Abstract
Invasive enteric bacteria must pass through the intestinal epithelium in order to establish infection. It is becoming clear that a common target for intestinal mucosa penetration is the specialized epithelial cell of Peyer’s patches, the M cell. In order to gain a better understanding of how bacteria interact with M cells, we have compared the interactions of Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells by using a murine ligated-loop model. Our results indicate that S. typhimurium possesses a highly efficient mechanism for M cell entry that targets and destroys these cells, while L. monocytogenes and S. flexneri appear to be internalized into M cells in a less disruptive fashion. Early uptake of Listeria or Shigella into M cells appeared to lead to the death of some cells, as evidenced by the appearance of holes in the intestinal epithelium. At later time points, the follicle-associated epithelium of animals infected with these bacteria displayed extensive destruction. These data indicate that enteric pathogens use different strategies to interact with M cells and initiate infection of a host.
Bacterial pathogens are specialized microorganisms that possess the ability to colonize a host by inducing cellular damage. As these bacteria enter a host, a variety of interactions are initiated that determine the success of the infection process (10). Following host entry, organisms must avoid host defense mechanisms and successfully compete with the commensal population. Successful pathogens have evolved to respond to these challenges in different ways, and each survival strategy used by bacterial pathogens takes advantage of a niche within the host that allows the organisms to multiply.
A number of pathogenic microorganisms have evolved strategies to cause disease following oral inoculation. These enteric pathogens include Vibrio cholerae, pathogenic Escherichia coli strains, Salmonella species, Shigella species, Yersinia species, Listeria monocytogenes, and Campylobacter species. Some infectious diseases such as cholera and E. coli-induced diarrhea and toxemia are caused by bacteria that remain primarily within the intestinal lumen and express enterotoxin(s) or colonize and alter the apical membrane of intestinal cells. Establishment of other enteric infections depends upon the ability of the pathogen to pass through the mucosa of the bowel. Bacterial pathogens that penetrate the intestinal epithelium as part of their infection strategy are able to occupy a variety of different locations within a host. Shigella species remain within human intestinal epithelial cells, where they cause the destruction of enterocytes and induce an inflammatory response. Following invasion, pathogenic Salmonella species are found either within vacuoles of intestinal epithelial cells or within vacuoles of cells that distribute systemically throughout the host, depending upon the host specificity of the strain. Other pathogenic species disseminate throughout the lymphatic system of a host and are found either primarily extracellularly (Yersinia species) or within cells of the lymphatic system (L. monocytogenes).
The mechanisms by which enteric pathogens pass through the intestinal epithelium have become the subject of considerable research interest. Many studies now indicate that enteric pathogens use specialized M cells as a primary portal of entry into the host (20, 33, 34). Some organisms, such as Mycobacterium bovis BCG (12), Streptococcus pneumoniae (45), and Yersinia enterocolitica (1, 16), have been shown to specifically attach to and pass through M cells as they initiate disease. Shigella flexneri (47) requires both an adhesive and invasive phenotype to efficiently colonize follicle-associated epithelium (FAE). Following Shigella infection, M cells begin to increase in size, which eventually disrupts the integrity of the epithelium. The effect of invasive Salmonella typhimurium on M cells is dramatic (7, 21). At the earliest stages of Salmonella invasion, large membrane ruffles appear on the apical surface of the M cell, and within a short period of time (30 to 60 min), the cell becomes necrotic and begins to die. These findings indicate that different enteric pathogens have evolved distinct mechanisms to interact with M cells of a host.
In an effort to more thoroughly characterize the mechanisms by which pathogenic bacteria enter M cells, we have compared the interactions that occur between pathogenic S. typhimurium, S. flexneri, or L. monocytogenes and the FAE of mice. We have performed experiments that have allowed us to examine the initial interactions that lead to uptake of each bacterial strain into M cells as well as the changes that occur in the FAE following bacterial penetration of the intestinal epithelium. We find that the interactions of different bacterial pathogens with M cells can differ. However, our results also suggest that passage through M cells is a common step in the pathogenic strategies of enteric pathogens.
MATERIALS AND METHODS
Mice.
Female BALB/c mice 8 to 12 weeks old purchased from the National Cancer Institute were used for all experiments. Mice were housed and maintained by the University of Iowa Department of Laboratory Animal Medicine.
Bacterial strains and growth conditions.
S. typhimurium SL1344 is an invasive mouse-virulent strain (49), and S. typhimurium BJ70 (38) is a noninvasive strain derived from SL1344 that contains a Tn5lacZY insertion in the invasion gene regulator hilA (2). L. monocytogenes 10403S is a virulent wild-type strain that has been described previously (3). L. monocytogenes DP-L2161 is an isogenic derivative of 10403S that carries a mutation in the listeriolysin O gene which does not affect cell entry but prevents cell-to-cell spread of the bacteria (22). S. flexneri 2a is a virulent strain that carries the genes required for entry into mammalian cells on a virulence plasmid (29).
To induce the invasive phenotype of S. typhimurium, strains were grown under oxygen-limiting conditions in L broth as previously described (24). Shigella and Listeria strains were grown with the same media and growth conditions as the Salmonella strains prior to inoculation into mice. Control experiments confirmed that S. flexneri and L. monocytogenes were as invasive when grown under these conditions as those previously published for Shigella (30) and Listeria (13).
Fluorescent labelling of bacteria.
Bacteria used in confocal microscopy experiments were labelled with fluorescein isothiocyanate (FITC) prior to introduction into the mouse intestine. To label the bacteria, the desired inoculum was pelleted, and the bacterial pellet was resuspended in phosphate-buffered saline (PBS) with FITC (500 μg per ml in PBS) for 1 h at 37°C in the dark. The labelled bacteria were pelleted and washed four times with PBS to remove unincorporated FITC. The final pellet was resuspended in a volume of PBS to achieve the desired concentration of bacteria.
