Abstract
Background:
Tissue trauma and hemorrhage result in pronounced activation of the innate immune system. Given known crosstalk between inflammation and coagulation, soluble inflammatory mediators could be associated with venous thromboembolisms (VTE) after major trauma.
Objectives:
Identify plasma inflammatory mediators that are independent predictors of VTE risk in trauma patients.
Methods:
We performed a secondary analysis of the Pragmatic Randomized Optimal Platelets and Plasma Ratios (PROPPR) study. Plasma levels of 27 cytokines/chemokines were measured by BioPlex at admission and 2, 4, 6, 12, 24, 48, and 72 hours later. Patients who died from exsanguination or within 24 hours were excluded. Mann-Whitney tests were performed to assess No-VTE and VTE groups at each time point. Multivariable logistic regression was used to determine the adjusted effects of inflammatory mediators on VTE risk.
Results:
86 (15%) of the 575 patients included developed VTE. Interleukins IL-1ra, IL-6, IL-8, IL-10, Eotaxin, granulocyte colony-stimulating factor (G-CSF), interferon-gamma inducible protein (IP-10), monocyte chemoattractant protein (MCP-1), and chemokine ligand 5 (RANTES) were all significantly increased among VTE patients. Multivariable analyses demonstrated that IL-6, IL-8, IP-10, and MCP-1 were independently associated with VTE. Cox proportional hazards modeling identified IL-6, IL-8, and MCP-1 as independent predictors of accelerated VTE development. We identified significant correlations between inflammation and markers of coagulation and endothelial activation.
Conclusions:
Sustained, systemic inflammation is a key driver of VTE risk after major trauma. Therapeutics targeting innate immune activation should be considered for development of future multimodal strategies to augment current VTE prophylaxis.
Keywords: trauma, hypercoagulability, inflammation, venous thromboembolism, thrombosis
Introduction
Traumatic injuries represent a significant cause of global mortality in individuals aged 1-44.1 Patients with combined tissue trauma and hemorrhagic shock (HS) are at highest risk of early, hemorrhage-related deaths, as well as late-stage deaths due to inflammatory and thrombotic complications.2 Venous thromboembolism (VTE) is the third most common cardiovascular disease worldwide and recognized as a leading cause of preventable hospital-associated mortality.3-5 Trauma patients are at notably elevated risk of VTE with reported incidence rates as high as 30%, representing an extremely prevalent complication that is associated with an elevated risk of in-hospital and post-discharge mortality.6-8
Several past studies have demonstrated blood hypercoagulability as a strong risk factor for trauma-related VTE, evidenced by systemic increases in thrombin generation, reduced endogenous anticoagulant factor levels, and elevated whole blood viscoelastic parameters.9-12 In agreement with this, implementation of institutional VTE prophylaxis protocols to improve early and consistent administration of anticoagulants, such as low molecular weight heparin, have reduced the overall incidence of VTE.6 However, recent evidence indicates trauma patients exhibit poor responsiveness to prophylactic anticoagulation and develop VTE in spite of anticoagulant dose escalations.13 These findings indicate that pharmacologic approaches to addressing hypercoagulability represent a significant clinical challenge and highlight a gap in our understanding of underlying mechanisms that contribute to sustained hypercoagulability and thrombus formation after trauma.
There is increasing evidence and appreciation that the coagulation and inflammatory systems are intricately linked, with activation of one system causing activation of the other in a feedback loop termed “thromboinflammation”.14,15 Inflammation triggers activation of coagulation through a number of mechanisms. Leukocytes secrete proteases that degrade endothelial anticoagulant proteins, neutrophil extracellular traps (NETs), procoagulant extracellular vesicles, as well as cytokines and chemokines. In particular, soluble proinflammatory cytokines and chemokines are important mediators of hypercoagulability. Cytokines such as tumor necrosis factor alpha and interleukins have been shown to potently activate ECs, altering their gene transcription and phenotype to support thrombin generation and fibrin deposition along the vessel wall.16,17 In addition, circulating cytokines can induce acute hepatic release of coagulation factors and also activate platelets.18,19 Past studies have linked elevated cytokine levels with VTE risk, particularly interleukin-6 (IL-6) and IL-8, which corroborates clinical data demonstrating an increased incidence of VTE among patients with chronic and acute inflammatory conditions.14,20-22
Tissue trauma, particularly in the presence of HS, elicits a profound systemic inflammatory response, hallmarked by significant increases in circulating inflammatory mediators with distinct temporal patterns.23 While past research has demonstrated an augmented proinflammatory response among trauma and HS patients who develop VTE,24 it remains unexplored whether distinct inflammatory mediators are independent predictors of VTE in this population, or whether inflammation is directly linked with hypercoagulability. The objectives of this study were to 1) determine the association between inflammation and VTE; 2) identify inflammatory mediators that are independent predictors of overall VTE incidence and time to VTE development; and 3) establish the correlation between inflammation and hypercoagulability.
Methods
Study Design
We conducted a secondary analysis of the Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial dataset, which was a multi-center clinical study of trauma patients with the primary objective of determining the efficacy of transfusing platelets, plasma, and red blood cells in a 1:1:1 versus a 1:1:2 ratio. The PROPPR trial was conducted between August 3, 2012 and December 2, 2013, predating the coronavirus pandemic. In addition, pregnant patients were excluded from enrollment in PROPPR, thus these conditions which are known risk factors for VTE were excluded. VTE was collected as a secondary study endpoint and defined as deep vein thrombosis (DVT) and/or pulmonary embolism (PE). DVT was diagnosed through duplex ultrasound and PE was diagnosed by computed tomography of the chest. In this analysis, patients who died of exsanguination or within 24 hours of admission were excluded as these patients do not have the opportunity to develop VTE.
