Abstract
Objectives:
Angiosarcoma is a rare complication of breast conserving therapy. This study evaluated the change in incidence between 1992–2016 of secondary breast angiosarcoma (SBA) in patients with a history of breast cancer and the impact of management strategies for the original breast carcinoma on angiosarcoma treatment.
Methods:
Breast cancer and angiosarcoma cases were abstracted from the Surveillance, Epidemiology, and End Result (SEER) database. SBA were defined as angiosarcomas located in the breast occurring after a prior breast cancer diagnosis. Primary breast angiosarcomas (PBA) were defined as an angiosarcoma diagnosis listed as “one primary only”. Incidence rates were estimated using a proportion of US total population. Survival was analyzed by the Kaplan-Meier method and Cox proportional hazard models were used to assess the association of clinicopathological characteristics on overall survival (OS).
Results:
Between 1992–2016, 193 cases of SBA were reported in the SEER dataset in patients with a prior history of breast cancer. The incidence of breast angiosarcoma in patients with a prior diagnosis of breast cancer increased three-fold from about 10 cases per 100,000 person-years to about 30 cases per 100,000 person-years over this same period (p=0.0037). For treatment of SBA (n=193), almost all (95%) had surgery. 9% received radiation (compared to 35% of patients with PBA, p <0.001) and 23% received chemotherapy (versus 45% for PBA, p=0.11).
Conclusions:
We demonstrate an increasing incidence of SBA over the study period. These data can help inform shared decision-making for optimal management of locoregional breast cancer and raise awareness of secondary angiosarcoma.
Keywords: Angiosarcoma, breast sarcoma, breast cancer, SEER, breast conserving therapy
INTRODUCTION:
Breast-conserving therapy, consisting of partial mastectomy (or lumpectomy) and radiation, is a cornerstone of management for locoregional breast cancer. Early-stage breast cancer is in many cases curable, but secondary cancers can be an infrequent, but serious, consequence of breast cancer treatment. Radiation-induced angiosarcomas can occur with an incidence of about 1 in 1000 patients at ten years.[1] A large retrospective cohort study confirmed radiation as a major risk factor; it also identified comorbidities of hypertension and diabetes as potential non-treatment risk factors for future angiosarcoma in patients treated for breast cancer.[2]
Further, for patients treated with axillary lymph node dissection, extremity and chest wall lymphedema are a known potential adverse outcome which may also increase the risk of secondary angiosarcoma (Stewart-Treves Syndrome).[3] Although the likely causative mechanism of oncogenesis is different, both radiation and lymphedema-associated angiosarcomas are characterized by MYC amplification, suggesting related pathogenetic mechanisms.[4, 5]
In spite of advances in our understanding of the molecular drivers of angiosarcoma[6–10], the optimal treatment strategy for secondary angiosarcoma of the breast remains undefined.[11] Management is often based on ad hoc multidisciplinary discussion with little prospective data to guide treatment planning. We therefore assessed the incidence of angiosarcoma after treatment for breast cancer over time and sought to identify if the management of patients with secondary breast angiosarcoma (SBA) is impacted by the antecedent management strategy for a patient’s original breast carcinoma.
MATERIALS AND METHODS:
We queried the Surveillance, Epidemiology, and End Result (SEER) database (data source: SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) - Linked To County Attributes - Total U.S., 1969–2017 Counties) and identified cases of angiosarcoma arising in the breast, either as a primary cancer or as a second diagnosis after a history of a separate breast cancer[12]. We first identified a group of potential cases that had multiple diagnosis entries and identical SEER patient ID. For this study, we defined SBA as an angiosarcoma diagnosis located on the breast with a prior breast cancer diagnosis. We defined primary breast angiosarcomas (PBA) as cases with an angiosarcoma diagnosis and were listed as “one primary only”. Patients with incomplete survival follow up, distant or missing SEER stage, and for PBA, cases diagnosed prior to year 1992 were excluded.
