Abstract
Background:
Thalamic volume loss is known to be associated with clinical and cognitive disability in progressive multiple sclerosis (MS).
Objective:
To investigate the treatment effect of Ibudilast on thalamic atrophy over 96 weeks in the phase 2 trial in progressive MS (SPRINT-MS).
Methods:
231 participants were randomized to either Ibudilast (n=114) or placebo (n=117). Thalamic volume change was computed using Bayesian Sequence Adaptive Multimodal Segmentation tool (SAMseg) incorporating T1, FLAIR, and fractional anisotropy maps and analyzed with a mixed-effects repeated-measures model.
Results:
There was no significant difference in thalamic volumes between treatment groups. On exploratory analysis, participants with primary progressive multiple sclerosis (PPMS) on placebo had a 0.004% greater rate of thalamic atrophy than PPMS participants on Ibudilast (p = 0.058, 95% CI −0.008 to <0.001). Greater reductions in thalamic volumes over 96 weeks were associated with worsening MSFC-4 scores (p = 0.002) and worsening performance on the SDMT (p<0.001).
Conclusion:
In a phase 2 trial evaluating Ibudilast in progressive MS, no treatment effect was demonstrated in preventing thalamic atrophy. Participants with PPMS exhibited a treatment effect. Longitudinal changes in thalamic volume were related to worsening of physical and cognitive disability, highlighting this outcome’s clinical importance.
Keywords: Ibudilast, multiple sclerosis, thalamus, atrophy, treatment outcome
Introduction
The thalamus is a deep gray matter (DGM) structure with extensive cortical and subcortical connectivity, existing as a relay center affected by the disease processes of multiple sclerosis (MS). The thalamus is comprised of multiple nuclei with specific functions ranging from relaying motor and sensory information to regulation of consciousness and alertness. The pathogenesis of thalamic damage in MS includes iron deposition,1 demyelination,2 and axon transection3 which contribute to thalamic neurodegeneration and structural atrophy occurring in both early and late stages of disease.4 Thalamic atrophy may result from a combination of effects from Wallerian degeneration from white matter disease in thalamocortical projections and direct insult to the thalamus. A gradient of “subependymal in” thalamic neuronal loss potentially emanating from adjacent CSF is identified.5 Thalamic atrophy is prevalent in progressive multiple sclerosis (PMS),6, 7 and data have shown strong associations between thalamic atrophy, cognitive impairment,8 and clinical disability progression.9 In vivo evidence of microglial activation in the thalamus is recognized, potentially to a similar extent, in both secondary progressive MS (SPMS) and primary progressive MS (PPMS).10
Ibudilast is a small molecule with mechanisms of action related to non-selective phosphodiesterase inhibition, macrophage migration inhibitory factor downregulation, and toll-like receptor 4 inhibition.11–13 These mechanisms regulate Th1/Th2 balance and increase the natural killer T cell subset.11 Ibudilast penetrates the blood brain barrier and may inhibit microglial activation, an important aspect of PMS pathogenesis with predilection to the thalamus.14–16 While Ibudilast did not exhibit a robust effect in reducing newly active white matter lesions in a phase 2 trial in RRMS, it did exhibit an effect on reducing brain atrophy, suggesting a stronger neuroprotective than anti-inflammatory effect and providing rationale for studying Ibudilast in PMS.17 Thus, the hypothesis is that Ibudilast would exhibit primarily an intrinsic therapeutic effect on the thalamus, rather than a secondary effect as a result of reducing inflammatory activity in connected white matter projections.
In contrast to whole brain atrophy, measures of brain atrophy quantifying volumetric changes in specific brain structures that are preferentially affected by MS have potential to result in larger effect sizes, resulting in greater disease specificity and smaller sample size requirements for clinical trials.18–20 Robust, semi-automated techniques of thalamic segmentation incorporating diffusion MRI are under development to measure outcomes in MS and other diseases.21–24
In this study, we use patient-level clinical and MRI outcome data from the SPRINT-MS trial, a large phase 2 study of Ibudilast treatment in PMS over 96 weeks, to evaluate thalamic volume as an outcome of interest in MS clinical trials aimed at neuroprotection. Specifically, the aims of this study are 1) to evaluate the treatment effect of Ibudilast on longitudinal changes in thalamic atrophy, 2) to analyze differences in thalamic atrophy by PMS subtype, and 3) to investigate the association of thalamic atrophy measures with progression of disability and cognitive decline over 96 weeks. Based on our aims, we hypothesize Ibudilast treatment will demonstrate a neuroprotective effect on thalamic atrophy, there will be no differences in rates of thalamic atrophy between PMS subtypes (PPMS and SPMS), and that thalamic atrophy will be associated with measures of physical and cognitive disability.
Methods
SPRINT-MS dataset
The SPRINT-MS data set and design has been described in detail previously.25 In brief it was a 96-week randomized controlled trial testing the effect of Ibudilast treatment on the primary outcome of whole brain atrophy by brain parenchymal fraction (BPF) in patients with PMS against placebo. The randomized sample included 255 participants (134 with PPMS and 121 with SPMS), of which 244 were included in their modified intention to treat primary analysis.
MRI acquisition and quality assurance
All MRIs were conducted using contemporary Siemens (Trio or Skyra) or GE (version 12X or higher) 3T systems. Image data collection was standardized across 23 participating sites.26 Best practices for multi-center imaging acquisition were used in the SPRINT-MS study to ensure high quality imaging for analysis. Scanner performance was monitored using monthly scans of the Biomedical Informatics Research Network (BIRN) phantom. Imaging physicists visited every imaging site prior to subject enrollment to review the study scanning protocol and phantom scan acquisition. In addition to analysis of monthly phantom scans, quality assurance assessments were conducted on every study subject scan to ensure adequate acquisition of high-quality images.