Murine ligated-loop model.
Prior to the initiation of each ligated-loop experiment, mice were fasted for 16 h to empty the intestinal contents. The surgical technique used was similar to that which has been previously described (21). Each mouse was injected with 1.5 to 2.0 mg of pentobarbital sodium (Nembutal; Abbott Laboratories, North Chicago, Ill.) intraperitoneally to reach the appropriate plane of surgical anesthesia. A midline incision was made into the abdominal cavity to expose the ileum. After grossly identifying Peyer’s patches, two ligatures were placed to create a loop that enclosed several Peyer’s patches. By using 4.0 silk, the intestine was ligated at the ileocecal junction and, following injection of the bacterial inoculum, at a location ∼3 to 5 cm proximal while maintaining the integrity of the blood supply. The bowel was returned to the abdominal cavity, which was closed with silk ligature. Mice were maintained under anesthesia for periods of time ranging from 30 to 120 min. The mice were sacrificed by cervical dislocation, and the intestinal tissue was processed for microscopy.
Preparation of tissue for SEM.
Following the specified incubation time of the bacteria with the murine intestinal tissue, the intestine was removed from the mouse, and transverse cuts were made with a razor blade on each side of the Peyer’s patches that were present in the ligated section of small bowel. The intestinal section was then cut longitudinally along the mesenteric border to produce a flat rectangle of tissue. The tissue was pinned onto dental wax, with the luminal surface facing up, placed immediately into vials containing ice-cold fixative (2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer [pH 7.2]), and stored overnight at 4°C. The following day, each tissue sample was immersed in 0.1 M sodium cacodylate for 60 min and secondarily fixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 1 to 2 h before being rinsed in 0.1 M sodium cacodylate buffer twice for 15 min. Samples were then rinsed in deionized water and dehydrated through a graded series of ethanol washes. Finally, the samples went through critical point drying, were mounted on aluminum stubs with colloidal silver (Ted Pella, Inc., Redding, Calif.), and sputter coated with gold palladium prior to examination. Samples were viewed with a Hitachi S-4000 field emission scanning electron microscope (SEM) at the University of Iowa College of Medicine Central Microscopy Research Facility.
Tissue preparation for confocal microscopy.
After inoculation of a ligated loop with FITC-labeled bacteria, the Peyer’s patches were removed as described above and pinned luminal side up to dental wax which had been previously melted onto a glass slide. Sample preparation was performed according to the method of Clark et al. (5). Briefly, the mounted tissue sample was immersed in cold methanol (−20°C) for 40 min and then incubated with 10 mg of tetramethyl rhodamine isothiocyanate (TRITC)-conjugated Ulex europaeus 1 (Sigma, St. Louis, Mo.) per ml for 60 min at room temperature. The sample was rinsed with PBS, and a coverslip was placed on top of the tissue sample to allow viewing with a Bio-Rad 1024 laser-scanning confocal microscope. Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, Calif.) was used to delay quenching of the fluorescent signal.
RESULTS
Specialized M cells within the follicle-associated epithelium can be distinguished from epithelial cells by SEM.
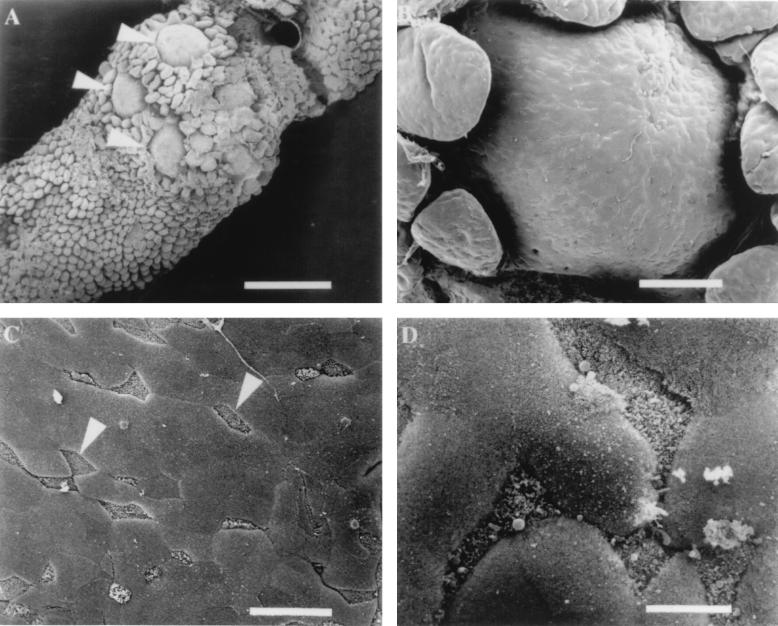
Peyer’s patches, or gut-associated lymphoid tissue (GALT) (35), can be grossly identified as white areas on the serosal surface of the murine small intestinal tract. Microscopic examination of the luminal surface of murine GALT reveals distinctive dome structures interspersed within a sea of absorptive villi which can be observed at low magnification (Fig. 1A), and higher magnification clearly demonstrates the difference in the lymphoid follicle dome and the surrounding villi (Fig. 1B). Beneath the epithelial surface of these domes reside numerous B lymphocytes, T lymphocytes, macrophages, and dendritic cells which provide the intestinal epithelium with localized immunity (15, 35). Examination of the epithelial surface of these domes at a higher magnification reveals that the cellular composition of the monolayer is heterogeneous. Figure 1C shows the presence of many cells (identified with arrows) with short, irregular microvilli and distinctive triangular shapes interspersed within the monolayer of enterocytes. These cells are the M cells that were originally described by two separate groups (4, 36) and appear to comprise approximately 10% of the cells in the murine FAE. At a higher magnification of the dome epithelium, the difference between the irregular apical surface of M cells and the highly ordered appearance of enterocyte microvilli is clearly discernible (Fig. 1D).
