in control interphase egg extracts that lack both APC/Fizzy and APC/Fizzy-Related activities (Figure 1C, lower panels). In contrast, cyclin B, a substrate of the APC/Fizzy-Related complex (Pfleger and Kirschner, 2000), was proteolysed within 60 min of addition to Fizzy-Related-containing extracts, indicating that the latter was functional. Similarly, [35S]Aurora-A was degraded 60 min after its addition (Figure 1C, upper panels), suggesting a role for APC/Fizzy-Related in Aurora-A proteolysis. In a similar experiment, we examined the endogenous levels of this kinase by western blotting. Endogenous Aurora-A was completely degraded ∼60 min after the addition of Fizzy-Related mRNA, which led to the synthesis of Fizzy-Related (Figure 1D). In its absence, endogenous Aurora-A levels did not vary (Figure 1D). Fizzy, another APC/Fizzy-Related substrate (Pfleger and Kirschner, 2000), also underwent complete degradation (Figure 1D).
To determine whether or not Aurora-A degradation depended upon APC, interphase extracts were immunodepleted with anti-CDC27 antibodies before the addition of the Fizzy-Related mRNA (Figure 2A, lower panel). This blocked degradation of endogenous Aurora-A, whereas this kinase was entirely proteolysed when non-specific antibodies were used for immunodepletion (Figure 2A). Thus, APC mediates degradation of Aurora-A induced by Fizzy-Related expression.
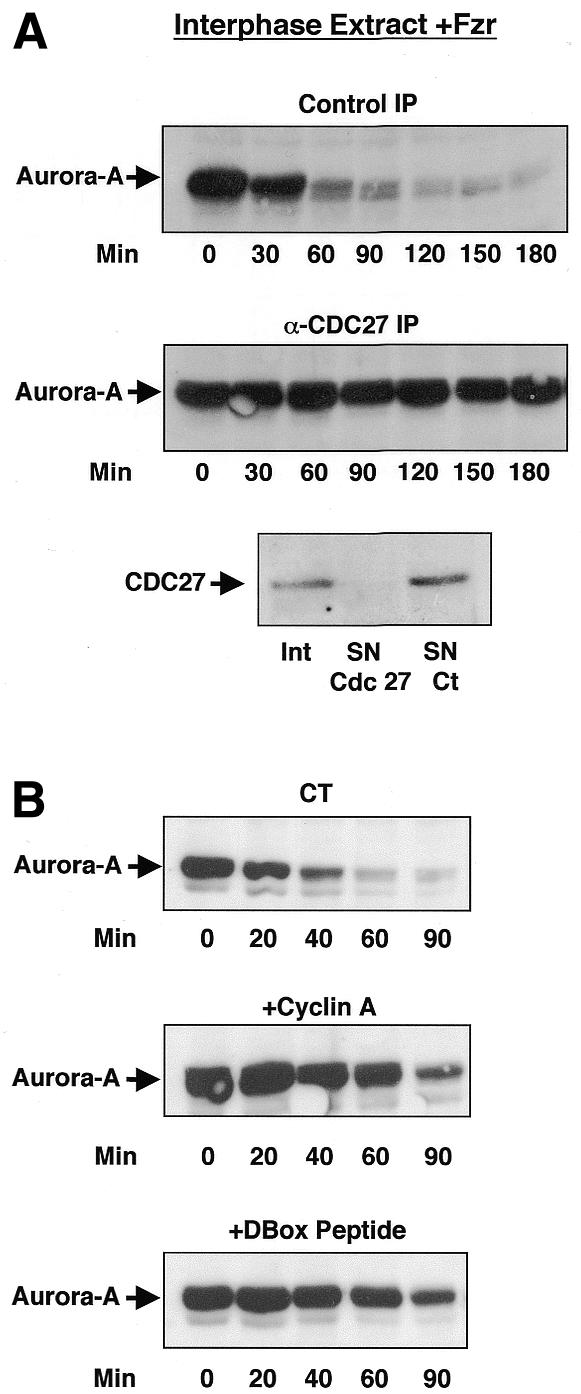
Fig. 2. The degradation of Aurora-A is mediated by the APC/Fizzy-Related complex and is blocked by the addition of a cyclin B D-Box-motif-containing peptide. (A) An interphase extract (50 µl) was first depleted with either α-CDC27 antibodies (α-CDC27 IP) or non-specific antibodies (Control IP) and then complemented with Fizzy-Related mRNA. A sample of 2 µl was then taken at different times, and endogenous Aurora-A levels were analysed by western blotting. The initial interphase extract (CDC27, Int) and the supernatants of α-CDC27 (CDC27, SN Cdc 27) and non-specific (CDC27, SN Ct) immunoprecipitations were analysed by western blotting with α-CDC27 antibodies to verify CDC27 depletion. (B) Fizzy-Related mRNA was added to interphase extracts (50 µl); 20 min later, we added a purified recombinant cyclin A protein (2 ng/µl, +Cyclin A), the cyclin B D-Box-containing peptide (7 µg/µl, +DBox Peptide) or buffer (CT). Endogenous levels of Aurora-A were then analysed by western blotting at different times.
