Abstract
The isolation of mutant cells with phenotypes caused by random mutagenesis has been hampered in mammalian cells because there are two alleles per gene and the disruption of both alleles is extremely rare. We describe a method for the efficient biallelic mutagenesis in embryonic stem cells. loxP sites were introduced near the centromeric regions of a pair of chromosome 1s. A mutant neo gene was inserted at the distal part of one of the loxP sites so that biallelic mutants would be selected by high-dose G418. Expression of Cre induced the recombination between homologous chromosomes and led to an elevation in the number of biallelic mutants. This system will facilitate phenotype-driven gene function study in the mammalian system.
INTRODUCTION
As we enter the post-genomic era, mutant resources will play a pivotal role in filling the gap between sequence information and understanding gene functions. In a mouse system, the gene-targeting method (Capecchi, 1989) opened the way to introduce desired mutations in any gene of choice and has proved to be a powerful method for analyzing gene function. However, a large-scale phenotype-driven genetic screening could not be achieved because mutational effects are masked until homozygous mutant mice are generated after a time-consuming breeding process.
It is known that homozygous mutant embryonic stem (ES) cells are generated spontaneously during the culture of heterozygous cells (Mortensen et al., 1992). This event can be selected by increasing the concentration of G418, although the proportion of homozygous cells is extremely small (1 in ∼105 of all cells). It is thought that mitotic recombination of homologous chromosomes during the 4N stage is one of the mechanisms of this event (Lefebvre et al., 2001). The frequency might be increased by using a site-specific recombination system. In fact, the FLP/FRT system was used for Drosophila to induce inter-chromosomal recombination, and mosaic animals consisting of heterozygous and homozygous cells were successfully generated (Xu and Rubin, 1993). In a mouse system, inter-chromosomal recombination using the Cre/loxP system has been reported (Ramirez-Solis et al., 1995; Smith et al., 1995), but it remains to be demonstrated whether homozygous cells can be generated as efficiently as shown in Drosophila.
In the present study, we utilized the Cre/loxP system to induce recombination of homologous chromosomes and demonstrated that homozygous ES cells could be obtained at high frequency. This approach will facilitate a large-scale genetic screening in mammalian systems.
RESULTS AND DISCUSSION
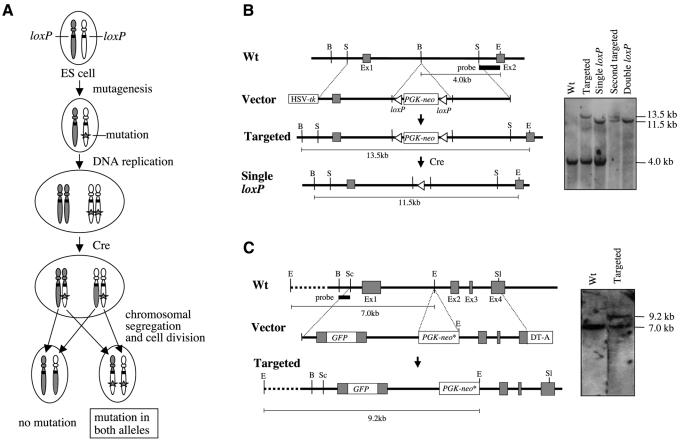
The basic principle of the strategy is shown in Figure 1A. loxP sites are introduced at the same location of a pair of homologous chromosomes, and the cells are subsequently mutagenized. When the recombination occurs at the 4N stage, cells bearing chromosomal homozygosity distal to the loxP sites are obtained after chromosomal segregation and cell division. As a result, the mutations are introduced in both alleles at the distal part of the loxP sites.
Fig. 1. Strategy to introduce biallelic mutations. (A) Principle for the mutagenesis. See text for details. (B) Introduction of loxP sites into the Oprk1 locus. The PGK-neo gene flanked by two loxP sites was inserted into intron 1 of the Oprk1 gene by homologous recombination. The PGK-neo gene was deleted by transient expression of Cre, leaving a single copy of the loxP site. The same procedure was repeated to introduce a loxP site into the other allele of the Oprk1 gene, and the genomic structure was confirmed by Southern blotting. (C) Introduction of a mutant neo gene (neo*) into the Fasl locus by homologous recombination. Wt, wild type; Ex, exon; B, BamHI; E, EcoRI; S, SpeI; Sc, SacI; Sl, SalI; HSV-tk, herpes simplex virus thymidine kinase gene; DT-A, diphtheria toxin A fragment.