MRI protocol
Cranial MRI scans were obtained at screening as well as weeks 24, 48, 72, and 96 of follow-up using contemporary Siemens or GE 3T systems. The image acquisition included T1-weighted (T1w) 3D spoiled gradient-recalled echo (TR=20ms, TE=6ms,1mm isotropic), 2D T2-weighted (T2w) Fluid-Attenuated Inversion Recovery (FLAIR, TR=9400ms, TE=77ms, TI=2500ms, slice thickness 3mm), and high angular resolution DTI (TR=8700ms, TE=80ms, b=700sec/mm2, slice thickness 2.5mm, 64 directions) that was utilized for this analysis. All DTI metrics showed good scan-rescan repeatability.27
Image processing
Images were preprocessed using tools from FreeSurfer (version 6.0, http://surfer.nmr.mgh.harvard.edu), FMRIB Software Library (FSL version 6.0.0, https://fsl.fmrib.ox.ac.uk), MATLAB (version 9.5, Natick, Massachusetts: The MathWorks Inc., 2018b), and custom in-house software. The segmentation tool (SAMseg) was released in FreeSurfer version 7.1.0 and sourced from that version.
First, the standard FreeSurfer recon-all pipeline was run for each timepoint, using the T1w and T2w images. This data was then processed using the FreeSurfer longitudinal stream in order to create an unbiased within-template image.28 In short, this process iteratively aligns all timepoints to their median image with a robust, inverse consistent registration method,29 simultaneously co-registering all timepoints and creating a template image. Each timepoint is then initialized with common information from the template image, significantly increasing reliability and statistical power.28 Diffusion scans were corrected for motion and eddy current distortion using FSL, and dtifit was used to generate fractional anisotropy (FA) maps.30 For each timepoint, T2w and B0 data were registered to the corresponding longitudinal T1w image using the FreeSurfer bbregister tool, which performs within-subject, cross-modal registration using a boundary-based cost function.31 FA maps were resampled to the longitudinal T1w image using the MRI vol2vol command.
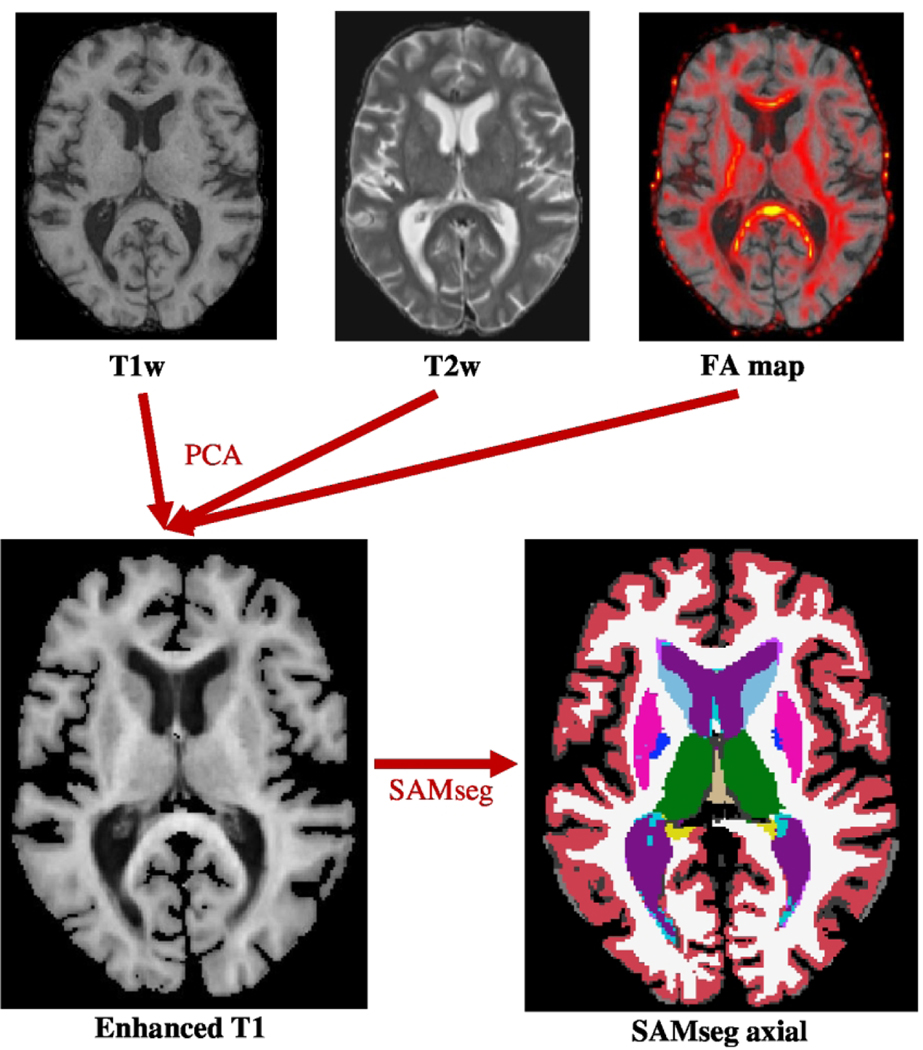
Subsequently, the T1w, T2w, and FA data were combined using principal component analysis (PCA)32 to generate a single image known as an “Enhanced T1.” This image has several advantages as it maintains the anatomical clarity of the T1w image while incorporating FA data localizing white matter tracts and T2w data accurately outlining the cerebrospinal fluid. This results in greater contrast between white and gray matter, which improves the delineation of the lateral border of the thalamic wall from white matter tracts (Figure 1).
Figure 1.
Overview of Preprocessing Stream for a Single Timepoint. Row 1: Axial cranial MRI images in T1w, T2w, and DTI-generated FA map sequences at the level of the lateral ventricles from the same subject. Row 2: Axial Enhanced T1 image (left) was used as an input for longitudinal SAMseg to produce the axial segmentation (right). FA: fractional anisotropy; PCA: principal component analysis; SAMseg: Bayesian Sequence Adaptive Multimodal Segmentation tool.