Phosphorylation of Fizzy-Related by cyclin/cdk complexes inhibits APC/Fizzy-Related activity (Jaspersen et al., 1999; Blanco et al., 2000; Kramer et al., 2000). To gain a deeper insight into the requirement of APC/Fizzy-Related complex to induce Aurora-A degradation, we added recombinant cyclin A to produce H1 kinase activity similar to that present in mitotic egg extracts. This led to a delay in Aurora-A proteolysis (Figure 2B, middle panel), indicating that cyclin-A/cdk inhibited Aurora-A proteolysis, most likely through phosphorylation of Fizzy-Related.
Together, these results strongly suggest that Aurora-A proteolysis requires the APC complex and its activator Fizzy-Related.
The addition of a D-Box-motif-containing peptide blocks Aurora-A degradation
Substrates recognized by the APC contain a destruction signal sequence, often located near the N-terminus of target proteins. Two types of destruction sequences have been identified: the D-Box (RxxL, where ‘x’ is any amino acid) and the KEN-Box. D-Box-containing substrates typically appear to be recognized by both APC/Fizzy and APC/Fizzy-Related (Pfleger and Kirschner, 2000). The KEN-Box-containing substrates are believed to be recognized exclusively by APC/Fizzy-Related. Recently, a novel extended D-Box has been reported to confer APC/Fizzy- and APC/Fizzy-Related-specific degradation signals to human cyclin A and Nek2A. This motif contains the core cyclin-B-type D-Box, followed by an additional short sequence at its extreme C-terminus (Hames et al., 2001).
N-terminal peptides of Xenopus and sea urchin cyclin B, containing the classic D-Box motif, inhibit the proteolytic activity of both APC/Fizzy and APC/Fizzy-Related by a competitive mechanism. Surprisingly, this works with substrates containing either the classic D-Box or KEN-Box motifs (Pfleger and Kirschner, 2000) but not those with the extended form of the D-Box motif (Hames et al., 2001). Therefore, we added a cyclin B D-Box-containing peptide to interphase extracts supplemented previously with the Fizzy-Related mRNA and monitored endogenous Aurora-A levels. The peptide strongly delayed APC/Fizzy-Related-dependent proteolysis of Aurora-A, suggesting that this degradation is mediated by either D-Box or KEN-Box motifs (Figure 2B, lower panel).
Aurora-A is degraded by a D-Box-dependent pathway
The Aurora-A protein sequence contains three potential D-Box motifs, D1 (212RVYL), D2 (293RTTL) and D3 (378RLPL), and one potential KEN-Box motif near its N-terminus (6KEN) (Figure 3A). The role of these destruction motifs on Aurora-A degradation has proved to be conflicting (Honda et al., 2000; Arlot-Bonnemains et al., 2001). By using deleted forms of Xenopus Aurora-A, Arlot-Bonnemains et al. (2001) concluded that this kinase contains a destruction motif required for its ubiquitylation and degradation. In contrast, Honda et al. (2000) showed that punctual mutations or deletions of the putative D-Box sequences did not alter human Aurora-A degradation. To clarify whether a functional D-Box sequence is present in Aurora-A, we developed a punctual mutation analysis of putative D-Box motifs in our assay, where the degradation of this kinase can selectively be induced by the addition of Fizzy-Related mRNA. Thus, the three arginine (212R, 293R and 378R) and leucine (215L, 296L and 381L) residues of each potential D-Box sequence were mutated to alanine (A). We then tested the degradation of the 35S-labelled mutated forms translated in reticulocyte lysates when added to Fizzy-Related-containing interphase extracts. We also analysed proteolysis of an Aurora-A kinase mutant lacking the KEN-Box motif (6KEN). The deletion of the KEN-Box motif did not affect Aurora-A stability (Figure 3B), nor did mutations of either D-Box 1 or D-Box 2 motifs (Figure 3C, upper and middle panels). However, a mutated D-Box 3 stabilized Aurora-A in this assay (Figure 3C, lower panel). We conclude that the D-Box 3 sequence of Aurora-A mediates its degradation. A Δ36-44 truncated form of Xenopus cyclin B has been reported to be ubiquitylated in vitro by APC/Fizzy-Related and not APC/Fizzy (Pfleger and Kirschner, 2000). This process is mediated by the recognition of the D-Box motif at position 7–15 of this protein. However, the authors found that a deleted form of cyclin B lacking this D-Box sequence was degraded to the same extent as wild-type protein by both APC/Fizzy and APC/Fizzy-Related. Therefore, the partial ubiquitylation dependent on APC/Fizzy-Related of cyclin B observed in vitro is not involved in physiological degradation of this protein. Thus, to our knowledge, this is the first example of a protein whose degradation is D-Box-dependent, at least in Xenopus egg extracts, and is exclusively mediated by APC/Fizzy-Related.
