Abstract
In toxigenic Vibrio cholerae, the CTX genetic element which carries the genes for cholera toxin (CT) is the genome of a lysogenic bacteriophage (CTXΦ). Clinical and environmental strains of V. cholerae O1 or O139 and stools that were culture positive for cholera were analyzed to study the induction and transmission of CTXΦ. To our knowledge, this is the first report of the examination of CTXΦ in clinical materials and in naturally occurring strains. DNA probe analysis revealed that 4.25% (6 of 141) of the isolated V. cholerae strains spontaneously produced a detectable level of extracellular CTXΦ particles in the culture supernatants whereas another 34.04% (48 of 141) produced CTXΦ particles when induced with mitomycin C. CTXΦ isolated from 10 clinical or environmental strains infected a CT-negative recipient strain, CVD103, both inside the intestines of infant mice and under laboratory conditions. All culture-positive stools analyzed were negative for the presence of CTXΦ both in the DNA probe assay and by in vivo assay for the infection of the recipient strain in infant mice. These results suggested that naturally occurring strains of toxigenic V. cholerae are inducible lysogens of CTXΦ but that cholera pathogenesis in humans is not associated with the excretion of CTXΦ particles in stools, indicating that induction of the phage may not occur efficiently inside the human intestine. However, in view of the efficient transmission of the phage under conditions conducive to the expression of toxin-coregulated pili, it appears that propagation of CTXΦ in the natural habitat may involve both environmental and host factors.
Cholera caused by toxigenic Vibrio cholerae is a major public health problem in developing countries. Epidemiological surveillance of cholera and comparative molecular analysis of strains collected during outbreaks have demonstrated clonal diversity among epidemic strains and a continual emergence of new clones of toxigenic V. cholerae (5–7, 21). The mechanisms involved in the emergence of new toxigenic clones have not been adequately explained, although it is assumed that a combination of genetic changes and natural selection caused by unidentified environmental factors as well as the immune status of the host populations is likely to influence the process.
The profuse secretory diarrhea characteristic of cholera is caused by an enterotoxin, cholera toxin (CT), produced by toxigenic V. cholerae when it colonizes the small intestine (20). The genes encoding CT (ctxAB) are part of a larger genetic element (CTX genetic element) consisting of at least six genes (ctxAB, zot, ace, cep, and orfU) comprising the “core” region that is flanked by two or more copies of a repeated sequence (19, 23). It has been demonstrated recently (24) that in the V. cholerae O1 strain P27459 (genetically modified by marker exchange and renamed SM44), the entire CTX element constituted the genome of a filamentous bacteriophage (CTXΦ). The phage could be propagated in recipient V. cholerae strains in which the CTXΦ genome either integrated chromosomally at a specific site, forming stable lysogens, or was maintained extrachromosomally as a replicative form (RF) of the phage DNA (24). Cultures of V. cholerae harboring the RF of CTXΦ produced high titers of the phage in their supernatants. This study indicated that the propagation of CTXΦ may be associated with horizontal gene transfer leading to the origination of novel toxigenic strains of V. cholerae. More recently, it has been reported that during passage of CTXΦ lysogens through the infant-mouse intestine, phage excision and replication occur in vivo (16). The present study was undertaken to analyze the induction of lysogenic CTXΦ in clinical and environmental isolates of toxigenic V. cholerae and to investigate whether cholera pathogenesis in humans is associated with the excretion of CTXΦ particles in the stools. Furthermore, this study investigated the ability of CTXΦ particles derived from naturally occurring V. cholerae strains to infect a CT-negative recipient strain of V. cholerae O1 inside the gastrointestinal tracts of infant mice and under laboratory conditions.
MATERIALS AND METHODS
V. cholerae strains and cholera stools.
Toxigenic V. cholerae strains analyzed in this study to investigate the induction of lysogenic CTXΦ included a total of 125 clinical isolates and 16 environmental isolates belonging to O1 or O139 serogroups. Clinical isolates and stools that were culture positive for cholera were obtained from patients who attended the treatment center of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) located in Dhaka. The clinical strains consisted of 78 strains from the culture collection of ICDDR,B (Table 1) and 47 strains isolated from freshly collected culture-positive stools, which were also analyzed in this study (Table 2). The environmental strains were obtained from surface waters in Dhaka. Strains were stored either in lyophilized form or in sealed deep nutrient agar at room temperature. Before use, the identities of the V. cholerae cultures were confirmed by biochemical reactions and serological test (27), and the presence of the CTX element was ascertained by using DNA probes (6). The relevant characteristics of V. cholerae strains used as controls or as a recipient of CTXΦ are listed in Table 3.
