Abstract
Purpose:
Penile cancer is rare, with significant morbidity and limited literature assessing utility of peripheral and deep en face margin assessment (PDEMA) versus traditional margin assessment (vertical sections) on treatment outcomes.
Materials and Methods:
32-year Retrospective Multicenter Cohort Study at three academic tertiary care centers. Cohort of 189 patients with histologic diagnosis of in situ or T1a cutaneous squamous cell carcinoma of the penis at Brigham and Women’s, Massachusetts General Hospital (1988–2020), and Memorial Sloan Kettering Cancer Center (1995–2020) treated with PDEMA surgical excision, excision/circumcision, or penectomy/glansectomy. Local recurrence, metastasis, and disease-specific death were assessed via multivariable Cox proportional hazard models.
Results:
The cohort consisted of 189 patients. Median age at diagnosis was 62 years. Median tumor diameter was 1.3 centimeters. The following outcomes of interest occurred: 30 local recurrences, 13 metastases, and 5 disease-specific deaths. Primary tumors were excised with PDEMA (N=30), excision/circumcision (N=110), or penectomy/glansectomy (N=49). Of patients treated with traditional margin assessment (non-PDEMA), 12% had narrow or positive margins. 5-year proportions were as follows with respect to local recurrence-free survival (LRFS), metastasis-free survival (MFS), and disease-specific survival/progression-free survival (DSS/PFS), respectively: 100%, 100%, 100% following PDEMA; 82%, 96%, 99% following excision/circumcision; 83%, 91%, 95% following penectomy/glansectomy. Limitations: multi-institutional cohort study not externally validated.
Conclusions:
Initial results are encouraging that PDEMA surgical management effectively controls early-stage penile squamous cell carcinoma.
Keywords: Penile Neoplasms/ surgery, Organ Sparing Treatments, Mohs Surgery, Penile Neoplasms / pathology, Penis / surgery, Penis / pathology
Introduction
A paradigm shift in penile cancer treatment has occurred with an increase in use of penile-sparing approaches and decreasing rates of penile amputation.1–5 Penile organ-sparing surgery (OSS) includes topical and laser therapy, wide local excision, glansectomy, and Mohs Micrographic Surgery (Mohs). Currently, National Comprehensive Cancer Network Version 1.2023 (NCCN) penile cancer guidelines state Tis, Ta, and T1 penile cancer may be amenable to conservative penile organ-sparing approaches,6 citing evidence that overall survival rates are comparable for patients with T1-T2 tumors treated with organ-sparing surgery compared to partial or total penectomy.7 Specifically, for Mohs, NCCN guidelines state the technique may have benefit for patients with low-risk, small, superficial tumors on the proximal shaft in order to avoid penectomy.6 NCCN guidelines Version 1.2023 for squamous cell skin cancer classify squamous cell carcinoma (SCC) of the anogenital region, regardless of size, as high risk.8 These guidelines recommend peripheral and deep en face margin assessment (PDEMA) for optimal tumor clearance and maximal tissue conservation.8 European Association of Urology and American Society of Clinical Oncology (EAU-ASCO) guidelines for stage-dependent local treatment of penile carcinoma recommend topical treatment, laser ablation, or glans resurfacing for in situ disease, and wide local excision (WLE) with circumcision, laser ablation with circumcision, laser ablation monotherapy, glansectomy, or radiotherapy for T1a tumors.9 OSS is now the preferred option, whenever possible, to preserve ability to void upright, sexual pleasure and sensation, aesthetic aspects, and masculinity.10, 11
PDEMA enables the surgeon to spare the maximal amount of uninvolved tissue while histologically visualizing the entire marginal surface of the tumor for highly accurate tumor clearance (Figure 1). Examples of PDEMA include Tubingen techniques and Mohs.8 PDEMA spares patients additional surgeries following narrow (<1 millimeter (mm)) or positive margins in an area where tissue preservation is especially important. In our previous work on penile squamous cell carcinoma in situ (PSCCis) we found surgical margins to be positive in 26% of non-PDEMA surgical cases, with two-thirds of these patients receiving subsequent treatment because of positive margins.12 Only a handful of studies have evaluated outcomes for PDEMA in PSCC.13–19 Recently, the largest cohort to date was published (63 PSCCis, 22 PSCC), showing low local recurrence rates (LRR) (1.2%, 1/85) and high patient-reported satisfaction with functional outcomes.17 A systematic review by Assaf et al. studying outcomes of Mohs excision for PSCC reported combined LRR of 7.7% for 246 tumors, lower than the published LRR for OSS of 21.2%.19 Four of seven included studies reported tumor staging, with authors noting a lower LRR for PSCCis treated with Mohs (4.1%) in comparison to invasive SCC (16.9%).19
Figure 1:
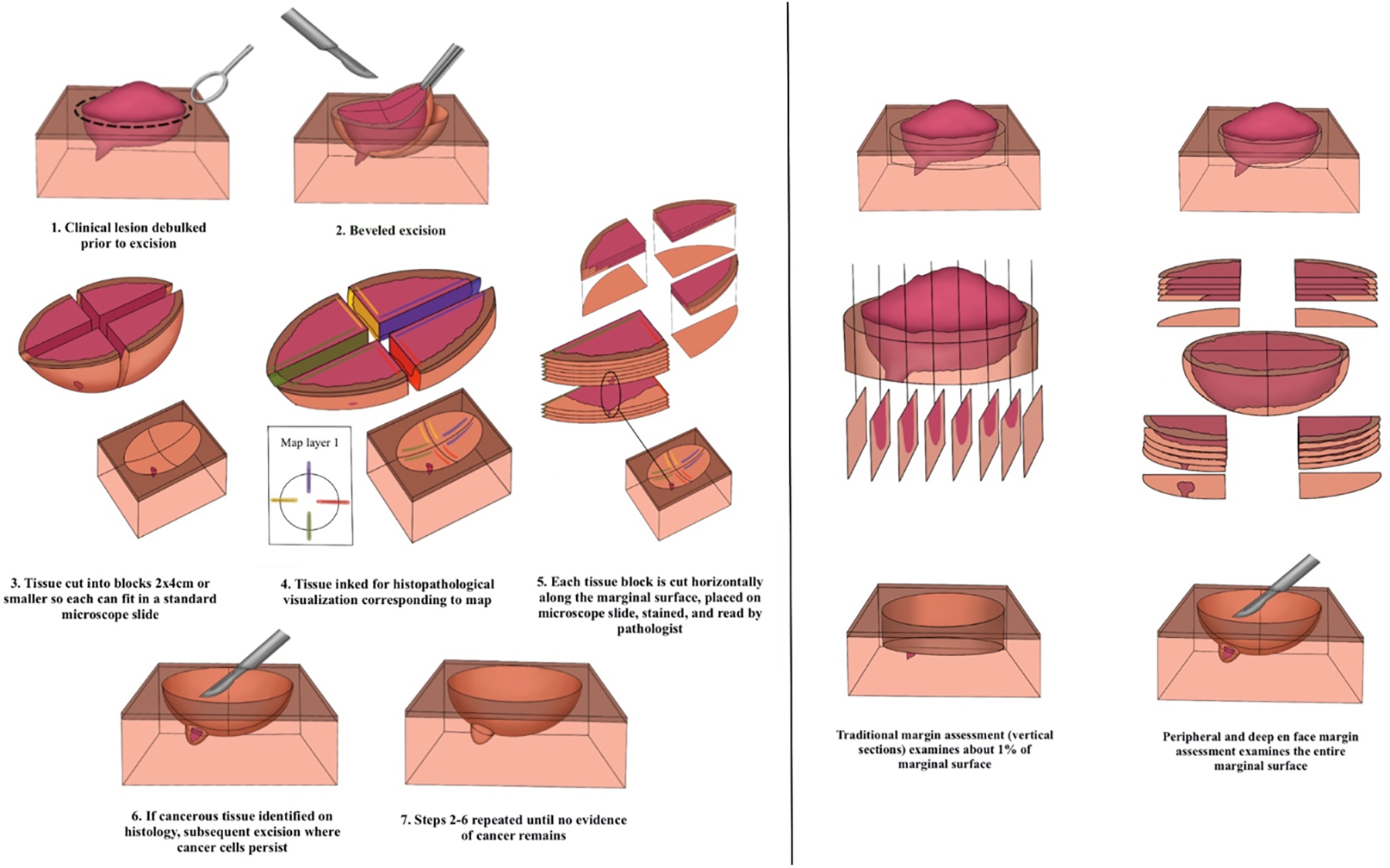
Peripheral and deep en face margin assessment
Currently no studies compare PDEMA to other surgical options for the treatment of PSCC. Based on data showing PDEMA to have superior outcomes for cutaneous SCC,20, 21 we sought to determine if there are differences in outcomes in patients with low-stage PSCC treated with PDEMA compared with other surgical treatment (circumcision/excision and penectomy/glansectomy).