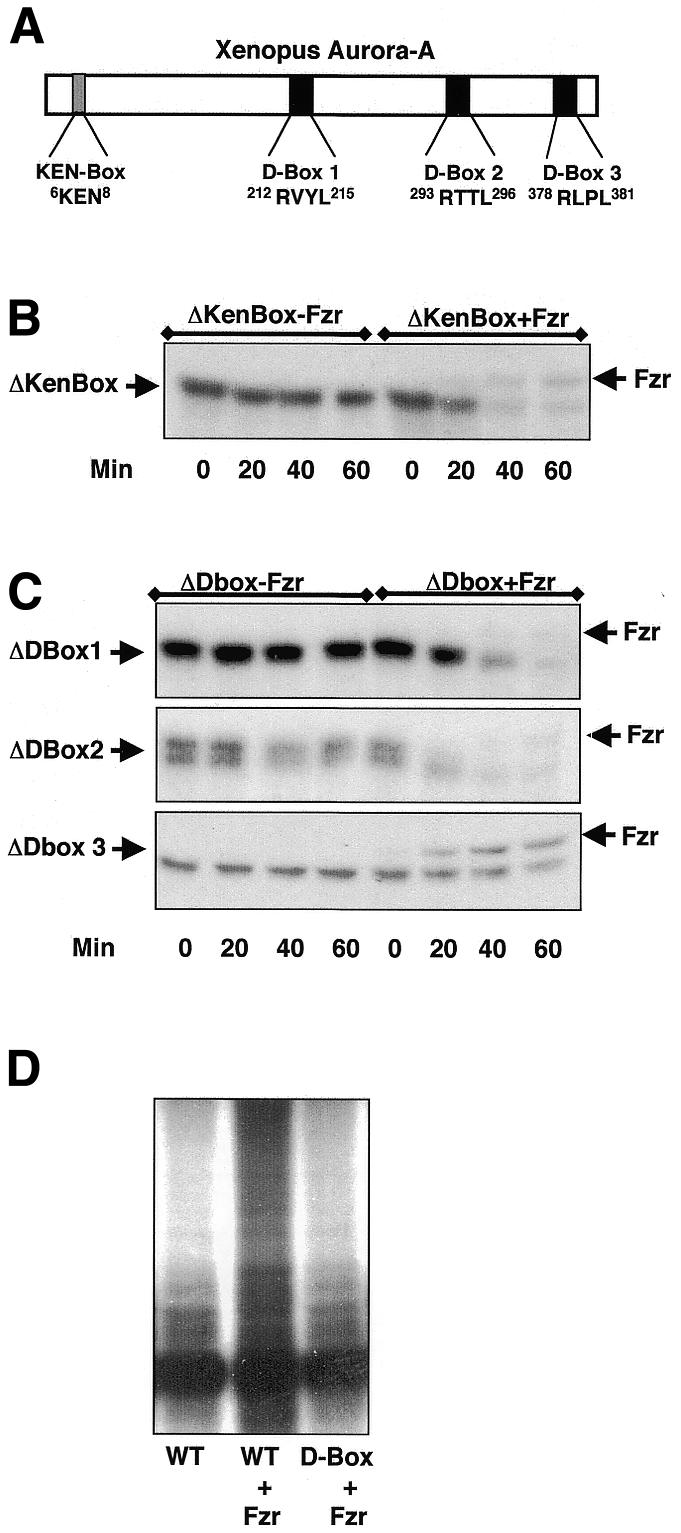
Fig. 3. Aurora-A targeting by the APC/Fizzy-Related complex requires a D-Box. (A) Schematic drawing of Xenopus Aurora-A depicting the presence of all the putative KEN-Box and D-Box motifs. (B) 35S-radiolabelled KEN-Box mutant of Aurora-A was added to interphase extracts containing Fizzy-Related mRNA. The degradation of this protein was then analysed by autoradiography. (C) Similar to (B), except for the addition of D-Box 1 (ΔDBox1), 2 (ΔDBox2) or 3 (ΔDBox3) mutants instead of KEN-Box mutant. (D) 35S-labelled wild type (WT and WT + Fzr) and D-Box 3 mutant (D-Box + Fzr) were added to an immunopurified APC in the presence (WT + Fzr) or absence (WT) of in vitro translated Fizzy-Related and assayed for ubiquitylation.