Sample Collection and Analysis
As part of the PROPPR study, citrated whole blood was collected from patients upon admission to the emergency department (time 0 hour) and 2, 4, 6, 12, 24, 48, and 72 hours after hospital admission. Biomarker assessment was conducted as part of secondary analyses to the PROPPR trial and markers of interest were selected a priori through the PROPPR Study Group. Additionally, whole blood was collected from 20 healthy donors (50% male) under an approved human use protocol at the University of Texas Health Science Center at Houston (HSC-MS-09-0314). Plasma was prepared and stored as previously described.25 Plasma cytokine and chemokine levels were measured using the Bio-Plex Pro Human Cytokine 27-plex Assay kit (Bio-Plex, Hercules, CA, USA) utilizing the Bio-Plex 200 system (Bio-Plex, Hercules, CA, USA). Thrombin generation was measured using calibrated automated thrombogram (CAT) (Thrombinoscope, Maastricht, the Netherlands) using platelet poor plasma as previously described. Parameters resulting from this analysis include peak (maximum thrombin concentration produced, nM), the endogenous thrombin potential (area under the curve, nM*time), and rate of thrombin generation (nM/min). Thrombin-antithrombin complex levels were measured by enzyme-linked immunosorbent assay (Abcam, Cambridge, United Kingdom). Markers of endothelial activation, including soluble syndecan-1 (Diaclone, Besancon Cedex, France), thrombomodulin (Abcam, Cambridge, United Kingdom), and endothelial protein C receptor (EPCR; Cloud Clone, Katy, TX, USA) were measured by enzyme-linked immunosorbent assay. All samples were run in duplicate.
Statistical Analysis
All continuous variables are presented as medians with interquartile ranges. Mann-Whitney U tests were performed to assess significance between no VTE and VTE groups for all continuous variables. Chi-square tests were used to assess differences in categorical variables. The mean of healthy donor values was determined for each inflammatory mediator. The fold change relative to healthy donors was established for each patient and each inflammatory mediator using a log2 transformation. Inflammatory mediators identified as being significantly different between groups underwent further analysis in multivariable modeling. To do this, the last available datapoint for each inflammatory mediator was determined; for patients who developed VTE, the last datapoint prior to VTE diagnosis was used. This was done in order to utilize only those values that were collected prior to the VTE diagnoses. Isolating data at this time for each patient who experienced in-hospital VTE best reflects the systemic milieu which preceded the thromboembolic event. For the no VTE group, the 72-hour value or last available prior to death or discharge was used. Patients were stratified into “high” or “low” groups using the median of the last available value for each marker, and defined as being above (“high”) or below (“low”) the median value. Multivariable logistic regression was used to determine the independent association between high versus low levels of inflammatory mediators and VTE incidence, while controlling for other known risk factors for VTE, including patient age, sex and injury severity scores. Given that the PROPPR study examined transfusion algorithms, we also controlled for the total number of units of red blood cells, plasma, and platelets administered within 24 hours of admission. All of these covariates were included as distinct explanatory variables with VTE in the logistic regression model. We performed Cox proportional hazards modeling to determine the independent association between high versus low inflammatory mediators and cause-specific hazard of VTE, controlling for age, sex, and injury severity. Specifically, the outcome in this model was VTE (presence or absence), time elapsed utilized VTE free days as a continuous variable, each cytokine was a categorical explanatory variable in which each patient was assigned a value of 0 or 1 according their status below (0) or above (1) the median last available value of that cytokine, sex was a categorical explanatory variable (male or female), age in years was a continuous explanatory variable, ISS was a continuous explanatory variable, and 24-hour transfusions of red blood cells (units), plasma (units), and platelets (units). Blood product components were all included in the model as distinct covariates. Cumulative incidence plots were generated to demonstrate time to VTE development between high versus low inflammatory mediator groups. Pairwise correlation tests were used to identify significant correlations between last available cytokine/chemokine values and last available thrombin production parameters. Two-sided p values are reported for all analyses and a p value of less than 0.05 was considered significant. Graphs were generated in GraphPad Prism (version 9, Boston, MA, USA) and statistical analyses were performed using Jamovi (version 2.31.21, Sydney, Australia).
Results
Patient Demographics and Outcomes
A total of 680 patients were enrolled in the PROPPR trial, of which 70% arrived to the emergency department in HS.26 Exclusion criteria for this analysis resulted in removal of 105 patients that died due to exsanguination or within 24 hours of admission. Of the remaining 575 patients, 489 did not develop VTE, while 86 did develop VTE (15%) (Table 1). The VTE cohort consists of 38 patients who developed DVT, 37 who developed PE, and 11 patients who developed both DVT and PE. The median time to VTE was 6 (4, 13) days. There were no significant differences in patient demographics, admission vitals, or injury scores between the VTE and no VTE groups. Compared to the no VTE group, VTE patients received significantly more transfusions of red blood cells [8 (5,13) vs. 9 (7,13), respectively; p=0.002] and plasma [5 (2, 9) vs. 7 (4, 12), respectively; p=0.001]. Furthermore, compared to no VTE patients, those who developed VTE had significantly fewer ventilator-free [27 (13, 29) vs 23.5 (12, 27); p<0.01], intensive care unit (ICU)-free [23 (9, 27) vs 16 (5, 23); p<0.001], and hospital-free days [10 (0, 20) vs 0 (0, 8); p<0.001]. Interestingly, we observed that patients who did not develop VTE had a significantly higher incidence of in-hospital mortality compared to VTE patients, which could be due to the presence of traumatic brain injury. We noted a trending increase in the incidence of head abbreviated injury scale (AIS) score ≥ 3 amongst non-VTE patients compared to VTE patients; however, this was not significant (p=0.05) (Table 1). Thus, other possible drivers of overall mortality cannot be excluded.
TABLE 1.
Patient demographics, injury, and outcomes in patients who did and did not develop VTE. Median and (Q1, Q3) are reported. Mann-Whitney or Pearson chi-square tests were performed to determine statistical significance between groups. Asterisk denotes statistical significance.