Methods
Study Population
After approval by Partners Human Research Committee and Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC), all records between 01/01/96–10/21/2020 at Brigham and Women’s Hospital, Massachusetts General Hospital, and MSKCC were searched for patients with confirmed histologic diagnosis of malignant neoplasm of penis. Duplicate records (N=43), tumors with insufficient primary tumor information (N=158), and diagnosis other than cutaneous SCC (N=141) were excluded. Records of all patients meeting inclusion criteria were reviewed.
Tumors were classified according to American Joint Committee on Cancer 8. Patients diagnosed with in situ or T1a penile squamous cell carcinoma were included. PSCC ≥T1b were excluded (N=122). Patients with evidence of nodal disease (N=74) or metastasis (N=8) at diagnosis (>N0) were excluded. Patients who did not undergo initial surgical nodal staging were considered to be N0 for overall staging. Patients treated non-surgically and those treated with laser-ablation techniques or therapy in addition to excision were excluded (e.g. those treated with laser ablation in the case of narrow or positive margins) (N=79).
Patients were grouped into three groups according to treatment modality: 1) PDEMA, 2) traditional margin assessment (vertical sections) receiving excision or circumcision, and 3) traditional margin assessment (vertical sections) receiving glansectomy, or partial or total penectomy.
Follow-up time was defined as time from initial treatment of PSCC to date of last visit with member of treatment team. If a patient had not been seen within the last 3 months, obituaries were searched to evaluate survival status. Information obtained from the medical record review was maintained in Research Electronic Data Capture (REDCap), a secure, web-based database application.
Outcomes
The primary outcome measure was recurrence free survival (RFS)/progression free survival (PFS). This was defined as time from resection to metastasis (nodal and/or distant metastasis). Secondary outcome measures were LR-free/progression-free survival and disease-specific death (DSD)-free/progression-free survival. Tumors with no clinical disease were analyzed together with those with narrow or positive margins via a combined endpoint. For tumors that did not result in any poor outcomes, follow-up time was censored on the date of death or the date of last follow-up if the patient was alive at the time of data collection.
Statistical Analysis
Baseline demographic characteristics and tumor characteristics were analyzed using descriptive statistics. The total cohort of patients treated with PDEMA, excision/circumcision, and glansectomy/penectomy were compared using chi-squared, Fisher exact, and Kruskal-Wallis H test. For variables with missing data and an “unknown” category, statistical tests excluded such observations.
Univariable cox proportional-hazard regression analysis was used to determine association of treatment type, patient characteristics, and tumor characteristics with development of primary and secondary end points: metastasis-free/progression-free survival, LR-free/progression-free survival, and DSD-free/progression-free survival. Subsequently, separate multivariable models were built and tested for each endpoint by incorporating different combinations of clinically important variables that were borderline or statistically significant (P≤.20) on univariable analysis. Given the number of events in the cohort was small, the number of variables included in the final multivariable models was limited. Ultimately, for each primary and secondary endpoint, multivariate Cox proportional hazard models with the lowest Akaike information criterion were reported and included the following variables: treatment modality, tumor diameter, tumor depth. Adjusted Kaplan-Meier (KM) curves were constructed to assess RFS/PFS for each endpoint by treatment type. KM curves were adjusted for tumor diameter and tumor depth. Pair-wise comparison (log-rank test) was conducted on adjacent KM curves within each figure to determine whether there was a significant difference between the treatment modalities.
Analyses were performed using STATA 15.1 software (StataCorp, College Station, TX). All reported P values were 2-sided, with type I error (α) <0.05 considered to be statistically significant.
Results
The study cohort of low stage tumors treated with surgical monotherapy included 189 patients, 94 with in situ disease and 95 with superficial invasion between epidermis and corpora (30 PDEMA;110 excision/circumcision;49 penectomy/glansectomy). Baseline patient characteristics are listed in Table 1. The cohort is generally typical of 62-year-old white persons with penises diagnosed with PSCC who were former or current smokers and followed in clinic for 57 months (Interquartile range (IQR):97). Only 28 patients (15%) had explicit mention of being tested for HPV DNA within their tumor or previous lesions, with 15 (54%) testing positive, and 26 total patients (14%) were reported to have history of HPV- associated premalignant penile lesion(s), i.e. genital warts. Similarly, a minority had documentation of being asked about history of STIs by their physician (22%), and of those asked 19/42 (45%) reported history of ≥1 STIs. A small number had associated immunosuppression (12%). Half were uncircumcised (49%) and one-third had a history of penile disease (37%) to include phimosis, balanitis, psoriasis, and urethral strictures.