Brain segmentations were generated using the Enhanced T1 image as an input into the Bayesian FreeSurfer tool SAMseg.33 By reducing the dimensionality of the T1w, T2w, and FA data with principal component analysis, the “Enhanced T1” encodes information from the three channels without creating an imbalance between the log-prior and the log-likelihood in the objective function of the Bayesian segmentation. We previously conducted a preliminary analysis comparing several thalamic segmentation methods and found that SAMseg with “Enhanced T1” input was highly accurate.34 Longitudinal SAMseg has been shown to produce more reliable segmentations and detect disease effects better than the cross-sectional method. It does not make any prior assumptions on the scanner, MRI protocol, or the number and timing of longitudinal follow-up scans, making it well suited to this dataset. Segmentation-based total intracranial volume, ventricular volume, and total thalamic volume (TTV) were extracted directly from the SAMseg statistical output files from each timepoint for each subject. Brain parenchymal fraction, gray matter fraction, white matter fraction, and T2 lesion volume were determined at Cleveland Clinic Foundation (OH) and NeuroRx Research (Montreal, Canada) as trial primary and secondary outcomes as previously described.35
Clinical outcomes
Patient demographics (age, sex, ethnicity), MS disease characteristics (disease duration, MS subtype), and treatment group were obtained at baseline. Clinical disability and cognitive decline were measured at baseline and every 24 weeks thereafter. Functional disability measures included raw scores for the Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk (T25FW), 9-Hole Peg Test (9HPT), Low-Contrast Visual Acuity (LCVA), and the Multiple Sclerosis Functional Composite Score (MSFC-4). Cognitive disability was determined by the Symbol Digit Modalities Test (SDMT). Sustained disability progression (SDP) was defined as an increase in EDSS by 1 point that was sustained for at least 24 weeks, or as an increase by 0.5 points if baseline EDSS was ≥ 5.5. Participants missing EDSS values were excluded from analysis.36
Study endpoints
The primary outcome was thalamic atrophy as determined by total thalamic volume (TTV). Secondary outcomes included relationships between thalamic atrophy and PMS subtype (PPMS vs. SPMS), the effect of baseline TTV and change in TTV on SDP, and correlation between TTV and clinical and cognitive disability.
Statistical Analysis
All statistical analyses and visualizations were completed in RStudio Version 1.4.1106.37 Analyses were conducted using auto-generated thalamic volumes from SAMseg as outcomes. First, we explored demographic and disease-measure differences between treatment groups using parametric Student’s T-tests and non-parametric Mann-Whitney U tests depending on whether the variable was normally distributed, or the normality assumption was not justified, respectively. We subsequently used a linear mixed effects model to analyze the longitudinal relationship between TTV, treatment group, and MS subtype over 96 weeks, as well as to identify any two-way interactions. Next, we performed an exploratory analysis using a mixed effects model with percent change in thalamic volume by MS subtype as the outcome. We then examined the relationship between baseline TTV as well as percent change of TTV and SDP using logistic regression models. Lastly, relationships between TTV and clinical and cognitive disability measures were explored both at baseline and over 96 weeks using linear regressions and mixed models, respectively. All mixed effects models in this study used a repeated measures analysis treating time as a continuous variable. Non-significant 3-way or 2-way interaction terms were removed from models unless otherwise noted. All analyses controlled for disease duration, age, sex, ethnicity, enrollment site, and normalized intracranial volume. Intracranial volume was normalized by centering and scaling by 100 in all models. Uncorrected p-values are reported, and the threshold for significance was obtained using the Bonferroni correction. Figures assumed a common intercept across treatment groups. Sensitivity models regarding outliers were evaluated and showed no change. Reference groups for dichotomous variables in all models were Ibudilast, PPMS, male sex, and Hispanic ethnicity; their comparison groups are shown in table parentheses.
Results
Baseline demographics and disease characteristics
231 of 244 participants were included in this study, as 13 had missing T1w, T2w and/or FA data at baseline. Their demographic and disease characteristics stratified by treatment group are shown in Table 1. There were no significant differences between treatment and placebo groups aside from the Ibudilast treatment group being slightly younger.
Table 1:
Baseline Demographics and Clinical Characteristics by Treatment Group
| Characteristic | Placebo (n = 117) | Ibudilast (n = 114) |
|---|---|---|
| Age — yrα | 58.5 (53.7 – 61.8) | 55.8 (50.9 – 60.4) |
| Female sex — no. (%) | 67 (57) | 59 (52) |
| Hispanic ethnic group — no. (%) | 4 (3.4) | 4 (3.5) |
| Disease duration — yrα | 14.8 (23.6 – 23.6) | 15.4 (8.9 – 23.4) |
| Primary Progressive disease — no. (%) | 62 (53) | 58 (51) |
| Expanded disability status scale1α | 6.0 (4.0 – 6.0) | 6.0 (4.5 – 6.0) |
| Timed 25-ft walk — secα | 9.9 (7.0 – 15.6) | 9.3 (6.9 – 15.0) |
| 9-Hole peg test — secα | 30.3 (25.8 – 41.5) | 28.6 (24.0 – 36.8) |
| Symbol digit modalities test2 — no. of correct answers | 41.83 ± 14.31 | 43.67 ± 14.53 |
| Low-contrast visual acuity test3 — no. of correct answersα | 30.0 (20.0 – 35.0) | 31.0 (24.0 – 39.0) |
| Multiple sclerosis functional composite score4α | −0.001 (−0.489 – 0.484) | 0.166 (−0.448 – 0.621) |
| Brain parenchymal fraction5α | 0.81 (0.79 – 0.82) | 0.81 (0.80 – 0.82) |
| Grey matter fraction5 | 0.46 ± 0.02 | 0.46 ± 0.02 |
| White matter fraction5α | 0.35 (0.33 – 0.36) | 0.35 (0.33 – 0.36) |
| T2 lesion volumeα — cm3 | 5.15 (2.24 – 16.01) | 6.60 (2.13–13.91) |
| Total intracranial volume — 10−6 mm2 | 10.8 ± 1.1 | 10.9 ± 1.1 |
| Normalized ventricular volumeα— scaled by 102 | 3.9 (3.2 – 5.1) | 3.9 (3.1 – 5.3) |
| Normalized thalamic volumeα— scaled by 103 | 9.9 (8.9 – 10.5) | 9.8 (9.0 – 10.4) |
Median (IQR) shown for variables with non-Gaussian distributions. Mean ± SD reported for Gaussian distributions. P-value for continuous variables computed using Student’s T-test. Wilcoxon Rank Sum test used for continuous variables with non-Gaussian distributions. Ventricular volume and thalamic volume normalized for head size using intracranial volume. P-value for Hispanic ethnic group computed using Fisher’s exact test. P > 0.05 for all variables except for age (p = 0.04).
indicates non-Gaussian distribution.