The APC/Fizzy-Related complex ubiquitylated wild type but not a D-Box 3 mutant of Aurora-A
To understand how mutation of the D-Box 3 motif blocks Aurora-A degradation, we tested whether this mutant is ubiquitylated in vitro by the APC/Fizzy-Related complex. Our in vitro ubiquitylation assay uses APC immunoprecipitated from interphase Xenopus egg extracts with α-CDC27 antibodies. This complex was incubated with 35S-labelled wild type or a D-Box 3 mutated form of Aurora-A translated in reticulocyte lysates, along with E2, Ubch5B and bovine ubiquitin. Finally, the ubiquitylation assay was performed in the presence or absence of in vitro translated Fizzy-Related activator. As shown in Figure 3D, wild-type Aurora-A became strongly ubiquitylated only when both APC and recombinant Fizzy-Related were present in the reaction mixture (compare the first two lanes). Thus, Fizzy-Related is required for efficient APC-mediated ubiquitylation of Aurora-A. Furthermore, mutation of D-Box 3 blocked Aurora-A ubiquitylation, indicating that an intact D-Box 3 motif is essential, in our assay system, for its in vitro ubiquitylation (Figure 3D, right-hand lane).
Ectopic expression of Fizzy-Related by mRNA microinjection induces Aurora-A degradation in G2-arrested oocytes
In quiescent Xenopus oocytes, Aurora-A is already present at stage VI oocytes, and no significant decrease in its levels has ever been observed during oocyte maturation. This is consistent with the lack of Fizzy-Related in Xenopus oocytes. We exploited this physiological situation to investigate whether the ectopic expression of Fizzy-Related could induce endogenous Aurora-A degradation in vivo. Stage VI oocytes were injected with Fizzy-Related mRNA. At different times, five oocytes were individually homogenized and analysed by western blotting for the presence or absence of Fizzy-Related and Aurora-A. We also followed the degradation of endogenous Fizzy protein as a positive control of APC/Fizzy-Related activation. Injection of Fizzy-Related mRNA induced the formation of a functional APC/Fizzy-Related complex, as endogenous Fizzy protein was clearly degraded (Figure 4, lower panel) in correlation with the appearance of Fizzy-Related (Figure 4, upper panel). Moreover, ectopic expression of Fizzy-Related in stage VI oocytes induced the degradation of endogenous Aurora-A protein, demonstrating that the proteolysis of this kinase is mediated in vivo by the APC/Fizzy-Related pathway (Figure 4, upper panel). This confirms our in vitro results in interphase Xenopus egg extracts. Thus, Xenopus oocytes contain the machinery required to induce Fizzy and Aurora-A degradation upon the expression of Fizzy-Related, confirming that this APC activator is a key regulator of the proteolysis of these proteins. Taken together, these results clearly show that degradation of Xenopus Aurora-A is mediated by the APC/Fizzy-Related complex in a D-Box-dependent way, where this motif acts by modulating Aurora-A ubiquitylation.