Table 1:
Patient Characteristics
| Patient Characteristics | PDEMA | Excision/Circumcision | Penectomy/Glansectomy | P-Value* |
|---|---|---|---|---|
| All patients | 30 | 110 | 49 | |
| BWH/MGH | 15 | 87 | 30 | |
| MSKCC | 15 | 23 | 19 | |
| Age at Diagnosis, years (median (Q1, Q3)) | 63 (52,68) | 58 (49, 72) | 68 (62,77) | 0.002a |
| Race, n (%) | 0.3 | |||
| White | 24 (80) | 98 (89) | 42 (86) | |
| Black | 3 (10) | 5 (4.5) | 5 (10) | |
| Asian | 2 (6.7) | 5 (4.5) | - | |
| Other/Unknown | 1 (3.3) | 2 (2.0) | 2 (4.0) | |
| Smoking, n (%) | 0.2 | |||
| Non-smoker | 18 (60) | 47 (43) | 16 (33) | |
| Current Smoker | 2 (7.0) | 13 (12) | 3 (6.0) | |
| Former smoker | 10 (33) | 46 (42) | 27 (55) | |
| Unknown | - | 4 (3.0) | 3 (6.0) | |
| Reported HPV infection, n (%) | 1 | |||
| No | 2 (7.0) | 10 (9.0) | 1 (2.0) | |
| Yes | 3 (10) | 11 (10) | 1 (2.0) | |
| Unknown | 25 (83) | 89 (81) | 47 (96) | |
| Reported STI, n (%) | 0.3 | |||
| No | 4 (13) | 14 (13) | 5 (10) | |
| Yes | 6 (20) | 12 (11) | 1 (2.0) | |
| Unknown | 20 (67) | 84 (76) | 43 (88) | |
| Previous Immunosuppression, n (%) | 6 (20) | 13 (12) | 3 (6.1) | 0.3 |
| Circumcision Status, n (%) | 1+ | |||
| No | 9 (30) | 57 (52) | 27 (55) | |
| Yes | 5 (17) | 32 (29) | 16 (33) | |
| Neonatal | 3 (60) | 25 (78) | 3 (19) | |
| Adult | 1 (20) | 2 (6.0) | 12 (75) | |
| Unknown | 1 (20) | 5 (16) | 1 (6.0) | |
| Unknown | 16 (53) | 21 (19) | 6 (12) | |
| Hx Penile Disease, n (%) | 8 (27) | 33 (30) | 28 (57) | 0.002± |
| Phimosis | 2 (6.7) | 11 (10) | 11 (23) | |
| Balanitis/posthitis | 3 (10) | 16 (15) | 13 (27) | |
| Psoriasis | 1 (3.3) | 6 (5.5) | - | |
| Urethral stricture | 1 (3.3) | - | 6 (12) | |
| Hx HPV-related Premalignant Lesion, n (%) | 3 (10) | 20 (18) | 3 (6.1) | 0.1 |
Abbreviations: PDEMA, peripheral and deep en face margin assessment; BWH, Brigham and Women’s Hospital; MGH, Massachusetts General Hospital; MSKCC, Memorial Sloan Kettering Cancer Center; Q1, first quartile; Q3, third quartile; HPV, human papilloma virus; STI, sexually transmitted infection; Hx, History
P-values represent the overall test for significance from Chi-squared test (Fisher’s exact test for values <5) unless otherwise specified
Kruskal-Wallis H test
P-value comparing presence of circumcision to absence of circumcision
P-value comparing history of any penile disease to no history of penile disease
Median tumor diameter was 1.3 cm (Quartile 1 (Q1):0.8, Quartile 3(Q3):2.4) (Range 0.2–7.4 cm) (Table 2). The cohort was evenly split between in situ (50%) and tumors invading between the epidermis and corpora (50%). Urethral invasion (3 tumors/1.6%) was uncommon. A small proportion of patients had documentation of staining with p16 (14%). Of these, 54% (14/26) stained positive. Most tumors were well differentiated/grade 1(76%).
Table 2:
Tumor Characteristics
| Tumor Characteristics | PDEMA | Excision/Circumcision | Penectomy/Glansectomy | P-Value* |
|---|---|---|---|---|
| All patients | 30 | 110 | 49 | |
| Tumor Diameter, cm (median (Q1, Q3)) | 1.2 (0.9,2.5) | 1.0 (0.6,1.8) | 2.4 (1.2,3.2) | <0.001a |
| In situ,± n (%) | 22 (73) | 69 (63) | 3 (6.1) | <0.001 |
| Urethral Invasion present, n (%) | - | - | 3 (6.1) | 0.02 |
| p16 Staining | 0.9 | |||
| No | 4 (13) | 6 (5.5) | 2 (4.0) | |
| Yes | 3 (10) | 9 (8.5) | 2 (4.0) | |
| Unknown | 23 (77) | 95 (86) | 45 (92) | |
| Well Differentiated,† n (%) | 30 (100) | 91 (83) | 23 (47) | <0.001 |
| Tumor Location | <0.001 | |||
| Glans | 9 (30) | 24 (22) | 29 (59) | |
| Foreskin | - | 23 (21) | - | |
| Shaft | 20 (67) | 45 (41) | 2 (4.0) | |
| Overlapping | 1 (3.0) | 18 (16) | 18 (37) | |
| Received imaging, n (%) | 11 (37) | 41 (37) | 31 (63) | 0.007• |
| MRI | 1 (3.3) | 13 (12) | 9 (18) | |
| CT | 6 (20) | 23 (21) | 20 (41) | |
| PET | 2 (6.7) | 1 (0.9) | - | |
| Ultrasound | 3 (10) | - | - | |
| CXR | - | 16 (15) | 11 (22) | |
| Received Lymph Node Dissection, n (%) | - | - | 4 (8.2) | 0.009 |
| Received Adjuvant Radiation, n (%) | - | 3 (2.7) | - | 0.7 |
| Final Margins, n (%) | 0.03 | |||
| Negative | 30 (100) | 90 (82) | 47 (96) | |
| Narrow (<1 mm) | 0 | 8 (7.0) | 1 (2.0) | |
| Positive | 0 | 12 (11) | 1 (2.0) |
Abbreviations: PDEMA, peripheral and deep en face margin assessment; Q1, first quartile; Q3, third quartile; MRI, magnetic resonance imaging; CT, computerized tomography; PET, positron emission tomography; CXR, chest radiograph
P-values represent the overall test for significance from Chi-squared test (Fisher’s exact test for values <5) unless otherwise specified
Kruskal-Wallis H test
Remaining tumors located between epidermis and corpora
Remaining tumors moderately differentiated
P-value comparing any imaging to no imaging
Statistically significant differences in patient characteristics between the PDEMA, excision/circumcision, and penectomy/glansectomy groups were median age at diagnosis, (Q1-Q3) (63 years, (52–68) vs 58 years, (49–72) vs. 68 years, (62–77) (P=0.002) and reported history of penile disease (27% vs.30% vs. 57%) (P=0.002), respectively. Statistically significant differences in tumor characteristics between PDEMA, excision/circumcision, and penectomy/glansectomy groups were median tumor diameter, (Q1-Q3) (1.2 cm, (0.9–2.5) vs. 1.0 cm, (0.6–1.8) vs. 2.4 cm, (1.2–3.2)) (P<0.001), tumor anatomic level of invasion (73% in situ vs.63% vs. 6%) (P<0.001), histologic differentiation (100% well differentiated vs. 83% vs. 47%) (P<0.001), and location (30% on glans vs.22% vs.59%) (P<0.001).