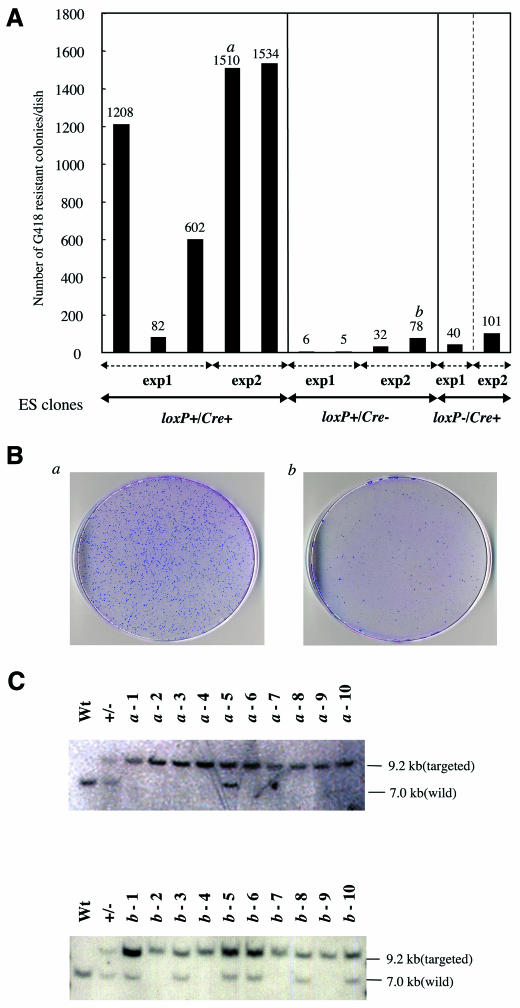
We tested this system in mouse chromosome 1 in ES cells. We inserted loxP sites in both alleles of the Opioid receptor, kappa 1 (Oprk1) locus (Nishi et al., 1994), which is located near the centromeric region of chromosome 1 (Figure 1B). The mutant neo gene (Yenofsky et al., 1990) was then inserted into the Fas antigen ligand (Fasl) locus (Takahashi et al., 1994) located in the middle of chromosome 1 (Figure 1C). The mutant neo gene serves as a marker for the efficiency of Cre-mediated recombination because the cells containing multiple copies of the mutant neo genes can be selected under high-dose G418 (Mortensen et al., 1992). To test the effect of the Cre/loxP-mediated inter-chromosomal recombination, we transfected the expression vector containing the Cre gene under the control of the PGK promoter into ES cells. Stable cell lines expressing Cre protein were established from cells bearing both the mutant neo gene and the loxP sites (loxP+/Cre+) and from cells bearing the mutant neo gene without the loxP sites (loxP–/Cre+). There were ∼1000 colonies from 2 × 106 loxP+/Cre+ ES cells after the selection with 750 µg/ml G418 (Figure 2A and B), and Southern blot analysis verified that the majority of G418-resistant clones had the mutant neo gene in both alleles of the Fasl locus (Figure 2C). Absence of either the Cre or loxP site resulted in a small number of colonies (5–100 colonies, Figure 2A), and the introduction of the biallelic mutations was not predominant (Figure 2C). The results showed that biallelic mutagenesis was induced by Cre/loxP-mediated inter-chromosomal recombination. Considering that the plating efficiency of ES cells bearing the mutant neo gene at both alleles of the Fasl locus was 20% under the condition of 750 µg/ml G418 (data not shown), we speculate that the absolute frequency of biallelic mutagenesis event was ∼0.25% [(1000/2 × 106)/0.2 = 0.0025].