| No VTE (N=489) |
VTE (N=86) |
p-value | |
|---|---|---|---|
| Age (years) | 33 (24, 49) | 39 (27, 47) | 0.10 |
| Male (n, %) | 396 (81.0%) | 70 (81.4%) | 0.93 |
| Race/Ethnicity | |||
| White (n, %) | 307 (62.6%) | 56 (65.1%) | 0.54 |
| Hispanic/Latino (n, %) | 89 (18.2%) | 16 (18.6%) | 0.93 |
| Admission Vitals | |||
| SBP (mmHg) | 105 (82, 126) | 100 (82, 124) | 0.86 |
| HR (bpm) | 115 (95, 143) | 114 (99, 130) | 0.83 |
| Base Deficit (mmol/L) | −7.6 (−11.8, −3.7) | −8.1 (−13.3, −4.1) | 0.20 |
| Injury Scores | |||
| GCS | 14 (3, 15) | 14 (3, 15) | 0.45 |
| ISS | 25 (16, 35) | 28 (19, 41) | 0.11 |
| Head AIS ≥ 3 (n, %) | 107 (22%) | 11 (13%) | 0.05 |
| Transfusions (24 hours) | |||
| Crystalloid (L) | 6.8 (4, 10.2) | 8.2 (4.4, 10.6) | 0.107 |
| Red Blood cells (units) | 8 (5, 13) | 9 (7, 13) | 0.002* |
| Plasma (units) | 5 (2, 9) | 7 (4, 12) | 0.001* |
| Platelets (units) | 6 (6, 12) | 6 (6, 18) | 0.12 |
| Patient Outcomes | |||
| Ventilator-free days | 27 (13, 29) | 23.5 (12, 27) | <0.01* |
| ICU-free days | 23 (9, 27) | 16 (5, 23) | <0.001* |
| Hospital-free days | 10 (0, 20) | 0 (0, 8) | <0.001* |
| In-hospital Mortality (n, %) | 56 (11.5%) | 3 (3.5%) | 0.03* |
| Deep vein thrombosis | - | 38 (44%) | - |
| Pulmonary embolism | - | 37 (43%) | - |
| Combined DVT and PE | - | 11 (13%) | - |
Inflammatory mediators are significantly elevated among patients who develop VTE
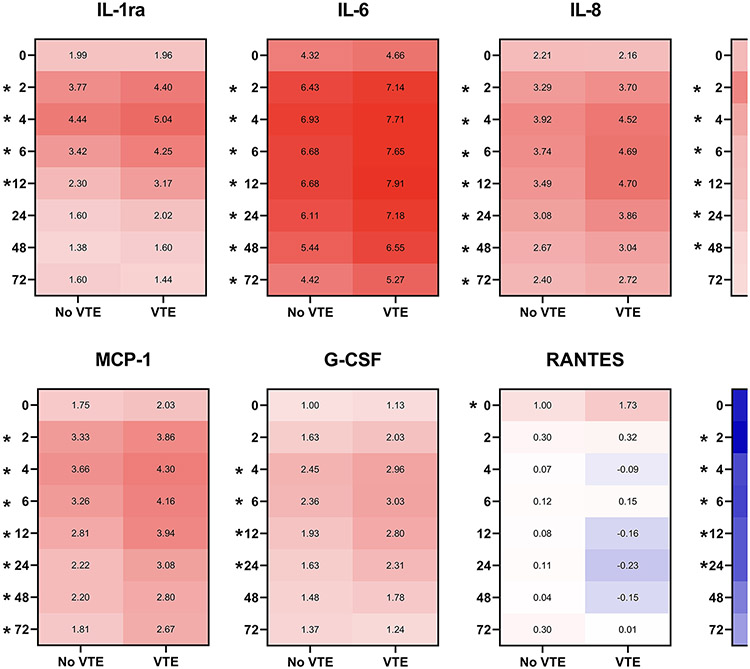
We observed significantly greater changes in plasma inflammatory mediators compared to healthy controls among patients who developed VTE compared to those who did not (Figure 1). In particular, significant elevations in VTE patients compared to non-VTE patients were identified in proinflammatory cytokines IL-6 and IL-8, chemokines monocyte chemoattractant protein-1 (MCP-1), chemokine ligand 5/regulated upon activation normal T cell expressed and presumably secreted (RANTES), interferon-inducible protein 10 (IP-10), the growth factor granulocyte-colony stimulating factor (G-CSF), and anti-inflammatory proteins IL-ra and IL-10 (Figure 1). No differences between groups were observed for other markers included in the 27-plex, such as fibroblast growth factor, eotaxin, granulocyte-macrophage colony stimulating factor, interferon γ, IL-1β, IL-1, IL-4, IL-5, IL-7, IL-9, IL-12, IL-13, IL-15, IL-17A, MIP-1α, MIP-1β, platelet-derived growth factor, TNFα, or vascular endothelial growth factor.
Figure 1.
Changes in plasma inflammatory mediators between no VTE and VTE groups, relative to healthy controls. Plasma cytokines and chemokines were measured using Bio-Plex at 0, 2, 4, 6, 12, 24, 48, and 72 hours from admission. Log2 transformations were performed on all values and data are expressed as median fold change relative to healthy controls. *denotes p<0.05 between No VTE and VTE groups.
Induction of IL-1ra was significantly increased in VTE patients at 2, 4, 6, and 12 hours post-admission. IL-1ra levels peaked similarly between groups, with no VTE patients demonstrating a peak 4.44-fold increase and VTE patients exhibiting a 5.04-fold increase (p<0.05) over healthy controls at 4 hours, following by a decrease over time (Figure 1). We observed consistent and sustained elevations in IL-6, IL-8, IL-10 and MCP-1 were observed among VTE patients, with significant differences observed at all time points after 0 hour (2, 4, 6, 12, 24, 48, and 72 hours post admission). Il-6 and IL-8 levels peaked at 4 hours for patients who did not develop VTE (6.93- and 3.92-fold increases over healthy; respectively), whereas VTE patients continued to increase until 12 hours (7.91-fold and 4.7-fold over healthy, respectively). Conversely, IL-10 and MCP-1 peaked at the same time between groups (2 and 4 hours, respectively). G-CSF levels increased slightly later as significant differences were observed between groups beginning at 4 hours and sustained through 24 hours. Significant differences in RANTES between groups were only identified at 0 hour, after which RANTES levels dropped to below healthy controls over time. In contrast to the other inflammatory mediators measured, IP-10 levels were decreased among patients compared to healthy controls; however, there was significantly less suppression of IP-10 among VTE patients at 2, 4, 6, 12, and 24 hours post admission (Figure 1).
IL-6, IL-8, MCP-1, and IP-10 are Independently Associated with VTE after Trauma
To identify inflammatory mediators that are predictive of a future VTE event, the last available values were identified for those inflammatory mediators that were significantly different between groups, as described above. Data were compared between no VTE and VTE groups (Table 2). Using Mann-Whitney U tests, we found that patients who developed VTE exhibited significantly increased plasma levels of IL-6 [128.25 (36.44, 195.07) vs. 72.815 (34.075, 187.08); p<0.001], IL-8 [42.02 (19.37, 58.81) vs. 29.64 (18.80, 60.01); p=0.007], IP-10 [538.4 (241.81, 847.27) vs. 427.825 (226.078, 825.998); p=0.018], MCP-1 [189.72 (59.14, 268.76) vs. 105.325 (55.453, 262.913); p<0.001] compared to patients who did not develop VTE (Table 2). Multivariable logistic regression analyses were performed to identify independent associations between last available IL-6, IL-8, IP-10, and MCP-1 and risk of VTE. When controlling for age, sex, injury severity score, and 24 hour transfusions of red blood cells, plasma, and platelets, we found that elevated plasma IL-6 (Odds Ratio [OR]=2.97, 95% CI 1.68, 5.24; p<0.001), IL-8 (OR=2.06, 95% CI 1.20, 3.56; p=0.009), IP-10 (OR=1.73, 95% CI 1.01, 2.95; p=0.044) and MCP-1 (OR= 3.20, 95% CI 1.80, 5.70; p<0.001) were all independently associated with the risk of VTE (Table 3).