Patients receiving penectomy/glansectomy were more likely to have had imaging (63%) vs. excision/circumcision: 37% vs. PDEMA: 37% (P=0.007) or undergone lymph node dissection (8%) vs. no patients in excision/circumcision and PDEMA groups (P=0.009). All margins were negative following PDEMA vs. 18% narrow (<1 millimeter)/positive in excision/circumcision group vs. 4.0% narrow/positive in penectomy/glansectomy group (P=0.03).
During the study period 30 patients developed LR, 13 metastasis, and 5 DSD. Median time to LR was 21 months (Q1:13, Q3:44). Most recurrences (77%) occurred <4 years following surgery (23 of 30). Median follow-up time for 155 patients without an event was 46 months. 5-year proportions were as follows with respect to local recurrence-free survival (LRFS), metastasis-free survival (MFS), and disease-specific survival/progression-free survival (DSS/PFS), respectively: 100%, 100%, 100% following PDEMA; 82%, 96%, 99% following excision/circumcision; 83%, 91%, 95% following penectomy/glansectomy.
Multivariable models were adjusted for tumor diameter (as a continuous variable) and depth (in situ versus T1a). For local recurrence (Figure 2), pairwise comparison testing showed PDEMA to have a statistically significantly higher local-recurrence free/progression-free survival as compared to penectomy/glansectomy (logrank P=0.048). PDEMA was also associated with a higher local-recurrence free/progression-free survival as compared to excision/circumcision with the difference falling short of the 0.05 threshold for statistical significance (logrank P=0.07). The hazard ratio (HR) for PDEMA vs excision/circumcision was HR:0.06 (95% CI 0.04, 0.1) and for PDEMA vs penectomy/glansectomy HR:0.06 (95% CI 0.03, 0.1). For metastasis (Figure 3), no significant differences were seen in metastasis-free/progression-free survival between surgical treatments groups. For metastasis, the HR for PDEMA vs excision/circumcision was HR:0.2 (95% CI 0.1, 0.6) and for PDEMA vs penectomy/glansectomy HR:0.1 (95% CI 0.04, 0.3). For DSD (Figure 4), no significant differences were seen in disease-specific death-free/progression-free survival between surgical treatments groups. For DSD, HR for PDEMA vs excision/circumcision was HR:0.9 (95% CI 0.1, 5) and for PDEMA vs penectomy/glansectomy HR:0.2 (95% CI 0.1, 0.8).
Figure 2:
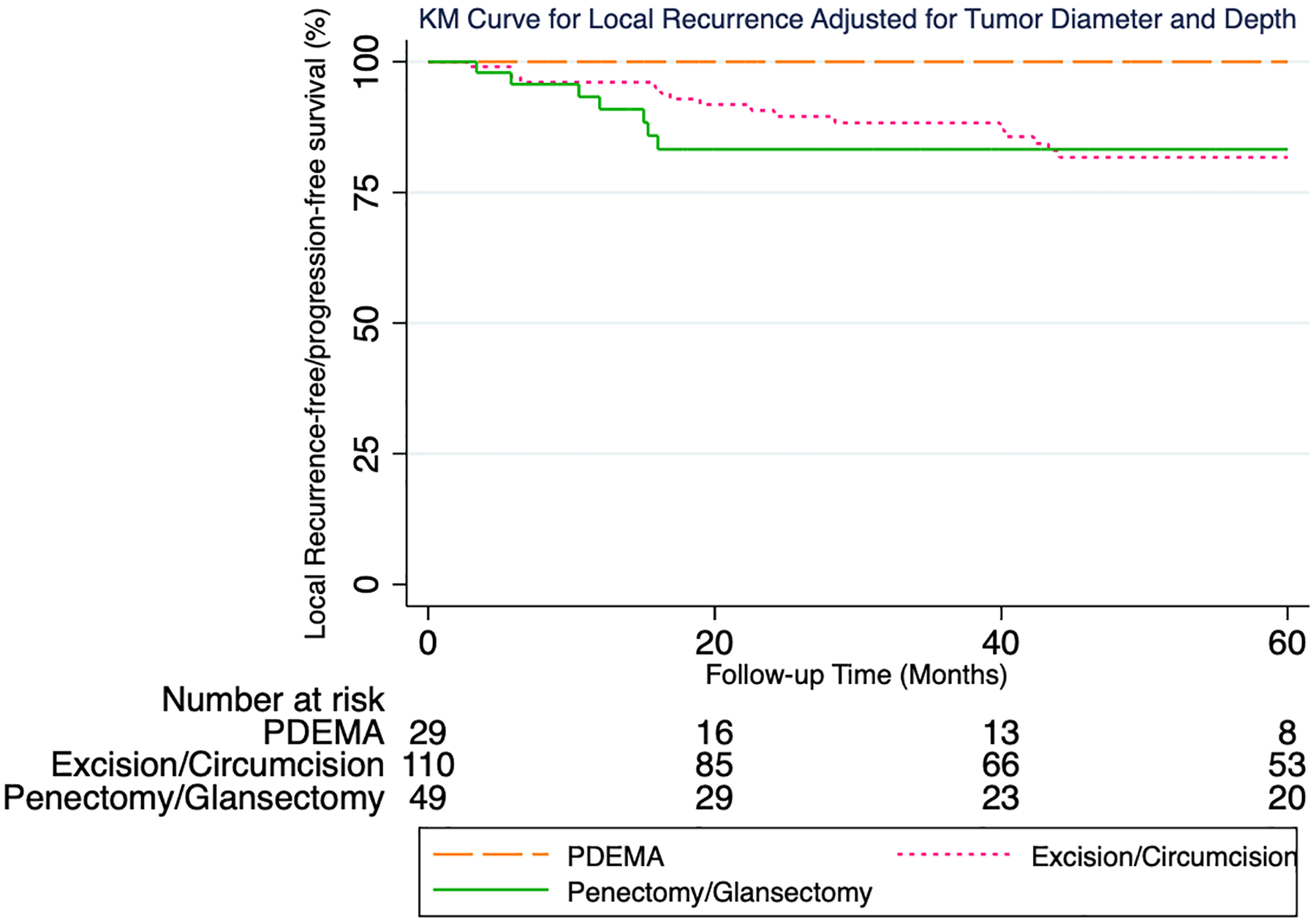
KM Curve for Local Recurrence Adjusted for Tumor Diameter and Depth
Figure 3:
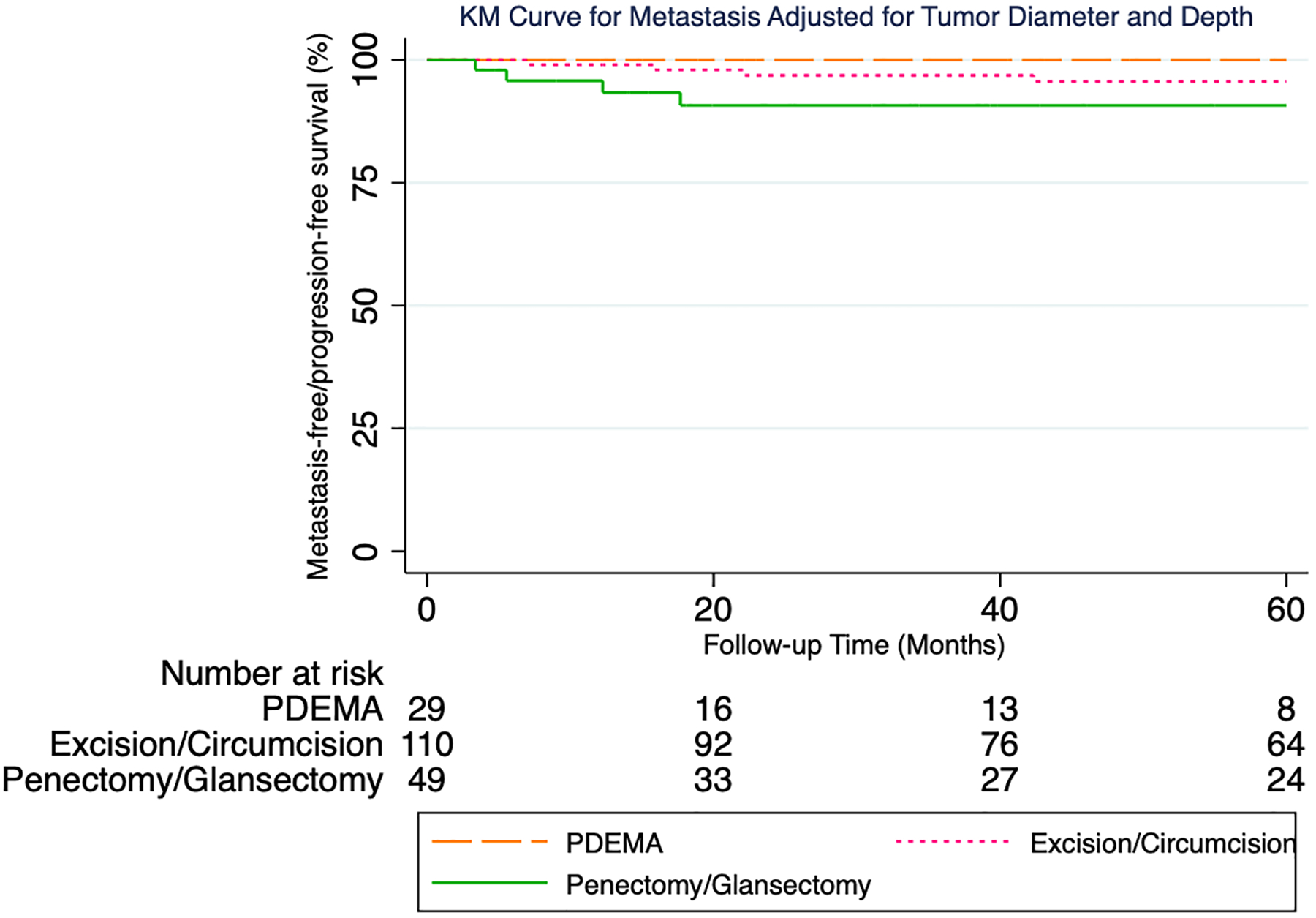
KM Curve for Metastasis Adjusted for Tumor Diameter and Depth
Figure 4:
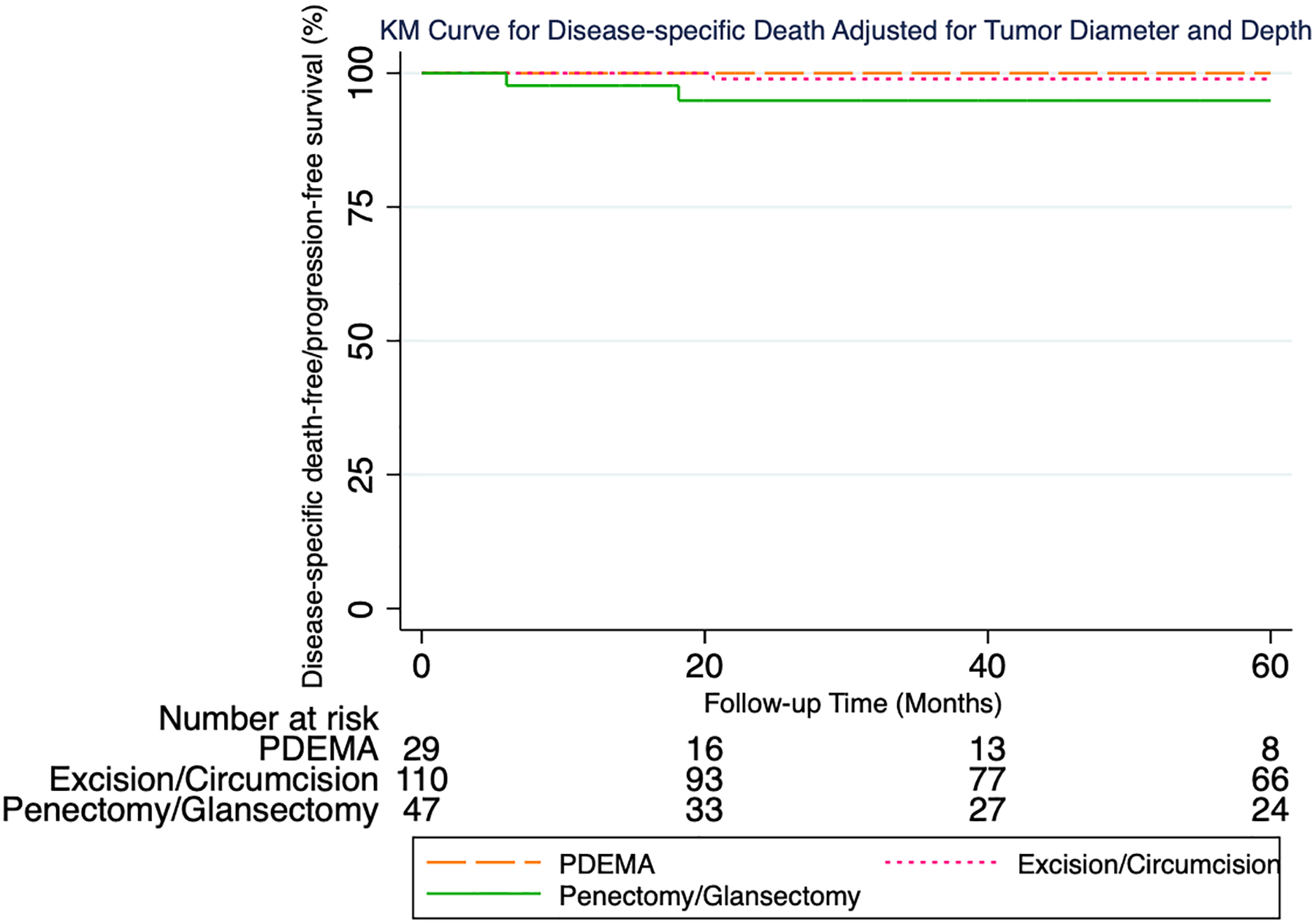
KM Curve for Disease-specific Death Adjusted for Tumor Diameter and Depth
Discussion
To the best of our knowledge, this study is the first comparison of a form of PDEMA (Mohs) against excision with standard vertical sections, and penectomy/glansectomy in the treatment of node-negative low-stage primary PSCC. Thirty tumors treated with PDEMA had no poor outcomes whereas other forms of excision carried elevated risks of local recurrence, metastasis, and DSD. This data suggests PDEMA treatment is a valid option for low-stage PSCC and is not inferior to more aggressive surgery. Herein we report LRRs following PDEMA, excision/circumcision, and