FIG. 1.
Scanning electron micrographs of murine Peyer’s patch tissue. (A) Luminal side of Peyer’s patch containing several domes (arrowheads) surrounded by villi. Bar, 700 μm. (B) Epithelial surface of a lymphoid follicle dome that contains numerous M cells and enterocytes randomly distributed. Bar, 100 μm. (C) Region of the FAE containing several M cells (arrowheads) that can be distinguished by short microvilli which give an irregular appearance. Bar, 20 μm. (D) Higher magnification of M cells and enterocytes. M cell microvilli create a rough irregular appearance in comparison to adjacent enterocytes which look smooth in texture. Bar, 6 μm.
Visualization of the effect of invasive S. typhimurium on murine M cells using SEM.
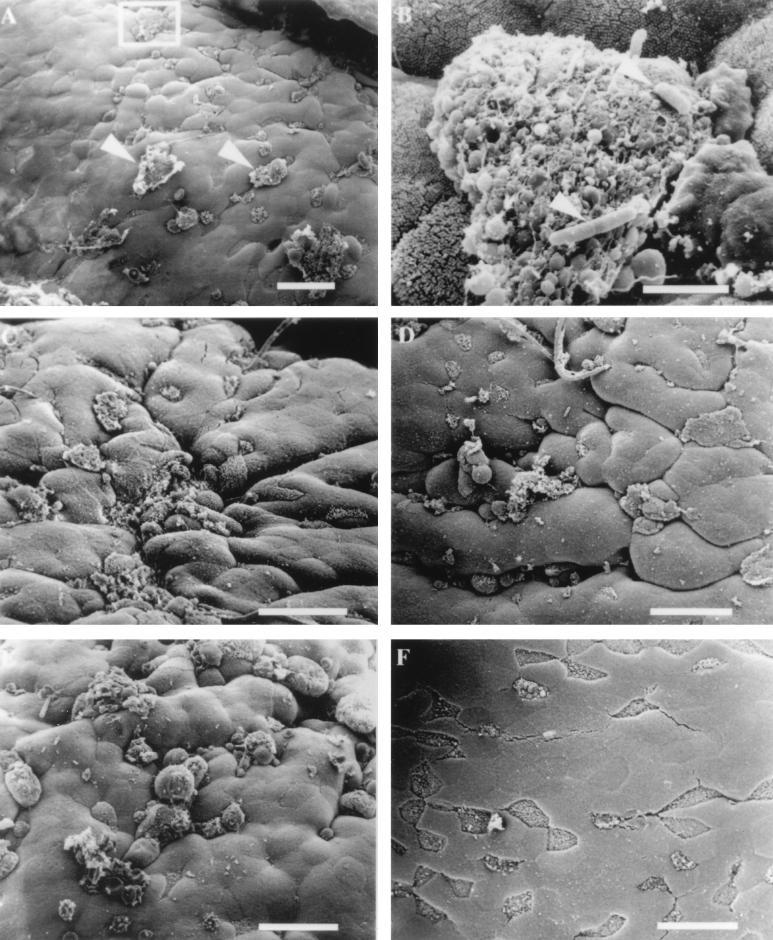
To establish a basis for comparison with other invasive bacterial strains and to evaluate our ability to visualize changes on the surface of the FAE, we infected murine ligated loops with ∼4 × 108 CFU of the invasive S. typhimurium strain SL1344 per ml. Within 30 min of infection, there were large membrane rearrangements scattered across the surface of the FAE which appeared to localize exclusively to the surfaces of M cells. In Fig. 2A, several disrupted regions can be seen, two of which are identified with arrows. In some instances, the effect on the M cell was so severe that the membrane ruffling was associated with cell lysis. Occasionally, organisms could be visualized associated with the cellular debris of these dead cells (Fig. 2B; magnified region of box in Fig. 2A). As described in earlier studies (7, 21), enterocytes within the epithelium were intact and unaffected by changes at the M cell surfaces at early time points of Salmonella infection. As the length of exposure of the intestinal tissue to invasive S. typhimurium increased, there was a qualitative increase in the size of the membrane rearrangements on M cell surfaces as well as a quantitative increase in the number of M cells displaying membrane alterations. At 60 and 90 min (Fig. 2C and D, respectively), most of the M cells in a given region of the FAE displayed membrane protrusions and disruptions. At these time points, it was difficult to identify M cells because their short, irregular microvilli were no longer discernible. By 120 min postinoculation, virtually all of the M cells displayed disruptions and were most easily identified by the presence of large bubbles or ruffles that were induced by invading S. typhimurium (Fig. 2E) rather than the short irregular microvilli, which is their signature in uninfected tissue (Fig. 1C and D). Even at the latest time point examined (120 min), the integrity of enterocytes appeared to be unaffected by the presence of invading S. typhimurium. In contrast to samples infected with invasive S. typhimurium, control loops inoculated with bacterial broth or an equivalent number of cells the noninvasive S. typhimurium strain BJ70 did not exhibit any notable changes in the cytoarchitecture of the M cells after incubation periods of up to 120 min (Fig. 2F).
FIG. 2.