TABLE 2.
Univariate analysis comparing the median last available cytokine values (pg/mL) between no VTE and VTE groups. Asterisk indicates statistical significance.
| Inflammatory Mediator | No VTE | VTE | p-value |
|---|---|---|---|
| IL-1b | 1.76 | 1.485 | 0.055 |
| IL-1ra | 120.465 | 115.995 | 0.138 |
| IL-5 | 2.24 | 1.75 | 0.133 |
| IL-2 | 6.485 | 6.68 | 0.513 |
| IL-6 | 72.815 | 128.25 | <.001* |
| IL-7 | 8.44 | 7.32 | 0.163 |
| IL-8 | 29.64 | 42.02 | 0.007* |
| IL-9 | 10.965 | 12.1 | 0.567 |
| IL-10 | 12.79 | 14.37 | 0.080 |
| IL-12 | 14.865 | 14.56 | 0.939 |
| IL-13 | 5.795 | 4.73 | 0.229 |
| IL-17 | 22.77 | 22.16 | 0.916 |
| Eotaxin | 35.71 | 37.43 | 0.114 |
| FGF basic | 32.16 | 28.99 | 0.715 |
| G-CSF | 292.45 | 277.28 | 0.789 |
| GM-CSF | 36.73 | 44.65 | 0.245 |
| IGNγ | 46.94 | 34.51 | 0.157 |
| IP-10 | 427.825 | 538.4 | 0.018* |
| MCP-1 | 105.325 | 189.72 | <.001* |
| MIP-1a | 3.75 | 3.65 | 0.496 |
| PDGFbb | 141.805 | 127.415 | 0.582 |
| MIP-1b | 43.625 | 50.025 | 0.075 |
| RANTES | 3996.86 | 3126.42 | 0.081 |
| TNFα | 26.98 | 22.16 | 0.383 |
| VEGF | 12.68 | 19.525 | 0.051 |
TABLE 3.
Multiple logistic regression analysis predicting VTE using last available cytokine values (pg/mL), while controlling for age, sex, injury severity score and 24-hour transfusions of red blood cells, plasma, and platelets as covariates. Asterisk indicates statistical significance.
| Inflammatory Mediator |
Odds Ratio | 95% Confidence Interval |
p-value |
|---|---|---|---|
| IL-6 ≥ 82.32 | 2.97 | 1.68, 5.24 | <0.001* |
| IL-8 ≤ 30.94 | 2.06 | 1.20, 3.56 | 0.009* |
| IP-10 ≥ 446.99 | 1.73 | 1.01, 2.95 | 0.044* |
| MCP-1 ≥ 114.81 | 3.20 | 1.80, 5.70 | <0.001* |
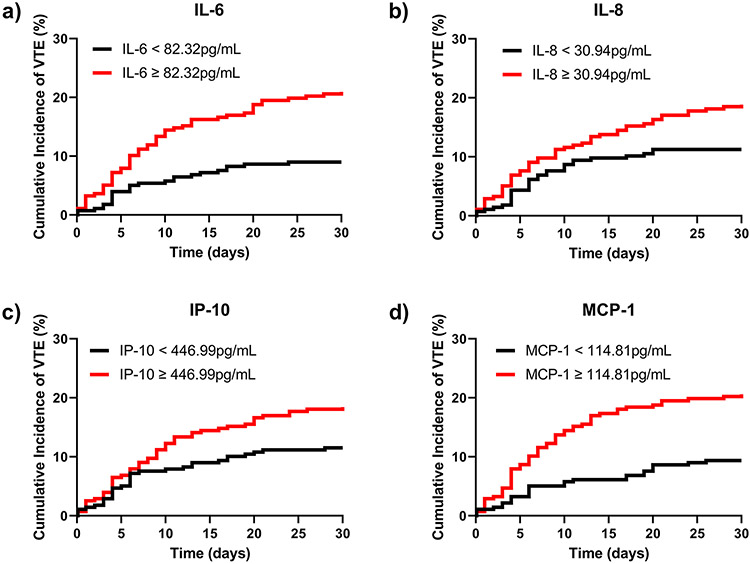
Elevated IL-6, IL-8, and MCP-1 Independently Drive Cumulative VTE Rates Over Time
In order to determine whether IL-6, IL-8, IP-10 and MCP-1 were independently associated with cumulative incidence of VTE over time, we next performed Cox Proportional Hazards Regression analysis, controlling for age, sex, injury severity scores, and 24-hour transfusion units of red blood cells, plasma, and platelets (Table 4). We found that IL-6 (Hazard Ratio [HR]=2.73, 95% CI 1.61, 4.64; p<0.001), IL-8 (HR=1.94, 95% CI 1.18, 3.21; p=0.009), IP-10 (HR=1.67, 95% CI 1.02-2.72; p=0.04) and MCP-1 (HR=2.97, 95% CI 1.75, 5.06; p<0.001) were all significant independent predictors of cumulative VTE rates over time. Cumulative incidence curves were plotted to demonstrate divergence in time to VTE development between groups above versus below the IL-6, IL-8, IP-10 and MCP-1 median last available cut points (Figure 2).
TABLE 4.