Mastectomy was defined for cases from 1992–1997 as any type of full mastectomy with surgery SEER code 30–70 and 90 and for cases from 1998–2016 SEER codes 30–80. Breast conservation surgery was defined for cases 1992–1997 SEER code 10, 20 or 80 and for cases 1998–2016 as any SEER code considered partial mastectomy, excisional biopsy, or lumpectomy (surgery SEER codes 20–24). To assess the involvement of axillary lymph node dissection in primary breast cancer surgery treatment, cases diagnosed between 1973–1997 with surgery code “10” and those diagnosed 1998 or later with surgery codes “22, 23, 24, 40, 41, 42, 45” were designated as no lymph node dissection. For cases with indications of lymph node dissection the surgery codes for years 1973–1997 were “20 and 50” and for years 1998 and later the surgery codes were “51 and 52”.
Incidence rate was calculated based on the projected total US population[13] with the total population at risk considered 27.8% of the total US population as defined by SEER[14]. Logistic regression and chi-square tests (with either Pearson’s or Fischer’s test for statistical significance) were used to compare categorical data. Cox proportional hazard models, and log-rank tests were used to assess the impact of clinical characteristics on overall survival (OS). A value of alpha<0.05 was designated for determination of statistical significance. Analyses were performed in Stata (versions 12 and 17.0. College Station, Texas: StataCorp LLC), GraphPad Prism (v7.03 La Jolla California USA) and Microsoft Excel (Microsoft Corporation: Redmond, WA).
Between 1992–2016, 193 cases of SBA were reported in the SEER dataset[12] in patients with a prior history of breast cancer; 145 cases of PBA were reported in patients without a prior history of breast cancer. In the same period, 1,081,311 cases of locoregional breast carcinoma (AYA site recode/WHO 2008= “8.4 carcinoma of breast”) were reported.
RESULTS:
The total US population incidence of a locoregional SBA increased from 0.0027 cases per 100,000 person years in 1995 to 0.014 cases per 100,000 person years in 2016, compared to a stable incidence of around 0.01 cases of PBA per 100,000 person years (Fig. 1A, B). The incidence of breast angiosarcoma in patients with a prior diagnosis of breast cancer, a more clinically significant measure, increased three-fold from about 10 cases per 100,000 person years to about 30 cases per 100,000 person years over this same period (p=0.0037, Fig. 1C). 92% of the patients were Caucasian. All were female. The median age at diagnosis was 75 years (range 40–94, Supplemental Table 1). The median interval between breast cancer and angiosarcoma diagnoses was 8 years (IQR: 6, 10). The interval between primary breast cancer diagnosis and secondary angiosarcoma diagnosis appears to be decreasing over time (Supplemental Table 2). 93 (48%) of patients had local disease at presentation, and 100 (52%) had regional disease. 4 (2%) had known lymph node involvement at diagnosis (Supplemental Table 1). The median age at diagnosis of the original breast cancer in patients that later developed a secondary breast angiosarcoma is 65 years, and the median age of diagnosis of all loco-regional breast cancer patients is 61.