Electron micrographs of mouse FAE infected with invasive S. typhimurium SL1344. (A) Membrane rearrangements are visible on the surfaces of some M cells (arrowheads) 30 min after infection with S. typhimurium SL1344. (B) Higher magnification of the region in the inset from panel A. Rod-shaped organisms (arrowheads) can be observed adhering to M cell debris. Bar, 3 μm. (C) Effects of S. typhimurium 60 min after infection. (D) Tissue infected for 90 min with invasive Salmonella. M cells with a normal apical appearance are difficult to find, and the majority of cells display membrane disruptions and blebbing. (E) Infection of ligated loop with invasive Salmonella for 120 min. A quantitative increase in the number of M cells displaying membrane changes and a qualitative increase in the size of apical membrane disruption are evident. (F) Mouse intestinal tissue inoculated with bacterial growth broth for 30 to 120 min was examined for the appearance of uninfected tissue. The tissue shown has been inoculated with broth for 120 min and appears completely normal. The M cells are clearly visible, and the microvilli have the typical appearance of uninfected tissue. Bar, 20 μm unless otherwise indicated.
Effect of L. monocytogenes on the murine Peyer’s patch epithelium.
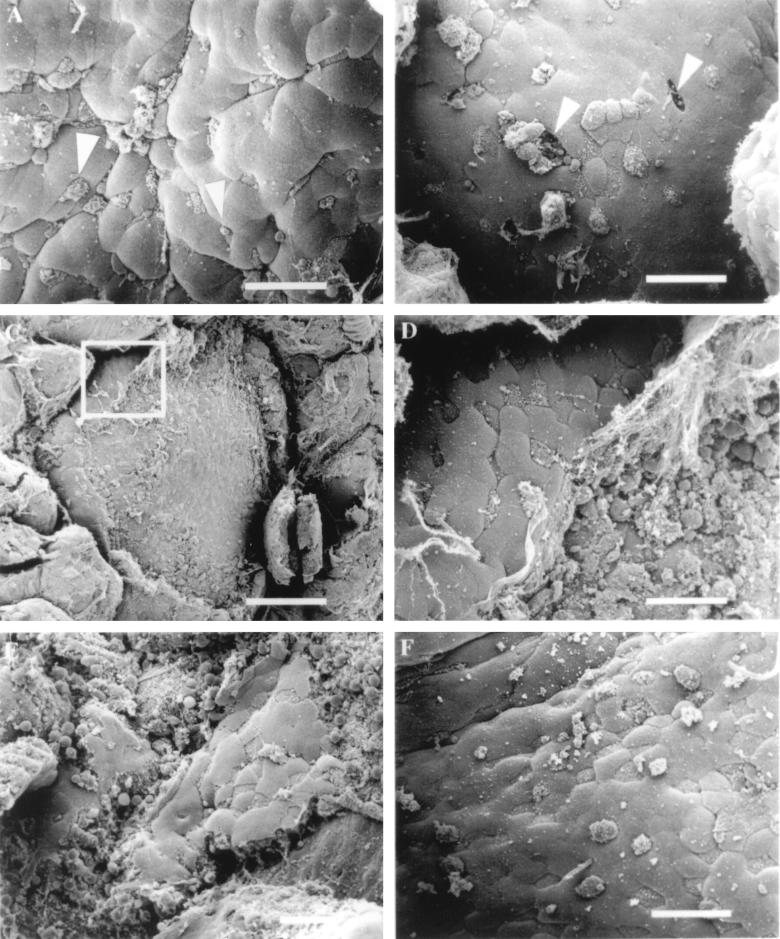
Despite evidence that L. monocytogenes localizes to Peyer’s patch tissue following oral inoculation (25, 27, 46), experiments have not been performed that demonstrate specific interactions between L. monocytogenes and M cells. To address this question directly, we infected murine ligated loops with the invasive L. monocytogenes strain 10403S, which has the ability to spread from cell to cell by actin-based motility. Murine intestinal infections were first performed with inocula of ∼1 × 109 CFU per ml. At this dose, which was 2.5× higher than that used for invasive S. typhimurium, the tissue infected with Listeria displayed no detectable changes at the apical surface of the M cells 30 and 60 min following infection and had an appearance similar to that shown in Fig. 1C and 2F. At later time points of infection (90 and 120 min), some changes in the apical membranes of M cells were occasionally detected, although the alterations were observed inconsistently (data not shown). This result suggested that we could increase the probability of observing Listeria-induced changes on M cell surfaces by increasing the bacterial inoculum in the ligated loop. We next performed experiments in which the inoculum of L. monocytogenes was increased to ∼4 × 109 CFU per ml. It has been reported that extremely high doses of L. monocytogenes, consistent with this level of inoculum, are required to cause clinical disease in immunocompetent humans infected orally (8). At this concentration of bacteria, changes that were observed 30 min following inoculation of intestinal loops were similar to those that were occasionally found at 120 min when a lower dose was used to infect murine intestines. At the earliest time point observed (30 min), many M cells displayed membrane alterations consisting of small protrusions that extended from the surface of the cell into the lumen (Fig. 3A). Confocal microscopy was also used to confirm that L. monocytogenes was physically interacting with murine M cells. Numerous FITC-labelled bacteria could be observed adhering to the surface of M cells that were stained with TRITC-conjugated U. europaeus 1 lectin, which is specific for M cells (6) (data not shown). In comparison to S. typhimurium infection of M cells, membrane changes related to M cell uptake of Listeria were substantially smaller and required approximately 10-fold more bacteria per ml of inoculum to observe on the surface of the cells. At 60 min of infection, we occasionally observed holes in the FAE (Fig. 3B). The precise cause of these breaks in the integrity of the epithelium is unknown, although one explanation is that an internalized L. monocytogenes organism has escaped from the internalization vacuole and induced the death of the M cell. No other significant changes were observed on the surface of the dome epithelium until 90 min following inoculation of Listeria into the murine intestinal loops. Extensive cellular destruction of a majority of the cells in the dome epithelium became apparent at this time point (Fig. 3C). Higher magnification of these damaged regions revealed margins of the FAE where areas of cellular necrosis and portions of the intact epithelium meet. Cellular debris and rounded up epithelial cells provide clear evidence of a destroyed epithelium (Fig. 3D and E).