Scores on Expanded disability status scale (EDSS) range from 0–10 in 0.5 point increments, with higher score indicating greater disability.
Scores on the Symbol digit modalities test range from 0–110 with higher scores indicating higher performance.
Scores on the low-contrast visual acuity test range from 0–60 with higher scores indicating greater ability to read letters on a 2.5% low-contrast eye chart.
Multiple sclerosis functional composite score is a cumulative z-score ranging from −1 to 1 with 0 indicating function at the level of the baseline mean of all participants.
Brain parenchymal fraction, gray matter fraction, and white matter fraction are measures of brain size, gray matter, and white matter, respectively, relative to the outer surface contour of the brain.
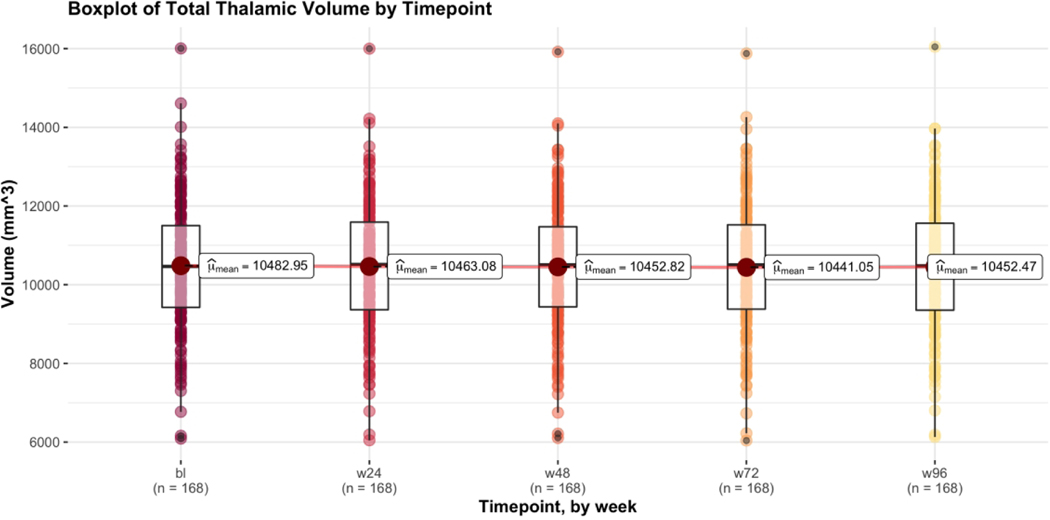
Figure 2 shows the trend in mean TTV of all study participants with MRI data at all 5 timepoints (n = 168) over the course of 96 weeks. At baseline, the mean TTV was 10,482.95 mm3. Volumes decreased by 19.87 mm3 (to 10,463.08 mm3) at week 24, 10.26 mm3 (to 10,452.82 mm3) at week 48, and 11.77 mm3 (to 10,441.05 mm3) at week 72. Week 96 volumes showed a slight increase by 11.42 mm3 (to 10,452.47 mm3).
Figure 2.
Boxplot of Total Thalamic Volume by Timepoint. Plot was produced using mean within-subject’s total thalamic volume of all participants with MRI data from all 5 timepoints. Includes both treatment groups (Placebo n = 86, Ibudilast n = 82) and both MS subtypes (PPMS n = 90, SPMS n = 78). Compared to the intention to treat analysis, if a participant was missing any sequence that precluded the generation of an Enhanced T1 image, they were excluded from the 168 with data at all 5 timepoints. Due to missing data, 0 participants were excluded at baseline, 10 participants at week 24, 23 participants at week 48, 31 participants at week 72, and 31 participants at week 96.
Treatment effect of Ibudilast on thalamic atrophy
Linear mixed effects models were used to examine the atrophy of the thalamus over the 96-week study period, including random intercepts for subjects. Mean TTV decreased by 0.188% annualized in the placebo group and 0.0146% annualized in the Ibudilast group. A 3-way interaction term between treatment group, time, and MS subtype was evaluated and found to be not significant. In the model including no interaction terms, both time and PPMS subtype were associated with significant decreases in total thalamic volume across all participants (Table 2). Notably amongst all participants, treatment group was not significantly associated with thalamic volume (p=0.842), however a small p-value of 0.105 for the two-way interaction between treatment group and time, in a model that included the 2-way interaction terms, prompted further investigation in an exploratory analysis.38
Table 2:
Results of the mixed effects model including all participants showing the effect of time, treatment group, MS subtype, and other predictors on changes in thalamic volume over 96 weeks. SPMS: Secondary progressive multiple sclerosis.
| Mixed Effects Model: Change in Thalamic Volume Over Time | ||||
|---|---|---|---|---|
|
| ||||
| Predictor | Estimate | P-value | 95% Confidence | Interval |
| Timepoint | −0.255 | 0.002 | −0.413 | −0.097 |
| Treatment Group (Placebo) | 34.600 | 0.842 | −279.518 | 348.727 |
| MS Subtype (SPMS) | −483.800 | 0.027 | −875.701 | −91.972 |
| Disease duration | −10.970 | 0.284 | −29.396 | 7.466 |
| Age | −8.135 | 0.528 | −31.403 | 15.132 |
| Sex (Female) | 120.000 | 0.543 | −236.265 | 476.226 |
| Ethnicity (Not Hispanic) | 402.900 | 0.416 | −490.115 | 1295.774 |
| Intracranial Volume | 0.966 | <0.001 | 0.802 | 1.130 |
Significant values shown in bold.