Cox proportional hazards model evaluating explanatory variables for VTE. IL-6, IL-8, IP10, and MCP-1 are dichotomized above and below their respective cut points (pg/mL) as defined by median last available value. Age, sex, injury severity score (ISS), and 24-hour transfusions of red blood cells, plasma, and platelets were included as covariates in the model. *Asterisk indicates p<0.05.
| Hazard Ratio |
95% Confidence Interval |
p-value | |
|---|---|---|---|
| IL-6 Association with Time to VTE | |||
| IL-6 (≥ 82.32 vs. < 82.32) | 2.73 | 1.61, 4.64 | <0.001* |
| Age (per 1 year increment) | 1.00 | 0.99, 1.02 | 0.51 |
| Sex (Female vs. Male) | 0.99 | 0.54, 1.81 | 0.96 |
| ISS (per 1 unit increment) | 1.02 | 1.00, 1.03 | 0.07 |
| 24-hour plasma transfused (units) | 1.03 | 0.96, 1.11 | 0.39 |
| 24-hour platelets transfused (units) | 0.98 | 0.94, 1.03 | 0.53 |
| 24-hour RBC transfused (units) | 0.99 | 0.94, 1.04 | 0.65 |
| IL-8 Association with Time to VTE | |||
| IL-8 (≥ 30.94 vs. < 30.94) | 1.94 | 1.18, 3.21 | 0.009* |
| Age (per 1 year increment) | 1.01 | 0.99, 1.02 | 0.45 |
| Gender (Female vs. Male) | 1.02 | 0.56, 1.88 | 0.94 |
| ISS (per 1 unit increment) | 1.02 | 1.00, 1.03 | 0.04* |
| Total plasma transfused (units) | 1.03 | 0.96, 1.10 | 0.46 |
| Total platelets transfused (units) | 0.99 | 0.94, 1.04 | 0.70 |
| Total RBC transfused (units) | 0.99 | 0.94, 1.04 | 0.63 |
| IP-10 Association with Time to VTE | |||
| IP-10 (≥446.99 vs. <446.99) | 1.67 | 1.02, 2.72 | 0.04* |
| Age (per 1 year increment) | 1.01 | 0.99, 1.02 | 0.47 |
| Gender (Female vs. Male) | 0.96 | 0.52, 1.77 | 0.90 |
| ISS (per 1 unit increment) | 1.02 | 1.00, 1.04 | 0.03* |
| Total plasma transfused (units) | 1.03 | 0.96, 1.10 | 0.48 |
| Total platelets transfused (units) | 0.99 | 0.94, 1.04 | 0.70 |
| Total RBC transfused (units) | 0.99 | 0.94, 1.04 | 0.70 |
| MCP-1 Association with Time to VTE | |||
| MCP-1 (≥ 114.81 vs. < 114.81) | 2.97 | 1.75, 5.06 | <0.001* |
| Age (per 1 year increment) | 1.01 | 0.99, 1.02 | 0.42 |
| Gender (Female vs. Male) | 0.92 | 0.50, 1.70 | 0.80 |
| ISS (per 1 unit increment) | 1.01 | 0.99, 1.03 | 0.18 |
| Total plasma transfused (units) | 1.03 | 0.96, 1.10 | 0.42 |
| Total platelets transfused (units) | 0.99 | 0.94, 1.04 | 0.65 |
| Total RBC transfused (units) | 0.98 | 0.93, 1.04 | 0.56 |
Figure 2.
Relationship between elevated plasma inflammatory mediators and cumulative incidence of VTE. The cumulative incidence of VTE over time was plotted to among patients below (black line) and above (red line) the last available cut points for IL-6 (a), IL-8 (b), IP-10 (c), and MCP-1 (d).
Inflammatory Mediators are Differentially Correlated with Thrombin Production and Endothelial Activation.
Last available values for peak, ETP, and rate of thrombin generation, as well as thrombin-antithrombin complex (TAT) and endothelial markers were determined as above. No significant correlations were identified between IL-6, IL-8, IP-10, or MCP-1 with peak, ETP, or rate of thrombin generation as measured by CAT (Table 5, all p>0.05). Weak yet significant positive correlations were found between TAT complex levels and both IL-8 (R value 0.21, p<0.0001) and MCP-1 (R value 0.09, p=0.03) (Table 5).
TABLE 5.
Correlations of last available IL-6, IL-8, IP-10, and MCP-1 with thrombin generation (TG), TEG, and endothelial markers. Asterisk indicates statistical significance. Asterisk indicates statistical significance.
| R value | p-value | |
|---|---|---|
| IL-6 | ||
| Peak TG | −0.02 | 0.64 |
| ETP | −0.01 | 0.78 |
| Rate of TG | −0.01 | 0.94 |
| TAT Complex | 0.05 | 0.23 |
| Syndecan-1 | 0.06 | 0.14 |
| Thrombomodulin | 0.07 | 0.09 |
| EPCR | 0.03 | 0.49 |
| IL-8 | ||
| Peak TG | −0.05 | 0.29 |
| ETP | −0.04 | 0.39 |
| Rate of TG | −0.05 | 0.24 |
| TAT Complex | 0.21 | <0.0001* |
| Syndecan-1 | 0.18 | <0.0001* |
| Thrombomodulin | 0.13 | <0.01* |
| EPCR | 0.15 | <0.01* |
| IP-10 | ||
| Peak TG | −0.05 | 0.23 |
| ETP | −0.03 | 0.52 |
| Rate of TG | −0.04 | 0.34 |
| TAT Complex | 0.04 | 0.33 |
| Syndecan-1 | 0.14 | <0.001* |
| Thrombomodulin | 0.17 | <0.0001* |
| EPCR | 0.14 | <0.01* |
| MCP-1 | ||
| Peak TG | −0.07 | 0.09 |
| ETP | −0.04 | 0.34 |
| Rate of TG | −0.06 | 0.17 |
| TAT Complex | 0.09 | 0.03* |
| Syndecan-1 | 0.29 | <0.0001* |
| Thrombomodulin | 0.18 | <0.0001* |
| EPCR | 0.17 | <0.0001* |
We also observed weak yet significant correlations between inflammatory mediators and markers of EC activation. IL-8 was correlated with syndecan-1 (R value 0.18, p<0.0001), thrombomodulin (R value 0.13, p<0.01), and EPCR (R value 0.15, p<0.01). IP-10 was also weakly correlated with syndecan-1 (R value 0.14, p<0.001), thrombomodulin (R value 0.17, p<0.0001), and EPCR (R value 0.14, p<0.01). MCP-1 was moderately associated with syndecan-1 (R value 0.29, p<0.0001) and weakly associated with both thrombomodulin (R value 0.18, p<0.0001) and EPCR (R value 0.17, p<0.0001). Notably, when we looked only at patients with VTE, the correlation between IL-6 and IL-8 with EPCR became stronger (R value 0.33, p<0.01; R value 0.49, p<0.0001; respectively). Similarly, we observed a stronger correlation between thrombomodulin and MCP-1 when restricting our analysis to VTE patients (R value 0.27, p=0.01).