FIG. 3.
Effect of L. monocytogenes on murine FAE. (A) Mouse tissue infected for 30 min with L. monocytogenes 10403S. Some M cells display aberrant microvilli that are rounded in appearance (arrowheads). (B) After 60 min of infection, the presence of the bacteria appears to have destroyed the integrity of the FAE by creating holes (arrowheads) in the dome epithelium where M cells once resided. (C) Listeria-induced destruction of the FAE 90 min postinfection. Bar, 100 μm. (D) Higher-magnification view of the inset in Fig. C. At the periphery of the dome, a few M cells and enterocytes are still intact. (E) Tissue infected for 120 min with L. monocytogenes. (F) Tissue infected with the L. monocytogenes listeriolysin O-negative mutant DP-L2161 did not induce epithelial necrosis after 120 min of infection. Bar, 20 μm (unless otherwise indicated).
Listeriolysin O is required for Listeria-induced destruction of the lymphoid follicle epithelium.
In an effort to understand the mechanism of Listeria-mediated destruction of the FAE, we examined the effect of L. monocytogenes DP-L2161 on murine Peyer’s patch tissue. This strain possesses the ability to induce internalization into cells but carries a mutation in the listeriolysin O gene (hly) which prevents the organism from spreading from cell to cell because it is unable to lyse the vacuolar membrane. This strain was inoculated into murine ligated intestinal loops at a concentration of ∼4 × 109 CFU per ml, and the effects of the bacteria on the tissue were examined at various time points by SEM. Observation of the infected tissue revealed the presence of many apparently normal M cells, while the appearance of a minority of the M cells was altered. These changes could be seen as early as 30 min postinfection and were also found at 120 min postinfection (Fig. 3F). Strikingly, we did not observe signs of epithelial destruction that were found in tissue infected with the wild-type L. monocytogenes strain, 10403S. The changes in the appearance of the M cells suggest that L. monocytogenes DP-L2161 can be internalized into M cells, but the listeriolysin O-negative mutant strain did not induce general destruction of the FAE, indicating that listerolysin O plays an important role in Listeria-mediated FAE necrosis.
Interactions between invasive S. flexneri 2a and the epithelium of lymphoid follicles results in extensive cellular damage.
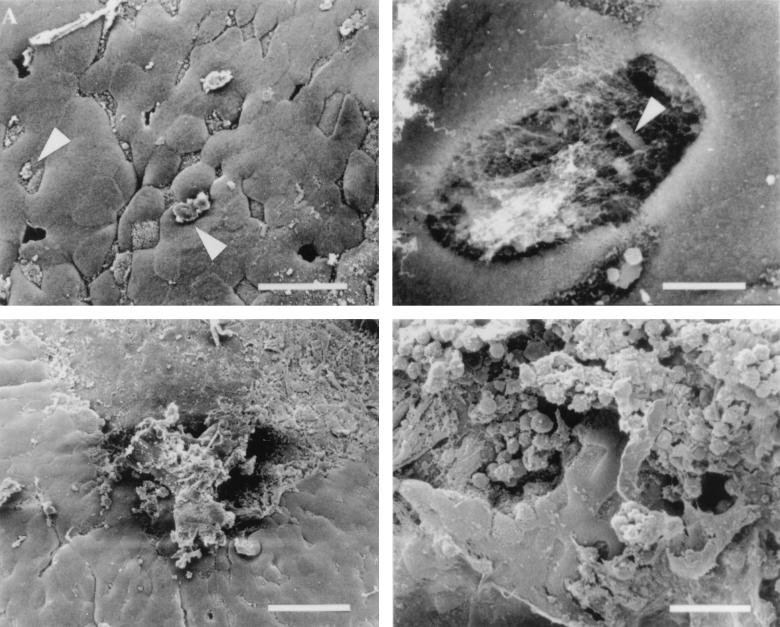
We next compared the effect of the human intestinal pathogen S. flexneri on murine FAE to that observed for S. typhimurium and L. monocytogenes. Two doses of S. flexneri, similar to those used for Listeria (∼1 × 109 and ∼4 × 109 CFU per ml), were inoculated into murine intestines, and the effects were observed by SEM. Tissue infected with the lower dose of organisms did not show significant M cell disruption at the various times examined and had the same appearance as the FAE shown in Fig. 1C and 2F. In contrast, at the higher dose, the interactions between S. flexneri and the epithelium of Peyer’s patches were similar to those observed for L. monocytogenes. At early stages of infection, some membrane perturbations could be found on M cells (Fig. 4A). As with L. monocytogenes, large numbers of FITC-labelled S. flexneri organisms could be detected adhering to TRITC-labelled M cells by using confocal microscopy (data not shown). Occasionally, samples of tissue contained epithelium with missing cells. In one instance, higher magnification of the region where a cell had been destroyed revealed a bacterium buried within the cellular debris and mucus (Fig. 4B). At later stages of the Shigella infection process, complete destruction of the epithelial surface was observed which appeared identical to that observed when wild-type L. monocytogenes was used to infect the murine intestinal tissue (Fig. 4C). Higher magnification of the destroyed regions revealed membrane blebs and a denuded epithelial surface (Fig. 4D) that closely resembled that observed for Listeria (Fig. 3E). Murine intestinal loops infected with noninvasive E. coli as a control induced no damage to the FAE and had an appearance similar to uninfected tissue (Fig. 1C) or that infected with noninvasive S. typhimurium (Fig. 2F). These data demonstrate that L. monocytogenes and S. flexneri possess the ability to induce massive destruction of FAE when inoculated into intestinal loops at inocula of ∼4 × 109 CFU per ml.