TABLE 1.
Analysis of toxigenic V. cholerae O1 and O139 strains isolated between 1969 and 1997 in Bangladesh for the induction of CTXΦ
| Strain | Yr of isolation | Source | No. of strains analyzed | No. of strains producing extracellular CTXΦ
|
|
|---|---|---|---|---|---|
| Induced with mitomycin Ca | Without mitomycin C | ||||
| V. cholerae O1, biotype El Tor | 1969–1976 | Patient | 5 | 4 | 0 |
| 1990–1992 | Patient | 6 | 3 | 2 | |
| 1995–1997 | Patient | 31 | 9 | 0 | |
| 1993–1996 | Surface water | 9 | 6 | 1 | |
| V. cholerae O139 | 1993 | Patient | 9 | 4 | 0 |
| 1995–1997 | Patient | 27 | 12 | 2 | |
| 1993–1997 | Surface water | 7 | 4 | 1 | |
These 42 strains included the 6 strains which spontaneously produced a low but detectable level of CTXΦ without treatment with mitomycin C.
TABLE 2.
Analysis of 47 culture-positive stools collected during June and July 1997 in Bangladesh for the presence of cell-free CTXΦa
| No. of stools analyzed | Serotype and biotype of V. cholerae strain isolated | No. of stoolsa positive for CTXΦ | No. (%) of strains producing extracellular CTXΦ
|
|
|---|---|---|---|---|
| Induced with mitomycin C | Without mitomycin C | |||
| 36 | O1, El Tor | 0 | 7 (19.44) | 0 |
| 11 | O139 | 0 | 5 (45.45) | 0 |
| 47 (total) | 0b | 12 (25.53) | 0 | |
All culture-positive stools were negative for the presence of CTXΦ both in the DNA probe assay and in the in vivo assay in infant mice (see the text for details).
TABLE 3.
Characteristics of V. cholerae O1 reference strains used in this study
| Strain | Relevant characteristic | Reference |
|---|---|---|
| SM44 | Derivative of El Tor strain P27459 in which the CTX genetic element was marked with a kanamycin resistance determinant by marker exchange disrupting the ctxAB operon | 12 |
| RV508 | Derivative of classical biotype strain 569B which is known to constitutively express CT, TCP, and other toxR-regulated gene products | 24 |
| CVD103 | Derivative of classical biotype strain 569B in which the ctxA gene was deleted to produce a vaccine prototype | 15 |
Probes and hybridization.
The gene probes used in this study to detect the CTXΦ genome were a 0.5-kb EcoRI fragment of pCVD27 (14), containing part of the ctxA gene, and an 840-bp region internal to the zot gene amplified by PCR from the recombinant plasmid pBB241 as described previously (8). Strand-specific oligonucleotide probes with the sequences 5′-TCTATCTCTGTAGCCCCTATTACG and 5′-CTCAGACGGGATTTGTTAGGCACG for probing the plus and minus strands, respectively, were also used to detect the presence of single-stranded DNA of CTXΦ. Colony blots or Southern blots were prepared with nylon filters (Hybond; Amersham International plc., Ayelesbury, United Kingdom) and processed by standard methods (17). The polynucleotide probes were labeled by random priming (10) with a random-primer DNA-labeling kit (Bethesda Research Laboratories, Gaithersburg, Md.) and [α-32P]dCTP (3,000 Ci/mmol; Amersham), and oligonucleotide probes were labeled by 3′ tailing with terminal deoxynucleotide transferase and [α-32P]dCTP. Southern blots and colony blots were hybridized with the labeled probes and autoradiographed as described by us previously (5–7).
Induction of CTXΦ lysogens.
Toxigenic V. cholerae strains were grown in Luria broth (LB) at 30°C to an absorbance at 540 nm of 0.2. The cells were collected by centrifugation, washed, and resuspended in fresh LB. The suspension was divided into aliquots, to which mitomycin C (Sigma Chemical Co., St. Louis, Mo.) was added at 20 ng/ml and incubated overnight at 30°C. The culture supernatants were analyzed for extracellular phage carrying the CTX element as described in the following section. Strains grown in a similar way but without mitomycin C were used as controls.