Fig. 2. Introduction of biallelic mutations by Cre/loxP. (A) Increase of high-dose G418-resistant colonies by Cre/loxP. Two million ES cells were plated per 100 mm dish and selected with 750 µg/ml G418 for 10 days. The presence or absence of loxP and Cre in each clone is indicated at the bottom. Two independent experiments (exp1 and exp2) were performed. (B) High-dose G418-resistant colonies stained with Giemsa’s solution. Plates a and b were obtained from the experiments marked in (A) as a and b, respectively. (C) Southern blotting of the high-dose G418-resistant clones at the Fasl locus. Clones a-1 to a-10 and b-1 to b-10 were derived from the experiments marked in (A) as a and b, respectively. Wt, wild-type ES cells; +/–, heterozygous ES clone bearing the neo* gene in a single allele of the Fasl locus. The conditions for Southern blotting were the same as in Figure 1C. Some lanes (b-1, b-5, b-6 and b-10) showed an intense upper band, suggesting that the neo gene was unusually amplified.
The rate at which resistant colonies appeared was examined by fluctuation analysis (Luria and Delbruck, 1943; Table I). The rate in the loxP–/Cre+ ES cells was 7.2 × 10–6/cell per generation, whereas that of the loxP+/Cre+ ES cells was 1.1 × 10–4/cell per generation. Therefore, the efficiency of biallelic mutagenesis was increased 15-fold in a Cre/loxP-dependent manner (Table I).
Table I. Fluctuation analysis of the resistance to high-dose G418.
| loxP+/Cre+ | loxP–/Cre+ | |
|---|---|---|
| Number of replicate cultures | 10 | 10 |
| Initial number of cells per culture | 1 | 1 |
| Final number of cells per culture (mean) | 4.6 × 106 | 4.3 × 106 |
| Number of cells per sample | 1 × 106 | 1 × 106 |
| Mean of resistant colonies per samplea | 1026 | 45 |
| The rate of appearance of resistant colonies | 1.1 × 10–4 | 7.2 × 10–6 |
aG418 (750 µg/ml) was used for selection.
To facilitate our phenotype-driven gene function study in mammalian cells, we developed a system to obtain a large number of homozygous mutant ES cells by utilizing Cre/loxP-mediated inter-chromosomal recombination. Two methods have been mainly used to obtain homozygous mutants in mammalian cells. One is sequential gene targeting, in which the standard gene targeting procedure is repeated to mutate each allele separately. This method can be applied only to pre-selected genes and cannot be used for a large number of genes. The other consists of the selection of high-dose G418 for isolating homozygous mutant cells without utilizing the Cre/loxP system (Mortensen et al., 1992). Although this is a straightforward approach, the number of homozygous cells is too small to accomplish a large-scale biallelic mutagenesis. We compared it with our approach and found that using the Cre/loxP system resulted in a 15-fold increase in the efficiency of biallelic mutagenesis. This is probably an underestimate, because only a fraction of the G418-resistant cells (4 out of 10) were homozygous mutants when the Cre/loxP system was not used, whereas a majority of high-dose G418-resistant clones (9 out of 10) were homozygous when the system was used (Figure 2C).
For the expression of Cre, we generated stable cell lines that express the Cre protein constitutively. We also tested the effect of the transient expression of Cre but found no increase in the number of high-dose G418-resistant clones (data not shown). Cre/loxP-mediated recombination must occur at the 4N stage to generate biallelic mutant cells (Figure 1A), and continuous expression of the Cre protein may be required to increase the chance of recombination at this specific stage.
Application of this method to mice would be of interest, as a similar method using FLP/FRT made it possible to generate biallelic mutation in Drosophila (Xu and Rubin, 1993). Combining our method with the recently developed transposon system may facilitate this approach. We (Horie et al., 2001) and others (Dupuy et al., 2001; Fischer et al., 2001) have reported that the Sleeping Beauty (SB) transposon reconstituted from fish transposes efficiently in mice, i.e. it preferentially transposes to the loci near the original site (Fischer et al., 2001; K. Horie, K. Yusa, J. Odajima and J. Takeda, unpublished data). This feature of the SB transposon is very useful for Cre/loxP-mediated biallelic mutagenesis, because, when the SB transposon is introduced into the chromosome bearing the loxP site, the genes on the same chromosome can be biallelically mutagenized. It remains to be determined, however, whether the frequency of biallelic mutagenesis in vivo is high enough to make the use of this system worthwhile.