FIG. 4.
Ligated loops inoculated with invasive S. flexneri 2a. (A) Like L. monocytogenes, S. flexneri induces small changes in the appearance of the M cell surface (arrowheads). In addition, it was possible to occasionally find holes in FAE infected with Shigella. (B) Higher-magnification view of a perforation in the intestinal epithelium with an organism (arrowhead) located within. Bar, 4 μm. (C) Shigella-induced destruction of the FAE. Bar, 40 μm. (D) Higher-magnification view of the damage induced by S. flexneri on the epithelium of the lymphoid follicle. Only a few intact M cells and enterocytes remain intact. Bar, 20 μm unless otherwise indicated.
DISCUSSION
We have examined by SEM the interactions between three invasive bacterial pathogens and the epithelium of lymphoid follicles in the small intestines of mice. Our results confirm previous observations that S. typhimurium specifically invades and destroys M cells of the ileal Peyer’s patches. In addition, we found that inoculation of S. flexneri or L. monocytogenes at high doses into ligated intestinal loops of mice resulted in significant damage and destruction of the FAE.
Lymphoid follicles (Peyer’s patches) are components of the mucosal immune system which are important in host defense against invasive enteric pathogens (17). This branch of the immune system inspects the antigenic content of the intestinal lumen and activates lymphocytes that circulate throughout the reticuloendothelial system of the host. M cells are a specialized component of this system which are at the interface of the intestinal lumen and the mucosal immune system. These cells are found exclusively within FAE and are usually surrounded by lymphocytes that have moved into and deformed the cytoplasm of the M cell. The association between M cells and lymphocytes suggests that a direct interaction, such as antigen presentation, occurs between the two types of cells.
Several studies have demonstrated that one function of M cells may be to engulf bacteria that do not actively invade host cells. Owen et al. (37) examined the ability of M cells to engulf Vibrio cholerae. This organism is a noninvasive pathogen that induces severe disease by inducing toxin-mediated fluid secretion from enterocytes of the intestine. When high bacterial inocula (∼109 CFU) of V. cholerae were introduced into rabbit ligated intestinal loops containing Peyer’s patches, single organisms could be observed within and beneath M cells by transmission electron microscopy. Bacteria were not detected within other intestinal cells. It is presumed that uptake of V. cholerae is an example of M cells engulfing antigen that would be used to prime a host immune response. Similarly, M cells were recently shown to engulf the gram-positive organism S. pneumoniae (45). A rabbit ligated-loop model was used to demonstrate that noninvasive S. pneumoniae cells are taken up and transported by M cells. The damaged appearance of the bacterial cell walls, as seen by electron microscopy, suggested that the M cells may have modified or processed the bacteria.
An expanding body of research indicates that many, if not all, enteric bacterial pathogens interact with M cells as part of their pathogenic strategy. Examination of rabbit intestinal tissue inoculated with BCG revealed that the bacteria specifically associated with M cells and could subsequently be found within macrophages of the Peyer’s patches (12). Another study found that Mycobacterium paratuberculosis cells inoculated into the intestinal lumen of calves were taken up by M cells and subsequently passed to intraepithelial macrophages (31). Yersinia species have also been shown to interact with M cells. Y. enterocolitica and Yersinia pseudotuberculosis initially colonize Peyer’s patch tissue by adhering to the apical surface and entering M cells of the FAE (1, 11, 16). Twenty-four hours later, microabscesses appear in the epithelium of the Peyer’s patches that increase in size over time and ultimately lead to the destruction of the entire FAE. Two groups have shown that the invasin gene plays an important role in facilitating the transport of Yersinia across the epithelium of Peyer’s patches (28, 39). The two invasive pathogens, S. flexneri and S. typhimurium, have been shown to specifically interact with M cells. At the earliest stages of S. flexneri infection, bacteria are found within and below M cells of rabbit FAE (40, 47). Within minutes of inoculation of S. typhimurium into murine ligated loops, bacteria inducing membrane rearrangements at the surface of the M cells are detected. Ultimately, the membrane changes induced during invasion appear to induce the death of the M cell and its removal from the epithelium.
The previously described research has helped to establish that many, if not all, enteric bacterial pathogens interact with M cells at an early stage of infection. Presumably, interactions between bacteria and Peyer’s patch tissue were first fostered by the host as a strategy for immune surveillance and host survival. However, it seems clear that while the host may have facilitated these interactions originally for its own advantage, many bacterial species have now evolved strategies to take advantage of and/or modify the normal function of the mucosal immune system.