Screening of stools and bacterial cultures for CTXΦ.
Freshly collected culture-positive stools and V. cholerae strains either isolated from the same stools (Table 2) or obtained from the culture collection (Table 1) were used. Aliquots of watery stools or cultures were centrifuged at 6,000 × g to precipitate solid particles and suspended bacteria, and the supernatants were sterilized by filtration through 0.22-μm-pore-size filters (Millipore Corp., Bedford, Mass.). To confirm that the filtrates from stools as well as the culture supernatants did not contain any bacterial cell, aliquots of the filtrates were streaked on Luria agar (LA) plates and incubated overnight at 37°C. The sterile filtrates were mixed with one-fourth volumes of a solution containing 20% polyethylene glycol 6000 and 10% NaCl and centrifuged at 12,000 × g to precipitate the phage particles. The precipitate was dissolved in a solution containing 20 mM Tris-Cl (pH 7.5), 60 mM KCl, 10 mM MgCl, and 10 mM NaCl and digested with pancreatic DNase I (100 U/ml) and RNase A (50 μg/ml) at 37°C for 1 h to remove possible nucleic acids carried over from lysed bacterial cells. The solution was extracted with phenol-chloroform to disrupt possible phage particles, and the total nucleic acids were precipitated with ethanol. Preparations containing CTXΦ DNA were identified by Southern blot hybridization (22). Aliquots of watery stools mixed with serial dilutions of CTX-KmΦ (101 to 104 particles/ml) isolated from a mitomycin C-induced culture of strain SM44 were used as positive controls for the assay of phage in stools, for both in vitro and in vivo assays. CTX-KmΦ derived from SM44 was quantified by incubating serially diluted filter-sterilized culture supernatants with strain RV508 and then determining the number of Kmr colonies, as described previously (24).
The infectious activity of possible CTXΦ present in the cell-free culture supernatants of toxigenic V. cholerae strains and extracts of culture-positive stools was assayed with strain CVD103 (15) as the recipient in vivo in suckling mice and in vitro under laboratory conditions as follows. Aliquots (10 ml) of filtered sterile supernatant fluids from mitomycin C-induced cultures or control cultures without mitomycin C or filtered stool extracts were used to precipitate phage particles. The pellet was suspended in 100 μl of TES buffer (20 mM Tris-HCl [pH 7.5], 10 mM NaCl, 0.1 mM disodium EDTA). The recipient strain was grown in LB at 37°C; the cells were precipitated by centrifugation and washed in fresh LB. Approximately 105 bacterial cells mixed with 10 μl of the phage preparation in a final volume of 50 μl were gastrointestinally inoculated into groups of 5-day-old Swiss Albino mice obtained from the breeding facilities of the Animal Resources Branch, ICDDR,B. For each phage preparation, at least five mice were inoculated. The animals were sacrificed after 24 h, and their intestines were removed and homogenized in 10 mM phosphate-buffered saline (pH 7.2). The homogenate was centrifuged at low speed to precipitate debris, the supernatant was then centrifuged to precipitate bacterial cells, and the pellet was resuspended in phosphate-buffered saline. Serial dilutions of an aliquot of the suspension were plated on taurocholate-tellurite-gelatin agar (18) to select V. cholerae colonies. All the colonies were screened for the presence of the CTXΦ genome by using the ctxA or zot probe. The ratio of probe-positive colonies to total colonies recovered was calculated and expressed as the percentage of recipient cells carrying the CTXΦ genome (Table 4). For in vitro assays, mixtures of phage and recipient cells as described above were prepared. Each mixture was inoculated into 5 ml of LB and incubated for 1 h at 30°C, and aliquots of the culture were plated on LA plates and incubated overnight at 30°C. For each strain and phage combination, five different in vitro assays were performed and at least 104 colonies of the recipient strain recovered from each of these assays were screened for the CTXΦ genome.
TABLE 4.