Differential rate of thalamic volume change by MS subtype
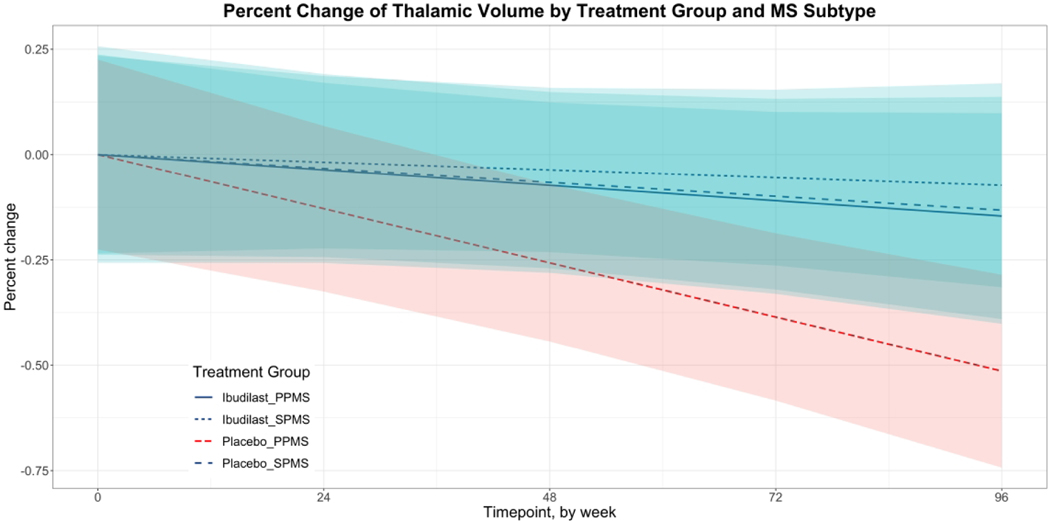
To better characterize the relationship between TTV and MS subtype by treatment group, we performed a subsequent analysis using a mixed effects model with percent change in thalamic volume by MS subtype as the outcome (Table 3). The interaction term between time and the PPMS placebo group approached significance (p = 0.058, CI −0.008 to <0.001). The difference between rates of thalamic atrophy between Ibudilast PPMS and placebo PPMS groups was −0.004% weekly, which represented a 0.21% greater thalamic volume loss in the PPMS placebo group each year (Figure 3). There were no remarkable differences in rates of thalamic atrophy between treatment groups in participants with SPMS.
Table 3:
Results of a mixed-effects model estimating the percent change in thalamic volume by treatment group and MS subtype.
| Mixed Effects Models: Effect of MS Subtype on Thalamic Atrophy | ||||
|---|---|---|---|---|
|
| ||||
| Predictor | Estimate | P-value | 95% CI | |
| Timepoint | −0.002 | 0.302 | −0.004 | 0.001 |
| Ibudilast SPMS | −0.073 | 0.685 | −0.401 | 0.258 |
| Placebo PPMS | −0.049 | 0.763 | −0.352 | 0.252 |
| Placebo SPMS | −0.014 | 0.942 | −0.363 | 0.334 |
| Timepoint * Ibudilast SPMS | 0.001 | 0.713 | −0.003 | 0.005 |
| Timepoint * Placebo PPMS | −0.004 | 0.058 | −0.008 | <0.001 |
| Timepoint * Placebo SPMS | <0.001 | 0.947 | −0.004 | 0.004 |
| Disease duration | 0.003 | 0.653 | −0.008 | 0.013 |
| Age | 0.009 | 0.239 | −0.004 | 0.021 |
| Sex (Female) | 0.099 | 0.368 | −0.097 | 0.296 |
| Ethnicity (Not Hispanic) | 0.163 | 0.570 | −0.357 | 0.684 |
| Intracranial Volume | <−0.001 | 0.112 | <−0.001 | <0.001 |
PPMS: Primary progressive multiple sclerosis. PPMS: Primary progressive multiple sclerosis. SPMS: Secondary progressive multiple sclerosis.
Figure 3.
Percent change of thalamic volume by MS subtype over 96 weeks estimated using an adjusted mixed effects model. 95% confidence intervals shown. PPMS: primary progressive multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
Associations between thalamic volumes and sustained disability progression
We used a logistic regression model to analyze the relationship between baseline TTV and SDP (Table 4 Model 1). We used a separate logistic regression model to examine the relationship between the percent change of TTV over 96 weeks and SDP (Table 4 Model 2). There were no significant relationships between SDP and TTV at baseline (p = 0.833) or the change in TTV over course of the study (p = 0.571).
Table 4:
Results of two adjusted logistic regression models showing associations between thalamic volume and sustained disability progression. Baseline thalamic volume used in Model 1. Percent change in thalamic volume over 96 weeks used in Model 2. TTV: total thalamic volume. SPMS: Secondary progressive multiple sclerosis. OR: Odds ratio.
| Logistic Regression: Thalamic Volumes and Sustained Disability Progression | |||
|---|---|---|---|
|
| |||
| Predictor | Model 1 | Model 2 | |
| Baseline thalamic volume | OR | 1.00 | - |
| P-value | 0.83 | - | |
| % Change in TTV over 96 weeks | OR | - | 0.88 |
| P-value | - | 0.57 | |
| Treatment Group (Placebo) | OR | 1.17 | 1.20 |
| P-value | 0.74 | 0.70 | |
| MS subtype (SPMS) | OR | 0.67 | 0.69 |
| P-value | 0.51 | 0.55 | |
| Disease Duration | OR | 0.99 | 0.99 |
| P-value | 0.72 | 0.86 | |
| Age | OR | 0.99 | 0.98 |
| P-value | 0.76 | 0.57 | |
| Sex (Female) | OR | 1.29 | 1.17 |
| P-value | 0.64 | 0.78 | |
| Ethnicity (Not Hispanic) | OR | 6.31E+07 | 7.94E+07 |
| P-value | 1.00 | 1.00 | |
| Intracranial Volume | OR | 1.00 | 1.00 |
| P-value | 0.91 | 0.62 | |
Differential effect of TTV on clinical and cognitive disability
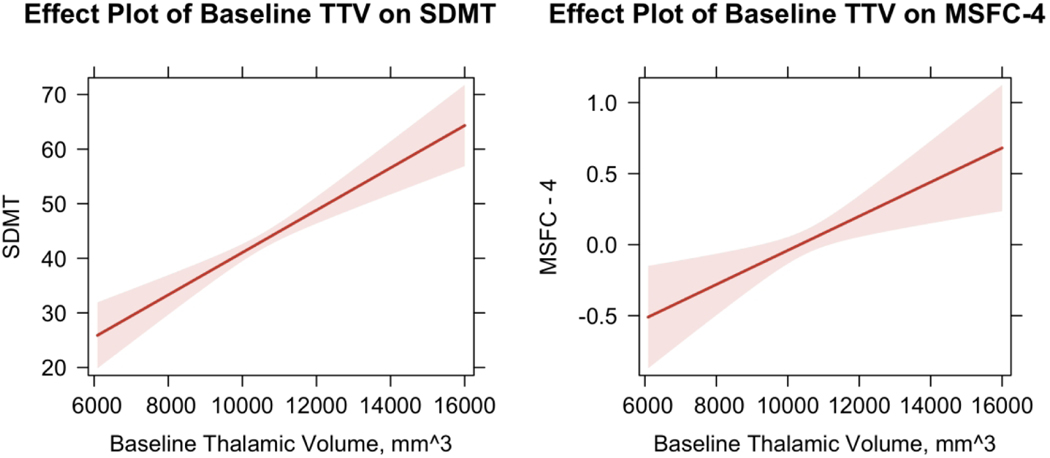
Using multiple regressions, we found significant relationships between baseline TTV and clinical disability. Specifically, smaller TTV at baseline was associated on average with a lower MSFC-4 score (β = 0.120, p = 0.003) and significantly poorer performance on the SDMT (β = 3.878, p < 0.0001), indicating greater clinical and cognitive disability, respectively.