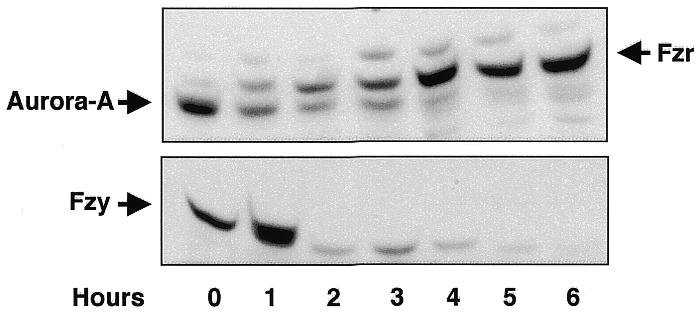
Fig. 4. APC/Fizzy-Related induces in vivo degradation of endogenous Aurora-A and Fizzy. Oocytes were microinjected with Fizzy-Related mRNA. Homogenates were prepared from individual oocytes hourly and analysed by coupled western blotting in the same nitrocellulose membrane for endogenous Aurora-A and Fizzy-Related. Fizzy degradation was followed by western blotting on a separate membrane.
METHODS
Immunization procedure, antibodies and immunofluorescence microscopy.
A wild-type Xenopus His6–Aurora-A fusion protein, produced in Escherichia coli, was used to immunize rabbits. Immune serum was affinity-purified on immobilized His6–Aurora-A. The Xenopus anti-cyclin B2, anti-Fizzy, anti-CDC27 and anti-Fizzy-Related antibodies have been described previously (Lorca et al., 1998; Castro et al., 2001). Immunofluorescence assays were developed as described previously (Roghi et al., 1998).
Translation and degradation in Xenopus egg extracts.
Interphase and CSF extracts, as well as extracts competent in translation of Xenopus Fizzy-Related mRNA, were prepared as described previously (Fesquet et al., 1997; Lorca et al., 1998). For protein degradation assays, 1 µl of either 35S-labelled cyclin B or Aurora-A was incubated at room temperature with 20 µl interphase extracts supplemented (1 h before) or not with Fizzy-Related mRNA.
In vitro ubiquitylation assay.
Wild-type and mutated (R398A–L401A) Aurora-A proteins were translated in vitro in rabbit reticulocyte lysates in the presence of [35S]methionine. To obtain APC/Fizzy-Related complexes, APC was immunopurified from interphase Xenopus egg extracts with anti-CDC27 antibodies and activated by reticulocyte-lysate-expressed Xenopus Fizzy-Related. The in vitro ubiquitylation reaction was performed as reported previously (Bembenek and Yu, 2001).
mRNA microinjection in Xenopus oocytes.
Fifty oocytes were microinjected with 20 ng Fizzy-Related mRNA. Each hour, five oocytes were homogenized individually in 10 µl homogenization buffer (20 mM Tris pH 7.5, 50 mM NaCl, 50 mM NaF, 10 mM β-glycerophosphate, 5 mM Na4P2O7, 1 mM EDTA). After extract centrifugation (13 000 r.p.m. for 3 min at 4°C), the clear supernatant was recovered and used for western blotting.
Site-directed mutagenesis.
Oligonucleotides D1 (5′-GGCTATTTCCACGATGCTTCCGCAGTCTACGCAATCCTGGATTATGCCC-3′) (5′−GGGCATAATCCAGGATTGCGTAGACTGCGGAAGCATCGTGGAAATAGCC-3′), D2 (5′-CATGCTCCATCCTCCAGGGCGACCACTGCGTGTGGAACACTGGAC-3′) (5′-GTCCAGTGTTCCACACGCAGTGGTCGCCCTGGAGGATGGAGCATG-3′) and D3 (5′-CACAACCCAAACCACGCGCTGCCAGCGAAAGGGGTTCTCGAAC-3′) (5′-GTTCGAGAACCCCTTTCGCTGGCAGCGCGTGGTTTGGGTTGTG-3′) were used for site-directed mutagenesis of D-Box 1, 2 and 3, respectively, according to the manufacturer’s recommendations (Stratagene). Deletion of the KEN-Box has been described previously (Arlot-Bonnemains et al., 2001). All constructs were verified by sequencing.
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Olivier Coux for the gift of the Ubch5B. We thank Dr Robert Hipskind for helpful comments on the manuscript. This work was supported by the Ligue Nationale Contre le Cancer (Equipe Labellisée). Y.A.-B. and C.P. are supported by the Région Bretagne, the Ligue Nationale Contre le Cancer and the Association pour la Recherche sur le Cancer. A.C. is a postdoctoral fellow supported by Beca de Formacion de Personal Investigador en el Extranjero, Ministerio de Educacion, Cultura y Deporte.