penectomy/glansectomy to be 0%, 19%, and 18% respectively. Current NCCN and EAU-ASCO guidelines recommend glansectomy, WLE, and laser therapy for treatment of low-stage disease, with NCCN citing LRRs of 9%, 25%, and 18% respectively.6, 9 While few studies have evaluated outcomes of PDEMA in PSCC,13–19 the largest cohort to date of 85 PSCC cases treated with Mohs reported a low LR rate of 1.2%. Authors of this cohort study called for consensus guidelines with clear indications for Mohs excision for persons with penises with genital cancer.17 Other studies evaluating PDEMA for PSCC have demonstrated local recurrence risks ranging from 6–32%.13, 14, 18, 19
Positive surgical margins in PSCC have been associated with both increased risk of local recurrence and death.7, 22 Study authors advise clinicians to strive for negative margins to decrease poor outcomes for patients. The 12% risk of narrow or positive margins with traditional margin assessment herein may be considered high but is lower than recent cohorts of nasal basal and squamous cell carcinoma (positive margin risk of 24%) and vulvar Extramammary Paget disease (positive margins in 97% despite vulvectomy) excised with traditional margin assessment.20, 23 Thus, the risk of positive margins may be quite high in cutaneous cancers when PDEMA is not employed. One study set out to quantify a safe clear margin following PSCC OSS. Authors found no significant difference in development of LR with clear margins (defined as ≤5 mm) compared to >5 mm margins (P=1) although margins clear by >1mm did show decreased risk in development of LR compared to margins clear ≤1 mm, P<0.001.24 Studies have reported higher rates of local recurrence following OSS when compared to penile amputation but no difference in overall survival.4, 7 However, in general, these cohorts include few, if any, patients treated with PDEMA excision.7 The data herein showed lower hazard ratios for patients treated with PDEMA versus both excision/circumcision and penectomy/glanesectomy for all poor outcomes (local recurrence, metastasis, and disease-specific death) after adjusting for tumor diameter and depth of invasion.
In a survey of patients treated with Mohs of the genital area, 98% reported satisfaction with the surgical outcome.17 This is noteworthy considering a systematic review by Maddineni et al found the treatment of penile cancer was associated with negative effects on well-being in up to 40% of individuals and psychiatric symptoms in approximately 50%.25 Aggressiveness of penile cancer surgery has been negatively correlated with quality of life.26 Increased rates of depression have been reported following penectomy.10 Additionally, urinary function is less often affected following OSS.27 A study comparing postoperative sexual function following wide local excision versus glansectomy for PSCC found wide local excision led to better sexual outcomes and less postoperative complications in comparison to glansectomy.28 In some cases patients may refuse partial or total penectomy. We speculate utilizing PDEMA may allow for decreased local recurrence rates, decreased post-surgical morbidity, and greater patient satisfaction.