The significant relationships between baseline TTV and disability measures are visualized in Figure 4. Pearson correlation coefficients were also computed to further examine the relationship between TTV and disability measures with significant change. There was a positive correlation between TTV and MSFC-4, r(229) = 0.328, p = <0.001 as well as between TTV and SDMT, r(229) = 0.377, p = <0.001 (Figure 4). There were no significant relationships between baseline TTV and EDSS, T25FW, and 9HPT scores. Our analysis also revealed confounders affecting participant clinical and cognitive disability scores including sex and MS subtype (Supplemental Table 1). The adjusted R-squared for all models ranged between 45.3% to 5.7%.
Figure 4.
Results of adjusted multivariate linear regressions showing significant relationship between baseline thalamic volume and disability measures. SDMT: symbol digit modalities test; MSFC-4: multiple sclerosis functional composite. Scores on the Symbol digit modalities test range from 0–110 with higher scores indicating higher performance. Multiple sclerosis functional composite score is a cumulative z-score ranging from −1 to 1 with 0 indicating function at the level of the baseline mean of all participants.
In addition to baseline TTV, we examined longitudinal associations between change in thalamic volume and changes in disability measures over the 96 weeks using mixed effects models. Similar to our baseline results, greater reductions in thalamic volumes over 96 weeks were associated with worsening in MSFC-4 scores (β = 0.120, p = 0.002) and worsening performance on the SDMT (β = 4.295, p<0.001; Table 5). Changes in other disability measures were not associated with thalamic volumes over time. Notably, we found time to be associated with incremental worsening in EDSS, LCVA-25, T25FW, and MSFC-4 (Table 5).
Table 5:
Longitudinal Associations Between TTV and Disability Measures. Significant p-values shown in bold, determined using Bonferroni correction for 6 outcomes (p<0.008). For interpretability, thalamic volumes are scaled by 103 for LCVA-25, SDMT, 9-HPT, and MSFC-4; and by 104 for EDSS and T25FW.
| Mixed Effects Model: Longitudinal Associations Between TTV and Disability Measures | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Predictor | EDSS | LCVA-25 | SDMT | T25FW | 9-HPT | MSFC-4 | |
| Thalamic volume — mm3 | β | −0.616 | 0.912 | 4.295 | 2.848 | −1.140 | 0.120 |
| P-value | 0.306 | 0.129 | <0.001 | 0.758 | 0.175 | 0.002 | |
| Timepoint | β | 0.002 | −0.020 | −0.007 | 0.028 | 0.011 | −0.001 |
| P-value | 0.001 | 0.001 | 0.233 | 0.007 | 0.128 | <0.001 | |
| Treatment group (Placebo) | β | −0.156 | −2.239 | −1.405 | 0.262 | 2.469 | −0.141 |
| P-value | 0.291 | 0.120 | 0.323 | 0.905 | 0.228 | 0.135 | |
| MS Subtype (SPMS) | β | 0.167 | −3.113 | 1.814 | 3.417 | 5.991 | −0.218 |
| P-value | 0.368 | 0.087 | 0.312 | 0.220 | 0.021 | 0.067 | |
| Disease duration | β | 0.008 | <0.001 | 0.019 | 0.072 | −0.031 | <0.001 |
| P-value | 0.364 | 0.132 | 0.820 | 0.579 | 0.795 | 0.978 | |
| Age | β | −0.009 | −0.161 | −0.219 | −0.256 | −0.207 | 0.003 |
| P-value | 0.422 | 0.132 | 0.039 | 0.118 | 0.172 | 0.693 | |
| Sex (F) | β | 0.096 | 1.520 | 7.631 | 2.488 | −10.363 | 0.371 |
| P-value | 0.567 | 0.350 | <0.001 | 0.319 | <0.001 | <0.001 | |
| Ethnicity (Not Hispanic) | β | 0.206 | 4.309 | 8.663 | −3.840 | −0.066 | 0.332 |
| P-value | 0.627 | 0.299 | 0.036 | 0.547 | 0.991 | 0.218 | |
| Intracranial volume1 — mm3 | β | <0.001 | <−0.001 | <0.001 | <−0.001 | −0.001 | <0.001 |
| P-value | 0.953 | 0.855 | 0.520 | 0.515 | 0.385 | 0.313 | |
Discussion
Our evaluation of thalamic volumes in the SPRINT-MS trial showed no treatment effect of Ibudilast compared to placebo in preventing thalamic atrophy over 96 weeks. However an interaction between treatment and time trending towards significance prompted an exploratory analysis which showed a slower trajectory of thalamic atrophy in participants with PPMS receiving Ibudilast. Lastly, we found thalamic volume at baseline and reductions over 96 weeks were associated with poorer performance on clinical and cognitive disability outcome measures and worsening of those outcomes during the trial, highlighting the clinical significance of thalamic atrophy in PMS.