Figure 1.
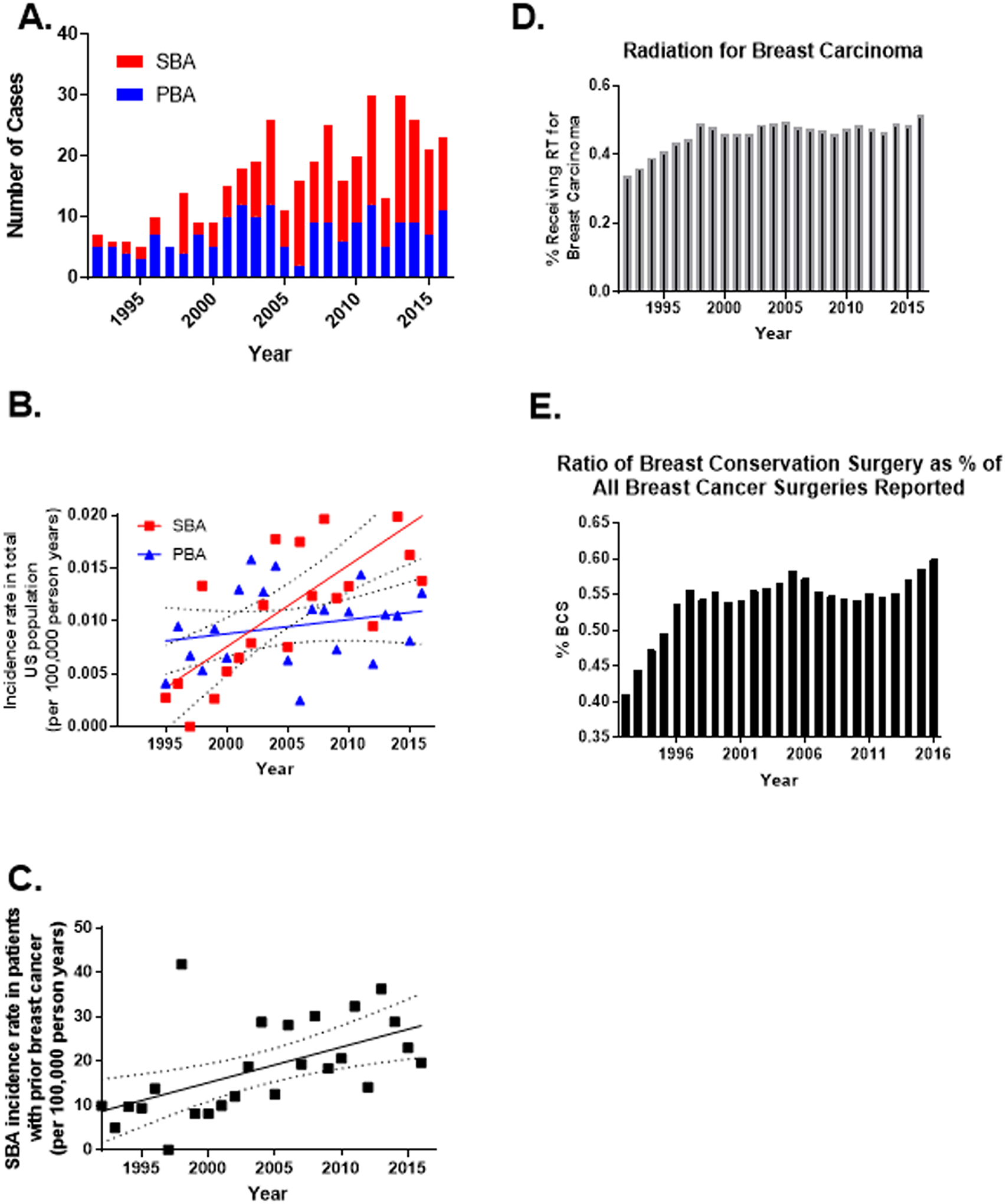
A) Number of primary (PBA) and secondary (SBA) breast angiosarcoma cases in SEER by year of diagnosis B) Incidence rate over time of primary and secondary breast angiosarcoma. The rate of increase of SBA cases was significantly different from that of PBA (p = 0.003). Data presented with best fit line (linear regression, SBA, R square = 0.549, F 24.4; PBA, R square = 0.0568, F = 1.21. C) Incidence rate in patients with a primary breast cancer of developing a secondary breast angiosarcoma (R square 0.312, F 10.4, p=0.0037). D) Percent of patients with breast carcinoma receiving radiation E) Of patients who had surgery for breast carcinoma, percent who had breast conservation surgery. Abbreviations: PBA, Primary breast angiosarcoma; SBA, secondary breast angiosarcoma. Dotted line = 95% confidence interval.
Noting the increasing incidence of SBA, we assessed the proportion of patients receiving radiation and breast conserving surgery or mastectomy over time for the primary breast carcinoma. Utilization of radiation and breast conservation surgery increased over time, especially in the early 1990s (Figure 1D, E). Most (89.8%) patients diagnosed with breast carcinoma prior to 1996 who later developed SBA had lymph node dissection for the primary breast carcinoma, while 11.8% of those diagnosed 1996 or later had lymph node dissection (Supplemental Table 3). Among patients who later developed SBA, there did not appear to be a clear pattern in likelihood of receiving prior radiation for the breast carcinoma by time period (Supplemental Table 4).
For treatment of SBA (n=193), almost all (95%) had surgery. 9% (n=17) received radiation (compared to 35% (n=51) of patients with PBA, p <0.001) and 23% (n=44) received chemotherapy (versus 45% (n=31) for PBA, p = 0.11).
To determine if the treatment modalities utilized for the primary breast cancer impacted treatment for the SBA, we compared the treatment modalities used for each cancer. 118 (61%) patients had surgery and radiation for the primary breast cancer, 39 (20%) had surgery, radiation, and chemotherapy, and 20 (10%) had surgery alone. 13 (7%) had surgery and chemotherapy and 3 (2%) had no reported treatment for their breast cancer. Most surgeries (94%) for the original breast carcinoma were breast conserving surgeries, while most surgeries (88%) for the SBA were mastectomies (Supplemental Fig. 1). Receipt of radiation therapy for treatment of a SBA was not associated with prior receipt of radiation therapy for breast cancer (p=0.744 Fischer’s exact test; Supplemental Table 5).