Methods of achieving complete margin assessment are outlined in NCCN guidelines Version 1.2023 for SCC.8 Mohs is widely available throughout the United States and in some European countries. The other forms of PDEMA include the Tubingen torte and muffin methods developed in Germany. Tubingen methods utilize rapid paraffin sections read within 24–48 hours of excision by a pathologist. Results are conveyed to the resecting surgeon with a mapping technique, allowing the surgeon to re-resect area(s) with positive margins (Figure 1).
Choice of reconstruction following PDEMA varies by extent of excision required and location. Urethral reconstruction via grafting is needed when there is significant urethral invasion. Although PDEMA may potentially be more time-consuming and requires a multi-disciplinary approach, it may confer a benefit for patients with regard to minimization of resection of uninvolved structures and less need for re-excision for narrow or positive margins. Increased margin visualization and accuracy of PDEMA has been associated with higher cure rates in cutaneous SCC, leading the NCCN to make it the preferred treatment option for “very high risk” SCC (>4 cm tumors, any location), a primary treatment option for “high risk” SCC which includes anogenital SCCs, and the recommended treatment in the case of positive margins after standard excision.8, 20, 21
Penectomy and glansectomy were increasingly less utilized over time, with only 4 cases performed after 2015 and a general temporal trend towards increased utilization of less invasive procedures (PDEMA, excision, circumcision). Unfortunately, a single patient received penectomy for in situ disease which highlights the need for physician education and further studies regarding minimally invasive therapies such as circumcision, total glans resurfacing or PDEMA for T1a disease, and topical therapies for in-situ disease.12 In our current practice we utilize surgical excision with PDEMA (Mohs) for penile in situ lesions that fail initial therapy with topical 5% 5-fluorouracil +/− calcipotriol and for penile SCC where the tumor can be cleared without penectomy. Randomized trials would be ideal and may possibly be conducted via a group of centers treating sufficient numbers of PSCC. Meanwhile PDEMA may be considered as an alternative to more radical forms of excision. Per NCCN and EAU-ASCO guidelines, patient surveillance for a minimum of five years via physical examination of the penis and groins is recommended.6, 9 In the absence of palpable inguinal lymphadenopathy, pathologic inguinal node staging is not necessary for in-situ and T1a PSCC per current guidelines, although surgical lymph node staging may be considered for grade 2/moderately differentiated T1a PSCC per EAU-ASCO guidelines. We agree with this guidance given that in our cohort, 4 of 144 well-differentiated tumors subsequently metastasized whereas 9 of 45 moderately-differentiated cases did so.6, 9 Individuals diagnosed with PSCC may have concurrent field dysplasia due to HPV and/or balanitis, warranting surveillance for new primary lesions and circumcision. Larger multicenter studies are needed to further assess outcomes for PDEMA excision vs. topical treatment for PSCCis and PDEMA compared to wide excision with traditional margin assessment treatment for T1a tumors.