Infection of strain CVD103 by extracellular CTXΦ isolated from the supernatant fluids of mitomycin C-induced cultures of clinical and environmental V. cholerae strains
| Strain | Source | CTXΦ genome-specific probe-positive colonies (% of total colonies recovered)a
|
|||
|---|---|---|---|---|---|
| In vitro
|
In mouse intestine
|
||||
| Median | Range | Median | Range | ||
| V. cholerae O1, biotype El Tor | |||||
| G755 | Patient | 2.31 | 1.62–5.76 | 25.61 | 10.59–44.82 |
| P27457 | Patient | 4.80 | 1.63–6.96 | 17.30 | 11.48–43.84 |
| AH806 | Patient | 3.45 | 3.07–5.81 | 40.82 | 16.88–51.15 |
| AM11726 | Patient | 3.94 | 1.19–6.05 | 27.49 | 9.48–44.38 |
| AM13860 | Patient | 4.25 | 1.35–5.56 | 23.90 | 12.16–38.13 |
| EV11 | Surface water | 2.38 | 1.29–3.05 | 21.60 | 11.74–44.98 |
| EV02 | Surface water | 5.23 | 4.43–7.17 | 24.85 | 9.20–41.67 |
| V. cholerae O139 | |||||
| AI885 | Patient | 4.70 | 1.06–8.03 | 19.14 | 13.48–49.04 |
| AL11089 | Patient | 9.73 | 4.07–16.14 | 12.23 | 9.77–56.46 |
| SB-Lake | Surface water | 4.35 | 2.70–5.81 | 18.66 | 9.82–32.13 |
Median and range of five different observations.
Representative probe-positive colonies of the recipient strain were further analyzed for the presence and integration of the phage genome. Total DNA or plasmid was extracted by standard methods (17), purified with microcentrifuge filter units (Ultrafree-Probind; Sigma), and analyzed by Southern blot hybridization. To test the stability of the CTXΦ genome in infected cells, representative probe-positive colonies of the infected recipient strain CVD103 were grown at 37°C in LB for several generations (6 to 24 h) and were tested for the presence of the CTX element by colony blot hybridization. Similarly, a colony of CVD103 infected with CTX-KmΦ derived from strain SM44 was grown for 6 to 24 h in aliquots of LB either containing kanamycin (50 μg/ml) or without kanamycin. Serial dilutions of the cultures were plated on LA plates containing kanamycin and on a duplicate set of LA plates devoid of the antibiotic to determine the proportion of cells retaining the phage genome.
RESULTS AND DISCUSSION
Induction and transmission of CTXΦ.
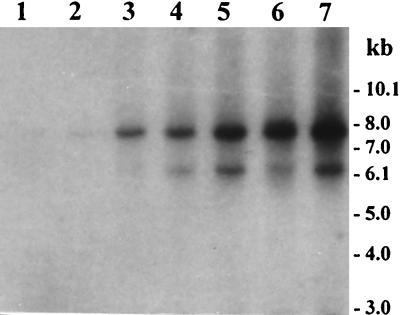
To investigate whether lysogenic CTXΦ present in toxigenic V. cholerae strains could be induced to produce extracellular infectious phage particles, we cultured the strains in the presence of a DNA-damaging agent, mitomycin C, and then screened the culture supernatant fluids for the presence of CTXΦ by using DNA probes. Similarly, freshly collected culture-positive stools were analyzed for the presence of CTXΦ to understand whether the phage is excreted in the stools of cholera patients. Another strategy for detecting the presence of infectious CTXΦ in culture supernatants as well as in culture-positive stools was to expose a suitable recipient strain, CVD103, to the phage preparations and then look for infection of the bacterial cells. None of the culture-positive stools analyzed was positive for the presence of a detectable level of CTXΦ either in the DNA probe assay or in the in vivo assay in infant mice. To determine the detection limits of our assays, we used reconstituted stools containing a known number of phage particles. This was done by using a genetically marked phage, CTX-KmΦ (derived from strain SM44), since CTX-KmΦ could be quantified by titration with a recipient strain RV508, which constitutively expresses toxin-coregulated pili (TCP) and hence is readily infected by the phage (24). In the case of stools mixed with exogenous CTX-KmΦ and used as positive controls, a minimum of 103 phage particles in 5 ml of stool could be distinctly detected by Southern blot hybridization (Fig. 1). When assayed in infant mice, the presence of 102 particles of CTX-KmΦ in 5 ml of reconstituted stools could be detected and the corresponding stool extract produced between one and nine colonies (recovered from five different mice) of Kmr transductants of CVD103.