The median OS for patients with locoregional SBA was 43 months (95% CI: 32–66 months). 1-, 5-, and 10-year OS were 82% (95% CI: 75%-86%), 45% (95% CI: 37%-53%), and 28% (95% CI: 20%-37%). Increasing age, tumor size, tumor grade, and SEER stage were associated with worse OS in univariable analyses (Table 1). The median OS for patients with PBA was 69 months (95% CI: 41-NR). 1-, 5-, and 10-year OS were 89% (95% CI: 83%-93%), 52% (95% CI: 43%-60%), and 47% (95% CI: 37%-55%). Increasing tumor size, tumor grade, lymph node involvement, and SEER stage were associated with worse OS (Table 1). Multivariable models were not done due to the relatively small sample sizes.
Table 1.
Univariable Cox analysis of factors associated with angiosarcoma overall survival. Abbreviations: PBA, Primary breast angiosarcoma; SBA, secondary breast angiosarcoma.
| SBA | PBA | |
|---|---|---|
| Characteristics | HR (95%CI), p-value | HR (95%CI), p-value |
| Race | ||
| White | referent | referent |
| Black | 0.97 (0.40–2.39), 0.953 | 1.50 (0.65–3.48), 0.346 |
| Other (American Indian/ AK Native, Asian/ Pacific Islander) | 0.85 (0.31–2.31), 0.746 | 0.86 (0.39–1.88), 0.698 |
| Age at Diagnosis | ||
| Continuous | 1.05 (1.03–1.06), <0.001 | 1.01 (1.00–1.02), 0.054 |
| Size | ||
| Continuous | 1.00 (1.00–1.01), 0.036 | 1.01 (1.01–1.01), <0.001 |
| Tumor Grade | ||
| I | 0.32 (0.11–0.89), 0.030 | 0.37 (0.17–0.78), 0.010 |
| II | 0.83 (0.44–1.59), 0.582 | 0.35 (0.17–0.70), 0.003 |
| III | 0.92 (0.56–1.49), 0.730 | 1.09 (0.58–2.05), 0.972 |
| IV | referent | referent |
| Missing | 0.79 (0.49–1.28), 0.340 | 0.39 (0.15–0.98), 0.046 |
| Lymph node involvement | ||
| Negative | referent | referent |
| Positive | 2.14 (0.67–6.82), 0.197 | 3.29 (1.30–8.30), 0.012 |
| Unknown | 1.52 (0.90–2.56), 0.120 | 1.19 (0.58–2.41), 0.637 |
| Tumor Stage | ||
| Local | referent | referent |
| Regional | 1.92 (1.31–2.82), 0.001 | 3.15 (1.84–5.40), <0.001 |
| Primary Site | ||
| Nipple | 2.11 (0.76–5.83), 0.152 | n/a |
| Central portion of breast | 1.46 (0.76–2.79), 0.252 | 0.78 (0.18–3.30), 0.736 |
| Upper-inner quadrant of breast | 0.84 (0.21–3.46), 0.815 | 0.35 (0.12–1.01), 0.052 |
| Lower- inner quadrant of breast | 1.62 (0.64–4.05), 0.306 | 0.37 (0.11–1.23), 0.104 |
| Upper- outer quadrant of breast | 0.47 (0.19–1.18), 0.107 | 0.55 (0.25–1.22), 0.140 |
| Lower- outer quadrant of breast | 1.11 (0.45–2.78), 0.820 | 0.33 (0.04–2.42), 0.275 |
| Overlapping lesion of breast | 1.42 (0.87–2.30), 0.161 | 1.06 (0.62–1.82), 0.831 |
| Breast, NOS | referent | referent |
| Radiation | ||
| No/Unknown | referent | referent |
| Yes | 0.72 (0.35–1.48), 0.374 | 1.21 (0.74–1.96), 0.445 |
| Chemotherapy | ||
| No/Unknown | referent | referent |
| Yes | 0.76 (0.47–1.21), 0.240 | 1.14 (0.68–1.90), 0.627 |
DISCUSSION:
In this registry-based analysis, we observed that SBA cases, while still extremely rare, appear to be increasing over time in patients previously treated for a primary locoregional breast cancer. This may be due to a rise in frequency of breast-conserving radiation therapy, as well as increased breast cancer screening and an associated growth in diagnosis of early-stage breast cancers. We suspect that the true rate of angiosarcoma after locoregional breast cancer treatment may be even higher than what we report due to our focus on patients with locoregional breast angiosarcomas and exclusion of cases with metastatic disease at presentation. We also excluded those tumors coded in SEER as being in the chest wall or extremity, which may have excluded some angiosarcomas arising in the skin or chest wall post-mastectomy and contributed to the finding that the majority of patients in our study had previously undergone breast conserving surgery rather than mastectomy for their primary breast cancer. In contrast, the incidence of PBA appears stable.