Although phase 3 randomized trials of Ibudilast in MS are limited to-date, phase 2 studies have previously shown Ibudilast to demonstrate neuroprotective potential with effect on whole brain atrophy in MS. In a phase 2 trial of relapsing MS, Ibudilast was associated with a dose-response reduction in whole-brain atrophy rate, while not demonstrating a strong anti-inflammatory effect on preventing newly active lesions.17 In the primary analysis of the SPRINT-MS trial in people with PMS, Ibudilast treatment was associated with a 48% decrease in BPF than treatment with placebo.25 While we measured a high relative reduction in the rate of thalamic atrophy for Ibudilast, this did not meet statistical significance. It is important to note that we excluded more participants than the primary study related to missing imaging data (primarily DTI), which limited power to detect a treatment effect. In addition, we observed a high degree of variability, both within subjects with volumetric measurements of the thalamus, and between subjects in the setting of neurodegeneration with PMS. Research methods to improve measurement error including higher resolution MRI scanning and automated analysis tools may decrease intra-subject variability and aid in the ability to detect true treatment effects. Assuming Ibudilast exerts a neuroprotective effect through reducing chronic inflammation in the innate immune response, it is not possible with the available data from this trial to assert whether that effect is greater in the cortical gray matter or deep gray matter structures such as the thalamus.
This trial enrolled both SPMS and PPMS participants using the same inclusion and exclusion criteria. Similar rates of deep gray matter atrophy in SPMS and PPMS has previously been reported in the literature with a slight increased rate of atrophy in PPMS.39 In a post-hoc analysis of the original SPRINT-MS trial, Goodman et al. detected a greater treatment effect in the PPMS group compared to SPMS for BPF as well as cortical thickness.40 Our results appear in line with what was previously reported for other imaging outcomes in the same trial as it relates to disease phenotype, suggesting these volumetric measures track together to some extent, both in relationship to treatment effect and in contrasting SPMS and PPMS subgroups. Although we found greater treatment effect in the PPMS group compared with SPMS in our exploratory analyses, these results should be validated in future studies given the high levels of variability of TTV in our sample and, hence, wide confidence internals of our estimates. Possible differences in biology between SPMS and PPMS to explain differential treatment effects cannot be ruled out but additional study in progressive MS trials enrolling both SPMS and PPMS participants are warranted.
Our findings align with another recent study by Arnold et al. that assessed the effect of ocrelizumab on thalamic volume as an outcome in PMS. That study found ocrelizumab contributed to a 35% reduction thalamic volume loss in PPMS that was sustained beyond the double-blind period.41 Measurement of thalamic volume was found to have the greatest effect size compared to whole brain, cortical gray matter, and white matter volume loss. Ocrelizumab’s mechanism action of B cell depletion results in a highly efficacious anti-inflammatory effect. The participants in the PPMS ORATORIO study were on average 10 years younger at the time of enrollment than SPRINT-MS, highlighting an important difference in baseline demographics. Given evidence that atrophy of the thalamus may be multifactorial with different mechanisms of atrophy being more prominent at different stages of in the disease course of MS, it is possible that ocrelizumab’s demonstrated effect on the thalamus may be more related to secondary effects on the thalamus from thalamocortical projections, rather than direct effects on the thalamus.
Our study demonstrates a cross-sectional association of thalamic atrophy with clinical and cognitive disability measures at baseline as well as a longitudinal association of changes in thalamic volume during the 96-week trial with changes in clinical and cognitive disability. This highlights the clinical importance of ongoing thalamic atrophy in PMS. However, we did not find a significant association of baseline thalamic atrophy as a predictor of future sustained disability progression determined by EDSS during the 96-week period. A similar lack of association between baseline thalamic volume and disability progression was also observed in the recently published evaluation of thalamic atrophy in the PPMS ORATORIO study.41 More investigation is needed to evaluate this relationship in PMS.
This study has a few limitations. In this study we used FA maps in an Enhanced T1 image to produce an automated thalamic segmentation with SAMseg. Although diffusion data provides useful information for segmentation, they are susceptible to EPI distortion. To obtain more structurally accurate diffusion-tensor imaging data, it would be beneficial to acquire a field map or reverse phase encoding b0 sequence in future studies.42 Future work is needed to determine the value of FA maps for improving thalamic segmentation. More studies are needed to determine the reproducibility and generalizability of our results as this was a post-hoc analysis and the trial was not specifically powered to evaluate thalamic volume changes. Suggestions for further research include the development and refinement of the production of accurate, automated thalamic segmentations incorporating FA maps, as well as investigation of atrophy in other deep gray matter structures in PMS.
In summary, while a therapeutic effect on thalamic volume was not demonstrated by Ibudilast in the SPRINT-MS study of PMS, there was support for continuing to develop improvements in thalamic segmentation techniques to potentially reduce within subject variability that may confound trial results. Subgroup analysis suggested the effect was greater in PPMS and highlighted the overall clinical relevance of changes in thalamic volume in PMS on measures of physical and cognitive disability. The results of these analyses may be used to power future progressive MS studies with a focus on neuroprotection using the thalamus as an outcome.
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of Conflicts of Interest
Eric Klawiter has received research funding from Abbvie, Biogen and Genentech and has received consulting fees from Banner Life Sciences, Galen/Atlantica, Genentech, Greenwich Biosciences, and TG Therapeutics.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Department of Defense (W81XWH-18-1-0372) and by a donation from Neil and Didi Ryland. The NN102 trial was supported by the National Institute of Neurological Disorders and Stroke (U01NS082329) and the National Multiple Sclerosis Society (RG 4778-A-6) and by MediciNova through a contract with the National Institutes of Health (NIH). We thank the Central Coordinating Center (Massachusetts General Hospital) and the Data Coordinating Center (University of Iowa) coordinating the trial and providing clinical data for analysis. We thank the imaging coordinating centers, the Cleveland Clinic and NeuroRx, for providing imaging data for analysis. We thank the NN102 study PI Dr. Robert Fox of the Cleveland Clinic and all the individual site PIs and study staff. We thank the persons with multiple sclerosis who made this trial possible by volunteering to participate, as well as their families and friends who supported their involvement in the trial.