FIG. 1.
Southern blot hybridization of bacteriophage DNA isolated from reconstituted culture-positive stools containing serial dilutions of the genetically marked phage CTX-KmΦ and probed with the zot probe. Lanes 1 through 7 correspond to extracts from 5 ml of stools containing 1 × 102, 5 × 102, 1 × 103, 1.5 × 103, 2 × 103, 2.5 × 103, and 5 × 103 phage particles, respectively. Numbers indicating the molecular sizes of bands correspond to the supercoiled DNA ladder (Bethesda Research Laboratories).
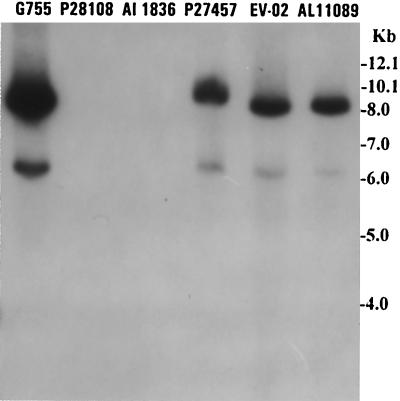
Of a total of 141 V. cholerae strains obtained from the culture collection and from fresh culture-positive stools, 6 strains spontaneously produced a low but detectable level of extracellular CTXΦ whereas another 48 strains (34.04%) produced extracellular CTXΦ when induced with mitomycin C, as shown by Southern blot hybridization (Fig. 2). These 54 strains included 12 strains isolated from fresh culture-positive stools which tested negative for the presence of CTXΦ. Strains that produced extracellular CTXΦ included 7 (19.44%) of 36 El Tor strains and 5 (45.45%) of 11 O139 strains obtained from freshly collected stools and 22 (43.13%) of 51 El Tor strains and 20 (46.51%) of 43 O139 strains analyzed from previous collections (Tables 1 and 2). As expected for a filamentous phage, Southern blot hybridization with strand-specific oligonucleotide probes revealed that the phage DNA was single stranded and hybridized with the probe specific for the plus strand but not with the probe for the corresponding minus strand. The bands corresponding to the CTXΦ DNA were not clearly visible on ethidium bromide-stained agarose gels (data not shown) but could be detected distinctly by hybridization of the Southern blots with the specific probes (Fig. 2).
FIG. 2.
Supernatant fluids of toxigenic V. cholerae O1 or O139 strains grown in the presence of mitomycin C (20 ng/ml) were sterilized by filtration through a 0.22-μm filter and were used to precipitate possible bacteriophage particles. The precipitates were dissolved in appropriate buffer and treated with DNase I and RNase A to remove contaminating exogenous DNA or RNA. Total phage nucleic acids were isolated as described in Materials and Methods and analyzed with the ctxA probe. Southern blot hybridization analyses of total phage nucleic acids derived from six different V. cholerae strains (indicated by strain numbers) are shown. Numbers indicating the molecular sizes of bands correspond to supercoiled DNA ladder (Bethesda Research Laboratories).
It has been shown previously that most of the genes in the core region of the CTXΦ genome play a crucial role in the morphogenesis of the phage (24). The reasons for the apparent inability of 87 of the 141 strains to produce CTXΦ particles on induction with mitomycin C (Table 1) may be that the integrated form of CTXΦ carried by these strains is defective due to possible mutations in one or more genes essential for phage morphogenesis. Strains analyzed in this study included both V. cholerae O1 and O139 carrying variable number of copies of the CTX element, and a difference was noted in terms of induction of CTXΦ in strains belonging to these serogroups. The phage was more often inducible in O139 strains (46.29%) than in El Tor strains (33.33%). Generally, O139 strains are known to carry more than one copy of the CTX element (9, 25), and this may account for the higher prevalence of inducible lysogens among O139 vibrios. In the present study, CTXΦ was induced by the DNA-damaging agent mytomycin C, which is known to induce many temperate bacteriophages. However, six strains including two environmental strains produced a detectable level of extracellular CTXΦ particles, even without treatment with mitomycin C (Table 1), indicating that unidentified environmental factors or possible mutations in the phage or the host bacteria might have caused induction of the CTXΦ in these strains. Further studies are required to define possible factors which may play a role in the induction of lysogenic CTXΦ in toxigenic V. cholerae in the natural habitat.