Treatment modalities utilized for the primary breast cancer appeared to impact treatment planning for a potential secondary angiosarcoma; patients with SBA were less likely to receive radiation therapy as part of their angiosarcoma treatment versus those with a PBA. Surgery remains the mainstay of treatment for locoregional SBA. Neither radiation nor chemotherapy was associated with OS, and any potential benefit for these modalities remains undefined based on these data. The lack of certain outcomes from SEER prevents consideration of other potential outcomes of interest, such as local relapse-free survival. The survival data presented here can inform and serve as a benchmark for future studies of SBA.
A separate large retrospective cohort analysis recently defined the incidence rate of secondary sarcomas that arise after breast cancer treatment[2], but did not address the metrics of how those sarcomas were treated. It has been suggested that patients with SBA may have inferior outcomes versus those with primary breast angiosarcoma, possibly due to a more advanced age and more aggressive tumor biology at presentation.[15] Another potential explanation for this finding is that treatment options may be more limited in patients who have already undergone treatment with radiation or chemotherapy for a primary breast cancer in the same anatomic site as the secondary angiosarcoma due to cumulative effects of prior cancer therapy. This may be especially true in the limited use of radiotherapy for secondary angiosarcoma.
Current recommendations for surgical management of breast angiosarcoma arising in a previously irradiated field call for removal of all radiated skin.[16, 17] Even with this aggressive surgical management, local recurrence rates are high with a median local recurrence free survival of 18 months.[18] For this reason, retrospective data suggests that reirradiation can be considered on an individualized basis in the neoadjuvant or adjuvant setting, with an informed discussion with regard to the potential for increased risk of treatment-related morbidity due to higher cumulative doses of radiotherapy.[19, 20] Although termed breast sarcomas, most secondary angiosarcomas that result from prior radiation are cutaneous and likely arise from more “benign” radiation induced vascular neoplasms.[21] Our data highlight the importance of educating patients who have completed treatment for breast cancer about continued screening and skin exams, even many years after they have completed treatment. In this dataset, angiosarcoma was identified as long as 27 (median 8 years, mean 8.29) years after primary breast cancer therapy.
A strength of this study is its use of a large national database (SEER) and matched diagnoses of primary breast cancer with secondary breast angiosarcoma. Weaknesses include the retrospective nature of the study and lack of specific dates or details of treatment such as specific chemotherapies or type of radiotherapy, and lack of potential underlying genetic risk mutations (e.g. BRCA1/2 mutation status) of the patients. Despite the large dataset, there are still relatively few cases of this very rare event. However, we observed that secondary angiosarcoma is increasing in incidence. These data can help inform shared decision making for optimal management of locoregional breast cancer and raise awareness of this rare but impactful event.
Supplementary Material
Funding:
Work supported in part by support to the Sarcoma Oncology Program of the University of Washington from Curt and Elizabeth Anderson and P30CA015704 (E.T.L., L.D.C., M.J.W.) and through the Paul G Allen Research Center at Swedish Cancer Institute (K.G.P.). Dr. Cranmer was supported in part by the Curt and Elizabeth Anderson Endowed Professorship in Sarcoma Research, and by funding from Steve and Jane Urner.