REFERENCES
- 1.Burgetova A, Dusek P, Vaneckova M, et al. Thalamic Iron Differentiates Primary-Progressive and Relapsing-Remitting Multiple Sclerosis. AJNR Am J Neuroradiol 2017;38:1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol 2005;64:1101–1107. [DOI] [PubMed] [Google Scholar]
- 3.Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 2002;52:650–653. [DOI] [PubMed] [Google Scholar]
- 4.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol 2018;83:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magliozzi R, Fadda G, Brown RA, et al. “Ependymal-in” Gradient of Thalamic Damage in Progressive Multiple Sclerosis. Ann Neurol 2022;92:670–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Diao W, Tian F, et al. Gray Matter Atrophy in the Cortico-Striatal-Thalamic Network and Sensorimotor Network in Relapsing-Remitting and Primary Progressive Multiple Sclerosis. Neuropsychol Rev 2021. [DOI] [PubMed] [Google Scholar]
- 7.Mesaros S, Rocca MA, Pagani E, et al. Thalamic damage predicts the evolution of primary-progressive multiple sclerosis at 5 years. AJNR Am J Neuroradiol 2011;32:1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batista S, Zivadinov R, Hoogs M, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 2012;259:139–146. [DOI] [PubMed] [Google Scholar]
- 9.Schoonheim MM, Pinter D, Prouskas SE, et al. Disability in multiple sclerosis is related to thalamic connectivity and cortical network atrophy. Mult Scler 2021:13524585211008743. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y, Pandya S, Zinger N, Michaelson N, Gauthier SA. Longitudinal change in TSPO PET imaging in progressive multiple sclerosis. Ann Clin Transl Neurol 2021;8:1755–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J, Misu T, Fujihara K, et al. Ibudilast, a nonselective phosphodiesterase inhibitor, regulates Th1/Th2 balance and NKT cell subset in multiple sclerosis. Mult Scler 2004;10:494–498. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y, Crichlow GV, Vermeire JJ, et al. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A 2010;107:11313–11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Perez D, Benito J, Polo G, et al. The Effects of the Toll-Like Receptor 4 Antagonist, Ibudilast, on Sevoflurane’s Minimum Alveolar Concentration and the Delayed Remifentanil-Induced Increase in the Minimum Alveolar Concentration in Rats. Anesth Analg 2016;122:1370–1376. [DOI] [PubMed] [Google Scholar]
- 14.Cooze BJ, Dickerson M, Loganathan R, et al. The association between neurodegeneration and local complement activation in the thalamus to progressive multiple sclerosis outcome. Brain Pathol 2022;32:e13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misin O, Matilainen M, Nylund M, et al. Innate Immune Cell-Related Pathology in the Thalamus Signals a Risk for Disability Progression in Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm 2022;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 2016;80:776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology 2010;74:1033–1040. [DOI] [PubMed] [Google Scholar]
- 18.Kim G, Chu R, Yousuf F, et al. Sample size requirements for one-year treatment effects using deep gray matter volume from 3T MRI in progressive forms of multiple sclerosis. Int J Neurosci 2017;127:971–980. [DOI] [PubMed] [Google Scholar]
- 19.Healy B, Valsasina P, Filippi M, Bakshi R. Sample size requirements for treatment effects using gray matter, white matter and whole brain volume in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2009;80:1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassiot B, Desgranges B, Eustache F, Defer G. Quantification and clinical relevance of brain atrophy in multiple sclerosis: a review. J Neurol 2009;256:1397–1412. [DOI] [PubMed] [Google Scholar]
- 21.Glaister J, Carass A, NessAiver T, et al. Thalamus segmentation using multi-modal feature classification: Validation and pilot study of an age-matched cohort. Neuroimage 2017;158:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stough JV, Glaister J, Ye C, Ying SH, Prince JL, Carass A. Automatic method for thalamus parcellation using multi-modal feature classification. Med Image Comput Comput Assist Interv 2014;17:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mang SC, Busza A, Reiterer S, Grodd W, Klose AU. Thalamus segmentation based on the local diffusion direction: a group study. Magn Reson Med 2012;67:118–126. [DOI] [PubMed] [Google Scholar]
- 24.Jonasson L, Hagmann P, Pollo C, et al. A level set method for segmentation of the thalamus and its nuclei in DT-MRI. 87:309–321-309–321. Available at: https://infoscience.epfl.ch/record/87233/files/sdarticle.pdf. Accessed 2007. [Google Scholar]
- 25.Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N Engl J Med 2018;379:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016;50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Sakaie KE, Debbins JP, Narayanan S, Fox RJ, Lowe MJ. Scan-rescan repeatability and cross-scanner comparability of DTI metrics in healthy subjects in the SPRINT-MS multicenter trial. Magn Reson Imaging 2018;53:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: A robust approach. NeuroImage 2010;53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 31.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009;48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci 2016;374:20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puonti O, Iglesias JE, Van Leemput K. Fast and sequence-adaptive whole-brain segmentation using parametric Bayesian modeling. Neuroimage 2016;143:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer KA. Improved automated thalamic segmentation using multiple MRI contrasts and machine learning in multiple sclerosis. ECTRIMS 2019 - Poster Session 3; 2019. [Google Scholar]
- 35.Naismith RT, Bermel RA, Coffey CS, et al. Effects of Ibudilast on MRI Measures in the Phase 2 SPRINT-MS Study. Neurology 2021;96:e491–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtzke JF. On the origin of EDSS. Mult Scler Relat Disord 2015;4:95–103. [DOI] [PubMed] [Google Scholar]
- 37.R: A Language and Environment for Statistical Computing [computer program]. Version 1.4.1106. Vienna, Austria: R Foundation for Statistical Computing, 2021. [Google Scholar]
- 38.Wasserstein RL, Schirm AL, Lazar NA. Moving to a World Beyond “p < 0.05”. The American Statistician 2019;73:1–19. [Google Scholar]
- 39.Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 2018;83:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman AD, Fedler JK, Yankey J, et al. Response to ibudilast treatment according to progressive multiple sclerosis disease phenotype. Ann Clin Transl Neurol 2021;8:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold DL, Sprenger T, Bar-Or A, et al. Ocrelizumab reduces thalamic volume loss in patients with RMS and PPMS. Mult Scler 2022:13524585221097561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallichan D, Andersson JL, Jenkinson M, Robson MD, Miller KL. Reducing distortions in diffusion-weighted echo planar imaging with a dual-echo blip-reversed sequence. Magn Reson Med 2010;64:382–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.