We used two different methods to determine the presence of infectious phage particles in culture supernatants or culture-positive stools. These included an in vitro laboratory method and the infant-mouse system, both of which have been previously described (24). However, in previous studies (16, 24), the transmission of CTXΦ was examined between a recipient strain and a donor strain of V. cholerae when the strains were grown in mixed culture or inoculated into infant mice, whereas in the present study cell-free phage particles were used to infect the recipient strain. In addition to adequate expression of TCP, which is the receptor for the phage, the infant-mouse system is known to cause significant enrichment of toxigenic V. cholerae strains (2). Hence, the infant-mouse assay was expected to detect the presence of a smaller number of infectious phage particles in the samples. On the other hand, the in vitro method was also chosen because, from the ecological perspective, it is important to understand the ability of CTXΦ to propagate independently of the mammalian host, in view of the possibility that propagation of CTXΦ in the natural habitat is associated with the origination of new toxigenic strains through lysogenic conversion (24).
The use of CVD103, which is a derivative of the V. cholerae O1 classical strain 569B with ctxA deleted, provided certain obvious advantages. Since CVD103 is a classical biotype strain, it was supposed to be more susceptible to CTXΦ than are El Tor strains, as described previously (24), and hence was expected to facilitate the detection of CTXΦ. Due to deletion of the ctxA gene, CVD103 would not hybridize with the ctxA probe which was used in colony blot hybridization to detect colonies infected by wild-type CTXΦ. Moreover, since CVD103 is a vaccine prototype strain, its use provided an opportunity to study the susceptibility of an attenuated vaccine strain to CTXΦ.
In the present study, strain CVD103 was infected by CTXΦ both under in vitro laboratory conditions and inside the intestines of infant mice (Table 4). The infant-mouse assay, which detected the presence of 102 CTX-KmΦ particles in 5 ml of reconstituted stool, was approximately 50 times more sensitive than the Southern hybridization assay, which detected 103 phage particles, since only one-fifth of the stool extract (10 μl of 50 μl of total extract) was used for each assay in mice. The high sensitivity of the assay in infant mice for both reconstituted stools and culture supernatants could also be due to the prolonged incubation of infected cells carrying the RF of CTXΦ inside the mouse intestine, leading to production of a larger number of infectious phage particles, in addition to adequate expression of TCP in vivo. It should be clarified that this study was not designed to compare the susceptibility of the recipient strain to CTXΦ in vivo and in vitro. Instead, we have successfully used the infant-mouse model in an assay to detect the presence of infectious CTXΦ in culture supernatants and clinical specimens. In addition, the observed ability of wild-type CTXΦ to transmit its genome into the vaccine prototype strain CVD103 suggested that there is a need to modify and redesign possible live vaccine candidates in view of the possible reacquisition of CT genes by attenuated strains.
Stability of the CTXΦ genome in the recipient strain.
CTXΦ is unusual among filamentous phages because it can either replicate as a plasmid or integrate into the V. cholerae chromosome at a specific attachment site (24). Analysis of representative probe-positive colonies for the presence of CTXΦ genome revealed the presence of the RF of the CTXΦ genome in freshly infected colonies. However, the concentration of the RF DNA was too low to be visible in ethidium bromide-stained gels, but it could be faintly detected by Southern blot hybridization (data not shown). The phage genome was rapidly lost from infected cells when the infected cells were cultured under laboratory conditions, and only 0.9% of the colonies recovered after 24 h of culture hybridized with the ctxA probe. The CTXΦ genome did not integrate into the chromosome of CVD103, as shown by Southern blot analysis of genomic DNA with the ctxA probe (data not shown) as well as presumed from the rapid loss of CTXΦ genome from infected cells. When CVD103 was infected with CTX-KmΦ derived from strain SM44, the infected cells retained the RF of the phage when cells were grown in the presence of kanamycin. However, when the infected cells were cultured in the absence of kanamycin, the RF of CTX-KmΦ was rapidly lost and 60.3% of viable cells recovered after 12 h of culture became susceptible to kanamycin (50 μg/ml). Previously, the CTXΦ genome was shown to integrate into the chromosome when an El Tor strain carrying a resident attRS sequence was used as the recipient (24). In the previous study, the phage was marked with kanamycin resistance and the transfectants were maintained in the presence of kanamycin. Similarly, in the present study, CVD103 cells infected with the CTX-KmΦ retained the RF DNA of the phage when the cells were grown in the presence of kanamycin but rapidly lost the RF of CTX-KmΦ in the absence of kanamycin. This indicated that there was a requirement for appropriate selection pressure for the retention of the extrachromosomal CTX element in V. cholerae. The lack of selection of cells carrying the RF of CTXΦ could account for the subsequent loss of the phage DNA. However, once the phage genome integrates into the chromosome of the recipient cell, it is likely to be more stable. There have been several studies which suggested that the gastrointestinal environment can cause a selective enrichment of toxigenic V. cholerae strains compared to nontoxigenic strains (2, 11, 13). It therefore seems possible that in addition to adequate expression of TCP, which is the receptor for the phage, the intestinal environment also contributes to selecting V. cholerae cells harboring the CTXΦ genome. Since the CTX element itself carries the necessary genes for its own integration at resident attRS sites of the host chromosome (26), the origination of novel toxigenic strains of V. cholerae and their selective enrichment are likely to occur in the intestinal environment.