Discussion
VTE is a common complication in trauma patients that poses a significant risk to patient recover and survival; however, the underlying mechanisms driving thrombus development in trauma patients remain poorly understood. Here, we show that while we observed significant increases in a multitude of inflammatory mediators over time between groups, only cytokines IL-6 and IL-8, as well as chemotactic proteins MCP-1 and IP-10 are independent predictors of time-varying VTE risk after trauma and HS.
These findings are in agreement with those of McCully et al who reported differences in inflammatory cytokine levels among VTE patients in PROPPR.24 We have added to these observations by including healthy donor samples to serve as reference values and multivariable modeling to identify inflammatory mediators that serve as independent predictors of VTE development. Our univariate analysis agreed with the findings of McCully et al24, demonstrating that fold change increases in IL-6, IL-8, and MCP-1 levels over healthy donors were uniquely elevated in VTE patients and all were identified as independent predictors of cumulative VTE incidence using multivariable logistic and Cox proportional hazards modeling. While we similarly observed significant differences in anti-inflammatory markers IL-1ra and IL-10 on univariate analyses, these differences were not retained in multivariable analyses, thereby diminishing a key role of overt dysregulation of anti-inflammatory mechanisms in trauma-related VTE. Using our analytical approach, we further identified IP-10 as a driver of VTE risk in this population, which was generally suppressed compared to healthy donors.
We have further added to our understanding of the link between inflammation and coagulation by partially defining the correlations between inflammatory mediators associated with VTE and markers of hypercoagulability. TAT complex levels are a commonly used surrogate for measuring ongoing thrombin production and hypercoagulability. We observed a weak yet significant positive correlation between inflammation and TAT complex levels. In contrast, we did not observe any significance correlations between inflammatory markers and kinetic thrombin generation as measured by CAT. We speculate that this could indicate inflammatory mediators are activating localized cellular processes that contribute to thrombin production in vivo and subsequent production of TAT complexes, that do not result in substantial increases in circulating procoagulants that remain in the plasma to induce further generation of thrombin upon recalcification.
In agreement with this, we observed several weak, yet significant positive correlations between inflammatory markers and markers of endothelial activation. Correlations were strongest when looking specifically at VTE patients. Interestingly, our group recently reported that EC activation markers are independently associated with trauma-related VTE.27 Combined, these data potentially suggest a scenario in which 1) pro-inflammatory cytokines and chemokines could be activating ECs, inducing a shift from an anticoagulant state to a persistent procoagulant state; and/or 2) the inflammatory mediators are being produced by ECs. IL-6 directly activates ECs through engagement of the IL-6 receptor to induce upregulation of prothrombotic genes, including plasminogen activator inhibitor (PAI-1).28 PAI-1 is the main inhibitor of fibrinolysis and elevated levels are associated with the risk of VTE.29 IL-6 also upregulates EC expression of leukocyte adhesion molecules and recruitment factors, like MCP-1, that could further augment thrombus formation.30 IL-8 has been shown to activate ECs and induce secretion of matrix metalloproteinases (MMPs) involved in barrier disruption31 and is a known trigger of monocyte adhesion to EC monolayers.32 Activated ECs produce a localized prothrombotic response that is largely restricted to the vessel wall where thrombus formation occurs. This could explain why we observed a correlation between inflammation and TAT complex levels, but not thrombin generation parameters by CAT; however, this is speculation.
Alternatively, our data demonstrating a link between inflammation and EC activation could indicate that elevated cytokines and chemokines in VTE patients are coming from ECs themselves. The literature supports an important role of ECs as active players in immune regulation and are considered conditional immune cells.33 ECs potently secrete IL-6 and IL-8 in response to stimulation by other cytokines, namely TNFα and IL-1. Activated ECs also express MCP-1 and IP-10, as well as E-selectin and adhesion molecules that regulate leukocyte recruitment to damaged tissues.34 Secretions of these cytokines and chemokines by ECs would facilitate adhesion of procoagulant neutrophils and monocytes to the vessel wall, where they participate in thrombus formation. Monocytes, a key source of tissue factor (TF), exhibit increased levels of monocyte-derived TF mRNA in patients with VTE.35 They also secrete TF-positive extracellular vesicles that can support thrombin generation and fibrin formation and are increased after trauma.36,37 Neutrophils also play an important role in thrombosis, in part, through their secretion of proteinases that degrade endogenous anticoagulants, like endothelial thrombomodulin and heparan sulfates. Neutrophils also release NETs, which are elevated after trauma and facilitate immunothrombus formation by binding and aggregating platelets, releasing histones that further activate ECs, and provide a scaffold for fibrin formation.14,38 Both IL-6 and IL-8 are well-described neutrophil chemotactic factors. In addition, IL-8 binds to CXCR1 and CXCR2 where it activates the MAPK and PI3K pathways in multiple cell types, resulting in increased TF, PAI-1, and MMP expression.39,40 MMPs play a key role in extracellular matrix remodeling and degradation during thrombus formation and directly upregulate leukocyte and endothelial cell activation at the site of vessel wall injury.41 IP-10 similarly activates the MAPK and PI3K pathways.42
Given the retrospective nature of this work, it is not possible to specify the exact contribution of cell type to post-trauma inflammation. Ultimately, it is very likely that both ECs and leukocytes, as well as other cellular sources, are responsible for elevated inflammatory mediators in this condition. Even platelets are important immune mediators and augment release of pro-inflammatory molecules after trauma.43 Leveraging in vitro and ex vivo model systems to scrutinize cellular sources of inflammation will be an important next step in this project.
Our analysis identified temporal patterns of inflammation that are uniquely sustained in VTE patients, with some cytokines, like IL-6 and IL-8, not reaching peak levels until 12 hours after injury. This highlights a potential screening window during which predictive inflammatory markers could be monitored to stratify risk. It also presents a future therapeutic opportunity in which targeting inflammation could augment current thromboprophylaxis protocols that largely only focus on anticoagulation. Current anticoagulants are not designed to directly inhibit inflammation. Several studies have demonstrated a potential for anti-inflammatory agents, like statins, to improve VTE prevention.44 In addition, other anti-inflammatory agents are currently in development for potential future use, including polyphosphate inhibitors or small molecules that inhibit the interaction between leukocytes and endothelial cells.15 A multi-modal prophylaxis regimen that addresses both coagulation and inflammation could be an important new avenue for mitigating the incidence of VTE after trauma and HS. Such an approach would need to balance addressing excessive inflammation with the beneficial role of immune processes after injury, such as thrombus resolution, wound healing, and clearance of infection. This could include, focusing on a limited therapeutic window (i.e. first 12 hours from injury), specifically targeting individual immune mediators that uniquely contribute to VTE using monoclonal antibody therapy, or development of tissue-specific therapeutics that limit localized inflammatory processes without crippling the systemic host immune and wound repair responses.