The interactions of S. typhimurium, L. monocytogenes, or S. flexneri and M cells have been examined and compared. We have noted several differences between the interactions of these organisms with the FAE and the M cells. First, in order to observe an effect on the appearance of the FAE, approximately 10-fold-more (∼4 × 109 CFU per ml) L. monocytogenes or S. flexneri organisms needed to be injected into the ligated loop of the mice than S. typhimurium (∼4 × 108 CFU per ml). Second, the effect of Listeria or Shigella on the apical membrane of M cells was significantly different from that observed for Salmonella. While apical membrane perturbations were observed following inoculation of either Listeria or Shigella, these changes were relatively minor compared to the large membrane ruffles induced by the invasion machinery of S. typhimurium. It seems likely that the M cell membrane changes that we observed with L. monocytogenes or S. flexneri were similar to membrane changes that would occur if an M cell were to engulf a large particle or a noninvasive bacterium. Finally, the effect of L. monocytogenes or S. flexneri on the integrity of the FAE was noted. While passage of these organisms through the FAE appeared dependent upon M cell macropinocytosis, following movement through the epithelium, the bacteria had a dramatic effect on the integrity of the epithelium. Within 90 min of inoculation, these pathogens induced extensive destruction of the FAE which appeared to begin at sites of M cell internalization before spreading outward. Similar damage has been observed for S. typhimurium with lower inocula (4 × 108 CFU per ml) after 120 and 180 min of infection (21). Importantly, in the case of Listeria, we found that FAE damage was dependent upon the ability of the bacteria to escape from the intracellular vacuole, since a L. monocytogenes mutant that lacks listeriolysin O did not induce the epithelial damage observed with the parent strain.
This is the first study to demonstrate that L. monocytogenes penetrates the intestinal mucosa through M cells. This finding extends the observations of Marco and coworkers (27), who found that L. monocytogenes, inoculated intragastrically, localized exclusively to Peyer’s patch mucosa. However, our results differ from those of two more recent studies (26, 43) in which no specific association of Listeria was observed with M cells. A significant finding in the study by Pron et al. (43) was that L. monocytogenes preferentially replicates within the Peyer’s patch tissue. Possible explanations for the difference in our research findings is that we used a somewhat higher inoculum of Listeria (5 × 109 CFU per ml) in our ligated-loop experiments compared to the other studies, in which the highest dose was 109 CFU per ml. In addition, we used SEM to examine the infected mouse tissue, which allowed us to examine virtually the entire FAE of several follicle domes from each mouse. However, our need to use a high bacterial inoculum to observe changes at the M cell surface agrees with the findings of the other studies, which concluded that Listeria passage through the intestinal mucosa is an inefficient process requiring large inoculum sizes.
A comparison of the interactions of Shigella and Salmonella with M cells is of value, since the invasive mechanisms of both pathogens involve export of effector proteins into target cells by a type III secretion system (14). One important consideration for these experiments is that Shigella is not a natural pathogen of mice. However, several studies have demonstrated that invasive Shigella species can invade murine cells (18, 19, 23). In addition, efforts are under way in several laboratories to develop mice as a model for Shigella infection (41, 42, 48), in addition to the rabbit, which is also not normally susceptible to this pathogen (9, 44, 47). Our results indicate that S. flexneri depends upon M cell uptake mechanisms to breach the intestinal epithelium of mice, but that following entry, the organisms lyse the vacuole and induce general epithelial damage by spreading from cell to cell. Perhaps it is not surprising that S. flexneri passage through the apical membrane of M cells is not more dramatic, since Mounier et al. (32) demonstrated that Shigella efficiently invaded cells from a basolateral side but only poorly through the apical surface. The inability of S. flexneri to facilitate its own uptake into M cells is likely not the explanation for its inability to cause disease in mice, since L. monocytogenes, which exhibits a similar interaction with M cells, can cause disease after oral inoculation (8). These observations indicate that S. flexneri strains possess the ability to initiate infection of mice, but they do not possess other host-specific factors, which prevents them from establishing long-term infection of murine intestinal tissue.
To our knowledge, this is the first attempt to compare the interactions of different enteric pathogens with M cells and the FAE in the same experimental system. Two distinct patterns of intestinal invasion were observed in our experiments. This approach should prove generally applicable in the analysis of the early interactions of a variety of microbial pathogens with the intestinal FAE. Such studies may reveal new strategies developed by enteric pathogens to breach the intestinal epithelium. In addition, this system provides an approach with which to analyze the impact of bacterial virulence factors on interactions with M cells and subsequent interactions with the intestinal epithelium as well as the opportunity to determine whether the immune status of the host can impact the earliest events of bacterial invasion.
ACKNOWLEDGMENTS
We thank Steve Clegg and Doug White for critically reading the manuscript and providing comments for improvement. Technical assistance with SEM and confocal microscopy was provided by the Central Microscopy Research Facility of the University of Iowa College of Medicine.
This work was supported by grants from the Roy J. Carver Charitable Trust (J.T.H. and B.D.J.) and grants AI38268 (to B.D.J.) and AI36864 (to J.T.H.) from the National Institutes of Health.