Liu et al. (2002) recently demonstrated that biallelic mutants could be obtained by means of Cre/loxP-mediated inter-chromosomal recombination, although the selection of mutant cells and chromosomes bearing loxP sites was different from ours. Their results, together with ours, validate the biallelic mutagenesis mediated by the Cre/loxP system.
Speculation
A large-scale biallelic mutagenesis would be possible in ES cells by combining the Cre/loxP-mediated inter-chromosomal recombination with the gene trap or chemical mutagenesis. In the gene trap (Friedrich and Soriano, 1991), the mutated neo gene is in the trap vector, and Cre-mediated recombinants are selected by means of high-dose G418. The current limitation is that the G418 resistance varies depending on the integration sites. Utilization of the insulator (Chung et al., 1997) and/or the negative feedback regulation system (Becskei and Serrano, 2000) may solve this problem by achieving constant levels of neo gene expression independent of the integration sites. In chemical mutagenesis (Chen et al., 2000; Munroe et al., 2000), ES cells carrying the mutant neo gene at a particular locus would then be mutagenized by chemicals. Cre-mediated recombinants can be easily selected under high-dose G418, as the conditions for selection can be predetermined (Figure 3).
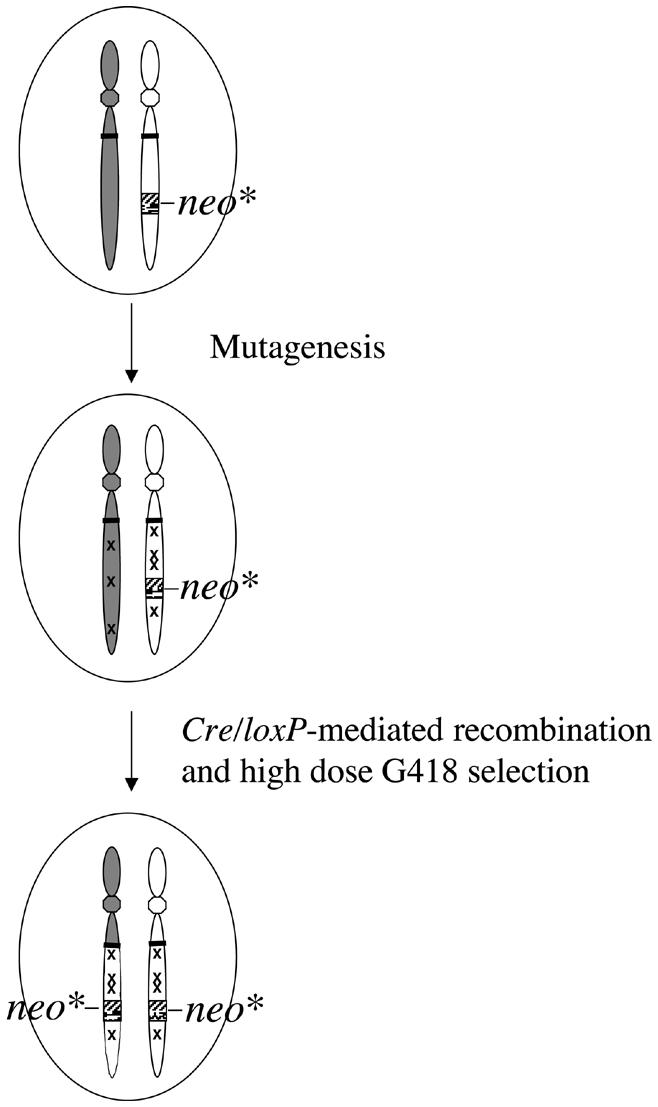
Fig. 3. Scheme of biallelic mutagenesis achieved by combining Cre/loxP-mediated inter-chromosomal recombination with chemical mutagenesis. ES cells bearing Cre, two loxP sites and one copy of the mutant neo gene are mutated by chemical mutagens. After selection of cells by high-dose G418, ES cells with biallelic mutations on the chromosomes bearing the neo gene can be expected to be obtained. ‘x’ indicates the mutation introduced by chemical mutagens.