Finally, consideration of these cytokines as potential biomarkers merits a careful review of existing clinical approaches to predicting VTE. A number of biomarkers have been identified to screen for the presence of VTE in other hypercoagulable conditions, including cancer, pregnancy, and COVID-19. Thrombin generation (kinetic and prothrombin fragment 1+2), D-dimer, and fibrinogen levels have all be used to screen suspected VTE during pregnancy. Interestingly, a recent cohort study showed that while values in these biomarkers differed between those with and without VTE, none were found to have diagnostic utility for selecting women for imaging based on sensitivity and specificity outcomes.45 Ay et al demonstrated that that the addition of D-dimer and soluble P-selectin, an adhesion molecule expressed by platelets and ECs, augmented risk prediction scoring systems for identifying cancer patients at high and low risk of VTE.46 Similarly, a recent study by Dujardin et al showed that using the combination of D-dimer and the global maker of inflammation, C-reactive protein, was more strongly predictive of VTE among critically ill COVID-19 patients than clinical markers of oxygenation.47 Taken together with our data, these past findings indicate that inclusion of inflammatory markers could improve VTE risk monitoring; however, the specific markers could vary by disease condition. The data from our study could be used in the future to determine which of the four candidate inflammatory markers improves currently available VTE risk prediction tools for trauma patients.48
Several limitations should be considered when interpreting the findings of this study. First, the research is a secondary analysis of the PROPPR trial, which specifically enrolled hemorrhaging patients anticipated to receive a massive transfusion. Therefore, the results may not be generalizable to the broader trauma population. Additionally, we were unable to distinguish patients presenting with pulmonary thrombosis from those with early pulmonary embolism development. Furthermore, variability in DVT screening practices across participating centers also introduced potential sources of bias, a topic which has been explored previously by others.49 It is also worth noting that the original study was not designed to understand risk of VTE and therefore VTE incidence is limited to in-hospital diagnosis and does not include patients who may have developed VTE after discharge. Precise data relating to frequency, timing and dosages of chemical thromboprophylaxis were not collected as part of PROPPR, and therefore we could not examine the associations between inflammation and responsiveness to VTE prophylaxis. Finally, the timing of VTE diagnosis and onset of development are distinct. Our approach utilizing the last available cytokine values for predictive modeling is limited by the VTE diagnosis time and could miss important information concerning PE development that occurred secondary to an undiagnosed DVT.
Conclusions
In conclusion, our study has demonstrated that systemic inflammation underlies the multifactorial pathogenesis of trauma-related VTE and has identified candidate pro-inflammatory mediators that can be used in conjunction with clinical factors and currently available biomarkers to improve risk stratification. In addition, our findings demonstrated that EC activation could be an important mechanism linking inflammation and hypercoagulability in trauma patients at high risk for VTE; however additional mechanistic studies are needed to examine specific cellular contributions to inflammation after trauma. Future studies should examine multi-modal thromboprophylaxis strategies that address both coagulation and inflammation for improving VTE prevention in this population.
Acknowledgments
Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group:
Clinical Coordinating Center: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD; Jeanette Podbielski, RN; Angela M. Beeler, BS.
Data Coordinating Center: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Hongjian Zhu, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; Michael O. Gonzalez, MS.
Core Laboratory: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; David Clark, BS.
Resuscitation Outcomes Consortium: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; Kellie Sheehan, BSN.
Systems Biology Committee: Alan Hubbard, PhD; Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD; Jeanne Callum, MD
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD; Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Christopher N. Miller, MD; Helen Hancock, BS, MT(ASCP); Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research - Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr, DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Jean-Francois Pittet, MD (Anesthesia Chair); Patrick L. Bosarge, MD; Albert T. Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS ; Marisa B. Marques, MD.
Oregon Health and Science University: Martin A. Schreiber, MD ; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD ; Richard M. Scanlan, MD; Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Sciences Centre: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; Connie Colavecchia, BSc, MLT.
Conflicts of Interest and Sources of Funding:
The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863), the U.S. Department of Defense, as well as Defense Research and Development Canada in partnership with the Canadian Institutes for Health Research (CIHR), Institute of Circulatory and Respiratory Health (CRR-120612). KES is supported by an NIH T32 fellowship (5T32GM8792-20).