Propagation of CTXΦ.
The existence of CTXΦ was discovered by using a genetically modified V. cholerae strain, SM44, in which the CTX element was marked with a kanamycin resistance (Kmr) determinant (12, 24). The presence of the Kmr marker facilitated the detection of the induction and transmission of the phage. The present study has investigated for the first time the prevalence of inducible lysogens of CTXΦ among naturally occurring strains of toxigenic V. cholerae and the potential of the extracellular phage particles to infect a CT-negative recipient strain of V. cholerae. This study is also the first to examine clinical materials for the presence of CTXΦ and to investigate whether cholera pathogenesis is normally associated with the excretion of the phage in stools. Since the CTX element was not marked with a phenotypically detectable marker in native strains, detection of the induction of CTXΦ and its transmission in vitro and in infant mice were studied with specific DNA probes. Although V. cholerae is known to be primarily a human pathogen, it has been suggested that the bacterium can persist in the aquatic environment in unexplained ecological associations (3). The natural habitat of toxigenic V. cholerae therefore seems to consist of two compartments: the gastrointestinal tract of the host and the aquatic environment outside the host. The host compartment is known to provide signals necessary for the expression of most ToxR-regulated virulence-associated factors, including CT and TCP (1, 4). To investigate whether induction of the CTXΦ prophage is also mediated by possible host factors in the human gastrointestinal tract, we screened culture-positive stools for the presence of the phage. That all the stools were negative for the phage suggested that induction of lysogenic CTXΦ was possibly not associated with cholera pathogenesis in humans, unless we assume that the CTXΦ particles were very unstable in the human intestine and rapidly degraded beyond the detection limit of our assays. However, the present study showed that when extracellular CTXΦ isolated from naturally occurring strains and the control strain SM44 were used to infect the recipient strain in vivo, CTXΦ particles were stable and remained infectious in the gastrointestinal environment of infant mice. It may be mentioned that induction of the CTXΦ prophage in infant mice has been reported recently based on observed transduction of a recipient strain by a CTXΦ lysogen inside the intestines of infant mice (16). The present study, however, did not provide any evidence in favor of the induction of lysogenic CTXΦ inside the human intestine in association with cholera pathogenesis.
The propagation of CTXΦ in its natural habitat is likely to involve the excision and replication of the lysogenic phage followed by infection of recipient V. cholerae strains, possibly mediated by appropriate signals in the host intestine. Since the present study did not provide any conclusive evidence to suggest that the induction of the phage occurs inside the intestine of the human host, we speculate that induction of CTXΦ in naturally occurring strains of toxigenic V. cholerae may also occur in the environmental habitat. Our efforts are at present directed toward understanding the role of possible environmental factors in the induction and propagation of the CTX phage.
ACKNOWLEDGMENTS
This research was funded by the U.S. Agency for International Development (USAID) under grant HRN-5986-A-00-6005-00 with the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). The ICDDR,B is supported by countries and agencies which share its concern for the health problems in developing countries.