METHODS
Construction of vectors. The targeting vector for the Oprk locus was generated as follows. A 10 kb SpeI fragment of the MORG17 genomic clone (Nishi et al., 1994) containing exon 1 and intron 1 of the Oprk gene was subcloned into the SpeI site of pBluescriptII (Stratagene) in which the BamHI site was disrupted. The 2 kb loxP-flanked PGK-neo cassette was inserted into the unique BamHI site of the Oprk region, resulting in pPGKneo-OpiR. An EcoRI–Asp718 fragment of the PGK-tk cassette was isolated from the plasmid, which was generated by cloning the KpnI–HindIII fragment of pPNT (Tybulewicz et al., 1991) into pBluescriptII, and was subcloned into the EcoRI–Asp718-digested pPGKneo-OpiR, resulting in the targeting vector for the Oprk locus.
The targeting vector for the Fasl locus was generated as follows. A 6.5 kb SalI–EcoRI (partially digested) fragment of Fasl genomic DNA was inserted into pBluescriptII, and the SalI site was used to clone a 1.2 kb XhoI–SalI DTA fragment of pBL-DTA (Adachi et al., 1995), resulting in pE/R-DTA. pPNT was digested by XhoI–BamHI and filled in, and the 1.8 kb mutant neo cassette was inserted into the SmaI site of pE/R-DTA, resulting in pE/R-DTAneo. A SacI–EcoRI (blunt-ended) fragment containing the Fasl exon 1 region with an in-frame EGFP gene was inserted into the SacI–NotI (blunt-ended) site of pE/R-DTAneo, resulting in the targeting vector for Fasl locus.
The Cre expression vector pPGK-Cre-IRES-Puro was generated as follows. The XhoI–SalI fragment of pPGK-puro containing the PGK promoter was inserted into the SalI site of pBS-Cre (Tarutani et al., 1997). As a result, the PGK promoter was placed upstream of the Cre gene. This plasmid was digested by SacII (blunt-ended), digested by NotI, and the PGK-Cre fragment was ligated to the XhoI(blunt-ended)–NotI fragment of pIRES-puro (Clontech), resulting in pPGK-Cre-IRES-Puro.
ES cell culture and electroporation. Linearized targeting vectors (10 µg) were electroporated into 1 × 107 R1 ES cells at 600 V/cm, 500 µF using Gene Pulser II (Bio-Rad). ES cells were selected by 150 µg/ml G418 for 7 days from the day after electroporation. GANC selection was performed in the targeting of the Oprk locus at a final concentration of 1 µM for 3 days from the second day of G418 selection. Resistant colonies were picked up, and homologous recombinants were analyzed by Southern blot hybridization.
For high-dose G418 selection, 2 × 106 cells were plated per 100 mm dish containing mouse embryonic fibroblasts, and G418 selection was started at the time of plating. G418-resistant colonies were counted after 10 days.
Isolation of stable clones expressing Cre. ES cells were transfected with pPGK-Cre-IRES-Puro vector using Transfast (Promega) according to the manufacturer’s instructions. ES cells were selected by 1 µg/ml puromycin for 7 days, and resistant colonies were expanded. Cre expression was examined by transient expression of the pCAG-CAT-EGFP reporter plasmid, in which Cre-mediated recombination of two loxP sites located between the GFP gene and its promoter creates a functional GFP expression unit.
Fluctuation analysis. The rate of biallelic mutagenesis was studied by Luria–Delbruck fluctuation analysis (Luria and Delbruck, 1943). One of the loxP+/Cre+ or loxP+/Cre– clones was plated on a 100 mm dish sparsely, and 10 subclones were picked up, expanded and selected under high-dose G418 (750 µg/ml). The calculations were carried out as described previously (Capizzi and Jameson, 1973).
Acknowledgments
ACKNOWLEDGEMENTS
We thank H. Takeshima and J. Miyazaki for providing the MORG17 genomic clone and the pCAG-CAT-EGFP reporter plasmid, and M. Takeda and Y. Sugita for encouragement of this research.