Footnotes
Disclosures: No additional disclosures.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. [DOI] [PubMed] [Google Scholar]
- 2.Oyeniyi BT, Fox EE, Scerbo M, Tomasek JS, Wade CE, Holcomb JB. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017;48(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregson J, Kaptoge S, Bolton T, et al. Cardiovascular Risk Factors Associated With Venous Thromboembolism. JAMA Cardiol. 2019;4(2):163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day ISCfWT. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931–938. [DOI] [PubMed] [Google Scholar]
- 5.Henke PK, Kahn SR, Pannucci CJ, et al. Call to Action to Prevent Venous Thromboembolism in Hospitalized Patients: A Policy Statement From the American Heart Association. Circulation. 2020;141(24):e914–e931. [DOI] [PubMed] [Google Scholar]
- 6.Teichman AL, Cotton BA, Byrne J, et al. Approaches for optimizing venous thromboembolism prevention in injured patients: Findings from the consensus conference to implement optimal venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2023;94(3):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godat LN, Kobayashi L, Chang DC, Coimbra R. Can we ever stop worrying about venous thromboembolism after trauma? J Trauma Acute Care Surg. 2015;78(3):475–480; discussion 480-471. [DOI] [PubMed] [Google Scholar]
- 8.Drake SA, Holcomb JB, Yang Y, et al. Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate. Ann Surg. 2020;271(2):375–382. [DOI] [PubMed] [Google Scholar]
- 9.Park MS, Spears GM, Bailey KR, et al. Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: A prospective cohort study. J Trauma Acute Care Surg. 2017;83(3):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahbar E, Cotton BA, Wade CE, Cardenas JC. Acquired antithrombin deficiency is a risk factor for venous thromboembolism after major trauma. Thromb Res. 2021;204:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCully BH, Connelly CR, Fair KA, et al. Onset of Coagulation Function Recovery Is Delayed in Severely Injured Trauma Patients with Venous Thromboembolism. J Am Coll Surg. 2017;225(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72(6):1470–1475; discussion 1475-1477. [DOI] [PubMed] [Google Scholar]
- 13.Vincent LE, Talanker MM, Butler DD, et al. Association of Changes in Antithrombin Activity Over Time With Responsiveness to Enoxaparin Prophylaxis and Risk of Trauma-Related Venous Thromboembolism. JAMA Surg. 2022;157(8):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branchford BR, Carpenter SL. The Role of Inflammation in Venous Thromboembolism. Front Pediatr. 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918. [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT, Esmon NL. The link between vascular features and thrombosis. Annu Rev Physiol. 2011;73:503–514. [DOI] [PubMed] [Google Scholar]
- 17.Byrnes JR, Wolberg AS. New findings on venous thrombogenesis. Hamostaseologie. 2017;37(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Gautam V, Naseem S. Acute-phase proteins: As diagnostic tool. J Pharm Bioallied Sci. 2011;3(1):118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590(5):1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. [DOI] [PubMed] [Google Scholar]
- 21.Gao Q, Zhang P, Wang W, et al. The correlation analysis of tumor necrosis factor-alpha-308G/A polymorphism and venous thromboembolism risk: A meta-analysis. Phlebology. 2016;31(9):625–631. [DOI] [PubMed] [Google Scholar]
- 22.Matos MF, Lourenco DM, Orikaza CM, Bajerl JA, Noguti MA, Morelli VM. The role of IL-6, IL-8 and MCP-1 and their promoter polymorphisms IL-6 -174GC, IL-8 -251AT and MCP-1 -2518AG in the risk of venous thromboembolism: a case-control study. Thromb Res. 2011;128(3):216–220. [DOI] [PubMed] [Google Scholar]
- 23.Bonaroti J, Billiar I, Moheimani H, et al. Plasma proteomics reveals early, broad release of chemokine, cytokine, TNF, and interferon mediators following trauma with delayed increases in a subset of chemokines and cytokines in patients that remain critically ill. Front Immunol. 2022;13:1038086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCully BH, Wade CE, Fox EE, et al. Temporal profile of the pro- and anti-inflammatory responses to severe hemorrhage in patients with venous thromboembolism: Findings from the PROPPR trial. J Trauma Acute Care Surg. 2021;90(5):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas JC, Wade CE, Cotton BA, et al. TEG Lysis Shutdown Represents Coagulopathy in Bleeding Trauma Patients: Analysis of the PROPPR Cohort. Shock. 2019;51(3):273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders KE, Holevinski S, Zhang X, Cotton BA, Cardenas JC. Soluble endothelial protein C receptor is an independent predictor of venous thromboembolism after severe injury: Secondary analysis of a prospective cohort study. Surgery. 2023;174(2):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A. 2020;117(36):22351–22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frischmuth T, Hindberg K, Aukrust P, et al. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J Thromb Haemost. 2022;20(7):1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhang Z, Wei R, et al. IL (Interleukin)-6 Contributes to Deep Vein Thrombosis and Is Negatively Regulated by miR-338-5p. Arterioscler Thromb Vasc Biol. 2020;40(2):323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170(6):3369–3376. [DOI] [PubMed] [Google Scholar]
- 32.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398(6729):718–723. [DOI] [PubMed] [Google Scholar]
- 33.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. [DOI] [PubMed] [Google Scholar]
- 35.Basavaraj MG, Braekkan SK, Brodin E, Osterud B, Hansen JB. Monocyte count and procoagulant functions are associated with risk of venous thromboembolism: the Tromso study. J Thromb Haemost. 2011;9(8):1673–1676. [DOI] [PubMed] [Google Scholar]
- 36.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9(11):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park MS, Xue A, Spears GM, et al. Thrombin generation and procoagulant microparticle profiles after acute trauma: A prospective cohort study. J Trauma Acute Care Surg. 2015;79(5):726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu FC, Chuang YH, Tsai YF, Yu HP. Role of neutrophil extracellular traps following injury. Shock. 2014;41(6):491–498. [DOI] [PubMed] [Google Scholar]
- 39.Chan LP, Liu C, Chiang FY, et al. IL-8 promotes inflammatory mediators and stimulates activation of p38 MAPK/ERK-NF-kappaB pathway and reduction of JNK in HNSCC. Oncotarget. 2017;8(34):56375–56388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghuwanshi SK, Su Y, Singh V, Haynes K, Richmond A, Richardson RM. The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions. J Immunol. 2012;189(6):2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T, Li Q, Wang L, Li G. Expression variations and clinical significance of MMP-1, MMP-2 and inflammatory factors in serum of patients with deep venous thrombosis of lower extremity. Exp Ther Med. 2019;17(1):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Mo Y, Jiang S, Shang C, Feng Y, Zeng X. CXC chemokine ligand-10 promotes the accumulation of monocyte-like myeloid-derived suppressor cells by activating p38 MAPK signaling under tumor conditions. Cancer Sci. 2023;114(1):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel S, Bodenstein R, Chen Q, et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J Clin Invest. 2015;125(12):4638–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H, Zheng H, Xu T, et al. Effects of statins in primary and secondary prevention for venous thromboembolism events: A meta analysis. Vascul Pharmacol. 2022;142:106931. [DOI] [PubMed] [Google Scholar]
- 45.Hunt BJ, Parmar K, Horspool K, et al. The DiPEP (Diagnosis of PE in Pregnancy) biomarker study: An observational cohort study augmented with additional cases to determine the diagnostic utility of biomarkers for suspected venous thromboembolism during pregnancy and puerperium. Br J Haematol. 2018;180(5):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. [DOI] [PubMed] [Google Scholar]
- 47.Dujardin RWG, Hilderink BN, Haksteen WE, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res. 2020;196:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park MS, Perkins SE, Spears GM, et al. Risk factors for venous thromboembolism after acute trauma: A population-based case-cohort study. Thromb Res. 2016;144:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers SP, Brown JB, Leeper CM, et al. Early versus late venous thromboembolism: A secondary analysis of data from the PROPPR trial. Surgery. 2019;166(3):416–422. [DOI] [PubMed] [Google Scholar]