Limitations of this study include a long time period, during which practice patterns have changed and the relatively small number of PDEMA cases, however, penile carcinoma is a rare disease with inherently small numbers. Over the study period, PDEMA use gradually increased to 11 cases performed in the latter 3 years of study as compared to penectomy/glansectomy where only 1 case was performed during this time. The study population was predominantly white reflecting the demographics of patients treated at the three contributing hospitals, which may not reflect the demographics of the PSCC population at large. Thus, further studies in broader populations reflective of the diverse PSCC population are necessary. Follow-up time was variable; however, median follow-up was 4 years and 77% of recurrences herein occurred within 4 years of surgery. This time to LR is in line with a meta-analysis of LR in OSS reporting 92% of LRs to occur within 5 years, so the effect of differential follow-up was likely minimal.29 Because the number of events in the cohort was small, the number of variables that could be included in multivariable analyses was limited. As in all cohort studies, it is possible that confounding factors exist that are unknown and are therefore not taken into account in the results. Histologic differentiation did not impact model fit via AIC in the present study. Other studies have shown moderate differentiation to be an independent prognostic factor relative to well differentiation.30, 31 The 3% vs 10% unadjusted 5-year metastasis risk in well vs moderately differentiated tumors in the present study indicates moderate differentiation may be a poor prognostic factor relative to well differentiation, though weaker than diameter and depth in these data, and a larger study would be needed to prove its contribution. Tumor diameter and depth are chief components of current staging and did improve the model’s AIC, so the models reported herein were adjusted for these factors. Another limitation is that reporting of HPV status and p16 staining was rare so its prognostic impact could not be assessed. Finally, PDEMA techniques may not be available in some countries with high PSCC incidence, such as Uganda and Eswatini.32 However, in 2022 Fu et al reported increasing age-standardized incidence rates (ASIR) of PSCC in 15 countries: 13 European countries, China, and Israel.32 To the authors knowledge, PDEMA is available in 13 of 15 of the reported countries with increasing PSCC ASIR. PDEMA is also available in Brazil and India, two countries with the highest number of PSCC incident cases in 2020.32 Where Mohs is unavailable, non-Mohs PDEMA (Tubingen methods) can be implemented with proper communication between surgeons and pathologists. See emerging guidance from members of the NCCN non-melanoma skin cancer panel.33
Conclusions
Surgical management utilizing peripheral and deep en face margin assessment (PDEMA which includes Mohs and Tubingen excision methods) effectively controls early-stage penile SCC. PDEMA allows opportunity for increased margin visualization, minimization of resection of uninvolved tissues without increasing risk of recurrence, and reduced need for re-excision. Further studies are needed comparing PDEMA versus traditional margin assessment for T1a penile SCC, and comparing PDEMA to topical treatment for in situ penile SCC.
Funding sources:
This research was funded, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748. The funder had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Conflicts of Interests:
KAO, JLT, FM, GZ, TNM, KA, AJX have no conflicts of interest to declare.
CDS is a steering committee member for Castle Biosciences; a steering committee member and consultant for Regeneron Pharmaceuticals; has received research funding from Castle Biosciences, Regeneron Pharmaceuticals, Novartis, Genentech, and Merck, and is a chair for NCCN.
PES is Vice-chair of NCCN bladder and penile cancer panel, President of the Global Society of Rare Genitourinary Tumors and on the ASCO/EAU Penile cancer panel.
MM: Speaker, AstraZeneca.
AMR disclosures:
Almirall: Consultant; Mavig: Travel; Merz: Consultant; Dynamed: Consultant; Canfield Scientific: Consultant; Allergan Inc: Advisory Board; Evolus: Consultant; Biofrontera: Consulatant; Quantia MD: Consultant; Lam Therapeutics; Consultant; Regeneron; consultant; Cutera, consultant; Skinfix, advisor; L’oreal, travel, DAR companies: Founder
ASLMS: A Ward Memorial Research Grant; Skin Cancer Foundation: Research Grant; Regen: Research / Study Funding; LeoPharma: Research / Study Funding; Biofrontera: Research Study Funding
Editorial Board: Lasers in Surgery and Medicine; CUTIS
Editorial Board: Journal of the American Academy of Dermatology (JAAD); Dermatologic Surgery
Board Member: ASDS
Committee Member and / or Chair: AAD; ASDS; ASLMS
GPS Disclosures in the past 36 months:
Advisory Board: BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, Lucence Health, IMV, Vial, Syapse
Consultant/Scientific Advisory Board (SAB): Suba Therapeutics
Research Support to institution: Sanofi (iaward), Astrazeneca, Gilead, Helsinn, Lucence, Predicine, BMS, EMD Serono, Jazz Therapeutics, Genecentric
Speaker: BIO – INFORMAÇÃO BRASILEIRA DE ONCOLOGIA Ltda, OLE Forum (Mexico), Seagen
Data safety monitoring committee honorarium: Mereo
Employment: Spouse employed by Myriad
Writing/Editor fees: Uptodate, Cancer Expert Now, Editor of Elsevier Practice Update Bladder Cancer Center of Excellence
Data Availability Statement:
The data sets generated during and/or analyzed during the current study are not publicly available due to confidentiality agreement but are available from the corresponding author on reasonable request
References
- 1.Campbell RA, Slopnick EA, Ferry EK, Zhu H, Kim SP, Abouassaly R. Disparity between pre-existing management of penile cancer and NCCN guidelines. Urol Oncol. 2017;35(8):531.e9–.e14. [DOI] [PubMed] [Google Scholar]
- 2.Veeratterapillay R, Teo L, Asterling S, Greene D. Oncologic Outcomes of Penile Cancer Treatment at a UK Supraregional Center. Urology. 2015;85(5):1097–103. [DOI] [PubMed] [Google Scholar]
- 3.Djajadiningrat RS, van Werkhoven E, Meinhardt W, van Rhijn BW, Bex A, van der Poel HG, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol. 2014;192(1):120–5. [DOI] [PubMed] [Google Scholar]
- 4.Kokorovic A, Duplisea J, Qiao W, McCormick B, Adibi M, Papadopoulos J, et al. Oncologic outcomes and subsequent treatment following organ sparing surgery for penile carcinoma: The University of Texas M.D. Anderson Cancer Center Experience. Urol Oncol. 2021;39(5):302.e19–.e27. [DOI] [PubMed] [Google Scholar]