REFERENCES
- 1.Baselski V, Briggs R, Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977;15:704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baselski V S, Medina R A, Parker C D. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1979;24:111–116. doi: 10.1128/iai.24.1.111-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colwell R R, Spira W M. The ecology of Vibrio cholerae. In: Barua D, Greenough III W B, editors. Cholera. New York, N.Y: Plenum Medical Book Co.; 1992. pp. 107–127. [Google Scholar]
- 4.DiRita V J. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol Microbiol. 1992;6:451–458. doi: 10.1111/j.1365-2958.1992.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 5.Faruque S M, Roy S K, Alim A R M A, Siddique A K, Albert M J. Molecular epidemiology of toxigenic Vibrio cholerae in Bangladesh studied by numerical analysis of rRNA gene restriction patterns. J Clin Microbiol. 1995;33:2833–2838. doi: 10.1128/jcm.33.11.2833-2838.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faruque S M, Ahmed K M, Alim A R M A, Qadri F, Siddique A K, Albert M J. Emergence of a new clone of toxigenic Vibrio cholerae biotype El Tor displacing V. cholerae O139 Bengal in Bangladesh. J Clin Microbiol. 1997;35:624–630. doi: 10.1128/jcm.35.3.624-630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faruque S M, Ahmed K M, Siddique A K, Zaman K, Alim A R M A, Albert M J. Molecular analysis of toxigenic Vibrio cholerae O139 Bengal strains isolated in Bangladesh between 1993 and 1996: evidence for the emergence of a new clone of the Bengal vibrios. J Clin Microbiol. 1997;35:2299–2306. doi: 10.1128/jcm.35.9.2299-2306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faruque S M, Comstock L, Kaper J B, Albert M J. Distribution of zonula occludens toxin (zot) gene among clinical isolates of Vibrio cholerae O1 from Bangladesh and Africa. J Diarrhoeal Dis Res. 1994;12:222–224. [PubMed] [Google Scholar]
- 9.Faruque S M, Alim A R M A, Roy S K, Khan F, Nair G B, Sack R B, Albert M J. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strain of toxigenic Vibrio cholerae O139 synonym Bengal. J Clin Microbiol. 1994;32:1050–1053. doi: 10.1128/jcm.32.4.1050-1053.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinberg A, Volgelstein B. A technique for radio labeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein R A, Vasil M L, Holmes R K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974;129:117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg I, Mekalanos J J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986;165:723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes R K, Vasil M L, Finkelstein R A. Studies on toxino-genesis in Vibrio cholerae. III. Characterization of nontoxigenic mutants in vitro and in experimental animals. J Clin Invest. 1975;55:551–556. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper J B, Morris J G, Jr, Nishibuchi M. DNA probes for pathogenic Vibrio species. In: Tenover F C, editor. DNA probes for infectious diseases. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 65–67. [Google Scholar]
- 15.Ketly J M, Michalski J, Galen J, Levine M M, Kaper J B. Construction of genetically-marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett. 1993;111:15–22. doi: 10.1111/j.1574-6968.1993.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 16.Lazar S, Waldor M K. ToxR-independent expression of cholera toxin from the replicative form of CTXΦ. Infect Immun. 1998;66:394–397. doi: 10.1128/iai.66.1.394-397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 18.Monsur K A. A highly selective gelatine-taurocholate tellurite medium for the isolation of Vibrio cholerae. Trans R Soc Trop Med Hyg. 1961;55:440–442. doi: 10.1016/0035-9203(61)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Pearson G D N, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabbani G H, Greenough W B. Cholera. In: Lebenthal E, Duffy M, editors. Text book of secretory diarrhea. New York, N.Y: Raven Press; 1990. pp. 233–253. [Google Scholar]
- 21.Sharma C, Nair G B, Mukhopadhyay A K, Bhattacharya S K, Ghosh R K, Ghosh A. Molecular characterization of Vibrio cholerae O1 biotype El Tor strains isolated between 1992 and 1995 in Calcutta, India: evidence for the emergence of a new clone of the El Tor biotype. J Infect Dis. 1997;175:1134–1141. doi: 10.1086/516453. [DOI] [PubMed] [Google Scholar]
- 22.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 23.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 25.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 26.Waldor M K, Rubin E J, Pearson G D, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. World Health Organization guidelines for the laboratory diagnosis of cholera. Geneva, Switzerland: Bacterial Disease Unit, World Health Organization; 1974. [Google Scholar]