REFERENCES
- Adachi M., Suematsu, S., Kondo, T., Ogasawara, J., Tanaka, T., Yoshida, N. and Nagata, S. (1995) Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genet., 11, 294–300. [DOI] [PubMed] [Google Scholar]
- Becskei A. and Serrano, L. (2000) Engineering stability in gene networks by autoregulation. Nature, 405, 590–593. [DOI] [PubMed] [Google Scholar]
- Capecchi M.R. (1989) Altering the genome by homologous recombination. Science, 244, 1288–1292. [DOI] [PubMed] [Google Scholar]
- Capizzi R.L. and Jameson, J.W. (1973) A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat. Res., 17, 147–148. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yee, D., Dains, K., Chatterjee, A., Cavalcoli, J., Schneider, E., Om, J., Woychik, R.P. and Magnuson, T. (2000) Genotype-based screen for ENU-induced mutations in mouse embryonic stem cells. Nature Genet., 24, 314–317. [DOI] [PubMed] [Google Scholar]
- Chung J.H., Bell, A.C. and Felsenfeld, G. (1997) Characterization of the chicken β-globin insulator. Proc. Natl Acad. Sci. USA, 94, 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy A.J., Fritz, S. and Largaespada, D.A. (2001) Transposition and gene disruption in the male germline of the mouse. Genesis, 30, 82–88. [DOI] [PubMed] [Google Scholar]
- Fischer S.E., Wienholds, E. and Plasterk, R.H. (2001) Regulated transposition of a fish transposon in the mouse germ line. Proc. Natl Acad. Sci. USA, 98, 6759–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G. and Soriano, P. (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev., 5, 1513–1523. [DOI] [PubMed] [Google Scholar]
- Horie K., Kuroiwa, A., Ikawa, M., Okabe, M., Kondoh, G., Matsuda, Y. and Takeda, J. (2001) Efficient chromosomal transposition of a Tc1/mariner-like transposon Sleeping Beauty in mice. Proc. Natl Acad. Sci. USA, 98, 9191–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L., Dionne, N., Karaskova, J., Squire, J.A. and Nagy, A. (2001) Selection for transgene homozygosity in embryonic stem cells results in extensive loss of heterozygosity. Nature Genet., 27, 257–258. [DOI] [PubMed] [Google Scholar]
- Liu P., Jenkins, N.A. and Copeland, N.G. (2002) Efficient Cre-loxP-induced mitotic recombination in mouse embryonic stem cells. Nature Genet., 30, 66–72. [DOI] [PubMed] [Google Scholar]
- Luria S.E. and Delbruck, M. (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics, 28, 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R.M., Conner, D.A., Chao, S., Geisterfer-Lowrance, A.A. and Seidman, J.G. (1992) Production of homozygous mutant ES cells with a single targeting construct. Mol. Cell. Biol., 12, 2391–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe R.J., Bergstrom, R.A., Zheng, Q.Y., Libby, B., Smith, R., John, S.W., Schimenti, K.J., Browning, V.L. and Schimenti, J.C. (2000) Mouse mutants from chemically mutagenized embryonic stem cells. Nature Genet., 24, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M., Takeshima, H., Mori, M., Nakagawara, K. and Takeuchi, T. (1994) Structure and chromosomal mapping of genes for the mouse κ-opioid receptor and an opioid receptor homologue (MOR-C). Biochem. Biophys. Res. Commun., 205, 1353–1357. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis R., Liu, P. and Bradley, A. (1995) Chromosome engineering in mice. Nature, 378, 720–724. [DOI] [PubMed] [Google Scholar]
- Smith A.J., De Sousa, M.A., Kwabi-Addo, B., Heppell-Parton, A., Impey, H. and Rabbitts, P. (1995) A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nature Genet., 9, 376–385. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka, M., Brannan, C.I., Jenkins, N.A., Copeland, N.G., Suda, T. and Nagata, S. (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell, 76, 969–976. [DOI] [PubMed] [Google Scholar]
- Tarutani M., Itami, S., Okabe, M., Ikawa, M., Tezuka, T., Yoshikawa, K., Kinoshita, T. and Takeda, J. (1997) Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc. Natl Acad. Sci. USA, 94, 7400–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V.L., Crawford, C.E., Jackson, P.K., Bronson, R.T. and Mulligan, R.C. (1991) Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell, 65, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Xu T. and Rubin, G.M. (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development, 117, 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yenofsky R.L., Fine, M. and Pellow, J.W. (1990) A mutant neomycin phosphotransferase II gene reduces the resistance of transformants to antibiotic selection pressure. Proc. Natl Acad. Sci. USA, 87, 3435–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]