REFERENCES
- 1.Autenrieth I B, Firsching R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 3.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 4.Bockman D E, Cooper M D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 5.Clark M A, Jepson M A, Simmons N L, Booth T A, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 6.Clark M A, Jepson M A, Simmons N L, Hirst B H. Differential surface characteristics of M cells from mouse intestinal Peyer’s and caecal patches. Histochem J. 1994;26:271–280. [PubMed] [Google Scholar]
- 7.Clark M A, Jepson M A, Simmons N L, Hirst B H. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 8.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 9.Etheridge M E, Hoque A T, Sack D A. Pathologic study of a rabbit model for shigellosis. Lab Anim Sci. 1996;46:61–66. [PubMed] [Google Scholar]
- 10.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimura Y, Kihara T, Mine H. Membranous cells as a portal of Yersinia pseudotuberculosis entry into rabbit ileum. J Clin Electron Microsc. 1992;25:35–45. [Google Scholar]
- 12.Fujimura Y. Functional morphology of microfold cells (M cells) in Peyer’s patches—phagocytosis and transport of BCG by M cells into rabbit Peyer’s patches. Gastroenterol Jpn. 1986;21:325–335. [PubMed] [Google Scholar]
- 13.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 14.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 15.Gebert A, Rothkotter H J, Pabst R. M cells in Peyer’s patches of the intestine. Int Rev Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- 16.Grutzkau A, Hanski C, Naumann M. Comparative study of histopathological alterations during intestinal infection of mice with pathogenic and non-pathogenic strains of Yersinia enterocolitica serotype O:8. Virchows Archiv A Pathol Anat Histopathol. 1993;423:97–103. doi: 10.1007/BF01606583. [DOI] [PubMed] [Google Scholar]
- 17.Heel K A, McCauley R D, Papadimitriou J M, Hall J C. Review: Peyer’s patches. J Gastroenterol Hepatol. 1997;12:122–136. doi: 10.1111/j.1440-1746.1997.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Hess C B, Niesel D W, Cho Y J, Klimpel G R. Bacterial invasion of fibroblasts induces interferon production. J Immunol. 1987;138:3949–3953. [PubMed] [Google Scholar]
- 19.Hess C B, Niesel D W, Holmgren J, Jonson G, Klimpel G R. Interferon production by Shigella flexneri-infected fibroblasts depends upon intracellular bacterial metabolism. Infect Immun. 1990;58:399–405. doi: 10.1128/iai.58.2.399-405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones B, Pascopella L, Falkow S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr Opin Immunol. 1995;7:474–478. doi: 10.1016/0952-7915(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 21.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapasi K, Inman R D. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992;148:3554–3559. [PubMed] [Google Scholar]
- 24.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald T T, Carter P B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marco A J, Altimira J, Prats N, Lopez S, Dominguez L, Domingo M, Briones V. Penetration of Listeria monocytogenes in mice infected by the oral route. Microb Pathog. 1997;23:255–263. doi: 10.1006/mpat.1997.0144. [DOI] [PubMed] [Google Scholar]
- 27.Marco A J, Prats N, Ramos J A, Briones V, Blanco M, Dominguez L, Domingo M. A microbiological, histopathological and immunohistological study of the intragastric inoculation of Listeria monocytogenes in mice. J Comp Pathol. 1992;107:1–9. doi: 10.1016/0021-9975(92)90090-h. [DOI] [PubMed] [Google Scholar]
- 28.Marra A, Isberg R R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurelli A T, Baudry B, d’Hauteville H, Hale T L, Sansonetti P J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurelli A T, Blackmon B, Curtiss R., III Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momotani E, Whipple D L, Thiermann A B, Cheville N F. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer’s patches in calves. Vet Pathol. 1988;25:131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 32.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neutra M R, Kraehenbuhl J P. Transepithelial transport and mucosal defense. I. The role of M cells. Trends Cell Biol. 1992;2:134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 34.Owen R L. M cells—entryways of opportunity for enteropathogens. J Exp Med. 1994;180:7–9. doi: 10.1084/jem.180.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen R L, Ermak T H. Structural specializations for antigen uptake and processing in the digestive tract. Springer Semin Immunopathol. 1990;12:139–152. doi: 10.1007/BF00197502. [DOI] [PubMed] [Google Scholar]
- 36.Owen R L, Jones A L. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 37.Owen R L, Pierce N F, Apple R T, Cray W J. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer’s patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986;153:1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- 38.Penheiter K L, Mathur N, Giles D, Fahlen T, Jones B D. Non-invasive Salmonella typhimurium mutants are avirulent because of an inability to enter and destroy M cells of ileal Peyer’s patches. Mol Microbiol. 1997;24:697–709. doi: 10.1046/j.1365-2958.1997.3741745.x. [DOI] [PubMed] [Google Scholar]
- 39.Pepe J C, Miller V L. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc Natl Acad Sci USA. 1993;90:6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perdomo O J J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phalipon A, Kaufmann M, Michetti P, Cavaillon J M, Huerre M, Sansonetti P, Kraehenbuhl J P. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phalipon A, Michetti P, Kaufmann M, Cavaillon J M, Huerre M, Kraehenbuhl J P, Sansonetti P J. Protection against invasion of the mouse pulmonary epithelium by a monoclonal IgA directed against Shigella flexneri lipopolysaccharide. Ann N Y Acad Sci. 1994;730:356–358. doi: 10.1111/j.1749-6632.1994.tb44291.x. [DOI] [PubMed] [Google Scholar]
- 43.Pron B, Boumaila C, Jaubert F, Sarnacki S, Monnet J-P, Berche P, Gaillard J-L. Comprehensive study of the intestinal stage of listeriosis in a rat ligated ileal loop system. Infect Immun. 1998;66:747–755. doi: 10.1128/iai.66.2.747-755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabbani G H, Albert M J, Rahman H, Islam M, Mahalanabis D, Kabir I, Alam K, Ansaruzzaman M. Development of an improved animal model of shigellosis in the adult rabbit by colonic infection with Shigella flexneri 2a. Infect Immun. 1995;63:4350–4357. doi: 10.1128/iai.63.11.4350-4357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regoli M, Borghesi C, Bertelli E, Nicoletti C. Uptake of a gram-positive bacterium (Streptococcus pneumoniae R36a) by the M cells of rabbit Peyer’s patches. Anat Anz. 1995;177:119–124. doi: 10.1016/S0940-9602(11)80057-9. [DOI] [PubMed] [Google Scholar]
- 46.Rouquette C, Berche P. The pathogenesis of infection by Listeria monocytogenes. Microbiologia. 1996;12:245–258. [PubMed] [Google Scholar]
- 47.Sansonetti P J, Arondel J, Cantey J R, Prevost M-C, Huerre M. Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Verg L L, Mallett C P, Collins H H, Larsen T, Hammack C, Hale T L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect Immun. 1995;63:1947–1954. doi: 10.1128/iai.63.5.1947-1954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wray C, Sojka W J. Experimental Salmonella typhimurium in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]