Conflicts of Interest:
Nothing to declare: KP, SKS, JK, HM, JS, BC
ETL – Clinical trial funding from BioAtla, Ayala, SpringWorks. LDC- Clinical trial funding from Eli Lilly, AADi, BluePrint Medicine, Iterion, Gradalis, Philogen, Advenchen Laboratories, and CBA Pharma. Honoraria or has served on advisory boards for Daaichi Sankyo. MJW- Clinical trial support from Deciphera, Adaptimmune, GSK, Athenex, Foghorn Therapeutics, Shaqsi, Presage Biosciences, Inhibrx, Incyte. Consulting fees from Adaptimmune, Epizyme, Aadi, Deciphera. HML- Research funding from Tolmar, EliLilly, GE Healthcare, Sanofi, Zymeworks, Consulting for GE Healthcare, Novartis, Sanofi, Eli Lilly. JMS Research funding from Celcuity, Genentech, Xencor, Pfizer, SeaGen, Merck, Carisma, Lyell. Consultant/advisory boards: A2 Biotherapeutics, Volastra Therapeutics, GE Healthcare, Sensei Therapeutics, GlaxoSmithKline
Footnotes
Consent for publication: All authors have consented for publication of this manuscript.
Availability of data and materials:
All data are publicly available through the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program (https://seer.cancer.gov/).
References
- 1.Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer 2009; 115: 4055–4063. [DOI] [PubMed] [Google Scholar]
- 2.Veiga LHS, Vo JB, Curtis RE et al. Treatment-related thoracic soft tissue sarcomas in US breast cancer survivors: a retrospective cohort study. Lancet Oncol 2022; 23: 1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Schwartz RA. Stewart-Treves syndrome: Pathogenesis and management. Journal of the American Academy of Dermatology 2012; 67: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Zhang L, Chang NE et al. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer 2011; 50: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manner J, Radlwimmer B, Hohenberger P et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 2010; 176: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner MJ, Ravi V, Menter DG, Sood AK. Endothelial cell malignancies: new insights from the laboratory and clinic. npj Precision Oncology 2017; 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Painter CA, Jain E, Tomson BN et al. The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med 2020; 26: 181–187. [DOI] [PubMed] [Google Scholar]
- 8.Boichard A, Wagner MJ, Kurzrock R. Angiosarcoma heterogeneity and potential therapeutic vulnerability to immune checkpoint blockade: insights from genomic sequencing. Genome Med 2020; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner MJ, Lyons YA, Siedel JH et al. Combined VEGFR and MAPK pathway inhibition in angiosarcoma. Sci Rep 2021; 11: 9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behjati S, Tarpey PS, Sheldon H et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet 2014; 46: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth GR, Cranmer LD, Smith BD et al. Radiation-induced sarcoma of the breast: a systematic review. Oncologist 2012; 17: 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying) - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. In.
- 13.Day Jennifer Cheeseman, Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050, U.S. Bureau of the Census, Current Population Reports, P25–1130, U.S. Government Printing Office, Washington, DC, 1996. [Google Scholar]
- 14.https://seer.cancer.gov/data-software/documentation/seerstat/nov2019/. Accessed March 11, 2023.
- 15.Yin M, Wang W, Drabick JJ, Harold HA. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer 2017; 17: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li GZ, Raut CP, Hunt KK et al. Breast Sarcomas, Phyllodes Tumors, and Desmoid Tumors: Epidemiology, Diagnosis, Staging, and Histology-Specific Management Considerations. Am Soc Clin Oncol Educ Book 2021; 41: 390–404. [DOI] [PubMed] [Google Scholar]
- 17.Li GZ, Fairweather M, Wang J et al. Cutaneous Radiation-associated Breast Angiosarcoma: Radicality of Surgery Impacts Survival. Ann Surg 2017; 265: 814–820. [DOI] [PubMed] [Google Scholar]
- 18.Morgan EA, Kozono DE, Wang Q et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol 2012; 19: 3801–3808. [DOI] [PubMed] [Google Scholar]
- 19.Scott MT, Portnow LH, Morris CG et al. Radiation therapy for angiosarcoma: the 35-year University of Florida experience. Am J Clin Oncol 2013; 36: 174–180. [DOI] [PubMed] [Google Scholar]
- 20.Feigenberg SJ, Mendenhall NP, Reith JD et al. Angiosarcoma after breast-conserving therapy: experience with hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 2002; 52: 620–626. [DOI] [PubMed] [Google Scholar]
- 21.Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol 2005; 29: 983–996. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are publicly available through the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program (https://seer.cancer.gov/).