REFERENCES
- Arlot-Bonnemains Y., Klotzbucher, A., Giet, R., Uzbekov, R., Bihan, R. and Prigent, C. (2001) Identification of a functional destruction box in the Xenopus laevis aurora-A kinase pEg2. FEBS Lett., 508, 149–152. [DOI] [PubMed] [Google Scholar]
- Bembenek J. and Yu, H. (2001) Regulation of the anaphase-promoting complex by the dual specificity phosphatase human Cdc14a. J. Biol. Chem., 276, 48237–48242. [DOI] [PubMed] [Google Scholar]
- Bischoff J.R. et al. (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J., 17, 3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco M.A., Sanchez-Diaz, A., de Prada, J.M. and Moreno, S. (2000) APC (ste9/srw1) promotes degradation of mitotic cyclins in G1 and is inhibited by cdc2 phosphorylation. EMBO J., 19, 3945–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A., Peter, M., Magnaghi-Jaulin, L., Vigneron, S., Galas, S., Lorca, T. and Labbe, J.C. (2001) Cyclin B/cdc2 induces c-Mos stability by direct phosphorylation in Xenopus oocytes. Mol. Biol. Cell, 12, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Fix O., Peters, J.M., Kirschner, M.W. and Koshland, D. (1996) Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev., 10, 3081–3093. [DOI] [PubMed] [Google Scholar]
- Fang G., Yu, H. and Kirschner, M.W. (1998) Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell, 2, 163–171. [DOI] [PubMed] [Google Scholar]
- Fesquet D., Morin, N., Doree, M. and Devault, A. (1997) Is Cdk7/cyclin H/MAT1 the genuine cdk activating kinase in cycling Xenopus egg extracts? Oncogene, 15, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Frank-Vaillant M., Haccard, O., Thibier, C., Ozon, R., Arlot-Bonnemains, Y., Prigent, C. and Jessus, C. (2000) Progesterone regulates the accumulation and the activation of Eg2 kinase in Xenopus oocytes. J. Cell Sci., 113, 1127–1138. [DOI] [PubMed] [Google Scholar]
- Hames R.S., Wattam, S.L., Yamano, H., Bacchieri, R. and Fry, A.M. (2001) APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J., 20, 7117–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K., Mihara, H., Kato, Y., Yamaguchi, A., Tanaka, H., Yasuda, H., Furukawa, K. and Urano, T. (2000) Degradation of human Aurora2 protein kinase by the anaphase-promoting complex–ubiquitin-proteasome pathway. Oncogene, 19, 2812–2819. [DOI] [PubMed] [Google Scholar]
- Jaspersen S.L., Charles, J.F. and Morgan, D.O. (1999) Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol., 9, 227–236. [DOI] [PubMed] [Google Scholar]
- Kimura M., Kotani, S., Hattori, T., Sumi, N., Yoshioka, T., Todokoro, K. and Okano, Y. (1997) Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J. Biol. Chem., 272, 13766–13771. [DOI] [PubMed] [Google Scholar]
- Kramer E.R., Scheuringer, N., Podtelejnikov, A.V., Mann, M. and Peters, J.M. (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell, 11, 1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Castro, A., Martinez, A.M., Vigneron, S., Morin, N., Sigrist, S., Lehner, C., Doree, M. and Labbe, J.C. (1998) Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J., 17, 3565–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger C.M. and Kirschner, M.W. (2000) The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev., 14, 655–665. [PMC free article] [PubMed] [Google Scholar]
- Roghi C. et al. (1998) The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci., 111, 557–572. [DOI] [PubMed] [Google Scholar]
- Sigrist S.J. and Lehner, C.F. (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell, 90, 671–681. [DOI] [PubMed] [Google Scholar]
- Visintin R., Prinz, S. and Amon, A. (1997) CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science, 278, 460–463. [DOI] [PubMed] [Google Scholar]
- Zachariae W. and Nasmyth, K. (1999) Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev., 13, 2039–2058. [DOI] [PubMed] [Google Scholar]
- Zhou H., Kuang, J., Zhong, L., Kuo, W.L., Gray, J.W., Sahin, A., Brinkley, B.R. and Sen, S. (1998) Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genet., 20, 189–193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