- 5.Sakalis VI, Campi R, Barreto L, Perdomo HG, Greco I, Zapala Ł, et al. What Is the Most Effective Management of the Primary Tumor in Men with Invasive Penile Cancer: A Systematic Review of the Available Treatment Options and Their Outcomes. Eur Urol Open Sci. 2022;40:58–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Penile Cancer (Version 1.2023) [Internet]. [updated 2023; cited 2023 Apr 30]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf
- 7.Kamel MH, Bissada N, Warford R, Farias J, Davis R. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J Urol. 2017;198(4):770–9. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 1.2023) [Internet]. [updated 2023; cited 2023 Apr 30]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf
- 9.Brouwer OR, Albersen M, Parnham A, Protzel C, Pettaway CA, Ayres B, et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur Urol. 2023. [DOI] [PubMed] [Google Scholar]
- 10.Thomas A, Necchi A, Muneer A, Tobias-Machado M, Tran ATH, Van Rompuy AS, et al. Penile cancer. Nat Rev Dis Primers. 2021;7(1):11. [DOI] [PubMed] [Google Scholar]
- 11.Raskin Y, Vanthoor J, Milenkovic U, Muneer A, Albersen M. Organ-sparing surgical and nonsurgical modalities in primary penile cancer treatment. Curr Opin Urol. 2019;29(2):156–64. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell KA, Thomas JL, Murad F, Zhou G, Rossi AM, Schmults CD. Non-Surgical Treatment with 5-Fluorouracil is Superior to Surgery for in situ Squamous Cell Carcinoma of the Penis: A 3-center, 24-year Retrospective Cohort Study. Under Review. 2022. [Google Scholar]
- 13.Machan M, Brodland D, Zitelli J. Penile Squamous Cell Carcinoma: Penis-Preserving Treatment With Mohs Micrographic Surgery. Dermatol Surg. 2016;42(8):936–44. [DOI] [PubMed] [Google Scholar]
- 14.Shindel AW, Mann MW, Lev RY, Sengelmann R, Petersen J, Hruza GJ, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178(5):1980–5. [DOI] [PubMed] [Google Scholar]
- 15.Mohs FE, Snow SN, Messing EM, Kuglitsch ME. Microscopically controlled surgery in the treatment of carcinoma of the penis. J Urol. 1985;133(6):961–6. [DOI] [PubMed] [Google Scholar]
- 16.Erlendsson AM, Wilson BN, Bellia P, Phillips W, Leddy L, Rossi AM. Mohs micrographic surgery for penile carcinoma with urethral invasion: A multidisciplinary approach. Journal of the American Academy of Dermatology. 2020;83(6):1803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukowiak TM, Perz AM, Aizman L, Kovell RC, Kovach S, Fischer JP, et al. Mohs micrographic surgery for male genital tumors: Local recurrence rates and patient-reported outcomes. Journal of the American Academy of Dermatology. 2021;84(4):1030–6. [DOI] [PubMed] [Google Scholar]
- 18.Brown MD, Zachary CB, Grekin RC, Swanson NA. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13(11):1163–7. [DOI] [PubMed] [Google Scholar]
- 19.Assaf J, Sarkis J, Lilly E, Ghazi R, Sarkis P, Stephan F. Latest updates in Mohs micrographic surgery for the management of penile squamous cell carcinoma. Int J Dermatol. 2022. [DOI] [PubMed] [Google Scholar]
- 20.Massey PR, Gupta S, Rothstein BE, Konnikov N, Mahalingam M, Ruiz ES, et al. Total Margin-Controlled Excision is Superior to Standard Excision for Keratinocyte Carcinoma on the Nose: A Veterans Affairs Nested Cohort Study. Ann Surg Oncol. 2021;28(7):3656–63. [DOI] [PubMed] [Google Scholar]
- 21.Gayre GS, Hybarger CP, Mannor G, Meecham W, Delfanti JB, Mizono GS, et al. Outcomes of excision of 1750 eyelid and periocular skin basal cell and squamous cell carcinomas by modified en face frozen section margin-controlled technique. Int Ophthalmol Clin. 2009;49(4):97–110. [DOI] [PubMed] [Google Scholar]
- 22.Lont AP, Gallee MP, Meinhardt W, van Tinteren H, Horenblas S. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol. 2006;176(2):575–80; discussion 80. [DOI] [PubMed] [Google Scholar]
- 23.Nitecki R, Davis M, Watkins JC, Wu YE, Vitonis AF, Muto MG, et al. Extramammary Paget Disease of the Vulva: A Case Series Examining Treatment, Recurrence, and Malignant Transformation. Int J Gynecol Cancer. 2018;28(3):632–8. [DOI] [PubMed] [Google Scholar]
- 24.Sri D, Sujenthiran A, Lam W, Minter J, Tinwell BE, Corbishley CM, et al. A study into the association between local recurrence rates and surgical resection margins in organ-sparing surgery for penile squamous cell cancer. BJU Int. 2018;122(4):576–82. [DOI] [PubMed] [Google Scholar]
- 25.Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sosnowski R, Wolski JK, Kulpa M, Ziętalewicz U, Kosowicz M, Kalinowski T, et al. Assessment of quality of life in patients surgically treated for penile cancer: Impact of aggressiveness in surgery. Eur J Oncol Nurs. 2017;31:1–5. [DOI] [PubMed] [Google Scholar]
- 27.Kieffer JM, Djajadiningrat RS, van Muilekom EA, Graafland NM, Horenblas S, Aaronson NK. Quality of life for patients treated for penile cancer. J Urol. 2014;192(4):1105–10. [DOI] [PubMed] [Google Scholar]
- 28.Sedigh O, Falcone M, Ceruti C, Timpano M, Preto M, Oderda M, et al. Sexual function after surgical treatment for penile cancer: Which organ-sparing approach gives the best results? Can Urol Assoc J. 2015;9(7–8):E423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgarten A, Chipollini J, Yan S, Ottenhof SR, Tang DH, Draeger D, et al. Penile Sparing Surgery for Penile Cancer: A Multicenter International Retrospective Cohort. J Urol. 2018;199(5):1233–7. [DOI] [PubMed] [Google Scholar]
- 30.Fankhauser CD, de Vries HM, Roussel E, Jakobsen JK, Issa A, Lee EWC, et al. Lymphovascular and perineural invasion are risk factors for inguinal lymph node metastases in men with T1G2 penile cancer. J Cancer Res Clin Oncol. 2022;148(9):2231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graafland NM, Lam W, Leijte JA, Yap T, Gallee MP, Corbishley C, et al. Prognostic factors for occult inguinal lymph node involvement in penile carcinoma and assessment of the high-risk EAU subgroup: a two-institution analysis of 342 clinically node-negative patients. Eur Urol. 2010;58(5):742–7. [DOI] [PubMed] [Google Scholar]
- 32.Fu L, Tian T, Yao K, Chen XF, Luo G, Gao Y, et al. Global Pattern and Trends in Penile Cancer Incidence: Population-Based Study. JMIR Public Health Surveill. 2022;8(7):e34874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu YG, Lim Y, Bordeaux JS, Aasi SZ, Alam M, Chen P, et al. Achieving Compliance with NCCN Non-Melanoma Skin Cancer Guidelines Regarding Peripheral and Deep En-Face Margin Assessment (PDEMA). JNCCN, Under Review. 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to confidentiality agreement but are available from the corresponding author on reasonable request


